Abstract
Purpose
HER2-targeted therapies are associated with cardiotoxicity which is usually asymptomatic and reversible. We report the updated cardiac safety assessment of patients with compromised heart function receiving HER2-targeted therapy for breast cancer, enrolled in the SAFE-HEaRt trial, at a median follow-up of 3.5 years.
Methods
Thirty patients with stage I-IV HER2-positive breast cancer receiving trastuzumab with or without pertuzumab, or ado-trastuzumab emtansine (T-DM1), with asymptomatic LVEF (left ventricular ejection fraction) 40–49%, were started on cardioprotective medications, with the primary endpoint being completion of HER2-targeted therapy without cardiac events (CE) or protocol-defined asymptomatic worsening of LVEF. IRB-approved follow-up assessment included 23 patients.
Results
Median follow-up as of June 2020 is 42 months. The study met its primary endpoint with 27 patients (90%) completing their HER2-targeted therapies without cardiac issues. Of the 23 evaluable patients at long-term f/u, 14 had early stage breast cancer, and 9 had metastatic disease, 8 of whom remained on HER2-targeted therapies. One patient developed symptomatic heart failure with no change in LVEF. There were no cardiac deaths. The mean LVEF improved to 52.1% from 44.9% at study baseline, including patients who remained on HER2-targeted therapy, and those who received prior anthracyclines.
Conclusions
Long-term follow-up of the SAFE-HEaRt study continues to provide safety data of HER2-targeted therapy use in patients with compromised heart function. The late development of cardiac dysfunction is uncommon and continued multi-disciplinary oncologic and cardiac care of patients is vital for improved patient outcomes.
Keywords: HER2-targeted therapy, Breast cancer, Cardiac dysfunction, Cardiac safety, Cardioprotective medications
Introduction
Human epidermal growth factor receptor 2 (HER2)-targeted therapies are associated with cardiotoxicity that is mostly asymptomatic and reversible. While the impact of withholding these therapies on breast cancer outcomes continues to be investigated [1-12], two recent retrospective studies suggest detrimental outcomes with increased risk of breast cancer relapse and death in patients with early stage HER2-positive breast cancer who did not complete adjuvant trastuzumab [13, 14]. Current guidelines limit the use of HER2-targeted therapies to patients with left ventricular ejection fraction (LVEF) within institutional limits of normal and recommend that significant changes in LVEF lead to temporary interruption or treatment discontinuation [15-20]. The SAFE-HEaRt trial (NCT01904903) was the first prospective study to evaluate and report the safety of HER2-targeted agents in patients with breast cancer with reduced LVEF receiving concomitant cardioprotective medications and close cardiac monitoring [21, 22]. We report the follow-up at 3.5 years.
Methods
Thirty-one patients with stage I-IV HER2-positive breast cancer receiving trastuzumab with or without pertuzumab, or ado-trastuzumab emtansine (T-DM1), with asymptomatic LVEF 40–49%, were enrolled in the SAFE-HEaRt trial and started on beta blockers and/or angiotensin-converting enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARBs) as tolerated. Eligible patients could either present with new asymptomatic LVEF decline during therapy or have preexisting LV dysfunction on evaluation prior to initiating therapy. The trial design and the algorithm detailing the cardiac medication titration in the study, as well as the primary aim results have been previously reported [21, 22]. Participants could be on study up to a maximum of 12 months, and were followed for 6 months after last dose of HER2-targeted therapy received while on study. The primary endpoint of the study was completion of HER2-targeted therapy without cardiac events (CE) or protocol-defined asymptomatic worsening of LVEF. CE was defined as symptomatic heart failure (HF); cardiac arrhythmia requiring pharmacological or electrical treatment; myocardial infarction (MI); sudden cardiac death or death due to MI, arrhythmia or HF. Protocol-defined asymptomatic worsening of LVEF was defined as an LVEF drop of more than 10% from baseline and/or an absolute decrease in LVEF to ≤35%. Participants underwent LVEF assessment via cardiac echocardiograms after last dose of HER2-targeted therapy on study—end of treatment (EOT) evaluation—and a final study LVEF assessment was performed 6 months later. After completion of trial participation, patients were allowed to continue on HER2-targeted therapies if clinically appropriate. Institutional Review Board (IRB) approval for long-term follow-up was obtained from the MedStar Health Research Institute (MHRI)-Georgetown University Oncology IRB and the Memorial Sloan Kettering Cancer Center IRB, after protocol amendment. Participants provided written informed consent for long-term follow-up, which involved chart review and telephone communication. There were no pre-defined study visits or imaging studies after trial completion. Off study follow-up cardiac evaluation including clinic visits and echocardiograms were done per routine clinical care. The difference in long-term LVEF between patients who remained on HER2-targeted therapy and those who did not was evaluated using Wilcoxon rank-sum test.
Results
Patients included in the long-term follow-up
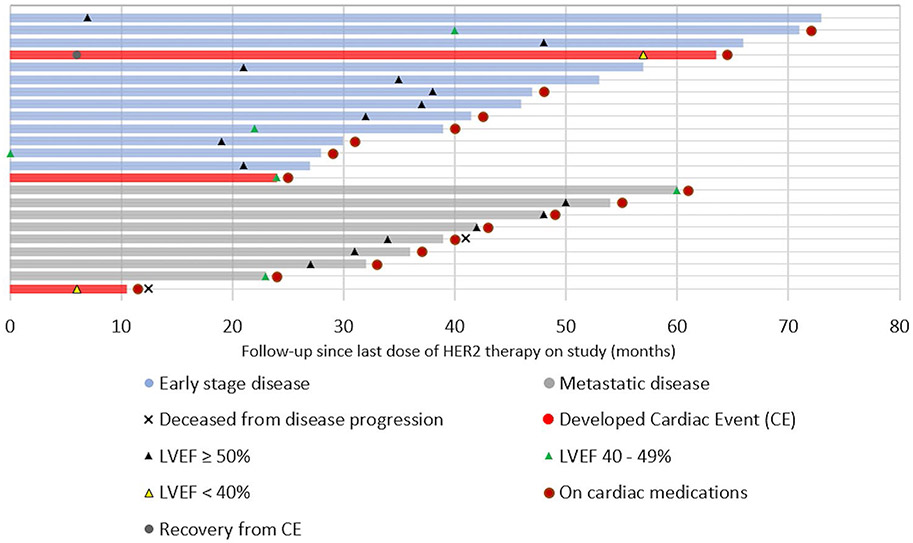
Patients were accrued from October 2013 to December 2017. Of 31 patients enrolled, 30 underwent at least one echocardiogram and were included in the analysis. At the time of consent for long-term follow-up in October 2017, 3 patients had died from disease progression, 2 patients declined consent, and 1 patient was lost to follow-up. Of the remaining 24 consented patients, 1 was lost to follow-up, and 23 were evaluable for long-term follow-up (Fig. 1). Nine (39.1%) had metastatic disease, and 14 (60.9%) had early stage breast cancer. Median overall follow-up from EOT evaluation was 42 months (10–73 months), as of June 2020.
Fig. 1.
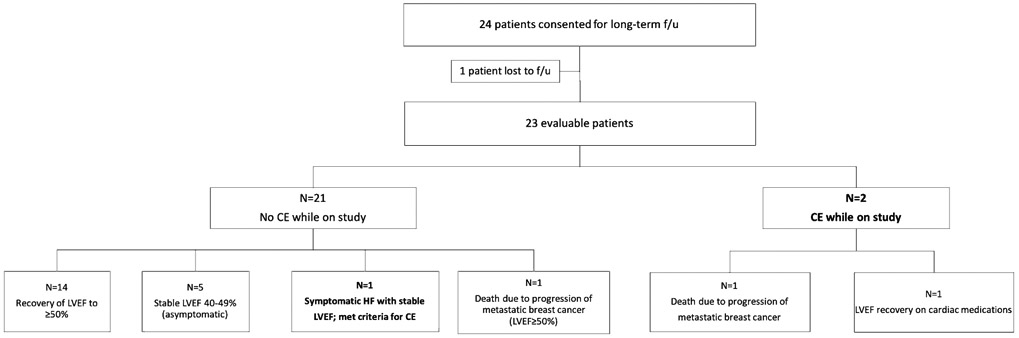
Outcomes of participants in the long-term follow-up of the SAFE-HEaRt trial (f/u: follow-up, CE: cardiac event, LVEF: left ventricular ejection fraction)
Follow-up of patients who experienced a cardiac event while on study
Three of the 30 (10%) patients enrolled on the initial study experienced a CE (n = 2) or protocol-defined asymptomatic worsening of LVEF (n = 1) while on study [22]. Of the two patients who developed CE, one died of progressive breast cancer 16 months after last study treatment, with improvement in her heart failure symptoms (dyspnea and orthopnea). The second patient had early stage disease and experienced LVEF recovery on cardiac medications at 6 months after coming off study, followed by a LVEF asymptomatic decline to 35% after self-discontinuing cardiac medications (beta-blocker and ARB), 58.5 months after the last dose of HER2-targeted therapy. Both patients were included in the long-term follow-up analysis. The patient who had an asymptomatic decline in LVEF to 35% while on study died of progressive breast cancer before the 6-month study follow-up, and was not included in the long-term follow-up analysis.
Cancer treatment and outcomes
After completing treatment on study, 8 out of 9 (88.9%) patients with metastatic disease included in the long-term follow-up remained on HER2-targeted therapies (2 on trastuzumab, 3 on trastuzumab and pertuzumab, and 3 on T-DM1). For these patients, the average duration of treatment with HER2-targeted agents since their last study treatment dose was 42 months (24–62 months). Seven of the 9 patients with metastatic disease included in the long-term follow-up evaluation were alive at the time of this analysis and two had died of progressive disease, at 16 and 39 months after last study treatment respectively. For the 13 patients with early stage disease who completed study treatment, none had disease recurrence at the time of the current analysis, and none received additional HER2-directed therapy. Fourteen of twenty-three (60.8%) patients had previously received systemic treatment with anthracyclines.
Cardiac function outcomes
LVEF data was available from 23 patients, with a median follow-up of 32 months (0–60 months) since last study treatment. The mean LVEF was 44.9% ± 2.6 at study baseline, 45.7% ± 6.3 at EOT, and improved to 52.1% ± 10.0 at long-term follow-up. For the 9 patients with metastatic disease, the mean LVEF improved from 44.9% ± 8.0 at EOT to 51.7% ± 10.7 at long-term follow-up. The LVEF of the 8 patients with metastatic disease who remained on HER2-targeted therapy improved from 47.1% ± 4.9 at EOT to 54.8% ± 6.1 at long-term follow-up. The LVEF of the 14/23 patients who received neo/adjuvant HER2-targeted therapy on study also improved to 52.4% ± 9.9 at long-term follow-up, from 45.2% ± 6.2 at EOT. There was no difference in the LVEF at long-term follow-up between patients who remained on HER2-targeted therapies after study completion and patients who did not (p = 0.436). The LVEF of the 14 patients who received prior treatment with anthracyclines improved to 51.5% ± 11.2 at long-term follow-up, from 43.9% ± 7.7 at EOT. The 9 patients who did not previously receive anthracyclines had improvement in LVEF to 53.0% ± 8.3 at long-term follow-up, from 41.7% ± 16.7 at EOT.
The outcomes of the patients who participated in the long-term follow up of the SAFE-HEaRt trial are summarized in Figs. 1 and 2. Seventy-one percent (15/21) of patients who completed study treatment without any cardiac event experienced recovery of their LVEF to ≥50%, including 6 patients with metastatic disease who continued on HER2-directed agents beyond study participation. Sixty percent (9/15) remained on cardiac medications, 6 of those having metastatic disease. Forty percent (6/15) were taken off cardiac medications, all with early stage disease. Twenty-four percent (5/21) of patients had stable LVEF of 40–49% and remained asymptomatic on cardiac medications, including 2 patients with metastatic disease on HER2-directed therapy. One patient (1/21, 5%) with early stage disease developed clinical symptoms of HF, 15 months after last dose of HER2-directed therapy and after being lost to follow-up. She has since re-established care with cardiology, with no change in her LVEF at 40–45%, and with progressive improvement in her symptoms with cardiac medication optimization. There were no new cardiac deaths at long-term follow-up.
Fig. 2.
Long-term follow-up for SAFE-HEaRt participants (LVEF: left ventricular ejection fraction)
Discussion
The SAFE-HEaRt study was the first prospective trial to provide safety information on the use of HER2-targeted therapies for patients with reduced LVEF and breast cancer [21, 22]. Since then, the results of the SCHOLAR trial, with a similar design, have also been reported confirming our findings in a group of 20 patients [23]. Nine-year follow-up of NSABP-B31, a large randomized phase 3 adjuvant trial, has shown that in patients without underlying cardiac disease at baseline, the addition of trastuzumab to adjuvant chemotherapy did not result in long-term worsening of cardiac function [9]. However, the NSABP-B31 follow-up cohort only included 20% of all patients enrolled in the study, and in that analysis, only two additional cardiac assessments were performed. Thus, that analysis may not be conclusive evidence regarding the long-term safety of trastuzumab, and long-term outcomes of exposure to trastuzumab in patients with already compromised cardiac function have not been reported. In contrast, in a retrospective cohort of almost 10,000 patients at a median follow-up of 5.4 years, trastuzumab was associated with a twofold increased risk of late HF compared to chemotherapy alone, although the absolute risk was low [24].
The long-term follow-up of the SAFE-HEaRt study is the first report of the cardiac safety of HER2-targeted therapy in patients with compromised cardiac function with close cardiac monitoring and use of cardioprotective agents. At 3.5-year follow-up, there was only one new cardiac event, no cardiac deaths, and 89% of patients with metastatic disease who participated on the study and consented for long-term follow-up were able to remain on therapy beyond 1 year. There was an improvement in mean LVEF overall, including in those patients who were maintained on HER2-targeted therapies, and in patients with prior anthracycline use. This observed improvement in LVEF is most consistent with the effect of treatment with carvedilol and/or ACE inhibitors and angiotensin receptor blockers in addition to lifestyle modifications and risk factor control, all of which were incorporated in this trial. Together, our results support the use of HER2-targeted therapies in patients with breast cancer and mildly reduced LVEF using a similar approach of concurrent cardiac therapy to the one used in the SAFE-HEaRt study.
Limitations of our study include the small sample size which significantly limited the number of patients who experienced a CE, and the relatively short period of follow-up, especially for patients with early stage disease where long-term cardiac consequences may have important clinical significance. Yet it is of significant relevance that the late development of cardiac events at 3.5-year follow-up in those with compromised cardiac function is uncommon. Another limitation of this study is selection bias, as LVEF at long-term follow-up was compared between patients who remained on HER2-targeted therapy and those who did not; patients who remain on HER2-directed therapy are more likely to have better cardiac function, or to have metastatic disease. Our study utilized dedicated cardio-oncology clinics which may not be widely available, thus potentially limiting its applicability to smaller community oncology practices. We believe that recent growth in awareness and education about cardio-oncology topics among larger cardiology community creates an opportunity for future large-scale pragmatic trials investigating the feasibility of a collaborative approach in management of patients with LV dysfunction and ongoing HER2-targeted treatment [25, 26].
Our study was the first to uniquely address the need of the small group of patients that may be at the highest risk for adverse oncology and cardiology outcomes, and has been included in recent proposals for comprehensive cardiac safety in oncology patients [27, 28].
In conclusion, continued multi-disciplinary oncologic and cardiac care of patients is essential for improved patient outcomes, given the large clinical benefit provided by HER2-targeted therapies in both early and advanced breast cancer. This long-term follow-up analysis continues to provide data that supports the use of HER2-targeted therapies in patients with breast cancer and mildly reduced LVEF, utilizing a cardiac regimen and monitoring strategy similar to the one used in the SAFE-HEaRt study.
Acknowledgments
Funding This work was supported by Genentech, Inc., a member of the Roche group [ML28685]; the National Cancer Institute at the National Institutes of Health [P30CA051008 to Site P.I., L.M. Weiner]; a Conquer Cancer Foundation of ASCO Young Investigator Award, supported by The Breast Cancer Research Foundation [F.L.]; a Georgetown-Howard Universities Center for Clinical & Translational Science Post-doctoral KL2 Award [A.B.]; and Genentech, Inc. provided study drugs: ado-trastuzumab emtansine (T-DM1), pertuzumab, and trastuzumab in-kind. Any opinions, findings, and conclusions expressed in this work are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology, the Conquer Cancer Foundation, The Breast Cancer Research Foundation, the National Cancer Institute at the National Institutes of Health, or the National Institutes of Health.
Footnotes
Conflict of interest K. Khoury, X. Geng, R. Warren, M. Srichai, M. Hofmeyer, and F. Asch, report no competing interests. F. Lynce reports consulting/advisory role from Bristol-Myers Squibb, Jounce Therapeutics (unpaid), AstraZeneca and Pfizer/EMD Serono, association with the speakers’ bureau for ASCO, travel, accommodations and expenses from Bristol-Myers Squibb and Genentech, has received research funding from Pfizer, Bristol-Myers Squibb and Inivata, and institution-associated research funding from Calithera Biosciences, Immunomedics, Chugai Pharma USA, Regeneron, Inivata, and Tesaro. A. Barac reports consulting/advisory role from Takeda Science Foundation. C. Dang reports consulting/advisory role and travel, accommodations and expenses and has received honoraria from Genentech/Roche, Puma Biotechnology, Lilly, and has received research institution-associated funding from Genentech/Roche and Puma Biotechnology. A. Yu reports consulting/advisory role from AstraZeneca and Genentech for himself and an immediate family member, and research funding from AstraZeneca received for an immediate family member. K. Smith reports stock and ownership interest in Abbvie and Abbott laboratories, and has received institution-associated funding from Pfizer. C. Gallagher reports consulting/advisory role from Seattle Genetics, and association with the speakers’ bureau for Daiichi-Sankyo. P. Pohlmann reports consulting/advisory role from Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, HERON, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, SeaGen and Juniper, association with the speakers’ bureau for Genentech/Roche, patents: United States Patent no. 8,486,413, United States Patent no. 8,501,417, United States Patent no. 9,023,362, United States Patent no. 9,745,377, stock/ownership and leadership in Immunonet BioSciences, received honoraria from ASCO and Dava Oncology, and institution-associated research funding from Genentech/Roche, Fabre-Kramer, Advanced Cancer Therapeutics, Caris Centers of Excellence, Pfizer, Pieris Pharmaceuticals, BOLT, SeaGen, Byondis, and Cascadian Therapeutics. R. Nunes reports consulting/advisory role from Agendia (unpaid), and has received research funding from bioTheranostics. P. Herbolsheimer is an employee of AstraZeneca, and reports travel, accommodations and expenses and stock/ownership from AstraZeneca. M. Tan reports consulting/advisory role from American Gene Technologies, has received honoraria from AstraZeneca, Incyte, Otsuka, and Sanofi Pasteur, travel and accommodations from Otsuka, and research funding from Genentech. C. Isaacs reports consulting/advisory role from Pfizer, Genentech/Roche, Novartis, AstraZeneca, Puma Biotechnology, Context Therapeutics, and Seattle Genetics, association with the speakers’ bureau for Genentech, Pfizer, and AstraZeneca, received honoraria from Genentech/Roche, AstraZeneca, and Pfizer, and institution-associated research funding from Novartis, Pfizer, Genentech, and Tesaro. S. Swain reports consulting/advisory or non promotional speaking role from Exact Sciences (Genomic Health), Genentech/Roche, Daiichi Sankyo, Athenex, Natura, and Silverback Therapeutics, IDMC from AstraZeneca, support for third party writing assistance from Genentech/Roche, and institution-associated research funding from Genentech and Kailos Genetics. The COI’s described above include competing interests of greater than 1 year.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Steingart RM, Yu AF, Dang CT (2019) Trastuzumab cures cancer and disrupts the practice of cardiology*. JACC: CardioOncol 1:11–13. 10.1016/j.jaccao.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzotta M, Krasniqi E, Barchiesi G et al. (2019) Long-term safety and real-world effectiveness of trastuzumab in breast cancer. J Clin Med 8:254. 10.3390/jcm8020254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan-Chiu E, Yothers G, Romond E et al. (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23:7811–7819. DOI:23/31/7811[pii] [DOI] [PubMed] [Google Scholar]
- 4.Perez EA (2008) Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clin Breast Cancer 8(Suppl 3):114. DOI:S1526-8209(11)70932-6[pii] [DOI] [PubMed] [Google Scholar]
- 5.Milano G, Chamorey E, Ferrero JM (2016) Trastuzumab and cardiac outcomes in breast cancer: a story we know by heart? JAMA Oncol 2:21–22. 10.1001/jamaoncol.2015.3866 [DOI] [PubMed] [Google Scholar]
- 6.Chien AJ, Rugo HS (2010) The cardiac safety of trastuzumab in the treatment of breast cancer. Expert Opin Drug Saf 9:335–346. 10.1517/14740331003627441 [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Jeong JH, Rastogi P et al. (2012) Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 30:3792–3799. 10.1200/JCO.2011.40.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seferina SC, de Boer M, Derksen MW et al. (2016) Cardiotoxicity and cardiac monitoring during adjuvant trastuzumab in daily dutch practice: a study of the southeast Netherlands breast cancer consortium. Oncologist 21:555–562. 10.1634/theoncologist.2015-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz PA, Romond EH, Cecchini RS et al. (2017) Long-term follow-up of cardiac function and quality of life for patients in NSABP protocol B-31/NRG oncology: a randomized trial comparing the safety and efficacy of doxorubicin and cyclophosphamide (AC) followed by paclitaxel with AC followed by paclitaxel and trastuzumab in patients with node-positive breast cancer with tumors overexpressing human epidermal growth factor receptor 2. J Clin Oncol 35:3942–3948. 10.1200/JCO.2017.74.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sendur MA, Aksoy S, Altundag K (2013) Cardiotoxicity of novel HER2-targeted therapies. Curr Med Res Opin 29:1015–1024. 10.1185/03007995.2013.807232 [DOI] [PubMed] [Google Scholar]
- 11.Jerusalem G, Lancellotti P, Kim SB (2019) HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat 177:237–250. 10.1007/s10549-019-05303-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lidbrink E, Chmielowska E, Otremba B et al. (2019) A real-world study of cardiac events in >3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat 174:187–196. 10.1007/s10549-018-5058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardesai S, Sukumar J, Kassem M et al. (2020) Clinical impact of interruption in adjuvant Trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardio-Oncology 6:26. 10.1186/s40959-020-00081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushton M, Lima I, Tuna M et al. (2020) Impact of stopping trastuzumab in early breast cancer: a population-based study in Ontario, Canada. J Natl Cancer Inst:djaa054. 10.1093/jnci/djaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barron CC, Alhussein MM, Kaur U et al. (2019) An evaluation of the safety of continuing trastuzumab despite overt left ventricular dysfunction. Curr Oncol 26. 10.3747/co.26.4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu AF, Yadav NU, Eaton AA et al. (2015) Continuous trastuzumab therapy in breast cancer patients with asymptomatic left ventricular dysfunction. Oncologist 20:1105–1110. 10.1634/theoncologist.2015-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barron CC, Tyagi NK, Alhussein MM et al. (2019) Adjuvant trastuzumab therapy: can we balance efficacy and safety? Oncologist 24:1405–1409. 10.1634/theoncologist.2019-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anonymous Herceptin [package insert]. San Francisco, CA: Genentech, Inc., 2015 [Google Scholar]
- 19.Anonymous Kadcyla [package insert]. San Francisco, CA: Genentech, Inc., 2016 [Google Scholar]
- 20.Anonymous Perjeta [package insert]. San Francisco, CA: Genentech, Inc., 2016 [Google Scholar]
- 21.Lynce F, Barac A, Tan MT et al. (2017) SAFE-HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2-positive breast cancer and reduced left ventricular function. Oncologist 22:518–525. 10.1634/theoncologist.2016-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynce F, Barac A, Geng X et al. (2019) Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat 175:595–603. 10.1007/s10549-019-05191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong DP, Cosman T, Alhussein MM et al. (2019) Safety of continuing trastuzumab despite mild cardiotoxicity: a phase I trial. JACC: CardioOncol 1:1–10. 10.1016/j.jaccao.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banke A, Fosbøl EL, Ewertz M et al. (2019) Long-term risk of heart failure in breast cancer patients after adjuvant chemotherapy with or without trastuzumab. JACC Heart Fail 7:217–224 [DOI] [PubMed] [Google Scholar]
- 25.Hayek SS, Ganatra S, Lenneman C et al. (2019) Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week. J Am Coll Cardiol 73:2226–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Cardona JA, Ray J, Carver J et al. (2020) Cardio-oncology education and training: JACC council perspectives. J Am Coll Cardiol 76:2267–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rassaf T, Totzeck M, Backs J et al. (2020) Onco-cardiology: consensus paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin Res Cardiol 109:1197–1222. 10.1007/s00392-020-01636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Fernández T (2020) Cardiac imaging in oncology patients in Europe: a model for advancement of CV safety and development of comprehensive CV care. J Cardiovasc Transl Res 13:490–494. 10.1007/s12265-020-10028-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.