Abstract
Objective:
(1) Assess the feasibility of 13 N-ammonia cardiac PET (13 N-ammonia-PET) imaging in radiotherapy (RT) treatment position in locally-advanced breast cancer (LABC) patients. (2) Correlate pre – /post-RT changes in myocardial flow reserve (MFR) with the corresponding radiation heart dose.
Methods:
Ten left-sided LABC patients undergoing Volumetric Modulated-Arc-Therapy (VMAT) to chest wall and regional lymph nodes underwent a rest/stress 13 N-ammonia-PET at baseline and (median) 13 months post-RT. Changes in cardiac functions and coronary artery Ca2+ scoring between baseline and follow-up were correlated with average RT dose to the myocardium,3 coronary territories, and 17 myocardial segments.
Results:
Eight (of 10) patients successfully completed the study. The average rest (stress) global MBF (ml.g-1.min-1) for baseline (follow-up) were 0.83 ± 0.25 (2.4 ± 0.79) and 0.92 ± 0.30 (2.76 ± 0.71), respectively. Differences in MBF, heart rate, blood pressure, and rate-pressure product (RPP) between baseline and follow-up were insignificant (P > 0.1).Strong (R = 0.79; P < 0.01) and moderate (R = 0.53; P = 0.37) correlation existed between MBF Rest and MBF Stress, and RPP respectively. Four patients showed a reduction in MFR of up to ~41% in follow-up studies, increasing to ~52% in myocardial segments close to high-radiation isodose lines in 5/8 patients. Agatston Ca + 2 scoring were zero in both baseline and follow-up in six patients; two patients exhibited mild increase in Ca + 2 on follow-ups (range:10–20).Rest and stress LVEF's were normal (> 50) for all patients in both studies.
Conclusion:
The feasibility of 13 N-ammonia-PET imaging in treatment position of LABC patients was demonstrated. MFR at 1-year post-irradiation of the heart decreased in 50% of the patients. MFR may be a potential index for early detection of cardiotoxicity in BC patients receiving RT to the chest wall.
Keywords: 13N-Ammonia PET, Radiotherapy, Cardiotoxicity
1. Introduction
Post-mastectomy radiotherapy (PMRT) reduces rates of recurrence and improves BC-specific mortality in locally advanced breast cancer (LABC) patients [1,2]. Previous clinical trials involving PMRT from 1960–70s showed, however, increased risk of premature coronary artery disease (CAD), presumably due to incidental radiation dose delivered to the heart [1,3]. Concerns due to cardiac toxicities induced by anthracycline-containing chemotherapy and anti-HER2 therapy, which has become routinely adopted into systemic regimens for BC, have been also raised.
Recent advances in radiotherapy regimens for BC yielded reduced doses to the myocardium. In most women, the mean heart doses from radiotherapy are currently 1–5 Gy [4-10]. Nevertheless, exposures at these levels could still cause ischemic heart disease [11-13]; the rate of coronary events was shown to increase by 7.4% per each Gy of radiation to the heart [14], with the increase starting within 5 years post-RT and continuing into the third decade [14].
In BC, VMAT may be used to treat BC patients receiving comprehensive nodal irradiation. VMAT provides excellent coverage of all target volumes at the cost of irradiating larger normal tissue volumes, in this case the heart and lungs, with low doses. A population-based Swedish study suggested that even low doses can cause ischemic heart disease [11-14]. Yet, the nature and magnitude of cardiac events induced by low radiation doses are not well understood [14].
Identifying means to determine radiation-induced cardiac toxicity soon after radiotherapy may allow early assessment of heart disease, and hence early intervention. Changes in myocardial functionality post-irradiation of the chest wall may be assessed with myocardial perfusion imaging (MPI). Left-Ventricle Ejection Fraction (LVEF) reduction to abnormal levels has been reported in 13% of 54 left-sided BC patients, 2.7 years post-chemo-radiotherapy [15]. Another prospective study involving pre- and serial post-radiation gated SPECT MPI to monitor heart function changes observed perfusion defects in 50–63% of left-sided BC patients, 6–24 months after radiotherapy [16]. The severity of perfusion defects correlated with the left ventricle (LV) irradiated volume; 10–20% (> 5% LV irradiated) versus 50–60% (< 5% LV irradiated) [17]. Wall motion defects (particularly anterior LV, i.e. heart region within radiation arc) also correlated with perfusion abnormalities [17].
PET MPI allows absolute quantitation of MBF, thus MFR, a key index for predicting adverse outcome in cardiomyopathies and CAD [18-21]. Cardiac events were shown to be more prominent in subjects with reduced MFR, regardless of myocardial perfusion findings [22]. MFR also allowed predict cardiovascular events independently of relative regional perfusion defects and improve risk stratification in patients assessed for myocardial ischemia [22].
This proof of principle study sought to assess; (1) the feasibility of performing serial rest/stress 13N-ammonia cardiac PET imaging in PMRT BC patients in-line with radiotherapy planning simulation, and (2) whether changes myocardial perfusion indices, especially MBF and MFR, may be detected as early as one-year post-radiation, and whether those correlate with heart radiation dose.
2. Methods & materials
2.1. Patients and treatments
Ten women with locally advanced BC receiving PMRT were prospectively enrolled into this IRB-approved protocol. All subjects received VMAT to the left chest wall and regional lymph nodes including the internal mammary nodes (IMN). Exclusion criteria included: age > 60 years, history of smoking, diabetes mellitus, hyperlipidemia, hypertension, obesity, and personal history of cardiac illness. Subjects received chemotherapy (5 neoadjuvant, 5 adjuvant) prior to RT. Chemotherapy regimens consisted of taxane, dose-dense adriamycin, and cyclophosphamide (TAC). Three of ten patients had HER2-positive disease and received concurrent trastuzumab, administered q3 weeks throughout radiation over a one-year period. Eight patients had immediate reconstruction with a tissue expander and 2 had permanent implant prior to RT. Radiotherapy was initiated ~4–9 weeks post-mastectomy. Each patient underwent 13N-ammonia Rest/Stress PET study at day of RT simulation (baseline) and ~1 year post-RT (follow-up).
2.2. Radiotherapy simulation and treatment planning
Treatment planning simulation was conducted on a GE Discovery ST PET/CT scanner (GEHC, Waukesha, WI). Patients laid supine and were immobilized with both arms up in a customized alpha cradle for CT simulation (CT-Sim) and treatment. Alignment tattoos were marked on patients to facilitate positioning for the cardiac-PET studies and radiation treatment. Deep-inspiration breath-hold (DIBH) techniques were utilized in 6 of 10 patients in order to displace the heart away from the chest wall (DIBH for remaining 4 patients did not show pronounced chest-wall displacement). Treatment plans were generated in the Eclipse v13.6 (Varian Medical Systems, Palo Alto, CA) treatment planning system using VMAT. The Planning Target Volume (PTV) included the chest wall, IMN, supraclavicular, and axillary lymph nodes. The treatment plan typically consisted of 4 arcs: 2 anterior, covering the medial PTV, and 2 lateral-posterior, avoiding entry through the arm and shoulder. The plans were normalized with 95% of the prescription dose being delivered to 95% of the PTV and dose inhomogeneity D05% < 115% of the prescription dose. The prescribed dose was 50 Gy in 25 fractions. Departmental guidelines on dose parameters for breast IMRT were followed. Treatment was delivered on a TrueBeam LINAC (Varian Medical Systems, Palo Alto, CA).
2.3. 13N-ammonia cardiac PET/CT imaging
2.3.1. Data acquisition
Upon RT-simulation completion, patients underwent a 13N-ammonia PET Rest/Stress MPI on a GE Discovery 690 PET/CT under supervision of a certified nuclear cardiologist. Patients were placed in treatment position using the immobilization hardware that was prepared during RT-simulation. The cardiac PET/CT study commenced with a prospectively-gated CT-scan of the chest for Ca2+ scoring (120 kV, 1.25 mm slice, 36 cm FOV, 512 × 512 matrix, and 250–450 mA optimized for patient size), followed by low-dose CT (10 mA, 120 kVp) for PET attenuation correction. The resting 13N-ammonia PET was then acquired in dynamic-mode (15x6sec, 6x15sec, 3x180sec), and started simultaneously with intravenous bolus injection of ~296 MBq of 13N-ammonia. Then, stress 13N-ammonia PET started simultaneously with intravenous injection of ~444 MBq of 13N-Ammonia, 30–60 s post-intravenous bolus injection of 0.4 mg/5 ml regadenoson (Astellas, US) followed by a 5 ml saline flush. The same rest image acquisition protocol was followed. The stress study was initiated 45 min post-injecting the rest dose. Heart rate (HR), blood pressure (BP), and 12-lead ECG were recorded continuously throughout the study. Patients underwent follow-up 13N-ammonia scans at a median of 13 months (range 10–17 months) post-RT.
2.3.2. Image processing
Three PET image sets were reconstructed by retrospective replay of ListMode data; 1) static, for myocardial perfusion assessment, 2) Gated (16 bins), for left ventricular ejection fraction (LVEF), and 3) dynamic, for MBF quantification. The first 2 min of data were discarded in the case of static and gated datasets to allow for clearance of activity from left ventricular blood-pool.
2.3.3. Image analysis
Ca+2 scoring assessment was performed with the SmartScore software on the GE Advantage Workstation v4.2 (GEHC, Waukesha, WI), using the Agatston model.
MPI visual assessment, as well as left-ventricular wall motion and thickening, and LVEF measurements were carried out using the 4DM software tool (Invia, Michigan, USA) by consensus of a nuclear medicine physician and a cardiologist.
2.3.4. Myocardial flow reserve
MBF was estimated using one-tissue, two-compartment model [23] by means of PMOD PCard module (PMOD v3.6). The plasma input function time-activity curve was derived from dynamic PET images as the average activity concentration within a region of interest placed within the left ventricular cavity, Clv(t), and with metabolite correction factor mCorr:
A value of mCorr = 0.077 (min−1) has been adopted [23]. The activity concentration in the myocardial, Cmyo, is described by
where K1 (surrogate of MBF) and k2 are the kinetic rate constants (min−1) between the two compartments. The model curve incorporates a cardiac dual spillover correction, given by
where the spill-over fractions are for blood activity in the left (VLV), and right (VRV) ventricles, CLV(t) and CRV(t) respectively. MFR was defined as
Rest MBF was normalized to the corresponding RPP, and multiplied by 10,000 [24] to account for blood-pressure and heart-rate changes between baseline and follow-up scans, with RPP defined as;
The myocardium in the static stress PET images was registered to that in the rest ones. The resulting registration matrix was then applied to the stress dynamic PET data, thus registering it to the rest dynamic PET. The myocardium in the rest images was then re-oriented in standard cardiac orientation and the resulting transformation matrix was applied to the stress dataset. The myocardium in the rest series was segmented in two steps; (1) epicardium (Vepi), and (2) endocardium (Vend), using the PMOD-PCARD's “hot” and “cold” region growing tools, respectively. Two additional volume-of-interests (VOIs) were drawn over LV (VLV) and RV (VRV) respectively, then copied to the stress study. In each case, the myocardium VOI, defined by the intersection of Vepi and Vend, was divided according to the 17 segments cardiac model. MBF and MFR were estimated for the whole myocardium, the three major territories (LAD, RCA, and LCX), and each of the 17 segments.
2.4. MFR-RT dose correlation
The myocardium in the CT-Sim images was registered to that in the static rest PET images. The corresponding transformation matrix was applied to the RT dose-map. The cardiac re-orientation matrix, previously determined for the rest study, was applied to RT dose-map, and VOIs (Vmyo, VLAD, VRCA, VLCX) copied to the re-oriented dose-map. The mean doses to Vmyo, the three territories, and the 17 myocardial segments were determined and correlated with the corresponding MFR.
2.5. Statistical analysis
Mean values are given with standard deviations. Changes in hemodynamics and MBF, between baseline and follow-up were compared using the Student's paired t-test. Correlations between the IMRT dose and the changes in, MBF (for the rest and stress respectively) and MFR, between the baseline and follow up studies, for each of the three coronary arteries respectively were assessed by means of least-squares regression. The corresponding correlations were also determined for the myocardial segments for each of the three coronary arteries. A P-value < 0.05 was considered significant.
3. Results
Eight patients successfully completed the pre- and post- (at average of 404 ± 79 days) RT 13N-ammonia PET studies.
3.1. Hemodynamic findings
The mean differences in the hemodynamics between baseline and follow-up studies were; rest HR (3.12 +/− 9.76), rest systolic BP (−6.62 ± 20.13), rest diastolic BP (−2.12 ± 10.34), rest rate-pressure product (−36.12 ± 1459.15), stress HR (0.5 ± 13.74), stress systolic BP (2.87 ± 26.44), stress diastolic BP (1.62 ± 13.77), stress rate-pressure product (341.75 ± 3489.22). None of the differences in the aforementioned was statistically significant (P > 0.3). The hemodynamic measurements obtained at baseline and follow-up are summarized in Table 1.
Table 1.
Summary of HR, BP, and RPP, for baseline and follow-up scans for each of the eight patients analyzed, and baseline-follow-up time. “1” = baseline and “2” = follow-up
| Patient # | Dt (days) | RHR1 | RBP1 (S/D) | RRPP1 | SHR1 | SBP1 (S/D) | SRPP1 | RHR2 | RBP2 (S/D) | RRPP2 | SHR2 | SBP2 (S/D) | SRPP2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 446 | 60 | 102 66 | 6120 | 123 | 121 74 | 14,883 | 63 | 109 75 | 6867 | 116 | 130 70 | 15,080 |
| 2 | 450 | 60 | 85 64 | 5100 | 93 | 88 58 | 8184 | 54 | 95 61 | 5130 | 85 | 76 48 | 6460 |
| 3 | 469 | 74 | 98 62 | 7252 | 116 | 104 64 | 12,064 | 57 | 122 68 | 6954 | 96 | 91 62 | 8736 |
| 4 | 525 | 71 | 97 60 | 6887 | 123 | 94 60 | 11,562 | 62 | 110 75 | 6820 | 150 | 107 72 | 16,050 |
| 5 | 308 | 58 | 93 61 | 5394 | 102 | 108 66 | 11,016 | 71 | 104 68 | 7384 | 103 | 110 70 | 11,330 |
| 6 | 364 | 62 | 122 93 | 7564 | 103 | 129 73 | 13,287 | 57 | 121 75 | 6897 | 99 | 142 78 | 14,058 |
| 7 | 322 | 94 | 163 93 | 15,322 | 123 | 172 96 | 21,156 | 100 | 125 88 | 12,500 | 131 | 112 66 | 14,672 |
| 8 | 350 | 98 | 100 69 | 9800 | 126 | 93 60 | 11,718 | 88 | 127 75 | 11,176 | 125 | 118 72 | 14,750 |
RHR: rest heart rate (bpm). SHR: stress heart rate (bpm). RPP: rate-pressure product (bpm × mmHg). RBP (S/D): rest blood pressure (mmHg) (systolic/diastolic). SBP (S/D): Stress blood pressure (mmHg) (systolic/diastolic). (1) and (2): baseline and follow up respectively.
3.2. Calcium scoring
One patient had no calcium scoring. The remaining seven had minimal or undetectable Ca+2 on both baseline and follow-up scans (range 0–14) (Table 2).
Table 2.
Calcium scores, MPI, and hemodynamics results for baseline and follow-up scans for each patients analyzed. “1” = baseline and “2” = follow-up.
| Patient# | CA1 | CA2 | MPI1 | MPI2 | LVEF1 | LVEF2 | SMBF1 | RMBF1 | SMBF2 | RMBF2 | MFR 1 | MFR 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | NL | NL | > 60 | > 60 | 2.97 | 0.76 | 3.23 | 0.85 | 3.89 | 3.79 |
| 2 | 0 | 0 | NL | ABNL | > 60 | > 60 | 0.804 | 0.36 | 1.40 | 0.60 | 2.22 | 2.35 |
| 3 | 0 | 1 | NL | NL | > 60 | > 60 | 2.646 | 0.71 | 2.94 | 0.77 | 3.75 | 3.83 |
| 4 | 0 | 0 | NL | ABNL | 52/56 | 50/55 | 2.654 | 0.92 | 1.97 | 0.83 | 2.88 | 2.37 |
| 5 | 4 | 14 | NL | NL | > 60 | > 60 | 2.482 | 0.71 | 3.17 | 0.72 | 3.52 | 4.41 |
| 6 | 3 | 12 | NL | NL | > 60 | > 60 | 3.056 | 1.01 | 2.73 | 0.98 | 3.04 | 2.77 |
| 7 | NA | NA | NL | NL | > 60 | > 60 | 1.762 | 0.99 | 3.19 | 1.04 | 1.78 | 3.07 |
| 8 | 1 | 1 | NL | ABNL | > 60 | > 60 | 3.13 | 1.17 | 3.45 | 1.57 | 2.66 | 2.20 |
CA: calcium score. MPI: myocardium perfusion imaging visual assessment. (1) and (2): baseline and follow up respectively. LVEF: left ventricle ejection fraction. RMBF: rest myocardial blood flow. SMBF: stress myocardial blood flow. MFR: myocardium flow reserve. NL: normal. ABNL: abnormal.
3.3. Myocardial perfusion imaging
All patients presented with normal perfusion images at baseline. New, reversible perfusion defects were identified in 3 patients on follow-up scans, including a moderate antero-apical defect (patient #2), a mild anterior wall defect (patient #4), and a mild lateral wall defect (patient #8).
3.4. Myocardial blood flow
Individual rest/stress MBF measurements are summarized in Table 2. The average global rest MBFs were 0.83 ± 0.25 ml.g−1 min−1 (range:0.36–1.17 ml.g−1 min−1) and 0.92 ± 0.30 ml.g−1 min−1 (range: 0.60–1.57 ml.g−1 min−1) for the baseline and follow-up respectively. The respective stress global MBF values were 2.4 ± 0.79 ml.g−1 min−1 (range: 0.80–3.13 ml.g−1 min−1) and 2.76 ± 0.71 ml.g−1 min−1 (range: 1.40–3.45 ml.g−1 min−1). Differences in MBF between baseline and follow-up were insignificant for both the rest (P = 0.146) and stress (P = 0.198). The insignificant difference in rest MBF persisted after normalizing it by RPP (P = 0.392). On the other hand, a strong (R = 0.79) and statistically significant correlation (P < 0.01) was observed between rest MBF and RPP, while a moderate insignificant (R = 0.53, P = 0.37) correlation was observed between the stress MBF and RPP (Fig. 1). Furthermore, insignificant correlation existed between the Rest (Stress) MBF and the radiotherapy dose for all the coronary arteries.
Fig. 1.
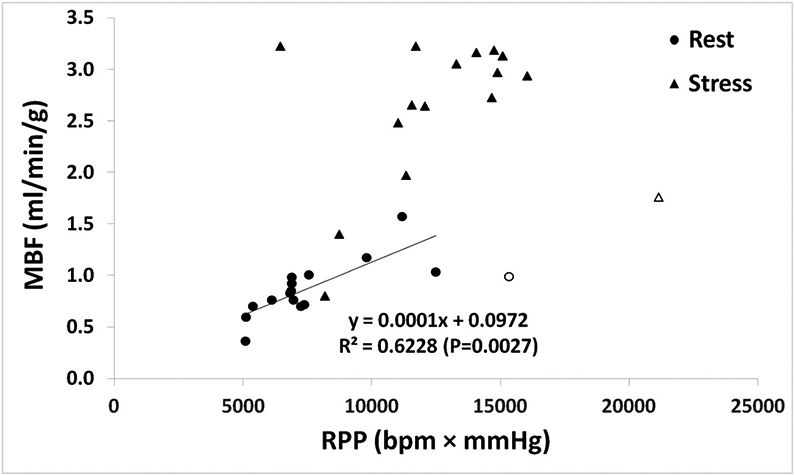
Relationship between MBF and RPP for rest (circle) and stress (triangle) data. MBF: Myocardial Blood Flow; RPP: Heart Rate-Blood Pressure Product.
3.5. Myocardial flow reserve
The individual rest and stress MFR measurements are summarized in Table 2. All patients exhibited a normal MFR at baseline (MFR > 2.5) except for patient#2 and patient#7 who had intermediate (MFR = 2.22) and low (MFR = 1.80) values respectively. The average global MFR's were 2.97 ± 0.74 ml.g−1 min−1 (range: 1.78–3.89 ml.g−1 min−1) and 3.10 ± 0.82 ml.g−1 min−1 (range: 2.20–4.41 ml.g−1 min−1) for the baseline and follow-up respectively. Insignificant difference between baseline and follow-up MFRs existed (P = 0.58). The average percent change in global MFR between baseline and follow-up was 7.34 ± 29.84% (range: −17.61-72.77%). The average percent changes in MFR for the LAD, RCA, and LCX, were 5.97% ± 27.90%, 7.96% ± 43.25%, and 90.05% ± 35.84% respectively. Among the 17 myocardial segments, the apical region (including apex, septal, anterior, lateral, inferior) exhibited the largest MFR reduction between baseline and follow-up. The largest reduction in MFR corresponded to the apex (average: −13.57% ± 22.50%; range: −50.87%–17.16%). However, the basal segments showed an increase in MFR between baseline and follow-up, with the maximum corresponding to the basal anteroseptal segment (average: 20.46% ± 52.63%). Fig. 2 summarizes the average percent differences for the different myocardial segments.
Fig. 2.
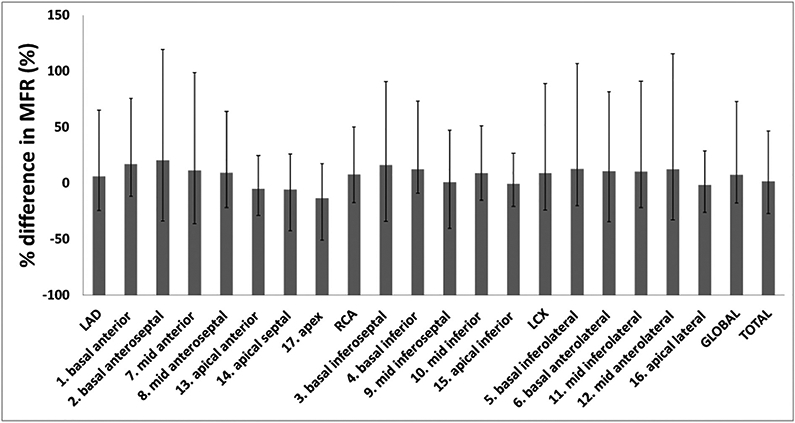
Average percent difference in MFR between the follow-up and baseline 13N-PET studies for the three major territories and 17 myocardial segments. Error bars refer to maximum and minimum corresponding values within the cohort. MFR was calculated after normalizing rest and stress MBF to the corresponding RPP. MFR: Myocardial Flow Rate; MBF: Myocardial Blood Flow; RPP: Heart Rate-Blood Pressure Product.
3.6. Correlation between MFR and myocardial radiation dose
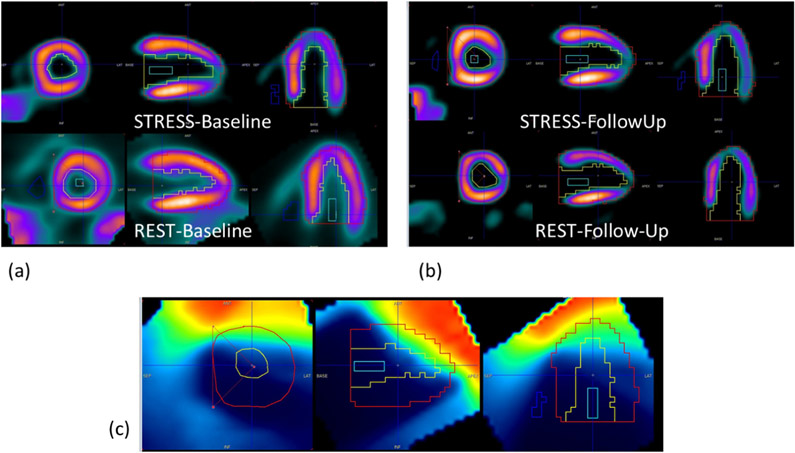
Fig. 3 shows an example of orthogonal views of (a) CT-Sim images fused with 13N-ammonia rest static PET images, and (b) corresponding RT dose-map. Fig. 4 shows representative images of stress and rest VOIs at baseline (a) and follow-up (b), that were used for MFR estimations and to calculate the average dose delivered to each myocardial segment (and the three major coronary arteries).
Fig. 3.
Representative (patient#8) orthogonal views of the (a) CT-Simulation, and (b) corresponding RT dose-map, each fused with the rest 13 N-Ammonia static PET images. Dose spill-in into the myocardium, particularly into the apex and anterior wall.
Fig. 4.
Stress and rest 13N-Ammonia PET images at (a) baseline and (b) 350 days post- radiotherapy. The left ventricular myocardial VOI is overlaid onto (c) the RT dose map, re-oriented into cardiac position. The mean dose delivered by radiotherapy was measured for the whole myocardium, the three coronary territories, as well as for each of the 17 segments.
Dose spill into the myocardium, particularly into the anterior wall and apex, was identified for all patients. The maximum dose was depicted in the apical anterior segment (average 2.09 cGy; range 0.96–3.23 cGy) (Fig. 5). An example of the RT dose overlap with the myocardium is tabulated in Fig. 4; radiation doses between ~2 Gy (green isodose) and ~ 4Gy (red isodose) overlapped with the apex and anterior wall.
Fig. 5.

Average of the 8 patient mean radiation doses to each of the three major cardiac territories and for each of the 17 myocardial segments. Error bars refer to maximum and minimum doses observed within the patient cohort.
The average radiation dose (across study patients) delivered by radiotherapy to the LAD, RCA, and LCX, each of the 17 segments, and, the global and total doses to the myocardium are summarized in Fig. 5. As expected, the apex and anterior wall - which are closest to the chest wall, and thus to radiotherapy radiation arcs - received the highest doses. The corresponding MFR percent changes are tabulated in Fig. 2.
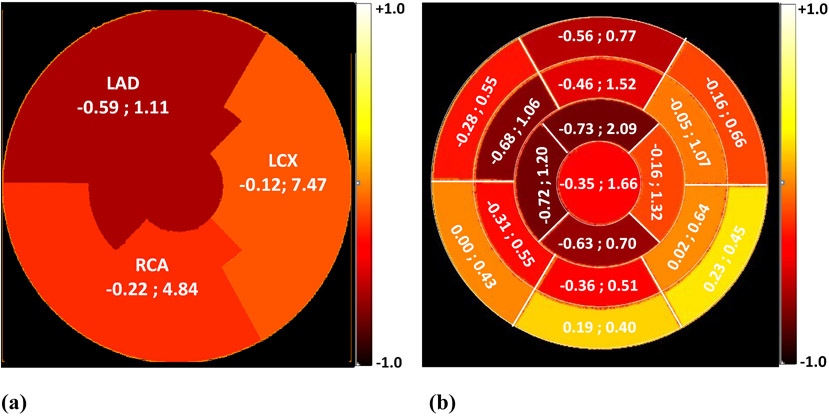
A negative, weak (R2 < 0.5), and insignificant (P > 0.05) correlation existed between the IMRT dose and the percent changes in MFR (averaged across the 8 patients) for the RCA and LCX respectively. Similar, yet moderate correlation (R2 = 0.34), was however observed in the case of LAD. The corresponding Pearson correlations and average radiation dose for the three coronary arteries as well as for each of the 17 myocardium segments are represented in Fig. 6(a) and (b) respectively.
Fig. 6.
Pearson correlation, R, between mean radiation dose and, the percent change in average MFR between baseline and follow-up studies (averaged over the 8 patients included in the study) and the corresponding radiotherapy radiation dose for the three coronary arteries (a), and each of the myocardium segments (b). The data are displayed as (Pearson R, Radiation dose in cGy). The color scale corresponds to the Pearson R values. All Pearson correlations were statistically insignificant (P > 0.05).
4. Discussion
Although MPI is commonly used to detect ischemia, its implementation in-line with RT-simulation to assess radiation-induced cardiotoxicity may be challenging mainly due to short half-life of 13N-Ammonia (~10 min) and patient tolerance to undergo imaging in treatment position (particularly for the stress cardiac session). In this pilot study, we have shown the feasibility of this procedure in 8 out of 10 patients accrued (two were withdrawn from the study), mainly as a result of a multidisciplinary effort between radiotherapy and nuclear medicine teams.
This pilot dataset was the basis our second aim, that is to develop methodology to assess the value of MFR in predicting radiation-induced cardiotoxicity in left-sided BC patients post-irradiation of the chest wall within 1 year post-RT.
Accelerated atherosclerosis is a major mechanism of radiation-induced cardiac damage [25], which can be detected in coronary arteries by measuring the amount of calcification (Agatston calcium score). However, previous studies showed evidence of augmented calcium score only decades post-radiation. In a study that included 236 BCE patients who were treated with four-field radiotherapy, Tjessem showed no correlation between the heart radiation-dose and Agatston follow-up score at an average of 12 years post-RT [26]. Similar results were reported from a study with 20 asymptomatic BC patients who underwent tangential RT. The investigators showed a significantly elevated score in only one out of 20 patients, while 15/20 had a calcium score of zero [25]. On the other hand, a pilot study that included nine Hodgkin lymphoma patients referred for CAD screening at a median of 28 years (range 12–35 years) post-radiation, Rademaker et al. showed a significant increase in calcium scores compared to other patients of the same age group [27]. In our study, no appreciable calcium was detected at baseline in our patients cohort, and insignificant change in Ca2+ score was seen at median follow-up of 13 months (range 10–17 months), possibly due to the short follow-up time.
The most significant radiation-induced functional damage in the heart appeared to be to the myocardium. This can manifest in reduced epicardial coronary artery blood flow, or as an effect on the microvasculature with diffuse interstitial fibrosis, which can contribute primarily to diastolic dysfunction [28]. Left ventricular dysfunction is a common side effect of cancer treatment [29], and a decrease in its value in patients with left ventricular dysfunction or CAD has been reported [15,30-32]. In our study, and at a median follow-up time of 13 months (range 10–17 months), there was no change in LVEF. Only patient#4 had both the baseline and follow-up LVEF values mildly below normal (52% and 50%, respectively – normal LVEF for female is between 54% and 74%) [33], the etiology of which was unknown. While isolating the cardiotoxic effects of chemotherapy from those induced by radiotherapy is not possible in our setting, one may still argue that lack of changes in left ventricular function may also be a result of reduced TAC toxicity compared to other chemotherapy regimens [34], the relatively short follow-up period, and the small patients sample size. Magne et al. showed a significant drop in LVEF (median decrease of 10%) in 33% of patients treated with doxorubicin/docetaxel/CMF regimens plus radiotherapy at a median follow-up of 6 years [35]. However, all patients regained normal cardiac function and LVEF during follow-up. Radiation-induced functional damage to the myocardium may also result in decreased coronary artery blood flow, or effect on microvasculature with diffuse interstitial fibrosis, which contributes primarily to diastolic dysfunction [36]. In our study, visual assessment of myocardial perfusion PET images was abnormal in three patients.
A spill-in of RT doses into the myocardium was observed in all 8 patients included in this study, with an average myocardium mean dose of 0.82 Gy (range: 0.56–1.13 Gy), and with the highest doses corresponding to the apex and anterior wall. The average percent change in global MFR between baseline and follow-up studies was 7.34 ± 29.84% (range: −17.61–72.77%). Additionally, the three major coronary arteries showed, on average, comparable and insignificant changes in MFR between baseline and follow-up. An inverse linear relationship, however insignificant between the average RT dose and the corresponding percent change in MFR was observed for the three coronary arteries (Fig. 6), suggesting a linear increased risk with the average corresponding RT dose. This effect was the most significant for the LAD, which shall be due to its closeness to the chest wall, thus to the radiation fields.
Collectively, these findings are encouraging and underscore the potential value of MFR in predicting radiation-induced cardiac toxicity as early as one-year post-RT.
There are three major limitations in this study; (1) patients received multimodality treatments (both chemo- and radio- therapy), which made it impossible to tease out the cardiotoxic effects of radiation from those of chemotherapy. (2) The follow up period of 1 year post-RT, which is not well-known whether it is adequate to capture the treatment-induced cardiotoxicities; (3) the small data sample; all correlations between the IMRT radiation doses and the changes in PET measurements were insignificant, which may be due to the small number of subjects (8 patients) that were included in the study.
5. Conclusion
In this proof-of-principle study, changes in MFR between baseline and one-year post-radiotherapy correlated with the radiotherapy heart dose. If substantiated in larger prospective studies, this technique may serve as an early predictor of radiation-induced cardiotoxicity, and hence allowing for interventional cardiac management.
References
- [1].Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366(9503): 2087–106. [DOI] [PubMed] [Google Scholar]
- [2].Darby S, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804): 1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cuzick J, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994;12(3):447–53. [DOI] [PubMed] [Google Scholar]
- [4].Schubert LK, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011;100(2):241–6. [DOI] [PubMed] [Google Scholar]
- [5].Jagsi R, et al. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys 2010;78(5):1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lohr F, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009;74(1):73–80. [DOI] [PubMed] [Google Scholar]
- [7].Ares C, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys 2010;76(3):685–97. [DOI] [PubMed] [Google Scholar]
- [8].Gulyban A, et al. Multisegmented tangential breast fields: a rational way to treat breast cancer. Strahlenther Onkol 2008;184(5):262–9. [DOI] [PubMed] [Google Scholar]
- [9].Aznar MC, et al. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol 2011;84(1004):743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor CW, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008;72(2):501–7. [DOI] [PubMed] [Google Scholar]
- [11].Carr ZA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys 2005;61(3):842–50. [DOI] [PubMed] [Google Scholar]
- [12].Shimizu Y, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 2010;340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Azizova TV, et al. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948-1958. Radiat Res 2010;174(2):155–68. [DOI] [PubMed] [Google Scholar]
- [14].Darby SC, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76(3):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perik PJ, van Veldhuisen DJ, Gietema JA. Natriuretic peptides in anthracycline-induced cardiotoxicity. Eur J Heart Fail 2005;7(5):940–1. [author reply 941]. [DOI] [PubMed] [Google Scholar]
- [16].Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract 2011;2011:317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marks LB, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005;63(1):214–23. [DOI] [PubMed] [Google Scholar]
- [18].Cecchi F, et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003;349(11):1027–35. [DOI] [PubMed] [Google Scholar]
- [19].Herzog BA, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54(2):150–6. [DOI] [PubMed] [Google Scholar]
- [20].Neglia D, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105(2):186–93. [DOI] [PubMed] [Google Scholar]
- [21].Tio RA, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med 2009;50(2):214–9. [DOI] [PubMed] [Google Scholar]
- [22].Ziadi MC, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58(7):740–8. [DOI] [PubMed] [Google Scholar]
- [23].DeGrado TR, et al. Estimation of myocardial blood flow for longitudinal studies with 13N-labeled ammonia and positron emission tomography. J Nucl Cardiol 1996;3(6 Pt 1):494–507. [DOI] [PubMed] [Google Scholar]
- [24].Campisi R, et al. Effects of long-term smoking on myocardial blood flow, coronary vasomotion, and vasodilator capacity. Circulation 1998;98(2):119–25. [DOI] [PubMed] [Google Scholar]
- [25].Chang M, et al. Coronary calcium scanning in patients after adjuvant radiation for early breast cancer and ductal carcinoma in situ. Front Oncol 2013;3:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tjessem KH, et al. Coronary calcium score in 12-year breast cancer survivors after adjuvant radiotherapy with low to moderate heart exposure - relationship to cardiac radiation dose and cardiovascular risk factors. Radiother Oncol 2015;114(3):328–34. [DOI] [PubMed] [Google Scholar]
- [27].Rademaker J, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol 2008;191(1):32–7. [DOI] [PubMed] [Google Scholar]
- [28].Gagliardi G, Lax I, Rutqvist LE. Partial irradiation of the heart. Semin Radiat Oncol 2001;11(3):224–33. [DOI] [PubMed] [Google Scholar]
- [29].Zamorano JL, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37(36):2768–801. [DOI] [PubMed] [Google Scholar]
- [30].Davidson NC, et al. Comparison of atrial natriuretic peptide B-type natriuretic peptide, and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol 1996;77(10):828–31. [DOI] [PubMed] [Google Scholar]
- [31].Omland T, Aakvaag A, Vik-Mo H. Plasma cardiac natriuretic peptide determination as a screening test for the detection of patients with mild left ventricular impairment. Heart 1996;76(3):232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Belkacemi Y, et al. Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: acute toxicity analyses from the French multicentric study. Ann Oncol 2008; 19(6):1110–6. [DOI] [PubMed] [Google Scholar]
- [33].Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39. [e14]. [DOI] [PubMed] [Google Scholar]
- [34].KI R TAC protocol in early breast cancer. Qatar Medical Journal 2011;20(1). [Google Scholar]
- [35].Magne N, et al. Special focus on cardiac toxicity of different sequences of adjuvant doxorubicin/docetaxel/CMF regimens combined with radiotherapy in breast cancer patients. Radiother Oncol 2009;90(1):116–21. [DOI] [PubMed] [Google Scholar]
- [36].Sardaro A, et al. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol 2012;103(2):133–42. [DOI] [PubMed] [Google Scholar]