Abstract
Background
Patients with atrial high‐rate episode (AHRE) are at higher risk of neurological events. This study aimed to identify the optimal cutoff threshold for AHRE duration in patients with dual chamber permanent pacemakers (PPM) without prior atrial fibrillation.
Methods
We included 355 consecutive patients receiving dual chamber pacemaker implantation. Primary outcome was composite endpoint of subsequent neurological events after various AHRE durations. AHRE was defined as >175 bpm (MEDTRONIC) or > 200 bpm (BIOTRONIK) for longer than 30 s. Cox regression analysis with time‐dependent covariates was conducted.
Results
The mean age of included patients was 75.6 ± 11.3 years. Among 355 included patients, some had multiple AHREs; 125 patients (35.2%) developed AHRE ≥2 min, 107 (30.1%) had ≥5 min, 55 (15.5%) had ≥6 h, and 37 (10.4%) had ≥24 h. The mean follow‐up was 42.1 ± 31.2 months. During follow‐up, 19 neurological events occurred. After adjustment for CHA2DS2‐VASc score and device type, multivariate Cox regression analysis indicated AHRE ≥2 min (HR 13.605, 95% CI 3.010–61.498), and AHRE ≥5 min (HR 5.819, 95% CI 2.056–16.470) were significantly associated with neurological events. Hence, the optimal AHRE cutoff value was 2 min with the highest Youden index (sensitivity, 89.5%; specificity, 67.8%; AUC, 0.823, 95% CI, 0.763–0.884; p < 0.001).
Conclusions
Patients with dual chamber PPM who develop AHRE have increased risk of neurological events. Comprehensive assessment of the risks and benefits of prescribing anticoagulants should be considered in PPM patients with AHRE ≥2 min.
Keywords: atrial fibrillation, atrial high‐rate episodes, dual chamber pacemakers, neurological events
1. INTRODUCTION
Atrial fibrillation (AF), despite good progress with its management, remains a common arrhythmia encountered in clinical practice and is a major cause of systemic thromboembolic diseases, such as stroke and systemic embolism. 1 AF is diagnosed by 12‐lead electrocardiography and may be transient and asymptomatic, leading to difficulty in its detection. The use of cardiac implantable electronic devices (CIEDs) is increased because of the technical ability to monitor long‐term atrial rhythm.
Recently, subclinical AF (SCAF), also called atrial high‐rate episode (AHRE), is detected by CIEDs. 2 Even in asymptomatic patients, AHRE has been shown to be associated with an elevated risk of neurological events, including stroke and transient ischemic attacks 3 ; however, this risk seems to be lower than in patients with diagnosed AF. 4 The optimal burden or cutoff value for AHRE contributing increasing risk of neurological events remains controversial. AHRE lasting ≥30 s, 5 ≥ 5 min, 6 ≥ 6 min, 2 ≥ 6 h, 7 and ≥ 24 h 8 have been shown to be related to an increased risk of systemic thromboembolic events. Currently, CIEDs should be interrogated on a regular basis for AHRE. 1 Patients with AHRE should undergo further assessment for systemic thromboembolic risk factors and for overt AF, including ECG monitoring. The recommended AHRE duration, for patients without known AF, as per 2016 guidelines, is >180 bpm lasting longer than 5–6 min, as detected by an implanted device. 1 Hence, we examined the associations between a range of cutoff durations of AHRE and the incidence rates of neurological events in Taiwanese patients with dual chamber permanent pacemakers (PPM).
2. METHODS
2.1. Study participants
We recruited patients older than 18 years old with dual chamber PPM (MEDTRONIC or BIOTRONIK) treated in the Cardiology Department of National Cheng Kung University Hospital, from January 2015 to August 2019. The protocol for this cohort study was reviewed and approved by the ethics committee of National Cheng Kung University Hospital (B‐ER‐108‐278), and was conducted according to the guidelines of the International Conference on Harmonization for Good Clinical Practice. We ensure that we have specified whether all data were fully anonymized before we accessed them and the ethics committee waived the requirement for informed consent.
2.2. Data collection and definitions
Patients' medical history, comorbidities, and echocardiographic parameters were collected from chart records for retrospective evaluation. Diabetes mellitus was defined by the presence of symptoms and a random plasma glucose concentration ≥ 200 mg/dl, fasting plasma glucose concentration ≥ 126 mg/dl, 2 h plasma glucose concentration ≥ 200 mg/dlL, from a 75 g oral glucose tolerance test, or taking medication for diabetes mellitus. 9 Hypertension was defined as in‐office systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg or taking antihypertensive medication. 10 Dyslipidemia was defined as low‐density lipoprotein ≥140 mg/dl, high‐density lipoprotein <40 mg/dl, triglycerides ≥150 mg/dl, or taking medication for dyslipidemia. 11 Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2. 12 Neurological events were defined as either ischemic stroke or transient ischemic attack (TIA), definitively diagnosed by an experienced neurologist. A TIA was defined as a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction. 13 Ischemic stroke was defined as acute focal or global disturbance of cerebral function due to vascular dysfunction, which lasted longer than 24 h or resulted in death. 14 AHRE were extracted from the devices via telemetry at each office visit every 3–6 months. AHRE electrograms were reviewed by at least one experienced electrophysiologist, who carefully considered the possibility that AHRE included lead noise or artifact, far‐field R‐waves, or paroxysmal supraventricular tachycardia and visually confirmed AF in the detected AHRE (Supplement Figure 1). Atrial sensitivity was programmed to 0.3 mV with bipolar sensing of MEDTRONIC and 0.2 mV with bipolar sensing of BIOTRONIK.
The primary endpoint for this study was the occurrence of neurological events after the date of implantation of a pacemaker. AHRE were defined as atrial rate > 175 bpm (MEDTRONIC) or > 200 bpm (BIOTRONIK) and lasting for at least 30 s of atrial tachyarrhythmia recorded by the devices on any day during the study period. AHREs were classified into six duration groups: ≥ 30 s, ≥ 1 min, ≥ 2 min, ≥ 5 min, ≥ 6 h and ≥ 24 h, to evaluate the cutoff threshold for neurological events. If the patient had multiple AHREs, the longest AHRE duration was used for analysis. Then, if the patient's longest AHRE duration was 6 min, this patient would be counted in AHRE ≥ 30 s, AHRE ≥ 1 min, AHRE ≥ 2 min, and AHRE ≥ 5 min.
2.3. Statistical analysis
Among baseline characteristics, categorical variables are presented as percentages. Continuous variables are presented as means and standard deviations. Chi‐square test or Fisher's exact test was used for categorical variables, and the two‐sample student's t‐test for continuous variables. Factors with significant differences (p < 0.10) in univariate analysis were then entered into multivariate Cox regression analysis. Cox regression analysis was used to identify variables associated with AHRE occurrence, reported as hazard ratios with 95% confidence intervals (CI). Indicators of AHRE ≥ 30 s, ≥ 1 min, ≥ 2 min, ≥ 5 min, ≥ 6 h, and ≥ 24 h were determined separately as time‐dependent covariates in multivariate Cox proportional hazards regression, and survival curves were generated for patients without neurological events. The receiver‐operating characteristic (ROC) area under the curve (AUC) from AHRE and their associated 95% confidence intervals (CI) were investigated for association with future neurological events. The optimal cutoff values were chosen based on the results of ROC curve analysis with the highest Youden index and used to evaluate the associated values of AHRE, in minutes, for determining end points. For all comparisons, p < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical package version 23.0 (SPSS Inc. Chicago, IL, USA).
3. RESULTS
3.1. Patient characteristics
From January 01, 2014 to August 31, 2019, a total of 498 consecutive patients receiving dual chamber PPMs at our hospital were initially recruited. Patients were excluded due to loss of follow‐up (10), or inadequate or missing data (3). Patients with a history of atrial fibrillation (130) were also excluded. After exclusions, 355 patients were included in this retrospective study.
Mean follow‐up was 42.1 ± 31.2 months after the implantation of a dual chamber PPM. Table 1 shows baseline characteristics and demographic data of all patients based on the occurrence of AHRE ≥30 s, ≥ 1 min, ≥ 2 min, ≥ 5 min, ≥ 6 h or ≥ 24 h. Mean age was 75.6 ± 11.3 years; 42.8% were women. The most common indication for dual chamber permanent pacemaker implantation was sick sinus syndrome (66.2%), followed by atrioventricular block (33.8%). High levels of hypertension (92.4%) and hyperlipidemia (90.4%) suggest a relatively high risk of neurological events for the entire study cohort (Table 1). During follow‐up, 162 (45.6%) patients developed AHRE ≥30 s, 145 (40.8%) developed AHRE ≥1 min, 125 (35.2%) developed AHRE ≥2 min, 107 (30.1%) developed AHRE ≥5 min, 55 (15.5%) developed AHRE ≥6 h, and 37 (10.4%) patients developed AHRE ≥24 h. Demographics, temporal data of the neurologic events, and type and incidence of neurological events are presented in Tables 2 and 3. Follow‐up was comprised of 1245.84 patient‐years of observation. The total number of neurological events that occurred was 19 (IR 1.53%/year, 95% CI 0.98–2.38), which includes TIA (total number 12, IR 0.96%/year, 95% CI 0.55–1.69) and ischemic stroke (total number 7, IR 0.56%/year, 95% CI 0.27–1.18). Incidence of atrial fibrillation and neurological events, stratified by AHRE durations, are shown in Tables 4 and 5. All patients with subsequent documented atrial fibrillation received anticoagulant therapy.
TABLE 1.
Baseline characteristics of the overall study group
| Variables | All patients (n = 355) | Neurological event | Univariate p valve | |
|---|---|---|---|---|
| Yes (N = 19) | No (N = 336) | |||
| Age (years) | 75.6 ± 11.3 | 77.3 ± 9.4 | 75.5 ± 11.4 | 0.502 |
| Gender | 0.057 | |||
| Male | 203 (57.2%) | 15 (78.9%) | 188 (56.0%) | |
| Female | 152 (42.8%) | 4 (21.1%) | 148 (44.0%) | |
| BMI (kg/m2) | 24.4 ± 2.3 | 24.3 ± 2.1 | 24.5 ± 2.3 | 0.795 |
| Device | 0.051 | |||
| MEDTRONIC | 220 (62.0%) | 16 (84.2%) | 204 (60.7%) | |
| BIOTRONIK | 135 (38.0%) | 3 (15.8%) | 132 (39.3%) | |
| Primary indication | 0.223 | |||
| Sinus node dysfunction | 235 (66.2%) | 16 (84.2%) | 219 (65.2%) | |
| Atrioventricular block | 120 (33.8%) | 3 (15.8%) | 117 (34.8%) | |
| CHA2DS2‐VASc score | 3.2 ± 1.3 | 3.8 ± 1.4 | 3.2 ± 1.3 | 0.056 |
| HAS‐BLED | 2.2 ± 1.2 | 2.6 ± 0.7 | 2.2 ± 1.2 | 0.165 |
| Hypertension | 328 (92.4%) | 19 (100.0%) | 309 (92.0%) | 0.381 |
| Diabetes mellitus | 185 (52.1%) | 14 (73.7%) | 171 (50.9%) | 0.061 |
| Hyperlipidemia | 321 (90.4%) | 19 (100%) | 302 (89.9%) | 0.236 |
| Prior stroke | 14 (3.9%) | 4 (21.1%) | 10 (3.0%) | 0.004 |
| Prior myocardial infarction | 72 (20.3%) | 4 (21.1%) | 68 (20.2%) | 1.000 |
| Heart failure | 0.322 | |||
| Preserved EF | 28 (7.9%) | 2 (10.5%) | 26 (7.7%) | |
| Reduced EF | 40 (11.3%) | 4 (21.1%) | 36 (10.7%) | |
| Chronic kidney disease | 18 (5.1%) | 8 (42.1%) | 125 (37.2%) | 0.668 |
| Chronic liver disease | 133 (37.5%) | 2 (10.5%) | 16 (4.8%) | 0.249 |
| Echo parameters | ||||
| LVEF (%) | 66.1 ± 12.8 | 63.2 ± 15.4 | 66.3 ± 12.7 | 0.308 |
| Mitral E/e′ | 12.4 ± 5.3 | 11.8 ± 4.8 | 12.4 ± 5.4 | 0.608 |
| LA diameter (cm) | 3.7 ± 0.6 | 3.8 ± 0.6 | 3.7 ± 0.6 | 0.297 |
| RV systolic function (s', m/s) | 12.6 ± 1.7 | 12.5 ± 2.0 | 12.6 ± 1.7 | 0.715 |
| Drug prescribed at baseline | ||||
| Antiplatelets | 128 (36.1%) | 12 (63.2%) | 116 (34.5%) | 0.011 |
| Anticoagulants | 32 (9.0%) | 2 (10.5%) | 30 (8.9%) | 0.685 |
| Beta blockers | 96 (27.0%) | 6 (31.6%) | 90 (26.8%) | 0.647 |
| Amiodarone | 44 (12.4%) | 3 (15.8%) | 41 (12.2%) | 0.717 |
| Propafenone | 15 (4.2%) | 0 (0%) | 15 (4.5%) | 1.000 |
| Digoxin | 4 (1.1%) | 0 (0%) | 4 (1.2%) | 1.000 |
| non‐DHP CCBs | 12 (3.4%) | 0 (0%) | 12 (3.6%) | 1.000 |
| RAAS inhibitors | 138 (38.9%) | 7 (36.8%) | 131 (39.0%) | 0.844 |
| Diuretics | 57 (16.1%) | 5 (26.3%) | 52 (15.5%) | 0.211 |
| Statins | 121 (34.1%) | 7 (36.8%) | 114 (33.9%) | 0.794 |
| Metformin | 57 (16.1%) | 4 (21.1%) | 53 (15.8%) | 0.523 |
| SGLT2 inhibitors | 4 (1.1%) | 0 (0%) | 4 (1.2%) | 1.000 |
| Follow duration (months) | 42.1 ± 31.2 | 29.5 ± 27.8 | 42.8 ± 31.3 | 0.070 |
| Follow times | 5.8 ± 4.4 | 4.1 ± 3.5 | 5.9 ± 4.4 | 0.071 |
| AHRE duration≥30 s | 162 (45.6%) | 19 (100%) | 143 (42.6%) | <0.001 |
| AHRE duration ≥ 1 min | 145 (40.8%) | 19 (100%) | 126 (37.5%) | <0.001 |
| AHRE duration ≥ 2 min | 125 (35.2%) | 17 (89.5%) | 108 (32.1%) | <0.001 |
| AHRE duration ≥ 5 min | 107 (30.1%) | 14 (73.7%) | 93 (27.7%) | <0.001 |
| AHRE duration ≥ 6 h | 55 (15.5%) | 6 (31.6%) | 49 (14.6%) | 0.046 |
| AHRE duration ≥ 24 h | 37 (10.4%) | 5 (26.3%) | 32 (9.5%) | 0.020 |
Note: Data are presented as mean ± SD or n (%).
Abbreviations: AF, atrial fibrillation; AHRE, atrial high‐rate episodes; BMI, body mass index; EF, ejection fraction; LA, left atrium; LVEF, left ventricular ejection fraction; RV, right ventricle; non‐DHP CCBs, non‐dihydropyridine calcium channel blockers; RAAS, renin‐angiotensin‐aldosterone system; SGLT2, sodium glucose co‐transporters 2.
TABLE 2.
Demographic data in all patients with ischemic stroke or TIA
| Number | Event | Age | Sex | Indication | CHA2DS2‐VASc score | Time from PPM to the first detection of AHRE (month) | Time from the first detection of AHRE to neurological events (month) | The longest AHRE (e.g., in hours) prior to neurologic events (hour) | Anti‐platelet | Anticoagulant |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TIA | 68 | M | SSS | 6 | 1 | 2 | 6.00 | Y | N |
| 2 | TIA | 83 | M | SSS | 7 | 6 | 6 | 816.00 | N | N |
| 3 | TIA | 74 | F | SSS | 4 | 8 | 28 | .50 | N | N |
| 4 | TIA | 64 | M | SSS | 3 | 4 | 2 | .06 | Y | N |
| 5 | IS | 89 | M | AVB | 4 | 3 | 5 | .05 | Y | N |
| 6 | TIA | 57 | F | AVB | 3 | 2 | 25 | .03 | N | N |
| 7 | TIA | 86 | M | SSS | 3 | 3 | 93 | .02 | Y | N |
| 8 | TIA | 83 | M | AVB | 3 | 2 | 10 | 2256.00 | N | Y |
| 9 | TIA | 84 | M | SSS | 5 | 2 | 9 | 3600.00 | N | N |
| 10 | IS | 71 | M | SSS | 4 | 1 | 10 | .06 | Y | N |
| 11 | TIA | 82 | M | SSS | 3 | 1 | 2 | 29520.00 | Y | N |
| 12 | IS | 69 | M | SSS | 2 | 1 | 1 | .15 | Y | N |
| 13 | TIA | 76 | F | SSS | 6 | 1 | 2 | 1.00 | Y | N |
| 14 | IS | 94 | F | SSS | 4 | 3 | 44 | 2.00 | Y | N |
| 15 | TIA | 79 | M | SSS | 4 | 2 | 23 | .24 | Y | N |
| 16 | IS | 68 | M | SSS | 2 | 1 | 24 | 10.00 | N | N |
| 17 | IS | 78 | M | SSS | 3 | 2 | 60 | 2.00 | Y | N |
| 18 | IS | 86 | M | SSS | 3 | 4 | 1 | 504.00 | Y | N |
| 19 | TIA | 77 | M | SSS | 3 | 3 | 2 | 3.00 | N | Y |
Abbreviations: AHRE, atrial high‐rate episodes; AVB, atrioventricular block; F, female; IS, ischemia stroke; M, male; N, no; PPM, permanent pacemaker; SSS, sick sinus syndrome; TIA, transient ischemic attack; Y, yes.
TABLE 3.
Type and incidence of neurological events in the cohort
| Types of neurological events | Number | Incidence rate (100 patient‐years) | CI 95% | Time to event (months) | Age (years) | Gender (female) | Prior stroke | Antiplatelets | Anticoagulant |
|---|---|---|---|---|---|---|---|---|---|
| TIA | 12 (3.4%) | 0.96 | 0.55–1.69 |
21.7 ± 25.7 (2–96) |
76.1 ± 8.9 | 3 (25%) | 3 (25%) | 6 (50%) | 2 (16.7%) |
| Ischemic stroke | 7 (2.0%) | 0.56 | 0.27–1.18 |
23.7 ± 22.6 (1–62) |
79.3 ± 10.5 | 1(14.3%) | 1(14.3%) | 6 (85.7%) | 0 (0%) |
| Total events | 19 | 1.53 | 0.98–2.38 |
Note: Data are presented as mean ± SD or n (%).
Abbreviations: AHRE, atrial high‐rate episodes; TIA, transient ischemic attack.
TABLE 4.
Incidence of atrial fibrillation among patients with different AHRE durations
| AHRE durations | Number | Incidence rate (100 patient‐years) | CI 95% |
|---|---|---|---|
| All patient | 32 (9.0%) | 2.57% | 1.82–3.62 |
| ≥ 30 s | 26 (16.0%) | 4.43% | 3.05–6.46 |
| ≥ 1min | 26 (17.9%) | 4.89% | 3.36–7.11 |
| ≥ 2 min | 23 (18.4%) | 4.97% | 3.33–7.41 |
| ≥ 5 min | 22 (20.6%) | 5.43% | 3.62–8.15 |
| ≥ 6 h | 14 (25.5%) | 6.95% | 4.19–11.51 |
| ≥ 24 h | 13 (35.1%) | 10.77% | 6.44–17.99 |
Abbreviations: AHRE, atrial high‐rate episodes.
TABLE 5.
Incidence of neurological events among patients with different AHRE durations
| AHRE durations | Number | Incidence rate (100 patient‐years) | CI 95% |
|---|---|---|---|
| ≥ 30 s | 19 (11.7%) | 3.24% | 2.08–5.04 |
| ≥ 1min | 19 (13.1%) | 3.57% | 2.30–5.55 |
| ≥ 2 min | 17 (13.6%) | 3.68% | 2.31–5.86 |
| ≥ 5 min | 14 (13.1%) | 3.46% | 2.07–5.78 |
| ≥ 6 h | 6 (10.9%) | 3.98% | 1.35–6.55 |
| ≥ 24 h | 5 (13.5%) | 4.14% | 1.76–9.77 |
Abbreviations: AHRE, atrial high‐rate episode.
3.2. Univariate analysis and multivariate Cox regression analysis of associations between duration of AHRE and neurological events in all patients
Univariate analysis found an association of gender, device type, CHA2DS2‐VASc score, and diabetes mellitus, with neurological events, to be only of borderline significance. Prior stroke, AHRE duration ≥30 s, AHRE duration ≥1 min, AHRE duration ≥2 min, and AHRE duration ≥5 min, ≥ 6 h and ≥ 24 h, were significantly associated with neurological events occurrence in all patients (Table 1). When CHA2DS2‐VASc score and device type were confounders, AHRE ≥2 min (HR 13.605, 95% CI 3.010–61.498, p = 0.001) and AHRE ≥5 min (HR 5.819, 95% CI (2.056–16.470, p = 0.001) were still independently associated with neurological events (Table 6). Multivariate Cox regression analysis revealed that, except for prior stroke, AHRE ≥2 min (HR 13.406, 95% CI 2.959–60.743, p = 0.001), AHRE ≥5 min (HR 5.725, 95% CI 1.960–16.720, p = 0.001), and AHRE ≥24 h (HR 2.950, 95% CI 1.008–8.634, p = 0.048) were all significantly associated with neurological events (Supplementary Table 1). However, AHRE ≥6 h (HR 2.401, 95% CI 0.862–6.687, p = 0.094) was not significantly associated with neurological events (Supplementary Table 1).
TABLE 6.
Multivariate Cox regression for neurological events
| Variables | Multivariate Cox regression | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||||||||
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| CHA2DS2‐VASc score | 1.669 | 1.144–2.433 | 0.008 | 1.614 | 1.114–2.339 | 0.029 | 1.587 | 1.093–2.305 | 0.015 | 1.669 | 1.144–2.433 | 0.008 | 1.614 | 1.114–2.339 | 0.029 | 1.587 | 1.093–2.305 | 0.015 |
| Device (Medtronic) | 1.131 | 0.305–4.188 | 0.854 | 1.075 | 0.102–1.298 | 0.119 | 0.682 | 0.181–2.571 | 0.572 | 0.399 | 0.112–1.27 | 0.158 | 0.306 | 0.086–1.083 | 0.066 | 0.317 | 0.089–1.134 | 0.077 |
| AHRE duration ≥ 30 s | 240 426 | 0.000–1969 | 0.905 | |||||||||||||||
| AHRE duration ≥ 1 min | 300 138 | 0.000–3201 | 0.905 | |||||||||||||||
| AHRE duration ≥ 2 min | 13.605 | 3.010–61.498 | 0.001 | |||||||||||||||
| AHRE duration ≥5 min | 5.819 | 2.056–16.470 | 0.001 | |||||||||||||||
| AHRE duration ≥6 h | 2.031 | 0.7575.454 | 0.160 | |||||||||||||||
| AHRE duration≥24 h | 2.277 | 0.791–6.553 | 0.127 | |||||||||||||||
Note: Data are presented as mean ± SD or n (%).
Abbreviations: AHRE, atrial high‐rate episodes.
3.3. ROC‐AUC determination of AHRE cutoff values for association with future neurological events
The optimal AHRE cutoff value for association with future neurological events was determined to be 2 min, with the highest Youden index of 1.573 (sensitivity, 89.5%; specificity, 67.8%; positive predictive value, 13.6%; negative predictive value, 99.1%; positive likelihood ratio, 2.79; negative likelihood ratio, 0.15; AUC, 0.823; 95% CI, 0.763–0.884; p < 0.001) (Figure 1). With AHRE of 5 min, we found: sensitivity, 73.7%; specificity, 72.3%; positive predictive value, 13.1%; negative predictive value, 98.0%; positive likelihood ratio, 2.66; negative likelihood ratio, 0.38. Figure 2 shows the Cox regression event‐free survival curves for neurological events.
FIGURE 1.
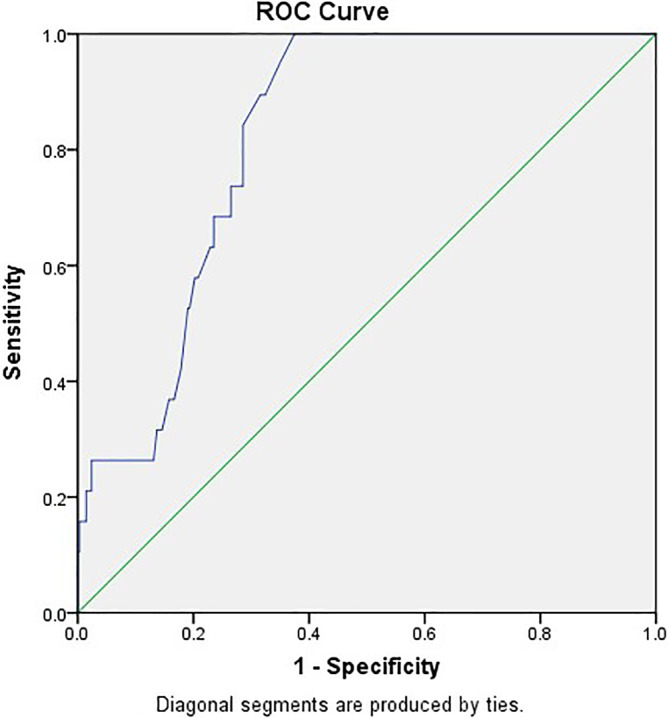
Atrial high‐rate episodes (minutes): cutoff value, 2 min; sensitivity, 89.5%; specificity, 67.8%; AUC, 0.823; 95% CI, 0.763–0.884; p < 0.001
FIGURE 2.
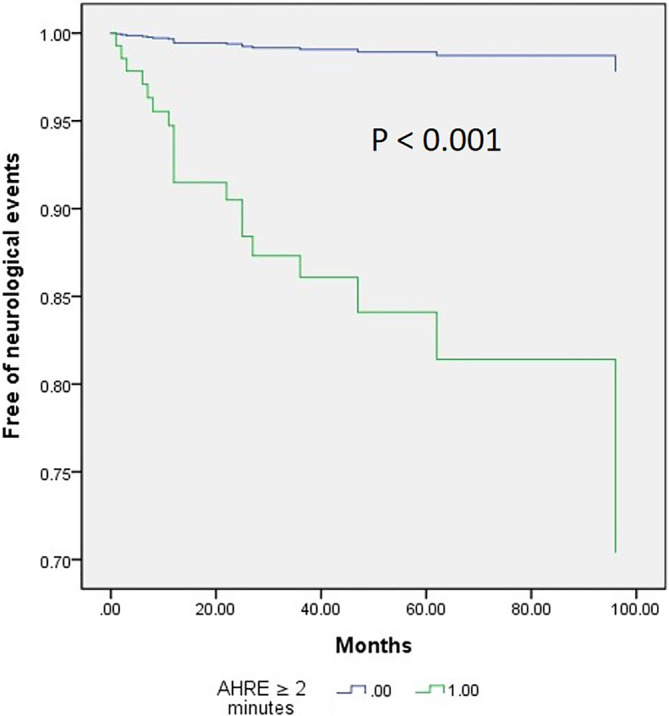
Cox regression event‐free survival curves from neurological events at 42.1 ± 31.2 months of follow‐up based on atrial high‐rate episode (AHRE) ≥2 min or not
4. DISCUSSION
The main finding of this study is that AHRE duration ≥2 min, as detected by dual chamber PPMs, was significantly associated with neurological events in a Taiwanese population that had no history of AF. However, further investigation is warrant to confirm the current findings and to implement early aggressive anti‐thromboembolic therapy to prevent future neurological events based on detection of AHRE ≥2 min in Taiwanese population.
The ASSERT study 15 is the only large, prospective trial to date to assess the relationship between AHRE (defined as an atrial rate of at least 190 beats/min lasting for ≥6 min) and systemic thromboembolic events in patients without a history of clinical AF. In the ASSERT study, stroke or systemic embolism occurred during follow‐up in 4.2% (1.7%/year) of patients in whom AHRE had been detected. 15 In our study, stroke or TIA occurred during follow‐up in 5.3% (1.53%/year) of patients. The MOde Selection Trial, in which AHRE was defined as an atrial rate > 220 beats/min lasting ≥5 min, 6 showed that patients with sinus node dysfunction in which AHRE was detected by pacemakers were more than twice as likely to die or have a stroke. A recent study showed that AHRE lasting ≥30 sec is a risk factor indicative of embolic stroke in a Japanese population with CIEDs. 5 AHRE lasting ≥30 s is the shortest cutoff point determined in studies thus far; however, AUC = 0.67 in the Japanese study 5 is relatively small compared to our result (AUC = 0.82).
In our study, the ROC curve showed that the best cutoff duration time of AHRE for predicting the risk of neurological events was 2 min. Compared to 5 min, our results showed that the cutoff value of 2 min had a higher positive likelihood ratio and negative predictive value, and lower negative likelihood ratio, indicating that 2 min is a more sensitive cutoff value for ruling out subsequent neurological events. Current guidelines 1 recommend that AF be diagnosed using a 12‐lead EKG for a duration of more than 30 s. Both artifacts and false detection of far‐field R‐wave by the atrial lead could misclassify AHRE if of too short a duration. Previously, the 5 min cutoff value excluded most episodes of over‐sensing due to mechanical problems and appropriately detected clinical AF. 16 In order to prevent over‐diagnosing SCAF we should focus on SCAF detected using our optimal cutoff value of AHRE ≥2 min confirmed by experienced electrophysiologists. Although both AHRE duration ≥ 6 h and AHRE duration ≥ 24 h are significantly different in patients with or without neurologic events in Table 1, however, in our multivariate analysis in Table 6, neither AHRE duration ≥ 6 h nor AHRE duration ≥ 24 h was independent predictor for neurological events. It may be related to relative small numbers of neurologic events in patients with AHRE duration ≥ 6 h (6, 10.9%) and AHRE duration ≥ 24 h (5, 13.5%) in Table 5, which were all less than AHRE duration ≥ 2 min (17, 13.6%).
Independent predictors for neurological events in our study were not only AHRE ≥2 min but also CHA2DS2‐VASc score. An increase in AHRE incidence with increasing CHA2DS2‐VASc score has been documented. The association was stronger with AHRE of increased duration, with CHA2DS2‐VASc demonstrating moderate accuracy as a predictor. 17 All patients with neurological events had AHRE ≥2 min, except for two patients with AHRE ≥1 min. CHA2DS2‐VASc scores for all patients were 3 and HAS‐BLED scores were all 2. The 2020 European Society of Cardiology Guidelines recommend that, prior to initiating oral anticoagulation therapy, patients with AHRE >5–6 min have further electrocardiogram monitoring to document overt AF. 1 The European Heart Rhythm Association, in a broadly endorsed 2017 consensus document regarding device‐detected AHRE, states that oral anticoagulation is recommended for patients with two additional risk factors: CHA2DS2‐VASc ≥2 in men, or ≥ 3 in women, and with AHRE burden >5.5 h/day. 18
Based on our results, we suggest that patients with dual chamber PPMs in Taiwan, with documented AHRE ≥2 min following dual chamber pacemaker implantation, or AHRE ≥1 min and CHA2DS2‐VASc score ≥ 3, be considered for prescribed anticoagulants for stroke prevention.
Two large‐scale randomized clinical trials of non‐vitamin K oral anticoagulant for patients with device‐detected AHRE are ongoing. 19 , 20 The results may help illuminate the critical role of AHRE in stroke prevention.
5. STUDY LIMITATIONS
The present study has several limitations. First, this study has a single‐center, retrospective, observational design with a relatively small number of patients with dual chamber PPM in a hospital‐based setting, with all patients being Taiwanese. As a result, causality as a general conclusion for other populations, cannot be stated between AHRE and neurological events, since results may have been affected by the stated confounding factors. Second, AHRE may have been underestimated due to different default settings for AHRE in devices designed by different companies. Prospective multicenter studies with larger samples are required to confirm results of the present study. Third, this study did not reach any conclusions about the nature of heart rhythms at the time of the onset of stroke or TIA. Fourth, not all patients with neurological events underwent brain magnetic resonance imaging/angiography to pursue the etiologies of embolic origin, however, the neurologists confirmed the all neurologic events. Finally, the number of neurological outcomes is relatively small; therefore, there is a problem of over‐fitting with the multivariable analyses.
6. CONCLUSIONS
Stroke or TIA events are relatively common in Taiwanese patients with dual chamber PPMs. AHRE lasting for ≥2 min is an independent risk factor for neurological events in this population. AHRE of different durations appear to be consistently associated with neurological events. When AHRE ≥2 min is detected in patients with dual chamber PPMs, a comprehensive assessment of the risks and benefits of prescribing an anticoagulant should be considered.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ju‐Yi Chen; data acquisition: Wei‐Da Lu, Ju‐Yi Chen; data analysis and interpretation: Wei‐Da Lu, Ju‐Yi Chen; statistical analysis: Wei‐Da Lu, Ju‐Yi Chen; drafting and finalizing the article: Ju‐Yi Chen; critical revision of the article for important intellectual content: Ju‐Yi Chen.
Supporting information
Figure S1
Supplementary Table 1 Multivariate Cox regression for neurological events
ACKNOWLEDGMENTS
The authors would like to thank Convergence CT for assistance with English editing of the manuscript.
Lu W‐D, Chen J‐Y. The optimal cutoff of atrial high‐rate episodes for neurological events in patients with dual chamber permanent pacemakers. Clin Cardiol. 2021;44(6):871–879. 10.1002/clc.23626
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Numbers: MOST 108‐2218‐E‐006‐019, MOST 109‐2218‐E‐006‐024
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Ju‐Yi Chen, MD, PhD.
REFERENCES
- 1. Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2020;2020:ehaa612. 10.1093/eurheartj/ehaa612. [DOI] [Google Scholar]
- 2. Uittenbogaart SB, Lucassen WAM, van Etten‐Jamaludin FS, de Groot JR, van Weert HCPM. Burden of atrial high‐rate episodes and risk of stroke: a systematic review. Europace. 2018;20:1420‐1427. 10.1093/europace/eux356. [DOI] [PubMed] [Google Scholar]
- 3. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120‐129. 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 4. Camm AJ, Simantirakis E, Goette A, et al. Atrial high‐rate episodes and stroke prevention. Europace. 2017;19:169‐179. 10.1093/europace/euw279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakano M, Kondo Y, Nakano M, et al. Impact of atrial high‐rate episodes on the risk of future stroke. J Cardiol. 2019;74:144‐149. 10.1016/j.jjcc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 6. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the atrial diagnostics ancillary study of the mode selection trial (MOST). Circulation. 2003;107:1614‐1619. 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 7. Boriani G, Glotzer TV, Santini M, et al. Device‐detected atrial fibrillation and risk for stroke: an analysis of > 10, 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508‐516. 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device‐detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339‐1344. 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Summary of Revisions: Standards of Medical Care in Diabetes. Diabetes Care. 2019;42:S4‐S6. 10.2337/dc19-Srev01. [DOI] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27:314‐340. 10.1080/08037051.2018.1527177. [DOI] [PubMed] [Google Scholar]
- 11. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140‐205. 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 12. Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, Levin A. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:5‐14. 10.1038/kisup.2012.77. [DOI] [PubMed] [Google Scholar]
- 13. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40:2276‐2293. 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 14. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58:113‐130. [PMC free article] [PubMed] [Google Scholar]
- 15. Healey JS, Connolly SJ, Gold MR, et al. ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120‐129. 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 16. Pollak WM, Simmons JD, Interian A Jr, et al. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001;24:424‐429. 10.1046/j.1460-9592.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 17. Rovaris G, Solimene F, D'Onofrio A, et al. Does the CHA2DS2‐VASc score reliably predict atrial arrhythmias? Analysis of a nationwide database of remote monitoring data transmitted daily from cardiac implantable electronic devices. Heart Rhythm. 2018;15:971‐979. 10.1016/j.hrthm.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 18. Chair GB, Bax J, Boriani G, et al. Device‐detected subclinical atrial tachyarrhythmias: definition, implications and management – an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca Y Electrofisiologia (SOLEACE). Europace. 2017;19:1556‐1578. 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 19. Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non‐vitamin K antagonist oral anticoagulants in patients with atrial high rate episodes (NOAH‐AFNET 6) trial. Am Heart J. 2017;190:12‐18. 10.1016/j.ahj.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the Reduction of Thrombo‐Embolism in Patients With Device‐Detected Sub‐Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137‐145. 10.1016/j.ahj.2017.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Supplementary Table 1 Multivariate Cox regression for neurological events
Data Availability Statement
The data that support the findings of this study are available from Ju‐Yi Chen, MD, PhD.


