Abstract
Animal models of addictive behaviors are useful for uncovering neural mechanisms involved in the development of dependence and for identifying risk factors for drug abuse. One such risk factor is biological sex, which strongly moderates drug self-administration behavior in rodents. Female rodents are more likely to acquire drug self-administration behaviors, consume higher amounts of drug, and reinstate drug-seeking behavior more readily. Despite this female vulnerability, preclinical addiction research has largely been done in male animals. The study of sex differences in rodent models of addictive behavior is increasing, however, as more investigators are choosing to include both male and female animals in experiments. This commentary is meant to serve as an introductory guide for preclinical investigators new to the study of sex differences in addiction. We provide an overview of self-administration models, a broad view of female versus male self-administration behaviors, and suggestions for study design and implementation. Inclusion of female subjects in preclinical addiction research is timely, as problem drug and alcohol use in women is increasing. With proper attention, design, and analysis, the study of sex differences in addiction has the potential to uncover novel neural mechanisms and lead to greater translational success for addiction research.
Keywords: addiction, alcohol, drug abuse, rodent, self-administration, sex differences
INTRODUCTION
There has been recent recognition of the importance of addressing sex differences in preclinical neuroscience studies. Although the field of neuroscience has long been and continues to be dominated by male-only studies (Beery & Zucker, 2011; Hughes, 2007; Will et al., 2017), recent policy changes from major funding agencies such as the National Institutes of Health (NIH Policy on Sex as a Biological Variable, n.d.; Lee, 2018) and growing recognition that female animals are not more variable or difficult to study than male animals (Becker, Prendergast, & Liang, 2016; Prendergast, Onishi, & Zucker, 2014) are helping to correct this bias (Mamlouk, Dorris, Barrett, & Meitzen, 2020). As such, investigators studying rodent models of addictive behaviors are increasingly likely to be including both male and female animals in their studies.
Inclusion of female animals is a positive development for the field of addiction research. Data from clinical populations suggest that women have particular vulnerabilities to drugs of abuse. For example, women who drink alcohol are more likely to suffer from alcohol-related health problems than men (Brady & Randall, 1999; Lynch, Roth, & Carroll, 2002). Moreover, although women on average use drugs and alcohol less and have lower rates of alcohol and substance use disorders (AUDs/SUDs) compared to men (Kranzler & Soyka, 2018; McHugh, Votaw, Sugarman, & Greenfield, 2018), there is evidence suggesting that women who do use drugs and alcohol may escalate their use faster over a shorter time period (Anglin, Hser, & McGlothlin, 1987; Piazza, Vrbka, & Yeager, 1989; Westermeyer & Boedicker, 2000). Women may also find it more difficult to achieve abstinence versus men (Perkins, 2001). At least some of these vulnerabilities appear to be driven by ovarian hormones (Moran-Santa Maria, Flanagan, & Brady, 2014). On top of these vulnerabilities, rates of drug use and AUDs/SUDs are currently increasing at a much faster rate in women than they are in men (Grant et al., 2017; Marsh, Park, Lin, & Bersamira, 2018; White et al., 2015).
Such statistics highlight the critical need for more studies of addictive behavior in female animals. Review of past research comparing female to male rodents demonstrates a clear vulnerability to drugs of abuse in females (Becker & Koob, 2016; Lynch et al., 2002). Females have a greater propensity for self-administration behaviors versus males, and as in humans, ovarian hormones contribute to these differences (Anker & Carroll, 2011). These vulnerabilities may be due to increased sensitivity to the rewarding effects of drugs of abuse (Anker & Carroll, 2011; Lynch et al., 2002). Females are also thought to be more likely to use drugs and alcohol to deal with stress and negative affect, demonstrating a potential vulnerability to negative reinforcement of addictive behavior (Pang, Zvolensky, Schmidt, & Leventhal, 2015; Peltier et al., 2019). As such, female rodents are a valuable model of addiction vulnerability. Although research investigating the neural mechanisms of this vulnerability in females has been limited thus far, the increasing inclusion of females in preclinical neuroscience studies has the potential to provide novel mechanistic insights regarding the development of addiction in both sexes.
This commentary is meant to serve as an introductory guide for preclinical investigators new to the study of sex differences in addiction. We first provide an introduction to the self-administration models used to study addictive behaviors in rodents and a broad overview of the state of the field regarding sex differences, as new investigators will need to be aware that female rodents often respond differently than males in drug self-administration paradigms. Next, we offer considerations and guidelines for experimental design and key questions for future studies. With proper attention, design, and analysis, the inclusion of sex as a biological variable in preclinical studies has the potential to uncover novel mechanisms and lead to greater translational success for addiction research.
FEMALE VULNERABILITY IN DRUG SELF-ADMINISTRATION STUDIES
Although cultural and societal confounds make it difficult to determine the influence of biological sex on drug abuse vulnerability in humans, rodent models avoid many of these confounds. Studies in rodents overwhelmingly suggest an influence of biological sex on addictive behavior, with females being more likely to consume and seek drugs in self-administration paradigms. These effects are consistent across factors such as species (i.e., rat vs. mouse) and genetic background, though they are more pronounced in certain types of paradigms. Below, we review the models used to assess addictive behavior in rodents and then present evidence suggesting that females are more likely to consume drugs across these paradigms.
Studying addictive behavior in practice
Self-administration of drugs has been used since at least the 1960s in the study of drug abuse (Schuster & Thompson, 1969) and has high predictive validity for identifying drugs with a liability for abuse (O’Connor, Chapman, Butler, & Mead, 2011; Spanagel, 2017). Self-administration can be accomplished by providing access to drugs in the home cage or in a standard operant chamber. These paradigms allow researchers to investigate behaviors related to motivation and reward-seeking (Sanchis-Segura & Spanagel, 2006), as well as to compare among groups based on factors such as sex, genetic differences, or other experimental manipulations (Schuster & Thompson, 1969; Panlilio & Goldberg, 2007; Schuster & Thompson, 1969). In mice and rats, self-administration can be used for a wide variety of drugs, including stimulants (Pickens, 1968), opioids (Ettenberg, Pettit, Bloom, & Koob, 1982), alcohol (Ulm, Volpicelli, & Volpicelli, 1995), cannabinoids (Fattore, Fadda, & Fratta, 2009), and nicotine (Donny, Caggiula, Knopf, & Brown, 1995). Depending on the drug and issues of experimental design, some types of administration (i.e., intravenous vs. oral) might be more appropriate than others.
When drugs are provided in the home cage, self-administration behavior is most typically operationalized as consumption or intake of the drug solution. When multiple solutions are presented, drug preference can also be calculated. With this type of experimental setup, drugs are available for oral consumption, making home-cage access best suited and most commonly employed for ethanol (EtOH) self-administration studies. A number of variations of such paradigms exist, including continuous-access models in which drug is available for 24 hr/day and intermittent-access models. Examples of the latter include the commonly employed two-bottle-choice intermittent-access (3 days/week) and limited-access (2 to 4 hr) “drinking in the dark” models of EtOH drinking (Becker, 2012; Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Simms et al., 2008; Thiele & Navarro, 2014). Aversion-resistant drinking (i.e., consumption that continues despite the risk of negative consequences) can also be assessed by pairing a punisher such as the aversive tastant quinine with drug intake (Hopf & Lesscher, 2014). The advantages of home-cage access models are that their implementation is straightforward and they can be employed without major investments in time or equipment.
Drug self-administration is also commonly assessed in an operant response chamber. In this setting, drugs may be provided orally or via intravenous infusion. Practically, intravenous administration is accomplished by implanting a catheter into a vein, typically the jugular. This is a fairly invasive procedure in which the animal is anesthetized, the vein is exposed, and a very small, delicate catheter is implanted (see Current Protocols article; Thomsen & Caine, 2005; Thomsen & Caine, 2007). This method of administration is particularly challenging given the necessity of flushing the catheter routinely with saline and antibiotics. Rodents may also pick at the catheter, and one major concern is ensuring that the catheter stays in place throughout the duration of the experiment (Thomsen & Caine, 2007). Nevertheless, this route of administration has been used for decades and has been an invaluable tool in studying drug self-administration. Oral self-administration paradigms are often used for drugs that are typically consumed orally, most commonly EtOH (Samson, Pfeffer, & Tolliver, 1988). In an operant chamber, oral drug solutions may be delivered via dipper-style cups (which are lowered and refilled each time the response requirement is met), fixed drinking cups, or a drinking spout (fixed or retractable) (Heyser, Roberts, Schulteis, & Koob, 1999; Samson & Czachowski, 2003; Sneddon, Ramsey, Thomas, & Radke, 2020). When a drinking spout is available, lickometers can be used to more precisely measure consumption in oral settings (Blegen et al., 2018; see Current Protocols article; Gaillard & Stratford, 2016). Models using inhalation of vaporized substances such as nicotine, alcohol, and fentanyl have also been developed and are growing in use (de Guglielmo, Kallupi, Cole, & George, 2017; Moussawi et al., 2020; Smith et al., 2020).
Rodents respond for drugs in the operant chamber via levers, nose-poke holes, or touch-sensitive screens. In this type of experiment, data are generally expressed as responses or the amount of drug consumed. Experimenters often assess performance across a range of drug doses, establishing a dose-response curve, and vary the length of the self-administration session [e.g., short (2-hr) vs. long (6-hr) access]. Once animals have acquired the self-administration behavior, it is common to assess a period of maintenance or escalation of intake. Reinstatement of drug-seeking following forced or voluntary abstinence is a widely used model of relapse-like behavior (Bossert, Marchant, Calu, & Shaham, 2013). Because drug consumption is not equivalent to drug addiction, researchers have also developed variants of simple fixed-ratio operant self-administration paradigms intended to more closely model addictive behavior (Banks, Hutsell, Schwienteck, & Negus, 2015; Deroche-Gamonet & Piazza, 2014; Goltseker, Hopf, & Barak, 2019; Radke et al., 2017). Progressive-ratio schedules, which assess motivation to obtain the drug by determining a response breakpoint, and choice paradigms in which animals choose between drug and another reward such as food, social interaction, or exercise are frequently used (Ahmed, 2018; Augier et al., 2018; Banks et al., 2015; Richardson & Roberts, 1996; Stafford, LeSage, & Glowa, 1998; Townsend, Negus, Caine, Thomsen, & Banks, 2019; Venniro et al., 2018). The addition of punishers to the operant box (e.g., footshock or adulteration of oral solutions with bitter compounds) is becoming a popular means of assessing drug use despite negative consequences (i.e., “aversion-resistant” or “punishment-resistant” drug-seeking) (Monroe & Radke, 2020; Radke et al., 2017; Seif et al., 2013; Sneddon et al., 2020).
Sex differences in addictive behaviors
In general, contemporary research indicates that female rats and mice are more susceptible to drug self-administration than males. This pattern of female vulnerability is observed in home-cage drinking (Rhodes et al., 2005; Sneddon, White, & Radke, 2019; Zanni et al., 2019) during the acquisition, maintenance/escalation, and reinstatement phases of operant self-administration (Anker & Carroll, 2011; Lynch et al., 2002), as well as in models of aversion resistance (Monroe & Radke, 2020; Radke, Held, Sneddon, Riddle, & Quinn, 2020; Sneddon et al., 2020; Radke, Sneddon, Frasier, & Hopf, 2021).
In studies of home-cage EtOH drinking, female rodents generally consume EtOH in higher amounts and exhibit greater preference for EtOH versus males. This pattern is well established in mice (Cailhol & Mormède, 2001; Hwa et al., 2011; Jury, DiBerto, Kash, & Holmes, 2017; Middaugh, Kelley, Bandy, & McGroarty, 1999; Tambour, Brown, & Crabbe, 2008) and rats (Almeida et al., 1998; Juárez & Barrios de Tomasi, 1999; Lancaster & Spiegel, 1992; Priddy et al., 2017; Rosenwasser, McCulley, & Fecteau, 2014) using a 24-hr, continuous-access paradigm. When EtOH is presented in an intermittent fashion (typically three 24-hr sessions/week), both male and female rodents will escalate their intake and reach higher levels of consumption than under conditions of continuous access (Hwa et al., 2011). Higher levels of EtOH consumption following intermittent-access procedures have been observed in females in many (Amodeo et al., 2018; Hwa et al., 2011; Li et al., 2019; Priddy et al., 2017), but not all (Radke et al., 2020; Schramm-Sapyta et al., 2014), studies, highlighting the importance of considering other factors, such as strain, age, and length of exposure, when studying sex differences in behavior. Females also drink more EtOH than males in limited-access paradigms (Grahame, Li, & Lumeng, 1999; Melón, Wray, Moore, & Boehm, 2013; Metten, Brown, & Crabbe, 2011; Rhodes et al., 2005; Sneddon et al., 2019). Finally, some recent studies using home-cage access paradigms to study oral opioid use have observed greater consumption in females versus males (Phillips et al., 2019; Zanni et al., 2019; but see Forgie, Beyerstein, & Alexander, 1988; Monroe & Radke, 2020).
When drug self-administration is performed in an operant conditioning box, both the rate at which the behavior is acquired and the level of consumption during a period of escalation or maintenance are measured. Female rats acquire self-administration of cocaine and heroin (Lynch & Carroll, 1999), cannabinoid CB1 receptor agonists (Fattore et al., 2009), and nicotine (Donny et al., 2000; Swalve, Smethells, & Carroll, 2016) more rapidly than males, though the effect appears to be dose dependent. Under fixed-ratio schedules, female rats respond more for opioids than males (Carroll, Campbell, & Heideman, 2001; Cicero, Aylward, & Meyer, 2003; Mavrikaki, Pravetoni, Page, Potter, & Chartoff, 2017). Studies using oral or vapor delivery of opioids have also observed greater consumption among female rodents (Klein, 2001; Fulenwider, Nennig, Hafeez, et al., 2019; Moussawi et al., 2020), though others have not observed a difference (Monroe & Radke, 2020). In addition, female rats and mice have been shown to maintain self-administration at higher levels than males for drugs such as cocaine (Lynch & Carroll, 1999), methamphetamine (Roth & Carroll, 2004), and EtOH (Sneddon et al., 2020). In some studies, these effects are concentration dependent, with females typically earning more drugs at higher concentrations (Mavrikaki et al., 2017; Sneddon et al., 2020). Breakpoints on progressive-ratio schedules are higher in female animals for opioids and nicotine (Carroll et al., 2001; Cicero et al., 2003; Donny et al., 2000). Females also escalate intake of psychostimulants faster than males (Reichel, Chan, Ghee, & See, 2012; Roth & Carroll, 2004).
Reinstatement of drug-seeking is studied by inducing a return to self-administration following a period of forced or voluntary abstinence via exposure to cues, drugs, or stress (Bossert et al., 2013). This type of relapse-like behavior appears to be more frequent in females versus males for a variety of drugs. For example, female rats exhibit enhanced drug- and stress-induced reinstatement behavior after self-administering cocaine (Anker & Carroll, 2010; Feltenstein, Henderson, & See, 2011; Lynch & Carroll, 2000). Females also exhibit greater methamphetamine reinstatement than males and require fewer priming injections than males (Reichel et al., 2012; Ruda-Kucerova et al., 2015). Additionally, female rats show greater reinstatement than males when responding for heroin (Smethells, Greer, Dougen, & Carroll, 2020), EtOH (Bertholomey, Nagarajan, & Torregrossa, 2016), or cannabinoids (Fattore, Spano, Altea, Fadda, & Fratta, 2010). These types of studies suggest a biological influence of sex on relapse-like behavior.
Recent evidence also suggests that female rodents are more likely to consume and respond for drugs despite the risk of negative consequences. This has been demonstrated for EtOH drinking under continuous-access conditions (Fulenwider, Nennig, Price, Hafeez, & Schank, 2019) and intermittent-access conditions (Radke et al., 2020). In the operant chamber, female mice demonstrated continued responding for EtOH mixed with quinine at quinine concentrations that reduced responding in males (Sneddon et al., 2020). Greater aversion resistance in female versus male mice has also been demonstrated for oral fentanyl consumption in the home cage, but not the operant chamber (Monroe & Radke, 2020). It is important to note that not all studies have found sex differences in aversion resistance (Bauer, McVey, & Boehm, 2021; DeBaker, Moen, Robinson, Wickman, & Lee, 2020; Sneddon et al., 2019), further demonstrating the importance of the behavioral paradigm in revealing female vulnerability to addictive behavior. See Table 1 for more information.
Table 1.
Summary of Findings Regarding Sex Differences in Drug Self-Administration Paradigms
| Paradigma | Description | Considerations for studying sex differences |
|---|---|---|
| Home-cage access | Drug solutions are provided in the home cage. Most commonly employed with alcohol, although other solutions (e.g., opioids or nicotine) are also used. Drug may be presented alone or alongside a bottle of drinking water. | Consumption is generally higher in females versus males. Drug preference (vs. water) is less often influenced by sex but sometimes higher in females versus males. |
| Chronic, continuous access | Drug solutions are provided without interruption. Consumption is typically measured every 24 or 48 hr. | Consumption is often higher in females versus males. |
| Intermittent access | Drug solutions are provided in 24-hr blocks separated by drug-free periods. Typically, drug is offered for three 24-hr sessions/week. | Produces escalation in males and females. Escalation is sometimes greater in females. |
| Limited access (e.g., “drinking in the dark”) | Drug solutions are provided during select hours of the day, typically for 1, 2, or 4 hr. Access during the dark phase of the light cycle can increase consumption. | For alcohol, consumption is higher in females versus males. |
| Aversion-resistant drinking | Drug solutions are mixed with an aversive, bitter-tasting compound (e.g., quinine). | Resistance to aversion is sometimes greater in females, depending on the access model/drug used. |
| Operant responding | Rodents respond for access to drug using a lever or at a nose-poke hole. Drug may be delivered orally or intravenously. | Females generally respond more for drug. |
| Fixed-ratio (FR) schedules | Rodents respond for drug during daily access sessions (varying from 30 min to 6 hr). The response requirement is fixed and does not vary within a session. Used to assess acquisition and escalation of drug intake. | Females often acquire responding sooner and maintain higher levels of intake. |
| Progressive-ratio (PR) schedules | Typically following training on an FR schedule, the response requirement is set to progressively increase following successful completion of the previous ratio. | PR responding and breakpoints are often higher in females versus males. |
| Extinction and reinstatement | During extinction, responding no longer results in drug delivery. Reinstatement behavior is assessed by delivering priming injections of the drug, cues previously paired with drug delivery, or exposure to a stressor. | Responding during reinstatement is often higher in females versus males. |
| Choice procedures | Rodents respond for drug versus access to an alternative reinforcer, such as food, sucrose, or social interaction. | Limited data suggest no sex differences. |
| Punishment procedures | Responding for drug is paired with probability of punishment, typically footshock. For oral paradigms, drug solutions may also be mixed with an aversive, bitter compound. | Limited data suggest female vulnerability. |
When sex differences in drug self-administration are observed, females tend to be more vulnerable than males. Female rodents typically consume more across home-cage access paradigms and sometimes prefer drug (vs. water) more than males as well. In the operant chamber, females acquire self-administration behavior more readily and will often respond for and/or consume drug at higher rates. Measures such as reinstatement and progressive-ratio responding have also been observed to be greater in female versus male animals. Some paradigms, such as those presenting drug versus the choice of an alternative reinforce, have not yet been widely tested in female rodents.
Ovarian hormones influence vulnerability for addictive behavior
There is some compelling evidence to suggest that many of the sex differences observed in self-administration behaviors are influenced by gonadal hormones (Finn, 2020). For instance, ovariectomized females show attenuated CB1 receptor agonist and EtOH self-administration compared to non-ovariectomized females (Fattore et al., 2007; Forger & Morin, 1982). Estradiol administration to ovariectomized females also enhances acquisition of cocaine and heroin self-administration (Fattore et al., 2009; Jackson, Robinson, & Becker, 2006; Roth, Casimir, & Carroll, 2002) compared to that in ovariectomized rats given vehicle. In adolescent female rats, cocaine self-administration responses are positively correlated with estradiol levels (Lynch, 2008). Further, estradiol treatment in ovariectomized rodents increases consumption and responding for EtOH (Ford, Eldridge, & Samson, 2002a; Rajasingh et al., 2007; Reid et al., 2002; Hubbell, & Reid, 2003; Satta, Hilderbrand, & Lasek, 2018).
Another way to investigate the influence of circulating hormones in female rodents is by monitoring the estrous cycle, which in rodents consists of four stages (proestrus, estrus, metestrus, and diestrus) and cycles every 4 to 5 days (Byers, Wiles, Dunn, & Taft, 2012; Marcondes, Bianchi, & Tanno, 2002). The results from studies of estrous effects on addictive behaviors are mixed. Many studies report no association between self-administration behaviors and estrous cycle phase (Amodeo et al., 2018; Donny et al., 2000; Fulenwider, Nennig, Price, et al., 2019; Li et al., 2019; Mavrikaki et al., 2017; Melón, Nolan, Colar, Moore, & Boehm, 2017; Priddy et al., 2017; Ruda-Kucerova et al., 2015). For EtOH, limited effects of the estrous cycle have been observed (Ford, Eldridge, & Samson, 2002b; Forger & Morin, 1982). For example, one study reported that consumption was lower during estrus, but only in rats with synchronized cycles (Roberts, Smith, Weiss, Rivier, & Koob, 1998). Studies with cocaine demonstrate that female rats will choose higher doses (Lynch, Arizzi, & Carroll, 2000) and reach higher breakpoints on a progressive-ratio schedule (Roberts, Bennett, & Vickers, 1989) during estrus and suggest differential regulation of cue-motivated cocaine-seeking during this phase of the cycle (Fuchs, Evans, Mehta, Case, & See, 2005; Johnson et al., 2019; Nicolas et al., 2019). Together, these data suggest that gonadal hormones in females contribute to sex differences in drug self-administration but that daily fluctuations in hormone levels may not be the primary driver of these effects for most drugs.
CONSIDERATIONS FOR EXPERIMENTAL DESIGN AND IMPLEMENTATION
Many preclinical addiction researchers find themselves overwhelmed at the prospect of including sex as a biological variable in their study designs. Traditional thinking in the field has led many to believe that studies with female rodents require extra investments of time and resources. Common themes echoed among our peers include concerns about having to test many more animals and perform unfamiliar procedures such as estrous-cycle monitoring and gonadectomy. Below, we hope to dispel some of these myths and encourage all addiction researchers to include both male and female animals in their studies. We also aim to provide practical advice for investigators as they design and implement studies of sex differences in addictive behavior. Finally, although we have focused the current article specifically on models of voluntary drug intake, it is important to recognize the existence of sex differences in the effects of drugs following passive exposure (Becker & Koob, 2016), including locomotor activation (e.g., Cailhol & Mormède, 1999; Harrod et al., 2004), development of place preferences (e.g., Russo et al., 2003; Yararbas, Keser, Kanit, & Pogun, 2010), effects on intracranial self-stimulation reward thresholds (e.g., Galankin, Shekunova, & Zvartau, 2010; Tan et al., 2019), and expression of withdrawal behaviors (e.g., Radke, Gewirtz, & Carroll, 2015; Radke, Holtz, Gewirtz, & Carroll, 2013, Varlinskaya & Spear, 2004). Many of the considerations discussed below are equally applicable to those models.
Should I include both female and male animals in my initial study?
When designing a new experiment, investigators may find themselves wondering whether they should include both male and female subjects. The answer here is very likely “yes” as there are few reasons to include only one sex in behavioral studies. Whereas sexually dimorphic behaviors (i.e., those that take one of two exclusive forms) are qualitative and can only be studied in one sex (e.g., postpartum care can only be studied in females), studies of addictive behaviors do not generally fall into this category. Instead, sex differences in addictive behaviors are likely to be quantitative in nature, with males and females varying in the degree or magnitude at which they express a behavior (McCarthy, Arnold, Ball, Blaustein, & De Vries, 2012). Some established manipulations are only effective in one sex, usually males, which we note is likely a legacy of male bias in behavioral neuroscience. In these cases, there is some justification to study only one sex, although parallel studies exploring mechanisms underlying resilience/vulnerability in the other sex may also be fruitful. Notably, the existence of previous studies using one’s behavioral model in only male or female rodents does not by itself justify continued study of only one sex. Finally, it is important to be aware of the possibility for sex convergence or latent sex differences (Beltz, Beery, & Becker, 2019; McCarthy et al., 2012) in behavior. In these cases, a behavioral endpoint that appears the same in males and females results from different neural mechanisms. Thus, the absence of a sex difference in behavior does not justify inclusion of only one sex in mechanistic investigations.
Will studying both sexes require a greater number of animals?
When determining group sizes for an experiment with male and female rodents, one recommended approach is to compose experimental groups of half males and half females and to test them concurrently (Shansky, 2019). This approach allows investigators to consider potential sex differences without increasing group size, experimenter time, or cost. Indeed, including female animals is potentially more efficient for labs maintaining an in-house breeding colony, as 100% of the pups born in each litter can be tested. Imbedded in this approach is the requirement for investigators to disaggregate data by sex during analysis to uncover potential trends driven by sex (Beery & Zucker, 2011). If these initial results suggest no difference between males and females, the study will have reached its endpoint. If trends driven by sex are observed in the data, the study should be continued by testing additional balanced cohorts of male and female animals. In this scenario, group sizes should be increased so that the study has sufficient statistical power to detect an influence of sex on the outcome. Importantly, balanced numbers of male and female animals should always be tested together to permit statistical comparison of results by sex.
In the case of many addictive behaviors, it is important to consider that sex differences may emerge as main effects but not interactions. As reviewed above, female rodents often consume or respond for drugs at higher levels than males. For example, we reported that female mice drink more EtOH than males using a limited-access “drinking in the dark” paradigm (Sneddon et al., 2019). In a recent follow-up study (Sneddon, Schuh, Frankel, & Radke, 2021), we also observed greater consumption in females versus males (i.e., a main effect of sex), but the two groups responded equally to treatment (i.e., no sex × treatment interaction). In such a case, it is acceptable to combine the data and analyze males and females as one experimental group (see the section on data analysis below). Future studies of how that treatment affects drinking behavior may also be done in mixed-sex cohorts. Thus, experimenters employing behavioral paradigms with known sex effects should always be alert to the possibility of sex differences in the results but do not need to double group sizes by default.
During study design, it is also the responsibility of the investigator to consider the literature on sex differences in the behavior or mechanism of interest. Many addictive behaviors are more pronounced in female rodents, but others are not affected by sex or have not yet been studied in both sexes. If there is strong a priori reason to believe that one’s measure is dependent on sex, it may be prudent to make initial plans for an experiment with statistical power to detect sex differences. If sex differences in the behavior of interest have not yet been explored, an initial study designed to answer this question alone may be warranted.
Do I need to monitor the estrous cycle in female rodents?
In contrast to the concerns of many researchers, it is not necessary to assess the effects of estrous cycle phase in most studies. Even when a sex difference in behavior or treatment outcome is found, estrous cycle phase is only one potential mediator of the result. As noted above, the effects of estrous cycle phase on addictive behaviors are mixed, with a number of studies finding no association with drug consumption or responding. Thus, investigators should consider published and preliminary data when deciding whether estrous cycle is a likely mediator of an experimental effect. Other factors to consider include the amount of variability in data collected from females versus males (McCarthy et al., 2012) and, for experiments that extend over multiple days, whether there are any apparent cyclical patterns in the data. Although data from female and male rodents are, on average, equally variable (Becker et al., 2016; Prendergast et al., 2014), increased variability in either sex may point to important hormonal effects (e.g., effects of reproductive cycle in females or dominance hierarchies in males) (McCarthy et al., 2012).
Although monitoring the estrous cycle is a relatively simple procedure, determining how an experimental result varies with cycle phase is not always as straightforward. Extended exposure to drugs of abuse can alter normal cycling in female rodents (e.g., King, Canez, Gaskill, Javors, & Schenken, 1993; Sanchis, Esquifino, & Guerri, 1985; Shuey, Stump, Carliss, & Gerson, 2008), as can group housing (McCarthy et al., 2012), an absence of males in the colony (Campbell, Ryan, & Schwartz, 1976), stress exposure (Grippo et al., 2005), food restriction (Bronson & Marsteller, 1985; Tropp & Markus, 2001), and some genetic manipulations (Arnold & Chen, 2009; Jablonka-Shariff, Ravi, Beltsos, Murphy, & Olson, 1999; Ng, Yong, & Chakraborty, 2010). The estrous cycle is also a dynamic process that causes hormonal changes on the order of hours. As a result, the timing of estrous monitoring can introduce variability within an experiment and between research groups (Becker et al., 2005). For these reasons, we recommend that investigations of estrous effects be conducted as dedicated, carefully controlled follow-up experiments and only when published or preliminary data suggest an influence. For a detailed commentary on designing and conducting these types of studies, readers are referred to an excellent review by Becker et al. (2005).
How should I analyze and report data from a study with both male and female animals?
Data from studies using equal numbers of male and female animals in each experimental group should always be analyzed with sex as a factor. If these analyses suggest no influence of sex (i.e., there are no trends suggesting that a sex difference may emerge with more statistical power), the data can be collapsed for further analysis and visualization. Any resulting publications or presentations of the data should report how many animals of each sex were included in the experimental groups and note that preliminary analyses included sex as a factor but that no differences were found. It is also important to indicate whether the study had the necessary statistical power to detect effects of sex (Beltz et al., 2019).
When a sex difference is found, it should be reported by including sex as a factor in statistical analyses and plotting data from males and females separately. We recommend including such analyses in the main body of research articles instead of in supplemental materials, which are easily overlooked by readers. If, as discussed above, a main effect of sex is revealed but without a treatment interaction, it may be appropriate to combine males and females in visualizations of the data, as long as the results concerning sex effects are clearly described in the text. Indications of the magnitude of the sex difference, for example by reporting effect sizes, are also useful in determining the practical (vs. statistical) significance of such findings (Beltz et al., 2019). Investigators should additionally consider including information about sex differences in the title, abstract, and/or keywords of a manuscript.
When interpreting the data, consideration should be given to how the observed sex difference may interact with other factors, such as age, genetic background, stress, housing environment, or context. It is also important to consider that male and female animals can exhibit different behavioral repertoires and that task deficits in one sex may actually reflect the use of sex-specific behavioral strategies (Shansky, 2018). Thus, in some cases, it may be necessary to interpret behavioral outcomes through a sex-specific lens.
If I find a sex difference, what types of follow-up studies should I consider?
When a sex difference in behavior or treatment response is discovered, a likely next step is to investigate the mechanisms driving the difference. Hormones are responsible for a majority of sex differences, and it is therefore common to begin with follow-up investigations designed to assess which hormones are important in the effect and during which phase of development they exert their influence (Becker et al., 2005; McCarthy et al., 2012). A common approach to determining whether a behavior is influenced by sex hormones is gonadectomy and replacement with exogenous hormone (Becker et al., 2005). This type of approach has demonstrated, for example, that ovariectomy (OVX) slows acquisition of intravenous heroin self-administration and that this deficit is restored by exogenous treatment with estradiol benzoate (Roth et al., 2002). Such data suggest that estrogens have a strong influence on heroin self-administration in female rats. Monitoring the estrous cycle in females or testosterone levels in male cage-mates can additionally provide insight into whether circulating hormones are driving differences. Sex hormones also have strong influences on brain development. Such “organizational” effects can be tested by treating animals with exogenous hormones or hormone receptor antagonists during the neonatal or pubertal periods (Becker et al., 2005).
In addition to sex hormones, the influence of sex chromosomes should also be considered, as the X and Y chromosomes each contain unique sets of genes that are known to contribute to sex differences in the brain and behavior (Arnold, 2004). The four core genotypes (FCG) model allows investigation of sex chromosome effects by dissociating gonadal development (driven by the Sry gene, normally on the Y chromosome) from inheritance of the XX versus XY genotype (Arnold & Chen, 2009; De Vries et al., 2002). This approach allows comparison of XX and XY genotypes in mice with both male (Sry+) and female (Sry−) gonads. FCG mice have been used to identify a role for sex chromosomes in behaviors such as habit formation (Barker, Torregrossa, Arnold, & Taylor, 2010; Quinn, Hitchcott, Umeda, Arnold, & Taylor, 2007), reward-seeking (Seu, Groman, Arnold, & Jentsch, 2014), and relapse-like EtOH drinking (Sneddon et al., unpub. observ.). Other approaches for testing sex chromosome effects in rodents can also be useful (for a review, see Arnold, 2009).
Finally, investigators should seek to uncover the neural mechanisms underlying sex differences in addictive behaviors. Although a thorough understanding of how chromosomal and hormonal influences contribute to behavior is important, most prior work has stopped at that level of analysis. To capitalize fully on the potential of work on sex differences, we recommend that investigators explore brain-based differences between males and females that drive female vulnerability to addiction. As an example, recent findings from Lasek and colleagues suggest that binge-like EtOH drinking in female mice is associated with enhanced ventral tegmental area neuron excitability driven by estrogen receptor α (ERα) and mGluR1 (Hilderbrand & Lasek, 2018; Vandegrift et al., 2020). Another notable line of work from Mermelstein and colleagues demonstrates that enhanced responsivity to cocaine in female rats involves the effects of ERα and mGluR5 coupling on dendritic spine plasticity in the nucleus accumbens (Martinez et al., 2016; Martinez, Peterson, Meisel, & Mermelstein, 2014; Peterson, Mermelstein, & Meisel, 2015). Additional studies exploring how divergences in gene or protein expression, neuron physiology, neurotransmitter signaling, or functional connectivity contribute to behavioral differences between males and females are needed. This approach requires identification of sex differences in brain structure and function and causal manipulations that link these observations back to the behavior of interest (Fig. 1).
Figure 1.
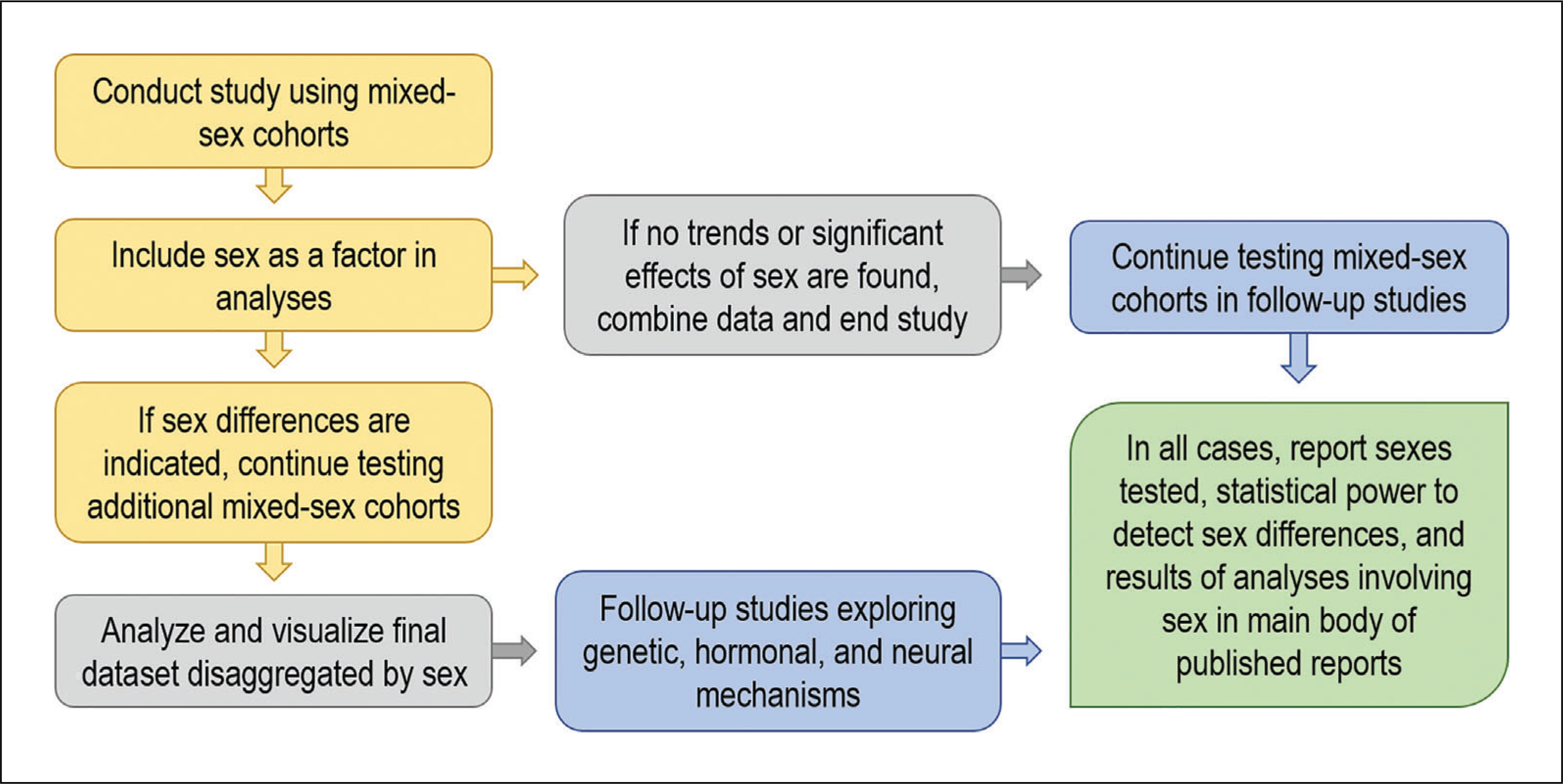
Considerations for studying sex differences in drug self-administration. Studies of rodent drug self-administration should generally include animals of both sexes, with equal numbers of male and female animals run concurrently. It is not necessary to increase the planned sample size of the study unless preliminary analyses reveal trends or statistically significant results that suggest an effect of sex on the measure of interest. When sex differences are not revealed, data from males and females can be combined for final analysis and visualization. When sex differences are present, additional animals should be tested to attain the statistical power to detect an effect of sex, and all data analyses and visualization should include sex as a variable. All analyses involving sex (including those that were not statistically significant) should be clearly reported in the main body of any published reports on the dataset.
CONCLUSIONS AND KEY QUESTIONS FOR FUTURE STUDIES
Understanding how sex influences the development of addiction is critical to achieve a full understanding of the disease and develop expedient therapeutics for individuals suffering from AUDs/SUDs. To date, preclinical studies on sex differences in addictive behaviors have revealed female vulnerability across drugs of abuse and self-administration models. Although ovarian hormones have been repeatedly linked to this vulnerability, investigations of the relevant neural mechanisms are limited.
As the field of addiction research enters what is hopefully a new era for studies of sex differences in addictive behaviors, there is much important work to be done. For example, it is critical that new behavioral models designed to better model the core features of addiction be validated in both male and female animals, and particular attention should be paid to whether animal models accurately reflect sex differences observed in clinical studies. Proper attention to sex in the validation of behavioral models has the potential to improve the translational efficacy of preclinical findings. Further, it will be important to determine how/if female vulnerability to addiction interacts with other vulnerability factors, such as age or exposure to stress. Discovery of mechanisms that contribute to such interactions could aid in the development of more personalized treatments for AUD/SUD patients. With regard to future studies of the neural underpinnings of addictive behavior, females should be used to uncover novel mechanistic insights. Determining how female animals differ on already established contributors to drug use and dependence will not advance the field sufficiently. Instead, if the study of sex as a biological variable in addiction neuroscience is to reach its full potential, all future studies must include both male and female animals (NIH Policy on Sex as a Biological Variable, n.d.). This approach will ensure that important contributors to addiction vulnerability are not overlooked or discounted.
We hope that this article can serve as guide for investigators newly considering issues of sex in addiction research. Although sex difference research has historically been avoided by many for various reasons, current investigators should be aware that studies with proper attention to sex as a biological variable do not necessitate increased group sizes and can therefore be performed in the same amount of time and for the same cost as traditional male-only studies. Determinations of whether a sex difference is under hormonal or chromosomal control (or an interaction of the two) can be done in dedicated follow-up studies. Importantly, novel mechanisms contributing to addiction vulnerability may be discovered by investigating the neural basis of drug self-administration in both male and female animals.
ACKNOWLEDGMENTS
This work was supported by NIH grants to A.K.R. (AA027915–01A1) and E.A.S. (NS118727–01A1).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable–no new data generated.
LITERATURE CITED
- Ahmed SH (2018). Trying to make sense of rodents’ drug choice behavior. Progress in Neuropsychopharmacology & Biological Psychiatry, 87, 3–10. doi: 10.1016/j.pnpbp.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, & Patchev VK (1998). Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. The Journal of Clinical Investigation, 101, 2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B, & Ehlers CL (2018). Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol, 73, 57–66. doi: 10.1016/j.alcohol.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin MD, Hser YI, & McGlothlin WH (1987). Sex differences in addict careers. 2. Becoming addicted. The American Journal of Drug and Alcohol Abuse, 13, 59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- Anker JJ, & Carroll ME (2010). Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug and Alcohol Dependence, 107, 264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, & Carroll ME (2011). Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. Current Topics in Behavioral Neurosciences, 8, 73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Arnold AP (2004). Sex chromosomes and brain gender. Nature Reviews. Neuroscience, 5, 701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Arnold AP (2009). Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. Journal of Neuroendocrinology, 21, 377–386. doi: 10.1111/j.1365-2826.2009.01831.x.Reviews approaches to studying sex chromosome effects in mice.
- Arnold AP, & Chen X (2009). What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in Neuroendocrinology, 30, 1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, … Heilig M (2018). A molecular mechanism for choosing alcohol over an alternative reward. Science, 360, 1321–1326. doi: 10.1126/science.aao1157. [DOI] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, & Negus SS (2015). Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Current Treatment Options in Psychiatry, 2, 136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, & Taylor JR (2010). Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. The Journal of Neuroscience, 30, 9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MR, McVey MM, & Boehm SL II. (2021). Three weeks of binge alcohol drinking generates increased alcohol front-loading and robust compulsive-like alcohol drinking in male and female C57BL/6J mice. Alcoholism, Clinical and Experimental Research, 45, 650–660. doi: 10.1111/acer.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2012). Animal models of excessive alcohol consumption in rodents. In Sommer WH & Spanagel R (Eds.), Behavioral neurobiology of alcohol addiction current topics in behavioral neurosciences (pp. 355–377). Berlin Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, … Young E (2005). Strategies and methods for research on sex differences in brain and behavior. Endocrinology, 146, 1650–1673. doi: 10.1210/en.2004-1142.Provides a thorough overview of approaches to studying hormonal influences on behavior, including consideration of estrous cycle effects.
- Becker JB, & Koob GF (2016). Sex differences in animal models: Focus on addiction. Pharmacological Reviews, 68, 242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, & Liang JW (2016). Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biology of Sex Differences, 7, 34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, & Zucker I (2011). Sex bias in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews, 35, 565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, & Becker JB (2019). Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology, 44, 2155–2158. doi: 10.1038/s41386-019-0524-3.Discusses many issues relevant to design and analysis of sex differences, including types of sex differences and approaches for analysis of each ptype.
- Bertholomey ML, Nagarajan V, & Torregrossa MM (2016). Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology, 233, 2277–2287. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blegen MB, da Silva E Silva D, Bock R, Morisot N, Ron D, & Alvarez VA (2018). Alcohol operant self-administration: Investigating how alcohol-seeking behaviors predict drinking in mice using two operant approaches. Alcohol, 67, 23–36. doi: 10.1016/j.alcohol.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, & Shaham Y (2013). The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology, 229, 453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, & Randall CL (1999). Gender differences in substance use disorders. The Psychiatric Clinics of North America, 22, 241–252. doi: 10.1016/S0193-953X(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Bronson FH, & Marsteller FA (1985). Effect of short-term food deprivation on reproduction in female mice. Biology of Reproduction, 33, 660–667. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, & Taft RA (2012). Mouse estrous cycle identification tool and images. PLOS One, 7, e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, & Mormède P (1999). Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Research, 842, 200–205. doi: 10.1016/S0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Cailhol S, & Mormède P (2001). Sex and strain differences in ethanol drinking: Effects of gonadectomy. Alcoholism, Clinical and Experimental Research, 25, 594–599. doi: 10.1111/j.1530-0277.2001.tb02255.x. [DOI] [PubMed] [Google Scholar]
- Campbell CS, Ryan KD, & Schwartz NB (1976). Estrous cycles in the mouse: Relative influence of continuous light and the presence of a male. Biology of Reproduction, 14, 292–299. doi: 10.1095/biolreprod14.3.292. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, & Heideman P (2001). Ketoconazole suppresses food restriction–induced increases in heroin self-administration in rats: Sex differences. Experimental and Clinical Psychopharmacology, 9, 307–316. doi: 10.1037/1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, & Meyer ER (2003). Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacology, Biochemistry, and Behavior, 74, 541–549. doi: 10.1016/S0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Cole MD, & George O (2017). Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology, 234, 2009–2018. doi: 10.1007/s00213-017-4608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, … Arnold AP (2002). A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of Neuroscience, 22, 9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaker MC, Moen JK, Robinson JM, Wickman K, & Lee AM (2020). Unequal interactions between alcohol and nicotine co-consumption: Suppression and enhancement of concurrent drug intake. Psychopharmacology, 237, 967–978. doi: 10.1007/s00213-019-05426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, & Piazza PV (2014). Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology, 76(Pt B), 437–449. doi: 10.1016/j.neuropharm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, & Brown C (1995). Nicotine self-administration in rats. Psychopharmacology, 122, 390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, … McCallum S (2000). Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology, 151, 392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, & Koob GF (1982). Heroin and cocaine intravenous self-administration in rats: Mediation by separate neural systems. Psychopharmacology, 78, 204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, & Fratta W (2009). Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology, 34(Suppl 1), S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, & Fratta W (2007). Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function: Sex differences in cannabinoid self-administration. British Journal of Pharmacology, 152, 795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, & Fratta W (2010). Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: Influence of ovarian hormones: Sex differences in relapse to cannabinoid-seeking. British Journal of Pharmacology, 160, 724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, & See RE (2011). Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: Sex differences and the role of the estrous cycle. Psychopharmacology, 216, 53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA (2020). The endocrine system and alcohol drinking in females. Alcohol Research: Current Reviews, 40, 02. doi: 10.35946/arcr.v40.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, & Samson HH (2002a). Ethanol consumption in the female Long–Evans rat: A modulatory role of estradiol. Alcohol, 26, 103–113. doi: 10.1016/S0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, & Samson HH (2002b). Microanalysis of ethanol self-administration: Estrous cycle phase-related changes in consumption patterns. Alcoholism, Clinical and Experimental Research, 26, 635–643. doi: 10.1111/j.1530-0277.2002.tb02585.x. [DOI] [PubMed] [Google Scholar]
- Forger NG, & Morin LP (1982). Reproductive state modulates ethanol intake in rats: Effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacology, Biochemistry, and Behavior, 17, 323–331. doi: 10.1016/0091-3057(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Beyerstein BL, & Alexander BK (1988). Contributions of taste factors and gender to opioid preference in C57BL and DBA mice. Psychopharmacology, 95, 237–244. doi: 10.1007/BF00174516. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, & See RE (2005). Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology, 179, 662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Hafeez H, Price ME, Baruffaldi F, Pravetoni M, … Schank JR (2019). Sex differences in oral oxycodone self-administration and stress-primed reinstatement in rats. Addiction Biology, 25, e12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, & Schank JR (2019). Sex differences in aversion-resistant ethanol intake in mice. Alcohol and Alcoholism, 54, 345–352. doi: 10.1093/alcalc/agz022. [DOI] [PubMed] [Google Scholar]
- Gaillard D, & Stratford JM (2016). Measurement of behavioral taste responses in mice: Two-bottle preference, lickometer, and conditioned taste-aversion tests. Current Protocols in Mouse Biology, 6, 380–407. doi: 10.1002/cpmo.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galankin T, Shekunova E, & Zvartau E (2010). Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Hormones and Behavior, 58, 827–834. doi: 10.1016/j.yhbeh.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Goltseker K, Hopf FW, & Barak S (2019). Advances in behavioral animal models of alcohol use disorder. Alcohol, 74, 73–82. doi: 10.1016/j.alcohol.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, & Lumeng L (1999). Limited access alcohol drinking in high- and low-alcohol preferring selected lines of mice. Alcoholism, Clinical and Experimental Research, 23, 1015–1022. doi: 10.1111/j.1530-0277.1999.tb04219.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, … Hasin DS (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry, 74, 911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, … Van de Kar LD (2005). Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology, 179, 769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, & Booze RM (2004). Sex differences and repeated intravenous nicotine: Behavioral sensitization and dopamine receptors. Pharmacology, Biochemistry, and Behavior, 78, 581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Roberts AJ, Schulteis G, & Koob GF (1999). Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcoholism, Clinical and Experimental Research, 23, 1468–1476. doi: 10.1111/j.1530-0277.1999.tb04669.x. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, & Lasek AW (2018). Estradiol enhances ethanol reward in female mice through activation of ERα and ERβ. Hormones and Behavior, 98, 159–164. doi: 10.1016/j.yhbeh.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, & Lesscher HMB (2014). Rodent models for compulsive alcohol intake. Alcohol, 48, 253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN (2007). Sex does matter: Comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behavioural Pharmacology, 18, 583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, & Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, Clinical and Experimental Research, 35, 1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Ravi S, Beltsos AN, Murphy LL, & Olson LM (1999). Abnormal estrous cyclicity after disruption of endothelial and inducible nitric oxide synthase in mice. Biology of Reproduction, 61, 171–177. doi: 10.1095/biolreprod61.1.171. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, & Becker JB (2006). Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology, 31, 129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, … Calipari ES (2019). Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology, 44, 1189–1197. doi: 10.1038/s41386-019-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez J, & Barrios de Tomasi E (1999). Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol, 19, 15–22. doi: 10.1016/S0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, & Holmes A (2017). Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol, 58, 53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, & Schenken RS (1993). Chronic cocaine disruption of estrous cyclicity in the rat: Dose-dependent effects. The Journal of Pharmacology and Experimental Therapeutics, 264, 29–34. [PubMed] [Google Scholar]
- Klein LC (2001). Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Experimental and Clinical Psychopharmacology, 9, 251–261. doi: 10.1037/1064-1297.9.3.251. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, & Soyka M (2018). Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA: The journal of the American Medical Association, 320, 815–824. doi: 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, & Spiegel KS (1992). Sex differences in pattern of drinking. Alcohol, 9, 415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lee SK (2018). Sex as an important biological variable in biomedical research. BMB Reports, 51, 167–173. doi: 10.5483/BMBRep.2018.51.4.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen P, Han X, Zuo W, Mei Q, Bian EY, … Ye J (2019). Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. International Journal of Physiology, Pathophysiology and Pharmacology, 11, 163–176. [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ (2008). Acquisition and maintenance of cocaine self-administration in adolescent rats: Effects of sex and gonadal hormones. Psychopharmacology, 197, 237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, & Carroll ME (2000). Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology, 152, 132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, & Carroll ME (1999). Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology, 144, 77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, & Carroll ME (2000). Reinstatement of cocaine self-administration in rats: Sex differences. Psychopharmacology, 148, 196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, & Carroll ME (2002). Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology, 164, 121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mamlouk GM, Dorris DM, Barrett LR, & Meitzen J (2020). Sex bias and omission in neuroscience research is influenced by research model and journal, but not reported NIH funding. Frontiers in Neuroendocrinology, 57, 100835. doi: 10.1016/j.yfrne.2020.100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, & Tanno AP (2002). Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian Journal of Biology, 62, 609–614. doi: 10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Marsh JC, Park K, Lin Y-A, & Bersamira C (2018). Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. Journal of Substance Abuse Treatment, 87, 79–85. doi: 10.1016/j.jsat.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, … Mermelstein PG (2016). Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro, 3, ENEURO.0140–16.2016. doi: 10.1523/ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, & Mermelstein PG (2014). Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behavioural Brain Research, 271, 39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, & Chartoff E (2017). Oxycodone self-administration in male and female rats. Psychopharmacology, 234, 977–987. doi: 10.1007/s00213-017-4536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, & De Vries GJ (2012). Sex differences in the brain: The not so inconvenient truth. The Journal of Neuroscience, 32, 2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012.Reviews experimental design and approaches to studying sex differences in neuroscience studies.
- McHugh RK, Votaw VR, Sugarman DE, & Greenfield SF (2018). Sex and gender differences in substance use disorders. Clinical Psychology Review, 66, 12–23. doi: 10.1016/j.cpr.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Nolan ZT, Colar D, Moore EM, & Boehm SL 2nd. (2017). Activation of extrasynaptic δ-GABAA receptors globally or within the posterior-VTA has estrous-dependent effects on consumption of alcohol and estrous-independent effects on locomotion. Hormones and Behavior, 95, 65–75. doi: 10.1016/j.yhbeh.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, & Boehm SL 2nd. (2013). Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacology, Biochemistry, and Behavior, 104, 177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Brown LL, & Crabbe JC (2011). Limited access ethanol drinking in the dark in adolescent and adult mice. Pharmacology, Biochemistry, and Behavior, 98, 279–285. doi: 10.1016/j.pbb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, & McGroarty KK (1999). Ethanol consumption by C57BL/6 mice: Influence of gender and procedural variables. Alcohol, 17, 175–183. doi: 10.1016/S0741-8329(98)00055-X. [DOI] [PubMed] [Google Scholar]
- Monroe SC, & Radke AK (2020). Aversion-resistant fentanyl self-administration in mice. Psychopharmacology, 238, 699–710. doi: 10.1007/s00213-020-05722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Flanagan J, & Brady K (2014). Ovarian hormones and drug abuse. Current Psychiatry Reports, 16, 511. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Ortiz MM, Gantz SC, Tunstall BJ, Marchette RCN, Bonci A, … Vendruscolo LF (2020). Fentanyl vapor self-administration model in mice to study opioid addiction. Science Advances, 6, eabc0413. doi: 10.1126/sciadv.abc0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KY, Yong J, & Chakraborty TR (2010). Estrous cycle in ob/ob and ovariectomized female mice and its relation with estrogen and leptin. Physiology & Behavior, 99, 125–130. doi: 10.1016/j.physbeh.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You Z-B, … Ikemoto S (2019). Incubation of cocaine craving after intermittent-access self-administration: Sex differences and estrous cycle. Biological Psychiatry, 85, 915–924. doi: 10.1016/j.biopsych.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Policy on Sex as a Biological Variable (n.d.). Retrieved from https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable.
- O’Connor EC, Chapman K, Butler P, & Mead AN (2011). The predictive validity of the rat self-administration model for abuse liability. Neuroscience and Biobehavioral Reviews, 35, 912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Pang RD, Zvolensky MJ, Schmidt NB, & Leventhal AM (2015). Gender differences in negative reinforcement smoking expectancies. Nicotine & Tobacco Research, 17, 750–754. doi: 10.1093/ntr/ntu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, & Goldberg SR (2007). Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction, 102, 1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, & McKee SA (2019). Sex differences in stress-related alcohol use. Neurobiology of Stress, 10, 100149. doi: 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (2001). Smoking cessation in women. Special considerations. CNS Drugs, 15, 391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, & Meisel RL (2015). Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Structure & Function, 220, 2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, … Root DH (2019). Oral prescription opioid-seeking behavior in male and female mice. Addiction Biology, 25, e12828. doi: 10.1111/adb.12828. [DOI] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, & Yeager RD (1989). Telescoping of alcoholism in women alcoholics. The International Journal of the Addictions, 24, 19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- Pickens R (1968). Self-administration of stimulants by rats. The International Journal of the Addictions, 3, 215–221. doi: 10.3109/10826086809042896. [DOI] [Google Scholar]
- Prendergast BJ, Onishi KG, & Zucker I (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews, 40, 1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, & Vendruscolo LF (2017). Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacology, Biochemistry, and Behavior, 152, 61–67. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, & Taylor JR (2007). Sex chromosome complement regulates habit formation. Nature Neuroscience, 10, 1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Radke AK, Gewirtz JC, & Carroll ME (2015). Effects of age, but not sex, on elevated startle during withdrawal from acute morphine in adolescent and adult rats. Behavioural Pharmacology, 26, 485–488. doi: 10.1097/FBP.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Held IT, Sneddon EA, Riddle CA, & Quinn JJ (2020). Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking. Addiction Biology, 25, e12829. doi: 10.1111/adb.12829. [DOI] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC, & Carroll ME (2013). Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology, 227, 117–126. doi: 10.1007/s00213-012-2945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, … Holmes A (2017). Chronic EtOH effects on putative measures of compulsive behavior in mice. Addiction Biology, 22, 423–434. doi: 10.1111/adb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Sneddon EA, Frasier RM, & Hopf FW (2021). Recent perspectives on sex differences in compulsion-like and binge alcohol drinking. International Journal of Molecular Sciences, 22, 3788. doi: 10.3390/ijms22073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Qin G, Ii M, Silver M, Hamada H, … Kishore R (2007). Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology, 148, 3618–3624. doi: 10.1210/en.2006-1357. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, & See RE (2012). Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences in rats. Psychopharmacology, 223, 371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Marinelli PW, Bennett SM, Fiscale LT, Narciso SP, Oparowski CJ, … Gianoulakis C (2002). One injection of estradiol valerate induces dramatic changes in rats’ intake of alcoholic beverages. Pharmacology, Biochemistry, and Behavior, 72, 601–616. doi: 10.1016/S0091-3057(02)00732-3. [DOI] [PubMed] [Google Scholar]
- Reid ML, Hubbell CL, & Reid LD (2003). A pharmacological dose of estradiol can enhance appetites for alcoholic beverages. Pharmacology, Biochemistry, and Behavior, 74, 381–388. doi: 10.1016/S0091-3057(02)01008-0. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior, 84, 53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richardson NR, & Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods, 66, 1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, & Koob GF (1998). Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism, Clinical and Experimental Research, 22, 1564–1569. doi: 10.1111/j.1530-0277.1998.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, & Vickers GJ (1989). The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology, 98, 408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, McCulley WD 3rd, & Fecteau M (2014). Circadian activity rhythms and voluntary ethanol intake in male and female ethanol-preferring rats: Effects of long-term ethanol access. Alcohol, 48, 647–655. doi: 10.1016/j.alcohol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, & Carroll ME (2004). Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology, 172, 443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, & Carroll ME (2002). Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacology, Biochemistry, and Behavior, 72, 313–318. doi: 10.1016/S0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, & Sulcova A (2015). Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Frontiers in Psychiatry, 6, 91. doi: 10.3389/fpsyt.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, & Quinones-Jenab V (2003). Sex differences in the conditioned rewarding effects of cocaine. Brain Research, 970, 214–220. doi: 10.1016/S0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, & Czachowski CL (2003). Behavioral measures of alcohol self-administration and intake control: Rodent models. International Review of Neurobiology, 54, 107–143. doi: 10.1016/S0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, & Tolliver GA (1988). Oral ethanol self-administration in rats: Models of alcohol-seeking behavior. Alcoholism: Clinical and Experimental Research, 12, 591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, & Guerri C (1985). Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels. Pharmacology, Biochemistry, and Behavior, 23, 221–224. doi: 10.1016/0091-3057(85)90560-X. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, & Spanagel R (2006). Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addiction Biology, 11, 2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, & Lasek AW (2018). Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcoholism, Clinical and Experimental Research, 42, 286–294. doi: 10.1111/acer.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, & Kuhn CM (2014). Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology, 231, 1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, & Thompson T (1969). Self administration of and behavioral dependence on drugs. Annual Review of Pharmacology, 9, 483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, … Hopf FW (2013). Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature Neuroscience, 16, 1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Groman SM, Arnold AP, & Jentsch JD (2014). Sex chromosome complement influences operant responding for a palatable food in mice. Genes, Brain, and Behavior, 13, 527–534. doi: 10.1111/gbb.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM (2018). Sex differences in behavioral strategies: Avoiding interpretational pitfalls. Current Opinion in Neurobiology, 49, 95–98. doi: 10.1016/j.conb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Shansky RM (2019). Are hormones a “female problem” for animal research? Science, 364, 825–826. doi: 10.1126/science.aaw7570. [DOI] [PubMed] [Google Scholar]
- Shuey DL, Stump DG, Carliss RD, & Gerson RJ (2008). Effects of the opioid analgesic oxymorphone hydrochloride on reproductive function in male and female rats. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 83, 12–18. doi: 10.1002/bdrb.20138. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, & Bartlett SE (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, Clinical and Experimental Research, 32, 1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Greer A, Dougen B, & Carroll ME (2020). Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology, 237, 453–463. doi: 10.1007/s00213-019-05381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LC, Kallupi M, Tieu L, Shankar K, Jaquish A, Barr J, … George O (2020). Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology, 45, 1909–1919. doi: 10.1038/s41386-020-0734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A, & Radke AK (2020). Increased responding for alcohol and resistance to aversion in female ice. Alcoholism, Clinical and Experimental Research, 44, 1400–1409. doi: 10.1111/acer.14384. [DOI] [PubMed] [Google Scholar]
- Sneddon EA, Schuh KM, Frankel JW, & Radke AK (2021). The contribution of medium spiny neuron subtypes in the nucleus accumbens core to compulsive-like ethanol drinking. Neuropharmacology, 187, 108497. doi: 10.1016/j.neuropharm.2021.108497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, White RD, & Radke AK (2019). Sex differences in binge-like and aversion-resistant alcohol drinking in C57BL/6J mice. Alcoholism, Clinical and Experimental Research, 43, 243–249. doi: 10.1111/acer.13923. [DOI] [PubMed] [Google Scholar]
- Spanagel R (2017). Animal models of addiction. Dialogues in Clinical Neuroscience, 19, 247–258. doi: 10.31887/DCNS.2017.19.3/rspanagel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, & Glowa JR (1998). Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacology, 139, 169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, & Carroll ME (2016). Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology, 233, 1005–1013. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL, & Crabbe JC (2008). Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcoholism, Clinical and Experimental Research, 32, 2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tan S, Xue S, Behnood-Rod A, Chellian R, Wilson R, Knight P, … Bruijnzeel AW (2019). Sex differences in the reward deficit and somatic signs associated with precipitated nicotine withdrawal in rats. Neuropharmacology, 160, 107756. doi: 10.1016/j.neuropharm.2019.107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48, 235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2005). Chronic intravenous drug self-administration in rats and mice. Current Protocols in Neuroscience, 32, 9.20.1–9.20.40. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2007). Intravenous drug self-administration in mice: Practical considerations. Behavior Genetics, 37, 101–118. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, & Banks ML (2019). Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology, 44, 2022–2029. doi: 10.1038/s41386-019-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropp J, & Markus EJ (2001). Effects of mild food deprivation on the estrous cycle of rats. Physiology & Behavior, 73, 553–559. doi: 10.1016/s0031-9384(01)00487-5. [DOI] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, & Volpicelli LA (1995). Opiates and alcohol self-administration in animals. The Journal of Clinical Psychiatry, 56(Suppl 7), 5–14. [PubMed] [Google Scholar]
- Vandegrift BJ, Hilderbrand ER, Satta R, Tai R, He D, You C, … Lasek AW (2020). Estrogen receptor α regulates ethanol excitation of ventral tegmental area neurons and binge drinking in female mice. The Journal of Neuroscience, 40, 5196–5207. doi: 10.1523/JNEUROSCI.2364-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2004). Acute ethanol withdrawal (Hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism, Clinical and Experimental Research, 28, 40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, … Shaham Y (2018). Volitional social interaction prevents drug addiction in rat models. Nature Neuroscience, 21, 1520–1529. doi: 10.1038/s41593-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, & Boedicker AE (2000). Course, severity, and treatment of substance abuse among women versus men. The American Journal of Drug and Alcohol Abuse, 26, 523–535. doi: 10.1081/ADA-100101893. [DOI] [PubMed] [Google Scholar]
- White A, Castle I-JP, Chen CM, Shirley M, Roach D, & Hingson R (2015). Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcoholism, Clinical and Experimental Research, 39, 1712–1726. doi: 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- Will TR, Proaño SB, Thomas AM, Kunz LM, Thompson KC, Ginnari LA, … Meitzen J (2017). Problems and progress regarding sex bias and omission in neuroscience research. eNeuro, 4, ENEURO.0278–17.2017. doi: 10.1523/ENEURO.0278-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, & Pogun S (2010). Nicotine-induced conditioned place preference in rats: Sex differences and the role of mGluR5 receptors. Neuropharmacology, 58, 374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Zanni G, Featherstone BS, DeSalle MJ, Deutsch HM, Barr GA, & Eisch AJ (2019). Development and validation of a novel oral oxycodone self-administration protocol for female and male rats. Cold Spring Harbor Laboratory, 690735. Retrieved from 10.1101/690735v1.abstract. [DOI]


