Supplemental Digital Content is available in the text.
Keywords: biomarker, cardiotoxicity, mortality, myocardium, troponin
Abstract
Background:
Our goal was to evaluate the ability of cardiovascular magnetic resonance for detecting and predicting cardiac dysfunction in patients receiving cancer therapy. Left ventricular ejection fraction, global and regional strain utilizing fast-strain-encoded, T1 and T2 mapping, and cardiac biomarkers (troponin and BNP [brain natriuretic peptide]) were analyzed.
Methods:
Sixty-one patients (47 with breast cancer, 11 with non-Hodgkin lymphoma, and 3 with Hodgkin lymphoma) underwent cardiovascular magnetic resonance scans at baseline and at regular intervals during 2 years of follow-up. The percentage of all left ventricular myocardial segments with strain ≤−17% (normal myocardium [%]) was analyzed. Clinical cardiotoxicity (CTX) and sub-CTX were defined according to standard measures.
Results:
Nine (15%) patients developed CTX, 26 (43%) had sub-CTX. Of the 35 patients with CTX or sub-CTX, 24 (69%) were treated with cardioprotective medications and showed recovery of cardiac function. The amount of normal myocardium (%) exhibited markedly higher accuracy for the detection of CTX and sub-CTX compared with left ventricular ejection fraction, T1, and T2 mapping as well as troponin I (Δareas under the curve=0.20, 0.24, and 0.46 for normal myocardium (%) versus left ventricular ejection fraction, troponin I, and T1 mapping, P<0.001 for all). In addition, normal myocardium (%) at baseline accurately identified patients with subsequent CTX (P<0.001), which was not achieved by any other markers.
Conclusions:
Normal myocardium (%) derived by fast-strain-encoded cardiovascular magnetic resonance, is an accurate and sensitive tool that can establish cardiac safety in patients with cancer undergoing cardiotoxic chemotherapy not only for the early detection but also for the prediction of those at risk of developing CTX.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03543228.
Clinical Perspective.
Advances in oncology have significantly improved the mortality rates of patients with cancer. However, these positive trends are offset by the possible cardiotoxic effects of chemotherapy. These adverse effects of chemotherapy on the cardiovascular system are summarized under the term cardiotoxicity and can be a common cause of death after successful treatment for cancer. The early detection and especially the prediction of cardiotoxicity, therefore, represent an unmet clinical need. In our study, we showed that cardiovascular magnetic resonance is an excellent tool for serial monitoring the cardiovascular health of patients with cancer. In this regard, we found that the amount of normal myocardium (%) measured using fast-strain-encoded and defined as percentage of segments with normal strain (≤−17%) can accurately identify patients who develop clinical and subclinical cardiotoxicity and is also the only independent predictor of cardiotoxicity. In addition, we found that this imaging marker, if measured just before the start of cardiovascular protective therapies (ie, ACE [angiotensin-converting enzyme]-inhibitors/angiotensin II receptor blockers or β-blocker) can accurately predict recovery from cardiotoxicity. In conclusion, normal myocardium (%) can fulfill a triple diagnostic function in such patients, aiding (1) early cardiotoxicity detection, (2) cardiotoxicity prediction before chemotherapy initiation using baseline normal myocardium (%) values, and (3) prediction of recovery from cardiotoxicity after established cardiotoxicity using normal myocardium (%) before the initiation of cardioprotective treatment. Because all cardiovascular magnetic resonance measures can be performed without the use of ionizing radiation and even using a purely noncontrast cardiovascular magnetic resonance protocol, the potential of our findings for translation into the clinical realm is high.
As a result of major advancements in cancer therapeutics over the past 2 decades, there has been substantial improvement in cancer-related mortality; however, there are important long-term sequelae from these treatments including cardiovascular dysfunction.1 Interestingly, cardiac damage because of cancer therapy can be a common cause of death, especially after ≥5 years of successful treatment for cancer.2 Thus, the early recognition or even the prediction of myocardial damage due to cardiotoxicity may allow the proactive modification of treatment regimens or accurate identification of those who would benefit from preventive cardioprotection. Indeed, this has become a top 10 priority within the discipline of Cardio-Oncology.3
Several imaging parameters and biomarkers were shown to be useful for the diagnostic workup and monitoring of cardiotoxicity.4 Measurement of left ventricular ejection fraction (LVEF) is the most commonly used parameter in clinical practice.5 Although LVEF is a widely accepted marker of global systolic performance, relevant reductions in LVEF are seen only when irreversible damage to the myocardium has occurred.6,7 Myocardial strain, however, can detect subclinical damage due to cancer therapy before LVEF changes occur.8 In this regard, the versatility of cardiovascular magnetic resonance imaging (CMR) provides the acquisition of LVEF, strain, and tissue characteristics, including T1 and T2 mapping in a single examination and without exposing the patients to contrast agents or radiation. This makes CMR an ideal tool for longitudinal studies in such patients.9 Several studies have demonstrated the ability of CMR to detect subclinical cardiotoxicity (sub-CTX).10 However, limited data are available comparing the ability of strain and T1 or T2 mapping, whereas the ability of such markers for the prediction of cardiotoxicity or recovery from cardiotoxicity (REC) of cardiac function during treatment with cardioprotective agents is largely unknown.
Therefore, the aim of this study was to prospectively evaluate and compare the ability of LVEF, myocardial strain using fast-strain-encoded (SENC), T1 and T2 mapping parameters, and cardiac biomarkers for the early detection and monitoring of cardiac dysfunction in patients who undergo chemotherapy due to breast cancer and lymphoma. Additionally, we sought to explore if baseline LV myocardial strain by CMR can accurately identify those patients at high risk for cardiotoxicity during chemotherapy for cancer.
Methods
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
The PREFECT Study (Proactive Evaluation of Function to Evade Cardiotoxicity) was a prospective, single-center, nonrandomized study that included patients with cancer scheduled to receive chemotherapy. Patients must have had a CMR examination (including fast-SENC–derived myocardial strain and T1 and T2 mapping acquisitions) before starting chemotherapy and at regular intervals of 3 to 6 months thereafter. The study was approved by the local ethics committee (PV5292) of the University Hospital Hamburg, all patients gave written informed consent, and the study complied with the Declaration of Helsinki.
Study Population
Patients with breast cancer, Non-Hodgkin, or Hodgkin lymphoma receiving chemotherapy, all being treated with curative intent, were included in this study. Exclusion criteria included age<18 or >80 years, inability to give informed consent, pregnancy, previous chemotherapy, contraindications to CMR examination, and renal failure with a glomerular filtration rate<30 mL/(kg·m2).
Before initiating chemotherapy, clinical, and demographic data, including cardiovascular risk factors, such as arterial hypertension, dyslipidemia, diabetes, family history of coronary artery disease, and smoking status were collected. Medications were recorded with a focus on cardioprotective medication, including ACE (angiotensin-converting enzyme)-inhibitors or angiotensin II receptor blockers (ARBs) and β-blockers. Blood samples were drawn and a complete blood count, creatinine, BNP (brain natriuretic peptide), and troponin I were measured at baseline. Before starting chemotherapy, a complete CMR examination was performed in all patients.
Chemotherapy
Anticancer treatments were managed at the discretion of the attending oncologist. Patients with breast cancer were treated with a total of 4 cycles of epirubicin and cyclophosphamide every 3 weeks followed by 12 weekly cycles of paclitaxel (epirubicin, cyclophosphamide, paclitaxel), with a few receiving dose-dense (every 2 weeks) epirubicin, cyclophosphamide, paclitaxel (total 8 cycles). Patients with HER2+ breast cancer were treated with trastuzumab. Radiation therapy was additionally performed based on current guidelines and at the discretion of the attending oncology team. Non-Hodgkin lymphoma patients were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone with the monoclonal antibody rituximab (rituximab - cyclophosphamide, doxorubicine, vincristien and prednison), whereas patients with Hodgkin lymphoma were treated with anthracycline-based therapy with or without radiation therapy.
CMR Examination
All CMR examinations were performed using a 1.5 Tesla Achieva MRI scanner (Philips Healthcare, Best, the Netherlands). A detailed CMR protocol for conventional, fast-SENC, and mapping is presented in Appendix A. Time spent was ≈10 to 12 minutes for both baseline and follow-up CMR scans. T1 maps were acquired using a standard Modified Look-Locker Inversion Recovery 5s(3s)3s T1-native sequence in standard midventricular short-axis views. T2 Maps were acquired using an mGRASE sequence with 9 echos. Lastly, 6 fast-SENC acquisitions were performed (3 short axes—basal, mid, and apical and 3 long axes—4 chambers, 2 chambers, and 3 chambers).
Analysis of CMR Data
CMR data were imported in a dedicated software (CVI 42, Circle Cardiovascular Imaging, Inc, Calgary, Canada). Conventional and morphological parameters were extracted from cine images as can be found in the appendix. T1 and T2 values were extracted from the septal segments of midventricular short-axis T1 and T2 mapping acquisitions. Fast-SENC images were assessed using dedicated software (Myocardial Solutions, Inc, Morrisville, NC). The analysis of the data for extracting longitudinal and circumferential strain can also be found in the appendix. Longitudinal strain (LS) was derived from the short-axis acquisitions and circumferential strain (CS) from long-axis acquisitions. Global LS (GLS) was calculated as an average of the 16 segments with longitudinal strain and global CS as an average of the 21 segments measuring circumferential strain, as described previously.11
As reported in previous studies, a value for LS or CS in any segment ≤−17% was considered normal.12,13 The percentage of normal myocardium in each patient is the ratio between the total number of segments expressing normal myocardium, that is, LS≤−17% (out of n=16) and CS≤−17% (out of n=21) divided by the total number of segments analyzed (37 segments in total), as described previously11:
 |
Strain values in longitudinal and circumferential directions are negative and are reported as such in our results section. Throughout the text, however, we refer to the absolute strain values (ie, higher strain values standing for increased deformation).
CMR and Clinical Follow-Up Examinations
Patients were followed at regular time intervals clinically and with CMR. For the CMR examination, patients were planned at regular intervals of 3 to 6 months, or when a cumulative dose of anthracycline greater than 150 mg/m2 was reached, or when clinically indicated.14 Clinical follow-up included medical history with New York Heart Association class assessment and standard clinical examination as well as blood testing to assess cardiac biomarkers (troponin I and BNP). With follow-up CMR studies, morphology and function, including strain by fast-SENC and T1 and T2 mapping were assessed.
Definitions and Clinical End Points for Cardiotoxicity and Sub-CTX
Cardiotoxicity was diagnosed based on current imaging recommendations for significant cardiac damage resulting from chemotherapy.5,15 Thus, cardiotoxicity was defined as a reduction of at least 10 percentage points of LVEF to below 53% in patients who develop symptoms of heart failure such as fatigue, dyspnea, or edema. Sub-CTX was defined as a reduction in the average GLS value of at least 15% compared with baseline GLS in patients who are asymptomatic. The group without cardiotoxicity (no cardiotoxicity) had neither a change in LVEF or GLS at these thresholds.
Initiation of Cardioprotective Therapies and Prediction of REC
Patients who developed Sub-CTX or cardiotoxicity (at clinical or CMR examination) were started on cardioprotective medications (ACE inhibitors or ARBs or β-blocker) at the discretion of the attending cardiologist. If improvement of myocardial function was reached, patients were classified as achieving REC. The ability of CMR variables, which were acquired before treatment initiation with cardioprotective therapies, such as β-blockers, ACE inhibitors, or the combination of both, was tested for the prediction for REC versus non-REC in patients with cardiotoxicity.
Statistical Analysis
Data are presented as mean±SD for normally distributed continuous variables, otherwise as median and interquartile range, or as counts and percentage for categorical variables. The Kolmogorov-Smirnov test was used to test for normal distribution. Differences between ≥3 groups of continuous variables were tested using ANOVA with the Scheffé test for post hoc analysis. The Mann-Whitney test was used to compare ordinal variables and Fisher test to compare nominal variables. Kruskal-Wallis tests were used to compare differences between patients with cardiotoxicity versus those without cardiotoxicity and REC. In addition, Conover post hoc analysis was used to compare the 3 different groups with each other. Receiver operating characteristic (ROC) analysis was performed to determine the optimal cutoff points for sensitivity and specificity in predicting the development of cardiotoxicity. Comparison of the areas under the curve of paired data ROC curves was performed using the DeLong method.16 Multiple regression analysis with an enter method was performed to test the ability of clinical and CMR variables and clinical status to predict patients who develop cardiotoxicity (both clinical and subclinical). In addition, the same type of analysis was employed to test the variables associated with REC in patients who experienced cardiotoxicity after initiation of chemotherapy. In addition, Cox proportional hazard regression models were constructed for clinical variables, CMR parameters, and biomarkers. Kaplan-Meyer analyses were used to predict the value of ROC-optimized cutoff values for the prediction of cardiotoxicity or sub-CTX. A P<0.05 was considered statistically significant. All statistical analyses were implemented using the MedCalc software version 18.11.6 (MedCalc, Ostend, Belgium, 2019).
Results
Clinical and CMR Data
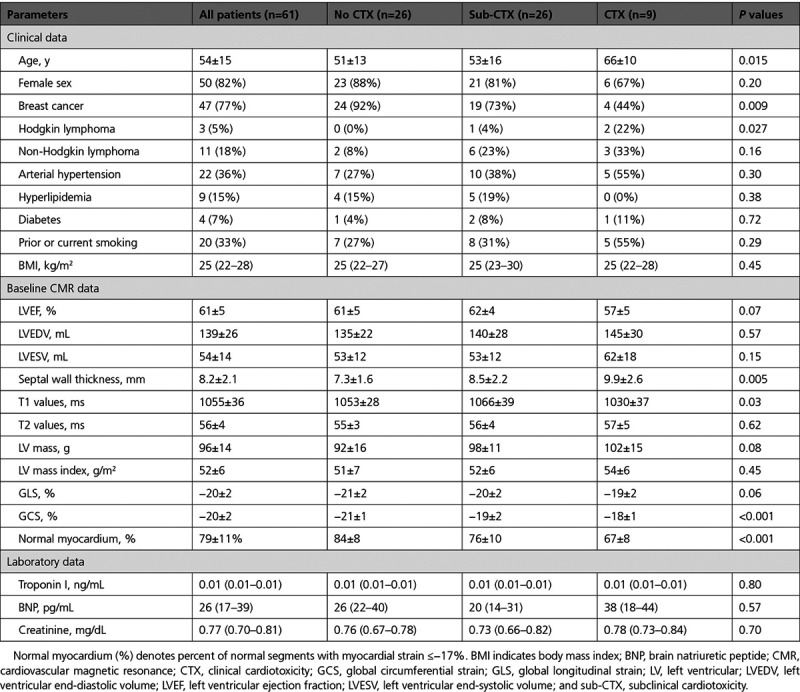
Out of 61 patients, 47 (77%) had breast cancer, 11 (18%) had non-Hodgkin, and 3 (5%) had Hodgkin lymphomas. Patients who developed cardiotoxicity were older (66±10, P=0.02), had higher T1 values (1030±37 ms), and lower percentage of normal myocardium (67%±8; Table 1). Chemotherapy included anthracyclines, taxanes, monoclonal antibodies, and alkylating agents in 60 (98%), 47 (77%), 18 (30%), and 17 (28%) patients, respectively. Fewer patients (8 %) received radiation therapy.
Table 1.
Clinical and Baseline CMR Data
Longitudinal CMR and Pharmacological Data
Nine (15%) patients went through phases of sub-CTX and cardiotoxicity, 26 (43%) showed only sub-CTX, whereas 26 (43%) exhibited no cardiotoxicity. Of 35 patients with cardiotoxicity or sub-CTX, 24 (69%) achieved REC in at least one of the follow-up CMR scans.
At least 2 follow-up CMR scans, after 5±2 months and 8±4 months, respectively, were available in all patients. Three, 4, and 5 follow-up CMR scans respectively were available in 58 (95%; 15±5 months), 48 (79%; 26±11 months), and 20 (33%) patients (29±7 months). β-Blockers and ACE inhibitors or ARBs were administrated in 24 (39%) and 32 (52%) patients, respectively. Twenty-one (34%) received combination therapy with β-Blockers and ACE inhibitors or ARBs.
Time Response of CMR Variables in Patients Without Cardiotoxicity and With Cardiotoxicity Versus Without REC
Looking at the time response of CMR variables during chemotherapy, normal myocardium (%) clearly differentiated between cardiotoxicity and non-cardiotoxicity (with and without subsequent REC) during the early stages after initiation of chemotherapy and simultaneously separated patients with cardiotoxicity and non-REC from those with REC during later stages. With LVEF, differences between the 3 groups were significant only during the first follow-up CMR scan and not throughout all other time points (Figure 1A and 1B).
Figure 1.
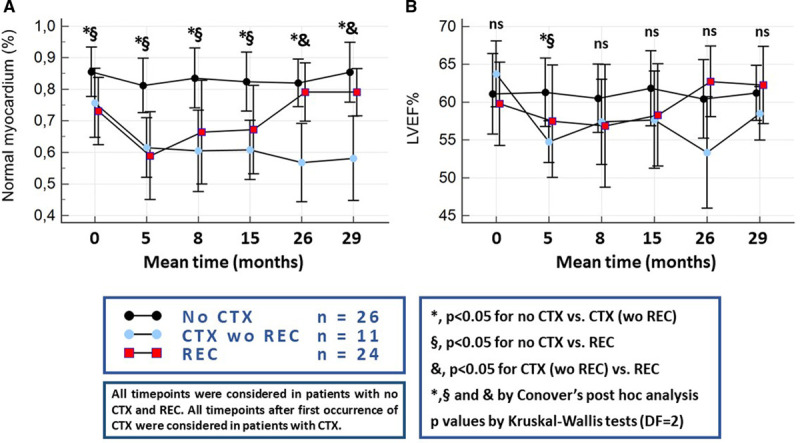
Evolution of the studied parameters over time in patients without clinical cardiotoxicity (no CTX), those with CTX without recovery from cardiotoxicity (CTX wo REC) and those who achieved REC. (A) Normal myocardium (%), (B) left ventricular ejection fraction. wo indicates without.
The time response for cardiac biomarkers troponin I and BNP are provided in Figure I in the Data Supplement.
Correlative Analysis and Detection of Cardiotoxicity
Good correlations were shown between global CS, GLS, and normal myocardium (%) Figure 2A and 2B. A moderate correlation, however, was observed between normal myocardium (%) and LVEF (Figure 2C). This was attributed to the presence of diminished strain with preserved LVEF≥53%, in patients with sub-CTX or CTX (green points within the dashed box in Figure 2C).
Figure 2.
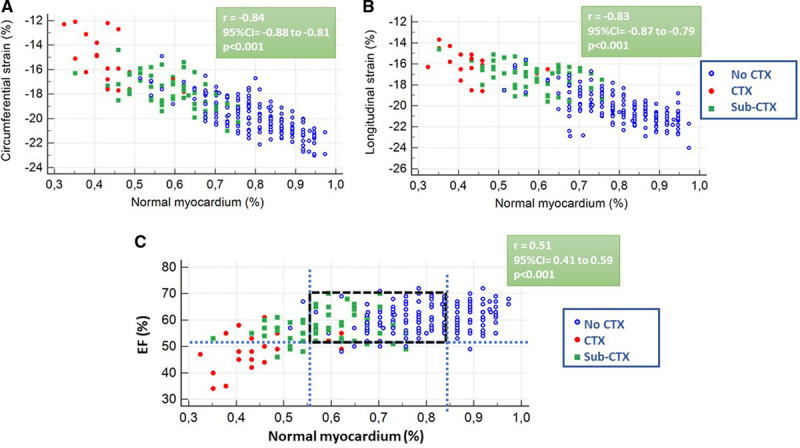
Correlation between strain, left ventricular ejection fraction (EF), and normal myocardium (%). (A) Correlation between circumferential strain and normal myocardium (%). (B) Correlation between longitudinal strain and normal myocardium (%). (C) Correlation between left ventricular EF and normal myocardium. CTX indicates clinical cardiotoxicity; and sub-CTX, subclinical cardiotoxicity.
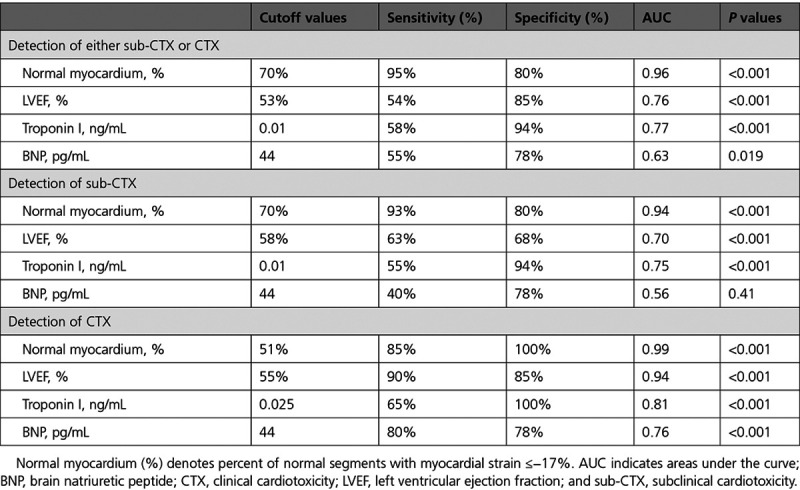
In addition, normal myocardium (%) demonstrated superior diagnostic value over T1, T2, BNP, and troponin I (Figure II in the Data Supplement). Corresponding sensitivities and specificities for sub-CTX and cardiotoxicity detection are shown in Table 2.
Table 2.
Sensitivities, Specificities, and AUC for the Sub-CTX and CTX Detection
Cardiotoxicity Prediction and Prediction of REC After Occurrence of Cardiotoxicity
Baseline normal myocardium (%) but not LVEF significantly differed between patients with subsequent sub-CTX and cardiotoxicity versus those without cardiotoxicity (Figure 3A and 3B).
Figure 3.
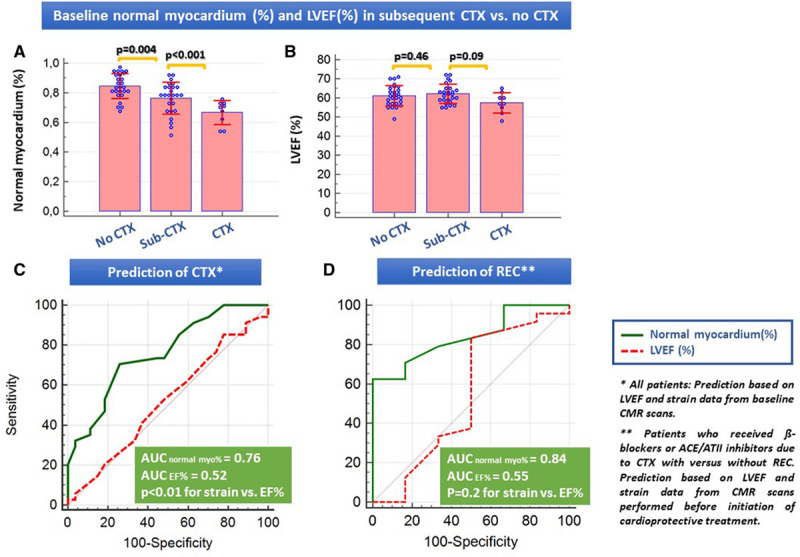
Evaluation of the baseline values of normal myocardium (%) and left ventricular ejection fraction and prediction of cardiotoxicity (CTX) and recovery (REC). A, Differences between baseline values of (A) normal myocardium (%) and (B) left ventricular ejection fraction (LVEF) in patients with versus without CTX. C, Receiver operating characteristic (ROC) analysis for CTX prediction based on baseline cardiovascular magnetic resonance (CMR) values. D, ROC analysis for the prediction of REC in patients who had CTX and received cardioprotective treatment with β-blockers, ACE (angiotensin-converting enzyme)/angiotensin II receptor blockers or combination of both (Note that pooled data were used for this analysis, derived from CMR examinations just before the initiation of the cardioprotective therapies). AUC indicates areas under the curve.
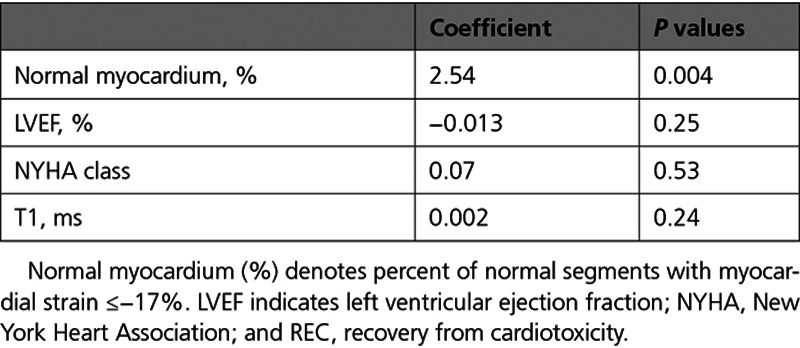
Thus, baseline normal myocardium (%) but not LVEF% enabled prediction of cardiotoxicity (Figure 3C), whereas normal myocardium (%) after occurrence of cardiotoxicity and before initiation of cardioprotective therapy accurately predicted REC (Figure 3D and Table 3). In addition, normal myocardium (%) was the only parameter independently related to cardiotoxicity and to REC prediction in multiple regression analysis (Tables 4 and 5).
Table 3.
Sensitivities, Specificities, and AUC for Sub-CTX and CTX Prediction
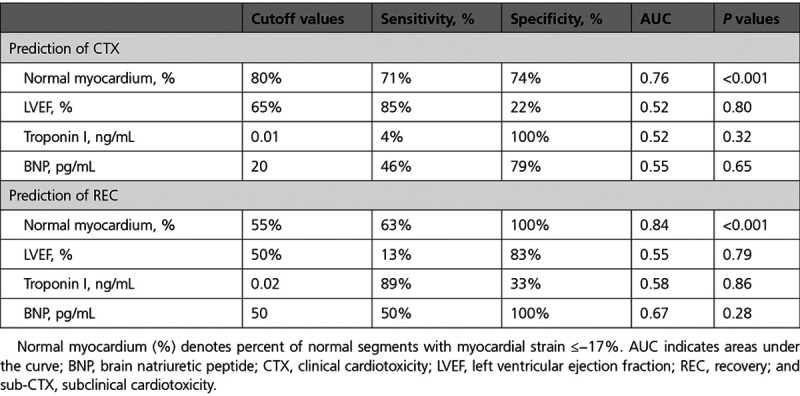
Table 4.
Multiple Regression Analysis for Predicting CTX Using Baseline Parameters
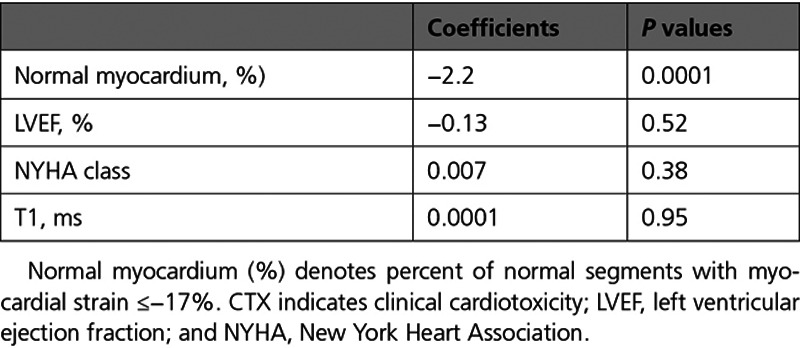
Table 5.
Multiple Regression Analysis for Predicting REC Using Parameters Before the Initiation of Cardioprotective Therapy
Kaplan-Meier Curves
Normal myocardium differentiated patients with from those without subsequent cardiotoxicity (χ2=15.3, P<0.001, hazard ratio, 4.2; Figures 4A and 4B) and simultaneously predicted REC after occurrence of cardiotoxicity during follow-up (χ2=4.6, P=0.03, hazard ratio, 6.6), which was not possible by any other CMR parameters or laboratory parameters (not shown).
Figure 4.
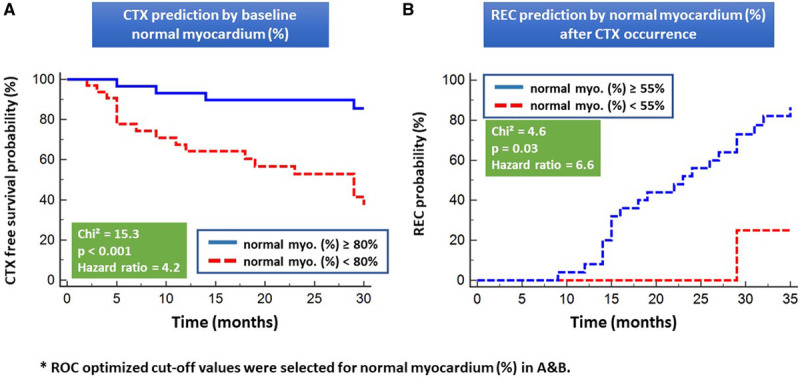
Normal myocardium (%) as predictor of cardiotoxicity. Normal myocardium<80% (A) at baseline and <55% (B) during follow-up cardiovascular magnetic resonance scans both accurately differentiated patients with from those without subsequent clinical cardiotoxicity (CTX) and patients with recovery from cardiotoxicity (REC) versus non-REC after CTX occurrence and initiation of cardioprotective treatment, respectively. Normal myo. (%) indicates normal myocardium (%); and ROC, receiver operating characteristic.
Representative images: Cine, fast-SENC, and T1 mapping images in the same patient at baseline (Figure IIIA through IIIE), after occurrence of cardiotoxicity (Figure IIIF through IIIJ), and during REC (Figure IIIK through IIIO) can be appreciated in Figure III in the Data Supplement.
Discussion
The following main findings were established in our study:
A short duration noncontrast CMR scan, lasting 10 to 12 minutes (Figure IIIP in the Data Supplement) can be serially performed in patients undergoing chemotherapy for cancer, contributing to early detection and prediction of cardiotoxicity and with zero radiation or contrast agent exposure.
Normal myocardium (%) promptly identifies changes during chemotherapy, allowing for the accurate detection of sub-CTX and cardiotoxicity and for strain normalization during cardioprotective medication. Corresponding changes in terms of LVEF, troponins, and BNP appear delayed compared with normal myocardium (%).
Normal myocardium (%) can predict cardiotoxicity in contrast to all other imaging data, clinical data, or laboratory markers.
Normal myocardium (%) also predicts responsiveness to cardioprotective treatment after occurrence of cardiotoxicity and initiation of cardioprotective therapy, thus identifying patients with higher chances for REC.
Identifying Cardiotoxicity
Recognizing the adverse effects of various chemotherapeutic agents on myocardial function led to the development of a multidisciplinary integrative approach, cardio-oncology, to assist with management of patients with cancer.3,17 Thus, as an increasing number of patients survive their cancers, their long-term prognosis will be adversely impacted by the risk of developing cardiovascular complications.12
A major concern for cardio-oncologists is the timely recognition of myocardial dysfunction in the context of administration of cardiotoxic agents. A significant proportion of patients, reaching up to 30%, have been shown to have reductions in LVEF without symptoms.18,19 Moreover, many patients who develop a reduced LVEF do not show significant improvements, even when the cardiotoxic therapy is stopped, and proper heart failure management is initiated.20 Thus, using LVEF as a marker for cardiotoxicity might prove insufficient for these patients. Our data displayed a similar pattern, where reductions in LVEF exhibited lower accuracy than normal myocardium (%) for the detection of cardiotoxicity.
CMR represents an excellent tool for monitoring the cardiovascular health of patients with cancer. Its lack of ionizing radiation and excellent reproducibility in assessing myocardial volumes and mass make this method suitable for the longitudinal evaluation of patients undergoing chemotherapy. CMR-derived myocardial strain using fast-SENC exhibits excellent inter and intraobserver variabilities.21,22 In addition, fast-SENC exhibits lower variability for the assessment of segmental strain compared with other CMR-based methods, such as feature tracking, thus increasing the consistency of the measurements in longitudinal studies.23 Recently, we demonstrated the ability of fast-SENC for the diagnostic classification and risk stratification of patients with heart failure in a large patient cohort of 1169 consecutive patients.11 Here, normal myocardium (%) precisely predicted the occurrence of heart failure in asymptomatic individuals and readily detected subclinical LV dysfunction even in patients at risk for heart failure without structural or functional heart disease, a subgroup which closely resembles to date healthy patients who are exposed to cardiotoxic agents.24
In previous studies using CMR, a decrease in LVEF and circumferential strain was noted in patients with breast cancer undergoing anthracycline therapy.25 Similarly, we found fast-SENC derived myocardial strain, and especially normal myocardium (%), to be an excellent parameter for identifying cardiotoxicity. CMR can also provide information related to the presence of inflammation and fibrotic tissue in the myocardium with the help of T1 and T2 mapping.26 This would be especially helpful in patients who receive anthracyclines because these compounds may produce edema and fibrosis.27 However, we failed to identify a significant role of T1 and T2 values in the diagnosis of sub-CTX and cardiotoxicity in our study. This is in concordance with a recent study by Altaha et al,28 who noted significant overlap, between patients with cardiotoxicity and healthy control in the measurement of T1 and T2 values, making it exceedingly difficult to derive cutoff values for the individual detection of sub-CTX.
Myocardial strain measured with echocardiography was also proven to be a valuable tool for the workup of patients with cancer.29,30 Despite its wide availability, good practicability, and cost-efficiency, however, echocardiography may be limited due to its dependency on the acoustic window of the patients and the skills of the operator. In addition, high intervendor, interobserver, and intraobserver variabilities have been mentioned previously with echocardiography.31,32 Moreover, the feasibility of 3-dimensional echocardiography derived myocardial strain in selected population can be as low as 85%.33
Cardiac biomarkers are also seen as possible good indicators of subclinical dysfunction. Indeed, the serial evaluation of cardiac troponin demonstrated promising results in patients undergoing anthracycline therapy.34 Moreover, increased levels of cardiac troponins appear to identify patients who would not recover LVEF after chemotherapy.35 Similarly, BNP and NT-proBNP (N-terminal pro-B-type natriuretic peptide) can help in the early recognition of cardiotoxicity.36 However, the optimal time point for such measurements is still unclear. Furthermore, it is unclear if cardiac biomarkers can promptly detect subtle myocardial damage, as experienced in sub-CTX. Indeed, in our cohort, increases in cardiac troponin and NT-pro BNP were seen firstly at the second visit, like the changes seen with LVEF, that is, at a time point where myocardial damage already may be irreversible.
Predicting Cardiotoxicity
Although identifying cardiotoxicity is an especially important end point in the workup of patients with cancer receiving cardiotoxic chemotherapy, predicting which patients would be at risk of developing cardiac dysfunction before starting the chemotherapeutic agent would significantly increase the likelihood of early cardiotoxicity recognition and aid in proper clinical management. To date, very few prediction models address the question of cardiotoxicity prediction in such patients.4 They are mostly based on clinical and demographic data and have a low sensitivity so that many patients who would not meet the criteria of high-risk patients may still develop cardiotoxicity.17 We were able to demonstrate a role of normal myocardium (%) for the identification of patients who subsequently develop cardiotoxicity. This is again in agreement with our recent findings, where asymptomatic patients at risk for heart failure and without structural or functional heart disorders exhibit reduced normal myocardium (%) by strain11 and may, therefore, potentially be at risk for developing overt heart failure in the long- or short term, respectively, if continuously exposed to risk factors such as diabetes or hypertension or to cardiotoxic agents. Interestingly, the ROC-optimized cutoff value of normal myocardium<80%, for the detection and for the prediction of subsequent cardiotoxicity in our present cohort, was identical to the one differentiating healthy subjects from those with subclinical LV dysfunction and thus at risk for future heart failure in recent studies.11 The predictive value of normal myocardium was independent of age, clinical heart failure symptoms, and cardiac biomarkers and is to our knowledge the first time, that an imaging marker achieves prediction of cardiotoxicity based on baseline scans. In addition, our study is the first to incorporate cine, strain, and T1 and T2 parameters, as well as biomarkers obtained before the initiation of therapy, to identify which tools and parameters are best suited for the clinician for the prediction and detection of cardiotoxicity. Previous CMR studies rather focused on conventional parameters, such as LV mass and LVEF.10
Role of Cardioprotection
All efforts invested in the prediction of cardiotoxicity and the early detection of sub-CTX aim at establishing an optimal time point of treatment with cardioprotective therapies. To date, few studies have evaluated the role of ACE inhibitors or ARBs and β-blockers in these patients. A study by Cardinale et al7 investigated the effect of enalapril and whenever possible carvedilol in 201 patients who developed overt LV dysfunction (LVEF<45%) during anthracycline therapy. They found an improvement in LVEF in 55% of patients over a period of 36 months. However, when the same group analyzed over 2000 patients with anthracycline-induced LV dysfunction over a period of over 5 years, they found that only 11% of patients achieved complete recovery.20 In both studies, the trigger for initiating cardioprotective medication was a clear reduction in LVEF, that is, a time point where a significant proportion of cardiomyocytes is already irreversibly affected. A more recent randomized study by Gulati et al37 showed the cardioprotective effect of candesartan when administrated in patients with breast cancer who received anthracycline-based regimes with or without adjuvant trastuzumab. In our study, and in concordance with the current recommendations, cardioprotective therapy was initiated early based on data provided from imaging studies, including strain.15,38 This resulted in a consecutive improvement of CMR parameters of LV-function and strain in over 2 out of 3 (69%) of our patients with sub-CTX and cardiotoxicity during follow-up. As the percentage of patients achieving REC is markedly higher, than that reported in the current literature, this implies that closer follow-up of such patients is clinically useful.17 In addition, normal myocardium (%) predicted REC after occurrence of cardiotoxicity in this context, which reaffirms the role of myocardial strain for the longitudinal workup of such patients. Interestingly, the ROC-optimized cutoff value of normal myocardium<55%, predicting lower chances for REC, was close to the one (<60%) predicting clinical heart failure end points in patients with subclinical LV dysfunction, as recently reported.11 Thus, normal myocardium (%) provides an additional diagnostic window to subtle changes in cardiac function, which can be used for monitoring cardiac safety during chemotherapy (Figure 5A). Based on these findings, we propose a potential algorithm for the management of patients with cancer undergoing chemotherapy (Figure 5B). This algorithm may help avoiding irreversible damage of the myocardium by the early initiation of cardioprotection and the potential adjustment of chemotherapy regimens after consultation of the cardio-oncology team.
Figure 5.
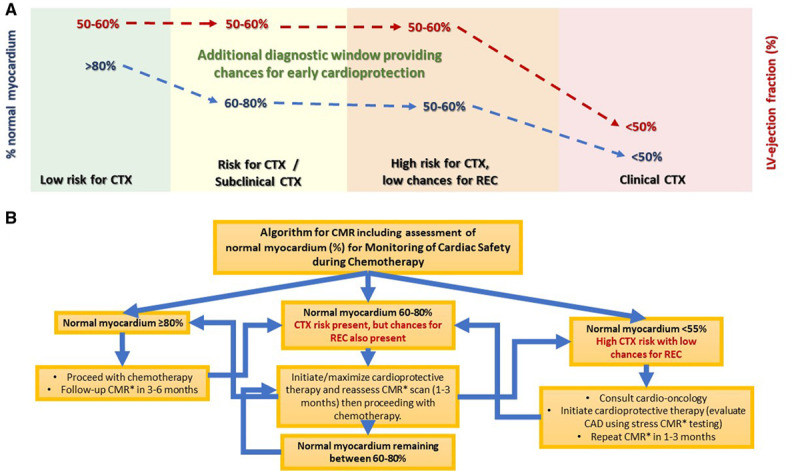
The role of normal myocardium (%) in the diagnostic evaluation of cancer patients. A, Additional diagnostic window provided by normal myocardium (%) versus left ventricular (LV) ejection fraction for the prediction of clinical cardiotoxicity (CTX) and for its early identification, allowing monitoring of cardiac safety during chemotherapy. B, Proposed clinical algorithm incorporating clinical and cardiovascular magnetic resonance (CMR) data for the management of patients who undergo chemotherapy. CAD indicates coronary artery disease; REC, recovery from cardiotoxicity; and ROC, recovery from cardiotoxicity.
Limitations
Several limitations of our study need to be addressed. First, this is a single-center study with a relatively low number of patients. Moreover, the decision to begin cardioprotective treatments was left at the discretion of the attending cardiologist. Furthermore, the study was nonrandomized, and no control group was present, whereas some freedom of timing needs to be acknowledged regarding the follow-up CMR visits. In addition, no standardized protocol was used for the administration of ACE inhibitors, ARBs, and β- blockers. However, all the cardioprotective drugs used are recognized in the heart failure guidelines for their beneficial effect in patients with reduced LVEF. Lastly, some patients received concomitantly radiotherapy, which can also be responsible for reduced regional myocardial performance, which is especially important in patients with breast cancer.30 However, the percentage of patients with concomitant radiotherapy was relatively low. Lastly, third-generation troponin I was used as marker of myocardial damage. It is conceivable that the use of high-sensitive troponin assays would have provided more consistent results.
Conclusions
Normal myocardium (%) derived by serial fast-SENC CMR acquisitions is an ideal tool for the longitudinal study of patients undergoing chemotherapy due to breast cancer or lymphoma. This parameter can both predict the incidence of cardiotoxicity and detect sub-CTX earlier and with higher accuracy than conventional CMR parameters and biochemical markers. The ability of this imaging marker to reduce the occurrence of cardiotoxicity merits further investigation in future randomized studies.
Acknowledgments
We thank Hayden Whayne for his help for addressing statistical questions with our article. We also thank Birgit Hoerig, Kirsten Falk, Ute Pfeiffer, and Silke Morgenstern for their excellent technical assistance with the acquisitions of all cardiovascular magnetic resonance imaging (CMR) scans.
Sources of Funding
This research was supported by an unrestricted grant from Myocardial Solutions, Inc.
Disclosures
Drs Steen and Kelle received research grants from Myocardial Solutions. Dr Kelle owns stock options of Myocardial Solutions. The other authors report no conflicts.
Supplementary Material
Footnotes
S. Giusca and G. Korosoglou contributed equally
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.121.012459.
This article was sent to Linda Dawn Gillam, MD, MPH, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1.Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, Dos-Santos-Silva I, Smeeth L, Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenihan DJ, Fradley MG, Dent S, Brezden-Masley C, Carver J, Filho RK, Neilan TG, Bales A, Melloni C, Herrmann J, et al. Proceedings from the global cardio-oncology summit. JACC CardioOncology. 2019;1:256–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teske AJ, Linschoten M, Kamphuis JAM, Naaktgeboren WR, Leiner T, van der Wall E, Kuball J, van Rhenen A, Doevendans PA, Cramer MJ, et al. Cardio-oncology: an overview on outpatient management and future developments. Neth Heart J. 2018;26:521–532. doi: 10.1007/s12471-018-1148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa D, Fallah-Rad N, Grenier D, Krahn M, Fang T, Ahmadie R, Walker JR, Lister D, Arora RC, Barac I, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat. 2009;117:357–364. doi: 10.1007/s10549-008-0260-6 [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 8.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;6325 pt A2751–2768. doi: 10.1016/j.jacc.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 9.Saunderson CED, Plein S, Manisty CH. Role of cardiovascular magnetic resonance imaging in cardio-oncology. Eur Heart J Cardiovasc Imaging. 2021;22:383–396. doi:10.1093/ehjci/jeaa345 [DOI] [PubMed] [Google Scholar]
- 10.Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6:1080–1091. doi: 10.1161/CIRCIMAGING.113.000899 [DOI] [PubMed] [Google Scholar]
- 11.Korosoglou G, Giusca S, Montenbruck M, Patel A, Lapinskas T, Götze C, Zieschang V, Al-Tabatabaee S, Pieske B, Florian A, et al. Fast strain-encoded cardiac magnetic resonance (fast-SENC CMR) for the diagnostic classification and risk stratification of patients with heart failure. JACC Cardiovasc Imaging. 2021. S1936-878X(20)31004-4. doi: 10.1016/j.jcmg.2020.10.024 [DOI] [PubMed] [Google Scholar]
- 12.Neizel M, Lossnitzer D, Korosoglou G, Schäufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2:116–122. doi: 10.1161/CIRCIMAGING.108.789032 [DOI] [PubMed] [Google Scholar]
- 13.Koos R, Altiok E, Doetsch J, Neizel M, Krombach G, Marx N, Hoffmann R. Layer-specific strain-encoded MRI for the evaluation of left ventricular function and infarct transmurality in patients with chronic coronary artery disease. Int J Cardiol. 2013;166:85–89. doi: 10.1016/j.ijcard.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Charbonnel C, Convers-Domart R, Rigaudeau S, Taksin AL, Baron N, Lambert J, Ghez S, Georges JL, Farhat H, Lambert J, et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. 2017;18:392–401. doi: 10.1093/ehjci/jew223 [DOI] [PubMed] [Google Scholar]
- 15.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, et al. ; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845 [PubMed] [Google Scholar]
- 17.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677 [PubMed] [Google Scholar]
- 19.Hussain Y, Drill E, Dang CT, Liu JE, Steingart RM, Yu AF. Cardiac outcomes of trastuzumab therapy in patients with HER2-positive breast cancer and reduced left ventricular ejection fraction. Breast Cancer Res Treat. 2019;175:239–246. doi: 10.1007/s10549-019-05139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 21.Giusca S, Korosoglou G, Zieschang V, Stoiber L, Schnackenburg B, Stehning C, Gebker R, Pieske B, Schuster A, Backhaus S, et al. Reproducibility study on myocardial strain assessment using fast-SENC cardiac magnetic resonance imaging. Sci Rep. 2018;8:14100. doi: 10.1038/s41598-018-32226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korosoglou G, Giusca S, Hofmann NP, Patel AR, Lapinskas T, Pieske B, Steen H, Katus HA, Kelle S. Strain-encoded magnetic resonance: a method for the assessment of myocardial deformation. ESC Heart Fail. 2019;6:584–602. doi: 10.1002/ehf2.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, Steen H, Stehning C, Pieske B, Pieske-Kraigher E, et al. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. 2020;7:523–532. doi: 10.1002/ehf2.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–2860. doi: 10.1161/CIRCULATIONAHA.105.600437 [DOI] [PubMed] [Google Scholar]
- 25.Drafts BC, Twomley KM, D’Agostino R, Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974 [DOI] [PubMed] [Google Scholar]
- 27.Ferreira de Souza T, Quinaglia A C Silva T, Osorio Costa F, Shah R, Neilan TG, Velloso L, Nadruz W, Brenelli F, Sposito AC, Matos-Souza JR, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc Imaging. 2018;11:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altaha MA, Nolan M, Marwick TH, Somerset E, Houbois C, Amir E, Yip P, Connelly KA, Michalowska M, Sussman MS, et al. Can quantitative CMR tissue characterization adequately identify cardiotoxicity during chemotherapy?: impact of temporal and observer variability. JACC Cardiovasc Imaging. 2020;13:951–962. doi: 10.1016/j.jcmg.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 29.Liu JE, Barac A, Thavendiranathan P, Scherrer-Crosbie M. Strain imaging in cardio-oncology. JACC CardioOncology. 2020;2:677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erven K, Jurcut R, Weltens C, Giusca S, Ector J, Wildiers H, Van den Bogaert W, Voigt JU. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;79:1444–1451. doi: 10.1016/j.ijrobp.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 31.Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, Voigt JU; EACVI-ASE-Industry Standardization Task Force. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging. 2018;11:25–34. doi: 10.1016/j.jcmg.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 32.Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, Voigt JU. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 33.Nabeshima Y, Seo Y, Takeuchi M. A review of current trends in three-dimensional analysis of left ventricular myocardial strain. Cardiovasc Ultrasound. 2020;18:23. doi: 10.1186/s12947-020-00204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinale D, Biasillo G, Salvatici M, Sandri MT, Cipolla CM. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev Mol Diagn. 2017;17:245–256. doi: 10.1080/14737159.2017.1283219 [DOI] [PubMed] [Google Scholar]
- 35.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC [DOI] [PubMed] [Google Scholar]
- 36.Lenihan DJ, Stevens PL, Massey M, Plana JC, Araujo DM, Fanale MA, Fayad LE, Fisch MJ, Yeh ET. The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: a feasibility study. J Card Fail. 2016;22:433–438. doi: 10.1016/j.cardfail.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 37.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


