Supplemental Digital Content is available in the text.
Keywords: autonomic nervous system, cohort studies, exercise test, heart rate, syncope
Abstract
Background:
Cardioneuromodulation is a cardioneuroablative approach aiming to create adequate vagolysis of the sinoatrial node through partial ablation of the anterior right ganglionated plexus.
Methods:
We performed an interventional study in patients with recurrent neurally mediated syncope (group A) or functional sinus node dysfunction (group B). Syncope burden, ECG, 24-hour rhythm data, tilt table test, exercise test, and pharmacological challenge with atropine were assessed at baseline and at regular intervals to 12 months.
Results:
Fifty patients (31 in group A and 19 in group B) underwent cardioneuromodulation. The mean number of syncopes during the previous 12 months was 9.7±18.2. The procedure was associated with a lower rate of syncope (−95%) and presyncope (−95%) at 12 months versus baseline (P<0.001). Thirty-seven patients remained entirely free of syncope at 12 months, and the syncope-free survival curve remained stable between the 12- and 30-month follow-up. After a mean ablation time of 8±4 minutes, the P-P interval shortened by 247±146 ms (P<0.001). Basal heart rate (HR) increased by 18% (P<0.001) and remained stable between 6 and 12 months. At 12 months, the mean HR increased by 12% in the entire cohort (P<0.001), reached 23% in patients with baseline mean HR <70 beats per minute (P<0.01), and did not increase in patients with baseline HR >70 beats per minute. Maximum HR during exercise decreased by 10 beats per minute at 1 month (P<0.001) and was restored at 12 months.
Conclusions:
Cardioneuromodulation is a safe and fast treatment giving rise to a long-term partial sinus node vagolysis with no apparent short- or long-term safety concerns or undesirable persisting modifications of the intracardiac autonomous nervous system. The impact on vasoplegia is less clear. Cardioneuromodulation is associated with a good clinical outcome in most patients with neurally mediated syncope or functional sinus node dysfunction. These promising data require confirmation in a multicenter randomized trial.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02954666.
What Is Known?
In an interim analysis of this prospective, open-label trial involving 50 patients with neurally mediated syncope or functional sinus node dysfunction, cardioneuromodulation, through a right-sided and computed tomography–guided procedure, was safe and effective, giving rise to a long-term partial and adequate sinus node vagolysis.
Cardioneuromodulation led to a good clinical outcome in most patients, with no apparent short- or long-term safety concerns or undesirable persisting modifications of the intrinsic cardiac autonomous nervous system.
As the only ganglionated plexus targeted during cardioneuromodulation is the anterior right ganglionated plexus, this particular type of cardioneuroablation has only a modest effect on atrioventricular node vagal innervation.
What the Study Adds?
Cardioneuromodulation is a fast procedure, exposing patients to fewer procedural risks than left-sided or biatrial multiple-site ablation strategies.
Our data do not support the use of cardioneuromodulation in patients with functional atrioventricular block.
Cardioneuromodulation may offer a new, alternative treatment approach in patients with recurrent neurally mediated syncope with a Vasovagal Syncope International Study group type 1, 2A, or 2B response during the tilt table test.
Neurally mediated syncope (NMS) is often refractory to routine treatments.1,2 There is, therefore, a need for a new treatment modality. In 2005, Pachon et al3,4 proposed cardiac vagal denervation to treat NMS, sinus node (SN) dysfunction, and functional atrioventricular block. Other research groups, using different multisite cardioneuroablation approaches, published good clinical results for different clinical conditions caused by an inadequate high vagal tone.5–12 While a (dual) pacemaker implantation is indicated in patients with a degenerative sick sinus syndrome having an intrinsic SN dysfunction, patients with a functional SN dysfunction (sinus bradycardia or sinoatrial pause) secondary to excessive high vagal tone could benefit from an intervention that can correct inadequate neural activity of their intrinsic cardiac autonomous nervous system (ICANS).
Cardioneuromodulation is a cardioneuroablative approach aiming to create adequate vagolysis of the sinoatrial node through partial ablation of the anterior right ganglionated plexus (ARGP).13–15 Ganglionated plexi contain large populations of colocalized sympathetic and parasympathetic neurons. Ablation of these plexi will, therefore, not only affect parasympathetic neural bodies and axons but will also interrupt sympathetic axons.3,16
In this article, we report the 12-month results of the CardNMH2 study in patients with NMS or functional SN dysfunction with no significant impairment of their intrinsic SN activity. We provide innovative data on changes in the ICANS physiology associated with cardioneuromodulation.
Methods
CardNMH2 (NCT02954666) is an investigator-initiated, prospective, open-label, interventional, single-center cohort study designed to assess the safety and efficacy of cardioneuromodulation in patients with NMS or functional SN dysfunction. The primary safety outcome was freedom from serious adverse events at 7 days, and the primary efficacy outcome was freedom from syncope at 12 months. Syncope and presyncope burden and electrocardiographic data were compared before and after cardioneuromodulation.
CardNMH2 was approved by the Ethical Committee of Imeldaziekenhuis, Bonheiden, Belgium, and has been conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent. An independent data and safety monitoring board reviewed data at regular intervals to safeguard the well-being of the participants.
The authors declare that all supporting data are available in the article.
Study Population
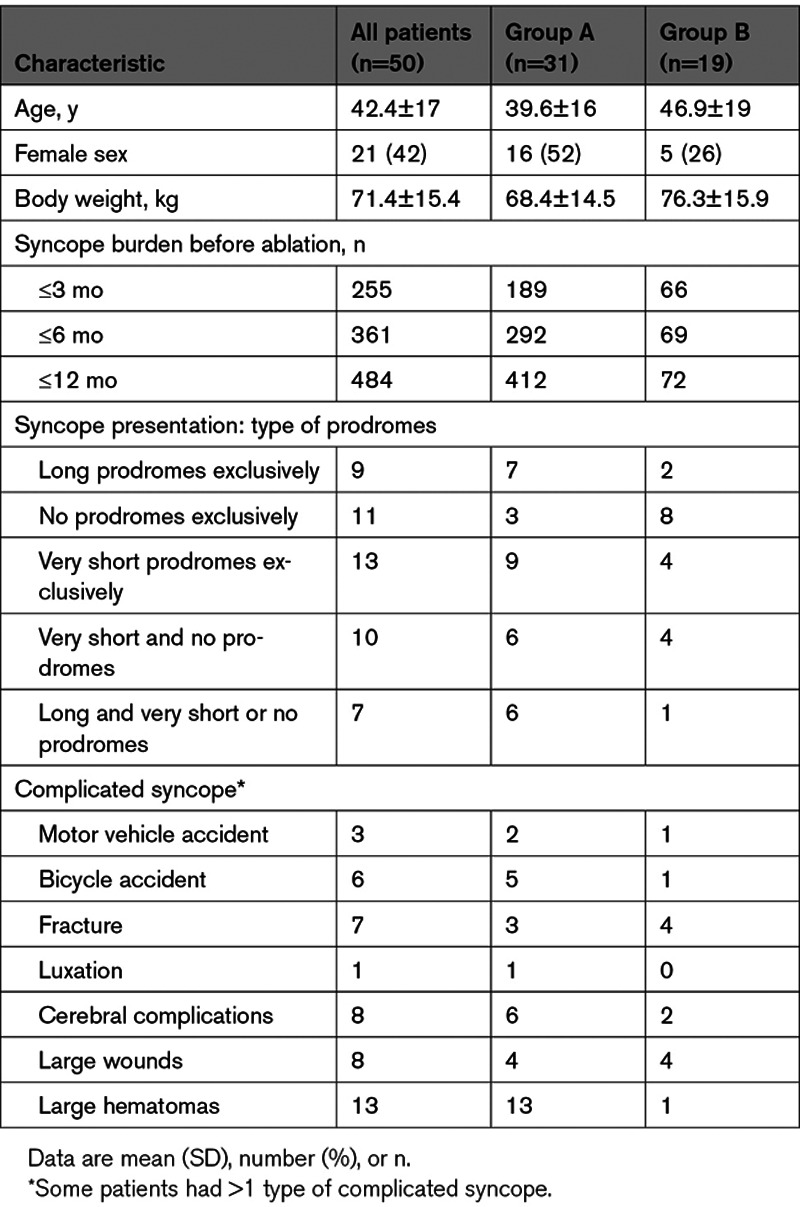
The study enrollment criteria have been published.14 Patients were assigned to group A if they were affected by neurally mediated syncope and had a type 1 -, type 2A- or type 2B -positive tilt table test (TTT) result according to the modified version of the classification of neurally mediated syncope proposed by the Vasovagal Syncope International Study group (VASIS) and to group B if they had a documented sinus pause ≥3 s.15,17 A P-P interval shortening ≥20% and <1000 ms after intravenous administration of 2 mg atropine (AT) was required for both groups. Patients in group A must have had ≥2 episodes of syncope (≥3 in patients <18 years of age) in their lifetime unless the last syncope was complicated by an injury or an accident. The main exclusion criteria were age <14 years, unstable medical condition, life expectancy <12 months, antiarrhythmic drugs, atrial fibrillation, bi- or trifascicular block, permanent PR prolongation, and valvular or myocardial disorders causing syncope. Patient baseline characteristics are summarized in Table 1.
Table 1.
Patient Baseline Characteristics and Syncope Presentation
Clinical and Electrocardiographic Data Collection
All patients underwent a 24-hour rhythm registration and a transthoracic echocardiography at baseline. A TTT was performed in patients in group A, tilting to 70° for 45 minutes without any pharmacological provocation, and was repeated following the same protocol during follow-up.
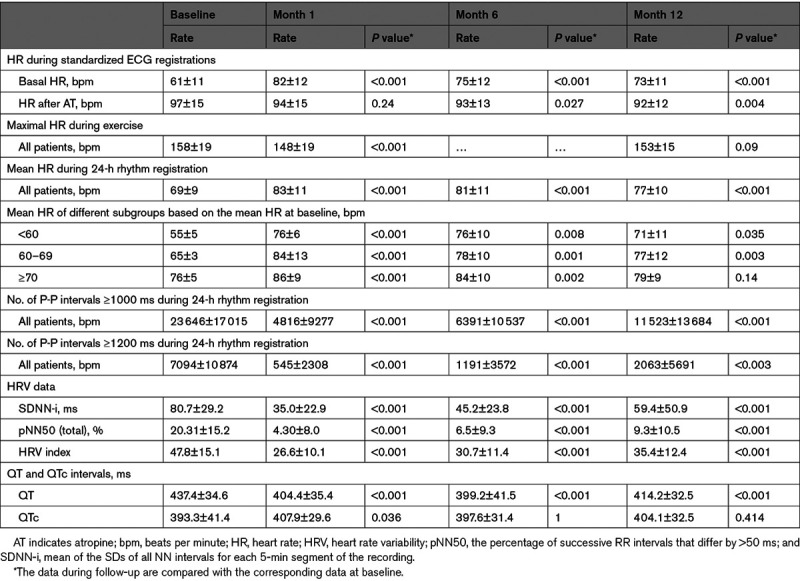
Data on syncope burden, presence of prodromes, and occurrence of injuries and accidents were recorded before cardioneuromodulation. An ECG was performed according to a highly standardized protocol, to establish the basal heart rate (HR) and the HR after 2 sequential intravenous injections of 1 mg AT. Initially, the second bolus was not administrated if the HR after 1 mg AT was >100 beats per minute. After a protocol amendment, a total dose of 2 mg was systematically administered, and the AT test was repeated on the day after the procedure. Basal HR and HR after AT injection were derived from the mean P-P interval of 5 consecutive P waves without extrasystole.
Therapeutic anticoagulation with a non–vitamin K antagonist oral anticoagulant was started >2 hours before cardioneuromodulation and was continued for 1 month afterward. Patients with a high bleeding risk were treated with clopidogrel. Use of medications with a chronotropic or dromotropic action was discouraged.
An in-hospital visit was planned at 1, 3, 6, and 12 months. All cases of recurrent syncope were tracked at each visit. The 24-hour rhythm registration and the ECG registration in resting conditions and after AT injection were repeated at 1, 6, and 12 months.
The TTT was repeated at 1 and 6 months in patients in group A using the same protocol used at baseline. A cycloergometry test was performed whenever feasible to evaluate SN chronotropic function. Cycloergometry was repeated at 1 and 12 months and transthoracic echocardiography at 12 months.
The number of patients who underwent each test is shown in Table I in the Data Supplement. The AT test was not repeated in patients with poor tolerance during a previous test.
During 24-hour rhythm registration, the mean HR and the number of P-P intervals >1200 and >1000 ms were determined, and data on HR variability (HRV) were collected. The presence of a pause or an atrioventricular block was tracked. Data on HRV were specifically the mean of the SDs of all NN intervals for each 5-minute segment of the recording, the percentage of successive RR intervals that differed by >50 ms (pNN50), and the integral of the density of the RR interval histogram divided by its height (HRV index). The QT interval was measured manually, and QTc was derived using the Sagie formula.
Cardiac Imaging and Invasive Procedure
Cardioneuromodulation was performed according to a right-sided ablation strategy under computed tomographic guidance.15 The endocardial site to target during ablation was annotated by a target line (TL) at the posteroseptal side of the junction between the right atrium and the superior vena cava, facing the mid and caudal parts of the right superior pulmonary vein antrum on the right heart cavities on a computed tomography image of the heart imported into the CARTO system (Biosense Webster, Diamond Bar, CA) before the procedure (Figure 1). Each procedure was performed with the patient under general anesthesia with sevoflurane in a steady state, favoring a high vagal tone.14 Electroanatomical mapping of the right atrium, the superior and inferior vena cava, and the coronary sinus was performed and merged with the computed tomography image. A right atrium activation map was performed at baseline and repeated after cardioneuromodulation. The crista terminalis was divided into 4 geometric zones (cranial, midsuperior, midinferior, and caudal). The distance between the TL and right phrenic nerve and the shortest distance between the TL and the earliest endocardial activation area in sinus rhythm were provided (Table 2).
Figure 1.
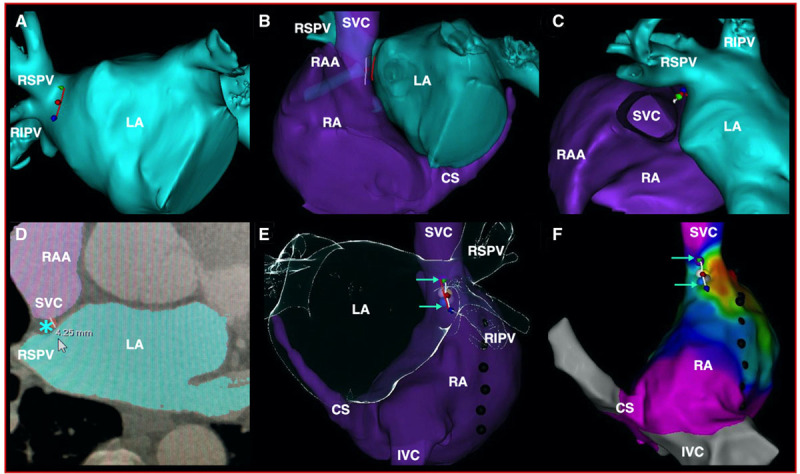
Computed tomography (CT) scan images and electroanatomical map indicating the region targeted during cardioneuromodulation (CardNM). CT scan images (A–E) and electroanatomical map (F) indicating the region targeted during CardNM. A, Red line delineating the mid and caudal parts of the right superior pulmonary vein antrum in an anteroposterior projection. B and C, Target line guiding CardNM indicated by the white line facing the first design line at the superior vena cava (SVC)–right atrium (RA) junction in a modified left anterior oblique (B) and cranial (C) projection. D, Transversal slice at the midportion of the target line showing the location and thickness of the fat pad containing the anterior right ganglionated plexus (ARGP) at this level (blue asterisk). E and F, Ablation tags along the target line in a posteroanterior CT scan view (E) and on the electroanatomical map (F) in the same projection after merging the two images. The region of the ARGP ablated is indicated by 2 green arrows. Each individual ablation is indicated using a colored tag. The phrenic nerve is tagged with black dots. CS indicates coronary sinus; IVC, inferior vena cava; LA, left atrium; RAA, right atrial appendage; RIPV, right inferior pulmonary vein; and RSPV, right superior pulmonary vein.
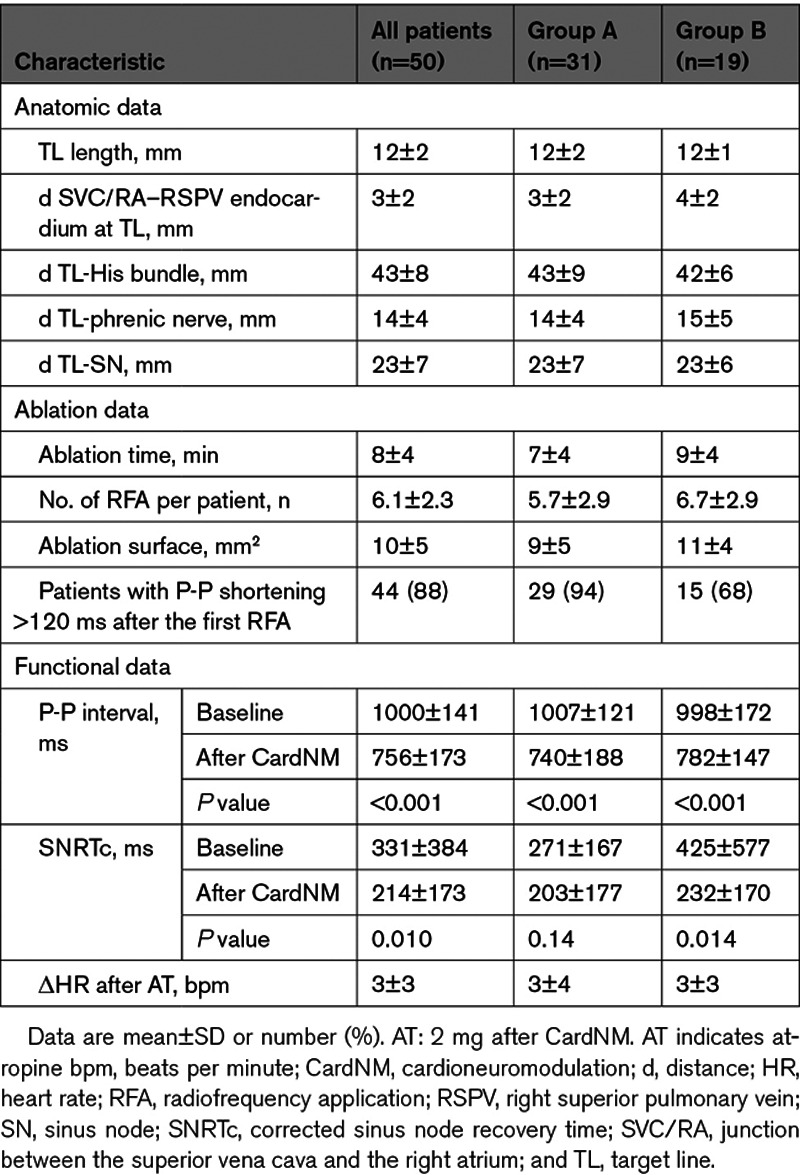
Table 2.
Procedural Characteristics
Radiofrequency applications were delivered along the TL with an irrigated tip catheter (SmartTouch Catheter; Biosense Webster, Diamond Bar, CA) with a power of 40 W and a contact force >8 g.15 They were interrupted if no significant shortening of the P-P interval was observed after 30 s or were prolonged when P-P interval shortened without exceeding 90 s. The ablation procedure was considered complete if the P-P interval shortened by >30%, if the P-P interval was <600 ms after 5 minutes of waiting, if 10 radiofrequency applications were delivered, or if the total ablation time reached 900 s. ECG registrations were obtained >30 min after the last ablation before and after an intravenous injection of 2 mg AT.
Statistical Analysis
The Wilcoxon rank-sum test was used for comparison of syncope and presyncope episodes. Proportions at baseline, 1 month, and 6 months were compared using the mid-P adjustment to McNemar exact test. TTT results were compared using the McNemar test with Bonferroni correction. To assess the correlation between TTT response at baseline and the probability of syncope recurrence, Fisher exact test was used. P-P interval and corrected SN recovery time were compared using the Wilcoxon rank-sum test. The Wilcoxon rank-sum test was used to compare P-P interval shortening between group A and group B and for comparison of other ablation data. The Bhapkar test was used for comparison of activation maps. ECG, exercise tests, and 24-hour rhythm registrations were compared using a paired Student t test or the ANOVA test with Bonferroni correction when multiple samples were compared. Data on HRV were compared using the Wilcoxon signed-rank test with Bonferroni correction. QT and QTc intervals were compared using the linear mixed-effect modeling. Time to first recurrence of syncope was provided by the Kaplan-Meier survival analysis. Continuous data are presented as mean±SD. All 2-tailed P<0.05 were considered statistically significant. The statistical analysis was performed using the SPSS (IBM, New York, NY) and the Stata (StataCorp, College Station, TX) softwares.
Results
Baseline Characteristics
Fifty patients were enrolled in the CardNMH2 study (Imeldaziekenhuis, Bonheiden, Belgium) between December 2016 and March 2019, 31 in group A and 19 in group B. All of the patients were in New York Heart Association class I, and there were no noteworthy abnormalities on transthoracic echocardiography. Patient characteristics and syncope presentation are provided in Table 1. Mean syncope burden in the previous 12 months was 9.7±18.2 (13.3±22.3 in group A and 3.8±3.3 in group B). Syncope was always preceded by long prodromes in 9 patients and never preceded by prodromes in 11 patients (3 in group A, of whom 2 had a documented pause ≥3 s during a spontaneous episode, and 8 in group B). In 22 patients (6 in group A and 16 in group B), a pause ≥3 s was documented during a spontaneous syncopal episode. Thirty-three patients had a history of complicated syncope. Among the patients with cerebral complications, 1 patient had a traumatic intracerebral hemorrhage, 1 patient had syncope complicated by urinary incontinence, and 6 patients had commotio cerebri. Five patients had an internal loop recorder (3 in group A and 2 in group B); among these, 2 (group B) had a documented pause ≥3 s. Over the 12 months preceding cardioneuromodulation, 2028 presyncope episodes were reported, corresponding to a mean of 56.2±107.6 in group A and 15.11±45.63 in group B.
According to the VASIS classification, the response in patients (group A) during the TTT was type 1 (mixed) in 20 patients, type 2A (cardioinhibition without asystole) in 3 patients, and type 2B (cardioinhibition with asystole) in 8 patients of whom 1 had an atrioventricular block. Second-degree atrioventricular block was documented during 24-hour rhythm registration in 2 patients. One patient in group A had idiopathic ventricular tachycardia. Seven patients were treated for arterial hypertension with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, combined with a diuretic in 2 patients and a calcium antagonist in 1 patient. Three patients were treated with β-blockers, 2 for intermittent inappropriate SN tachycardia and 1 for migraine. One patient was treated with midodrine, 1 with fludrocortisone, and 1 with etilefrine. Two patients in group B were dependent on intravenous isoprenaline before the procedure. The patients did not take any other noteworthy medications.
Procedural Data
Procedural data are summarized in Table 2. After 6.1±2.9 applications of radiofrequency energy and a mean ablation time of 8±4 minutes, baseline procedural P-P interval shortened by a mean of 248±146 ms (P<0.001), resulting in an increase in the basal HR of 22±13 beats per minute. AH (73±17 ms) and HV (45±9 ms) intervals were normal. The Wenckebach point seemed similar before and after ablation (392±80 versus 395±70 ms, respectively; P=0.58). The earliest SN activation zone was found in the cranial zone in 18 patients before cardioneuromodulation and in 24 patients after cardioneuromodulation (P=0.21). The proportion of patients with a shift in the earliest activation zone toward the cranial zone after cardioneuromodulation did not appear to differ significantly in patients with and without syncope recurrence (61.5% versus 57.1%, respectively; P=1.00).
Whereas the HR after cardioneuromodulation was higher in patients with versus without syncope recurrence (90±8.1 versus 80.1±17.0 beats per minute, respectively; P=0.007), the absolute increase in HR (25.8±9.2 versus 20.5±14.6 beats per minute; P=0.22) did not differ significantly. The HR increase following AT injection was not significantly higher in patients with versus without syncope recurrence (3.5±4.3 versus 2.9±4.5 beats per minute, respectively; P=0.68). The HR before cardioneuromodulation (64.5±5.9 versus 59.7 beats per minute; P=0.78), the endocardial distance between the right atrium/superior vena cava junction and LA/RSPV at the TL (2.83±1.23 versus 3.60±2.18 mm; P=0.16), and the other procedural data were not statistically different in patients with or without syncope recurrence.
Clinical Follow-Up
One patient developed a pseudoaneurysm >1 week after the procedure. No other adverse events were reported during follow-up. No patients were excluded or lost to 6-month follow-up.
Four patients were considered to have a treatment failure: 1 patient (age, 17 years) in group A, reluctant to pacemaker implantation, who underwent an additional ablation procedure motivated by atrioventricular block and prolonged asystole during TTT at 6 months, and 3 patients in group B (age, 29, 70, and 75 years) with syncope recurrence and documentation of a sinus pause who underwent pacemaker implantation after 6 months.
β-Blockers were initiated in 3 patients: for idiopathic fast ventricular tachycardia present before cardioneuromodulation in 1 patient (β-blockers had worsened the NMS episodes before cardioneuromodulation), for palpitations without arrhythmia documentation in 1 patient, and for headache in 1 patient. Flecainide was initiated in 2 patients for supraventricular extrasystoles and ventricular extrasystoles. Midodrine, fludrocortisone, and etilefrine were interrupted after cardioneuromodulation. No other relevant medication changes occurred during follow-up.
Two patients underwent internal loop recorder implantation after cardioneuromodulation. Of the 7 patients with an internal loop recorder, 5 had syncope recurrence, but no cases of bradyarrhythmia were documented.
One patient in group A without syncope recurrence failed to attend 12-month follow-up. All patients remained in New York Heart Association class I, and none presented significant changes on transthoracic echocardiography. Thirty-seven patients remained free of syncope at 12 months (P<0.001). Additional follow-up data beyond the 12-month final evaluation were obtained in 47 patients: 34 patients had 18-month data, 23 had 24-month data, and 18 had 30-month data. The syncope-free survival rate did not change between the 12- and 30-month follow-up (Figure 2).
Figure 2.
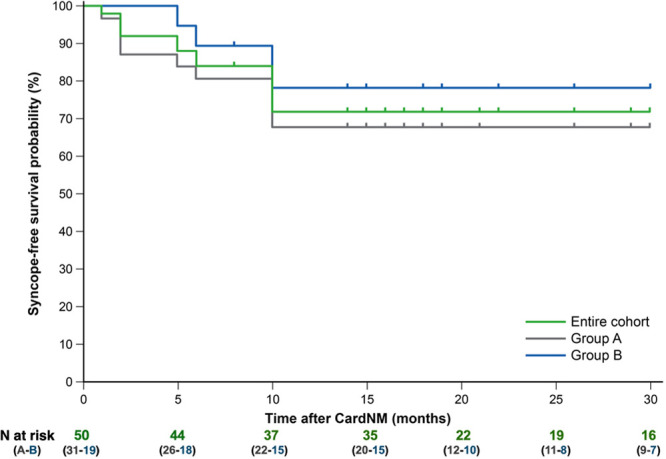
Syncope-free survival curves. CardNM indicates cardioneuromodulation.
Syncope burden was lower (−95%) at 12-month follow-up compared with baseline (P<0.001; Figure 3). No cases of complicated syncope occurred during the 96 patient-years of follow-up in the study. The 9 patients in group A with syncope recurrence cumulated 17 episodes during the 12 months after the procedure compared with 254 episodes at baseline (−93%; P=0.008). Eight of these patients had exclusively syncope with long prodromes compared with 2 at baseline. The procedure was associated with a lower presyncope burden during follow-up compared with baseline (−95%; P<0.001). With the exception of the patient with atrioventricular block during TTT at baseline, no patients developed atrioventricular block during follow-up.
Figure 3.
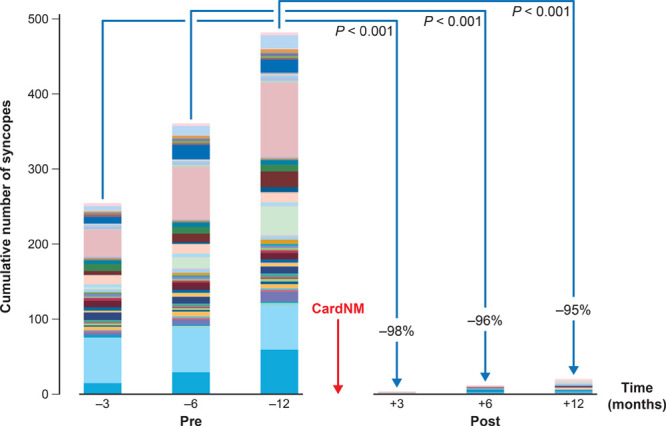
Syncope burden at 3, 6, and 12 mo before and after cardioneuromodulation (CardNM). Each patient is represented by a different color.
Tilt Table Test
The results of the TTT were negative in 24 patients at both 1- and 6-month follow-up (P<0.001). Seven patients had a positive TTT result at 1 month (VASIS 1 in 2, VASIS 2A in 1, VASIS 2B in 1, and VASIS 3 in 3). Three patients with a VASIS 1 response at baseline had a positive test at 6 months (VASIS 1 in 1 patient and VASIS 3 in 2 patients), and 3 with a cardioinhibitory response at baseline had a positive test (VASIS 1 in 1 patient and VASIS 2B in 2 patients). Eighty-two percent of the patients with syncope recurrence in group A had a VASIS 1 response during the TTT at baseline and, 18% had a VASIS 2 response (P=0.11). One patient with an atrioventricular block at baseline presented an atrioventricular block at 6 months.
Electrocardiographic Data
The basal HR data and the HR data after AT injection are shown in Figure 4 and Table 3. During cardioneuromodulation, the basal HR was reset to 73±13 beats per minute, corresponding to a 20% mean increase in basal HR (P<0.001). In the patients who underwent pacemaker implantation after 6 months of follow-up, the basal HR was reset to 74±14 beats per minute.
Figure 4.
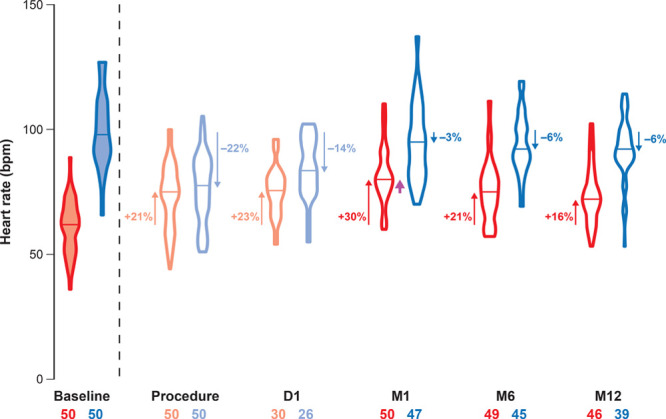
Basal heart rate (HR) and HR after atropine injection. Basal HRs are represented by red violin plots. Reset basal HRs after ablation and 1 d later are represented by pale-red violin plots. Corresponding HRs after atropine are shown by the blue and pale-blue violin plots. The median value is mentioned for each violon plot by a horizontal line. The number of patients involved (X–X) and level of the median HR modification compared with baseline (%) are shown. HR acceleration (upward arrows) or deceleration (downward arrows) were all significant compared with baseline (P<0.001). The median basal HR at M6 and M12 was comparable with the postprocedural basal HR while the median basal HR at M1 was significantly higher (purple arrow; P<0.001). D indicates day; and M, month.
Table 3.
Ambulatory Electrocardiographic Data
Compared with this postprocedural reset of basal HR, basal HR remained similar the day after the procedure (75±10 beats per minute; P=0.92), increased significantly at 1 month (82±12 beats per minute; P<0.001), and was identical at 12 months (73±11 beats per minute; P=0.93). The basal HR at baseline, the day after the procedure, and at 12-month follow-up was similar in patients with and without syncope recurrence.
At baseline, basal HR increased from 61±11 to 97±15 beats per minute after AT injection (P<0.001). Twenty-one patients (15 in group A and 6 in group B) had an HR >100 beats per minute, and 16 had an HR >90 beats per minute (10 in group A and 6 in group B). In 13 patients (6 in group A and 7 in group B), HR remained <90 beats per minute. After AT, basal HR increased minimally after ablation (from 73±13 to 77±14 beats per minute; P<0.001) and modestly the day after the procedure (from 75±10 to 85±12 beats per minute; P<0.001). In the patients who underwent pacemaker implantation after 6 months of follow-up, HR increased by 2±5 beats per minute after AT injection after cardioneuromodulation.
In the 39 patients who also underwent an AT test at 12 months, HR post-AT was 99±15 beats per minute at baseline, tended to be lower at 1 month (94±15 beats per minute; P=0.24), and was significantly lower at 6 months (93±12 beats per minute; P=0.027) and 12 months (92±12 beats per minute; P<0.004). The mean HR after AT administration at the 12-month follow-up was identical when using the HR value corresponding to a total dose of 2 mg in all patients or using the HR corresponding to the dose of AT administered at baseline in all patients. There was no statistical difference between the HR post-AT at 6- and 12-month follow-up compared with baseline (P=1.00). The basal HR at baseline (61.1±14.1 versus 60.8±9.8 beats per minute; P=1.00), the day after the procedure (78.9±9.7 versus 73.7±9.7 beats per minute; P=0.71), and at 12-month follow-up (74.7±13.4 versus 72.5±10.9 beats per minute; P=1.00) did not differ significantly in patients with and without syncope recurrence. At baseline, the basal HR in the patients who underwent pacemaker implantation after 6 months of follow-up was 44±11 beats per minute.
HRs after AT administration at baseline (103.4±12.4 versus 94.4±15.4 beats per minute; P=0.28) and on the day after the procedure (88.7±9.4 versus 82.0±13.1 beats per minute; P=0.69) did not differ significantly in patients with and without syncope recurrence and tended to be higher in patients with syncope recurrence at 12 months (98.4±10.6 versus 89.6±12.21 beats per minute; P=0.07). At baseline, the HR after AT in the patients who underwent pacemaker implantation after 6 months of follow-up was 83±12 beats per minute (74 and 78 beats per minute in patients aged 70 and 75 years, respectively).
Twenty-Four–Hour Rhythm Registration Data
Holter data are summarized in Table 3.
The number of P-P intervals ≥1000 ms during 24-hour rhythm registration decreased from 23 646±17 015 beats at baseline to 11 523±13 684 beats at 12 months (P<0.001). Equivalent data for P-P intervals ≥1200 ms decreased from 7094±10 874 beats at baseline to 2063±5691 beats at 12 months (P<0.003).
Changes in mean HR during 24-hour rhythm registration are detailed in Figure 5. Mean HR of the whole cohort increased from 69±9 beats per minute at baseline to 77±10 beats per minute at 12 months, corresponding to mean HR accelerations of 12% (P<0.001). Patients with a mean HR <70 beats per minute at baseline increased their mean HR from 61±6 to 75±12 beats per minute at 12 months (P<0.001), whereas patients with a mean HR ≥70 beats per minute at baseline did not increase their HR significantly (P=0.14).
Figure 5.
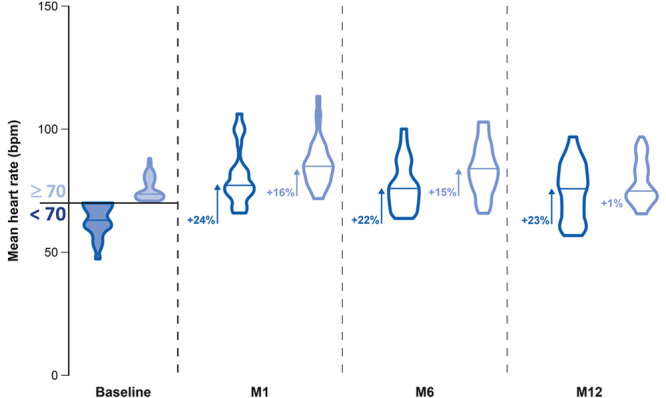
Mean heart rate (HR) during 24-h rhythm registration of patients with a mean HR at baseline under or above 70 beats per minute (bpm) at baseline, M1, M6, and M12. The blue violin plots indicate patients with a mean HR at baseline <70 bpm, and the light blue violin plots indicate these with a mean HR ≥70 bpm. The data are presented at baseline, M1, M6, and M12. The median values are indicated by the horizontal line in each violin plot. The level of HR acceleration compared with baseline is indicated in percentage. Significant values (P<0.001) compared with baseline are indicated by arrows. M indicates month.
The mean of the SDs of all NN intervals for each 5-minute segment of the recording decreased from 80.7±29.2 ms at baseline to 59.4±50.9 ms at 12 months and the HRV index from 47.8±15.1 at baseline to 35.4±12.4 at 12 months. Mean HR and HRV data did not differ between patients with and without syncope recurrence.
The mean HR at baseline was 69±9 beats per minute in patients without syncope recurrence and 56±9 beats per minute in patients who underwent pacemaker implantation after 6 months. The mean HR at 1-month follow-up was 83±11 versus 79±6 beats per minute. At 6 months, Holter monitoring was performed in 2 patients who underwent later a pacemaker implantation and revealed a mean HR of 73±9 beats per minute compared with 81±11 in patients without a recurrence. The HRV data were in line with the ECG and Holter data.
Exercise Test
The maximal HR decreased transiently by 10 beats per minute at 1 month (P<0.001). The maximal workload at baseline (202±64 W) and at 1 month (197±58 W) appeared similar (P=0.75). Maximal HR changes are detailed in Table 3.
Discussion
In this article, we report 12-month data from 50 patients with syncope included in the CardNMH2 study, 31 with NMS, and 19 with a pause ≥3 s. These results confirm our intermediate analysis and show that cardioneuromodulation is a reproducible and fast procedure and was associated with a lower syncope and presyncope burden (−95%) compared with baseline and was without safety concerns in this patient cohort. The treatment was considered to have failed in 1 patient in group A and in 3 patients in group B. Of these 3 patients, 2 were ≥70 years of age and hence were at higher risk of having intrinsic SN impairment. Of note, their HRs accelerated moderately after AT at baseline, possibly pointing to the beginnings of intrinsic SN dysfunction. Based on this, we believe that the criteria used for group B need to be more selective to improve our ability to identify with greater specificity patients with a functional SN dysfunction. The remaining 9 patients in group A who were not free of syncope had a lower rate of syncope during follow-up (−93%); in all except 1 patient, syncope recurred only after long prodromes. Limited medication changes occurred during the study and are unlikely to have affected the conclusions.
At baseline, the HR after AT injection indicates to which level the basal HR could be reset by any procedure aiming at complete SN vagal denervation. Had long-term complete vagal denervation been achieved, the basal HR would have been >100 beats per minute in 21 patients and >90 beats per minute in 37 patients. We discuss further why this was not observed.
A marked shortening in the P-P interval was observed during cardioneuromodulation. At 12 months, the basal HR and the mean HR were reset to a higher level compared with baseline. Interestingly, the HR acceleration was more pronounced in patients with a slow HR at baseline. Twenty-four−hour rhythm registration data confirmed the marked diminution in the number of beats <60 per minute after cardioneuromodulation.
The basal HR, maximum HR during exercise, and mean 24-hour rhythm data showed dynamic changes during follow-up due to modifications in SN innervation. Whereas basal HR at 12 months was similar to postprocedural reset HR, HR after AT injection was lower at 12 months versus baseline. How do we explain this? HR increased significantly more after AT injection at 12 months compared with post-procedure, probably due to vagal reinnervation. Despite this, the basal HR at 12 months remained similar to postprocedural reset basal HR. This apparent paradox could be explained by a concomitant sympathetic reinnervation. As the sympathetic activity does affect the basal HR, we link the transient higher basal HR at 1 month versus the postprocedural basal HR to the fact that SN sympathetic reinnervation should have occurred faster than SN vagal reinnervation.18 We assume that SN sympathetic reinnervation after cardioneuromodulation is essentially due to axonal regrowth of neurons penetrating the ARGP, whereas SN vagal reinnervation after cardioneuromodulation is due to axonal regrowth from nontargeted ganglionated plexi. As these ganglionated plexi are located further from the SN area, sympathetic and vagal SN reinnervation processes are not complete at the same moment. The average rate of axonal regeneration in the peripheral nervous system is 1 mm/day.19 We have not found similar data in the literature concerning the ICANS, but axonal regeneration could be in the same range. Indeed, assuming that our data pointed to SN sympathetic reinnervation at 1 month, sympathetic axonal regeneration should be ≥0.8 mm/day as the distance between the TL and the SN area was 23±7 mm.
At 12 months, vagal and sympathetic reinnervation effects appeared to antagonize each other, resulting in a basal HR at 12 months similar to the postprocedural basal HR. As ganglionated plexi contain both vagal and sympathetic fibers, it is reasonable to assume that cardioneuromodulation induced not only SN vagal denervation but also SN sympathetic innervation modifications. Although SN sympathetic reinnervation predominated at 1 month, sympathetic reinnervation was then not complete, as the maximal HR was lower than that at baseline and fortunately was restored at 6 months.
During cardioneuromodulation, ARGP ablation reset the basal HR to a higher level. During follow-up, modified ICANS physiology resulted from dynamic modifications of both the vagal and the sympathetic SN innervation. Twelve months after ARGP ablation, the persisting effect corresponded to a partial SN vagolysis, which was the goal of the procedure.
Assuming the intrinsic SN function was unaffected by the cardioneuromodulation procedure and remained unchanged during follow-up, the decrease in HR during the AT test at 12 months versus baseline could be due to a higher catecholaminergic status at baseline despite the highly standardized ECG registration protocol. Alternatively, it may indicate a residual nonclinical impairment of SN sympathetic denervation. Regarding this, one should also note that, although not statistically significant (P=0.09), the maximal HR during exercise tended to be lower at 12 months (153±15 beats per minute) versus baseline (158±19 beats per minute).
Partial vagal reinnervation after any cardioneuroablation procedure is desirable. Indeed, any ablation procedure achieving a persisting long-term complete SN vagal denervation will give rise to an inappropriate high basal HR in most patients, unless the procedure downgrades the intrinsic SN function or compromises the sympathetic SN innervation in the long term. This should be kept in mind when assessing, developing, proposing, or performing any cardioneuroablation approach.
The goal of cardioneuroablation procedures is to modify the ICANS physiology to improve patient outcome. Ganglionated plexi have been proposed as complex integration centers modulating cardiac responses to autonomic input.20 Changes in ICANS physiology induced by cardioneuroablation procedures may differ greatly according to the sites being targeted and hence have different short- and long-term consequences. Ganglionated plexi contain not only large populations of colocalized sympathetic and parasympathetic neurons but also afferent neurons, motor neurons, interconnecting local neurons, and, interestingly, some neurons expressing both cholinergic and adrenergic activity.15 Any cardioneuroablation strategy will interfere specifically with this complex system. After cardioneuroablation, the interactions of the different neurons of the ICANS can change substantially. This effect could partially explain the apparent efficacy of these approaches even in the long term when vagal reinnervation is thought to be complete. Although the global level of vagal reinnervation after a procedure can be assessed, the fine interactions between the different components of the ICANS will probably be difficult to assess in humans. Standardization of the lesion set is important, to be able to investigate the physiological changes associated with the different ablation strategies. Targeting a single ganglionated plexus selectively offered us a unique opportunity to describe the short- and long-term physiological changes associated with ARGP ablation and enabled us to demonstrate that the ARGP belonged to the final common pathway of SN vagal innervation.14
Our opinion is that total syncope burden and nonprodromic and complicated syncope-free survival curves are the most important clinical variables when assessing the efficacy of any cardioneuroablation approach for syncope. Moreover, the effect on syncope should always be put into perspective with the collateral effects and risks. The ideal cardioneuroablation approach for a specific clinical problem should be safe, fast, and reproducible, with minimal physiological changes, but still able to predict a good clinical outcome.21 Cardioneuromodulation is a fast procedure, exposing patients to fewer procedural risks than left-sided or biatrial multiple-site ablation strategies. The long-term clinical results were good, with a persisting adequate partial SN vagolysis, while the chronotropic SN function seems only transiently and modestly affected by cardioneuromodulation. Interestingly, Hu et al9 pointed out that ARGP could be the cornerstone of cardioneuroablation procedures.
In this study, the level of SN vagolysis achieved during cardioneuromodulation was similar in patients with or without syncope recurrence. The 24-hour rhythm registration data did not reveal a higher level of vagal reinnervation at 12 months in patients with syncope recurrence compared with patients without recurrence.
While our data suggest that cardioneuromodulation could be useful for most patients with NMS (having a VASIS 1 or 2 response during the TTT) and for patients with functional SN dysfunction, they do not suggest that cardioneuromodulation could be useful for treating functional atrioventricular block. Additional ablation steps, or a panablation strategy, could be of use for this particular clinical problem. Importantly, in this cohort of 50 patients, none of the patients without an atrioventricular block at baseline developed a block during follow-up (12–30 months). Vasoplegia could be the underlying mechanism of syncope recurrence in patients with NMS without documented bradycardia.
Limitations
As the AT test the day after the procedure was not included in the first version of the protocol, the corresponding data are limited. The dose of AT used has been adjusted at the beginning of the trial. Although this protocol amendment could be considered as a limitation, it apparently did not affect the results.
This is a single-center, nonrandomized, unblinded trial with no control arm (sham procedure) as with previous trials on cardioneuroablation. There was no other active arm such as a pacemaker with a dedicated algorithm or another ablation strategy targeting other parts of the ICANS or all the structures of the ICANS. Therefore, we cannot determine whether these therapeutic strategies would have given different results. All procedures were performed by the same operator. The current results need to be confirmed in a multicenter randomized trial.
Conclusions
Cardioneuromodulation is a safe and fast treatment that gives rise to a long-term partial SN vagolysis with no apparent short- or long-term safety concerns or undesirable persisting modifications of the intracardiac autonomous nervous system. The impact on vasoplegia is more questionable. It is associated with a good clinical outcome in most patients with NMS or functional SN dysfunction. These promising data require confirmation in a multicenter randomized trial.
Acknowledgments
We are grateful to our institution and our Cardiology Department who have made this project possible and to all the physicians who referred patients for cardioneuromodulation. We thank Inge Benoy (Biosense Webster) for professional support during the procedure. We are grateful for the valuable contributions of Margareta Badts and Sofie Albertijn, the study nurses of our Cardiology Department, who were dedicated to this trial. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications, London), provided editorial assistance in manuscript preparation, under supervision of the lead author, and was funded by the Cardiology Department of Imeldaziekenhuis.
Sources of Funding
The study is being supported by grants from Biosense Webster, Biotronik, and Abbott. The companies had no role in the design of the study or in the collection or management of the data or in the writing of the manuscript.
Disclosures
None.
Supplemental Materials
Supplemental Table I
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- ARGP
- anterior right ganglionated plexus
- AT
- atropine
- HR
- heart rate
- HRV
- heart rate variability
- ICANS
- intrinsic cardiac autonomous nervous system
- NMS
- neurally mediated syncope
- SN
- sinus node
- TL
- target line
- TTT
- tilt table test
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.009747.
For Sources of Funding and Disclosures, see page 576.
Contributor Information
Tom Rossenbacker, Email: Tom.Rossenbacker@imelda.be.
Luc Janssens, Email: Luc.Janssens@imelda.be.
Christine Collienne, Email: christine.bazelmans@biotronik.com.
Joris Ector, Email: jorisector@gmail.com.
Peter Haemers, Email: peter.haemers@uzleuven.be.
Jean-Benoît le Polain de Waroux, Email: Jean-Benoit.LePolainDeWaroux@azsintjan.be.
Christine Bazelmans, Email: christine.bazelmans@biotronik.com.
Tim Boussy, Email: tboussy@gmail.com.
William Wijns, Email: William.Wyns@nuigalway.ie.
References
- 1.Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e25–e59. doi: 10.1161/CIR.0000000000000498 [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martin A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 3.Pachon JC, Pachon EI, Pachon JC, Lobo TJ, Pachon MZ, Vargas RN, Jatene AD. “Cardioneuroablation”–new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Pachon JC, Pachon EI, Cunha Pachon MZ, Lobo TJ, Pachon JC, Santillana TG. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–1242. doi: 10.1093/europace/eur163 [DOI] [PubMed] [Google Scholar]
- 5.Yao Y, Shi R, Wong T, Zheng L, Chen W, Yang L, Huang W, Bao J, Zhang S. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465 [DOI] [PubMed] [Google Scholar]
- 6.Aksu T, Golcuk E, Yalin K, Guler TE, Erden I. Simplified crdioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Zheng L, Qiao Y, Shi R, Hou B, Wu L, Guo J, Zhang S, Yao Y. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. 2016;5:e003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivarola EW, Hachul D, Wu T, Pisani C, Hardy C, Raimundi F, Melo S, Darrieux F, Scanavacca M. Targets and end points in cardiac autonomic denervation procedures. Circ Arrhythm Electrophysiol. 2017;10:e004638. doi: 10.1161/CIRCEP.116.004638 [DOI] [PubMed] [Google Scholar]
- 9.Hu F, Zheng L, Liang E, Ding L, Wu L, Chen G, Fan X, Yao Y. Right anterior ganglionated plexus: the primary target of cardioneuroablation? Heart Rhythm. 2019;16:1545–1551. doi: 10.1016/j.hrthm.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Jiang W, Zhou L, Wang Y, Zhang X, Wu S, Xu K, Liu X. Atrial autonomic denervation for the treatment of long-standing symptomatic sinus bradycardia in non-elderly patients. J Interv Card Electrophysiol. 2015;43:151–159. doi: 10.1007/s10840-015-9981-8 [DOI] [PubMed] [Google Scholar]
- 11.Qin M, Zhang Y, Liu X, Jiang WF, Wu SH, Po S. Atrial ganglionated plexus modification: a novel approach to treat symptomatic sinus bradycardia. JACC Clin Electrophysiol. 2017;3:950–959. doi: 10.1016/j.jacep.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 12.Aksu T, Guler TE, Bozyel S, Golcuk SE, Yalin K, Lakkireddy D, Gopinathannair R. Medium-term results of cardioneuroablation for clinical bradyarrhythmias and vasovagal syncope: effects on QT interval and heart rate. J Interv Card Electrophysiol. 2021;60:57–68. doi: 10.1007/s10840-020-00704-2 [DOI] [PubMed] [Google Scholar]
- 13.Debruyne P. “Cardio-neuromodulation” with a multielectrode irrigated catheter: a potential new approach for patients with cardio-inhibitory syncope. J Cardiovasc Electrophysiol. 2016;27:1110–1113. [DOI] [PubMed] [Google Scholar]
- 14.Debruyne P, Wijns W. Cardio-neuromodulation: the right-sided approach. JACC Clin Electrophysiol. 2017;3:1056–1057. doi: 10.1016/j.jacep.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 15.Debruyne P, Rossenbacker T, Collienne C, Roosen J, Ector B, Janssens L, Charlier F, Vankelecom B, Dewilde W, Wijns W. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction under computed tomographic guidance. Circ Arrhythm Electrophysiol. 2018;11:e006604. doi: 10.1161/CIRCEP.118.006604 [DOI] [PubMed] [Google Scholar]
- 16.Hanna P, Rajendran PS, Ajijola OA, Vaseghi M, Andrew Armour J, Ardell JL, Shivkumar K. Cardiac neuroanatomy - Imaging nerves to define functional control. Auton Neurosci. 2017;207:48–58. doi: 10.1016/j.autneu.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N, Bartoli P, Sutton R. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace. 2000;2:66–76. doi: 10.1053/eupc.1999.0064 [DOI] [PubMed] [Google Scholar]
- 18.Zaglia T, Mongillo M. Cardiac sympathetic innervation, from a different point of (re)view. J Physiol. 2017;595:3919–3930. doi: 10.1113/JP273120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066 [DOI] [PubMed] [Google Scholar]
- 21.Debruyne P. Letter by Debruyne Regarding Article, “Targets and end points in cardiac autonomic denervation procedures”. Circ Arrhythm Electrophysiol. 2017;10:e005261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


