l-Theanine is widely used in food additives and dietary supplements. Industrial production of l-theanine uses the toxic and highly flammable precursor ethylamine, raising production costs. In this study, we used Escherichia coli to engineer two biosynthetic pathways that produce l-theanine from glucose and ammonia in the absence of supplemental ethylamine. This study establishes a foundation for safely and economically producing l-theanine.
KEYWORDS: l-theanine, Escherichia coli, ω-transaminase, γ-glutamylmethylamide synthetase, theanine hydrolase, alanine decarboxylase
ABSTRACT
l-Theanine is a nonproteinogenic amino acid present almost exclusively in tea plants and is beneficial for human health. For industrial production, l-theanine is enzymatically or chemically synthesized from glutamine/glutamate (or a glutamine/glutamate derivative) and ethylamine. Ethylamine is extremely flammable and toxic, which complicates and increases the cost of operational procedures. To solve these problems, we developed an artificial biosynthetic pathway to produce l-theanine in the absence of supplemental ethylamine. For this purpose, we identified and selected a novel transaminase (NCBI:protein accession number AAN70747) from Pseudomonas putida KT2440, which catalyzes the transamination of acetaldehyde to produce ethylamine, as well as γ-glutamylmethylamide synthetase (NCBI:protein accession number AAY37316) from Pseudomonas syringae pv. syringae B728a, which catalyzes the condensation of l-glutamate and ethylamine to produce l-theanine. Expressing these genes in Escherichia coli W3110S3GK and enhancing the production capacity of acetaldehyde and l-alanine achieved successful production of l-theanine without ethylamine supplementation. Furthermore, the deletion of ggt, which encodes γ-glutamyltranspeptidase (EC 2.3.2.2), achieved large-scale production of l-theanine by attenuating its decomposition. We show that an alanine decarboxylase-utilizing pathway represents a promising route for the fermentative production of l-theanine. Our study reports efficient methods to produce l-theanine in the absence of supplemental ethylamine.
IMPORTANCE l-Theanine is widely used in food additives and dietary supplements. Industrial production of l-theanine uses the toxic and highly flammable precursor ethylamine, raising production costs. In this study, we used Escherichia coli to engineer two biosynthetic pathways that produce l-theanine from glucose and ammonia in the absence of supplemental ethylamine. This study establishes a foundation for safely and economically producing l-theanine.
INTRODUCTION
The compound l-theanine (γ-glutamylethylamide), which is a unique amino acid present in green tea, is a major constituent of the umami taste (1, 2). l-Theanine promotes relaxation without drowsiness (3), reduces stress and anxiety (4, 5), improves the quality of sleep (6), enhances cognition (7), promotes concentration (5), diminishes the symptoms of premenstrual syndrome (8), and suppresses elevated blood pressure (9). In Japan, l-theanine was designated a food additive in 1964, before other countries, and in 2007, the Food and Drug Administration of the United States designated l-theanine as generally recognized as safe. These beneficial properties account for the wide use of l-theanine as a dietary supplement and food additive, which is reflected by its globally increasing 6-year compound annual growth rate (7.7%) (10).
To meet the increasing demand, various methods were developed for producing l-theanine, including extraction from tea leaves, chemical synthesis, enzymatic synthesis, and microbial fermentation (11–13). Other methods, such as tea callus cultivation (14) and submerged fermentation of the mushroom Xerocomus badius (15), are in their initial stages of development. l-Theanine accounts for approximately 1.5% of the dry weight of tea leaves, which contain other significant amounts of soluble substances, such as caffeine and polyphenols (16). Accordingly, the isolation of l-theanine from tea leaves is laborious and lengthy, and the yield is low (11). Although the synthetic product is often a racemic mixture of l- and d-forms, chemical synthesis is simple and now the most cost-effective method that achieves high yields (10, 11). In contrast, biosynthetic methods are preferred, because they are environmentally friendly, and chemically synthesized compounds tend to be avoided as they are “unnatural” (11). Thus, many studies on biological production have been performed as cited below.
Methods for enzymatic synthesis of l-theanine are classified as ATP-dependent or ATP-independent reactions. γ-Glutamyltranspeptidase (GGT; EC 2.3.2.2) and glutaminase (EC 3.5.1.2) catalyze the synthesis of l-theanine in an ATP-independent manner. GGT catalyzes the transfer of the γ-glutamyl moiety from γ-glutamyl compounds to amino acids and oligopeptides, which is reversible, and the substrate specificity is broad (17). GGT therefore can be employed to produce l-theanine from l-glutamate (Glu) and ethylamine (18, 19). Although glutaminase natively catalyzes the hydrolysis of the γ-amido bond of l-glutamine (Gln) to produce Glu and ammonia, glutaminases derived from Pseudomonas nitroreducens strains catalyze the synthesis of l-theanine (20, 21). Further, evidence indicates that the fungus Trichoderma koningii produces a glutaminase that also converts Gln and ethylamine to l-theanine (22). However, the reaction using GGT and glutaminase produces the hydrolytic by-product Glu, which reduces l-theanine yields (17, 23). Furthermore, a high concentration of ethylamine and highly alkaline pH are required for efficient synthesis (21, 24).
l-Theanine synthetase (TS; EC 6.3.1.6), γ-glutamylcysteine synthetase (γ-GCS; EC 6.3.2.2), l-glutamine synthetase (GS; EC 6.3.1.2), and γ-glutamylmethylamide synthetase (GMAS; EC 6.3.4.12) produce l-theanine using the cofactor ATP. l-Theanine has long been thought to be synthesized from Glu and ethylamine in tea plants through reactions catalyzed by the TS isoforms TS1 and TS2, which generate few side reactions but are very labile (25, 26). Recently, it was shown that TS1 functions mainly in shoot tissue and that Camellia sinensis TSI (CsTSI), which shares high homology with GS from Pseudomonas taetrolens (PtGS), is the key enzyme involved in the production of l-theanine in root tissue, where l-theanine is mainly generated in tea plants (27, 28). γ-GCS, which catalyzes the first step in glutathione biosynthesis in Escherichia coli, synthesizes low yields of l-theanine (29). This problem was addressed by the conversion of γ-GCS to an l-theanine synthase through directed evolution, whereas the original catalytic activity remained (30). GS derived from Corynebacterium glutamicum ATCC 13032 was first reported to synthesize l-theanine from Glu and ethylamine in the presence of ATP (31). However, the relative ligation activity of GS, such as PtGS, toward ethylamine is low compared with that toward the original substrate, free ammonia (32). GMASs from Methylophaga sp. strain AA-30 and Methylovorus mays No. 9 efficiently synthesize l-theanine (33, 34). GMAS was originally found to be required for the biosynthesis of γ-glutamylmethylamide, which is an intermediate in methylamine anabolism and more efficiently accepts ethylamine than GS as the ligation partner of Glu (35). Although enzymatic synthesis using GS or GMAS shows promise, it requires ATP, which is expensive for commercial purposes. To overcome this problem, an ATP-regeneration system using glucose and cells such as Saccharomyces cerevisiae is often applied (34). ATP regeneration using polyphosphate kinase (EC 2.7.4.1), which catalyzes the transfer of phosphates from polyphosphate to ADP, serves as an alternative approach (36).
Despite such attempts, enzymatic production essentially involves complex processes. Therefore, the fermentative production of l-theanine was accomplished using a C. glutamicum strain that expresses recombinant GMAS derived from M. mays No. 9 (MmGMAS), supplemented with ethylamine (12). Under these conditions, the enzymes and substrates, except ethylamine, are simultaneously produced. This strain produced 42 g/liter of l-theanine in 48 h. A contemporaneously published study employed a similar fermentation technique using an E. coli strain that produced 40 g/liter of l-theanine in 20 h (13). Although the yields of these fermentation methods are lower than the highest achieved using an enzymatic method (110 g/liter in 48 h) (34), the simplicity of the former is greatly advantageous when applied to manufacturing.
Although efficient biological and chemical methods are available to synthesize l-theanine as mentioned above, each uses ethylamine, which is highly flammable and toxic, as a starting material. Ethylamine boils at 16.6°C, and its vapor adversely affects the health of employees of manufacturing plants and is harmful to the external environment (37). Moreover, to improve reaction efficiency, special equipment is required to perform reactions at temperatures higher than the boiling point of ethylamine (37). There is therefore a need for a novel production method that avoids using concentrated ethylamine solutions.
To address this problem, use of alanine decarboxylase (AlaDC) in a fermentation method was attempted (12). AlaDC decarboxylates l-alanine (Ala) to produce ethylamine, which plays an important role in l-theanine biosynthesis (38). Further, AlaDC identified in the tea plant Camellia sinensis (CsAlaDC) was reported to lack AlaDC activity (12). Therefore, suitable l-theanine production methods independent of ethylamine as a raw material are unavailable.
In this study, we investigated direct fermentation of l-theanine from glucose in the absence of supplemental ethylamine via the development of artificial biosynthetic pathways in E. coli. First, we designed a novel pathway composed of transaminase (TA) and GMAS that produced acceptable yields of l-theanine in vitro. Then we constructed E. coli strains harboring this pathway and demonstrated direct fermentation of l-theanine. We subsequently investigated the possibility of using CsAlaDC to produce l-theanine. In contrast to a previous study (12), CsAlaDC efficiently supplied ethylamine in vivo, and E. coli strains coexpressing CsAlaDC (i.e., the gene encoding CsAlaDC), GMAS, and the gene encoding alanine dehydrogenase produced l-theanine. In a 3-liter culture vessel, l-theanine decomposed late during cultivation, which was eliminated by inactivation of GGT. Finally, we established fed-batch fermentation processes to synthesize l-theanine from glucose and ammonia via two new pathways: the transaminase-utilizing pathway and the AlaDC-utilizing pathway.
RESULTS
Designing a biosynthetic pathway that produces l-theanine from glucose.
To accomplish the fermentative production of l-theanine in the absence of supplemental ethylamine, we first planned to establish a genetically engineered E. coli strain by designing an artificial pathway utilizing transaminase (Fig. 1). In this pathway, acetaldehyde, which is synthesized by catabolizing glucose, is converted to ethylamine by a transamination reaction catalyzed by transaminase. Next, ethylamine and Glu are condensed to produce l-theanine using a theanine-synthesizing enzyme such as GMAS. We call this the TA pathway. For this purpose, we first identified suitable transaminases.
FIG 1.
Artificial l-theanine biosynthetic pathways mediated by transaminase (TA) or alanine decarboxylase (AlaDC) in E. coli. l-Theanine is synthesized via the TA-utilizing pathway (orange and blue) or the AlaDC-utilizing pathway (green and blue). In the TA pathway, acetaldehyde is supplied by acetyl-CoA via EutE, and subsequently, PpTA catalyzes the transamination reaction that converts acetaldehyde to ethylamine. The amino donor l-alanine (Ala) is regenerated by Bacillus subtilis 168 alanine dehydrogenase (BsAld). In the AlaDC pathway, pyruvate is converted to Ala through the reaction catalyzed by BsAld, and Ala is decarboxylated to produce ethylamine via CsAlaDC. Finally, l-theanine is synthesized from ethylamine and l-glutamate (Glu) via γ-glutamylmethylamide synthetase (GMAS) in both pathways. Genes, their products, and their species of origin are as follows: eutE, acetaldehyde dehydrogenase, E. coli; PpTA, transaminase, Pseudomonas putida; BsAld, alanine dehydrogenase, Bacillus subtilis; CsAlaDC, alanine decarboxylase, Camelia sinensis; GMAS, γ-glutamylmethylamide synthetase; gdhA, glutamate dehydrogenase, E. coli; and gltA, citrate synthase, E. coli. TCA, tricarboxylic acid.
Identification of transaminases that convert acetaldehyde to ethylamine.
Transaminase catalyzes the transfer of an amino group from an amino donor to a ketone or aldehyde. In particular, ω-transaminase transfers the amino group from a carbon atom without a carboxyl group and often exhibits broad substrate capacity (39, 40), which raised the possibility of an acetaldehyde transaminase in this family. Pseudomonas putida, a Gram-negative bacterium, decomposes diverse molecules in its environment, indicating that P. putida promiscuously utilizes carbon, nitrogen, or both from various compounds (41, 42). In nitrogen utilization, in particular, deamination of nitrogen sources catalyzed by transaminases may be involved, because the genome of P. putida encodes numerous transaminases. Therefore, we cloned and expressed in E. coli BL21(DE3) 32 transaminase-encoding genes from P. putida KT2440 and assessed their abilities to convert acetaldehyde to ethylamine (see Table S1 in the supplemental material). To ensure efficiency, the most preferred amino donor is free ammonia, which is inexpensive and is readily available as a raw material. However, aminating enzymes whose amino donor is ammonia are limited to the amino acid dehydrogenase families with strict substrate specificities (43). We chose Ala as an amino donor for transaminase because Ala can be supplied easily exogenously and endogenously, and it serves as a typical amino donor for ω-transaminase. We performed in vitro assays of crude extracts of transaminase-expressing E. coli strains with the substrates Ala and acetaldehyde (Fig. 2). Consequently, we identified two candidate ω-transaminases with efficient activity, P. putida TA3 (PpTA3) (NCBI:protein accession number AAN67793) and PpTA8 (NCBI:protein accession number AAN70747).
FIG 2.
In vitro assay of transaminases derived from Pseudomonas putida KT2440. The rates of conversion of acetaldehyde to ethylamine represent the averages of two biological replicates. Standard deviations between replicates are shown as error bars.
Acquisition of GMAS and in vitro constitution of the TA pathway.
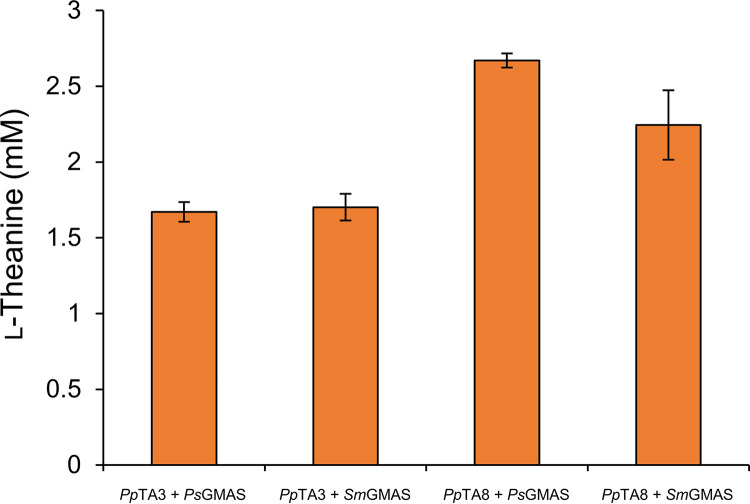
To convert ethylamine to l-theanine in E. coli, Glu is a more desirable partner than Gln, because Gln is the downstream metabolic product of Glu and requires ATP for its synthesis from Glu, suggesting that GS or GMAS is appropriate. To construct the TA pathway, we therefore selected GMAS, because the optimum pH range for GS activity is 8.5 to 11.0 (32), which will not support the viability of E. coli (intracellular pH range, 7.0 to 8.0) (44). In contrast, the pH optimum range of GMAS is 7.0 to 8.0 (32). Characterized GMAS homologs were not available from our genome collection (33, 34, 45), and we therefore attempted to molecularly clone homologs. When we used MmGMAS (NCBI:protein accession number A9ZPH9) as a query for BLAST searches, we identified the GMAS homologs in our genome collection as follows: P. syringae pv. syringae B728a, Sinorhizobium meliloti 1021, and Mesorhizobium loti MAFF303099. The amino acid sequences of the homologs PsGMAS (NCBI:protein accession number AAY37316), SmGMAS (WP_0109147491), and MlGMAS (NCBI:protein accession number WP_003532529) are 62.0%, 58.1%, and 39.6% identical to that of MmGMAS, respectively. We expressed each of these homologs in BL21(DE3) and assayed crude extracts for enzyme activity (Fig. 3). GshA (γ-GCS) from E. coli W3110 served as a positive control (30). Each extract synthesized l-theanine, and the activities of PsGMAS and SmGMAS were highest. Thus, we selected PsGMAS and SmGMAS for further analysis. The trace activity of the negative control BL21(DE3) harboring the empty vector was likely caused by endogenous GshA.
FIG 3.
In vitro assay of γ-glutamylmethylamide synthetase homologs. The rates of conversion of ethylamine to l-theanine represent the averages of four biological replicates. Standard deviations between replicates are shown as error bars.
We subsequently determined whether the combination of transaminase and GMAS converted acetaldehyde to l-theanine in the presence of Ala, Glu, and ATP (Fig. 4). We found that the combination of PpTA8 and PsGMAS produced the highest concentration of l-theanine. Therefore, we selected PpTA8 and PsGMAS as components of the TA pathway.
FIG 4.
In vitro constitution of the TA pathway. The conversion of acetaldehyde to l-theanine by the combination of TA and GMAS was measured in vitro. The concentrations of l-theanine represent the averages of four biological replicates. Standard deviations between replicates are shown as error bars.
Metabolic engineering of E. coli harboring the TA pathway for l-theanine production.
Although we constructed an artificial pathway of l-theanine biosynthesis in vitro, when we introduced PpTA8 and PsGMAS into E. coli W3110S3GK (TEA1) and cultivated TEA1 in a test tube, accumulation of l-theanine in the culture was not detected (data not shown), indicating an insufficient supply of acetaldehyde and Ala. When we added acetaldehyde and Ala to the medium (final concentrations = 10 mM), 22.9 mg/liter of l-theanine was produced (Fig. 5). To enhance the effect of Ala, we introduced the gene encoding alanine dehydrogenase derived from Bacillus subtilis 168 (BsAld), which is often used to increase Ala production in several hosts (46), into W3110S3GK (TEA2). TEA2 supplemented with acetaldehyde produced 24.1 mg/liter of l-theanine, which was equivalent to that of TEA1 supplemented with acetaldehyde and Ala (Fig. 5), indicating that the expression of BsAld successfully complemented 10 mM Ala supplementation. Overexpression of eutE, encoding aldehyde dehydrogenase, which catalyzes the reaction between acetyl coenzyme A (acetyl-CoA) and acetaldehyde, increases the production of acetaldehyde in E. coli (47). We therefore overexpressed eutE to increase the endogenous amount of acetaldehyde. The resulting strain, TEA3, when supplemented with 10 mM Ala, produced higher concentrations of l-theanine than TEA1 supplemented with acetaldehyde and Ala, indicating that TEA3 produced sufficient amounts of acetaldehyde. Accordingly, we constructed strains transformed with plasmids that coexpressed BsAld and eutE along with PsGMAS and PpTA8, which produced >300 mg/liter of l-theanine (TEA4 and TEA5). Because TEA4 had the highest level of l-theanine production, we selected this strain for further analysis.
FIG 5.
l-Theanine production using the TA pathway in recombinant E. coli W3110S3GK strains harboring the plasmids listed in Table 2. Cells were cultured in TT medium at 30°C for 24 h. Supplemental acetaldehyde and l-alanine were added to the media as required. The yields of l-theanine represent the averages of three biological replicates. Standard deviations between replicates are shown as error bars.
l-Theanine production using AlaDC.
AlaDC has long been considered to be involved in l-theanine synthesis in tea plants (C. sinensis) (48). In this pathway, AlaDC decarboxylates Ala to generate ethylamine, which is then condensed with Glu to produce l-theanine by theanine-synthesizing enzymes (28, 48). While we were studying the TA pathway, CsAlaDC was finally identified in C. sinensis (38). Contrary to this report, this enzyme was subsequently reported to lack alanine decarboxylase activity (12). To be certain, we decided to attempt to construct the AlaDC pathway in E. coli comprising BsAld, CsAlaDC, and PsGMAS to produce l-theanine (Fig. 1). Using a synthetic gene identical to native CsAlaDC (MN241445), we constructed three types of plasmids expressing BsAld, CsAlaDC, and PsGMAS to optimize the expression of these genes and cultivated W3110S3GK strains harboring the respective plasmids (TEA6, TEA7, and TEA8). l-Theanine was produced by each strain, and TEA6 yielded the highest l-theanine concentration (1.53 g/liter) (Fig. 6), which was higher than that of TA pathway, suggesting that CsAlaDC works well in E. coli. We therefore selected TEA6 for further analysis.
FIG 6.
l-Theanine production using the AlaDC pathway in recombinant E. coli W3110S3GK strains harboring the plasmids listed in Table 2. Cells were cultivated in TT medium at 30°C for 24 h. The yields of l-theanine represent the averages of three biological replicates. Standard deviations between replicates are shown as error bars.
Fed-batch fermentation of l-theanine using E. coli harboring the TA or AlaDC pathway.
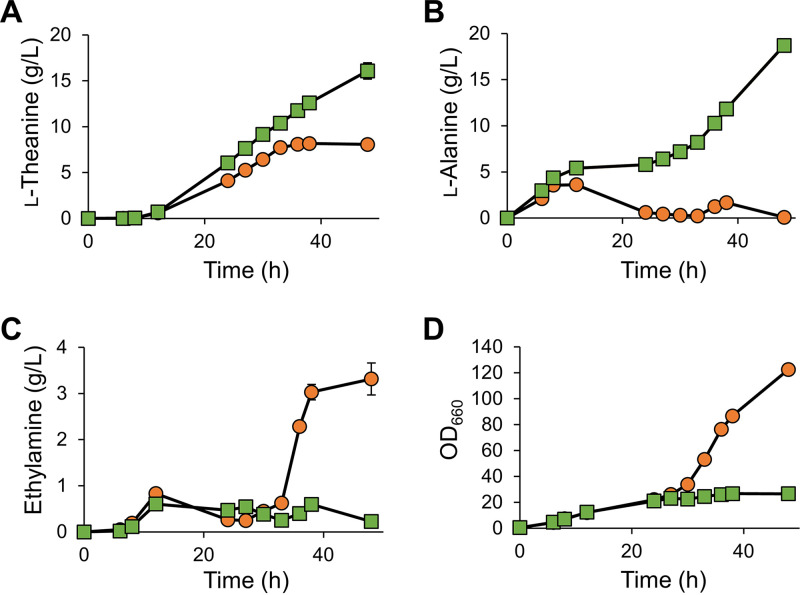
To evaluate the industrial potential of the TA and AlaDC pathways to produce l-theanine, fed-batch fermentation (3-liter jar) was performed. When we first employed TEA4, the yield of l-theanine was low after 48 h, because it decomposed late during fermentation (Fig. S1). We therefore developed a strategy to prevent the degradation of l-theanine. Although C. glutamicum does not catabolize l-theanine (12), it was indicated that E. coli expresses an enzyme with this activity, which is produced during fermentation. We focused on GGT as a candidate because it hydrolyzes γ-glutamyl-containing molecules, including l-theanine (49). In C. sinensis, l-theanine hydrolase (EC 3.5.1.65) decomposes l-theanine (50), and evidence indicates that it is identical to CsPDX2.1, which is a homolog of pyridoxine biosynthesis 2 (PDX2) (51). The genome of E. coli does not harbor a gene encoding a homolog of CsPDX2.1 (52). We therefore constructed a ggt-deficient mutant of W3110S3GK (W3110S3GK Δggt) and evaluated its ability to decompose l-theanine (Fig. S2). W3110S3GK Δggt showed almost no l-theanine decomposition activity. Therefore, the ggt-deficient strains TEA4 and TEA6 (TEA4 Δggt and TEA6 Δggt, respectively) were constructed and employed for fed-batch fermentation (Fig. 7). TEA4 Δggt and TEA6 Δggt produced 8.07 g/liter and 16.1 g/liter of l-theanine in 48 h, respectively, and the yields of l-theanine relative to glucose were 0.053 g/g and 0.133 g/g, respectively, which proves that the TA and AlaDC pathways synthesize l-theanine in a large-scale fermentation. Moreover, the ethylamine concentration produced by both pathways remained low (>1 g/liter) during the production phase. These findings provide compelling evidence that our strategy effectively mitigated the toxic effects of ethylamine compared with existing methods for producing l-theanine. Notably, TEA6 Δggt produced up to 18.7 g/liter of Ala, and the concentration of ethylamine, when TEA4 Δggt was cultivated, increased after the l-theanine production phase, suggesting that further optimization of metabolic flux of both pathways will lead to more efficient synthesis of l-theanine.
FIG 7.
Fed-batch fermentation using TEA4 Δggt and TEA6 Δggt. Orange circles represent TEA4 Δggt, and green squares represent TEA6 Δggt. The yields of l-theanine (A), l-alanine (B), and ethylamine (C) and OD660 (D) represent the averages of two biological replicates. Standard deviations between replicates are shown as error bars.
DISCUSSION
Here, we describe the construction of novel synthetic pathways that robustly synthesize l-theanine while minimizing the toxic effects of ethylamine. For this purpose, we first designed the TA pathway in E. coli comprising transaminase and GMAS (Fig. 1). Then we identified novel ω-transaminases derived from P. putida KT2440 that catalyze the transfer of an amino group from Ala to acetaldehyde and catalytically active GMAS homologs of bacterial species (P. syringae and S. meliloti) associated with plants. When we introduced these genes into E. coli, manipulated intermediary metabolism, and deleted a gene related to decomposition of l-theanine, we achieved high-level production of l-theanine in the absence of supplemental ethylamine in a large-scale fermentation.
Production of l-theanine by the TA pathway ceased after 33 h, at which time cell growth and ethylamine production concomitantly began to increase (Fig. 7), suggesting that the supply of Glu stopped and that the cell used the tricarboxylic acid cycle to generate energy to support growth. However, these processes did not occur in cells harboring the AlaDC pathway, likely because of the use of eutE and PpTA8, whose products are not components of the AlaDC pathway. Further studies are therefore required to understand this finding. In this context, pyruvate decarboxylase (PDC; EC 4.1.1.1) can be substituted for EutE. PDC is derived from ethanol-producing Zymomonas mobilis, which efficiently decarboxylates pyruvate to produce acetaldehyde (53, 54). In contrast, bacterial strains such as E. coli produce aldehyde reductases (ALRs) that convert aldehydes to alcohols (55), which may catabolize acetaldehyde to ethanol. We therefore predict that deleting genes encoding ALRs will increase the concentration of l-theanine by eliminating the waste product acetaldehyde.
In the present study, we also found that CsAlaDC was catalytically active in E. coli and that the AlaDC pathway synthesized l-theanine (Fig. 1). This pathway produced equal amounts of Ala and l-theanine, while the ethylamine concentration remained low throughout fermentation (Fig. 7), indicating that AlaDC activity was insufficient. The reaction catalyzed by AlaDC is regarded as the rate-limiting step in the l-theanine synthetic pathway of C. sinensis (56), and Bai et al. reported that CsAlaDC has low catalytic efficiency (38). We therefore conclude that protein engineering will increase the activity of CsAlaDC. However, our knowledge of the enzymatic properties of AlaDC is insufficient, and further functional and structural studies are therefore required. Alternatively, E. coli may not serve as a suitable host for the expression of CsAlaDC because of the difference between the intracellular environments of prokaryotes and eukaryotes. Thus, employing other hosts, such as S. cerevisiae, may achieve higher solubility and appropriate posttranslational modifications of CsAlaDC.
We were unconcerned about the supply of Glu to both pathways, because insignificant amounts of Glu were detected in the fermentation experiments, and we were unable to determine if the Glu supply was insufficient. For example, similar efforts to improve l-theanine productivity employed the Glu-overproducing strain C. glutamicum GDK-9, in which the gene encoding the Glu exporter was disrupted (12). Others knocked out sucCD of E. coli W3110, which encodes succinyl-CoA synthetase, to increase the metabolic flux of 2-oxoglutarate, the precursor of Glu (13). We conclude from these studies that enforced expression of Glu-synthetic genes such as gdhA, deletion of sucCD, and inactivation of Glu efflux genes may increase the production of l-theanine.
l-Theanine-producing Luteibacter spp., isolated from plantlets of C. sinensis cultivars grown in vitro, synthesize l-theanine more efficiently from Gln and l-lysine than from Glu and Ala (57), suggesting that its biosynthesis does not require AlaDC and that a novel enzyme mediates l-theanine synthesis. Identification of this putative enzyme may provide another solution that will achieve fermentative production of l-theanine without ethylamine supplementation.
In summary, we successfully developed artificial synthetic pathways in E. coli that synthesize l-theanine from glucose and ammonia. Our findings provide a foundation for producing l-theanine in a manner that mitigates the toxic effects of the indispensable precursor ethylamine as well as reducing the considerable expense required for this purpose. Further optimization of metabolic flux of both pathways will likely lead to higher productivity. We are currently developing an industrial method on the basis of our present findings to realize the commercial production of l-theanine.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. E. coli W3110S3GK is registered as NBRC114657 to the NITE Biological Resource Center (NBRC; Tokyo, Japan) and was used to produce l-theanine. E. coli DH5α was used as a molecular cloning host for plasmid preparation. E. coli BL21(DE3) was used as a host to produce recombinant enzymes.
TABLE 1.
E. coli strains used in this study
| Strains | Description | Source or reference |
|---|---|---|
| DH5α | deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| BL21(DE3) | B dcm ompT hsdS(rB− mB−) gal | Invitrogen |
| W3110S3GK | NBRC 114657 | NBRC |
| W3110S3GKΔggt | W3110S3GK ggt-deficient mutant | This study |
| TEA0 | W3110S3GK harboring plasmid pTrc99a | This study |
| TEA1 | W3110S3GK harboring plasmid pTEA1 | This study |
| TEA2 | W3110S3GK harboring plasmid pTEA2 | This study |
| TEA3 | W3110S3GK harboring plasmid pTEA3 | This study |
| TEA4 | W3110S3GK harboring plasmid pTEA4 | This study |
| TEA5 | W3110S3GK harboring plasmid pTEA5 | This study |
| TEA6 | W3110S3GK harboring plasmid pTEA6 | This study |
| TEA7 | W3110S3GK harboring plasmid pTEA7 | This study |
| TEA8 | W3110S3GK harboring plasmid pTEA8 | This study |
| TEA4 Δggt | W3110S3GKΔggt harboring plasmid pTEA4 | This study |
| TEA6 Δggt | W3110S3GKΔggt harboring plasmid pTEA6 | This study |
| BL21(DE3)/pET21a(+)-PpTA1 to -32 | BL21(DE3) strains each harboring one of the plasmids from pET-21a(+)-PpTA1 to pET-21a(+)-PpTA32 | This study |
| BL21(DE3)/pET21a(+) | BL21(DE3) harboring plasmid pET-21a(+) | This study |
Construction of plasmids and strains.
The plasmids used in this study are listed in Table 2. The primers used for gene amplification and recombinant plasmid construction are listed in Tables S2 and S3. Genes encoding transaminases, γ-glutamylmethylamide synthetases, and γ-glutamylcysteine synthase were PCR amplified from genomic DNA using PrimeSTAR MAX DNA polymerase (TaKaRa Bio, Shiga, Japan) with programs set according to the manufacturer’s instructions. The gene encoding CsAlaDC was synthesized by GenScript Biotech (Nanjing, China). Amplicons were inserted into the pET-21a(+) vector using the In-Fusion cloning system (TaKaRa Bio), and the resulting plasmids were introduced into E. coli BL21(DE3) using electroporation. The PpTA8, PsGMAS, BsAld, eutE, and CsAlaDC genes were ligated to the pTrc99A vector, and the resulting plasmids were electrophoretically introduced into E. coli W3110S3GK.
TABLE 2.
Plasmids used in this study
| Plasmids | Description | Source/reference |
|---|---|---|
| pET-21a(+)-PpTA1 to -32 | Expressing one of the proteins from PpTA1 to PpTA32 | This study |
| pET-21a(+) | Replication origin of pBR322; T7 promoter; lacI; ampicillin resistant | Novagen |
| pTEA1 | l-Theanine production, pTrc99A-PsGMAS-PpTA8 | This study |
| pTEA2 | l-Theanine production, pTrc99A-PsGMAS-PpTA8-BsAld | This study |
| pTEA3 | l-Theanine production, pTrc99A-PsGMAS-PpTA8-eutE | This study |
| pTEA4 | l-Theanine production, pTrc99A-PsGMAS-PpTA8-BsAld-eutE | This study |
| pTEA5 | l-Theanine production, pTrc99A-PsGMAS-PpTA8-eutE-BsAld | This study |
| pTEA6 | l-Theanine production, pTrc99A-PsGMAS-AlaDC-BsAld | This study |
| pTEA7 | l-Theanine production, pTrc99A-PsGMAS-BsAld-AlaDC | This study |
| pTEA8 | l-Theanine production, pTrc99A-AlaDC-PsGMAS-BsAld | This study |
| pTrc99A | Replication origin of pBR322; trc promoter; lacI; ampicillin resistant | GE Healthcare |
| pCatSac | pHSG299 cat sacB; chloramphenicol resistant; kanamycin resistant | 58 |
| pKD46 | oriR101 repA101(ts) araBp-gam-bet-exo; ampicillin resistant | 59 |
Gene deletion.
Gene deletion was performed using the modified phage lambda Red recombinase system as previously described (58–60). Primers are listed in Table S4. pKD46 was obtained from the E. coli Genetic Stock Center. DNA fragments for gene deletion were prepared as follows. The 5′-flanking region of the target DNA was amplified using ggt_1 and ggt_2, while the 3′-flanking region of the target DNA was amplified using ggt_3 and ggt_4. These fragments were fused to a cat-sacB cassette using PCR, which was used for the first recombination step. Subsequently, the 5′-flanking region of the target DNA was amplified using ggt_1 and ggt_5, while the 3′-flanking region of the target DNA was amplified using ggt_6 and ggt_4. These fragments were then combined using PCR, and the resulting fragments were used for the second recombination step.
Expression of transaminases and γ-glutamylmethylamide synthetases.
Recombinant E. coli BL21(DE3) was cultivated in 2 ml of LB medium (10 g/liter of Bacto tryptone, 5 g/liter of yeast extract, and 5 g/liter of NaCl) containing 100 mg/liter of ampicillin in a test tube at 30°C with overnight shaking (300 rpm). Fifty microliters of the preculture was transferred to 5 ml of TB medium (24 g/liter of yeast extract, 12 g/liter of tryptone, 4 ml/liter of glycerol, 2.31 of g/liter KH2PO4, and 12.54 g/liter of K2HPO4) in a test tube and incubated at 30°C with shaking (300 rpm). After 5 h, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM, and cultivation was continued for 19 h. Cells were then harvested using centrifugation at 5,000 × g for 10 min. The cell pellet was suspended in 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.8) and disrupted using sonication at 4°C with a Bioruptor II (Cosmo Bio, Tokyo, Japan). Cellular debris was removed using centrifugation at 15,000 × g for 10 min, and the supernatant was used to test for enzyme activity.
Conditions for in vitro reactions.
To measure transaminase activity, a reaction mixture (100 μl) containing 10 μl of crude extract, 10 mM acetaldehyde, 10 mM respective amino donors, 50 mM MOPS (pH 7.8), 10 mM MgCl2, and 0.1 mM pyridoxal phosphate (PLP) was prepared and then incubated at 30°C for 1 h. The reaction mixture (30 μl) for assaying γ-glutamylmethylamide synthetase activity contained 15 μl of crude extract, 50 mM Glu, 50 mM ethylamine, 10 mM ATP, 30 mM MgCl2, and 50 mM MOPS (pH 7.8). Incubation was performed at 30°C for 3 h. To constitute the TA pathway in vitro, 3 μl of crude extract containing transaminase and 15 μl of crude extract containing GMAS were incubated with 50 mM MOPS (pH 7.8), 30 mM MgCl2, 50 mM acetaldehyde, 50 mM Glu, 50 mM Ala, 10 mM ATP, and 0.1 mM PLP (total volume 30 μl) at 30°C for 3 h.
Product analysis.
To measure the concentrations of products, the liquid sample was derivatized using 9-fluorenylmethyloxycarbonyl (Fmoc-Cl), the sample (5 μl) was transferred to a 1.5-ml tube, and then 45 μl of boric acid buffer (0.1 M borate adjusted to pH 9.0) and 50 μl of Fmoc-HCl solution (1.5 mg/ml of Fmoc-HCl in acetone) were added. The reaction mixture was placed in the dark at room temperature for 40 min, at which time 500 μl of quench buffer (25% [vol/vol] acetonitrile and 0.25 M borate adjusted to pH 5.5) was added to terminate the reaction. The resulting mixture was analyzed using a high-performance liquid chromatography (HPLC) system (Prominence; Shimadzu, Kyoto, Japan) equipped with an RF-20A fluorescence detector and a Develosil ODS-HG-5 column (5 μm, 4.6 [inside diameter] by 250 mm) (Nomura Chemical, Aichi, Japan). Solvent A [20 mM (NH4)2HPO4] and solvent B (90% [vol/vol] acetonitrile) were used as eluents. The HPLC program included initial column elution with 30% solvent B for 5 min and then with a linear gradient of 30% to 75% solvent B for 25 min, followed by a linear gradient of 75% to 95% solvent B for 3 min. The column was further washed with 95% solvent B for 4 min and then reequilibrated with 30% solvent B for 5 min. The column temperature was maintained at 35°C, the flow rate was 0.42 ml/min, and the products were detected using a fluorimeter (excitation, 254 nm; emission, 630 nm).
In the fermentation experiments, the culture supernatant was analyzed using HPLC after postcolumn derivatization with the o-phthalaldehyde reaction phase (18.5 g/liter of borate, 11 g/liter of sodium hydroxide, 3 ml/liter of Brij 35, 0.6 g/liter of o-phthalaldehyde, 4.7 g/liter of N-acetyl-l-cysteine). An aliquot of the diluted supernatant was analyzed using an HPLC system (Prominence; Shimadzu) equipped with a SUMIPAX Z-CLUE column (3 μm, 4.6 [inside diameter] by 250 mm; SCAS, Tokyo, Japan) (mobile phase: 2.94 g/liter of trisodium citrate dihydrate, 1.42 g/liter of anhydrous sodium sulfate, 80 ml/liter of 1-propanol, and 3 g/liter of sodium lauryl sulfate adjusted to pH 1.8 with sulfuric acid). The flow rates of the mobile and reaction phases were 0.7 ml/min and 0.3 ml/min, respectively (column temperature, 40°C). After separation, fluorescence was measured at 345-nm excitation and 455-nm emission.
Analyses of bacterial growth and glucose concentrations.
To measure the cell growth, optical density at 660 nm (OD660) was measured using a UV-visible spectrophotometer (UV-1800; Shimadzu, Kyoto, Japan). The concentration of residual glucose was measured using an automatic glucose analyzer (GA05; A&T Corporation, Kanagawa, Japan).
Laboratory-scale fermentation.
Cultures of recombinant E. coli W3110S3GK were incubated in 2 ml of LB medium containing 100 mg/liter of ampicillin in a test tube at 30°C overnight with shaking (300 rpm). The preculture (50 μl) was transferred to 5 ml of TT medium [30 g/liter of glucose, 16 g/liter of K2HPO4, 14 g/liter of KH2PO4, 2 g/liter of (NH4)2SO4, 5 g/liter of Casamino Acids, 1 g/liter of citric acid, 2 g/liter of MgSO4·7H2O, 50 mg/liter of FeSO4·7H2O, 10 mg/liter of MnSO4·7H2O, and 10 mg/liter of thiamine hydrochloride] in a test tube and then was shaken (300 rpm) at 30°C. After 5 h, IPTG was added to a final concentration of 1.0 mM, and cultures were shaken (300 rpm) at 30°C for 19 h.
l-Theanine production in a fed-batch culture.
Cultures of recombinant E. coli W3110S3GK were incubated in 250 ml of seed medium (10 g/liter of peptone, 5 g/liter of yeast extract, 5 g/liter of NaCl, 15 g/liter of glucose, and 3 g/liter of CaCO3) containing 100 mg/liter of ampicillin in a 1-liter Erlenmeyer flask at 30°C for 16 h with shaking (220 rpm). The culture (37.5 ml) was transferred into a 3-liter jar fermentor (Mitsuwa Frontech, Osaka, Japan) containing 750 ml of main medium [20 g/liter of glucose, 6 g/liter of Na2HPO4, 3 g/liter of KH2PO4, 5 g/liter of NaCl, 3 g/liter of (NH4)2SO4, 5 g/liter of yeast extract, 10 mg/liter of MnSO4·7H2O, 2 g/liter of MgSO4·H2O, 200 mg/liter of FeSO4·7H2O, 10 mg/liter of thiamine hydrochloride, and 0.2 ml of defoamer LG109; Adeka, Tokyo, Japan] with 200 mg/liter of carbenicillin and cultivated at 30°C with aeration (0.75 liter/min) and agitation (800 rpm). After 8 h, IPTG (1.0 mM final concentration) was added to induce the expression of recombinant proteins. When the initial glucose was depleted, continuous feeding of a glucose solution (500 g/liter) was begun. Glucose solution was supplied at a controlled rate (10 ml/h for TEA4 Δggt and 4 ml/h for TEA6 Δggt) so as not to exceed 50 g/liter of glucose in the main culture. During cultivation, the pH was maintained at 7.0 with 14% (vol/vol) NH4OH. At each time point (Fig. 7), a sample (5 ml) was taken, and a portion (1 ml) was used to measure optical density at 660 nm. The remainder of the sample was immediately centrifuged, and the supernatant was used to determine the concentrations of glucose, amino acids, and l-theanine.
Data availability.
E. coli W3110S3GK was deposited in the NITE Biological Resource Center under accession number NBRC114657.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shinichiro Shoji and Kaho Yamada for experimental support, Kento Koketsu for revising the manuscript, and Edanz Group for editing a draft of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sakato Y. 1950. Studies on the chemical constituents of tea; III. A new amide theanine. Nippon Nōgeikagaku Kaishi 23:262–267. (In Japanese.) 10.1271/nogeikagaku1924.23.262. [DOI] [Google Scholar]
- 2.Narukawa M, Morita K, Hayashi Y. 2008. l-Theanine elicits an umami taste with inosine 5′-monophosphate. Biosci Biotechnol Biochem 72:3015–3017. 10.1271/bbb.80328. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K, Ozeki M, Juneja LR, Ohira H. 2007. l-Theanine reduces psychological and physiological stress responses. Biol Psychol 74:39–45. 10.1016/j.biopsycho.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Lu K, Gray MA, Oliver C, Liley DT, Harrison BJ, Bartholomeusz CF, Phan KL, Nathan PJ. 2004. The acute effects of l-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum Psychopharmacol 19:457–465. 10.1002/hup.611. [DOI] [PubMed] [Google Scholar]
- 5.Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, Kunugi H. 2019. Effects of l-theanine administration on stress-related symptoms and cognitive functions in healthy adults: a randomized controlled trial. Nutrients 11:2362. 10.3390/nu11102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozeki M, Juneja LR, Shirakawa S. 2004. The effects of l-theanine on sleep using the actigraph. Nihon Seirijinruigaku Kaishi 9:143–150. (In Japanese.) [Google Scholar]
- 7.Yamada T, Terashima T, Honma H, Nagata S, Okubo T, Juneja LR, Yokogoshi H. 2008. Effects of theanine, a unique amino acid in tea leaves, on memory in a rat behavioral test. Biosci Biotechnol Biochem 72:1356–1359. 10.1271/bbb.70669. [DOI] [PubMed] [Google Scholar]
- 8.Ueda T, Ozeki M, Okubo T, Chu D, Juneja LR, Yokogoshi H, Matsumoto S. 2001. Improving effect of l-theanine on premenstrual syndrome. J Jpn Soc Psychosom Obstet Gynecol 6:234–239. 10.18977/jspog.6.2_234. (In Japanese.) [DOI] [Google Scholar]
- 9.Yoto A, Motoki M, Murao S, Yokogoshi H. 2012. Effects of l-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J Physiol Anthropol 31:28. 10.1186/1880-6805-31-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.QY Research. 2019. Global l-theanine Market Report, History and Forecast 2014–2025, Breakdown Data by Manufacturers, Key Regions, Types and Application. QY Research, Beijing, China. [Google Scholar]
- 11.Mu W, Zhang T, Jiang B. 2015. An overview of biological production of l-theanine. Biotechnol Adv 33:335–342. 10.1016/j.biotechadv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Ma H, Fan X, Cai N, Zhang D, Zhao G, Wang T, Su R, Yuan M, Ma Q, Zhang C, Xu Q, Xie X, Chen N, Li Y. 2020. Efficient fermentative production of l-theanine by Corynebacterium glutamicum. Appl Microbiol Biotechnol 104:119–130. 10.1007/s00253-019-10255-w. [DOI] [PubMed] [Google Scholar]
- 13.Fan X, Zhu YX, Cao H, Xu Q, Zhang T, Xie P, Liu B, Chen N, Xie X, Zhang C. 2018. Genetically engineered bacterium for producing l-theanine and fermentation method thereof. CN patent 109370966.
- 14.Matsuura T, Kakuda T. 1990. Effects of precursor, temperature, and illumination on theanine accumulation in tea callus. Agric Biol Chem 54:2283–2286. [Google Scholar]
- 15.Li J, Li P, Liu F. 2008. Production of theanine by Xerocomus badius (mushroom) using submerged fermentation. LWT 41:883–889. 10.1016/j.lwt.2007.05.020. [DOI] [Google Scholar]
- 16.Miwa E, Takayanagi H, Nakakawa M. 1978. Distribution of chemical constituents in different position of tea shoot. Chagyo Kenkyu Hokoku 47:48–52. (In Japanese.) 10.5979/cha.1978.48. [DOI] [Google Scholar]
- 17.Suzuki H, Izuka S, Miyakawa N, Kumagai H. 2002. Enzymatic production of theanine, an “umami” component of tea, from glutamine and ethylamine with bacterial γ-glutamyltranspeptidase. Enzyme Microb Technol 31:884–889. 10.1016/S0141-0229(02)00213-2. [DOI] [Google Scholar]
- 18.Suzuki H, Miyakawa N, Kumagai H. 2002. Enzymatic production of γ-l-glutamyltaurine through the transpeptidation reaction of γ-glutamyltranspeptidase from Escherichia coli K-12. Enzyme Microb Technol 30:883–888. 10.1016/S0141-0229(02)00038-8. [DOI] [Google Scholar]
- 19.Li Z, Zhu R, Liu Y, Li J, Gao H, Hu N. 2020. γ-Glutamyltranspeptidase from Bacillus amyloliquefaciens: transpeptidation activity enhancement and l-theanine production. Enzyme Microb Technol 140:109644. 10.1016/j.enzmictec.2020.109644. [DOI] [PubMed] [Google Scholar]
- 20.Tachiki T, Yamada T, Ueda M, Naemura Y, Imamura N, Hamada Y, Shiode J. 1996. Purification and some properties of glutaminase from Pseudomonas nitroreducens IFO 12694. Biosci Biotechnol Biochem 60:1160–1164. [DOI] [PubMed] [Google Scholar]
- 21.Pu H, Wang Q, Zhu F, Cao X, Xin Y, Luo L, Yin Z. 2013. Cloning, expression of glutaminase from Pseudomonas nitroreducens and application to theanine synthesis. Biocatal Biotransform 31:1–7. 10.3109/10242422.2012.749462. [DOI] [Google Scholar]
- 22.Alemzadeh I, Sakhaei M. 2017. Enzymatic synthesis of theanine in the presence of l-glutaminase produced by Trichoderma koningii. Appl Food Biotechnol 4:113–121. [Google Scholar]
- 23.Okada Y, Ozeki M, Aoi N. 2005. Theanine production method. JP patent 4874105.
- 24.Yao YF, Weng YM, Hu HY, Ku KL, Lin LL. 2006. Expression optimization and biochemical characterization of a recombinant gamma-glutamyltranspeptidase from Escherichia coli novablue. Protein J 25:431–441. 10.1007/s10930-006-9037-0. [DOI] [PubMed] [Google Scholar]
- 25.Abe I, Aoi N, Koseki M, Okada Y, Shu S. 2005. Theanine synthetase. JP patent JP2006254780.
- 26.Sasaoka K, Kito M, Onishi Y. 1965. Some properties of theanine synthesizing enzyme in tea seedings. Agric Biol Chem 29:984–988. 10.1271/bbb1961.29.984. [DOI] [Google Scholar]
- 27.Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, Xia E, Lu Y, Tai Y, She G, Sun J, Cao H, Tong W, Gao Q, Li Y, Deng W, Jiang X, Wang W, Chen Q, Zhang S, Li H, Wu J, Wang P, Li P, Shi C, Zheng F, Jian J, Huang B, Shan D, Shi M, Fang C, Yue Y, Li F, Li D, Wei S, Han B, Jiang C, Yin Y, Xia T, Zhang Z, Bennetzen JL, Zhao S, Wan X. 2018. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci U S A 115:E4151–E4158. 10.1073/pnas.1719622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu X, Liao Y, Cheng S, Xu X, Grierson D, Yang Z. 2021. Nonaqueous fractionation and overexpression of fluorescent-tagged enzymes reveals the subcellular sites of l-theanine biosynthesis in tea. Plant Biotechnol J 19:98–108. 10.1111/pbi.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyake K, Kakita S. 2009. A novel catalytic ability of gamma-glutamylcysteine synthetase of Escherichia coli and its application in theanine production. Biosci Biotechnol Biochem 73:2677–2683. 10.1271/bbb.90538. [DOI] [PubMed] [Google Scholar]
- 30.Yao J, Li J, Xiong D, Qiu Y, Shi G, Jin JM, Tao Y, Tang S. 2020. Development of a highly efficient and specific l-theanine synthase. Appl Microbiol Biotechnol 104:3417–3431. 10.1007/s00253-020-10482-6. [DOI] [PubMed] [Google Scholar]
- 31.Tachiki T, Wakisaka S, Suzuki H, Kumagai H, Tochikura T. 1983. Variation of properties of Micrococcus glutamicus glutamine synthetase brought about by divalent cations. Agric Biol Chem 47:287–292. 10.1271/bbb1961.47.287. [DOI] [Google Scholar]
- 32.Yamamoto S, Uchimura K, Wakayama M, Tachiki T. 2004. Purification and characterization of glutamine synthetase of Pseudomonas taetrolens Y-30: an enzyme usable for production of theanine by coupling with the alcoholic fermentation system of baker’s yeast. Biosci Biotechnol Biochem 68:1888–1897. 10.1271/bbb.68.1888. [DOI] [PubMed] [Google Scholar]
- 33.Kimura T, Sugahara I, Hanai K, Tonomura Y. 1992. Purification and characterization of γ-glutamylmethylamide synthetase from Methylophaga sp. AA-30. Biosci Biotechnol Biochem 56:708–711. 10.1271/bbb.56.708. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto S, Wakayama M, Tachiki T. 2008. Cloning and expression of Methylovorus mays No. 9 gene encoding γ-glutamylmethylamide synthetase: an enzyme usable in theanine formation by coupling with the alcoholic fermentation system of baker’s yeast. Biosci Biotechnol Biochem 72:101–109. 10.1271/bbb.70462. [DOI] [PubMed] [Google Scholar]
- 35.Kung HF, Wagner C. 1969. Gamma-glutamylmethylamide. A new intermediate in the metabolism of methylamine. J Biol Chem 244:4136–4140. 10.1016/S0021-9258(17)36394-9. [DOI] [PubMed] [Google Scholar]
- 36.Pan X, Yu J, Du Q, Zeng S, Liu J, Jiao Q, Zhang H. 2020. Efficient synthesis of γ-glutamyl compounds by co-expression of γ-glutamylmethylamide synthetase and polyphosphate kinase in engineered Escherichia coli. JIndMicrobiol Biotechnol 47:573–583. 10.1007/s10295-020-02305-4. [DOI] [PubMed] [Google Scholar]
- 37.Doi T, Koseki M, Tachiki T. 2008. Method for producing theanine. JP patent JP2009225705.
- 38.Bai P, Wei K, Wang L, Zhang F, Ruan L, Li H, Wu L, Cheng H. 2019. Identification of a novel gene encoding the specialized alanine decarboxylase in tea (Camellia sinensis) plants. Molecules 24:540. 10.3390/molecules24030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin JS, Kim BG. 1997. Kinetic resolution of alpha-methylbenzylamine with omicron-transaminase screened from soil microorganisms: application of a biphasic system to overcome product inhibition. Biotechnol Bioeng 55:348–358. . [DOI] [PubMed] [Google Scholar]
- 40.Coscolin C, Katzke N, García-Moyano A, Navarro-Fernández J, Almendral D, Martínez-Martínez M, Bollinger A, Bargiela R, Gertler C, Chernikova TN, Rojo D, Barbas C, Tran H, Golyshina OV, Koch R, Yakimov MM, Bjerga GEK, Golyshin PN, Jaeger KE, Ferrer M,. The INMARE Consortium. 2019. Bioprospecting reveals class III ω-transaminases converting bulky ketones and environmentally relevant polyamines. Appl Environ Microbiol 85:e02404-18. 10.1128/AEM.02404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luengo JM, Olivera ER. 2020. Catabolism of biogenic amines in Pseudomonas species. Environ Microbiol 22:1174–1192. 10.1111/1462-2920.14912. [DOI] [PubMed] [Google Scholar]
- 42.Thompson MG, Incha MR, Pearson AN, Schmidt M, Sharpless WA, Eiben CB, Cruz-Morales P, Blake-Hedges JM, Liu Y, Adams CA, Haushalter RW, Krishna RN, Lichtner P, Blank LM, Mukhopadhyay A, Deutschbauer AM, Shih PM, Keasling JD. 2020. Fatty acid and alcohol metabolism in Pseudomonas putida: functional analysis using random barcode transposon sequencing. Appl Environ Microbiol 86:e01665-20. 10.1128/AEM.01665-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohls H, Steffen-Munsberg F, Höhne M. 2014. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr Opin Chem Biol 19:180–192. 10.1016/j.cbpa.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Wilks JC, Slonczewski JL. 2007. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J Bacteriol 189:5601–5607. 10.1128/JB.00615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto S, Wakayama M, Tachiki T. 2007. Characterization of theanine-forming enzyme from Methylovorus mays no. 9 in respect to utilization of theanine production. Biosci Biotechnol Biochem 71:545–552. 10.1271/bbb.60590. [DOI] [PubMed] [Google Scholar]
- 46.Tabata K, Hashimoto S. 2007. Fermentative production of l-alanyl-l-glutamine by a metabolically engineered Escherichia coli strain expressing l-amino acid alpha-ligase. Appl Environ Microbiol 73:6378–6385. 10.1128/AEM.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H, Gonzalez R, Bobik TA. 2011. Coproduction of acetaldehyde and hydrogen during glucose fermentation by Escherichia coli. Appl Environ Microbiol 77:6441–6450. 10.1128/AEM.05358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeo T. 1974. l-Alanine as a precursor of ethylamine in Camellia sinensis. Phytochemistry 13:1401–1406. 10.1016/0031-9422(74)80299-2. [DOI] [Google Scholar]
- 49.Suzuki H, Kumagai H, Tochikura T. 1986. γ-Glutamyltranspeptidase fromEscherichia coli K-12: purification and properties. J Bacteriol 168:1325–1331. 10.1128/jb.168.3.1325-1331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsushida T, Takeo T. 1985. An enzyme hydrolyzing l-theanine in tea leaves. Agric Biol Chem 49:2913–2917. 10.1080/00021369.1985.10867191. [DOI] [Google Scholar]
- 51.Fu X, Cheng S, Liao Y, Xu X, Wang X, Hao X, Xu P, Dong F, Yang Z. 2020. Characterization of l-theanine hydrolase in vitro and subcellular distribution of its specific product ethylamine in tea (Camellia sinensis). J Agric Food Chem 68:10842–10851. 10.1021/acs.jafc.0c01796. [DOI] [PubMed] [Google Scholar]
- 52.Ehrenshaft M, Daub ME. 2001. Isolation of PDX2, a second novel gene in the pyridoxine biosynthesis pathway of eukaryotes, archaebacteria, and a subset of eubacteria. J Bacteriol 183:3383–3390. 10.1128/JB.183.11.3383-3390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Algar EM, Scopes RK. 1985. Studies on cell-free metabolism: ethanol production by extracts of Zymomonas mobilis. J Biotechnol 2:275–287. 10.1016/0168-1656(85)90030-6. [DOI] [Google Scholar]
- 54.Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF. 1987. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53:2420–2425. 10.1128/AEM.53.10.2420-2425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez GM, Atsumi S. 2014. Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab Eng 25:227–237. 10.1016/j.ymben.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng S, Fu X, Wang X, Liao Y, Zeng Y, Dong F, Yang Z. 2017. Studies on the biochemical formation pathway of the amino acid l-theanine in tea (Camellia sinensis) and other plants. J Agric Food Chem 65:7210–7216. 10.1021/acs.jafc.7b02437. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, Chang M, Li H, Zhang Z, Chen Q, Chen Y, Yao Y, Pan A, Shi C, Wang C, Zhao J, Wan X. 2019. Endophytic bacteria as contributors to theanine production in Camellia sinensis. J Agric Food Chem 67:10685–10693. 10.1021/acs.jafc.9b03946. [DOI] [PubMed] [Google Scholar]
- 58.Mikiro H, Kazuhiko T. 2013. Metabolic engineering for l-glutamine overproduction by using DNA gyrase mutations in Escherichia coli. Appl Environ Microbiol 79:3033–3039. 10.1128/AEM.03994-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiroshi M, Kimie T-M, Hideo M. 2007. A simple method for multiple modification of the Escherichia coli K-12 chromosome. Biosci Biotechnol Biochem 71:2905–2911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
E. coli W3110S3GK was deposited in the NITE Biological Resource Center under accession number NBRC114657.