Borrelia persica is a spirochete that causes tick-borne relapsing fever in humans in an area that spans from India to the Mediterranean. Until now, it was thought that the soft tick vector of this infection, Ornithodoros tholozani, is also its main reservoir and it transmits B. persica mostly transovarially between tick generations.
KEYWORDS: Ornithodoros tholozani, Borrelia persica, relapsing fever, Argasidae, blood meal
ABSTRACT
Borrelia persica, transmitted by the argasid tick Ornithodoros tholozani, causes human tick-borne relapsing fever in the Middle East and Central Asia. Infection is acquired often when visiting tick-infested caves and reported to be transmitted mainly transovarially between ticks, occasionally infecting humans. To study the epidemiology of this infection, ticks were trapped in 24 caves in 12 geographic zones covering all of Israel and identified morphologically. DNA was extracted from larvae, nymphs, and adult stages from each location and PCR followed by DNA sequencing was performed to identify Borrelia infection, tick species, and tick blood meal sources. We collected 51,472 argasid ticks from 16 of 24 caves surveyed. We analyzed 2,774 O. tholozani ticks, and 72 (2.6%) from nine caves were PCR positive for B. persica. Infection rates in male, female, and nymphal ticks (4.4%, 3%, and 3.2%, respectively) were higher than in larva (P < 0.001), with only 3 (0.04%) positive larvae. Presence of blood meal was associated with B. persica infection in ticks (P = 0.003), and blood meals of golden jackals, red foxes, and Cairo spiny mouse were associated with infection (P ≤ 0.043). PCR survey of 402 wild mammals revealed B. persica infection with the highest rates in social voles (22%), red foxes (16%), golden jackals (8%), and Cairo spiny mice (3%). In conclusion, although transovarial tick transmission of B. persica occurs at low levels, ticks apparently acquire infection mainly from wildlife canid and rodents and may eventually transmit relapsing fever borreliosis to humans who enter their habitat.
IMPORTANCE Borrelia persica is a spirochete that causes tick-borne relapsing fever in humans in an area that spans from India to the Mediterranean. Until now, it was thought that the soft tick vector of this infection, Ornithodoros tholozani, is also its main reservoir and it transmits B. persica mostly transovarially between tick generations. This study showed that tick infection with B. persica is associated with feeding blood from wild jackals, foxes, and rodents and that transovarial transmission is minimal. Since O. tholozani ticks are found in isolated caves and ruins, it is assumed that wild canids who migrate over long distances have a major role in the transmission of B. persica between remote tick populations, and it is then maintained locally also by rodents and eventually transferred to humans during tick bites. Prevention of human infection could be achieved by restricting entrance of canines and humans to habitats with O. tholozani populations.
INTRODUCTION
Tick-borne relapsing fever (TBRF) is transmitted to humans by the bite of infected argasid ticks (1). TBRF was reported initially in Israel under the British Mandate in 1919 in British troops and subsequently studied by Adler, who made a detailed clinical description of the disease and pointed out that visiting caves was a common element in the medical history of infected patients (2, 3). TRBF caused by Borrelia persica and transmitted by the tick Ornithodoros tholozani is common in Israel and other countries in the Near East, extending from India and Central Asia to Egypt (4, 5). The disease is characterized by spirochetemia with episodes of fever, separated by afebrile intervals. Complications with fatal consequences include neurological manifestations, liver pathology, and cardiac dysfunction (5–7). Relapsing fever is a notifiable disease in humans in Israel, and its incidence was reported to be 0.11/100,000 population among civilians and 6.4/100,000 population among military personnel (8); however, the disease is probably underreported, as B. persica is susceptible to many types of antibiotics, and patients with high fever are often treated before diagnosis is complete (9). From 2004 to 2018, 92 patients, including 30 children, were diagnosed with relapsing fever caused by B. persica in two hospitals in Jerusalem (10), and 47 patients were diagnosed with this infection in a single Jerusalem hospital between 2009 and 2019 (11). The transmission of B. persica in O. tholozani has been reported to be transovarial, as described also for other TBRF Borrelia spp., and it has been suggested that the tick itself may act as a reservoir for infection, passing it on between generations of ticks and occasionally transmitting it to humans (12, 13). Rodents have been considered the main reservoirs of TBRF caused by other relapsing fever borreliae, such as Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae in North America and Borrelia crocidurae and Borrelia hispanica in Africa and Spain (14–17). The presence of an animal reservoir for B. persica has not been demonstrated previously, and although experimental infection was described in laboratory mice and guinea pigs (18, 19), no records of natural rodent infection have been published. We have recently described infection and clinical disease with B. persica in domestic dogs and cats and infection in wild rock hyraxes (Procavia capensis) from Israel (20, 21). Natural infection in rock hyraxes constituted the only report of B. persica in wild animals in Israel. Ornithodoros tholozani usually takes a short blood meal of 15 to 60 min (22), and it is not found attached to animals in tick surveys (4). Its ecological niche is typically in shady, moist, and relatively cool environments such as caves, ruins, and rock crevices (4).
The study of arthropod blood meals is a useful method that can help elucidate the identity of their hosts and understand transmission cycles of vector-borne pathogens (23). Mitochondrial genes have been used as reliable targets for identification of vertebrate blood meal sources of hematophagous arthropods, including ticks, due to the variation in their nucleotide sequences between vertebrate host species and the high number of mitochondrial gene copies found per cell, which increases the likelihood of detecting host DNA in the vector’s blood meal (23–27).
In order to better understand the epidemiology and transmission of TBRF caused by B. persica, we planned a study to assess the presence and identity of soft ticks in caves in Israel, detect infection with Borrelia spp. in these ticks, assess the presence and source of blood meals in ticks at various life stages, survey wild mammals such as those living near locations of infected tick hosts for infection with relapsing-fever Borrelia spp., and determine possible associations between tick and wildlife infection.
RESULTS
Ticks.
Altogether, 51,472 argasid ticks were collected from November 2013 to August 2015 from 16 out of 24 (67%) cave sites surveyed in Israel (Table 1; Fig. 1). The total number of ticks trapped in each cave where ticks were found varied from 1 tick (cave 10) to 26,782 (cave 5), with a median of 181. We analyzed 2,777 ticks (nymphs, n = 1,036; larvae, n = 787; adult males, n = 525; and adult females, n = 429) from 16 caves.
TABLE 1.
Locations and number of argasid ticks collected in caves in Israel from November 2013 to August 2015
| Cave no. | Cave name | Cave coordinates | No. of female ticks (no. of positive ticks) | No. of male ticks (no. of positive ticks) | No. of nymph ticks (no. of positive ticks) | No. of larval ticks (no. of positive ticks) | Total no. of ticks (no. of positive ticks) |
|---|---|---|---|---|---|---|---|
| 1 | Kerem Ben Zimra | 33°01′58.93″N, 35°27′12.08″E | 1 (0) | 1 (0) | 1 (0) | 52 (0) | 55 (0) |
| 2 | Lavi | 32°46′35.85″N, 35°25′ 50.94″E | 7 (0) | 3 (0) | 103 (2) | 0 | 115 (2) |
| 3 | Shh'in 1 | 32°46′06.30″N, 35°16′32.37″E | 1,208 (1) | 1,507 (0) | 12,568 (3) | 3,201 (1) | 18,484 (5) |
| 4 | Shh'in 2 | 32°45′ 47.35″N, 35°16′18.28″E | 25 (1) | 12 (1) | 123 (9) | 5 (1) | 165 (12) |
| 5 | Nurit | 32°32′04.62″N, 35°21′46.32″E | 306 (5) | 144 (5) | 4,989 (6) | 21,343 (0) | 26,782 (16) |
| 6 | Avinadav | 32°27′14.32″N, 35°26′04.39″E | 69 (3) | 127 (12) | 1,010 (2) | 139 (0) | 1,345 (17) |
| 7 | Herzeliya | 32°10′04.12″N, 34°48′50.73″E | 0 | 0 | 0 | 0 | 0 |
| 8 | Afeka | 32°07′46.56″N, 34°48′32.88″E | 0 | 0 | 0 | 0 | 0 |
| 9 | Ras el-Amud | 31°44′08.95″N, 35°03′ 47.74″E | 0 | 0 | 0 | 0 | 0 |
| 10 | Emek Hamatzlevah | 31°46′18.31″N, 35°12′22.14″E | 0 | 0 | 1 (0) | 0 | 1 (0) |
| 11 | Beit Guvrin 1 | 31°35′52.28″N, 34°53′27.47″E | 3 (0) | 5 (0) | 42 (0) | 315 (0) | 365 (0) |
| 12 | Beit Guvrin 2 | 31°36′2.88″N, 34°53′26.29″E | 22 (0) | 31 (1) | 156 (5) | 178 (0) | 387 (6) |
| 13 | Gvaram | 31°34′50.28″N, 34°36′35.85″E | 1 (0) | 1 (0) | 19 (0) | 0 | 21 (0) |
| 14 | Ruchama | 31°30′08.66″N, 34°41′12.75″E | 0 | 1 (0) | 3 (0) | 0 | 4 (0) |
| 15 | Yatir | 31°20′03.01″N, 35°02′09.06″E | 0 | 2 (0) | 30 (0) | 0 | 32 (0) |
| 16 | Arad 1 | 31°16′08.33″N, 35°13′51.04″E | 13 (0) | 21 (0) | 125 (1) | 35 | 196 (1) |
| 17 | Arad 2 | 31°14′37.21″N, 35°13′13.51″E | 0 | 0 | 0 | 0 | 0 |
| 18 | Dimona | 31°07′26.29″N, 35°07′20.44″E | 0 | 1 (0) | 38 (0) | 0 | 39 (0) |
| 19 | Ashalim | 30°56′37.07″N, 34°44′20.76″E | 28 (1) | 42 (0) | 531 (0) | 96 (1) | 697 (2) |
| 20 | Nitzana | 30°51′50.41″N, 34°25′40.20″E | 67 (2) | 127 (4) | 2,111 (5) | 479 (0) | 2,784 (11) |
| 21 | Ovdat | 30°47'35.47″N, 34°46′23.29″E | 0 | 0 | 0 | 0 | 0 |
| 22 | Nafha | 30°41'55.36″N, 34°47′08.17″E | 0 | 0 | 0 | 0 | 0 |
| 23 | Timna | 29°47'27.15″N, 34°55′58.64″E | 0 | 0 | 0 | 0 | 0 |
| 24 | Eilat | 29°30'27.60″N, 34°55′06.91″E | 0 | 0 | 0 | 0 | 0 |
| Total | 1,753 (13) | 2,025 (23) | 21,847 (33) | 25,810 (3) | 51,472 (72) |
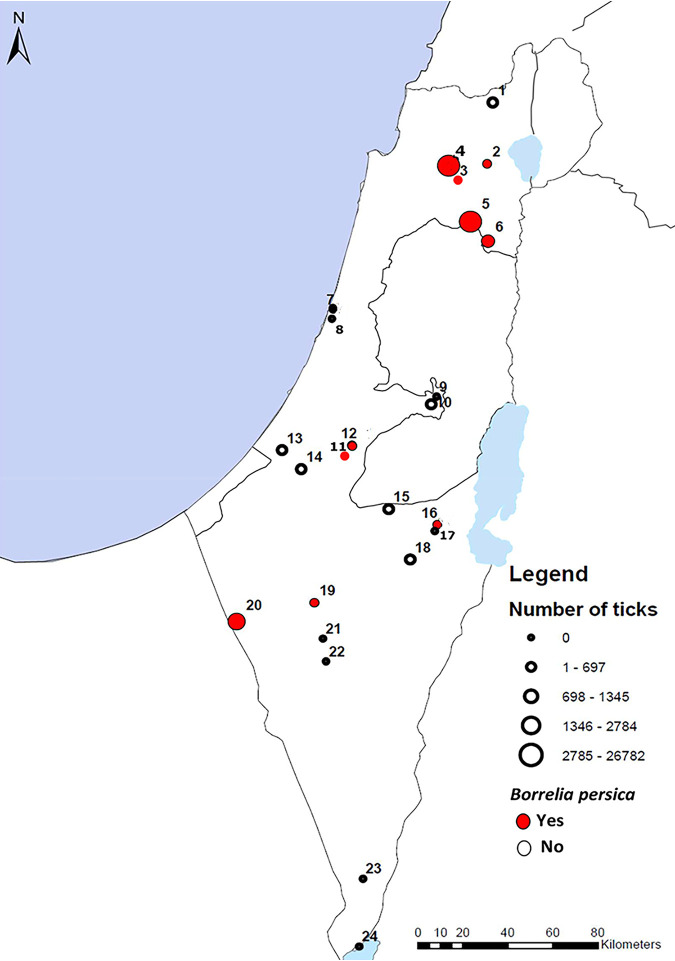
FIG 1.
Map showing the tick collection sites represented with circles, where red circles symbolize sites where B. persica-infected ticks were found and circle size represents the number of ticks collected in each site. Numbers in the map represent the cave identity number.
Tick infection by Borrelia spp.
flaB DNA segments of 270 to 273 bp with 100% identity to B. persica sequences available in GenBank were amplified from 72 ticks (13 females, 23 males, 33 nymphs, and 3 larvae) out of a total of 2,777 ticks analyzed. Infection was detected in 9 of the 16 caves (56%) where ticks were found (Table 1; Fig. 1). The prevalence of B. persica infection among ticks in each cave ranged from 0.6 to 8.6% (standard deviation [SD], 2.1). There was no significant difference between the infection rates in males, females, and nymphs (4.4%, 3%, and 3.2%, respectively; chi-square P = 0.4); however, the infection rate in larvae was significantly lower, with only 3 positive larvae (2 individuals and 1 pool) out of 787 analyzed (0.04%; chi-square P < 0.001). The pools included 5 larvae each, and due to the low percentage of infection found in single larvae (2/391; 0.5%) and considering the minimal infection rate (MIR), calculated according to the ratio of positive pools to the total number of larvae analyzed (28), the positive pool was considered to stem from one infected larva.
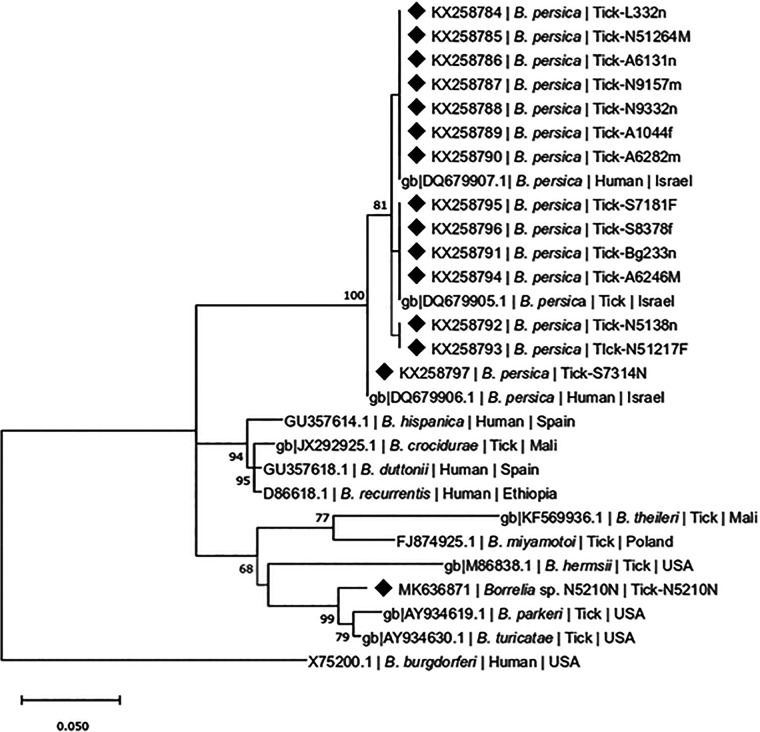
The 270- to 273-bp flaB DNA segments of B. persica amplified from ticks were 98 to 100% identical to each other and clustered together in a phylogenetic analysis with B. persica from a tick and 2 human patients from Israel (GenBank accession numbers DQ679905.1, DQ679906.1, and DQ679907, respectively) (Fig. 2). In addition, 19 ticks, from which 270- to 273-bp-long B. persica flaB sequences were amplified (29), were positive also by PCR for an overlapping 720-bp segment of flaB (30), and 14 707- to 710-bp-long flaB sequences from ticks in the current study were deposited in GenBank (accession numbers KX258784 to KX258797). Of the 72 ticks positive for B. persica flaB, 67 were also positive by the glpQ PCR (31). Analysis of the glpQ sequences revealed that they all clustered together with B. persica sequences from a tick and a human from Israel (GenBank accession numbers HM161656 and HM161657, respectively) (Fig. S1 in the supplemental material). Three glpQ sequences from ticks in this study were deposited in GenBank (MH923346 to MH923348). In addition, one tick from cave 5 harbored DNA that was 100% identical (flaB gene) to Borrelia sp. strain ZFB15_113, designated “Candidatus Borrelia fainii” (GenBank accession no. LC375836.1), a spirochete found to infect a febrile human patient in Zambia and transmitted by the soft tick Ornithodoros faini, which also infests wild bats (Rousettus aegyptiacus) (32) (Fig. 2). This sequence was deposited in GenBank as accession number MK636871.
FIG 2.
Maximum-likelihood phylogram comparing 270- to 273-bp DNA sequences of the flaB gene from B. persica detected in O. tholozani ticks included in the study to sequences corresponding to other B. persica GenBank accession numbers and from other Borrelia spp.; B. burgdorferi (GenBank accession no. X75200.1) was used as outgroup. The Tamura 3-parameter model was chosen with a pairwise deletion procedure. Numbers at nodes correspond to a percentage confidence level higher than 70% in a bootstrap test performed on 1,000 replicates. New sequences derived from this study are marked with black diamond squares and deposited in GenBank. The scale bar corresponds to a distance of 0.05 nucleotide substitution per site.
Of the nine caves where infected ticks were found (Fig. 1), four were sites where human cases of TBRF were reported to be acquired (cave no. 2, 12, 16, and 19), an additional four were suspected of being infected (no. 3, 4, 5, and 6), and one (no. 20) was from a location where human infection was not reported before. Of the six caves where no infection was found in ticks, one cave (no. 10) with one tick was located in an area with a TBRF report, two were caves suspected of infection (no. 11 and 18), and the other four were from locations where human infection was not reported before (no. 1, 13, 14, and 15). Of the nine caves where no ticks were trapped, one was a cave located in an area from which cases of TBRF have been reported (no. 21), and seven were sites from which no report of previous human infection is known (no. 7, 8, 9, 17, 22, 23, and 24).
Tick blood meal identification.
Vertebrate blood meals were found in 1,415 ticks out of the 2,777 analyzed (51%). Blood meals were detected in all tick life stages (females, n = 355; males, n = 422; nymphs, n = 622; and larvae, n = 16) from all the caves analyzed where ticks were found, with the exception of two caves, no. 10, where only a single tick was collected and analyzed, and no. 14, where two ticks were analyzed and none were engorged (Table 1). The prevalence of adult females and males harboring blood meals was significantly higher than nymphs and larvae (82.8%, 80.2%, 60%, and 2%, respectively; chi-square P < 0.0001). The prevalence of ticks carrying a blood meal varied significantly between the different caves (range, 6 to 76.7%; chi-square P < 0.001), with the highest prevalence in caves 15 and 20 (76.7 and 66.5%, respectively) and the lowest in caves 1 and 11 (6 and 9%, respectively).
DNA of 25 different vertebrate species was detected in ticks (Table 2). The most prevalent blood meal found was from the Indian crested porcupine (Hystrix indica) (71.5%), followed by the rock hyrax (Procavia capensis), the European badger (Meles meles) (6.8% and 4.3%, respectively), and 22 other species (Table 2). In addition, mixed blood meals with more than one blood source were found in 53 ticks (3.7% of all ticks with blood meals) (Table 2). The Indian porcupine was the most abundant host blood meal source in male, female, and nymphal ticks (77.6, 78.6, and 70%, respectively); however, in larvae, the most abundant blood meal was from humans (Homo sapiens) (43.8%), followed by the domestic dog (Canis lupus familiaris) (18.7%). The presence of rock hyrax blood was the second most prevalent blood meal in males, females, and nymphs (6, 6.7, and 7.8%, respectively).
TABLE 2.
Identified animal sources of blood meal from ticks according to life stage and number of ticks infected with B. persica in each stagea
| Source of blood meal (Latin species name) | Total no. of ticks | No. of adult male ticks (no. of infected ticks) | No. of adult female ticks (no. of infected ticks) | No. of nymphs (no. of infected ticks) | No. of larvae (no. of infected ticks) |
|---|---|---|---|---|---|
| Single blood meal source | |||||
| Indian crested porcupine (Hystrix indica) | 1,088 | 347 (8) | 291 (5) | 450 (11) | 0 |
| Rock hyrax (Procavia capensis) | 103 | 27 (1) | 25 (1) | 50 (1) | 1 |
| European badger (Meles meles) | 66 | 18 | 16 | 30 (1) | 2 |
| Wild goat (Capra aegagrus) | 41 | 5 | 7 | 29 (1) | 0 |
| Red fox (Vulpes vulpes) | 48 | 23 (5) | 20 (2) | 5 | 0 |
| Human (Homo sapiens) | 39 | 7 | 1 | 24 | 7 |
| Black rat (Rattus rattus) | 19 | 5 | 1 | 13 (1) | 0 |
| Golden jackal (Canis aureus) | 17 | 5 (2) | 2 (1) | 10 (2) | 0 |
| Cairo spiny mouse (Acomys cahirinus) | 10 | 5 (2) | 3 | 1 | 1 |
| Domestic cat (Felis catus) | 7 | 1 | 1 | 5 | 0 |
| Sheep (Ovis aries) | 5 | 1 | 0 | 4 | 0 |
| Domestic dog (Canis lupus familiaris) | 5 | 2 | 0 | 0 | 3 |
| Turkey (Meleagris gallopavo) | 4 | 0 | 0 | 3 | 1 |
| Cow (Bos taurus) | 3 | 0 | 1 | 2 | 0 |
| Striped hyena (Hyaena hyaena) | 3 | 0 | 0 | 3 | 0 |
| Mongoose (Herpestes sp.)b | 3 | 0 | 0 | 3 (1) | 0 |
| Egyptian fruit bat (Rousettus aegyptiacus) | 3 | 1 | 0 | 2 | 0 |
| Wild boar (Sus scrofa) | 2 | 0 | 0 | 1 | 1 |
| Caracal (Caracal caracal) | 1 | 0 | 0 | 1 | 0 |
| Macedonian mouse (Mus macedonicus) | 1 | 0 | 0 | 1 | 0 |
| Leschenault's rousette (Rousettus leschenaultii) | 1 | 0 | 1 | 0 | 0 |
| Mouse (Mus musculus) | 1 | 0 | 0 | 1 | 0 |
| Yellow-necked mouse (Apodemus flavicollis) | 1 | 0 | 0 | 1 | 0 |
| Etruscan shrew (Suncus etruscus) | 1 | 0 | 1 | 0 | 0 |
| Pigeon (Columba livia) | 1 | 0 | 0 | 1 | 0 |
| Mixed blood meals in the same tick | |||||
| Indian crested porcupine + rock hyrax | 18 | 7 (2) | 4 | 7 | 0 |
| Indian crested porcupine + rock hyrax + red fox | 13 | 8 (2) | 4 | 1 | 0 |
| Indian crested porcupine + European badger | 4 | 1 | 1 | 2 (1) | 0 |
| Indian crested porcupine + red fox | 3 | 1 | 2 | 0 | 0 |
| Human+ European badger | 1 | 0 | 0 | 1 | 0 |
| Wild goat + human | 2 | 0 | 0 | 2 | 0 |
| Indian crested porcupine + human | 5 | 0 | 0 | 5 | 0 |
| Turkey + Indian crested porcupine | 1 | 0 | 0 | 1 | 0 |
| Wild goat + Indian crested porcupine | 2 | 0 | 0 | 2 | 0 |
Identification of blood meals was based on PCR and sequencing. Amplicons of more than 200 base pairs and successful identification of the host with >98% identity of first hit by BLAST compared to sequences available in GenBank were obtained.
95% identity to Herpestes javanicus was obtained.
Borrelia sp. infection and blood meals in ticks.
Of the 72 B. persica-infected ticks, 50 were positive for the presence of a blood meal. The rate of infected ticks with blood meal was significantly higher than those infected without blood meal (68% and 32%, respectively; chi-square P = 0.003). The rates of infected ticks with blood meals according to their life stage were 95.6%, 69.2%, 54.5%, and 0%, for males, females, nymphs, and larvae, respectively. The rate of infection among engorged ticks was significantly higher in males compared to nymphs and larvae (likelihood ratio chi-square P = 0.002 and P = 0.001, respectively). No significant differences in infection rates were observed between engorged males and females and between engorged females, nymphs, and larvae.
The blood meal sources identified in the 50 B. persica-infected ticks included the Indian crested porcupine (n = 24; 2.3% of the total ticks with Histrix indica blood meal), red fox (Vulpes vulpes) (n = 7; 22.6%), golden jackal (Canis aureus) (n = 5; 29.4%), rock hyrax (P. capensis) (n = 3; 4.3%), Cairo spiny mice (Acomys cahirinus) (n = 2; 20%), and one infected tick with blood from each of the following species: mongoose (Herpestes sp.) (33%), European badger (Meles meles) (3%), goat (Capra aegagrus) (2.4%), and black rat (Rattus rattus) (5.3%) (Table 3). There were also infected ticks with blood meals from two animals, i.e., porcupine and hyrax (n = 2) and badger and porcupine (n = 1), and 2 ticks with infected blood from three animal species, i.e., porcupine, hyrax, and red fox.
TABLE 3.
Infected ticks according to the animal sources of blood meal and life stage
| Source of blood meal (Latin species name) | Total no. of infected ticks (%)a | No. of infected adult male ticks (%) | No. of infected adult female ticks (%) | No. of infected nymphs (%) | No. of infected larvae (%) |
|---|---|---|---|---|---|
| Single blood meal source | |||||
| Indian crested porcupine (Hystrix indica) | 24 (2.2) | 8 (2.3) | 5 (1.7) | 11 (2.4) | 0 |
| Rock hyrax (Procavia capensis) | 3 (4) | 1 (3.7) | 1 (4) | 1 (2) | 0 |
| European badger (Meles meles) | 1 (2.7) | 0 | 0 | 1 (3.3) | 0 |
| Wild goat (Capra aegagrus) | 1 (2.7) | 0 | 0 | 1 (3.4) | 0 |
| Red fox (Vulpes vulpes) | 7 (22)b | 5 (21.7) | 2 (10) | 0 | 0 |
| Black rat (Rattus rattus) | 1 (5.5) | 0 | 0 | 1 (7.7) | 0 |
| Golden jackal (Canis aureus) | 5 (29)b | 2 (40) | 1 (50) | 2 (20) | 0 |
| Cairo spiny mouse (Acomys cahirinus) | 2 (20)c | 2 (40) | 0 | 0 | 0 |
| Mongoose (Herpestes sp.) | 1 (33.3) | 0 | 0 | 1 (33.3) | 0 |
| Mixed blood meals | |||||
| Indian crested porcupine + rock hyrax | 2 (11.1) | 2 (28.6) | 0 | 0 | 0 |
| Indian crested porcupine + rock hyrax + red fox | 2 (15.3) | 2 (25) | 0 | 0 | 0 |
| Indian crested porcupine + European badger | 1 (25) | 0 | 0 | 1 (50) | 0 |
To calculate the percentage of infection, only animals with one blood meal source were evaluated.
Statistical significance; P < 0.001.
Statistical significance; P = 0.043.
Infected tick blood meals from the red fox and golden jackal were significantly more frequent than the rest of the animal blood meal sources in infected ticks with a single blood meal (chi-square and Fisher’s test, P < 0.001). Blood meals from the Cairo spiny mouse were also more frequent, with a more moderate level of significance (Fisher’s test, P = 0.043). Porcupine blood meal was significantly less frequent than other sources of blood meal in B. persica-infected ticks (chi-square P = 0.008).
Tick sequence analysis.
We analyzed 216 ticks using the mitochondrial 16S rRNA gene PCR, which produced DNA sequences of 408 bp. Molecular alignment revealed an identity of 98.3 to 99.0% with sequences of O. tholozani present in GenBank (accession numbers NC039830.1 and MF818023.1), which were also the first match by BLAST analysis. Eight tick 16S rRNA gene sequences from O. tholozani ticks were deposited in GenBank as accession numbers MF615990 to MF615997, and the tick’s genetic variability was analyzed (Fig. S2).
Borrelia sp. infection in animals.
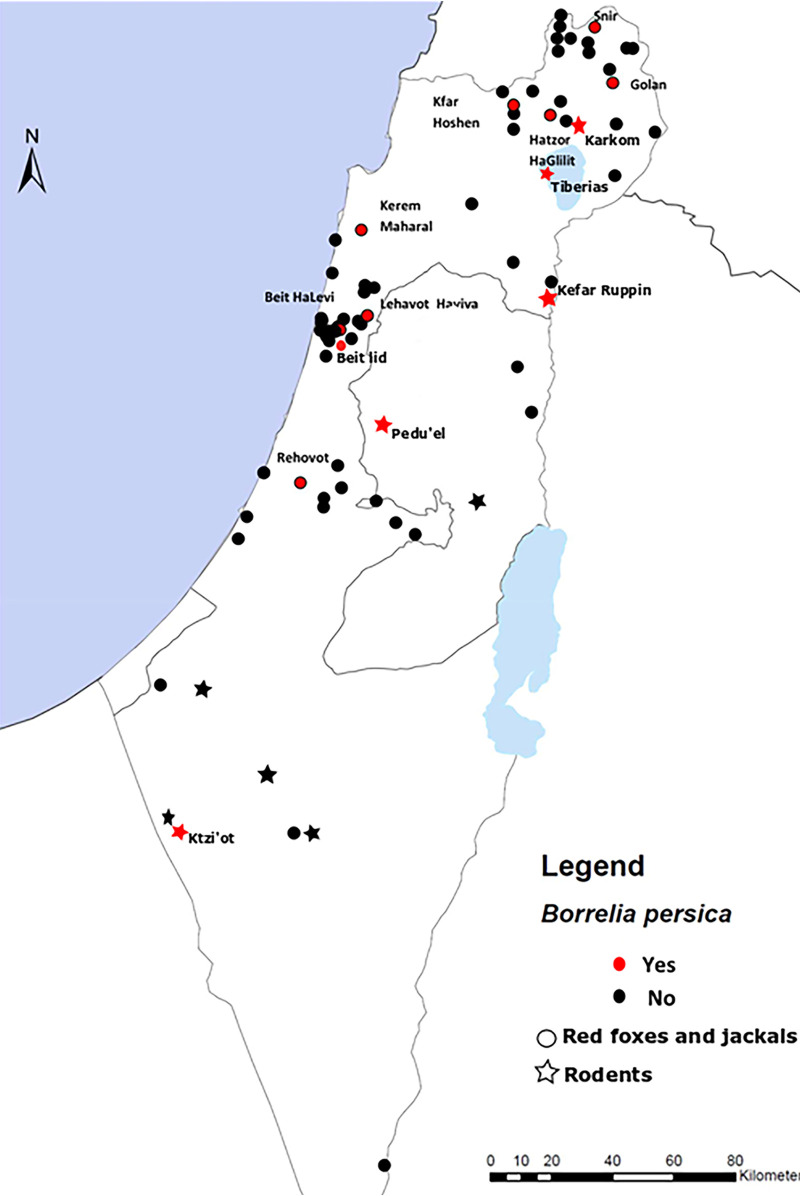
The study included 402 animals belonging to 13 species and 4 mammalian families (Table 4). In total, 459 samples were analyzed (402 blood and 57 spleen samples). Samples of rodents and insectivores were collected from 11 sites, and red fox and golden jackal samples were obtained from 23 and 45 sites, respectively (Fig. 3). Twenty-eight animals were positive for B. persica infection by PCR, comprising 7% of the animals tested. These included 13 social voles (22.4% of the total number of social voles), 5 red foxes (15.6%), 5 golden jackals (8%), 4 Cairo spiny mouse (3%), and one fat sand rat (8.3%) (Table 4).
TABLE 4.
Animal species sampled, type of sample (blood or spleen), and infection with B. persica
| Family | Species | No. of animals tested | No. of B. persica-infected animals | No. of positive blood samples/total no. of total blood samples | No. of positive spleen samples/total no. of spleen samples | % infected with B. persica |
|---|---|---|---|---|---|---|
| Cricetidae | Social vole (Microtus socialis) | 58 | 13 | 10/58 | 9/57 | 22.4 |
| Muridae | Black rat (Rattus rattus) | 1 | 0 | 0 | 0 | 0 |
| Cairo spiny mouse (Acomys cahirinus) | 133 | 4 | 4/133 | 0 | 3.0 | |
| Tristram's jird (Meriones tristrami) | 48 | 0 | 0 | 0 | 0 | |
| House mouse (Mus musculus) | 43 | 0 | 0 | 0 | 0 | |
| Indian crested porcupine (Hystrix indica) | 2 | 0 | 0 | 0 | 0 | |
| Eastern broad-toothed field mouse (Apodemus mystacinus) | 3 | 0 | 0 | 0 | 0 | |
| Fat sand rat (Psammomys obesus) | 12 | 1 | 1/12 | 0 | 8.3 | |
| Anderson's gerbil (Gerbillus andersoni) | 3 | 0 | 0 | 0 | 0 | |
| Wagner's gerbil (Gerbillus dasyurus) | 1 | 0 | 0 | 0 | 0 | |
| Canidae | Red fox (Vulpes vulpes) | 32 | 5 | 5/32 | 0 | 15.6 |
| Golden jackal (Canis aureus) | 63 | 5 | 5/63 | 0 | 8 | |
| Erinaceidae | Southern white-breasted hedgehog (Erinaceus concolor) | 3 | 0 | 0 | 0 | 0 |
| Total | 402 | 28 | 25/402 | 9/57 | 7.2 |
FIG 3.
Locations where insectivores and wild canids were sampled, indicating the presence or absence of infection with B. persica. Locations where B. persica was detected are noted in red, and their names are included. Twelve collections sites corresponding to 12 wild canid samples were unknown, including an area where a positive sample was collected.
Thirty-four samples were positive for B. persica by PCR, including 25 blood and 9 spleen samples. Six social voles were positive both in blood and spleen, 4 only in blood, and 3 only in the spleen (Table 4). All flaB sequences were 99% to 100% identical to B. persica sequences available in GenBank and clustered in a phylogram separately from other TBRF Borrelia spp. (Fig. S3).
DISCUSSION
This study of B. persica infection combines data on tick infection with findings on tick blood meals, identification of the animal species which infected ticks have fed on, and the wildlife animal hosts which harbor infection. The study has clearly identified an association between the presence of a blood meal and B. persica infection in O. tholozani ticks and has also shown that red fox and golden jackal blood meals are significantly associated with tick infection. These were supported by the relatively high rate of B. persica infection found in these two wild canine species surveyed along with other wildlife in this study. Only a very small number of unfed O. tholozani larvae were found to be infected with B. persica, while infection rates were significantly increased in the nymph and adult stages, in particular in those with a blood meal. These findings strengthen the idea that although transovarial transmission of B. persica exists, as previously reported (13), transmission associated with an animal reservoir probably plays an important role in the epidemiology of this infection, its maintenance, and its dispersal between remote tick foci. The existence of animal reservoirs for B. persica and the fact that its transmission is mainly transstadial and not transovarial were not known previously. Other TBRF Borrelia spp. are transmitted mostly transovarially in the tick, with some exceptions, such as B. duttonni transmitted by Ornithodoros moubata and B. hermsii transmitted by Ornithodoros hermsi (33–35). Borrelia crucidurae was shown to have a transovarial transmission rate of 33.3 to 53.3% in the soft tick Ornithodoros erraticus (36), and Borrelia miyamotoi, a TBRF transmitted by the hard tick Ixodes scapularis, was shown to have a transovarial transmission rate of 90.9% (37).
The collection of more than 51,000 ticks in multiple locations all over Israel has enabled this study to take an in-depth look at the natural history of B. persica infection. We documented a prevalence of B. persica in 2.6% in the soft ticks examined, ranging from 0.6 to 8.6% in different caves. A limited previous study reported a higher prevalence of B. persica in 8 of 94 (8.5%) O. tholozani ticks collected from 5 caves in Israel (30). Studies on argasid tick infection with other Old World relapsing fever Borrelia species have generally also reported higher infection rates, ranging between 4.6% to 20% (16, 38).
In contrast to some other relapsing fever borreliae, B. persica in Israel is prevalent in O. tholozani tick populations located in isolated niches of caves, ruins, or rock crevices, found distantly from each other with presumably no direct contact between these populations. This may explain the genetic variability observed between O. tholozani in this study as demonstrated by the phylogenetic analysis (Fig. S2). The detection of O. tholozani ticks in the surveyed locations indicated that they were present in 67% of the surveyed caves. Ticks were not found in the arid part of southern Israel represented by four caves (no. 21 to 24), probably due to incompatible climate conditions in agreement with previous studies on the prevalence of human TBRF in Israel and previous cave tick surveys (4, 9).
Regarding the association of certain animal blood meals in ticks and B. persica infection, since O. tholozani may take multiple blood meals from different animals in the same developmental stage, it may be difficult to confirm the association between a certain blood meal and a reservoir host. We therefore analyzed the association between blood meal and B. persica infection only in ticks in which a single host species blood meal was identified. Blood meal analysis revealed that although 74% of the blood meals found were from porcupines, only fox and jackal blood meals were strongly positively associated with infection with B. persica, and Cairo spiny mouse blood meals were also significantly associated with B. persica in ticks. The detection of a tick infected with “Candidatus Borrelia fainii,” which is associated with bats and has been reported to be pathogenic to a human (32), was unexpected. Surprisingly, the infected tick harbored blood meal of a porcupine; however, several of the ticks examined from the same cave had bat blood meals (Rousettus leschenaultia and R. aegyptiacus). Thus, it is possible that the tick acquired this particular Borrelia sp. infection during a previous bat blood meal. The study of natural B. persica infection in wild animals revealed infection with B. persica in the social vole, Cairo spiny mouse, fat sand rat, red fox, and the golden jackal. Rodents serve as hosts for other TBRF Borrelia agents, including B. caucasica, B. graingeri, B. hispanica, B. merionesi, B. coriaceae, B. hermsii, B. parkeri, B. turicatae, B. miyamotoi, and Borrelia crocidurae (16, 39, 40). There are no records of natural rodent infection with B. persica; however, successful experimental infections of B. persica in laboratory guinea pigs and mice have been reported (18, 19). The highest rate of B. persica infection in rodents in our study was found in the social vole (22.4%), followed by the fat sand rat (8.3%) and Cairo spiny mouse (3%). Infection with B. persica was detected in 15.6% and 7.9% of the red foxes and golden jackals tested, respectively. Positive golden jackals originated from four locations, one from northern and three from central Israel, whereas positive red foxes originated from five sites, four from northern and one from central Israel. These findings further substantiate the significant association found between red fox and golden jackal tick blood meals and tick infection with B. persica, and they suggest that these wild canid species in Israel do not only harbor infection at relatively high rates but also contribute to infection of O. tholozani ticks and to the transmission from infected ticks to naive ticks.
While rodents tend to have a sedentary behavior and a relatively small habitat range for foraging and digging of burrows used for shelter, protection, and reproduction (41–43), golden jackals and red foxes show high geographical dispersal ranges in search of food in natural surroundings or waste deposited near human settlements (44). Thus, despite the fact that wild canids did not show the highest infection rates, it is highly likely that they have a main role in the transmission of B. persica to new and distant locations, infecting new ticks, which may infect other animals and humans who enter O. tholozani habitats.
An interaction between rodent and wild canid hosts of B. persica may allow the dissemination of B. persica locally and interregionally, with wild canids disseminating infection between distantly located O. tholozani populations and rodents maintaining permanent sources for transmission within a cave or a small area.
In conclusion, this study has mapped the widespread presence of B. persica infection in Israel, confirmed the status of O. tholozani as its tick vector, and found that transovarial transmission of this pathogen is limited and that infection of O. tholozani is related to feeding blood on animal hosts with foxes, jackals, and several rodent species as putative reservoirs for this infection. Control of B. persica TBRF may include enforcing inclusive measures to prevent entrance of animals to caves and other locations of O. tholozani abundance and warn visitors of the presence of dangerous ticks in order to minimize the risk of infection.
MATERIALS AND METHODS
Tick collection and study sites.
Israel was divided into 12 study zones of 60 by 40 km representing the study areas (Fig. S4 in the supplemental material). Soft ticks were collected during 2014 and 2015 from a total of 24 caves (numbered 1 to 24), with two caves in each study area. Caves were selected as the sites of collection, since O. tholozani ticks are known to inhabit caves in Israel, and humans are typically infected when entering cave sites (4, 5). The caves selected for sampling included locations where human disease cases were reported to acquire TBRF (n = 8), locations which were suspected of being associated with human infection (where a clinical case of human TBRF was acquired after visiting the cave’s area) (n = 4), and locations from which there was no history of infection (n = 12). The locations of the caves were obtained from the Israeli Cave Research Center at the Hebrew University (https://www.malham.info/), from the Israeli Health Ministry, and from the Amudanan website (http://amudanan.co.il/) (Fig. 1). CO2 traps were used to trap ticks as described previously by Assus and Wilamowski (5). Tree collector traps connected to a cool box emitting CO2 from dry ice were buried in the soil in each sampling site and left overnight. The next morning, all trapped ticks were collected, kept in vials with 70% alcohol, and brought to the laboratory for analysis. All the tick specimens were then identified morphologically as described by Filipova (45), counted, and sorted according to their life stage and gender if in the adult stage.
Animal samples.
Samples from wildlife mammals, including canids, rodents, and hedgehogs, were collected from 2006 to 2018 by the Israeli Nature and Parks Authority as part of an epidemiological study on infection with Leishmania tropica and Leishmania major in wild animals and a rabies surveillance program as part of the Israeli national program of wildlife disease surveillance. All trapping locations were recorded with coordinates and plotted on a map. As trapping was made for reasons not related to the TBRF study, trapping locations were not necessarily in close proximity to tick collection sites; however, they covered all areas of Israel, including the northern, central, and southern parts. Small mammals were trapped using Tomahawk live box traps (Tomahawk Live Trap, Hazelhurst, WI, USA). Trapped animals were anesthetized by intramuscular injection of ketamine-HCl (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA, USA) at 10 mg/kg. Small rodents were euthanized using CO2 chambers, and larger animals were euthanized by intravenous sodium pentobarbital injection and necropsied by the Israeli Nature and Parks Authority.
Blood was collected in EDTA tubes for DNA extraction and kept at −20°C for further analysis. Spleen tissue samples were collected during necropsy and kept frozen at −20°C until DNA extraction. Trapping of wild animals was performed by wardens of the Israel Nature and Park Authorities with an approved permit from this institution, and the study was conducted following the Hebrew University’s guidelines for use of animals in research.
Molecular analysis.
DNA from ticks was extracted using a commercial kit (DNeasy blood and tissue kit; Qiagen, Germany) following the manufacturer's protocol. PCR was performed on DNA extracted from 100 males, 100 females, and 100 nymphs from each cave. Larvae were analyzed both individually (50 per cave) and by pools (10 pools of five larvae each per cave). If a smaller number of ticks was available from a certain cave, then all individual ticks collected from each of the tick's life cycle stages from that cave were studied. DNA from animal blood (200 μl) was extracted using the Illustra blood genomicPrep mini spin kit (GE Healthcare, Buckinghamshire, UK), and DNA from splenic tissue was extracted by the guanidine thiocyanate technique, with some modifications as previously described (46).
PCR for Borrelia spp.
All tick and animal DNA samples were screened for Borrelia infection by a real-time PCR targeting a 346-bp fragment of the Borrelia flagellin (flaB) gene using primers Fbpbu and Fbpcr (29) (Table 5). Ticks positive for Borrelia spp. were further tested for additional genotyping by PCR targeting a 750-bp segment of the same gene by conventional PCR with primers Bor1 and Bor2 (30) and by real-time PCR targeting a 280-bp fragment of the glycerophosphodiester phosphodiesterase gene (glpQ) using primers GlpQ-510f and GlpQ-770r (31) (Table 5). A conventional PCR mixture (25 μl) with DNA polymerase (Syntezza, Jerusalem, Israel) contained 4 μl target DNA, 0.4 μM of primers (Syntezza), and distilled water to reach the final volume. Real-time PCR assays targeting the flaB and glpQ genes were carried out in 20 μl Maxima Hot Start PCR master mix (Thermo Scientific, Loughborough, UK) with 0.25 μM each primer and 0.6 μl SYTO9 (Invitrogen, CA). The reactions were done at an annealing temperature of 52°C or 58°C for tick and animal samples, respectively, and 50 cycles followed by a melting phase. Plasmids (Topo TA cloning kit; Life Technologies, Grand Island, NY, USA) containing a B. persica flaB or glpQ gene insert, nontemplate control (NTC), and Borrelia-negative tick or animal DNA were employed as positive and negative controls, respectively.
TABLE 5.
PCR primers used for tick identification, detection of Borrelia spp., and identification of blood meal sources
| Primer | Target gene | Target species | 5′–3′ primer sequence | Amplicon length (bp) | Reference no. or source |
|---|---|---|---|---|---|
| Primers for tick identification | |||||
| 16S+1 | 16S rRNA | Ticks | CTGCTCAATGATTTTTTAAATTGCTGTGG | 480 | 47 |
| 16S-1 | 16S rRNA | Ticks | CCGGTCTGAACTCAGATCAAGT | 480 | 47 |
| Primers for Borrelia sp. identification | |||||
| Bfpbu | flaB | Borrelia | GCTGAAGAGCTTGGAATGCAACC | 346 | 29 |
| Bfpcr | flaB | Borrelia | TGATCAGTTATCATTCTAATAGCA | 346 | 29 |
| BOR1 | flaB | Borrelia | TAATACGTCAGCCATAAATGC | 750 | 30 |
| BOR2 | flaB | Borrelia | GCTCTTTGATCAGTTATCATTC | 750 | 30 |
| GlpQ-510f | glpQ | Borrelia | AAAACCCTTTTGGCATAAACAACA | 280 | 31 |
| GlpQ-770r | glpQ | Borrelia | CCAGGGTCCAATTCCGTCAG | 280 | 31 |
| Primers for identification of blood meal | |||||
| 12S-16SF | 12S and 16S rRNA | All vertebrates | ACACCGCCCGTCACCCTCC | 500 | 27 |
| 12S-16SR | 12S and 16S rRNA | All vertebrates | AACCAGCTATCACCAGGCTCG | 500 | 27 |
| 16S24F | 16S rRNA, inner primer for nested PCR | All vertebrates | AAGTCGTAACATGGTAAGCA | 366 | This study |
| 16S366R | 16S rRNA, inner primer for nested PCR | All vertebrates | GGTAGCTCGTCTGGTTTCGG | 366 | This study |
| Por100f | 16S rRNA | Porcupine | CAGAAGACTTCAGGTAATACTGGC | 311 | This study |
| Por391r | 16S rRNA | Porcupine | GGGTTGGCTCAATTTAGCTGT | 311 | This study |
| FOX131f | 16S rRNA | Fox | ACGAAAGCTAGCCCAATCGAC | 303 | This study |
| FOX410r | 16S rRNA | Fox | CACTATTTTGCCACATAGACGAGT | 303 | This study |
| BADG45f | 16S rRNA | Badger | ACATCATGACCACTTTGAACCA | 302 | This study |
| BADG345r | 16S rRNA | Badger | TGAGTTTATCCCTGTGGATTGT | 302 | This study |
| Jack134f | 16S rRNA | Jackal | GCTAGCCCAACTAACCCCAA | 287 | This study |
| Jack401r | 16S rRNA | Jackal | TTTTGCTACATAGATGAGTTGATCC | 287 | This study |
| Hyrax102f | 16S rRNA | Hyrax | AACCCCATTGACCACTTTGAACT | 305 | This study |
| Hyrax407r | 16S rRNA | Hyrax | GGGTTGGTTCTGTAAACTGCTC | 305 | This study |
| Cat95f | 16S rRNA | Cat | TCATATTAAACTGACCATCTTGAGC | 318 | This study |
| Cat412r | 16S rRNA | Cat | CAACATAGACGAGTTCATCCTGTAA | 318 | This study |
| goat 162F | 16S rRNA | Goat | TTACCAAAACAGTCTAAAACAA | 238 | This study |
| Goat380r | 16S rRNA | Goat | AACTGCCCATGAGTAGCTCG | 238 | This study |
| Unrev1025 | Cytochrome b | Mammal | GGTTGTCCTCCAATTCATGTTA | 334/680c | 26 |
| Human741Fa | Cytochrome b | Human | GGCTTACTTCTCTTCATTCTCTCCT | 334 | 26 |
| Dog368Fa | Cytochrome b | Dog | GGAATTGTACTATTATTCGCAACCAT | 680 | 26 |
| 12S-13R | 12S rRNA | All vertebrates | AGGAGGGTGACGGGCGGT | 326/369d | 24 |
| RodentFb | 12S rRNA | Muridae, Cricetidae | GGCGGTACTTTATATCCAT | 326 | 24 |
| BirdFb | 12S rRNA | Birds | TACGAGCACAAACGCTTAA | 369 | 24 |
Forward primers used with UNREV1025.
Forward primers used with 12S-13R.
Reverse primer UNREV amplifies a 334-bp amplicon with the forward primer Human741F when amplifying a human blood meal and a 680-bp amplicon with the forward primer Dog368F when amplifying a dog blood meal.
Reverse primer 12S-13R amplifies a 326-bp amplicon with the forward primer RodentF when amplifying a rodent blood meal and a 369-bp amplicon with the forward primer BirdF when amplifying a bird blood meal.
PCR for host blood meal identification.
The identification of tick blood meals was done as outlined in Fig. S5. General vertebrate primers (12S-16SF and 12S-16SR) targeting a 500-bp sequence that includes segments of the mitochondrial 12S and 16S rRNA genes were used for screening for the presence of blood meals in DNA extracted from tick samples (27) (Table 5). The DNA products of the positive ticks were sequenced, and the blood source was identified as described in the DNA sequencing and analysis section. Positive samples from this screening PCR, which showed an unknown host sequence or ambiguous DNA chromatography, were analyzed further for the possibility of a host whose sequences for the particular amplified DNA segments were missing from GenBank or for a sample containing a mixed blood meal. This was done using additional PCRs with species-specific primers targeting the mammal cytochrome b, mitochondrial 12S rRNA genes, or the 16S rRNA genes (Table 5). The products of these PCRs were also sequenced and identified. If the screening PCR showed a band which did not sequence well due to insufficient amplified DNA, an additional primer set (16S24F and 16S366R) targeting a 366-bp internal segment of the 16S rRNA gene designed for this study based on alignments of the segment previously described (500-bp segment, including the mitochondrial 12S and 16S rRNA gene) was used for a nested PCR (Table 5). Conventional PCRs for tick blood meal identification were performed in a mixture (25 μl) with DNA polymerase (Syntezza) containing 3 μl of target DNA and 0.4 μM concentrations of primers (Syntezza). For nested PCR, 1 μl of DNA product was used as the template. The protocol used for the PCR screening of blood meals targeting mitochondrial 12S and 16S rRNA genes was performed as described by Valinsky et al. (27), with a modified annealing temperature of 60°C. The cycling parameters for the nested PCR were 94°C for 2 min followed by 35 cycles of 40 s at 94°C, 40 s at 62°C, and 40 s at 72°C and 4 min at 72°C. PCR using species-specific primers was performed as for the detection of mitochondrial genes in a blood meal (27). NTCs as negative and mammal positive controls were included in each PCR.
The validation of efficacy of detection of different blood meals was performed by testing the primers against DNA of the following mammals from which DNA was present in our laboratory: dog (Canis lupus familiaris), cow (Bos taurus), cat (Felis catus), camel (Camelus dromedaries), rock hyrax (Procavia capensis), red fox (Vulpes vulpes), golden jackal (Canis aureus), porcupine (Hystrix indica), gerbil (Meriones unguiculatus), Cairo spiny mouse (Acomys cahirinus), sand rat (Psammomys obesus), human, and the house mouse (Mus musculus), as well as DNA from engorged field-collected soft ticks. All of the primers used successfully amplified the animal species tested.
PCR for tick characterization.
All Borrelia-positive tick DNA samples and 10 Borrelia-negative samples from each cave were analyzed by PCR targeting a 460-bp segment of the tick mitochondrial 16S rRNA gene using primers 16S+1 and 16S-1 (Table 5). The PCR protocol for tick 16S rRNA gene detection was performed according to a previous publication with a modified annealing temperature set at 60°C (47). The PCR mixture (25 μl) contained 3 μl of target DNA and 0.4 μM of primers (Syntezza). NTC and O. tholozani tick DNA used as negative and positive controls, respectively, were included in each PCR.
New primers for tick blood meal identification were designed using the Primer-BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The primers were tested with DNA of mammals from which DNA was present in our laboratory as detailed earlier. The other primers used were from previously published articles (Table 5). Nevertheless, all primers were standardized with the DNA polymerase and thermocycler used in this study, and all positive samples were sequenced to confirm the identity of the blood meal source.
DNA sequencing and analysis.
All samples positive by PCR in this study underwent DNA sequencing. Phylograms were constructed using the maximum-likelihood algorithm in the MEGA software, version X (48). Amplified conventional PCR products were detected by electrophoresis on 2% agarose gel stained with ethidium bromide. PCR amplicons of conventional PCR and positive real-time products were purified using a PCR purification kit (ExoSAP; New England Biolabs, Inc., Ipswich, MA) and subsequently sequenced using the Sanger technique at the Center for Genomic Technologies (The Hebrew University, Jerusalem, Israel). Species-level identification was obtained when the sequences were the first match by BLAST and showed percentage identity higher or equal to 98%. All DNA sequences were aligned using MUSCLE in the MEGA software version X. The sequences were further compared with sequences from GenBank using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic analysis was performed by MEGA software, version X. Phylograms were constructed using the maximum-likelihood, Tamura 3-parameter model for the Borrelia flaB, Tamura-Nei model for glpQ sequences, and the Hasegawa-Kishino-Yano model for tick 16S rRNA sequences. Phylogram robustness was determined by bootstrap (BS) percentages with 1,000 replicates.
Data source and statistical analysis.
Chi-square, Fisher’s test, and Student's t test were used for univariable models for nominal and ordinal variables, respectively. These tests were used to find an association between tick B. persica infection, tick life stage, presence of blood meal, and blood meal source. Statistical analysis was performed using WinPepi (version 11.65) and SPSS software (version 20; IBM, New York, NY, USA).
Data availability.
DNA sequences generated in this study were deposited in GenBank under the following accession numbers: KX258784 to KX258797 for B. persica flaB partial sequences and MK636871 for an uncultured Borrelia sp. flaB partial sequence from ticks, MK138356 and MK138357 for flaB partial sequences from wildlife, MH923346 to MH923348 for B. persica glpQ partial sequences from ticks, and MF615990 to MF615997 for O. tholozani tick 16S rRNA gene partial sequences.
Supplementary Material
ACKNOWLEDGMENTS
We thank Roi Lapid from the Israel Wildlife Disease Surveillance, who helped to collect wildlife samples for this study, and Kostas Mumcuoglu from the Hebrew University for his help in morphologic identification of ticks.
This study was supported by funds from USAID MERC program grant no. TA-MOU-12-M32-038 and grant 2014·52146 from the Netherlands Ministry of Foreign Affairs, The Hague, Netherlands.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Goubau PF. 1984. Relapsing fevers, a review. Ann Soc Belg Med Trop 64:335–364. [PubMed] [Google Scholar]
- 2.Nicholson FD. 1919. Tick fever in Palestine. Br Med J 2:811. 10.1136/bmj.2.3077.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler S, Theodor O, Schieber H. 1937. Observations on tick-transmitted human spirochaetosis in Palestine. Ann Trop Med Parasitol 31:25–35. 10.1080/00034983.1937.11684964. [DOI] [Google Scholar]
- 4.Avivi A, Warburg M, Galun R. 1973. Ecological studies on the cave tick Ornithodoros tholozani and its distribution in Israel. Israel J Entomol 8:109–129. [Google Scholar]
- 5.Assous MV, Wilamowski A. 2009. Relapsing fever borreliosis in Eurasia–forgotten, but certainly not gone! Clin Microbiol Infect 15:407–414. 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 6.Yagupsky P, Moses S. 1985. Neonatal Borrelia species infection (relapsing fever). Am J Dis Child 139:74–76. 10.1001/archpedi.1985.02140030076034. [DOI] [PubMed] [Google Scholar]
- 7.Hasin T, Davidovitch N, Cohen R, Dagan T, Romem A, Orr N, Klement E, Lubezky N, Kayouf R, Sela T, Keller N, Derazne E, Halperin T, Yavzori M, Grotto I, Cohen D. 2006. Postexposure treatment with doxycycline for the prevention of tick-borne relapsing fever. N Engl J Med 355:148–155. 10.1056/NEJMoa053884. [DOI] [PubMed] [Google Scholar]
- 8.Sidi G, Davidovitch N, Balicer RD, Anis E, Grotto I, Schwartz E. 2005. Tickborne relapsing fever in Israel. Emerg Infect Dis 11:1784–1786. 10.3201/eid1111.050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilamowski A, Assous M, Anis E. 2005. Tick-borne relapsing fever (RF) in the civilian population of Israel, 1980–2002, p 399–407. In Fifth International Conference on Urban Pests, Singapore. [Google Scholar]
- 10.Hashavya S, Gross I, Gross M, Hurvitz N, Weiser G, Temper V, Megged O. 2020. Tickborne relapsing fever, Jerusalem, Israel, 2004–2018. Emerg Infect Dis 26:2420–2423. 10.3201/eid2610.181988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breuer A, Megged O, Kashat L, Assous MV. 2021. Quantitative real-time PCR in Borrelia persica tick-borne relapsing fever demonstrates correlation with the Jarisch-Herxheimer reaction. Eur J Clin Microbiol Infect Dis 10.1007/s10096-020-04148-4. [DOI] [PubMed] [Google Scholar]
- 12.Pavlovskiy EN, Skruinnik AN. 1956. Contribution to the biology of the tick Ornithodoros papillipes. Dokl Akad Nauk SSSR 3:1403–1405. (In Russian.) [Google Scholar]
- 13.Balashov JS. 1968. Transovarial transmission of the spirochaete borrelia by the tick Ornithodoros papillipes and its effect on biological peculiarities of the pathogen. Parazitologiia 2:193–201. [Google Scholar]
- 14.Burgdorfer W, Mavros AJ. 1970. Susceptibility of various species of rodents to the relapsing fever spirochete, Borrelia hermsii. Infect Immun 2:256–259. 10.1128/IAI.2.3.256-259.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieto NC, Teglas MB. 2014. Relapsing fever group Borrelia in Southern California rodents. J Med Entomol 51:1029–1034. 10.1603/me14021. [DOI] [PubMed] [Google Scholar]
- 16.Trape J-F, Diatta G, Arnathau C, Bitam I, Sarih M, Belghyti D, Bouattour A, Elguero E, Vial L, Mané Y, Baldé C, Prugnolle F, Pugnolle F, Chauvancy G, Mahé G, Granjon L, Duplantier J-M, Durand P, Renaud F. 2013. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS One 8:e78473. 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynns HL, Beck MD. 1935. Epidemiological studies on relapsing fever in California. Am J Public Health 25:270–276. 10.2105/ajph.25.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour G, Hayes SF. 1986. Biology of Borrelia species. Microbiol Rev 50:381–400. 10.1128/MMBR.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarzer S, Overzier E, Hermanns W, Baneth G, Straubinger R. 2016. Borrelia persica infection in immunocompetent mice – a new tool to study the infection kinetics in vivo. PLoS Negl Trop Dis 10:e0004404. 10.1371/journal.pntd.0004404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baneth G, Nachum-Biala Y, Halperin T, Hershko Y, Kleinerman G, Anug Y, Abdeen Z, Lavy E, Aroch I, Straubinger RK. 2016. Borrelia persica infection in dogs and cats: clinical manifestations, clinicopathological findings and genetic characterization. Parasit Vectors 9:244. 10.1186/s13071-016-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinerman G, King R, Nachum-Biala Y, Baneth G. 2018. Borrelia persica infection in rock hyraxes. Ticks Tick Borne Dis 9:382–388. 10.1016/j.ttbdis.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Vial L. 2009. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 16:191–202. 10.1051/parasite/2009163191. [DOI] [PubMed] [Google Scholar]
- 23.Kent RJ. 2009. Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour 9:4–18. 10.1111/j.1755-0998.2008.02469.x. [DOI] [PubMed] [Google Scholar]
- 24.Humair PF, Douet V, Cadenas FM, Schouls LM, Van De Pol I, Gern L. 2007. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J Med Entomol 44:869–880. 10.1603/0022-2585(2007)44[869:MIOBSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN. 2007. Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes 7:544–548. 10.1111/j.1471-8286.2007.01748.x. [DOI] [Google Scholar]
- 26.Kent RJ, Norris DE. 2005. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg 73:336–342. 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valinsky L, Ettinger G, Bar-Gal GK, Orshan L. 2014. Molecular identification of bloodmeals from sand flies and mosquitoes collected in Israel. J Med Entomol 51:678–685. 10.1603/me13125. [DOI] [PubMed] [Google Scholar]
- 28.Gu W, Lampman R, Novak RJ. 2004. Assessment of arbovirus vector infection rates using variable size pooling. Med Vet Entomol 18:200–204. 10.1111/j.0269-283X.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaga M, Ushijima Y, Aoki Y, Talbert A. 2001. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector Borne Zoonotic Dis 1:331–338. 10.1089/15303660160025949. [DOI] [PubMed] [Google Scholar]
- 30.Assous MV, Wilamowski A, Bercovier H, Marva E. 2006. Molecular characterization of relapsing fever Borrelia, Israel. Emerg Infect Dis 12:1740–1743. 10.3201/eid1211.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potkonjak A, Kleinerman G, Gutierrez R, Savic S, Vracar V, Nachum-Biala Y, Jurišić A, Rojas A, Petrović A, Ivanović I, Harrus S, Banet G. 2016. Occurrence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks with first identification of Borrelia miyamotoi in Vojvodina, Serbia. Vector Borne Zoonotic Dis 16:631–635. 10.1089/vbz.2016.2008. [DOI] [PubMed] [Google Scholar]
- 32.Qiu Y, Nakao R, Hang'ombe BM, Sato K, Kajihara M, Kanchela S, Changula K, Eto Y, Ndebe J, Sasaki M, Thu MJ, Takada A, Sawa H, Sugimoto C, Kawabata H. 2019. Human borreliosis caused by a New World relapsing fever Borrelia-like organism in the Old World. Clin Infect Dis 69:107–112. 10.1093/cid/ciy850. [DOI] [PubMed] [Google Scholar]
- 33.Estrada-Peña A, Álvarez-Jarreta J, Cabezas-Cruz A. 2018. Reservoir and vector evolutionary pressures shaped the adaptation of Borrelia. Infect Genet Evol 66:308–318. 10.1016/j.meegid.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Tabuchi N, Kataoka-Ushijima Y, Talbert A, Mitani H, Fukunaga M. 2008. Absence of transovarial transmission of Borrelia duttonii, a tick-borne relapsing fever agent, by the vector tick Ornithodoros moubata. Vector Borne Zoonotic Dis 8:607–613. 10.1089/vbz.2007.0279. [DOI] [PubMed] [Google Scholar]
- 35.Schwan TG, Piesman J. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis 8:115–121. 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaber MS, Khalil GM, Hoogstraal H, Aboul-Nasr AE. 1984. Borrelia crocidurae localization and transmission in Ornithodoros erraticus and O. savignyi. Parasitology 88:403–413. 10.1017/S0031182000054676. [DOI] [PubMed] [Google Scholar]
- 37.Han S, Lubelczyk C, Hickling GJ, Belperron AA, Bockenstedt LK, Tsao JI. 2019. Vertical transmission rates of Borrelia miyamotoi in Ixodes scapularis collected from white-tailed deer. Ticks Tick Borne Dis 10:682–689. 10.1016/j.ttbdis.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, McCoy BN, Safronetz D, Sogoba N, Maïga O, Traoré SF. 2012. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Negl Trop Dis 6:e1924. 10.1371/journal.pntd.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diatta G, Duplantier JM, Granjon L, Bâ K, Chauvancy G, Ndiaye M, Trape JF. 2015. Borrelia infection in small mammals in West Africa and its relationship with tick occurrence inside burrows. Acta Trop 152:131–140. 10.1016/j.actatropica.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Talagrand-Reboul E, Boyer PH, Bergström S, Vial L, Boulanger N. 2018. Relapsing fevers: neglected tick-borne diseases. Front Cell Infect Microbiol 8:98. 10.3389/fcimb.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elvert R, Kronfeld N, Dayan T, Haim A, Zisapel N, Heldmaier G. 1999. Telemetric field studies of body temperature and activity rhythms of Acomys russatus and A. cahirinus in the Judean Desert of Israel. Oecologia 119:484–492. 10.1007/s004420050811. [DOI] [PubMed] [Google Scholar]
- 42.Gromov VS. 2017. The evolution of sociality in rodents: a family affair. Russ J Theriol 16:47–65. 10.15298/rusjtheriol.16.1.05. [DOI] [Google Scholar]
- 43.Krasnov B, Shenbrot G, Khokhlova I, Ivanitskaya E. 1996. Spatial structure of rodent community in the Ramon erosion cirque, Negev Highlands (Israel). J Arid Environ 32:319–327. 10.1006/jare.1996.0026. [DOI] [Google Scholar]
- 44.Cohen TM, King R, Dolev A, Boldo A, Lichter-Peled A, Kahila Bar-Gal G. 2013. Genetic characterization of populations of the golden jackal and the red fox in Israel. Conserv Genet 14:55–63. 10.1007/s10592-012-0423-1. [DOI] [Google Scholar]
- 45.Filippova NA. 1966. Argasid ticks (Argasidae). Fauna SSSR: Paukoobraznye, Tom IV Vypusk 3. Book on Demand, India. (In Russian.) [Google Scholar]
- 46.Marciano O, Gutiérrez R, Morick D, King R, Nachum-Biala Y, Baneth G, Harrus S. 2016. Detection of Bartonella spp. in wild carnivores, hyraxes, hedgehog and rodents from Israel. Parasitol 143:1232–1242. 10.1017/S0031182016000603. [DOI] [PubMed] [Google Scholar]
- 47.Black WC, Piesman J. 1994. Phylogeny of hard, and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A 91:10034–10038. 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences generated in this study were deposited in GenBank under the following accession numbers: KX258784 to KX258797 for B. persica flaB partial sequences and MK636871 for an uncultured Borrelia sp. flaB partial sequence from ticks, MK138356 and MK138357 for flaB partial sequences from wildlife, MH923346 to MH923348 for B. persica glpQ partial sequences from ticks, and MF615990 to MF615997 for O. tholozani tick 16S rRNA gene partial sequences.