Abstract
Background
The output of many healthy physiological systems displays fractal fluctuations with self-similar temporal structures. Altered fractal patterns are associated with pathological conditions. There is evidence that patients with bipolar disorder have altered daily behaviors.
Methods
To test whether fractal patterns in motor activity are altered in patients with bipolar disorder, we analyzed two-week actigraphy data collected from 106 patients with bipolar disorder type I in a euthymic state, 73 unaffected siblings of patients, and 76 controls. In addition, to examine the link between fractal patterns and symptoms, we analyzed 180-day actigraphy and mood symptom data that were simultaneously collected from 14 bipolar patients.
Results
Compared to controls, bipolar patients showed excessive regularity in motor activity fluctuations at small time-scales (<1.5h) as quantified by a larger scaling exponent (α1>1), thus indicating a more rigid motor control system. α1 values of siblings were between those of patients and controls. Further examinations revealed that the group differences in α1 were only significant in females. Sex also affected the group differences in fractal patterns at larger time scales (>2h) as quantified by scaling exponent α2. Specifically, female patients and siblings had a smaller α2 as compared to female controls, indicating more random activity fluctuations at >2h; while male patients had a larger α2 as compared to male controls. Interestingly, within bipolar patients, a higher weekly depression score was associated with a lower α1 in the subsequent week.
Conclusions
Our results show sex- and scale-dependent alterations in fractal activity regulation in patients with bipolar disorder. The mechanisms underlying the alterations are yet to be determined.
Keywords: bipolar disorder, actigraphy, scale invariance, sleep-wake rhythm, fractal patterns, mood disorder
Introduction
Bipolar disorder is one of the leading causes of disability worldwide (Murray et al. 2012). Patients with bipolar disorder experience characteristic episodes of mood symptoms, ranging from depressive episodes to manic episodes (Association 2013). With a delay of 5–10 years between illness onset and diagnosis, finding diagnostic biomarkers to allow earlier detection and therapeutic interventions is one of the key clinical challenges in bipolar disorder (Baldessarini et al. 2007; Phillips & Kupfer 2013). It is desirable that these diagnostic biomarkers are non-invasive, reliable and cost-efficient.
Many physiological signals, such as motor activity, exhibit fractal fluctuations as characterized by self-similar temporal patterns or statistical properties across a wide range of time scales from seconds to hours (Hu et al. 2004). The fractal fluctuations are very complex, being neither too random nor too regular (Goldberger et al. 2002; Pittman‐Polletta et al. 2013). It is believed that fractal regulation reflects the integrity and adaptability of biological systems (i.e., maintaining the internal stability while being able to respond to changes in external influences) and that degraded fractal regulation indicates a loss of functional resilience and increased vulnerability to disruptive events. Supporting this concept, many studies showed that fractal regulation is robust in healthy human physiology but is disrupted with aging or in diseases (Goldberger et al. 2002). For instance, fractal motor activity regulation in humans is degraded in older people and in people with Alzheimer’s disease, leading to distinct patterns over two different time scale regions with a boundary at ~1.5–2 hours (i.e., different temporal correlations as quantified by different scaling exponents, α1 at 1.5–90 min and α2 between 2 and 10 hours) (Hu et al. 2009, 2013, 2016). More interestingly, our recent study showed that degradations in fractal motor activity regulation predict frailty, disability, mortality, and the risk for developing Alzheimer’s disease many years before the clinical onset of the disease (Li et al. 2018, 2019).
Recent studies have provided evidence for a key role of the circadian control system in fractal regulation. An example is the breakdown of fractal patterns in rodent motor activity following the lesioning of the suprachiasmatic nucleus (SCN)—the master circadian clock located in the hypothalamus responsible for the coordination of circadian rhythms in various physiological processes (Hu et al. 2007, 2013). Specifically, motor activity fluctuations of SCN-lesioned rats became almost completely random at larger time scales, as indicated by a lower value of α2 close to 0.5 (reflective of randomness, or ‘white noise’). In humans, disrupting circadian rhythms appears to affect also the scaling exponent of motor activity fluctuations at smaller time scales, α1, as observed in night shift workers (Li et al., 2017). In addition, Aybek et al. showed that patients with major depressive disorder have a larger α1 than controls (>1), indicating rigidity in motor activity regulation (Aybek et al. 2012). Importantly, fractal patterns appear to be more sensitive to the neuronal changes in the human SCN as compared to traditional circadian measures estimated by daily rhythms (7). Because circadian dysfunction is believed to be linked to bipolar disorder, fractal patterns may serve as a promising diagnostic biomarker of bipolar disorder (Harvey 2008). In the current study, we tested the hypothesis that bipolar patients have altered fractal regulation compared to healthy controls, and that unaffected siblings of bipolar patients share a milder alteration. We also tested whether there are any differences in fractal regulation between a euthymic state and a depressed or manic state within bipolar patients. Moreover, we examined the temporal relationship between changes in fractal regulation and mood fluctuations in a small group of bipolar patients during a follow-up of 180 days.
Methods and materials
Participants
Two different samples were used for the analyses.
Cross-sectional sample
For the cross-sectional analysis in a euthymic sample, participants were included from the Dutch Bipolar Cohort (DBC) study, a collaboration between the University Medical Center Utrecht, University Medical Center Groningen, various other mental health care providers in the Netherlands, and the University of California, Los Angeles (Bergen et al. 2018). The DBC study was designed to investigate genetic and (endo)phenotypic vulnerability factors for bipolar disorder. The medical ethical committees of the three University Medical centers approved the study and their follow-up studies. These studies were in accordance to the Declaration of Helsinki. The DBC study consisted of a baseline study and several follow-up studies, including an actigraphy study of 14 days to investigate the daily rhythm and sleep disturbances in patients with bipolar disorder. All participants were at least 18 years old, and did not suffer from major self-reported somatic illness or pregnancy. The inclusion criterion for patients was a bipolar type I diagnosis, which was verified using the Structured Clinical Interview for DSM-IV (SCID-I) (First & Gibbon 2004). The exclusion criterion for siblings of bipolar patients and control subjects was diagnosis of bipolar disorder or other psychotic disorders. In addition, control subjects had no first- or second-degree relative with any psychotic disorder. Both siblings and controls were assessed using the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al. 1998). Further selection procedures, dropout rates and comparisons to the original cohort have been previously published (Verkooijen et al. 2017). Each participant was monitored for up to 14 days. Participants with valid actigraphy and sleep diary data for at least 8 days were included in the analysis. Non-wear periods as documented in the sleep diary were excluded from the analysis. Data from 106 patients, 73 unaffected siblings and 76 control participants were included. Medication use was checked, and mood stabilizer use (lithium, carbamazepine, lamotrigine or valproate acid) was specifically noted due to its known effect on the circadian system (Gould & Manji 2002; Milhiet et al. 2011). Disease characteristics (age of onset, number of years suffering from the disease, psychotic symptoms, presence/absence of rapid cycling bipolar disorder, number of depressed and manic episodes and suicide attempt [yes or no]) were also considered for post-hoc analyses.
Longitudinal sample
Participants for the longitudinal sample were from the Sleep-Wake patterns In the CHange of mood in Bipolar Disorder (SWITCH-BD) study, a pilot study that was conducted in the University Center for Psychiatry in Groningen to examine the temporal relation between sleep, daily rhythm and mood changes in a naturalistic setting. Patients were recruited from the outpatient clinic of the University Center for Psychiatry of the University Medical Center Groningen through a newsletter in the patient society for manic depressive illness. Patients visited the hospital 5 times. The first time was for a baseline interview with the M.I.N.I. (Sheehan et al. 1998) in which patients received instructions about the actiwatch (a wrist-worn motion detection device used to assess activity, see below) and the online diaries. During the other 4 visits, actigraphy data were downloaded from the actiwatch, the battery was changed, and any questions were addressed. Patients were followed for 180 days with continuous monitoring of actigraphy, a sleep diary was filled out every morning, and a mood diary was filled out every evening. The diaries consisted of questions on affect, agitation and energy on a visual analog scale. Every week, patients also received two validated questionnaires for assessment of mood status: the Inventory for Depressive Symptomatology (Self-Report) (IDS-SR) (Rush et al. 2000) and the Altman Self-Rating Mania scale (ASRM) (Altman et al. 1997). A total of 15 patients started the protocol. One patient dropped out due to the time burden of the study.
Mood episodes were assessed using validated weekly questionnaires combined with the Life-Chart method, which includes a daily visual analog scale ranging from depressed (0) to manic (100) (LC-self) (Altman et al. 1997; Rush et al. 2000; Born et al. 2014). The definition of a mood episode wasmainly based o n modified DSM-IV criteria (American Psychiatric Association, 2000). Certain modifications were introduced during a team meeting with two experienced psychiatrists (RFRvdL and RAS) and the first author (SEK) for the following considerations, prior to initiating the fractal analyses. (1) While the items on the Altman Self-Rating Mania Scale (ASRM) require subjects to provide the rating reflecting the past 7 days (Altman et al., 1997), it has been shown that the rating is influenced by recency, i.e. the response will mostly reflect the mental state on the day of completing the ASRM with less influences from the mental state several days ago (Aan het Rot, Hogenelst, & Schoevers, 2012). In order to circumvent this limitation, we decided that to define a manic episode, the score on the ASRM had to remain above 5 for two consecutive weeks. In addition, patients were required to have daily mood above the midline on the Life-Chart for at least 75% of the 2 weeks, again to ensure remaining in the same state for a sufficient percentage of the time. The threshold of 75% was also chosen by the authors, as there are currently no agreed-upon criteria for this type of threshold. (2) For the definition of a depressed episode, a patient had to score above 26 on the IDS-SR on at least 3 consecutive weeks, again to ensure at least a full 2 weeks of symptoms, following the same logic as described above for the ASRM. Patients also had to report their daily mood below the midline on the LC-self for at least 75% during that time period. (3) A stable euthymic episode was defined as at least 5 consecutive weeks without scoring above the threshold for manic or depressive symptoms. A minimum of 5 weeks was chosen to encompass a larger period in order to achieve a reliable measure of a period without symptoms. In addition, the standard deviation on the LC-self of this period had to be lower than that for the full 180 days for each individual to further ensure a period of relatively stable mood. Episode characterizations according to these criteria were selected by two independent raters and any discrepancies were discussed.
Actigraphy acquisition
Motor activity data were collected using Actiwatch 2 (Philips Respironics) for the cross-sectional sample, and the Motionwatch 8 (CamNTech) for the longitudinal sample. Activity counts were stored every minute.
Fractal analysis
The correlation across different time scales (i.e., a scaling behavior) is commonly used to assess temporal fractal regulation in time series. To assess the temporal correlation, we performed detrended fluctuation analysis (DFA) (Peng et al. 1994; Hu et al. 2001). This method involves the following four steps: i) integrating the activity counts after removing the global mean, i.e., cumulative summation from the first to the last epoch; ii) dividing the integrated signal into non-overlapping windows of the same window size n (i.e., time scale); iii) removing the trend within each window of the integrated signal (estimated using polynomial fitting) to obtain residuals; and iv) calculating the root mean square of residuals in all windows to obtain the fluctuation amplitude F(n). The last three steps are repeated for different time-scales. To reliably estimate F(n) at a specific time scale n, at least 6 windows of size n without gaps are required. Otherwise, F(n) at that time scale and larger time scales will not be calculated. In Step ii, 2nd order polynomial functions were used to extract the trend within each window (Hu et al. 2001). A power-law form of F(n), i.e., F(n)~nα, indicates a fractal structure in the fluctuations. The parameter α, called the scaling exponent, quantifies the temporal correlation as follows: if α = 0.5, there is no correlation in the fluctuations (“white noise”); if α > 0.5, there are positive correlations, where large values are more likely to be followed by large values (and vice versa for small values). If α is greater than 1 and becomes closer to 1.5, it indicates that the control system becomes more rigid or excessive regular. Note that α=1 indicates the most complex fluctuation patterns (i.e., not too regular while not being random). The α values close to 1.0 have been observed in many physiological outputs under healthy conditions (Peng et al. 1995, 2002; Hausdorff et al. 1997; Hu et al. 2004).
Previous human studies showed that degraded fractal regulation, as occurred with aging and in dementia, lead to different behaviors of F(n) over two distinct time scale regions with the boundary at ~1.5–2 hours (i.e., different α values) (Hu et al. 2009, 2013, 2016). We thus calculated α values over two non-overlapping time scale regions: (i) α1 from 1.5–90 min, and (ii) α2 from 2 up to 10 hours. The transition region from 90 min to 2 hours was excluded (Hu et al. 2009).
To ensure signal quality, all actigraphic recordings were checked with the assistance of a self-designed MATLAB GUI program (Ver. R2016b, the MathWorks Inc., Natick, MA, USA). The following types of quality issues were considered: i) isolated spikes with values outside of 10 standard deviations (SD) from the individual global mean level; and ii) consecutive zeros of >60 minutes during the daytime (likely occurred when participants took the device off but did not document the incident). The episodes with those issues were marked as gaps and were skipped when performing the DFA in order to avoid any potential effects of interpolating missing data and/or manipulating the signal (e.g., stitching the rest data after removing the missing data) on F(n) (Hu et al. 2001, 2004). With gaps, it is possible that F(n) could not be calculated at all time scales up to 10 hours. To assure reliable estimation of the scaling exponent from power-law fitting, no α2 value was calculated if the maximum time scale was smaller than 6 hours. Furthermore, we excluded those α (usually α2) values that were based on power-law fitting of F(n) with goodness smaller than 0.8.
Due to the sensitivity of activity monitors, the signal-to-noise ratio is low during the sleep episodes when activity levels are low. It has been shown that high levels of noise may contaminate the DFA results (Hu et al. 2001). Thus, we decided to focus on only the data during the periods of wakefulness for the fractal analysis. Because not all sleep records were available, we excluded all the data during or close to sleep episodes using the following rigorous method. Specifically, mean and standard deviation of bedtime/get-up time were calculated for each participant, and data within two standard deviations from mean bedtime/get-up time in each day were excluded.
Statistical analyses
Group differences in continuous variables were tested using analysis of variance (ANOVA). Group differences in categorical variables were tested using chi-squared tests. To determine group differences in scaling exponents, analysis of (co)variance (ANOVA/ANCOVA) and post-hoc Student T-tests were used. First, an unadjusted model was performed with group as the only predictor. Second, an adjusted model was performed while adjusting for sex and age. Extra analyses of covariance were performed to test if depressive or manic symptoms were related to the scaling exponents. Post hoc analyses were conducted to examine the differences between male and female patients in mood stabilizer use and in disease characteristics (age of onset, duration of disease in years, presence of psychotic symptoms, presence of rapid cycling, number of depressed episodes, number of manic episodes and presence of suicide attempt). Daily rhythm variables, as quantified by the non-parametric circadian variables (intradaily variability, interdaily stability, activity levels, timing of the most active 10 hours, timing of the least active 5 hours, and relative amplitude) were also studied (Van Someren et al. 1999).
State differences in the longitudinal study were tested using multiple regressions with participant as a random effect to test within-subject differences. To assess the lag in the relation between mood ratings and the scaling components, a linear regression model was performed using the scaling component as a continuous outcome, with depression score of the previous days as an independent variable.
Statistical analyses were performed using JMP Pro 11 (SAS Institute, Cary, NC). Statistical significance was accepted at alpha level of 0.05.
Results
Cross-sectional sample
The characteristics, mood symptoms, and medication use of the 255 participants in the cross-sectional analysis are provided in table 1. Age differed between the groups, with the siblings being somewhat older than the patients and controls (post-hoc analysis, Student’s t-test, sibling vs controls p < 0.001, sibling vs patient p = 0.046). As expected, scores on the manic and depressive scales were higher and psychotropic medication use more frequent in patients compared to the other two groups (table 1).
Table 1.
Cross-sectional sample characteristics.
| Patient N = 106 |
Sibling N = 73 |
Control N = 76 |
p | |
|---|---|---|---|---|
| Sex (female, %) | 62 (59%) | 44 (60%) | 41 (54%) | 0.718 |
| Age, years (sd) | 50.3 (11.3) | 54.3 (12) | 47 (16.3) | 0.004 |
| Employment (yes, %) | 70 (67%) | 52 (74%) | 43 (58%) | 0.118 |
| Children (yes, %) | 25 (24%) | 30 (41%) | 21 (28%) | 0.037 |
| ASRM score (sd) | 1.9 (1.8) | 1.2 (1.4) | 1.6 (2.2) | 0.046 |
| IDS-SR score (sd) | 15 (10.8) | 7 (6.6) | 5.9 (4.9) | < 0.001 |
| Mood stabilizer1 (yes, %) | 75 (71%) | 1 (1%) | 1 (1%) | < 0.001 |
Lithium, valproate acid, carbamazepine and lamotrigine
Fractal patterns in cross-sectional group
Patients with bipolar disorder showed a larger α1 (β = 0.009, p = 0.028, table 2) in an unadjusted model compared to healthy controls (figure 1). When the model was adjusted for age and sex, patients still showed a larger α1 (β = 0.009, p = 0.04, table 2). Post-hoc tests in the adjusted model showed that the group difference was significant in female subjects (t(1,246) = 3.26, p = 0.001), but not in male subjects (figure 2). For α2, in the unadjusted and adjusted model including both females and males, patients and siblings did not show significant differences compared to the control group. However, there was a significant sex interaction effect. Specifically, males patients had larger α2 compared to male controls (post-hoc Student’s t-test, t(1,102) = 2.22, p = 0.028); and both female patients (post-hoc Student’s t-test, t(1,141) = -2.78, p = 0.006) and female siblings (t(1,141) = -2.66, p = 0.009) had smaller α2 compared to female controls (figure 2).
Table 2.
Multivariate analysis for α1 and α2 in bipolar patients, their siblings, and controls
| α1 |
α2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Basic model | Adjusted model | Basic model | Adjusted model | |||||
| Estimate | p | Estimate | p | Estimate | p | Estimate | p | |
| Intercept | 1.02124 | 0.96837 | 0.80378 | 0.79903 | ||||
| Group: control | Ref | Ref | Ref | Ref | ||||
| Group: patient | 0.0094 | 0.0277 | 0.0086 | 0.0395 | 0.001899 | 0.8615 | 0.007906 | 0.4606 |
| Group: sibling | −0.001272 | 0.7850 | −0.00563 | 0.2284 | −0.01162 | 0.3301 | −0.0133 | 0.2659 |
| Sex (male) | −0.00998 | 0.0016 | 0.0283 | 0.0005 | ||||
| Age | 0.00101 | <0.0001 | 0.00015 | 0.8077 | ||||
| Group:Patient*age | −0.000235 | 0.4945 | −0.00157 | 0.0772 | ||||
| Group:Sibling*age | 0.000113 | 0.7758 | 0.00187 | 0.0477 | ||||
| Group:Patient*sex (male) | −0.00562 | 0.1820 | 0.02520 | 0.0203 | ||||
| Group:Sibling*sex (male) | 0.00160 | 0.7255 | 0.01479 | 0.2095 | ||||
Figure 1.
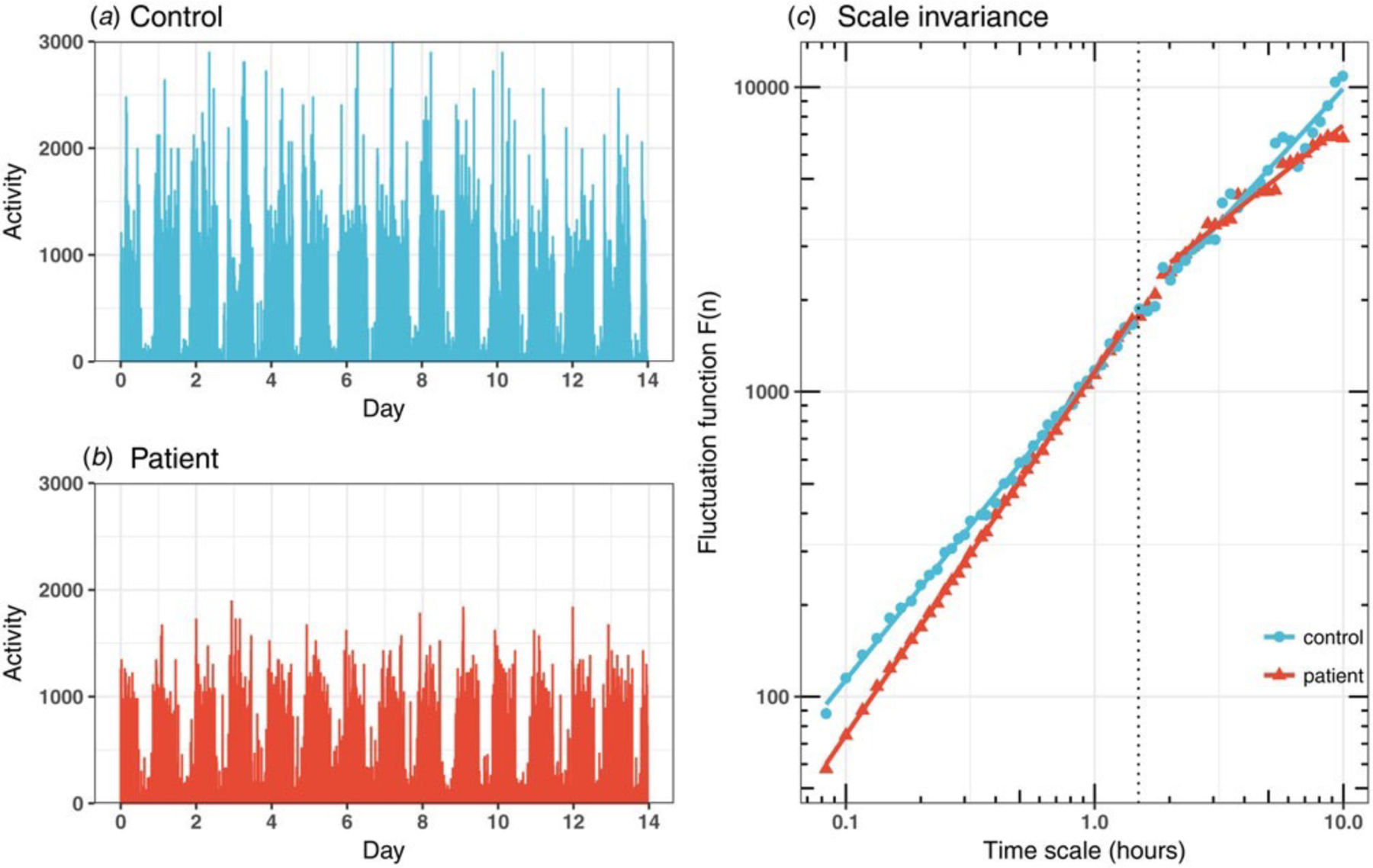
(A-B) Representative 14-day actigraphy recordings of a control participant (A) and a bipolar patient (B). (C) The scaling behaviors in the fluctuations of the two recordings in A and B. The fluctuation amplitudes at different time scales were shown in the log-log plot. Scaling exponents were obtained from power-law fitting (i.e., coefficients of the fits). The scaling exponent, α1, was calculated from the power-law fit at timescales <90 min (left to vertical dotted line), and the scaling exponent, α2, from the fit at timescales >2h (the right to the dotted line).
Figure 2.
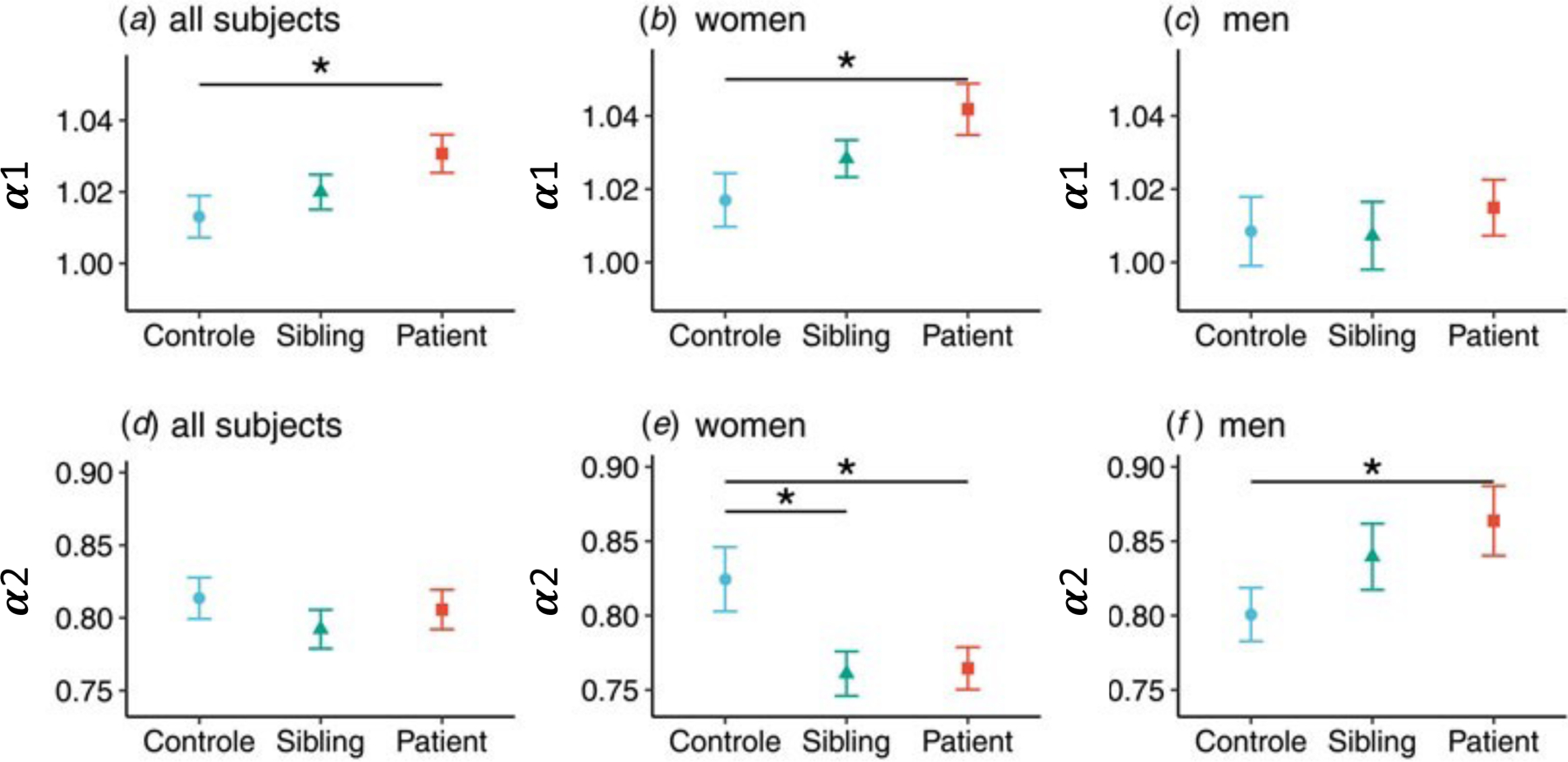
Differences in scaling exponents between groups and between men and women.
The group differences in α1 and α2 appeared to not be caused by depressive or manic symptoms because α1 and α2 were not significantly associated with depressive or manic scores (table S1 and S2).
To further investigate the sex effect, post-hoc tests were conducted to explore disease-related differences between men and women within the patient group. Medication use was not different between male and female patients (table S3). For daily rhythm variables, male patients showed lower interdaily stability (IS) compared to female patients (p = 0.003, table S4). But this was unlikely an explanation for the larger α2 in male patients because a larger α2 is linked to more robust circadian function and, thus, would correlate with higher IS (instead of the observed lower IS). Disease characteristics between male and female patients were studied as well. Although male patients had a later age of onset (p = 0.007, table S5), this was not related to α2.
Fractal patterns in the longitudinal group
For the longitudinal analysis, 14 patients with bipolar disorder type 1 (11 women and 3 men, mean age ± SD 44.7 ± 10.7 years) were included. No differences in the weekly scaling exponents were found between stable, depressive or manic states. There were no associations between the scaling exponents (either α1 or α2) and mood scores (ASRM and IDS-SR) within the same week. However, when a lag effect was tested, a significant negative correlation was found between the IDS score during one week and α1 of the subsequent week, i.e., a higher depressive symptom rating predicted a lower α1 next week (mixed model, β = -0.0007, p = 0.047). To better understand the time-lagged temporal relationship with higher temporal resolution, day-to-day depression score (measured by the visual analog scale from the question “I am feeling down”) and α1 computed on a daily basis were analyzed. A significant association was found with a lag of 5 and 7 days, supporting the approximately one-week delay between changes in depressive symptoms and the change in α1 (figure 3).
Figure 3.
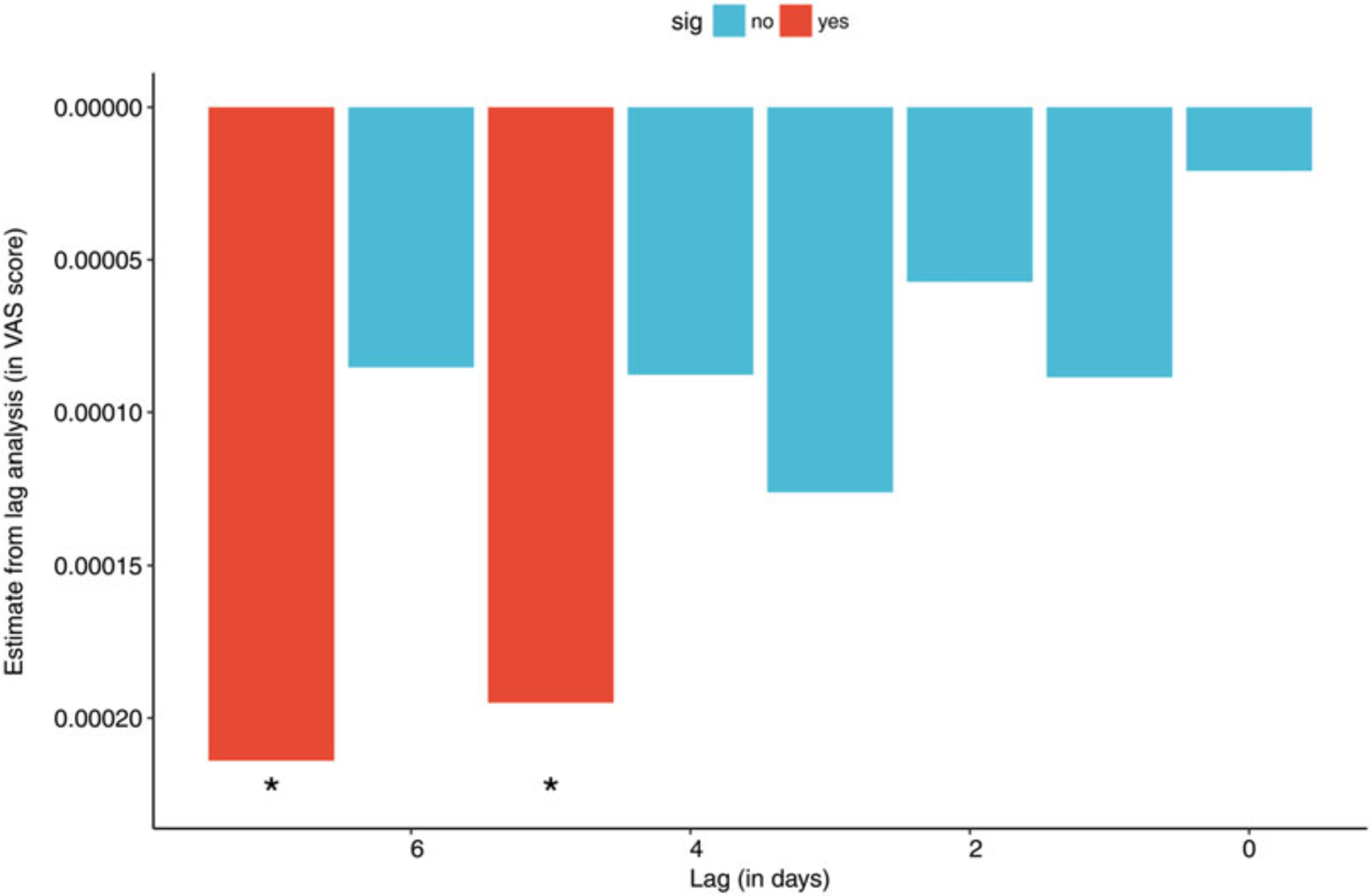
Time-lagged effect of “I’m feeling down” on α1. Note that the effect was significant for time lags of 5 and 7 days. Because the score of “I’m feeling down” question was obtained using a visual analog scale with a range from 0 to 100, the estimates are very small.
Discussion
This is, to our knowledge, the first study showing altered fractal activity regulation in patients with bipolar disorder. Patients with bipolar disorder showed a larger α1 compared to controls, indicating a more regular activity fluctuation pattern at time scales < 1.5 hours. Interestingly, this effect was sex-specific, i.e., female bipolar patients showed a larger α1 compared to female controls while no such effect was observed in men. The sex difference was also present at larger time scales (> 2 hours, α2). Specifically, we showed that female patients had a smaller α2 (below 1, indicating more random activity fluctuations) as compared to female controls, while male patients had a larger α2 than male controls.
The female unaffected siblings of bipolar patients also showed a smaller α2, similar to the female patients, which was not expected and requires further studies to understand its underlying mechanism. One possible explanation is that bipolar patients and their siblings share certain common genetic variants with effects on fractal activity regulation at larger time scales (α2). Previous studies showed that both altered α2 and bipolar disorder are associated with disturbances in circadian regulation. Thus, one testable hypothesis is that the genetics of circadian control may underlie the similar alterations in fractal regulation in bipolar patients and their siblings (Hu et al. 2007, 2013; Bradley et al. 2017).
We found no differences in fractal regulation between euthymic periods v. depressed or manic episodes in the longitudinal study. This suggest that the fractal pattern may be a trait feature in bipolar patients, being present independent of major mood episodes. However, when longitudinal analyses were conducted to assess week-to-week fluctuations, we did find a time-lagged, negative association between depression score and fractal regulation at small time scales (<1.5h) with a lag of one week. We confirmed this finding using data with a higher time resolution (i.e., day-to-day fluctuations of mood and α1), showing that depressed mood was associated with α1 determined 5 and 7 days later. This temporal association within bipolar patients appears to be robust because it persisted even when depressed mood was based on a single question instead of a questionnaire. Note that this negative association (i.e., higher depression rating associated with lower α1) seemingly contradicts our observation of larger α1 in bipolar patients and a previous observation of larger α1 in patients with major depression (Aybek et al. 2012). Clearly the group effect is not immediately translated to within-individual patient variations across time. One potential explanation is that the patients respond to the depressive symptoms with coping behavior, activating themselves and implementing learned techniques from psychotherapy. This is possible because the patients were familiar with their disease status and they had the motivation to participate in such a long-term study. This selection bias is likely in intensive studies as previously reported (Bos et al. 2015).
Indic and colleagues have also found significantly altered temporal structures in motor activity fluctuations of patients with bipolar disorder using a different scaling analysis (Indic et al. 2011). Here we reported the effect of bipolar disease on fractal activity regulation in two specific time-scale regions (i.e., <1.5h and 2–10 h). In addition, the observed sex-specific effect of bipolar disorder on fractal activity regulation has never been shown prior to this study. The sex difference in α2 is interesting because (i) the central circadian clock plays a critical role in α2, and (ii) sex differences in fundamental properties of the human circadian system and in human clock gene expression have been demonstrated (Duffy et al. 2011; Lim et al. 2013; Swanson et al. 2017). Our additional analyses refuted the differences in medication use, interdaily stability, or disease characteristics as potential explanations for the observed sex differences. Further studies are warranted to reveal this link between α and sex in patients with bipolar disorder.
Fractal regulation is believed to reflect system integrity and adaptability (i.e., the ability to respond to external changes while maintaining certain stability for orchestrated internal physiological functions). The balance between regularity and flexibility can be estimated by the scaling exponent (i.e., α) derived from the detrended fluctuation analysis (Goldberger et al. 2002; Pittman‐Polletta et al. 2013). When α is larger than 1 and increases toward 1.5 as observed in female patients, the fluctuations become overly regular, suggesting that the system might be less responsive to external changes. This loss of responsiveness has been reported previously in the studies of network structures of depressive symptoms, in which depressed patients showed decreased resilience to counter an external challenge (Wichers 2014; Van Borkulo et al. 2015). This rigidity, or loss of responsiveness, as reflected by both the fractal patterns and network structures, might be a core characteristic within mood disorders. Future studies are required to examine the responsiveness in both symptom networks and fractal motor control in order to determine whether they might be a suitable biomarker of mood disorders.
The current study has certain limitations. In the cross-sectional analysis, although we considered medication use, controlling for all different medications was impossible to do because patients used many different types of medications with different mechanisms of action. In addition, side effects relevant to activity measures, such as extrapyramidal symptoms, were not examined. The power of the longitudinal analysis was limited by the small sample size of patients. For both cross-sectional and longitudinal samples, there might be a selection bias of well-functioning patients with bipolar disorder, considering that they were able to complete an intense and long protocol (Bos et al. 2015). Furthermore, there were mainly depressive symptoms in the patients such that it is yet to be determined how manic symptoms and fractal patterns are related. Future work should focus on replicating our findings in other samples, ideally with a larger sample size and/or in twin studies. Less well-functioning patients should also be studied to better illustrate the longitudinal temporal relationship between depressive symptoms and the scaling exponent.
In summary, both our cross-sectional and longitudinal studies indicated that there is a relationship between fractal regulation and bipolar disorder. The assessment of fractal regulation is based on motor activity data that can be collected with actigraphy. The unobtrusive nature of actigraphy makes this approach feasible to be applied in clinical practice (Kaplan et al. 2012; Nicholas et al. 2017). Thus, our results raise the possibility that fractal activity regulation may serve as an additional cost-effective and non-invasive biomarker for bipolar disorder and can be used for long-term monitoring of patients with bipolar disorder.
Supplementary Material
Acknowledgements
Financial support:
This project has been supported by the Foundation “De Drie Lichten” in The Netherlands. The cross-sectional study was funded by the National Institute of Mental Health (NIMH), Grant number: R01 MH090553. The NIMH had no role in the design and conduct of the study, collection, management, analysis, or interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication. FAJLS was partially supported by NIH grants R01HL118601 and RF1AG059867. KH was partially supported by the NIH grants: R01AG048108, RF1AG059867, and RF1AG064312.
Footnotes
Disclosures
FAJLS received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer. SEK, PL, RFRvdL, SV, MPMB, RAS and KH reported no biomedical financial interests or potential conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
References
- Aan het Rot M, Hogenelst K, & Schoevers RA. (2012). Mood disorders in everyday life: A systematic review of experience sampling and ecological momentary assessment studies. Clinical Psychology Review, 32, 510–523. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM (1997). The Altman Self-Rating Mania Scale [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR® Arlington County, Virginia, US: American Psychiatric Pub. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub. [Google Scholar]
- Aybek S, Ionescu A, Berney A, Chocron O, Aminian K, Vingerhoets FJG (2012). Fractal temporal organisation of motricity is altered in major depression. Psychiatry Research 200, 288–293. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L, Baethge CJ, Lepri B, Bratti IM (2007). Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Denmark Bipolar disorders 9, 386–393. [DOI] [PubMed] [Google Scholar]
- Born C, Amann BL, Grunze H, Post RM, Schärer L (2014). Saving time and money: a validation of the self ratings on the prospective NIMH Life-Chart Method (NIMH-LCM). BMC psychiatry 14, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos FM, Schoevers R a., aan het Rot M (2015). Experience sampling and ecological momentary assessment studies in psychopharmacology: A systematic review. Elsevier European Neuropsychopharmacology, 1–12. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Webb-Mitchell R, Hazu A, Slater N, Middleton B, Gallagher P, McAllister-Williams H, Anderson KN (2017). Sleep and circadian rhythm disturbance in bipolar disorder. Psychological Medicine, 1–12. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A-M, Phillips AJK, Munch MY, Gronfier C, Wyatt JK, Dijk D-J, Wright KP, Czeisler CA (2011). Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proceedings of the National Academy of Sciences 108, 15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) John Wiley & Sons Inc [Google Scholar]
- Goldberger AL, Amaral LAN, Hausdorff JM, Ivanov PC, Peng C, Stanley HE (2002). Fractal dynamics in physiology : Alterations with disease and aging 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Manji HK (2002). The Wnt signaling pathway in bipolar disorder. Sage Publications Sage CA: Thousand Oaks, CA: The Neuroscientist 8, 497–511. [DOI] [PubMed] [Google Scholar]
- Harvey AG (2008). Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. The American journal of psychiatry 165, 820–9. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL (1997). Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. Journal of Applied Physiology (Bethesda, Md.: 1985) 82, 262–269. [DOI] [PubMed] [Google Scholar]
- Hu K, Harper DG, Shea SA, Stopa EG, Scheer FAJL (2013). Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Scientific Reports 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ivanov PC, Chen Z, Carpena P, Stanley HE (2001). Effect of trends on detrended fluctuation analysis. Physical Review E 64, 011114. [DOI] [PubMed] [Google Scholar]
- Hu K, Ivanov PC, Chen Z, Hilton MF, Stanley HE, Shea SA (2004). Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A: Statistical Mechanics and its Applications 337, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Riemersma-van der Lek RF, Patxot M, Li P, Shea SA, Scheer FA, Van Someren EJ (2016). Progression of Dementia Assessed by Temporal Correlations of Physical Activity: Results From a 3.5-Year, Longitudinal Randomized Controlled Trial. Sci Rep 6, 27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FAJL, Ivanov PC, Buijs RM, Shea SA (2007). The suprachiasmatic nucleus functions beyond circadian rhythm generation. Elsevier Neuroscience 149, 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Van Someren EJW, Shea SA, Scheer FAJL (2009). Reduction of scale invariance of activity fluctuations with aging and Alzheimer ‘ s disease : Involvement of the circadian pacemaker. PNAS 106, 2490–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indic P, Salvatore P, Maggini C, Ghidini S, Ferraro G, Ross J, Murray G (2011). Scaling Behavior of Human Locomotor Activity Amplitude : Association with Bipolar Disorder 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K a, Talbot LS, Gruber J, Harvey AG (2012). Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar disorders 14, 870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Morris CJ, Patxot M, Yugay T, Mistretta J, Purvis TE, Scheer FAJL, Hu K (2017). Reduced Tolerance to Night Shift in Chronic Shift Workers: Insight From Fractal Regulation. Sleep 40, zsx092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Yu L, Lim ASP, Buchman AS, Scheer FAJL, Shea SA, Schneider JA, Bennett DA, Hu K (2018). Fractal regulation and incident Alzheimer ‘ s disease in elderly individuals. Elsevier Inc. Alzheimer’s & Dementia, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Y Li P, Lim ASP, Gao L, Hu C, Yu L, Bennett DA, Buchman AS, Hu K (2019). More random motor activity fluctuations predict incident frailty, disability, and mortality. Science Translational Medicine doi: 10.1126/scitranslmed.aax1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Myers AJ, Yu L, Buchman AS, Duffy JF, De Jager PL, Bennett DA (2013). Sex difference in daily rhythms of clock gene expression in the aged human cerebral cortex. Journal of biological rhythms 28, 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhiet V, Etain B, Boudebesse C, Bellivier F (2011). Circadian biomarkers, circadian genes and bipolar disorders. Journal of physiology, Paris 105, 183–9. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Abdulhak A Bin, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng ATA, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, De Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380, 2197–2223. [DOI] [PubMed] [Google Scholar]
- Nicholas J, Boydell K, Christensen H (2017). Beyond symptom monitoring: Consumer needs for bipolar disorder self-management using smartphones. Elsevier Masson SAS European Psychiatry 44, 210–216. [DOI] [PubMed] [Google Scholar]
- Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL (1994). Mosaic organization of DNA nucleotides. Physical Review. E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics 49, 1685–1689. [DOI] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL (1995). Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. Journal of Electrocardiology 28 Suppl, 59–65. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus JE, Liu Y, Lee C, Hausdorff JM, Stanley HE, Goldberger AL, Lipsitz LA (2002). Quantifying fractal dynamics of human respiration: age and gender effects. Annals of Biomedical Engineering 30, 683–692. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Kupfer DJ (2013). Bipolar Disorder 2 Bipolar disorder diagnosis : challenges and future directions. Elsevier Ltd The Lancet 381, 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman‐Polletta BR, Scheer FAJL, Butler MP, Shea SA, Hu K (2013). The role of the circadian system in fractal neurophysiological control. Wiley Online Library Biological Reviews 88, 873–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz P (2000). The Inventory of Depressive Symptomatology (IDS): Clinician (IDS‐C) and Self‐Report (IDS‐SR) ratings of depressive symptoms. Wiley Online Library International Journal of Methods in Psychiatric Research 9, 45–59. [Google Scholar]
- Sheehan D V, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Physicians Postgraduate Press PO BOX 240008, MEMPHIS, TN 38124 USA. Journal of Clinical Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Van Bergen AH, Verkooijen S, Vreeker A, Abramovic L, Hillegers MH, Spijker AT, Hoencamp E, Regeer EJ, Knapen SE, Der Lek RFR, Schoevers R, Stevens AW, Schulte PFJ, Vonk R, Hoekstra R, Van Beveren NJ, Kupka RW, Sommer IEC, Ophoff RA, Kahn RS, Boks MPM (2018). The characteristics of psychotic features in bipolar disorder. Cambridge University Press; Psychological medicine, 1–13. [DOI] [PubMed] [Google Scholar]
- Van Borkulo C, Boschloo L, Borsboom D, Penninx BWJH, Lourens JW, Schoevers RA (2015). Association of symptom network structure with the course of longitudinal depression. JAMA Psychiatry 72, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall W V, Rosenquist PB (1999). Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiology international 16, 505–518. [DOI] [PubMed] [Google Scholar]
- Swanson LM, Burgess HJ, Huntley ED, Bertram H, Mooney A, Zollars J, Dopp R, Ho R, Armitage R, Arnedt JT (2017). Relationships between circadian measures , depression , and response to antidepressant treatment : A preliminary investigation 252, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkooijen S, van Bergen AH, Knapen SE, Vreeker A, Abramovic L, Pagani L, Jung Y, Riemersma-van der Lek R, Schoevers RA, Takahashi JS, Kahn RS, Boks MPM, Ophoff RA (2017). An actigraphy study investigating sleep in bipolar I patients, unaffected siblings and controls. Journal of Affective Disorders 208, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M (2014). The dynamic nature of depression: a new micro-level perspective of mental disorder that meets current challenges. Psychological medicine 44, 1349–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


