Abstract
Despite early diagnosis and established protocols, a subset of prostate cancer patients will eventually be categorized as castration resistant prostate cancer. Recently it has been reported that these multi-modal therapy cases may harbor a special subset of cancer cells termed as polypoidal giant cancer cells (PGCC). These cells are phenotypically described either as possessing highly irregular polylobated nuclei or multiple pleomorphic nuclei. To identify and characterize the distribution of these cells we created a cohort of 5 randomly selected cases of multi-modal therapy failure prostate cancer (16 selected non-osseous and osseous tumor sites) enrolled in Michigan Legacy Tissue Program. In all cases specific “regions of interest” or “hot spots” within tumor areas showing an increased proportion of these multi-nucleated/polylobated cells under light microscopy were labeled as PGCC rich area. On microscopic evaluation, overall PGCC count was 42.4 ± 3.91 with case 2 in the study cohort with the highest number of average PGCC count of 17 ± 4.04. Site wise analysis showed retroperitoneal lymph node as the tissue with highest number of average PGCC number/site (5.0 ± 0.32). On correlating the average number of PGCC recorded with the time elapsed from last dose of chemotherapy administered to autopsy, the spearman correlation value (R) was 0.67 but the result was not statistically significant (p=0.22). A systematic assessment of PGCC in a large stratified cohort of prostate cancer patients integrated with various histopathological and clinical parameters along with discovery of specific biomarkers for PGCC, are the future studies suggested.
Summary
The study documents the presence of PGCC in a small cohort of prostate cancer patients with a lethal clinical phenotype and enrolled in a rapid autopsy program (MLTP). Future studies aimed at systematic assessment of PGCC in a large stratified cohort of prostate cancer patients integrated with various histopathological parameters along with other clinical and radiological features of disease could reveal associative information between PGCC and prostate cancer progression and therapy resistance.
Keywords: Castration resistant, Polypoidal giant cancer cells, Prostate cancer, Rapid autopsy
Introduction
Prostate cancer continues to be a major cause of morbidity and mortality across the world, and is a common cancer in American men with 174,650 new cases and 31,620 deaths reported in 2019 [1]. Although many prostate cancer patients are diagnosed early with good survival outcomes from treatment with prostatectomy and/or radiation therapy, a subset of these cases become metastatic. Androgen deprivation therapy (ADT) is started, but resistance in inevitable and the patients may transition from hormone-sensitive to the therapy resistant and lethal phase of the disease, castration resistant prostate cancer (CRPC). Recent studies suggest that the therapy resistant tumors may be enriched with particular type of cancer cells called polypoidal giant cancer cells (PGCC) [2]. PGCC are defined as cancer cells with morphological appearance of highly irregular polylobated nuclei or multiple pleomorphic nuclei. It was reported that the presence of PGCC in prostate cancer may be associated with disease progression and metastasis along with resistance to therapy [2–6]. Based on this data, we investigated a cohort of 5 patients with CRPC enrolled in our Michigan Legacy Tissue Program (MLTP) at Michigan Medicine. We characterized the PGCC and their distribution in 16 selected non-osseous and osseous tumor sites from patient specimens and evaluated the association between PGCC frequency, the sites of metastasis and clinico-pathological parameters.
Materials and Methods
Case Selection
The rapid autopsy program at Michigan Medicine or Michigan Legacy Tissue Program (MLTP) was approved by the Institutional Review Board of University of Michigan and is supported by Specialized Program of Research Excellence (SPORE) in Prostate Cancer (National Cancer Institute grant 2P50CA186786–06). The goal of MLTP has been to maximize the sample number and diversity of metastatic prostate cancer tissue available for research purposes. Patients were identified with CRPC by the Medical Oncology Service of the Comprehensive Cancer Center at the University of Michigan Hospitals as described previously [7, 8]. Autopsies were performed on patients with CRPC as part of the MLTP program- these autopsies have been referred to as “rapid” or “warm” because of the short time interval (average 3 hours) between patient death and starting the autopsy.
We randomly selected 5 CRPC patients enrolled in MLTP for this study who were treated with multimodality therapies (various combination of radical prostatectomy, radiation, chemotherapy and anti-androgen agents). The formalin fixed paraffin embedded (FFPE) blocks representing procured autopsy tissue were retrieved from the MLTP archival tissue core (n=16) and selected blocks were sectioned at 5-micron thickness and stained with hematoxylin and eosin (H&E) for histologic evaluation. A minimum of 3 different metastatic sites from each patient were selected for assessment that included both non-osseous (liver, lung, pancreas, retro-peritoneal lymph nodes, urinary bladder, peri-vesical region and dura) and osseous sites (femur, rib and thoracic vertebra) (Table 1). Tumor within the prostate was evaluated when available (i.e., in the absence of previous prostatectomy).
Table 1:
Clinico-Pathological Parameters of the selected Cohort
| Case No. | Age at death (years) | Disease Extent | Gleason score at initial diagnosis | Prostate Directed Therapy Delivered | Systemic Therapy | Duration between last Chemotherapy and Autopsy (Days) |
|---|---|---|---|---|---|---|
| 1 | 55 | Urinary Bladder, Seminal Vesicle, Lung (Lt), Iliac Soft Tissue, Liver (multifocal), Adrenal (Lt), Pancreas, Peri-tracheal and Peri-aortic soft tissue, Pituitary, Both Femur and Humerus, and Thoracic and Lumbar Vertebra | 4 + 5 | Adjunct RT | ADT and CT | 44 |
| 2 | 69 | Dura, Sternum, Ribs, liver, Retro-peritoneum, Humerus (Rt), Femur, Retroperitoneal and Mediastinal lymph nodes, Lumbar and Thoracic Vertebra | 5 + 5 | NA | ADT and CT | 932 |
| 3 | 76 | Skull base, Vertebra, Ribs, Extremities, Pelvis, Cerebellum, Liver, Lungs, Diaphragm, Spleen and Pancreas, | 5 + 5 | Transurethral prostatic resection | CT | 170 |
| 4 | 60 | Sternum, Ribs, Femur, Humerus, Lumbar Vertebra, Thoracic Vertebra, Humerus, Mediastinal/ Retroperitoneal/Supra-Clavicular Lymph Nodes, Liver, Spleen, Dural and Leptomeningeal | 5 + 5 | Adjunct RT | ADT and CT | 569 |
| 5 | 65 | Bilateral Femur, Liver, Lung, Thoracic lymph nodes, Ribs, Vertebral column | 5 + 5 | Radical Prostatectomy | CT | 413 |
ADT- Androgen deprivation therapy; CT-Chemotherapy; RT-Radiotherapy
Histopathological Evaluation and Inclusion Criteria
Polypoidal giant cancer cells (PGCC) may present as either multi-nucleated/polylobated cancer cells or as relatively larger cells characterized by a “giant” nucleus that is three times larger than that of the neighboring diploid cancer cell [2–5]. For the purpose of this study, only multi-nucleated/ polylobated cancer cells were classified as PGCC; the larger cells were not included in the analysis to maintain a high degree of diagnostic and recognition specificity for PGCC. We identified specific “regions of interest” or “hot spots” within tumor areas showing an increased proportion of these multi-nucleated/polylobated cells under light microscopy at 200x magnification and labeled these as PGCC rich areas. PGCC were assessed in 5 consecutive PGCC rich areas by two pathologists including a genitourinary pathologist (RM and RM) at intermediate power (200x) and high power magnification/field (400x or HPF).
Since the main focus of our study was to interrogate the presence and characterization of PGCC from a qualitative/quantitative perspective, we counted and recorded the total number of PGCC per metastatic site from 5 topographically separate PGCC rich areas (“hot spots”) utilizing high power evaluation (400x) in each case of our cohort. In total, we assessed 16 different anatomical sites with each site sampled through 5 PGCC rich areas (“hot spots”) thereby leading to final assessment of 80 PGCC rich areas of this cohort (Supplementary Table 1).
For statistical purposes, an average number of PGCC per each of the 5 cases (Table 2) and average number of PGCC per site in each individual case were recorded (Supplementary Table 1). We also calculated the overall total average PGCC number (calculated by summing up the individual PGCC recorded in all 80 PGCC rich fields in 16 metastatic sites of the 5 cases in the study); this was performed to compare and assess features with the overall PGCC recorded in some non-prostate cancer studies published in the literature.
Table 2:
Comparison of overall and site wise average number of PGCC with maximum and minimum PGCC noted in CRPC cohort
| Case # | No of sites assessed/Case | Total PGCC rich areas assessed/Case | Total No of PGCC/Case | Average PGCC/Case | Site with Maximum PGCC No | Site with Minimum PGCC No |
|---|---|---|---|---|---|---|
| 1 | 4 | 20 | 51 | 12.7 ± 1.11 | Peri-vesical Region | Urinary Bladder |
| 2 | 3 | 15 | 51 | 17.0 ± 4.04 | Retroperitoneal Lymph Node | Liver- Site 2 |
| 3 | 3 | 15 | 34 | 11.3 ± 0.33 | Prostate | Liver & Pancreas |
| 4 | 3 | 15 | 43 | 14.33 ± 1.76 | Dura | Lung |
| 5 | 3 | 15 | 33 | 11 ± 0.57 | Rib | Thoracic vertebra |
Results
Clinico-Pathological Parameters
Most patients in our cohort had high-grade disease with Grade Group 5 and an average Gleason score of 4 + 5 = 9 or 5 + 5 = 10 (Table 1). Autopsy findings in the patients recapitulated the known phenomenon of pro-skeletal disease progression of metastatic prostate cancer. Skeletal metastasis, especially in lower extremities, vertebra and ribs were observed in all cases. In the non-osseous sites, liver was the most common site for metastasis (seen in all 5 cases), followed by lung and lymph nodes (seen in 3/5 cases). In case 3, tumor within prostate was available for evaluation, remainder of the sites comprised of metastatic tumor (Supplementary Table 1).
Morphological Assessment
Histopathological evaluation by light microscopy revealed the presence of isolated to small groups of relatively larger and hyperchromatic appearing cells admixed within a population of tumor cells in PGCC rich areas (“regions of interest”/” hot spots”). When reviewed under higher magnification these cells were proportionally much larger than the neighboring tumor cell population exhibiting two types of identifiable and reproducible morphology. The first morphologic phenotype comprised of cells with multi-nucleated, hyperchromatic nuclei showing irregular nuclear membrane, presence of multiple nucleoli and having minimal amount of cytoplasm with indistinct cytoplasmic membrane. The second phenotype we observed of PGCC was that of larger cells with high nuclear: cytoplasmic ratio, hyperchromatic pleomorphic nuclei showing irregular nuclear membrane exhibiting indentations and polylobation, multiple nucleoli and indistinct cytoplasmic membrane (Fig. 1).
Fig. 1:
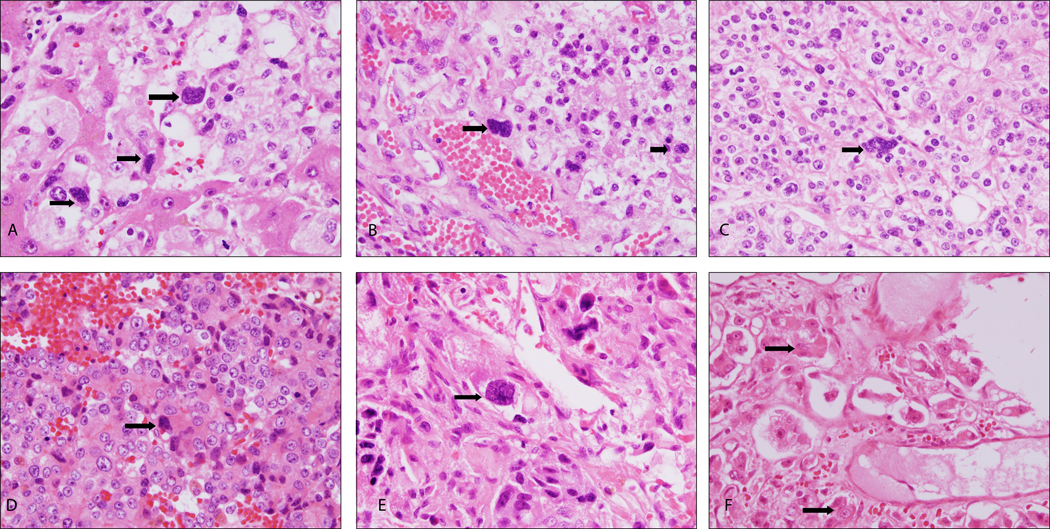
Polypoidal giant cancer cells (PGCC, indicated by arrows) in [A] Prostate and in various representative metastatic sites: [B] Liver; [C] Dura; [D] Peri-Vesical Tissue; [E] Femur; [F] Thoracic Vertebra
Overall average PGCC count
Based on morphologic assessment, PGCC were identified in all the cases evaluated (5/5) and at all metastatic sites, characterized by the presence of large multi-nucleated/ polylobated cancer cells as described above. Overall, the average number of PGCC per case (calculated by summing up the individual PGCC recorded in all the five consecutive hot spot areas in 16 sites of all the 5 cases in the study) was 42.4 ± 3.91.
Case Wise average PGCC count
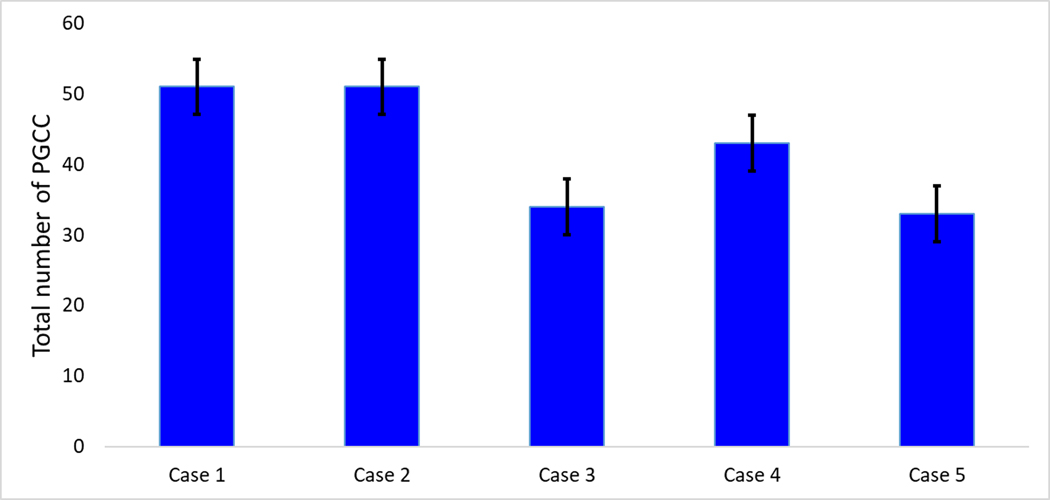
Sub-dividing the cases in the study cohort individually, the case 2 in the study cohort showed the highest number of average PGCC count of 17 ± 4.04 with a range of 2 to 6 PGCC / HPF at different anatomical sites, followed by case 4 with an average PGCC count of 14.33 ± 1.76 with a range of 2 to 4 PGCC / HPF at different representative sites. Case 5, which had only osseous metastatic disease with no non-osseous organ involvement by metastatic tumor, demonstrated the lowest recorded PGCC with an average count of 11 ± 0.57 with a range of 2 to 3 PGCC / HPF at various anatomical sites (Table 2, Fig. 2).
Fig. 2:
Case wise distribution of PGCC in the study cohort
Site wise average PGCC count
Systematic site wise analysis showed that retroperitoneal lymph node metastasis in case 2 exhibited the highest average PGCC number/site (5.0 ± 0.32). Thoracic vertebral metastasis recorded the least number of average PGCC/site (2.0 ± 0.0). The detailed description of site wise (n=16) and corresponding field wise (n=80) PGCC observed are provided in Supplementary Table 1.
Correlation of presence of PGCC with most recent chemotherapy administration
On correlating the average number of PGCC recorded with the time elapsed from last dose of chemotherapy administered to autopsy (Table 1 and 2), the spearman correlation value (R) was 0.67 but the result was not statistically significant (p=0.22) (Supplementary Fig. 1).
Discussion
The development of therapy resistant metastatic prostate cancer accounts for the majority of deaths related to prostate cancer. Our group has previously investigated castration resistance mechanisms, ERG gene rearrangement, ERG protein expression discordance and other clinico-pathological and genomic sequencing based studies in patients with CRPC [7–9]. A recent and interesting report/review by Amend et al. has described the presence of polypoidal giant cells (PGCC) in tumor from patients who failed multimodal therapies and proposed these as the “keystone species” in the prostate cancer ecosystem [2]. Citing multiple pertinent studies available in the published literature the authors have also proposed that these rare subpopulation of cells may be able to survive harsh tumor ecosystem of low oxygen tension, low pH and low nutrients and therefore drive metastasis and therapeutic resistance [2]. Interestingly, it has been reported and shown that both therapeutic intervention (chemotherapy and radiation) and experimental induction of hypoxia reportedly simulate the tumor microenvironment, and may result in the generation of PGCC [10–12].
Here, we interrogated the presence of PGCC in a small cohort of CRPC patients (representing a lethal phenotype of prostate cancer) enrolled in MLTP. Our observations support the identification of sparse and focal topographical presence of these cells within the tumor landscape of prostate cancer, similar to previous findings from Amend et al [2]. The cells exhibited two types of morphology on light microscopy- multi-nucleated and polylobated- thus also corroborating earlier work in non-prostate malignancies such as ovarian, breast, melanoma, lung, pancreas, urinary bladder, kidney and thyroid, amongst others [3–6]. Recently, a study by Alharbi et al. noted the presence of pleomorphic giant cells (possibly akin to PGCC) in their selected cohort of 30 cases of prostate cancer with pleomorphic giant cells visualized in less than 5% of the tumor area [13]. This is relatively consistent with our observations that PGCC are localized in a few PGCC rich areas or hot spots and are not present diffusely within the tumor. We did observe that every tumor we examined though had PGCC within them, supporting their potential role in metastasis formation or maintenance. Also, the microenvironment of the resident tissue for a specific metastasis may interact with PGCC development or maintenance, indicated by the variable amounts we see between tissues/tumor sites. Comparison of the average number of PGCC in the present study of 42.4 ± 3.91 to the other studies in different non-prostate malignancies, we noted a wide range reported in literature as 22.61 ± 1.15 in breast cancer [5], 18.12 ± 8.70 in serous epithelial ovarian tumors [6] and 32.75 ± 22.21 in anorectal malignant melanoma [15].
Various studies of PGCC in non-prostate tumors have also attempted to correlate PGCC with clinical and histopathological variables, vasculogenic mimicry and poor survival [14–17]. Such a parametric description and systematic analysis in context of standardized clinico-pathological evaluation regarding PGCC in prostate cancer is currently lacking [2, 13]. The samples included in the present study were tissues harvested from the patients who died due to the complications of metastatic CRPC. In this cohort of patients with highly aggressive disease showing wide spread multiple non-osseous and osseous metastasis, high Gleason scores (9 and 10) and high PSA levels, PGCC could be identified in all the investigated cases and sites. Similarly, other cancer sub-types have showed an increase in number of PGCC at metastatic sites and in higher tumor grade areas [6, 15–16]. We hypothesized that chemotherapy may maintain PGCC number, thus comparing time from last chemotherapy to amount of PGCC observed at autopsy. The correlation value of R=0.67 is intriguing, but clearly not statistically significant possibly from small cohort size or long mean time from chemotherapy of 426 days.
The current challenges to studying PGCC include the lack of a universally acceptable and reproducible definition of PGCC, and lack of specific biomarkers and approaches to harvest a significant population of PGCC from both solid tumor tissue and liquid biopsies [2, 18]. Tissue banks from rapid autopsy programs such as MLTP with access to specimens from metastatic and lethal prostate cancers may provide an opportunity to further study and assess PGCC, from a pathology, functional and mechanistic standpoint. Since 1996, MLTP at Michigan Medicine has conducted systematic studies of morphology, immunophenotype, genomic alterations, transcriptome profiling and clinical outcomes through a large number of rapid autopsies performed on men who died from complications of metastatic CRPC [8, 19–20]. Like other rapid autopsy programs, this has aided in elucidating the molecular mechanisms underlying hormone resistance as well as the rare presenting phenotypic/genotypic prostate cancer subtypes [7, 9, 21]. It has also allowed us to obtain biospecimens from inaccessible sites that could aid in the isolation of pure PGCC population and discovery of specific biomarkers for PGCC, which we consider as aims for future projects.
The present morphology driven study to ascertain PGCC on routine H&E stained sections was aimed at identifying the cell of interest (PGCC) in castration resistant prostate cancer. Despite utilizing strict morphological criteria, assessing PGCC on histological sections is associated with challenges; the low percentage of PGCC observed in each ‘hot spot’ further demands a rigorous microscopic evaluation. The cell membranes in H&E stained sections are often if not frequently indistinct thereby making a comprehensive distinction of a true PGCC from group of cells overriding and overlapping and pseudo multi-nucleation (due to artefact of sectioning) difficult at times. Companion immunohistochemical membranous markers such as EpCAM and E-Cadherin may be of assistance in identification and confirmation of PGCC, which would be aims for future studies.
Although the molecular, cellular and genetic biology behind pathogenesis of PGCC have yet to be fully elucidated, recording the histopathological observations in prostate cancer could have future clinical significance when integrated with other laboratory and clinical data. Finally, it is conceivable that the unique biology of PGCC may make them susceptible to novel pharmacological agents [2].
Supplementary Material
Spearman correlation plot between the average number of PGCC/Case versus the duration of last chemotherapy to autopsy (in days
Acknowledgements:
Sathiya P Narayanan PhD, Jyoti Athanikar PhD
Funding: None
Footnotes
Disclosures: None Declared
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019. January;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Amend SR, Torga G, Lin KC, Kostecka LG, de Marzo A, Austin RH, Pienta KJ. Polyploid giant cancer cells: Unrecognized actuators of tumorigenesis, metastasis, and resistance. The Prostate. 2019. September;79(13):1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33(1):116–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Mercado-Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer Lett. 2013;333(2):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fei F, Zhang D, Yang Z, Wang S, Wang X, Wu Z, Wu Q, Zhang S. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. Journal of Experimental & Clinical Cancer Research. 2015. December;34(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Ding P, Lv H, Zhang D, Liu G, Yang Z, Li Y, Liu J, Zhang S. Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor. BioMed Research International. 2014;2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, MacVicar GR, Varambally S, Harwood J, Bismar TA, Kim R. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer research. 2004. December 15;64(24):9209–16. [DOI] [PubMed] [Google Scholar]
- 8.Udager AM, Shi Y, Tomlins SA, Alva A, Siddiqui J, Cao X, Pienta KJ, Jiang H, Chinnaiyan AM, Mehra R. Frequent discordance between ERG gene rearrangement and ERG protein expression in a rapid autopsy cohort of patients with lethal, metastatic, castration‐resistant prostate cancer. The Prostate. 2014. September;74(12):1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weindorf SC, Taylor AS, Kumar-Sinha C, Robinson D, Wu YM, Cao X, Spratt DE, Kim MM, Lagstein A, Chinnaiyan AM, Mehra R. Metastatic castration resistant prostate cancer with squamous cell, small cell, and sarcomatoid elements—a clinicopathologic and genomic sequencing-based discussion. Medical Oncology. 2019. March 1;36(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Niu N, Zhang J, Qi L, Shen W, Donkena KV, Feng Z, Liu J. Polyploid Giant Cancer Cells (PGCCs): The Evil Roots of Cancer. Current cancer drug targets. 2019. May 1;19(5):360–7. [DOI] [PubMed] [Google Scholar]
- 11.Mittal K, Donthamsetty S, Kaur R, Yang C, Gupta MV, Reid MD, Choi DH, Rida PC, Aneja R. Multinucleated polyploidy drives resistance to Docetaxel chemotherapy in prostate cancer. British journal of cancer. 2017. April;116(9):1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin KC, Torga G, Sun Y, Axelrod R, Pienta KJ, Sturm JC, Austin RH. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clinical & experimental metastasis. 2019. April 15;36(2):97–108. [DOI] [PubMed] [Google Scholar]
- 13.Alharbi AM, De Marzo AM, Hicks JL, Lotan TL, Epstein JI. Prostatic adenocarcinoma with focal pleomorphic giant cell features: a series of 30 cases. Am J Surg Pathol. 2018; 42(10): 1286–1296. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Wang Y, Fei F, Wang X, Li C, Liu K, Du J, Cao Y, Zhang S. Clinical characteristics and preliminary morphological observation of 47 cases of primary anorectal malignant melanomas. Melanoma research. 2018. December 1;28(6):592–9 [DOI] [PubMed] [Google Scholar]
- 15.Fei F, Qu J, Liu K, Li C, Wang X, Li Y, Zhang S. The subcellular location of cyclin B1 and CDC25 associated with the formation of polyploid giant cancer cells and their clinicopathological significance. Laboratory Investigation. 2019. April;99(4):483. [DOI] [PubMed] [Google Scholar]
- 16.Lv H, Shi Y, Zhang L, Zhang D, Liu G, Yang Z, Li Y, Fei F, Zhang S. Polyploid giant cancer cells with budding and the expression of cyclin E, S-phase kinase-associated protein 2, stathmin associated with the grading and metastasis in serous ovarian tumor. BMC cancer. 2014. December;14(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukács G, Balázs G, Zs-Nagy I, Juhász F. Prognostic significance of nuclear DNA content in highly malignant thyroid tumors. Wiener Klinische Wochenschrift. 1990. April;102(9):253–6. [PubMed] [Google Scholar]
- 18.Mirzayans R, Andrais B, Murray D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers. 2018. April;10(4):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra R, Kumar-Sinha C, Shankar S, Lonigro RJ, Jing X, Philips NE, Siddiqui J, Han B, Cao X, Smith DC, Shah RB. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clinical Cancer Research. 2011. June 15;17(12):3924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H. Integrative clinical genomics of advanced prostate cancer. Cell. 2015. May 21;161(5):1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012. July;487(7406):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman correlation plot between the average number of PGCC/Case versus the duration of last chemotherapy to autopsy (in days