Abstract
Background:
Despite an increasing trend of onychomycosis caused by Candida species in recent years, there is a scarcity of published data.
Objective:
To determine the epidemiological and clinical characteristics of Candida onychomycosis, to identify the prevalent, and perform in-vitro antifungal susceptibility testing (AFST) of the isolates.
Methodology:
A total of 506 consecutive patients with a clinical suspicion of onychomycosis were included in a cross-sectional clinical study. Nail scrapings and clippings were subjected to KOH examination and culture. Species identification and antifungal drug sensitivity testing were done for Candida isolates using Vitek 2YST Compact system using Vitek 2 cards.
Results:
Out of 384 (75.88%) culture-positive cases, dermatophytes were isolated in 58.08%, yeast in 26.30%, and NDM in 12.24%. Of the yeast, Candida albicans was isolated in 59.4% and non-albicans species in 40.59%. AFST showed that most of Candida species exhibited 100% susceptibility to most of the antifungal drugs tested, while intermediate resistance to fluconazole and flucytosine was seen in some non-albicans species (C. krusei, C. glabrata, and C. guilliermondii). Time taken for species identification was 14–18 h (average 15.5 h), while determination of minimum inhibitory concentration took 9–27 h (average 13 h).
Conclusions:
Our study showcases the present scenario of Candida distribution and the resistance patterns of various species afflicting the nail unit. Furthermore, our findings clearly indicate that the carriage of this pathogenic yeast is seen in both healthy individuals as well as with immunosuppression.
Key Words: Antifungal susceptibility test, candida, onychomycosis, Vitek 2YST
Introduction
Despite an increasing trend of onychomycosis (OM) caused by Candida species in recent years, there is a lack of published literature. Candida onychomycosis (CO) is listed as a separate entity in the new classification and is subdivided into three categories: (1) Candida paronychia, (2) Candida granuloma, and (3) Candida onycholysis.[1,2] Virulence factors of Candida species associated with OM include adhesion, filamentation, and secretion of extracellular enzymes and the development of resistance to antifungals.[3,4] Due to the predominantly endogenous nature, ability to colonize and evident opportunism of yeasts, CO is mostly thought to be restricted to patients with chronic mucocutaneous candidosis (CMC), as secondary invaders in chronic paronychia or prevalent amongst elderly, immunodeficient, and diabetic patients.[3,4] But the significance of Candida in the pathogenesis of nail dystrophy needs to be reconsidered in some patients of nail dystrophy not associated with the aforesaid risk factors. Therefore, the present study was designed to determine the epidemiological and clinical pattern of CO, to identify the prevalent strains using culture and Vitek 2YST Compact (Biomeurix France) system, approved by FDA in 2006, and perform antifungal susceptibility testing (AFST) of the isolated species in vitro.
Materials and Methods
A total of 506 consecutive patients with a clinical suspicion of OM were included in a cross-sectional clinical study carried over a span of 2 years from November 2016 to October 2018 in the out-patient department of Dermatology, Venereology and Leprosy in a tertiary care hospital of Kashmir. Patients who had taken antifungals for their nail infection in the preceding 6 months, those with concurrent dermatoses like psoriasis or lichen planus, and those not willing to comply with the protocols of our study were excluded.
After an approval from Institutional Ethics Committee and an informed written consent from each patient, clinic-demographic data was collected, followed by general physical, systemic examination, and a detailed examination of the nail unit. Scrapings and nail clippings were collected from the deepest part of the nail. One part was subjected to direct microscopic examination (DME) in 10% KOH and another inoculated for duplicated culture on Sabouraud Dextrose Agar and chloramphenicol with and without cycloheximide. Chloramphenicol was added to reduce bacterial contamination making the medium selective for fungi while cycloheximide addition helps in isolation of dermatophytes. The colonies were identified by routine microbiological tests, colonial characterization, germ tube test, biochemical tests, and microscopic morphology on corn-meal agar with tween 80. To attribute OM to Candida infection or to differentiate colonization from true infection, a set of diagnostic criteria utilized by earlier studies were followed.[5]
Four criteria used in our study:
Identification of yeast in the nail samples by DME
Isolation of candida in culture
Exclusion of dermatophytes in culture
Repeated isolation of candida in culture.
The samples were further processed by automated isolate identification and sensitivity testing system (Vitek 2YST Compact, Biomeurix France) which has an identification probability of more than 95%. The system uses ID-YST card, designed for identification of yeast and yeast-like organisms and performs by biochemical analysis using the colorimetric method. Antifungal susceptibility was performed using amphotericin B (AMB), fluconazole (FLC), itraconazole (ITZ), flucytosine (5FC), voriconazole (VRC), and caspofungin (CSF), micafungin (MCF), and posaconazole (PSC). Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as the quality control strains for identification and antifungal susceptibility testing. The minimum inhibitory concentrations (MIC) and AFST were determined by broth microdilution method (BMD) as per Clinical and Laboratory Standards Institute (CLSI) guidelines. The interpretation of the sensitivity test results was categorized as susceptible (S), intermediate (I), resistant (R), sensitive dose dependent, or no interpretation.[6]
The compiled data was analyzed using descriptive statistics in MS Office and the categorical variables were summarized as frequencies and percentages.
Results
Out of 506 cases, 384 (75.88%) were found to be culture positive. Of these, dermatophytes were isolated in 223 (58.08%), yeast in 101 (26.30%), and NDM in 47 (12.24%) and mixed infection was seen in 13 (3.38%) of cases.
The mean age of patients with CO was 42.11 ± 8.30 years (range: 14–56). Male to female ratio was 1:1.2. Majority of the cases (47.5%) were in the age group of 21–40 years. Duration of disease ranged from 3 months to 3 years. Most of our patients (75.25%) belonged to rural background. Various predisposing factors included trauma (5), diabetes mellitus (5), concurrent chemotherapy (2), and HIV (1). Baseline data, occupational status, and clinical patterns observed in culture-positive cases of CO are shown in Table 1 [Figures 1 and 2].
Table 1.
Clinical and demographic data of patients with candidal onychomycosis (n=101)
| Characteristics | Number (n) | Percentage (%) |
|---|---|---|
| Mean age | 42.11±8.24 years | |
| Males/Females | 42/59 | 41.58%/58.41% |
| Residence | ||
| Rural/Urban | 76/25 | 75.25%/24.75% |
| Nail Involved | ||
| Fingernails | ||
| Single | 39 | 38.61% |
| Multiple | 11 | 10.89% |
| Toenails | ||
| Single | 35 | 34.66% |
| Multiple | 9 | 8.91% |
| Both | ||
| Single | 5 | 4.95% |
| Multiple | 2 | 1.98% |
| Occupation | ||
| Males | ||
| Farmers | 24 | 57.15% |
| Manual Laborers | 8 | 19.04% |
| Students | 5 | 11.90% |
| Office workers | 5 | 11.90% |
| Females | ||
| Housewives | 35 | 59.32% |
| Working Females | 13 | 22.04% |
| Students | 11 | 18.64% |
| Clinical Patterns | ||
| Paronychia with trachyonychia | 11 | 11.00% |
| Paronychia with DLSO | 24 | 24.00% |
| DLSO | 32 | 32.00% |
| TDO | 16 | 15.84% |
| Erosion of distal and lateral nail plate | 18 | 18.16% |
| Total | 101 | 100% |
Figure 1.
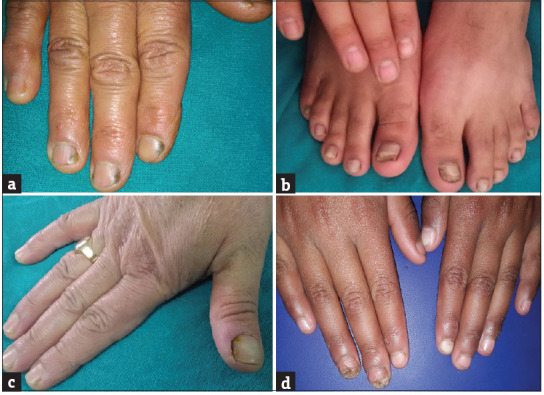
Distolateral onychomycosis. (a) Fingernail involvement, (b) toenail involvement, (c) associated paronychia, (d) tracyonychia
Figure 2.
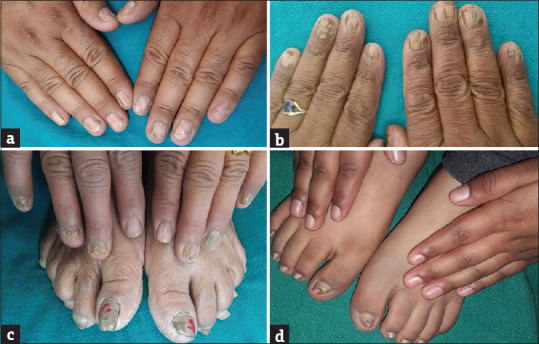
(a) Erosion of distal and lateral nail plates, (b) total dystrophic onychomycosis of all twenty nails, (c) fingernail dystrophy, (d) pincer nail deformity
Out of 101 isolates of Candida species, 60 cases (59.41%) were caused by C. albicans and rest 41 (40.59%) cases by non-albicans species. Species distribution of Candida on Vitek 2YST is highlighted in Table 2. Certain clinical presentations were more frequently associated with particular species. Table 3.
Table 2.
Susceptibility pattern of various candida isolates to antifungal drugs (n=101)
| Candida Spp. | C. albicans 59.41% (n=60) | C. krusei 24.39% (n=10) | C. parapsilosis 26.82% (n=11) | C. glabrata 17.07% (n=7) | C. tropicalis 12.19% (n=5) | C. lipolytica 7.31% (n=3) | C. guilliermondii 12.19% (n=5) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| Fluconazole | ||||||||
| S | 60 | 6 | 10 | 3 | 5 | 3 | 2 | 88(%) |
| I | 0 | 4 | 1 | 4 | 0 | 0 | 3 | 12(%) |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Amphotericin | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Flucytosine | ||||||||
| S | 60 | 8 | 11 | 5 | 5 | 3 | 3 | 95(%) |
| I | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 6(%) |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Micafungin | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Voriconazole | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Caspofungin | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Itraconazole | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| Posaconazole | ||||||||
| S | 60 | 10 | 11 | 7 | 5 | 3 | 5 | 100% |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
| R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 00 |
S: Susceptible, I: Intermediate, R: Resistant
Table 3.
Various morphological patterns associated with the type of candida species
| Clinical presentation | Number of patients | Type of species | ||||||
|---|---|---|---|---|---|---|---|---|
| C.alb (60) | C.Para (11) | C.krus (10) | C.glab (7) | C.guillr (5) | C.tropi (5) | C. lipo (3) | ||
| DLSO | 32 | 18 | 7 | - | 1 | 3 | 2 | 1 |
| Paronychia associated with trachyonychia | 11 | 7 | - | 2 | 2 | |||
| Paronychia associated with DLSO | 24 | 18 | 1 | 2 | 3 | |||
| TDO | 16 | 8 | 3 | - | - | 2 | 2 | 1 |
| Erosions of distal and lateral nail plate | 18 | 9 | - | 6 | 1 | - | 1 | 1 |
DLSO: distolateral onychomycosis, TDO: total dystrophic onychomycosis, C. alb: candida albicans, C.para: candida parapsilosis, C.krus: candida krusei, C.glab: candida glabrata, C. guill: candida guillermondii, C.tropi: candida tropicalis, C. lipo: candida lipolytica
Antifungal susceptibility testing results showed that most of C. albicans species exhibited 100% susceptibility to the antifungal drugs tested. Intermediate resistance to fluconazole and flucytosine was seen in some non-albicans species (C. krusei, C. glabrata, and C. guilliermondii). Species distribution and antifungal sensitivity pattern of various Candidal isolates on Vitek 2YST Compact are summarized in Table 2. Time taken for species identification after incubation for all species of candida was 14–18 h (average 15.5 h), while as the time taken for MIC determination was 9–27 h (average: 12–14 h).
Discussion
Although the primary cause for onychomycosis (OM) is dermatophytes, Candida species have emerged as second-line pathogens. Breach in local immunity at the nail complex due to trauma, chronic exposure to moisture, wet work, and chemicals including detergents, soaps, etc., are considered to be the main contributing factors to CO.[7] Most of our patients belonged to rural background (76%) and were involved in agricultural activities, especially paddy work. This would require an average of 6 to 8 h of constant immersion of hands and feet into water, exposure to mud, cowdung, and wastewater, predisposing them to various fungal infections. Cohen et al. pointed out in their study that increased exposure to plants, animals, and soil in agricultural workers and laborers, while as the continued exposure to wet work in housewives leading to macerated cuticles could serve as a gateway for nail infections.[7] CO has been increasingly reported in individuals with defective immunity consequent to aging, diabetes mellitus, vascular diseases, HIV infection and drug therapies such as immunosuppressives and broad-spectrum antibiotics.[3,4,8] However, we did not find any such association as the majority of our CO patients were without underlying immunosuppression.
Bokhari et al.[9] observed a female preponderance in their study, citing chronic exposure to water in housewives, harboring of organism in their intestine or vagina and greater cosmetic concern in females as the basis for this observed difference, whereas Elewski[10] mentioned male gender as a general risk factor for onychomycosis. No male or female predominance was found in our study. Fingernails have been shown to be affected more than toenails in various studies.[4,5,7] Gupta et al.[11] and Venugopal and Venugopal[12] reported an exceptionally high incidence of CO of toenails and suggested that this phenomenon might be related to the Muslim religious practice of washing feet five times a day, use of occlusive footwear associated with increased perspiration and trauma. However, our study could not relate to any such findings.
The percentage of Candida isolated in 26.05% of OM patients in our study is similar to the findings of Gianni et al. (25.55%).[13] Shenoy et al.[14] isolated Candida in only 6%. In the studies conducted by Bokhari et al., Gupta et al., and Foster et al.,[9,11,15] Candida was the most common pathogen in 46%, 40.8%, and 40.4% cases, respectively, which is much higher in comparison to our study. The clinical spectrum of nail disease caused by Candida is usually indistinguishable from that of dermatophytes. Recent trends have shown a change in the epidemiological behavior of this disease whereby emergence of less frequent species such as C. parapsilosis, C. tropicalis, C. krusei, and C. glabrata acting as etiological agents in these processes has been observed.[5,6,16,17,18] However, like most of the previous studies, we observed a higher prevalence of C. albicans, followed by C. krusei and C. tropicalis.[4,10,12,15]
There are a small number of studies focused on MIC determination for antifungal agents against Candida spp. that are responsible for nail infection.[5,6,19] Methods for testing susceptibility have been standardized in order to make the determination of resistance more efficient, to have better guidance for the treatment and, consequently, to have more effective clinical outcomes.[19,20] We performed in vitro susceptibility tests with fluconazole, itraconazole, voriconazole, caspofungin, amphotericin B, flucytosine, micafungin, and posaconazole employing Clinical and Laboratory Standards Institute broth microdilution method. Using this method, the determination of MICs for all drugs after 24 h of incubation was feasible. C. albicans isolates showed 100% susceptibility to all of the antifungals tested, whereas dose-dependent sensitivity was observed in some non-albicans species. Our findings correlated with those observed by Kashid et al. and Shivanand et al. in their studies conducted on the susceptibility pattern of Candida isolates in different clinical specimens.[21,22]
Limitations
Histological evidence of Candida in tissues using PAS staining to differentiate colonization from true infection could not be carried out due to financial constraints. Evaluation of VITEK 2 yeast susceptibility test was carried out without a reference method.
Conclusion
Our study showcases the present scenario of Candida distribution and the resistance patterns of various species afflicting the nail unit. With the change in the pattern of Candida species causing OM, identification of the exact fungal species and knowledge of the MIC values of various antifungals become mandatory as they have a significant impact in the disease management. Furthermore, our findings clearly indicate that the carriage of this pathogenic yeast is not only associated with immunosuppression as it can be isolated from healthy individuals as well.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998;139:567–71. doi: 10.1046/j.1365-2133.1998.02449.x. [DOI] [PubMed] [Google Scholar]
- 2.Jayatilake JA, Tilakaratne WM, Panagoda GJ. Candidal onychomycosis: A mini-review. Mycopathologia. 2009;168:165–73. doi: 10.1007/s11046-009-9212-x. [DOI] [PubMed] [Google Scholar]
- 3.Hay RJ, Baran R, Moore MK, Wilkinson JD. Candida onychomycosis-an evaluation of the role of Candida species in nail disease. Br J Dermatol. 1988;118:47–58. doi: 10.1111/j.1365-2133.1988.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 4.Ellabib MS, Agaj M, Khalifa Z, Kavanagh K. Yeasts of the genus Candida are the dominant cause of onychomycosis in Libyan women but not men: Results of a 2-year surveillance study. Br J Dermatol. 2002;146:1038–41. doi: 10.1046/j.1365-2133.2002.04688.x. [DOI] [PubMed] [Google Scholar]
- 5.Ding CH, Rahman MM, Tzar MN, Yusoff H, Satim H. Non-dermatophyte moulds and yeasts as agents of onychomycosis in a Malaysian medical centre. Bangladesh J Med Sci. 2017;16:380–3. [Google Scholar]
- 6.Figueiredo VT, de Assis Santos D, Resende MA, Hamdan JS. Identification and in vitro antifungal susceptibility testing of 200 clinical isolates of Candida spp. responsible for fingernail infections. Mycopathologica. 2007;164:27–33. doi: 10.1007/s11046-007-9027-6. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JL, Scher RK, Pappert AS. The nail and fungus infections. In: Elewski B, editor. Cutaneous Fungal Infections. New York: Lgaku-Shoin; 1992. pp. 106–22. [Google Scholar]
- 8.Gupta AK, Jain HC, Lynde CW, MacDonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244–8. doi: 10.1067/mjd.2000.104794. [DOI] [PubMed] [Google Scholar]
- 9.Bokhari MA, Hussain I, Jahangir M, Haroon TS, Aman S, Khurshid K. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591–5. doi: 10.1046/j.1365-4362.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- 10.Elewski BE. Onychomycosis: Pathogenesis, diagnosis, and management. Clinl Microbiol Rev. 1998;11:415–29. doi: 10.1128/cmr.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta M, Sharma NL, Kanga AK, Mahajan VK, Tegta GR. Onychomycosis: Clinico-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389–92. doi: 10.4103/0378-6323.37055. [DOI] [PubMed] [Google Scholar]
- 12.Venugopal PV, Venugopal TV. Superficial mycoses in Saudi Arabia. Australas J Dermatol. 1992;33:45–8. doi: 10.1111/j.1440-0960.1992.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 13.Gianni C, Morelli V, Cerri A, Greco C, Rossini P, Guiducci A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283–8. doi: 10.1159/000051659. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy MM, Teerthanath S, Vimal KK, Girisha BS, Krishna Prasad MS, Pinto J. Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid-Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol. 2008;74:226–9. doi: 10.4103/0378-6323.39584. [DOI] [PubMed] [Google Scholar]
- 15.Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748–52. doi: 10.1016/s0190-9622(03)02117-0. [DOI] [PubMed] [Google Scholar]
- 16.Kwok YK, Thai YK, Goh CL, Kamarudin A, Koh MT, Seow CS. Epidemiology and in vitro activity of antimycotics against candida vaginal/skin/nail infections in Singapore. Int J Dermatol. 1998;37:145–9. doi: 10.1046/j.1365-4362.1998.00038.x. [DOI] [PubMed] [Google Scholar]
- 17.Fich F, Abarzua-Araya A, Pierez M, Nauhm Y, Leon E. Candida parapsilosis and candida guillermondi: Emerging pathogens in nail candidiasis. Indian J Dermatol. 2014;59:24–9. doi: 10.4103/0019-5154.123485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilkit M. Onychomycosisin Adana, Turkey: A 5-year study. Int J Dermatol. 2005;44:851–4. doi: 10.1111/j.1365-4632.2005.02265.x. [DOI] [PubMed] [Google Scholar]
- 19.Posteraro B, Martucci R, Sorda ML, Fiori B, Sanglard D, Carolis ED, et al. Reliability of the Vitek 2 yeast susceptibility test for detection of in vitro resistance to fluconazole and voriconazole in clinical isolates of Candida albicans and Candida glabrata. J Clin Microbiol. 2009;47:1927–30. doi: 10.1128/JCM.02070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanguinetti M, Porta R, Sali M, Sorda ML, Pecorini G, Fadda G, et al. Evaluation of VITEK 2 and RapID yeast plus systems for yeast species identification: Experience at a large clinical microbiology laboratory. J Clin Microbiol. 2007;45:1343–6. doi: 10.1128/JCM.02469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashid RA, Belawadi S, Devi G, Indumati Characterization and antifungal susceptibility testing for Candida species in a tertiary care hospital. J Res Health Sci. 2011;2:1–7. [Google Scholar]
- 22.Shivanand D, Dominic S. Species identification of Candida isolates in various clinical specimens with their anti-fungal susceptibility patterns. J Clin Diagn Res. 2011;5:1177–81. [Google Scholar]


