Abstract
Objectives:
To evaluate and compare the efficacy of MMR vaccine and MIP vaccine for resolution of Cutaneous warts (Cw).
Methods:
The hospital-based prospective randomized interventional study was done where a total of 60 patients of Cw were divided into two groups of 30 patients each: Group A received 0.1 ml of intralesional injection of MIP vaccine and Group B received 0.5 ml of MMR vaccine. The treatment protocol involved three intralesional injection of vaccines at intervals of 3 weeks (maximum of three injections). The follow-up was done every 4 weeks for at least 24 weeks for the comparison of the two groups. The primary outcomes were the decrease in size of the wart or clearance of primary warts. The secondary outcomes were the improvement in the distant warts and any complications related to the use of vaccines. The data were entered in MS Excel and analyzed using SPSS 17.0 version. A P value of <0.05 was considered statistically significant.
Results:
The baseline demographic and wart characteristics were comparable between the two groups (P > 0.05). As compared to MMR, MIP showed an early (9.41 vs 11.71 weeks, P = 0.027), and a significantly higher complete response (90% vs 76.67%) with P < 0.05. The less duration of the warts was significantly associated with the higher complete response (P < 0.05) in both the groups. The common side effects were erythema/inflammation [19 (63.34%)] in Group A and pain during the injection [19 (63.34%)] in Group B with P < 0.0001.
Conclusion:
In conclusion, MIP intralesional injections have a quicker response and are more efficacious compared to MMR in the treatment of Cw, though each vaccine carries its own sets of side effects.
Key Words: Cutaneous warts, immunotherapy, MIP, MMR
Introduction
Cutaneous warts (Cw) are hyperkeratotic benign papillomas, which are caused by the human papilloma virus (HPV) infection. The prevalence rate among adults varies from 1.2% (reported in UK) to 3.5% (reported in the USA). The usual sites involved are the hands and feet.[1]
Cw may resolve spontaneously in 15%–63% of the cases.[2,3,4,5,6] Age, stress, sleep quality, socio-economic status, and immune system of an individual are the factors affecting the spontaneous resolution.[1,7]
The mechanism of Cw primarily involves derangement of the immune mechanisms, that is, inhibition of helper T cells stimulated release of interleukins, which is similar to various viruses such as human immunodeficiency virus (HIV) and HTLV-4.[8,9,10]
Thus, enhancing the immune system to clear HPV and achieve a long-lasting effective response against Cw is an active field of exploration.[11]
Recently, intralesional vaccines (BCG vaccine, measles, mumps, rubella virus or MMR vaccine, and Mycobacterium w vaccine) have been tried for the treatment of common warts with promising results.[8,11,12,13,14,15,16] Their mechanism is based on the local stimulation of cell-mediated and humoral immunity using different antigens to clear HPV and the host's infected cells.[17,18] However, selection among existing immunotherapeutic modalities remains challenging as none of them have proven 100% efficacious.
Literature review of the primary researches[8,12,14,15,16] and a meta-analysis[18] shows that the MMR vaccine is one of the most effective modalities for Cw clearance at primary and distant sites. Though BCG vaccine (live attenuated) has also been found to be equally effective as MMR,[8] lately the use of Mycobacterium indicus pranii (MIP or Mw) vaccine has been found to be more promising due to its strong antigenic nature.[11,12] It differs from the BCG vaccine as it is a killed vaccine derived from atypical Mycobacterium. Its effectiveness for boosting immunity has been confirmed in diseases such as leprosy, HIV infection, and tuberculosis.[19] It was developed at the National Institute of Immunology, New Delhi, India, and clinical trials by Singh S et al.,[12] and Gupta S et al.,[11] have shown its efficacy on Cw and anogenital warts.
To date, no randomized comparative trial has been done on MMR and MIP vaccines. We, in this study, aimed to evaluate and compare the efficacy of MMR and MIP vaccines for resolution of Cw.
Methods
The hospital-based prospective randomized interventional study was done in the Department of Dermatology, Venereology and Leprology at a medical college cum hospital in Faridkot from June 2018 to June 2019. Informed consent was taken from the patients with Cw who were included in this study as per the following criteria.
Inclusion criteria
Patients aged between 18 and 65 years of both sexes.
Patients diagnosed with multiple Cw (>2) based on clinical examination.
Exclusion criteria
Immunocompromised patients or patients on steroids or other immunosuppressive drugs.
Pregnant or lactating females.
Patients with fever or signs of any inflammation or infection, meningitis, and convulsions.
Patients with any history of asthma, allergic skin disorders, hypersensitivity, or allergy to vaccines.
Patients with any history of concurrent systemic, or topical treatment of warts within the past 4 weeks.
Patients with genital/mucosal warts.
The sample size was calculated based on the study of Chauhan et al.,[13] who observed that 48% of patients got complete clearance at 4 weeks after dose after MMR vaccine. Taking these values as a reference and assuming a difference of 35% in complete clearance between MMR vaccine and MIP vaccine, the minimum required sample size with 80% power of study and 5% level of significance is 26 patients in each study group. To reduce the margin of error, the total sample size taken was 60 (30 patients per group).
Demographic and clinical details for the number and size of warts, duration, and sites involved were recorded. Photographic records were made prior to treatment (at baseline) and at each subsequent visit. No other treatment for warts was allowed for concurrent use. The study was approved by the Institutional Protocol Review Board and Institutional Ethics Committee.
Randomization
A total of 60 patients were divided into two groups of 30 patients each. Patients with odd last digit of OPD slip were placed in Group A (MIP vaccine) and those with even last digit of OPD slip were placed in Group B (MMR vaccine) after matching for age (± 5 years) and sex. There was no concealment or blinding of the treatment. The study design flow has been shown as a CONSORT diagram [Figure 1].
Figure 1.
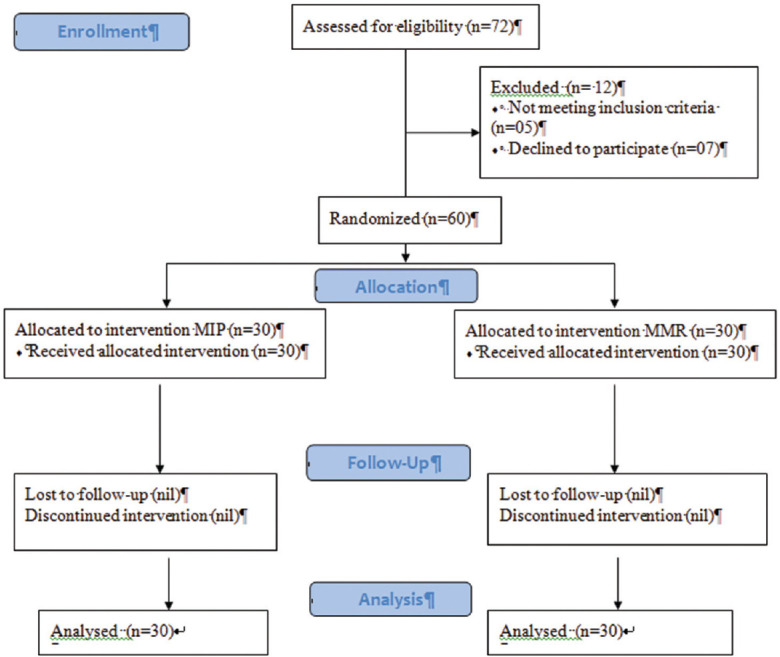
Participant flow algorithm
Treatment protocol and outcome evaluation
MIP is available commercially as a multidose vial of 0.5 ml containing 500 million heat-killed bacilli, in a buffered solution with thiomersal as preservative. MMR vaccine is available commercially as live attenuated, single dose vial of freeze-dried vaccine. It was reconstituted with 0.5 ml of diluent (water for injections). Both vaccines were stored at 2–8°C temperature and a cold chain was maintained.
Group A
They received 0.1 ml of intralesional injection of MIP (Mw) vaccine using an insulin syringe at the base of single Cw. In case of multiple warts, intralesional injections were given in the largest wart and the injections were repeated at the same site.
Group B
They received 0.5 ml of intralesional injection of reconstituted MMR vaccine using an insulin syringe at the base of Cw or into 2–3 largest warts in case of multiple warts.
In both the groups, intralesional injections were repeated at intervals of 3 weeks, until complete clearance of all the warts or maximum of three injections. The unused vaccine was discarded.
After the completion of the treatment, patients were followed up every 4 weeks for at least 24 weeks. The size of warts was measured with Vernier caliper. Grades of improvement were decided based on the decrease in size and number of the lesions.
It was graded as
G0 = no reduction in size
G1 = <25% reduction in size
G2 = 26%–50% reduction in size
G3 = 51%–75% reduction in size
G4 = >76% reduction in size.
The patients were evaluated clinically and by comparing with baseline clinical photographic records at each treatment session for resolution of the treated wart and distant warts, reduced size and number of warts, and any immediate or late adverse effects, if any.
The primary outcomes were the decrease in size of the wart or clearance of primary warts. The secondary outcomes were the improvement in the distant warts and any complications related to the use of vaccines.
Statistical evaluation
The data were entered in MS Excel and analyzed using SPSS 17.0 version. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. Qualitative variables were compared using Chi-square test. A P value <0.05 was considered as statistically significant.
Results
The baseline demographic characteristics were comparable between the two groups as shown in Table 1. In Group A, the mean age was 28.87 years, with 50% males and 50% females. Among them, 56.67% were married and 43.33% were unmarried; 83.33% resided in urban areas and 16.67% in rural areas. Professionally, 13.33% were farmers, 33.34% students, 30% TP, and 23.33% other professionals.
Table 1.
Demographic comparison of the two groups
| Demographic characteristics | Group A (n=30) | Group B (n=30) | Test performed | P |
|---|---|---|---|---|
| Age group (years) | ||||
| ≤ 20 | 7 (23.33%) | 6 (20%) | Chi-square test; 0.288 | 0.962 |
| 21-30 | 11 (36.67%) | 12 (40%) | ||
| 31-40 | 7 (23.33%) | 6 (20%) | ||
| 41-50 | 5 (16.67%) | 6 (20%) | ||
| Mean±SD | 28.87±9.17 | 29.43±10.21 | Independent t-test; 0.224 | 0.824 |
| Gender | ||||
| Males | 15 (50%) | 16 (53.33%) | Chi-square test; 0.067 | 0.7957 |
| Females | 15 (50%) | 14 (46.67%) | ||
| Marital status | ||||
| Married | 17 (56.67%) | 16 (53.33%) | Chi-square test; 0.067 | 0.7657 |
| Unmarried | 13 (43.33%) | 14 (46.67%) | ||
| Profession | ||||
| Farmer | 4 (13.33%) | 4 (13.33%) | Chi-square test; 0.5 | 0.918 |
| Other profession | 7 (23.33%) | 9 (30%) | ||
| Student | 10 (33.34%) | 10 (33.34%) | ||
| TP | 9 (30%) | 7 (23.33%) | ||
| Residence | ||||
| Rural | 5 (16.67%) | 7 (23.33%) | Chi-square test; 0.417 | 0.518 |
| Urban | 25 (83.33%) | 23 (76.67%) |
In Group B, the mean age was 29.43 years, with 53.33% males and 46.67% females. Among them, 53.33% were married and 46.67% were unmarried; 76.67% resided in urban areas and 23.33% in rural areas. Professionally, 13.33% were farmers, 33.34% students, 23.33% TP, and 30% other professionals.
Compared to Group B, Group A patients had significantly more number of warts (8 vs 2, P = 0.0001); comparable mean size of warts (5.37 vs 6.13, P = 0.155); comparable symptoms such as I (26.7% vs 26.7%), I, P (16.67% vs 20%), NI (53.33% vs 43.33%), and P (3.33% vs 0%); comparable duration (11.3 ± 6.02 vs 12.32 ± 5.94 months, P = 0.512) and comparable site of warts such as foot (40% vs 53.33%), H/F (13.33% vs 6.67%), hand (30% vs 23.33%), and others (16.67% vs 16.67%) (P > 0.05) [Table 2].
Table 2.
Comparison of warts characteristics of the two groups
| Characteristics of warts | Group A(n=30) | Group B(n=30) | Test performed | P |
|---|---|---|---|---|
| Warts | ||||
| Median(25th-75th percentile) | 8(6-14) | 2(1-5) | Mann Whitney test;183.5 | 0.0001 |
| Size of warts | ||||
| Mean ± SD | 5.37 ± 2.04 | 6.13 ± 2.05 | Independent t test;1.439 | 0.155 |
| Symptom | ||||
| I | 8(26.7%) | 8(26.7%) | ||
| I, P | 5(16.67%) | 6(20%) | Chi- square test;1.401 | 0.73 |
| NI | 16(53.33%) | 13(43.33%) | ||
| P | 1(3.33%) | 3(10%) | ||
| Duration of warts(months) | ||||
| Mean ± SD | 11.3 ± 6.02 | 12.32 ± 5.94 | t test;0.659 | 0.512 |
| Site | ||||
| Foot | 12(40%) | 16(53.33%) | ||
| H/F | 4(13.33%) | 2(6.67%) | Chi- square test;1.488 | 0.694 |
| Hand | 9(30%) | 7(23.33%) | ||
| Others | 5(16.67%) | 5(16.67%) |
I: Itching, P: Pain, NI: No symptoms (nil)
The adverse effects of the two interventions were significantly different as shown in Figure 2. The patients undergoing MIP treatment in Group A experienced erythema/inflammation in 19 (63.34%) patients, pain/erythema in 3 (10%), and abscess and pain in 1 (3.33%) patient each. No adverse event was seen in 6 (20%) patients. A representative photo of the abscess in a patient treated with MIP is shown in Figure 3.
Figure 2.
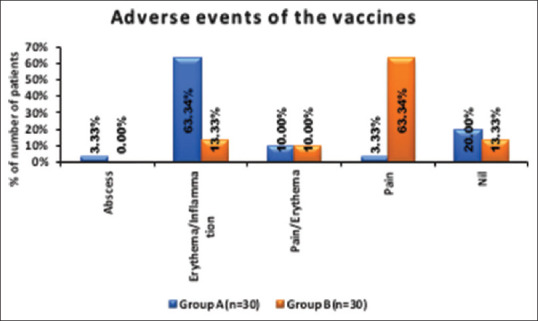
Adverse effects of the vaccines
Figure 3.
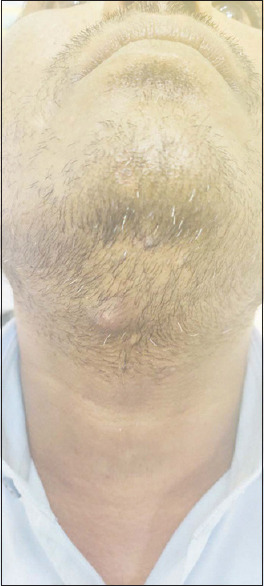
A case of an abscess formation after MIP vaccine injection at 2 weeks
The patients undergoing MMR treatment in Group B experienced pain in 19 (63.34%) patients, erythema/inflammation in 4 (13.33%) patients, and pain/erythema in 3 (10%) patients.
In Group A, the MIP vaccine effect was visible even at the time of the second dose infusion itself where none of the patients had G0 (no improvement) as compared with the MMR vaccine where 7 (23.33%) had G0 (P < 0.0001). In Group A, The maximal effects of MIP vaccine (G4) had been attained among 13 (43.33%) patients at the time of the third dose infusion as compared with 6 (20%) among those with MMR vaccine (P = 0.003). The treatment outcome for a patient with the MIP vaccine has been shown in Figure 4a-c.
Figure 4.
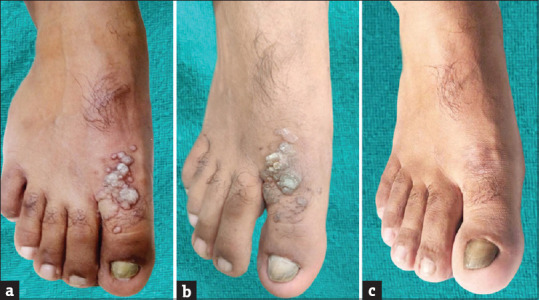
Treatment response of Cw with Mw vaccine (a) at the time of the first injection, (b) at the time of the second injection, (c) at 16 weeks after the third dose
After the complete course of three doses, at the first follow-up visit (4 weeks after the last dose), 80% of the patients had achieved G4 improvement with MIP and only 36.67% had achieved G4 improvement with MMR (P = 0.003). Thereafter in Group A, there was continuous improvement in the follow-ups with the maximal of 90% patients achieving G4 improvement and 10% achieving G3 improvement by the end of 16 weeks after the last dose, further to which no improvement was seen till 24 weeks of follow-up (P < 0.0001); whereas in Group B, though there was a continuous improvement in the follow-ups with the maximal of 76.67% patients achieving G4 improvement, 10% achieving G3 improvement, 6.67% achieving G2, and 6.67% having no improvement by the end of 20 or 24 weeks after the last dose (P < 0.0001), the overall response was slow and less as compared with Group A. The treatment outcome for a patient with MMR vaccine has been shown in Figure 5a-c.
Figure 5.
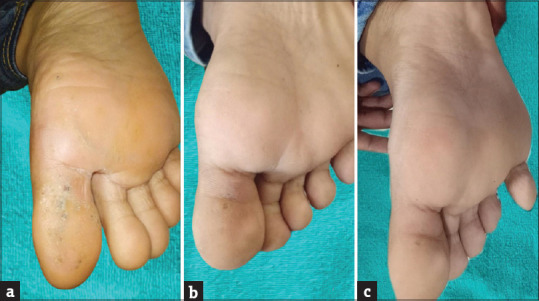
Treatment response of Cw with MMR vaccine (a) at the time of the first injection, (b) at the time of the third injection, (c) at 20 weeks after the third dose
The mean duration to show complete response in wart resolution was 9.41 weeks with MIP and 11.74 weeks with MMR; with a statistical significant difference between them (P = 0.027). However, the effects of the vaccines in the primary and distant warts were comparable with both the treatments [Table 3].
Table 3.
Comparison of the Treatment response for Cw
| Treatment response | Group A (n=30) | Group B (n=30) | Test performed | P |
|---|---|---|---|---|
| Time for complete response (in weeks) | ||||
| 6 | 12 (40%) | 6 (20%) | Chi-square test; 6.378 | 0.195 |
| 10 | 8 (26.7%) | 5 (16.67%) | ||
| 14 | 6 (20%) | 8 (26.7%) | ||
| 18 | 1 (3.33%) | 4 (13.33%) | ||
| Not complete response | 3 (10%) | 7 (23.33%) | ||
| Mean±SD | 9.41±3.63 | 11.74±4.32 | Independent t-test; 2.262 | 0.027 |
| Effect on wart | ||||
| Primary wart | ||||
| Yes | 30 (100%) | 28 (93.33%) | Chi-square test; 2.069 | 0.492 |
| No | 0 (0%) | 2 (6.67%) | ||
| Distant wart | ||||
| Yes | 29 (96.67%) | 25 (83.33%) | Chi-square test; 2.82 | 0.195 |
| No | 1 (3.33%) | 5 (16.67%) |
Overall, the treatment effects and the responses with both interventions showed significant improvements with both the vaccines during various patient visits and follow-ups.
Among the various demographic (age, gender) and wart-related factors (wart number, size, and duration), duration of the warts showed significant association with complete response (11.33 ± 6.35 months in complete response vs 14.2 ± 2.25 months in partial response, P = 0.016) as shown in Table 4.
Table 4.
Association of parameters with the response
| Parameters | Complete response (n=50) | Incomplete response (n=10) | Total | P | Test performed |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean±SD | 28.84±9.83 | 30.7±8.84 | 29.15±9.63 | 0.581 | t-test; 0.554 |
| Gender | |||||
| Female | 25 (50%) | 4 (40%) | 29 (48.33%) | 0.732 | Fisher’s exact test |
| Male | 25 (50%) | 6 (60%) | 31 (51.67%) | ||
| Duration of warts (months) | |||||
| Mean±SD | 11.33±6.35 | 14.2±2.25 | 11.81±5.95 | 0.016 | t-test; 2.505 |
| Number of warts | |||||
| Mean±SD | 10.04±12.04 | 10.5±17.91 | 10.12±13.01 | 0.919 | t-test; 0.101 |
| Size of warts (mm) | |||||
| Mean±SD | 5.78±2.16 | 5.59±1.55 | 5.75±2.06 | 0.794 | t-test; 0.261 |
Discussion
The treatment of Cw has been debated for a long time. Destructive therapies like electrocautery, cryotherapy and many are ineffective for distant lesions and often need multiple, often painful sessions with a risk of scarring. Evidence showing that cellular immune responses play a critical role in wart clearance has inspired the development of topical and intralesional immunotherapy regimens for patients with multiple and/or persistent warts. To the best of our knowledge, this is the only study comparing the efficacy and safety of MIP versus MMR vaccine as immunotherapy in the treatment of Cw.
Both the study groups were comparable in respect to baseline patient demographic characteristics and wart-related characteristics like number, size, location, duration, and symptoms associated with wart which eliminated any bias.
MIP vaccine
MIP vaccine appears to be an efficacious and safe treatment modality. In the present study, MIP vaccine effect was visible even at the time of the second dose infusion itself with a mean duration of 9.41 weeks to show complete response in wart resolution. Various previous studies have given reported clearance time of 9.7, 5.9, and 6.75 weeks.[11,12,20] All these studies gave weekly to fortnightly injections following a sensitization dose of 0.1 ml at each deltoid. In a study by Garg and Baveja where no sensitization dose was given, the clearance rate was higher (93.33%), which was similar to our study.[14]
With MIP vaccine, there was continuous improvement in the follow-ups; with 90% of patients achieving G4 improvement by 16 weeks after the last dose, further to which no improvement was seen till 24 weeks of follow-up. Previous studies reported a complete clearance of 33 (83%) patients of Cw,[20] and 88.9% patients of anogenital warts.[11]
MIP therapy was relatively well tolerated with minor side effects like erythema, abscess, and pain, and no systemic adverse event. The side effects reported among previous studies were ulcer and scar at the deltoid region; erythema, swelling, and superficial ulceration at the site of the warts; low-grade fever and tenderness and swelling of the submandibular lymph node.[11,12,20] In our study, as no sensitization dose was given, no side effects like nodule and scar formation were seen at the deltoid region.
Since most of the Indian population is vaccinated with BCG and has a latent infection with M.tb, (which is similar to MIP), a sensitization dose seems unnecessary which can avoid local complications at the deltoid region and limit the amount of vaccine injected to be 0.1 ml only. Through previous literature[8,14] and our study, we advocate avoiding any sensitization dose in the Indian population. However, internationally, MIP vaccination may cause a positive Mantoux reaction leading to a false impression of TB infection-causing diagnostic dilemma and sometimes unnecessary treatment; thus MMR is considered more appropriate immunotherapy treatment for Cw outside India.[18]
MMR vaccine
With MMR vaccine[Figure 4], the mean duration to show complete response in wart resolution was 11.74 weeks. Among all, 76.67% of patients achieved G4 improvement but 6.67% had G0 improvement by the end of 20 or 24 weeks after the last dose (P < 0.0001), the overall response being slow and less compared to Group A. As compared, Nofal and Nofal[2] reported cure rates of 81.4% of patients as compared with 27.5% in the placebo group with intralesional MMR vaccine and antigens. Similar results were also reported by Mohamad et al.[21] and Zamanian et al.[22] separately observing complete clearance in 82%, partial response in 6%, and no response in 12% patients of plantar warts, and complete cure of common warts in 75%, relative cure in 16.66% and no cure in 8.33% patients, respectively. Saini et al.[23] reported >75% improvement in only 49.43% patients, where 26.44% patients had a complete resolution from MMR immunotherapy.
The results have been variable but comparable with our study because there is no consensus for a minimum dose of MMR vaccine, dosing frequency, and duration of therapy to treat warts.[15,22,24] Invariably, three to six doses of 0.1–0.5 mL administered at intervals of 2‒3 weeks have been used with the varied outcome.
Also, The response rate in the present study (76.67% G4 improvement) was higher than that reported by Kus et al.[25] (29.4%) and Clifton and others[26] (47%), who used intralesional antigen immunotherapy (tuberculin and mumps or Candida, respectively) for the treatment of recalcitrant non-genital warts in open-label trials. The higher response rate in our study may have been caused by different antigens in MMR, which makes the probability of sensitivity to the injected antigen very high. In addition, live vaccines such as MMR are more immunogenic than skin test antigens such as mumps, Candida, and tuberculin.
The patients undergoing MMR treatment-experienced pain, erythema/inflammation without any systemic symptoms. The similar side effects have been seen in the studies by Singh P et al.,[12] Na CH et al.,[15] and Nofal A et al.[16]
MIP versus MMR
Both MIP and MMR local injections may hold significant advantages to the patients such as (1) inexpensive, (2) showing good response in less visits of the primary and distant noninjected warts, (3) less side effects, (4) allowing early resumption of normal daily activities, and (5) to be free of residual scars.
As compared with MMR, MIP showed an early (9.41 vs 11.71 weeks), and a significantly higher response (90% G4 vs 76.67% G4) with P < 0.05.The similar response of both the distant and the target warts in the MIP-treated group with the less favorable response of the former in the MMR-treated group points to a less systemic immune response induced by MMR. It implies that MMR therapeutic modality is better to be used in treating single warts or to be injected in each wart. These results were against our expectations of a better response of distant warts with MMR treatment, which, being composed of more than one antigen, was anticipated to impose a better systemic immunostimulant effect. In addition, it must be stressed here that MIP being a killed vaccine can also be advantageous to population who are immunocompromised or where live vaccines is contraindicated.
The adverse effects of the two vaccines were also significantly different (P < 0.05); erythema/inflammation being most common with MIP (63.34%) and pain during injection as most common with MMR (63.34%). The painful injections of MMR carry an additional disadvantage which may be due to the different nature of the vaccine. This has also been confirmed in other studies.[2,3,8]
Limitations of the Study
No follow-up was done for recurrences.
Placebo group was not taken.
Immunotherapy involves long follow-ups and thus may not be an acceptable modality for patients who want a quick response.
HPV serotyping was not done in the study.
Relevant Th1 cytokine profile and antibody titers were not done.
Conclusion
MIP intralesional injections have a quicker response and are more efficacious compared to MMR in the treatment of Cw, though each vaccine carries its own sets of side effects.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liu J, Li H, Yang F, Ren Y, Xia T, Zhao Z, et al. Epidemiology and clinical profile of cutaneous warts in Chinese college students: A cross-sectional and follow-up study. Sci Rep. 2018;8:15450. doi: 10.1038/s41598-018-33511-x. doi: 10.1038/s41598.018.33511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166–70. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 3.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with Mumps, Candida and Trichophytin skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson L, Leijonhufvud I, Aronsson A, Mossberg AK, Svanborg C. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. N Engl J Med. 2004;350:2663–72. doi: 10.1056/NEJMoa032454. [DOI] [PubMed] [Google Scholar]
- 5.Varnavides CK, Henderson CA, Cunliffe WJ. Intralesional interferon: Ineffective in common viral warts. J Dermatol Treat. 1997;8:169–72. [Google Scholar]
- 6.Bruggink SC, Gussekloo J, Berger MY, Zaaijer K, Assendelft WJ, de Waal MW, et al. Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: Randomized controlled trial. CMAJ. 2010;182:1624–30. doi: 10.1503/cmaj.092194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006:24:S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen MA, Salem SA, Fouad DA, El-Fatah AA. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194–200. doi: 10.1111/dth.12230. [DOI] [PubMed] [Google Scholar]
- 9.Abud-Mendoza C, Cuevas-Orta E, Santillán-Guerrero EN, Martínez-Martínez MU, Hernández-Castro B, Estrada-Capetillo L, et al. Decreased blood levels of B lymphocytes and NK cells in patients with systemic lupus erythematosus (SLE) infected with papillomavirus (HPV) Arch Dermatol Res. 2013;305:117–23. doi: 10.1007/s00403-012-1258-9. [DOI] [PubMed] [Google Scholar]
- 10.Chow LT, Broker TR. Human papillomavirus infections: Warts or cancer? Cold Spring Harb Perspect Biol. 2013;5:a012997. doi: 10.1101/cshperspect.a012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80:509–14. doi: 10.4103/0378-6323.144145. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan PS, Mahajan VK, Mehta KS, Rawat R, Sharma V. The efficacy and safety of intralesional immunotherapy with measles, mumps, rubella virus vaccine for the treatment of common warts in adults. Indian Dermatol Online J. 2019;10:19–26. doi: 10.4103/idoj.IDOJ_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203–8. doi: 10.4103/0974-2077.150740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na CH, Choi H, Song SH, Kim MS, Shin BS. Two-year experience of using the measles, mumps and rubella vaccine as intralesional immunotherapy for warts. Clin Exp Dermatol. 2014;39:583–9. doi: 10.1111/ced.12369. [DOI] [PubMed] [Google Scholar]
- 16.Nofal A, Nofal E, Yosef A, Nofal H. Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: A promising approach. Int J Dermatol. 2015;54:667–71. doi: 10.1111/ijd.12480. [DOI] [PubMed] [Google Scholar]
- 17.Rezai MS, Ghasempouri H, Asqary Marzidareh O, Yazdani Cherati J, Rahmatpour Rokni G. Intralesional injection of the Measles-Mumps-Rubella vaccine into resistant palmoplantar warts: A randomized controlled trial. Iran J Med Sci. 2019;44:10–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Salman S, Ahmed MS, Ibrahim AM, Mattar OM, El-Shirbiny H, Sarsik S, et al. Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J Am Acad Dermatol. 2019;80:922–30.e4. doi: 10.1016/j.jaad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Zaheer SA, Mukherjee R, Ramkumar B, Misra RS, Sharma AK, Kar HK, et al. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167:401–10. doi: 10.1093/infdis/167.2.401. [DOI] [PubMed] [Google Scholar]
- 20.Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol. 2013;149:237–9. doi: 10.1001/jamadermatol.2013.866. [DOI] [PubMed] [Google Scholar]
- 21.Mohamad NS, Badran F, Yakout E. Evaluation of the efficacy of a combination – Measles, mumps and rubella vaccine in the treatment of plantar warts. Our Dermatol Online. 2013;4:463–7. [Google Scholar]
- 22.Zamanian A, Mobasher P, Jazi GA. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomed Res. 2014;3:107. doi: 10.4103/2277-9175.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saini S, Dogra N, Dogra D. A prospective randomized open label comparative study of efficacy and safety of intralesional measles, mumps, rubella vaccine versus 100% trichloroacetic acid application in the treatment of common warts. Int J ResMed Sci. 2016;4:1529–33. [Google Scholar]
- 24.Gonçalves MA, Donadi EA. Immune cellular response to HPV: Current concepts. Braz J Infect Dis. 2004;8:1–9. [PubMed] [Google Scholar]
- 25.Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515–6. doi: 10.1111/j.1468-3083.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- 26.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]


