Abstract
Numerous studies have implicated involvement of the hippocampus in the etiology and expression of schizophrenia-spectrum psychopathology, and reduced hippocampal volume is one of the most robust brain abnormalities reported in schizophrenia. Recent studies indicate that early stages of schizophrenia are specifically characterized by reductions in anterior hippocampal volume; however, studies have not examined hippocampal volume reductions in subclinical schizotypy. The present study was the first to examine the associations of positive, negative, and disorganized schizotypy dimensions with hippocampal subfield volumes in a large sample (n = 195) of nonclinically ascertained young adults, phenotyped using the Multidimensional Schizotypy Scale (MSS). Hippocampal subfields were analyzed from high-resolution 3 Tesla structural magnetic resonance imaging scans testing anatomical models, including anterior vs posterior regions and the cornu ammonis (CA), dentate gyrus (DG), and subiculum subfields separately for the left and right hemispheres. We demonstrate differential spatial effects across anterior vs posterior hippocampus segments across different dimensions of the schizotypy risk phenotype. The interaction of negative and disorganized schizotypy robustly predicted left hemisphere volumetric reductions for the anterior and total hippocampus, and anterior CA and DG, and the largest reductions were seen in participants high in negative and disorganized schizotypy. These findings extend previous early psychosis studies and together with behavioral studies of hippocampal-related memory impairments provide the basis for a dimensional neurobiological hippocampal model of schizophrenia risk. Subtle hippocampal subfield volume reductions may be prevalent prior to the onset of detectable prodromal clinical symptoms of psychosis and play a role in the etiology and development of such conditions.
Keywords: schizophrenia, schizotypy, hippocampus, magnetic resonance imaging (MRI), volume, subfields
Introduction
Current models of schizophrenia conceptualize it as the most extreme manifestation of a dynamic continuum of clinical and subclinical symptoms and impairment referred to as schizotypy.1,2 Schizotypy, like schizophrenia, is heterogeneous in terms of etiology, course, and presentation. This heterogeneity can be captured within a multidimensional structure, including positive, negative, and disorganized schizotypy dimensions, similar to those observed in schizophrenia.3 Positive schizotypy is characterized by unusual beliefs (including delusions), aberrant perceptual experiences (including hallucinations), and paranoia. Negative schizotypy involves diminished functioning, including anhedonia, affective flattening, avolition, and alogia. Disorganized schizotypy is characterized by disruptions in cognition, communication, and behavior, including formal thought disorder and grossly disorganized behavior.
Schizotypy offers a useful and unifying framework for understanding the etiology, development, and expression of schizophrenia-spectrum psychopathology. Furthermore, schizotypy allows us to examine the etiological and developmental pathways underlying schizophrenia-spectrum psychopathology while minimizing the confounding and consequential effects of these disorders. This is important when considering structural and functional neurobiological measures as it is often difficult to disentangle whether neurological anomalies in patients represent relevant etiological processes or sequelae of the disorders. Furthermore, the multidimensional structure of schizotypy offers a promising approach for addressing the heterogeneity of schizophrenia-spectrum psychopathology.
Schizophrenia, Schizotypy, and the Hippocampus
Reduced hippocampal volume is one of the most robust brain abnormalities in schizophrenia.4,5 Patients with chronic schizophrenia average an 8% reduction in hippocampal volume compared with healthy adults.6 Recent findings indicate that overall hippocampal volumes might correlate with schizotypal personality traits.7 However, only recently have imaging studies considered functional partitions within the hippocampus.8The hippocampus can be subdivided along the anterior-posterior longitudinal axis into the head (anterior), and the body and tail (posterior) segments.9,10 This parallels recent findings of a molecular gene expression gradient along this axis with changing connectivity patterns.11 Previous studies indicate robust volume reductions across the length of the hippocampus in chronic schizophrenia, including reductions in anterior (eg, 12–14) and posterior sections (eg, 15–17). However, several studies suggest that reductions in patients with early schizophrenia are limited to anterior regions.12,18,19
The transverse axis of the hippocampus can also be subdivided into 3 subfields: dentate gyrus (DG), the cornu ammonis (CA) sectors 1 to 4, and the subiculum. Structural and functional disruptions in these subregions likely play a central role in the development and expression of schizophrenia-spectrum psychopathology. These include hyperactivity in CA1 region,20 GABAergic dysfunction in CA2/CA3,21 disruption in the DG,22 and hyperactivity in the subiculum.23
A recent study by McHugo et al19 was the first to examine whether there are volumetric reductions along the longitudinal and transverse hippocampal axes in large samples of patients with chronic or early schizophrenia and matched control participants. They reported that, as hypothesized, early psychosis patients only exhibited volumetric reductions in the anterior hippocampus relative to control participants, whereas chronic psychosis exhibited both anterior and posterior reductions compared with controls. In terms of subfields, patients with chronic psychosis exhibited volume reductions in the CA head and body, but not the subiculum or DG. However, early psychosis patients only showed reductions in the CA subfield of the hippocampal head. Thus, these findings are consistent with the model that volumetric changes in the anterior hippocampus occur in the early stages of psychosis and raise questions of whether such deficits predate the development of initial psychotic episodes. The findings also suggest that posterior volumetric reductions may represent progressive degenerative consequences of psychotic illnesses. This is consistent with recent studies showing progressive volume loss during the course of schizophrenia across hippocampal subfields,24 ultra-high-risk subjects with persisting symptoms,25,26 and genetic high-risk subjects.27
Multidimensional schizotypy offers a promising approach for examining hippocampal volume reductions, whereas McHugo et al19 provide a useful framework for considering hippocampal subfield volumes across anterior and posterior regions. However, to our knowledge, no previous studies examined associations of schizotypy dimensions with volumes of hippocampal subfields or subregions in non-patients. If reductions in hippocampal volume simply represent disease markers or neurodegenerative sequelae of the disorder, we would not expect reductions in subclinical schizotypy. However, if hippocampal reductions are part of the etiology of such disorders, they may provide useful risk markers, and we would expect such reductions in young adults with elevated schizotypy. Nevertheless, it is expected that effect sizes will be relatively small in non-patients compared with patients, as many schizotypes will never transition into schizophrenia-spectrum disorders, and patients may also experience neurodegenerative hippocampal reductions in addition to neurodevelopmental volume loss.
The schizophrenia literature does not provide specific guidance about the extent to which schizotypy dimensions are differentially associated with hippocampal subfield or subregion reductions. This is not due to lack of studies examining associations of symptom dimensions with hippocampal volume in patients, but rather reflects the heterogeneity/inconsistency of findings. For example, some studies reported that positive symptoms are associated with a reduction in certain subfields or subregions (eg, 28–30), others found associations with negative symptoms (eg, 31,32), and few others did not find any such associations (eg, 33). This heterogeneity in part reflects that examination of symptom dimensions in patients often occurs in a post hoc, exploratory manner, rather than with a priori designs to recruit patients with specific symptom characteristics. Thus, patient studies are often limited in terms of the extent to which symptom dimensions are represented and are also often limited in power to detect such associations (but see, 29 for an exception). Finally, positive symptoms may be overrepresented in patient studies compared with other dimensions, given the central role they have in schizophrenia-spectrum diagnoses.
The hippocampus is uniquely involved in relational memory,34–39 and recent behavioral studies demonstrated that negative, and to a lesser extent disorganized, schizotypy are associated with relational memory impairments.40 Therefore, we tentatively expect that negative and disorganized schizotypy, and their interaction, will be associated with hippocampal volume reductions in non-patients. Following McHugo et al,19 we expect that these effects will be especially notable in the anterior hippocampus, and especially in the anterior CA regions. We also expect these schizotypy dimensions will be associated with reductions in anterior DG, given prominent involvement of the DG in pattern separation41–46 and findings of pattern separation deficits in schizophrenia47,48 and negative and disorganized schizotypy.49
The goal of the present study, the first of its kind, is to examine the extent to which positive, negative, and disorganized schizotypy are associated with hippocampal subfield volume reductions (and in particular anterior vs posterior subregions) in a large non-patient sample of young adults. Following McHugo et al,19 we focused our analyses on examining the association of the 3 schizotypy dimensions with (a) total hippocampal volume, (b) anterior vs posterior hippocampal volume, and (c) anterior and posterior CA, DG, and subiculum volume. We performed these analyses separately by hemisphere given that many studies found reductions only in the left hippocampus (eg, 50–52), whereas others found bilateral hippocampal reductions (for a review, see 53). Specifically, we hypothesized that negative and disorganized schizotypy should be broadly associated with anterior hippocampal volume reductions, and specifically reductions in the volume of the anterior CA and DG.
Methods
Sample
We initially assessed 232 psychiatrically and neurologically healthy participants recruited using circular e-mails at Philipps-University Marburg and local advertisements in Marburg, Germany. Thirty-seven participants were omitted for quality assurance reasons (see below), resulting in 195 participants with usable data, including 132 women and 63 men; Mage = 23.7 years, SD = 3.9. Note that dropped and retained participants did not differ on demographic characteristics or on schizotypy subscale scores. Participants provided informed consent and the study protocol was approved by the Ethics Committee of the School of Medicine, Philipps-University Marburg, following the latest version of the Declaration of Helsinki.54 We included native German speakers with Central European origin and ages 18–40 years.
We screened subjects using a standardized protocol with the Structured Clinical Interview for DSM-IV Axis I Disorders screening questionnaire,55 German version,56 to exclude participants with current or past psychiatric disorders, psychotherapeutic treatment, and substance use disorders. Participants were free from traumatic brain injury or neurological disorders, psychotropic medication, common magnetic resonance imaging (MRI) contraindications, and physical disorders that could interfere with scanning procedures. We excluded subjects with BMI <18 or >35 or intelligence quotient <80, estimated with the German Mehrfach-Wortschatz-Intelligenztest B test.57,58The IQ criterion was chosen to exclude both subjects with learning disabilities (normally <70) and subjects in the 70–80 range, where the accuracy of the test used might lead to false-negative findings (however, none of the recruited subjects scored 80 or below). Subjects received financial compensation following participation.
MRI Data Acquisition
MRI data were obtained with a 3T MRI scanner (Tim Trio, Siemens) with Syngo MR B17 software, using a 12-channel head matrix RX-coil. We used a 3D MP-RAGE sequence consisting of 176 sagittal slices with an in-plane field-of-view of 256 × 256 mm and a matrix of 256 × 252 resulting in isotropic voxels of 1 × 1 × 1 mm. Further acquisition parameters were as follows: relaxation time = 1900 ms; time to echo = 2.26 ms; inversion time = 900 ms; flip angle 9°; parallel imaging factor 2 (GRAPPA), sequence bandwidth 200 Hz/Px; acquisition duration = 4:26 minutes. Before preprocessing, scans were manually inspected for the absence of artifacts and anatomical abnormalities, resulting in the exclusion of data from one participant.
Imaging Data Processing
T1-weighted images were processed using Freesurfer software version 6.0 (https://surfer.nmr.mgh.harvard.edu/). For preprocessing, the main reconstruction pipeline (“recon-all”) was used for volumetric segmentation. This processing includes motion correction, removal of non-brain tissue, automated Talairach transformation, tessellation of the gray matter/white matter boundary, and automated topology correction.59,60 Hippocampal structures were further parcellated using the Hippocampal Subfields protocol, automatically segmenting the hippocampal formation into subfields (“head” and “body” of presubiculum, subiculum, CA regions [CA1, CA3, CA4], molecular layer, and GCMLD [granule cell and molecular layers of the DG], as well as HATA [hippocampal amygdala transition area], fimbria, parasubiculum, and hippocampal fissure) for each hemisphere and calculating their volumes, using a probabilistic brain atlas.61 The validity and reliability of this procedure have been demonstrated in previous studies.62
We used a combined quality assurance protocol for MRI images. First, all images were visually inspected to exclude those with visible artifacts (eg, gross subject motion and ghosting). Second, we processed and compared for each individual the original (“raw”) T1 image as well as the scan using the “prescan normalize” function, a function included in the scanner’s software, which is intended to provide additional image homogeneity correction. Based on our previous work on segmentation reliability,63 we excluded those scans where subfield segmentation results between those 2 variations of the protocol differed by more than 3% in regional volumes, as a conservative means of quality assurance (resulting in final inclusion of 195 scans).
Hippocampal Subfield Segmentation
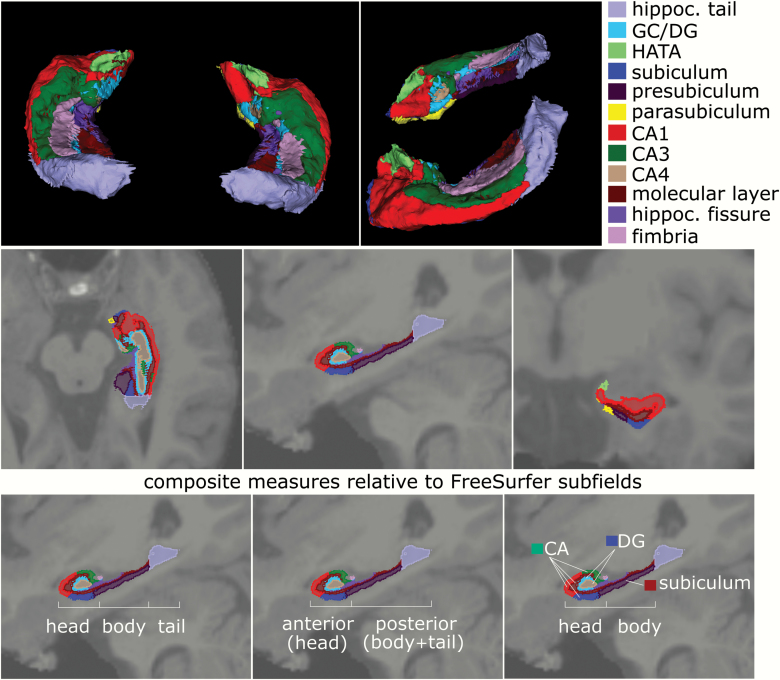
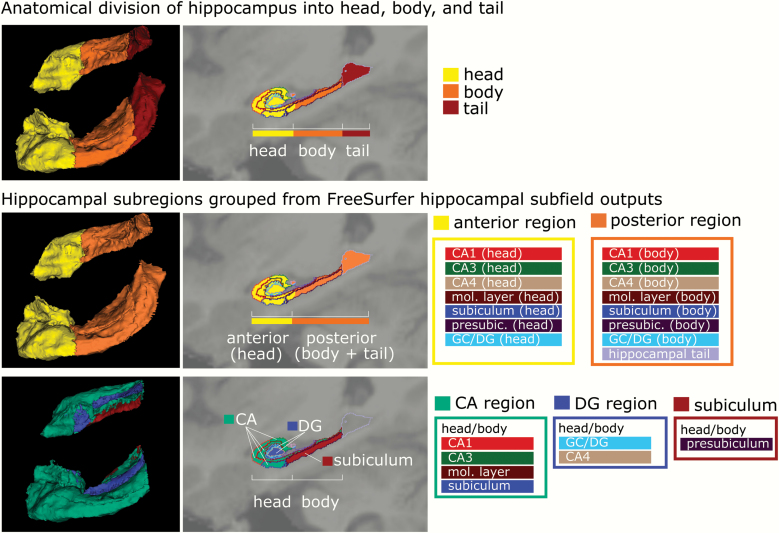
Following the previous segmentation approaches,19 we computed composite measures from the 12 subregions of hippocampal formation segmented by Freesurfer (figure 1). Anterior region (head) was defined as the sum of the volumes for the following subfields within the hippocampal head: CA1, CA3, CA4, molecular layer, GC/DG, subiculum, and presubiculum. The posterior region (body + tail) included the sum of these same subfields within the hippocampal body plus the tail (figure 2, middle). Within the head and body of the hippocampus of each hemisphere, we also defined composite regions for the CA, DG, and subiculum (figure 2, bottom). The CA composite region consisted of the sum of the volumes for CA1, CA3, subiculum, and the molecular layer. The DG region consisted of the sum of the CA4 and GC/DG subfields. The subiculum was defined as Freesurfer’s presubiculum subfield.
Fig. 1.
Description of hippocampal subfields derived from original FreeSurfer 6.0 segmentation. Top row shows 3D model of the different segmented hippocampal subfields with top and lateral views on the segmented hippocampus (color legend of individual subfield segments on right); middle row shows transverse, sagittal, and coronal sections of the hippocampus with subfields superimposed on T1-weighted MRI scan; the bottom row shows the relation of these original FreeSurfer 6.0 hippocampal subfields to functional models separating the hippocampus into either (from left to right) head, body, and tail components, or an anterior vs posterior part, or finally a model grouping cornu ammonis (CA), dentate gyrus (DG), and subiculum subfields within the head-body separation.
Fig. 2.
Grouping of hippocampal subfields into functional subregion models. Each row shows (from left to right) a 3D model (superior-lateral views), T1-superimposed sagittal section, and color symbol legend of re-grouping original FreeSurfer 6.0 hippocampal subfield outputs into newly computed hippocampal subregions based on functional models, and in particular the previous study of McHugo et al.19 Note that in the sagittal sections (middle images) in each row, the original FreeSurfer 6.0 hippocampal subfields are indicated by lines to illustrate the grouping of FreeSurfer outputs into new subregions. The top row shows the division of the hippocampus into 3 parts (head, body, and tail), a commonly used functional anatomical model (also implemented in FreeSurfer). The second row shows the re-grouping into anterior (=head) and posterior (=body + tail) subfields into an anterior-posterior model of the hippocampus; note that the color symbol boxes on the right indicate which initial FreeSurfer 6.0 subfields have been combined (colors of these FreeSurfer subfields correspond to colors used in figure 1). The bottom row shows the regrouping of subfields into a CA-DG-subiculum model; note that this model does not include the hippocampal tail segment (which is therefore only included in outline).
Phenotyping for Schizotypy
The Multidimensional Schizoytpy Scale (MSS; 64) was completed as part of a larger online survey (www.soscisurvey.de) within the week of MRI scanning. Each participant received a unique individualized access ID to complete the questionnaire; completeness of responses was controlled automatically. The MSS includes subscales assessing positive (26 items), negative (26 items), and disorganized schizotypy (25 items). The MSS includes true-false items such as: “I have sometimes felt that strangers were reading my mind” (positive schizotypy), “Having close friends is not as important as people say” (negative schizotypy), and “Most of the time I find it is very difficult to get my thoughts in order” (disorganized schizotypy). The MSS has good psychometric properties64,65 and construct validity.66,67
To examine the volumetric differences along the longitudinal axis of the hippocampus, we computed linear regression analyses on overall hippocampal volume in each hemisphere, followed by examination of anterior and posterior volume regions. Afterward, we assessed subfield volumes along the transverse axis in CA, DG, and subiculum in the head and the body of the hippocampus of each hemisphere. In each hierarchical regression analyses, we entered age, sex (women = 0, men = 1), and intracranial volume at step 1, followed by the MSS positive, negative, and disorganized schizotypy subscales at step 2, and the 2-way and 3-way schizotypy interactions at step 3. Simple slopes analyses were computed to disentangle statistically significant interactions by examining the effect of one predictor at low (−1 SD), medium (0 SD or mean), and high (+1 SD) levels of the other predictors.
Results
Descriptive statistics for the MSS subscales are shown in table 1. Note that correlations among the subscales were minimal, consistent with the literature, suggesting that multicollinearity was not a problem in the regression analyses.
Table 1.
Descriptive Statistics for MSS Subscales, Reliability Coefficients, and Correlations Among the Subscales
| Mean | SD | Range | Cronbach’s α | |
|---|---|---|---|---|
| MSS-Positive schizotypy | 0.72 | 1.64 | 0–10 | .78 |
| MSS-Negative schizotypy | 2.65 | 2.77 | 0–18 | .78 |
| MSS-Disorganized schizotypy | 1.11 | 2.11 | 0–13 | .80 |
| Correlations | ||||
| Positive and Negative schizotypy | r = .02 | |||
| Positive and Disorganized schizotypy | r = .28 | |||
| Negative and Disorganized schizotypy | r = .13 |
Note: MSS, Multidimensional Schizotypy Scale.
We initially examined the association of the schizotypy dimensions and their interactions with the left hemisphere total hippocampus, subregions, and subfield volumes (table 2). Contrary to expectations, none of the main effects of positive, negative, or disorganized schizotypy with left hemisphere subregions or subfields were significant. However, several significant 2-way interactions of the schizotypy dimensions emerged. Specifically, as hypothesized, a significant negative × disorganized schizotypy interaction was observed in the prediction of whole left hippocampal volume. Simple slopes analysis indicated that negative schizotypy was associated with reduced left hippocampal volume at high levels of disorganized schizotypy (+1 SD), but not at the mean or at low levels (−1 SD) of disorganized schizotypy (figure 3, upper left).
Table 2.
Prediction of Left (L) Hemisphere Hippocampal Volume by Positive (P), Negative (N), and Disorganized (D) Schizotypy
| Step 1 | Step 2 | Step 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | ICV | Negative | Disorganized | Positive | N × D | P × D | P × N | N × D × P | |
| (L) Whole HC | .06 (.315) | .09 (.173) | .62 (<.001) | −.02 (.713) | −.05 (.394) | −.01 (.833) | −.29 (.044) | −.31 (.114) | −.12 (.511) | −.39 (.129) |
| (L) anterior HC | .01 (.851) | .16 (.017) | .54 (<.001) | −.03 (.611) | −.01 (.884) | −.02 (.698) | −.35 (.020) | −.41 (.044) | −.20 (.288) | −.49 (.066) |
| (L) posterior HC | .09 (.161) | −.02 (.826) | .56 (<.001) | .01 (.877) | −.08 (.145) | .01 (.897) | −.19 (.221) | −.21 (.329) | −.06 (.770) | .29 (.301) |
| (L) CA anterior | .01 (.808) | .16 (.019) | .54 (<.001) | −.03 (.617) | −.02 (.780) | −.02 (.764) | −.33 (.026) | −.40 (.050) | −.22 (.239) | −.49 (.068) |
| (L) DG anterior | −.03 (.617) | .16 (.028) | .46 (<.001) | −.04 (.583) | .04 (.512) | −.06 (.389) | −.37 (.021) | −.47 (.032) | −.18 (.381) | −.53 (.062) |
| (L) SUB anterior | .07 (.287) | .11 (.130) | .49 (<.001) | −.01 (.888) | −.05 (.464) | .01 (.821) | −.24 (.129) | −.17 (.455) | −.02 (.929) | .17 (.558) |
| (L) CA posterior | .09 (.189) | .01 (.967) | .51 (<.001) | −.05 (.439) | −.10 (.139) | −.01 (.836) | −.14 (.391) | −.07 (.745) | −.06 (.763) | .10 (.719) |
| (L) DG posterior | .08 (.202) | −.08 (.278) | .59 (<.001) | −.08 (.235) | .01 (.984) | −.05 (.414) | −.09 (.578) | −.11 (.620) | .05 (.796) | .11 (.688) |
| (L) SUB posterior | .01 (.904) | −.03 (.694) | .52 (<.001) | −.02 (.796) | .04 (.543) | .01 (.972) | −.01 (.970) | −.02 (.946) | .33 (.113) | −.19 (.531) |
Note: ICV, intracranial volume. Beta values with P-values in parentheses; boldface indicates significant effects at α ≤ .05 or less.
Fig. 3.
Prediction models of refined extended psychosis phenotype (based on negative, positive, and disorganized schizotypy, as assessed with the Multidimensional Schizotypy Scale, MSS) in different subsections of the hippocampus. Left to each of the 4 models, a 3D reconstruction indicating the hippocampal region to which the plots refer.
We next examined associations of the schizotypy dimensions with the left anterior and posterior hippocampus. There were significant negative × disorganized schizotypy and positive × disorganized schizotypy interactions in the anterior subregion. Simple slopes analyses indicated that negative schizotypy was associated with reduced volume in the anterior hippocampus at high levels of disorganized schizotypy, but not at the mean or at low levels of disorganized schizotypy (figure 3, lower left). Simple slopes analyses indicated that there was a trend for positive schizotypy to be associated with reduced volume in the left anterior subregion at high levels of disorganized schizotypy (P = .057) but not at the mean or at low levels of disorganized schizotypy (figure 3, lower left). As hypothesized, neither the schizotypy main effects nor their interactions predicted left posterior hippocampal volume.
We next examined the prediction of left anterior CA, DG, and subiculum subfields. Both the negative × disorganized schizotypy and the positive × disorganized schizotypy interactions significantly predicted left anterior CA volume. Simple slopes revealed that, consistent with our predictions, negative schizotypy was associated with reduced volume in left anterior CA at high levels of disorganized schizotypy but not at the mean or at low levels of disorganized schizotypy (figure 3, top right). Simple slopes indicated that there was a trend for positive schizotypy to be associated with reduced volume in left anterior CA at high levels of disorganized schizotypy (P = .087) but not at the mean or at low levels (figure 3, top right). Similarly, there were significant negative × disorganized schizotypy and the positive × disorganized schizotypy interactions predicting left anterior DG volume. Simple slopes revealed that, consistent with our predictions, negative schizotypy was associated with reduced volume in anterior DG at high levels of disorganized schizotypy but not at the mean or at low levels of disorganized schizotypy (figure 3, lower right). Likewise, positive schizotypy was associated with reduced volume in left anterior DG volume at high levels of disorganized schizotypy but not at the mean or at low levels (figure 3, lower right). The schizotypy dimensions were not associated with the left hemisphere anterior subiculum volume. As expected, we did not find any significant associations of the schizotypy dimensions or their interactions with posterior CA, DG, or subiculum volume.
With only the exception of the posterior subiculum, we did not find any effects in the right hemisphere and report all the findings from the right hemisphere in the supplementary materials.
Discussion
The present study adds 3 major aspects to the understanding of the hippocampus in the schizophrenia spectrum. First, it shows that the variation in hippocampal subfield volumes is influenced by psychosis proneness (schizotypy) in nonclinical subjects, ie, even in the absence of manifest disease or a high-risk status. Second, it lays out the regional selectivity on these effects in different hippocampal subfields, across the anterior-posterior axis of the hippocampus, which coincides with recent models of differential functional and structural connectivity of hippocampus segments,8,68,69 and gradients of gene expression reflecting connectivity patterns.11 Third, it demonstrates considerable divergence of phenotypic dimensions within schizotypy (negative, positive, and disorganized) in their effect on hippocampal subfields, thus providing an approach to integrate basic behavioral and cognitive models of hippocampal function with alterations seen in clinical schizophrenia.22 This provides empirical evidence for a continuum model of schizophrenia/psychosis and hippocampal (dys)function.
The present study extended the literature on hippocampal volume reductions in schizophrenia by examining differential associations of positive, negative, and disorganized schizotypy with regional hippocampal volume in nonclinically ascertained young adults. Numerous studies indicate that hippocampal volume is reduced in patients with schizophrenia,70 including ENIGMA analyses.4 [Studies on hippocampal subfields have also provided evidence for volume reductions; however, these appeared rather nonselective in (predominantly) chronic patient samples.71,72 High-risk subjects show intermediate volume reductions in subfields,73 and possibly progressive reductions in (anterior) CA1 segments,25 overlapping with findings in first-episode psychosis/early vs chronic schizophrenia.19,33 Our findings specifically support an anterior-to-posterior gradient effect, previously reported in a case-control study,19 suggesting that this pattern is already present in nonclinical subjects with varying degrees of psychosis proneness/schizotypy and thus different genetic/environmental risk profiles. Importantly, this spatial pattern of variation is related to dimensional aspects of the phenotype (ie, positive vs negative vs disorganized schizotypy, rather than overall schizotypy, and does not reflect disease [onset] or treatment effects).
Genetic effects on the schizotypy phenotype are likely contributors to this pattern. Following previous twin studies showing heritability of subfield volumes,74 more recent studies have demonstrated not only considerable genetic impact on hippocampal subfields but also some of the common genetic variants identified map on to neuronal differentiation as well as schizophrenia risk.75 More importantly, gene expression profiling and connectivity analyses confirm an anterior-posterior gradient within these subfields.11 Based on our new findings, refined phenotyping considering symptom dimensions (rather than case-control status) in larger samples could provide an additional advance in future studies.
The heterogeneity of both the etiology and clinical course of schizophrenia (eg, 76) has had a confounding impact on our understanding of schizophrenia-spectrum psychopathology. Current models of schizophrenia (eg, 77) and schizotypy1 indicate that this heterogeneity can be captured using a multidimensional structure that includes positive, negative, and disorganized symptom dimensions. However, studies of schizophrenia often do not examine associations of symptom dimensions with outcome measures, and when they do, they often assess them as a secondary aspect of studies, which may not have adequate representation of the symptom dimensions in their sample, and/or have insufficient power to capture those effects. Furthermore, studies that do consider symptom dimensions often do so in a limited fashion because they do not recruit patients with comparable levels of these symptom dimensions and often have an overabundance of positive symptoms, given these symptoms prominent role in psychotic disorders. A powerful but largely untapped potential of schizotypy is aiding the identification of neurobiological bases of schizophrenia-spectrum psychopathology by extending the phenotype beyond diagnosed disorders.
Although functional differences between the anterior and posterior hippocampus have been well established, the specific implications for schizophrenia pathophysiology, especially cognitive deficits, are not well understood.69,78,79 Some studies suggested that the anterior hippocampus is related to coarse gist-like memory, whereas the posterior hippocampus is more involved in detailed memories.69,80–83 For example, initially “locating” a memory implicates engagement of anterior hippocampus, whereas later elaboration of its details implicates posterior hippocampus.84–86 Behavioral studies from our lab revealed deficits in negative schizotypy in initially locating memories through context reinstatement,40,87,88 consistent with the involvement of anterior hippocampus in mental reinstatement of context. Other work shows that the anterior hippocampus is specialized for memory encoding,89,90 and consistent with these findings, we reported encoding deficits in negative schizotypy.91 Anterior hippocampus is also involved in relational memory,92 and our behavioral studies revealed that negative schizotypy involves deficits in relational memory.40 Thus, memory deficits in negative schizotypy in behavioral studies are consistent with observed reductions in anterior hippocampal volume in the current study. Finally, DG is prominently involved in pattern separation,41–44,46,93 and an influential model links schizophrenia to disruption in the DG22. Our recent behavioral work revealed deficits in pattern separation in negative and disorganized schizotypy.49 Thus, current findings of reduced volume in DG are consistent with our behavioral findings in negative and disorganized schizotypy, and also models linking schizophrenia to DG disruptions. Other groups have used habituation paradigms to identify cognitive deficits associated with anterior hippocampal function94 and blood flow95 in early psychosis, but so far this approach has not been extended to risk phenotypes in nonclinical samples, while other recent pilot studies on hippocampal blood flow and schizotypy96 have not considered the anterior-posterior gradient or subfield distinction, which is of high relevance.
Hippocampal volume reduction in schizophrenia is observed early in the disease process.50,52,97 It is found in unaffected first-degree relatives of patients98,99 and in at-risk populations,100,101 suggestive of genetic risk factors.101,102 Twin studies also reveal smaller hippocampal volumes in discordant co-twins of schizophrenia patients.103 Decreased hippocampal volume is not found in all patients with schizophrenia and other disorders involve reduced hippocampal volume. However, recent studies have increasingly supported the model that decreased hippocampal volume reveals something unique about the pathology of schizophrenia, rather than simply representing sequelae of the disorder. Nevertheless, there is considerable heterogeneity in hippocampal findings, with many studies finding reductions bilaterally, some finding hippocampal volume reduction only in the left hemisphere,104–106 and yet others not finding any evidence of hippocampal reduction at all.107 In addition, some studies find reductions in the anterior hippocampus (eg, 12–14,78), and others find a reduction in posterior regions (eg, 15–17).
The present findings should be interpreted in light of some limitations. First, levels of schizotypy in the sample were relatively low, especially positive schizotypy, as we did not oversample high schizotypy scorers. Nevertheless, consistent with Mathew et al,29 we did find several significant interactions involving positive schizotypy. The present study employed a university and a community-recruited sample. Concerns are raised about using university samples for studying subclinical expressions of psychopathology and risk for disorders. However, university students are an ideal age for assessing schizotypy as they are just entering the window of greatest risk for developing schizophrenia-spectrum disorders. Furthermore, university students readily experience schizophrenia-spectrum psychopathology (as well as other forms of psychopathology),66,108 and schizotypy scales identify students at heightened risk for developing schizophrenia-spectrum disorders.109
In summary, the present study builds on previous support for the pathophysiological model, in which the hippocampus plays an important role in the development and expression of schizophrenia-spectrum psychopathology. It supports a dimensional model of disease expanding to include subclinical risk traits. Furthermore, it highlights the importance of considering multidimensional risk phenotypes. Future studies might build on the present findings by oversampling high schizotypy scorers and provide a comparison with high-risk and prodromal subjects. Longitudinal reassessments would allow us to examine the extent to which multidimensional schizotypy, hippocampal volume and functioning, and cognitive performance predict the development of schizophrenia-spectrum disorders.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin.
Supplementary Fig. S1. Subiculum body (right hemisphere).
Supplementary Table S1. Prediction of Right (R) Hemisphere Hippocampal Volume by Positive (P), Negative (N), and Disorganized (D) Schizotypy
Acknowledgment
None of the authors report any financial interests or potential conflicts of interest.
Funding
This study was supported by a research grant of the University Medical Center Giessen and Marburg (UKGM) grants (11/2017 MR to I.N. and 05/2018 MR to Sarah Grezellschak and I.N.). This work was partially supported through Forschungscampus Mittelhessen (FCMH) FlexiFunds grants (2017_2_1_5 and 2018_2_1_1 to I.N.).
References
- 1. Kwapil TR, Barrantes-Vidal N. Schizotypy: looking back and moving forward. Schizophr Bull. 2015;41 (Suppl 2):S366–S373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lenzenweger MF. Current status of the scientific study of the personality disorders: an overview of epidemiological, longitudinal, experimental psychopathology, and neurobehavioral perspectives. J Am Psychoanal Assoc. 2010;58(4):741–778. [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 4. van Erp TG, Hibar DP, Rasmussen JM, et al. . Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21 (4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist 2012;18(2):180–200. [DOI] [PubMed] [Google Scholar]
- 6. Velakoulis D, Wood SJ, Wong MT, et al. . Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139–149. [DOI] [PubMed] [Google Scholar]
- 7. Derome M, Zoller D, Modinos G, Schaer M, Eliez S, Debbane M. Developmental trajectories of subcortical structures in relation to dimensional schizotypy expression along adolescence. Schizophr Res. 2020;218:76–84. [DOI] [PubMed] [Google Scholar]
- 8. Grady CL. Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis. Hippocampus 2020;30(5):456–471. [DOI] [PubMed] [Google Scholar]
- 9. Malykhin NV, Bouchard TP, Ogilvie CJ, Coupland NJ, Seres P, Camicioli R. Three-dimensional volumetric analysis and reconstruction of amygdala and hippocampal head, body and tail. Psychiatry Res. 2007;155(2):155–165. [DOI] [PubMed] [Google Scholar]
- 10. Rajah MN, Kromas M, Han JE, Pruessner JC. Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia 2010;48(14):4020–4030. [DOI] [PubMed] [Google Scholar]
- 11. Vogel JW, La Joie R, Grothe MJ, et al. . A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat Commun. 2020;11(1):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szeszko PR, Goldberg E, Gunduz-Bruce H, et al. . Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(12):2190–2197. [DOI] [PubMed] [Google Scholar]
- 13. Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr Res. 2003;60(2-3):105–115. [DOI] [PubMed] [Google Scholar]
- 14. Goldman MB, Torres IJ, Keedy S, Marlow-O’Connor M, Beenken B, Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus 2007;17(7):554–562. [DOI] [PubMed] [Google Scholar]
- 15. Benes FM, Sorensen I, Bird ED. Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull. 1991;17(4):597–608. [DOI] [PubMed] [Google Scholar]
- 16. Narr KL, Thompson PM, Szeszko P, et al. . Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 2004;21(4):1563–1575. [DOI] [PubMed] [Google Scholar]
- 17. Maller JJ, Daskalakis ZJ, Thomson RH, Daigle M, Barr MS, Fitzgerald PB. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in de-tail. Hippocampus 2012;22(1):9–16. [DOI] [PubMed] [Google Scholar]
- 18. Kalmady SV, Shivakumar V, Arasappa R, et al. . Clinical correlates of hippocampus volume and shape in antipsychotic-naive schizophrenia. Psychiatry Res Neuroimaging. 2017;263:93–102. [DOI] [PubMed] [Google Scholar]
- 19. McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. Neuroimage Clin. 2018;20:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lieberman JA, Girgis RR, Brucato G, et al. . Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23(8):1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46(5):589–599. [DOI] [PubMed] [Google Scholar]
- 22. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. [DOI] [PubMed] [Google Scholar]
- 23. Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18(3-4):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ho NF, Iglesias JE, Sum MY, et al. . Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22(1):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho NF, Holt DJ, Cheung M, et al. . Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology 2017;42(6):1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho NF, Holt DJ, Cheung M, et al. . Correction: progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology 2019;44(12):2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mancini V, Sandini C, Padula MC, et al. . Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22q11DS. Mol Psychiatry 2020. (in press). doi: 10.1038/s41380-019-0443-z. [DOI] [PubMed] [Google Scholar]
- 28. Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl Psychiatry. 2012;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathew I, Gardin TM, Tandon N, et al. . Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71(7):769–777. [DOI] [PubMed] [Google Scholar]
- 30. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain 2013;136(Pt 3):804–814. [DOI] [PubMed] [Google Scholar]
- 31. Anvari AA, Friedman LA, Greenstein D, Gochman P, Gogtay N, Rapoport JL. Hippocampal volume change relates to clinical outcome in childhood-onset schizophrenia. Psychol Med. 2015;45(12):2667–2674. [DOI] [PubMed] [Google Scholar]
- 32. Kawano M, Sawada K, Shimodera S, et al. . Hippocampal subfield volumes in first episode and chronic schizophrenia. PLoS One. 2015;10(2):e0117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baglivo V, Cao B, Mwangi B, et al. ; GET UP Group . Hippocampal subfield volumes in patients with first-episode psychosis. Schizophr Bull. 2018;44(3):552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88(2):982–990. [DOI] [PubMed] [Google Scholar]
- 35. Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus 2004;14(1):5–8. [DOI] [PubMed] [Google Scholar]
- 36. Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia 2013;51(12):2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen NJ, Eichenbaum H.. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: The MIT Press; 1993. [Google Scholar]
- 38. Eichenbaum H, Cohen NJ.. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 39. Slotnick SD. Does the hippocampus mediate objective binding or subjective remembering? Neuroimage 2010;49(2):1769–1776. [DOI] [PubMed] [Google Scholar]
- 40. Sahakyan L, Kwapil TR, Lo Y, Jiang L. Examination of relational memory in multidimensional schizotypy. Schizophr Res. 2019;211:36–43. [DOI] [PubMed] [Google Scholar]
- 41. Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 2007;315(5814):961–966. [DOI] [PubMed] [Google Scholar]
- 42. Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 2008;319(5870):1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumaran D, McClelland JL. Generalization through the recurrent interaction of episodic memories: a model of the hippocampal system. Psychol Rev. 2012;119(3):573–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res. 2013;254:1–7. [DOI] [PubMed] [Google Scholar]
- 46. Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci. 2013;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinelli C, Shergill SS. Clarifying the role of pattern separation in schizophrenia: the role of recognition and visual discrimination deficits. Schizophr Res. 2015;166(1-3):328–333. [DOI] [PubMed] [Google Scholar]
- 48. Das T, Ivleva EI, Wagner AD, Stark CE, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159(1):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sahakyan L, Wahlheim C, Kwapil T. Pattern separation deficits in multidimensional schizotypy consistent with findings in schizophrenia. (in press)
- 50. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35(1):1–13. [DOI] [PubMed] [Google Scholar]
- 51. Kubicki M, Shenton ME, Salisbury DF, et al. . Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002;17(4):1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Velakoulis D, Pantelis C, McGorry PD, et al. . Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56(2):133–141. [DOI] [PubMed] [Google Scholar]
- 53. Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10(2):160–184. [DOI] [PubMed] [Google Scholar]
- 54. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 55. First MB, Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV AXIS II disorders (SCID-II). In: Hilsenroth MJ, Segal DL, eds. Comprehensive Handbook of Psychological Assessment, Vol. 2: Personality Assessment. Hoboken, NJ: John Wiley & Sons Inc; 2004:134–143. [Google Scholar]
- 56. Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M.. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
- 57. Lehrl S, Merz J, Erzigkeit H, Galster V. [MWT-A–a repeatable intelligence short-test, fairly independent from psycho-mental disorders]. Nervenarzt. 1974;45(7):364–369. [PubMed] [Google Scholar]
- 58. Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91(5):335–345. [DOI] [PubMed] [Google Scholar]
- 59. Fischl B. FreeSurfer. Neuroimage 2012;62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fischl B, Salat DH, van der Kouwe AJ, et al. . Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23 (Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- 61. Van Leemput K, Bakkour A, Benner T, et al. . Model-based segmentation of hippocampal subfields in ultra-high resolution in vivo MRI. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iglesias JE, Augustinack JC, Nguyen K, et al. ; Alzheimer’s Disease Neuroimaging Initiative . A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 2015;115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One. 2012;7(9):e45081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kwapil TR, Gross GM, Silvia PJ, Raulin ML, Barrantes-Vidal N. Development and psychometric properties of the Multidimensional Schizotypy Scale: a new measure for assessing positive, negative, and disorganized schizotypy. Schizophr Res. 2018;193:209–217. [DOI] [PubMed] [Google Scholar]
- 65. Kemp KC, Gross GM, Kwapil TR. Psychometric properties of the Multidimensional Schizotypy Scale and Multidimensional Schizotypy Scale-Brief: item and scale test-retest reliability and concordance of original and brief forms. J Pers Assess. 2020;102(4):508–515. [DOI] [PubMed] [Google Scholar]
- 66. Kemp KC, Bathery AJ, Barrantes-Vidal N, Kwapil TR. Positive, negative, and disorganized schizotypy predict differential patterns of interview-rated schizophrenia-spectrum symptoms and impairment. Assessment 2020:1073191119900008. [DOI] [PubMed] [Google Scholar]
- 67. Kwapil TR, Kemp KC, Mielock A, et al. . Association of multidimensional schizotypy with psychotic-like experiences, affect, and social functioning in daily life: comparable findings across samples and schizotypy measures. J Abnorm Psychol. 2020;129(5):492–504. [DOI] [PubMed] [Google Scholar]
- 68. Chase HW, Clos M, Dibble S, et al. . Evidence for an anterior-posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: focus on the subiculum. Neuroimage 2015;113:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. [DOI] [PubMed] [Google Scholar]
- 70. Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74(11):1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Haukvik UK, Tamnes CK, Söderman E, Agartz I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–226. [DOI] [PubMed] [Google Scholar]
- 72. Nakahara S, Matsumoto M, van Erp TGM. Hippocampal subregion abnormalities in schizophrenia: a systematic review of structural and physiological imaging studies. Neuropsychopharmacol Rep. 2018;38(4):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vargas T, Dean DJ, Osborne KJ, et al. . Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44(5):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Patel S, Park MTM, Devenyi GA, et al. . Heritability of hippocampal subfield volumes using a twin and non-twin siblings design. Hum Brain Mapp. 2017;38(9):4337–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Meer D, Rokicki J, Kaufmann T, et al. . Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatry. 2020. (in press). doi: 10.1038/s41380-018-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tsuang MT, Lyons MJ, Faraone SV. Heterogeneity of schizophrenia. Conceptual models and analytic strategies. Br J Psychiatry. 1990;156:17–26. [DOI] [PubMed] [Google Scholar]
- 77. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1–23. [DOI] [PubMed] [Google Scholar]
- 78. Small SA. The longitudinal axis of the hippocampal formation: its anatomy, circuitry, and role in cognitive function. Rev Neurosci. 2002;13(2):183–194. [DOI] [PubMed] [Google Scholar]
- 79. Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. [DOI] [PubMed] [Google Scholar]
- 80. Bonne O, Vythilingam M, Inagaki M, et al. . Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69(7):1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hayes JP, LaBar KS, McCarthy G, et al. ; VISN 6 Mid-Atlantic MIRECC workgroup . Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45(5):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron 2011;72(6):931–937. [DOI] [PubMed] [Google Scholar]
- 83. Poppenk J, Walia G, McIntosh AR, Joanisse MF, Klein D, Köhler S. Why is the meaning of a sentence better remembered than its form? An fMRI study on the role of novelty-encoding processes. Hippocampus 2008;18(9):909–918. [DOI] [PubMed] [Google Scholar]
- 84. Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 2009;47(11):2222–2238. [DOI] [PubMed] [Google Scholar]
- 85. Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, Maguire EA. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J Neurosci. 2012;32(47):16982–16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Holland AC, Addis DR, Kensinger EA. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia 2011;49(12):3164–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sahakyan L, Kwapil TR. Positive schizotypy and negative schizotypy are associated with differential patterns of episodic memory impairment. Schizophr Res Cogn. 2016;5:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sahakyan L, Kwapil TR. Moving beyond summary scores: decomposing free recall performance to understand episodic memory deficits in schizotypy. J Exp Psychol Gen. 2018;147(12):1919–1930. [DOI] [PubMed] [Google Scholar]
- 89. Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 2009;47(8-9):1765–1779. [DOI] [PubMed] [Google Scholar]
- 90. Woollett K, Maguire EA. Exploring anterograde associative memory in London taxi drivers. NeuroReport 2012;23(15):885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sahakyan L, Kwapil TR. Hits and false alarms in recognition memory show differential impairment in positive and negative schizotypy. J Abnorm Psychol. 2019;128(6):633–643. [DOI] [PubMed] [Google Scholar]
- 92. Ongür D, Cullen TJ, Wolf DH, et al. . The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63(4):356–365. [DOI] [PubMed] [Google Scholar]
- 93. Leutgeb JK, Moser EI. Enigmas of the dentate gyrus. Neuron 2007;55(2):176–178. [DOI] [PubMed] [Google Scholar]
- 94. Avery SN, McHugo M, Armstrong K, Blackford JU, Woodward ND, Heckers S. Disrupted habituation in the early stage of psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(11):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McHugo M, Talati P, Armstrong K, et al. . Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176(12):1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Modinos G, Egerton A, McMullen K, et al. . Increased resting perfusion of the hippocampus in high positive schizotypy: a pseudocontinuous arterial spin labeling study. Hum Brain Mapp. 2018;39(10):4055–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hirayasu Y, Shenton ME, Salisbury DF, et al. . Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155(10):1384–1391. [DOI] [PubMed] [Google Scholar]
- 98. Seidman LJ, Faraone SV, Goldstein JM, et al. . Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46(7):941–954. [DOI] [PubMed] [Google Scholar]
- 99. Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54(11):1234–1240. [DOI] [PubMed] [Google Scholar]
- 100. Keshavan MS, Dick E, Mankowski I, et al. . Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58(2-3):173–183. [DOI] [PubMed] [Google Scholar]
- 101. Phillips LJ, Velakoulis D, Pantelis C, et al. . Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58(2-3):145–158. [DOI] [PubMed] [Google Scholar]
- 102. Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001;11(5):520–528. [DOI] [PubMed] [Google Scholar]
- 103. Baaré WF, van Oel CJ, Hulshoff Pol HE, et al. . Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58(1):33–40. [DOI] [PubMed] [Google Scholar]
- 104. Hulshoff Pol HE, Schnack HG, Mandl RC, et al. . Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–1125. [DOI] [PubMed] [Google Scholar]
- 105. Shenton ME, Kikinis R, Jolesz FA, et al. . Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327(9):604–612. [DOI] [PubMed] [Google Scholar]
- 106. Woodruff PW, Wright IC, Shuriquie N, et al. . Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med. 1997;27(6):1257–1266. [DOI] [PubMed] [Google Scholar]
- 107. Marsh L, Sullivan EV, Morrell M, Lim KO, Pfefferbaum A. Structural brain abnormalities in patients with schizophrenia, epilepsy, and epilepsy with chronic interictal psychosis. Psychiatry Res. 2001;108(1):1–15. [DOI] [PubMed] [Google Scholar]
- 108. Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: further validation of the psychometric high-risk strategy. J Abnorm Psychol. 2005;114(1):170–175. [DOI] [PubMed] [Google Scholar]
- 109. Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol. 2013;122(3):807–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.