Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, intubation; mechanical ventilation, mortality, retrospective cohort, severe acute respiratory syndrome coronavirus-2
Abstract
Objectives:
There has been controversy about the timing and indications for intubation and mechanical ventilation in novel coronavirus disease 2019. This study assessed the effect of early intubation and mechanical ventilation on all-cause, inhospital mortality for coronavirus disease 2019 patients.
Design:
Multicenter retrospective cohort study.
Setting:
Eleven municipal hospitals in New York City from March 1, 2020, to December 1, 2020.
Patients:
Adult patients who tested positive for coronavirus disease 2019 in the emergency department were subsequently admitted. Patients with do-not-intubate orders at admission were excluded.
Interventions:
Intubation within 48 hours of triage and intubation at any point during hospital stay.
Measurements and Main Results:
Data from 7,597 coronavirus disease 2019 patients were included; of these, 1,628 (21%) were intubated overall and 807 (11%) were intubated within 48 hours of triage. After controlling for available confounders, intubation rates for coronavirus disease 2019 patients varied significantly across hospitals and decreased steadily as the pandemic progressed. After nearest neighbor propensity score matching, intubation within 48 hours of triage was associated with higher all-cause mortality (hazard ratio, 1.30 [1.15–1.48]; p < 0.0001), as was intubation at any time point (hazard ratio, 1.62 [1.45–1.80]; p < 0.0001). Among intubated patients, intubation within 48 hours of triage was not significantly associated with differences in mortality (hazard ratio, 1.09 [0.94–1.26]; p = 0.26). These results remained robust to multiple sensitivity analyses.
CONCLUSIONS:
Intubation within 48 hours of triage, as well as at any time point in the hospital course, was associated with increased mortality in coronavirus disease 2019 patients in this observational study.
Early and continued reports of the management of respiratory failure in patients with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (coronavirus disease 2019 [COVID-19]) suggest that mechanical ventilation is the mainstay of therapy (1, 2), and the mortality rate of COVID-19 patients receiving mechanical ventilation is high (3, 4). There has been controversy about the optimal timing of mechanical ventilation and selection of COVID-19 patients who require invasive mechanical ventilation (5–8). Delayed invasive mechanical ventilation in patients with acute respiratory distress syndrome from sepsis and pneumonia has been shown to be associated with increased mortality compared with those intubated within 48 hours (9). The optimal treatment of respiratory failure from COVID-19 has yet to be established, and it has been suggested that COVID-19 patients should have an earlier threshold for receiving invasive mechanical ventilation due to high inspiratory efforts and transpulmonary pressures causing self-inflicted lung injury (8, 10). However, there are no robust data on the timing of tracheal intubation and its relationship to mortality for COVID-19 patients, and the existing studies have inconsistent findings (11–13), suggesting either no relationship between time to invasive mechanical ventilation and mortality (11) or that early intubation may affect mortality (12, 13).
In this observational study using data from 11 public hospitals in New York City gathered from March to December 2020, we examined the relationship between invasive mechanical ventilation and inhospital mortality, using propensity score matching and adjustment to reduce confounding. We looked at the effect on mortality of intubation within 48 hours of hospital triage, as well as the effect of intubation at any time point.
MATERIALS AND METHODS
Data Extraction
We conducted a retrospective chart review of 11 New York City Health and Hospitals (H + H), public hospitals for all adult patients, seen in emergency departments (EDs) between March 1, 2020, and December 1, 2020, who were tested with a polymerase chain reaction test for SARS-CoV-2 (COVID-19) during their time in the ED and subsequently admitted. Patients with negative, discontinued, or indeterminate tests were excluded, as were patients that were transferred to hospitals outside of the New York City H + H system. As the same patient may have presented to the ED multiple times, we used only the earliest visit resulting in admission so that each patient contributed unique, noncorrelated data. We obtained institutional review board (IRB) approval for this study both from the Lincoln Medical Center IRB and from the New York City H + H IRB (IRB approval number: 20-013).
We extracted a range of demographic and clinical data for each patient, including initial labs obtained within 24 hours of triage. For each patient, we extracted their inhospital exposure to invasive ventilation (endotracheal intubation and mechanical ventilation). Data were extracted automatically from the Epic electronic medical record system (14). For a subset of 300 patients, all variables were extracted manually by three authors (A.J.P., D.Y., T.M.J.), and Cohen kappa was calculated to the assess accuracy of the automatic extraction. For continuous variables where 30% or fewer patients had missing values, we used multiple imputation with predictive mean matching to estimate the missing numerical data (15); variables with more than 30% missing values were not used in any models.
We excluded patients that died within 48 hours of triage. We also excluded all patients that had do-not-intubate (DNI) orders placed within the first 48 hours of triage. To reduce the effect of immortal time bias, in the primary analysis, we compared patients that were intubated within 48 hours of ED triage with all other patients, including those intubated after this time point (16, 17).
Statistical Analysis
The primary outcome was all-cause, inhospital mortality; patients that were discharged, left against medical advice, or were still in the hospital as of December 1, 2020, were censored. We calculated the hazard ratio (HR) for mortality using the Cox proportional hazards model (18) and estimated median survival times using Kaplan-Meier analysis (19). We calculated propensity scores for intubation with multivariate logistic regression and used 1:1 nearest neighbor propensity score matching without replacement (20) to balance covariates between those that were intubated and those that were not (21). Additionally, we used propensity score adjustment (22) and overlap propensity score weighting (23) as alternative methods to estimate the primary outcome.
We included in the propensity score model variables considered likely to be related to the intervention (intubation) and primary outcome (mortality) (24). The variables that were included were as follows: age, sex, body mass index, ever-smoker, ethnicity, race, hospital that the patient was admitted to, admission date, total hospital census (number of inpatients) at admission date, whether the patient was a Medicare or Medicaid recipient, history of diabetes mellitus, hypertension, asthma, chronic obstructive pulmonary disease, chronic kidney disease, end-stage renal disease, congestive heart failure, coronary artery disease or cancer, triage vitals (including initial Spo2, heart rate, respiratory rate, mean arterial pressure, and temperature), emergency severity index, altered mental status at triage, initial labs (ferritin, lactate dehydrogenase, d-dimer, procalcitonin, anion gap, absolute lymphocyte count, absolute neutrophil count, creatinine, blood urea nitrogen, hemoglobin, platelet count, and Pco2 in venous or arterial blood gas), presence of bilateral opacities or infiltrate on initial chest radiograph, and whether the patient received each of the following supplemental oxygen therapies: nasal cannula, nonrebreather mask, high-flow nasal cannula (HFNC), or noninvasive positive-pressure ventilation (NIPPV).
We carried out two main analyses: 1) the effect of intubation within 48 hours of triage on inhospital mortality, with the control group consisting of both patients that were never intubated and those that were intubated after 48 hours and 2) the effect of intubation at any point in time on mortality, with the control group consisting only of patients that were never intubated. We also carried out a sensitivity analysis comparing those that were intubated within 48 hours with those that were intubated at a later point in their hospital course, excluding patients that were never intubated.
We analyzed the rate of intubation and mortality over time by plotting the data and by calculating the per-week odds ratio (OR) for mortality and for intubation. We attempted to isolate the effect of time on intubation and mortality by adjusting this per-week OR for age, sex, race, ethnicity, hospital location, smoking status, and comorbidities. We analyzed the effect of admission hospital on rate of intubation by using mixed-effects logistic regression and treating admission hospital as a random effect (25).
For all statistical tests, nominal statistical significance was set at α = 0.05. A post hoc power analysis was carried out, and power was estimated using the Schoenfeld model (26). All statistical analyses were carried out using Version 4.0.0 of the R programming language (R Core Team, 2020, https://www.R-project.org/). Propensity score matching was carried out using the MatchIt package in R (Vienna, Austria) (27).
RESULTS
Between March 1, 2020, and December 1, 2020, a total of 82,578 adult patients were tested for COVID-19 in the ED; of these tests, 12,902 (15.6%) were positive. Of these patients, 3,377 (26.2%) were discharged home, 487 (3.8%) were transferred to another facility outside the hospital system, 129 (1.0%) left against medical advice, 272 (2.1%) died before admission, and 8,637 (66.9%) were admitted. Of these, 8,510 (98.5%) represented a unique patient admission. Of these unique patients, 263 (3.1%) died within 48 hours of triage and were excluded. Of the remaining 8,247 patients, 650 (7.9%) had DNI orders placed within 48 hours of triage and were excluded, leaving 7,597 patients in the primary data set for this study. The agreement between the manually and automatically extracted data was substantial (see Supplementary Table 1, http://links.lww.com/CCX/A673). The overall flowchart for data acquisition and inclusion is summarized in Supplementary Figure 1 (http://links.lww.com/CCX/A674).
As of December 1, 2020, 1,628 of these patients (21.4%) had been intubated and 1,898 had died (25.0%). Of the 1,628 intubations, 807 (49.6%) occurred within 48 hours of triage. The distribution of days until death or discharge and days between triage and intubation are summarized in Figure 1.
Figure 1.

Left: Hospital length of stay. Right: Days until intubation. A, Distribution of days until death or hospital discharge for 7597 coronavirus disease 2019 patients without do-not-intubate (DNI) orders; median 6.6 d (interquartile range [IQR], 3.2–12.2 d). B, Distribution of number of days between triage and intubation for 1,628 intubated patients without DNI orders; median 2.1 d (IQR, 0.4–4.9 d). For both plots, the median is displayed as a vertical black line.
Rate of Intubation Over Time and Across Hospitals
Intubation rates varied significantly from hospital to hospital, with as few as 11.4% of admitted patients being intubated to as many as 35.3%. After treating location as a random effect and treating the variables summarized above for the propensity score model as fixed effects, location was significantly associated with intubation in multilevel logistic regression (median OR, 1.60; p = 0.0013).
The dynamics of mortality and intubation over the course of the pandemic from March 1, 2020, to December 1, 2020, are summarized in Figure 2, and the associated data are included in Supplementary Table 2 (http://links.lww.com/CCX/A673). Mortality decreased over time; the per-week OR for inhospital mortality was 0.93 (0.92–0.94) (p < 0.0001). This effect remained after adjusting for age, sex, race, ethnicity, hospital location, smoking status, hospital census at admission date, intubation, and comorbidities (per-week OR, 0.96 [0.95–0.98]; p < 0.0001). Similarly, the effect remained when only including deaths that occurred within 7 days of admission (per-week OR, 0.94 [0.92–0.97]; p < 0.0001).
Figure 2.

Mortality rate and intubation rate from March 1, 2020, to December 1, 2020 (n = 7597 coronavirus disease 2019 patients without early do-not-intubate orders; 1628 intubations, 1,898 deaths). Data reported as percentages with vertical error bars representing percentage ± se; gray shading represents 95% CIs from locally estimated scatterplot smoothing.
Intubation rates also decreased with time (per-week OR, 0.96 [0.95–0.97]; p < 0.0001), an effect that remained after adjusting for the aforementioned factors (per-week OR, 0.97 [0.96 –0.98]; p < 0.0001) and when only including intubations that occurred within 7 days of admission (per-week OR, 0.96 [0.94–0.97]; p < 0.0001).
Intubation and Mortality
Before matching, intubation within 48 hours of triage was associated with increased inhospital mortality (HR, 2.26 [2.05–2.49]; p < 0.0001). After matching, intubation was still associated with increased mortality (HR, 1.30 [1.15–1.48]; p < 0.0001). Propensity score adjustment yielded similar results (HR, 1.46 [1.28–1.61]; p < 0.0001) as did overlap weighting (HR, 1.22 [1.07–1.38]; p = 0.0024). The covariate balance before and after matching is summarized in Supplementary Table 3 (http://links.lww.com/CCX/A673) and Supplementary Figure 2 (http://links.lww.com/CCX/A675); the distribution of propensity scores is summarized in Supplementary Figure 3 (http://links.lww.com/CCX/A676) and Supplementary Figure 4 (http://links.lww.com/CCX/A677).
Before matching, median survival was 10.8 days (9.8–12.1 d) for intubated patients versus 25.8 days (24.3–27.8 d) for controls; after matching, median survival was 10.8 days (9.8–12.1 d) for intubated patients versus 15.3 days (14.2–17.7 d) for controls. The survival curves for intubation within 48 hours of triage, and before and after matching are shown in Figure 3.
Figure 3.
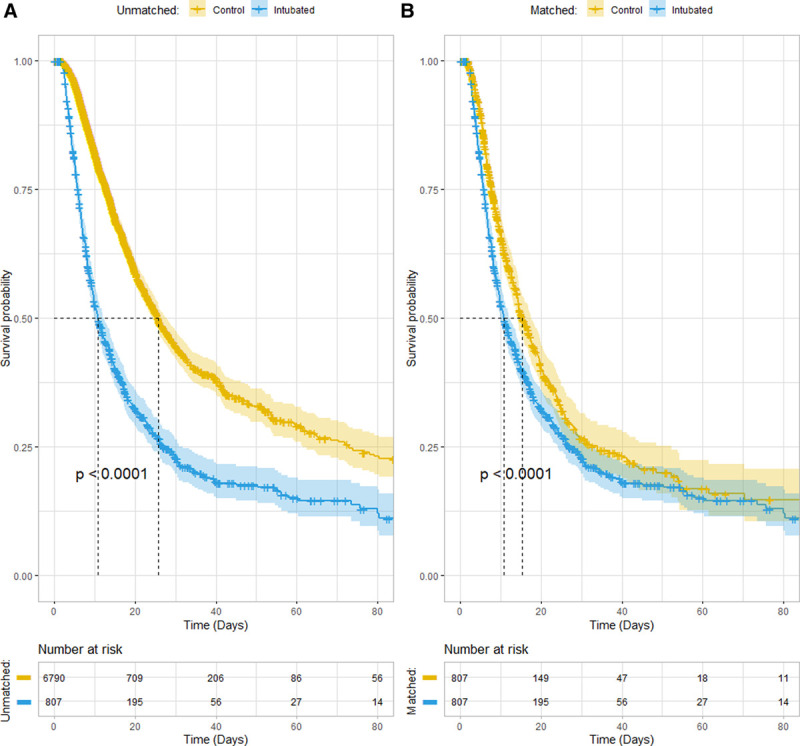
Left: Survival before matching. Right: Survival after matching. All-cause inhospital mortality Kaplan-Meier survival curves before (A) and after (B) 1:1 nearest neighbor propensity score matching without replacement, for 7,597 coronavirus disease 2019 patients without early do-not-intubate orders, 807 (10.6%) of whom were intubated within 48 hr of emergency department triage. For the unmatched group, the Cox hazard ratio (HR) was 2.26 (2.05–2.49) (p < 0.0001); after matching, the HR was 1.30 (1.15–1.48) (p < 0.0001).
Sensitivity Analyses
The association between intubation and mortality was robust to several sensitivity analyses (summarized in Supplementary Table 4, http://links.lww.com/CCX/A673). Notably, not excluding patients with DNI orders and restricting only to patients with significant oxygen requirements (those receiving HFNC, NIPPV, or mechanical ventilation) and/or those admitted to the ICU did not change the direction of the effect. In addition to the effect of intubation within 48 hours, we found intubation at any time point to still be associated with increased mortality after matching (HR, 1.62 [1.45–1.80]; p < 0.0001).
Notably, we also found that when restricting to intubated patients (n = 1628), intubation within 48 hours of triage (n = 807) was not associated with significantly increased mortality compared with those intubated later in their hospital course (HR, 1.09 [0.94–1.26]; p = 0.26).
Post Hoc Power Analysis
Given 1,898 (25.0%) deaths and 807 (10.6%) intubations within 48 hours of triage, assuming α = 0.05 and a power of 80%, our study was adequately powered to detect an HR of approximately 0.811 or an HR of approximately 1.23. For the primary effect size detected (HR, 1.30 [1.15–1.48]), our study had a post hoc power of approximately 94.1% (46.6–100.0%).
DISCUSSION
In this work, we examined patterns of invasive ventilation in COVID-19 patients admitted during the New York City coronavirus outbreak. Previous studies have focused on recommendations for safe intubations (28), reported data regarding intubation success and safety (29, 30), or analyzed the effect of timing of intubation on mortality in COVID-19 patients (13). To our knowledge, this is the first study that has directly compared outcomes for intubated and nonintubated COVID-19 patients using propensity score matching.
We found rates of intubation to vary significantly from hospital to hospital, with providers at certain hospitals having intubated significantly more patients than others, even after adjusting for a variety of available confounders such as age or markers of disease severity. This difference may be due to local practice standards (such as lower clinical thresholds to intubate) that may have been amplified by the uncertainty of a novel viral pandemic. We found intubation to have decreased significantly over time, alongside mortality, and relationships that again remained stable after controlling for potential confounding variables. A portion of this change may be due to less severe cases presenting later in the pandemic; some amount, however, may be due to changes in clinical practice as understanding of the disease and treatment options grew.
In the early stages of the pandemic, it was uncertain which patients would benefit from early intubation and mechanical ventilation. Some researchers and groups argued for early intubation (3, 13, 31), whereas others cautioned against it (2, 7, 32). The potential benefit of adjunctive airway treatments such as HFNC or NIPPV (33, 34) or awake proning (35, 36) was uncertain. Hence, even within one hospital system and after controlling for confounders, there was significant variation in rates of intubation.
After propensity score matching, we found intubation to be associated with increased mortality for COVID-19 patients, and this relationship remained robust to several sensitivity analyses. Intubation and mechanical ventilation carry a variety of risks. Early complications of tracheal intubation include cardiovascular collapse (37), hypoxemia (38), and aspiration (39). Longer term complications include ventilator-induced lung injury (40) and ventilator-associated pneumonia (41). Mortality in intubated COVID-19 patients is remarkably high (42). There have been reports of increased rates of barotrauma (43), high rates of ventilator-associated pneumonia (44), as well as severe acute kidney injury resulting from intubation in these patients (45). Additionally, mechanically ventilated patients require substantially increased nursing and provider time, resources that may become increasingly limited during a pandemic surge (46). A recent study found that greater ICU patient load was associated with increased mortality for COVID-19 patients (47).
Notably, we did not find a significant association between mortality and timing of intubation (within 48 hr of triage vs later in hospital course), when restricting only to intubated patients. This differs from the results of Hyman et al (13), who found that earlier intubation was associated with significantly reduced mortality, with each additional day increasing the hazard of death by 3%. The differences between our results and theirs may be due to baseline differences in populations, differing clinical decision thresholds for intubation, the inclusion of early laboratory markers of disease severity in our propensity score model (such as ferritin and d-dimer), or different study timeframes (from March 1, 2020, to December 1, 2020, in our study vs from January 30, 2020, to April 30, 2020, in theirs). Their study also focused on timing of intubation and did not compare intubated patients with nonintubated patients. Further studies from other populations and locations investigating this issue are needed.
Our study has several advantages. We drew data from a large, heterogeneous population across multiple centers in a city with significant racial, ethnic, and socioeconomic diversity. We used nearest neighbor propensity score matching and adjustment, which has theoretical advantages over multivariate regression (48). We carefully selected the covariates included in the propensity score model and achieved good covariate balance. We attempted to reduce the effect of immortal time bias (16, 17) by treating as exposure patients that were intubated within 48 hours of triage. We performed several sensitivity analyses and found the primary effect to be robust to them.
This work also has multiple limitations. As a retrospective observational study, unmeasured confounding cannot be eliminated, and the design precludes us from drawing causal conclusions about intubation and mortality. The association between intubation and mortality after propensity matching/adjustment may still be due to more severely ill patients being intubated. Although there was substantial agreement between automatically and manually extracted chart data, there was little available information on whether patients were receiving oxygen therapy at the exact time the initial vitals were recorded. Similarly, we did not have access to exact information about these therapies, such as the effective Fio2 being delivered. By restricting to patients that were intubated within 48 hours of triage, the power of our study was reduced. However, one of the sensitivity analyses we performed did not restrict to intubation within 48 hours, and in post hoc power analysis, we still had sufficient power to detect the effects identified in this study. Our analysis did not use time-varying covariates, as we only used data available at the time of triage, or the exposure (intubation) and outcome (mortality).
CONCLUSIONS
Given the wide variation of intubation rates across hospitals and time points in this cohort, alongside the robust effect of increased mortality in intubated patients, there may have been patients who were intubated prematurely or unnecessarily. The decision to intubate is complex, even more so in face of an unprecedented viral pandemic. Future prospective studies should further explore the impact of intubation on mortality in COVID-19 patients and other techniques that may reduce or delay the need for intubation in these patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Parish helped in study design, data acquisition, data processing, statistical analysis, and article writing. Dr. West helped in study design, statistical analysis, and article writing and editing. Drs. Caputo and Singer contributed to study design, and article writing and editing. Drs. Janus and Yuan helped in data acquisition, and article writing and editing. Dr. Zhang contributed to data acquisition, data processing, study design, and article editing.
Research was supported, in part, by a grant from the Patient Care Trust Fund of the Committee of Interns & Residents.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schünemann HJ, Khabsa J, Solo K, et al. Ventilation techniques and risk for transmission of coronavirus disease, including COVID-19: A living systematic review of multiple streams of evidence. Ann Intern Med. 2020; 173:204–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rola P, Farkas J, Spiegel R, et al. Rethinking the early intubation paradigm of COVID-19: Time to change gears? Clin Exp Emerg Med. 2020; 7:78–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020; 201:1319–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020; 17:787–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016; 44:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020; 323:2329–2330 [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. ; and the Emory COVID-19 Quality and Clinical Research Collaborative. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: A single-center cohort study. Crit Care Med. 2020; 48:e1045–e1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Shen J, Chen L, et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. 2020; 89:1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman JB, Leibner ES, Tandon P, et al. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit Care Explor. 2020; 2:e0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epic Systems Corporation: Epic Electronic Medical Records. 2020. Available at: https://www.epic.com/software. Accessed December 22, 2020
- 15.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009; 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lévesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ. 2010; 340:b5087. [DOI] [PubMed] [Google Scholar]
- 17.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008; 167:492–499 [DOI] [PubMed] [Google Scholar]
- 18.Cox D. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972; 34:187–202 [Google Scholar]
- 19.Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc. 1988; 83:414–425 [Google Scholar]
- 20.Austin P. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2013; 33:1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009; 28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vansteelandt S, Daniel RM. On regression adjustment for the propensity score. Stat Med. 2014; 33:4053–4072 [DOI] [PubMed] [Google Scholar]
- 23.Thomas LE, Li F, Pencina MJ. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020; 323:2417–2418 [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol. 2006; 163:1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006; 60:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983; 39:499–503 [PubMed] [Google Scholar]
- 27.Ho DE, Imai K, King G, et al. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011; 42:1–28 [Google Scholar]
- 28.Mahdavinia M, Foster KJ, Jauregui E, et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020; 8:2388–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad I, Jeyarajah J, Nair G, et al. A prospective, observational, cohort study of airway management of patients with Covid-19 by specialist tracheal intubation teams. Can J Anaesth. 2020; 68:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao W, Wang T, Jiang B, et al. ; collaborators. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br J Anaesth. 2020; 125:e28–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewster DJ, Chrimes N, Do TB, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020; 212:472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, Choi K, Choi S, et al. Clinical significance of timing of intubation in critically ill patients with Covid-19: A multi-center retrospective study. J Clin Med. 2020; 9:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhazzani W, Møller M, Arabi Y, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (Covid-19). Intensive Care Med. 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: Systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020; 67:1217–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubosh N, Wong M, Grossestreuer A, et al. Early, awake proning in emergency department patients with Covid-19. Am J Emerg Med. 2020 Dec 3. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albert RK, Keniston A, Baboi L, et al. ; Proseva Investigators. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014; 189:494–496 [DOI] [PubMed] [Google Scholar]
- 37.De Jong A, Rolle A, Molinari N, et al. Cardiac arrest and mortality related to intubation procedure in critically ill adult patients: A multicenter cohort study. Crit Care Med. 2018; 46:532–539 [DOI] [PubMed] [Google Scholar]
- 38.Bodily JB, Webb HR, Weiss SJ, et al. Incidence and duration of continuously measured oxygen desaturation during emergency department intubation. Ann Emerg Med. 2016; 67:389–395 [DOI] [PubMed] [Google Scholar]
- 39.Driver BE, Klein LR, Schick AL, et al. The occurrence of aspiration pneumonia after emergency endotracheal intubation. Am J Emerg Med. 2018; 36:193–196 [DOI] [PubMed] [Google Scholar]
- 40.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 41.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002; 165:867–903 [DOI] [PubMed] [Google Scholar]
- 42.Fried M, Crawford J, Mospan A, et al. Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States. Clin Infect Dis. 2020; 72:e558–e565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuinness G, Zhan C, Rosenberg N, et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020; 297:E252–E262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gamberini L, Tonetti T, Spadaro S, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: Multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020; 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaibi K, Dao M, Pham T, et al. Severe acute kidney injury in patients with COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020; 202:1299–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020; 382:2049–2055 [DOI] [PubMed] [Google Scholar]
- 47.Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs Hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: Propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018; 53:1112–1117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


