Abstract
Background
We developed iodine-coated titanium implants to suppress microbial activity and prevent periprosthetic joint infection (PJI); their efficacy was demonstrated in animal and in vitro models. The iodine content in iodine-coated implants naturally decreases in vivo. However, to our knowledge, the effect of reduced iodine content on the implant’s antimicrobial activity has not been evaluated to date.
Questions/purposes
(1) How much does the iodine content on the implant surface decrease after 4 and 8 weeks in vivo in a rat model? (2) What effect does the reduced iodine content have on the antimicrobial effect of the implant against multiple bacteria in an in vitro model?
Methods
This experiment was performed in two parts: an in vivo experiment to determine attenuation of iodine levels over time in rats, and an in vitro experiment in which we sought to assess whether the reduced iodine content observed in the in vivo experiment was still sufficient to deliver antimicrobial activity against common pathogens seen in PJI. For the in vivo experiment, three types of titanium alloy washers were implanted in rats: untreated (Ti), surface-anodized to produce an oxide film (Ti-O), and with an iodine layer on the oxidation film (Ti-I). The attenuation of iodine levels in rats was measured over time using inductively coupled plasma-mass spectrometry. Herein, only the Ti-I washer was used, with five implanted in each rat that were removed after 4 or 8 weeks. For the 4- and 8-week models, two rats and 15 washers were used. For the in vitro study, to determine the antibacterial effect, three types of washers (Ti, Ti-O, and Ti-I) (nine washers in total) were implanted in each rat. Then, the washers were removed and the antibacterial effect of each washer was examined on multiple bacterial species using the spread plate method and fluorescence microscopy. For the spread plate method, six rats were used, and five rats were used for the observation using fluorescence microscopy; further, 4- and 8-week models were made for each method. Thus, a total of 22 rats and 198 washers were used. Live and dead bacteria in the biofilm were stained, and the biofilm coverage percentage for quantitative analysis was determined using fluorescence microscopy in a nonblinded manner. Ti-I was used as the experimental group, and Ti and Ti-O were used as control groups. The total number of rats and washers used throughout this study was 24 and 213, respectively.
Results
Iodine content in rats implanted with Ti-I samples decreased to 72% and 65% after the in vivo period of 4 and 8 weeks, respectively (p = 0.001 and p < 0.001, respectively). In the in vitro experiment, the Ti-I implants demonstrated a stronger antimicrobial activity than Ti and Ti-O implants in the 4- and 8-week models. Both the median number of bacterial colonies and the median biofilm coverage percentage with live bacteria on Ti-I were lower than those on Ti or Ti-O implants for each bacterial species in the 4- and 8-week models. There was no difference in the median biofilm coverage percentage of dead bacteria. In the 8-week model, the antibacterial activity using the spread plate method had median (interquartile range) numbers of bacteria on the Ti, Ti-O, and Ti-I implants of 112 (104 to 165) × 105, 147 (111 to 162) × 105, and 55 (37 to 67) × 105 of methicillin-sensitive Staphylococcus aureus (Ti-I versus Ti, p = 0.026; Ti-I versus Ti-O, p = 0.009); 71 (39 to 111) × 105, 50 (44 to 62) × 105, and 26 (9 to 31)× 105 CFU of methicillin-resistant S. aureus (Ti-I versus Ti, p = 0.026; Ti-I versus Ti-O, p = 0.034); and 77 (74 to 83) × 106, 111 (95 to 117) × 106, and 30 (21 to 45) × 106 CFU of Pseudomonas aeruginosa (Ti-I versus Ti, p = 0.004; Ti-I versus Ti-O, p = 0.009). Despite the decrease in the iodine content of Ti-I after 8 weeks, it demonstrated better antibacterial activity against all tested bacteria than the Ti and Ti-O implants.
Conclusion
Iodine-coated implants retained their iodine content and antibacterial activity against methicillin-sensitive S. aureus, methicillin-resistant S. aureus, and P. aeruginosa for 8 weeks in vivo in rats. To evaluate the longer-lasting antibacterial efficacy, further research using larger infected animal PJI models with implants in the joints of both males and females is desirable.
Clinical Relevance
Iodine-coated titanium implants displayed an antibacterial activity for 8 weeks in rats in vivo. Although the findings in a rat model do not guarantee efficacy in humans, they represent an important step toward clinical application.
Introduction
The incidence of periprosthetic joint infection (PJI) after THA and TKA is 1% [5] and 1% to 2% [19], respectively, whereas that of postoperative infection for spinal surgery and pin-site infection for external fixation is 2% to 13% [8] and 50% to 65% [6, 21], respectively. Treating PJI is often challenging due to the biofilm on the implant surface. Bacteria in biofilms have ≥ 1000 times more resistance to antibiotics than do planktonic bacteria [24], and poor vascularization of the bone and implant interface makes infections extremely difficult to treat [18]. Gristina et al. [10] proposed the phrase “race for the surface,” suggesting there is a competition between host tissue cell integration and bacterial cell attachment to the implant surface. Hence, it is desirable to impart antimicrobial activity to the implant for preventing biofilm formation.
We therefore developed iodine-coated titanium implants to suppress microbial activity and prevent PJI and postoperative infection, and their efficacy was demonstrated in animal and in vitro models [29, 34]. Inoue et al. [15] showed that iodine-coated implants had an in vitro biofilm inhibition effect against methicillin-sensitive Staphylococcus aureus (MSSA) and had good antimicrobial effects against methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, methicillin-sensitive S. epidermidis, and Candida albicans [14]. Iodine-coated implants were partly incorporated into the guidelines at the 2018 International Consensus Meeting on Musculoskeletal Infection [1] because of their potential to prevent infection.
Several antimicrobial implants with silver [35], copper [9], magnesium [12], antibiotic [20, 33], chitosan [3], and other surface treatments have been reported. However, for most antibacterial coating surfaces reported previously [9, 12, 20, 33], only the initial antimicrobial effect of the implant was examined, and there was a lack of data on the residual amount of coating after implantation. In addition, the duration of the antimicrobial effect was very short for silver and antibiotic coatings [20, 33, 35]. The advantage of iodine over other antimicrobial agents is its relatively long duration of antibacterial activity and broad-spectrum antimicrobial activity. Small amounts of free iodine are constantly released, exerting the antimicrobial characteristic of iodine until the available iodine is exhausted [37].
We therefore suggested that iodine-coated implants can have a longer-lasting antimicrobial effect than conventional antimicrobial implants in multiple species of bacteria that form biofilms.
Therefore, this study addressed the following questions: (1) How much does the iodine content on the implant surface decrease after 4 and 8 weeks in vivo in a rat model? (2) What effect does the reduced iodine content have on the antimicrobial effect of the implant against multiple bacteria in an in vitro model?
Materials and Methods
Experimental Overview
This experiment was performed in two parts: an in vivo experiment to determine attenuation of iodine levels over time in rats, and an in vitro experiment in which we sought to assess whether the reduced iodine content observed in the in vivo experiment still was sufficient to deliver antimicrobial activity against common pathogens seen in PJI (Fig. 1).
Fig. 1.
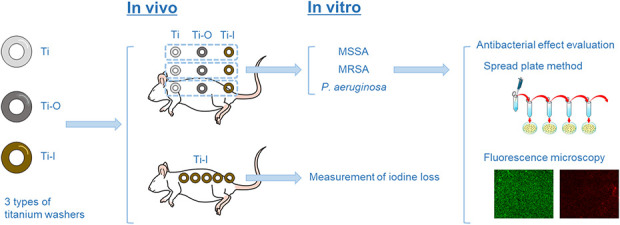
In our experimental design, three types of Ti-6Al-4V titanium washer implants were used: untreated (Ti), surface-anodized to produce an oxide film (Ti-O), and with an iodine layer on the oxidation film (Ti-I); MSSA= methicillin-sensitive Staphylococcus aureus; MRSA = methicillin-resistant Staphylococcus aureus; P. aeruginosa = Pseudomonas aeruginosa. A color image accompanies the online version of this article.
Study Design
In the in vivo experiment, titanium alloy (Ti-6Al-4V) washers (diameter: 6 mm, thickness: 0.5 mm, and mean weight: 0.044 g) were implanted in rats. The washers were of three types: untreated (Ti), surface-anodized to produce an oxide film (Ti-O), and with an iodine layer on the oxidation film (Ti-I). To measure the attenuation of iodine levels in rats over time, only the Ti-I washer was used; five were implanted in each rat and removed after 4 or 8 weeks. To determine the antibacterial effect of each implant using the spread plate method and fluorescence microscopy, a total of nine washers (three washers of each type) were implanted in each rat. Then, each washer was removed and its antibacterial effect on three bacterial species (MSSA, MRSA, P. aeruginosa) was examined using the spread plate method and fluorescence in a nonblinded in vitro study. Here, Ti-I was used as the experimental group, and Ti and Ti-O were used as control groups.
Experimental Procedures and Animals
Twenty-four male 10-week-old Sprague-Dawley rats (Japan Charles River), weighing 300 to 350 g each, were used in this study, as in the previous study [15]. An all-male group was used to keep the animals’ sizes relatively consistent during the experiment. Surgery was performed under aseptic conditions while the rats were under general anesthesia (medetomidine: 0.15 mg/kg, midazolam: 2.0 mg/kg, and butorphanol: 2.5 mg/kg body weight, injected intraperitoneally). The implants were sterilized by ethylene oxide gas sterilization before use. The Ti-I samples were implanted for 4 or 8 weeks (one rat at each point) subcutaneously on the back of the rats to make the iodine attenuation model in vivo. Similarly, Ti and Ti-O samples were subcutaneously implanted near the Ti-I samples as control samples to ensure a similar condition.
The rats were euthanized at 4 and 8 weeks postoperatively using intravenously administered thiopental sodium (100 mg/kg body weight), and the three types of implants (Ti, Ti-O, and Ti-I) were removed under aseptic conditions. These washers were placed in 24-well plates containing 1 mL of each bacterium. The solution was incubated for 24 hours at 37°C to permit the bacteria to form biofilms. After 24 hours, the solution containing MSSA or MRSA was replaced with fresh tryptic soy broth medium, and the solution containing P. aeruginosa was replaced with fresh lysogeny broth medium. Then, each solution was incubated for another 24 hours at 37°C to form a matured biofilm, which was examined by the standard spread plate method for quantitative evaluation and fluorescence microscopy for quantitative and qualitative evaluation.
Housing and Husbandry
All the rats used in this study lived in a cage under a controlled temperature (23 ± 3°C) and humidity (55% ± 10%), had a certified diet (CRF-1; Oriental Yeast Co) and water (chlorine concentration; 10 ppm), and 12 hours of daily light.
Sample Size
In total, we used 24 rats and 213 washers during this study. Two rats, each with five Ti-I washers implanted for 4 and 8 weeks, were used to investigate iodine attenuation. Five Ti-I washers were used at each point before implantation and 4 and 8 weeks after implantation, for a total of 15 Ti-I washers. Six rats were used for the spread plate method and five for the observation using the fluorescence microscope. Further, 4- and 8-week models were made for each method, for a total of 22 rats. Each of the 22 rats was implanted with three washers of each type (Ti, Ti-O, and Ti-I); nine washers were implanted in each rat, for a total of 198 washers.
Primary and Secondary Study Outcomes
Our primary study goal was to determine how much the iodine content on the surface of the implant decreased after 4 and 8 weeks in vivo in a rat model. Our secondary study goal was to determine what effect the reduced iodine content has on the antimicrobial effect of the implant against three bacteria in an in vitro model.
Preparation of Implants
The titanium washer implants used in this study had the same specifications as in a previous study [15]. The iodine-coated processing was conducted using a technique described previously [13]. First, a 5-μm to 10-μm-thick anodic oxide film with more than 50,000 pores/mm2 was formed on the surface of the titanium implants. This treatment formed a porous structure on the surface of the Ti-O and Ti-I samples. Then, ionized iodine was electrodeposited in the pores of the Ti-I sample to impart antimicrobial activity. The mean iodine content was approximately 10 µg/cm2 to 12 µg/cm2. The formation of porous oxide films on the surfaces of Ti-O and Ti-I by iodine-coated processing was observed via scanning electron microscopy (SEM) (Fig. 2). All implants were processed by the Promedical Instruments Company.
Fig. 2.
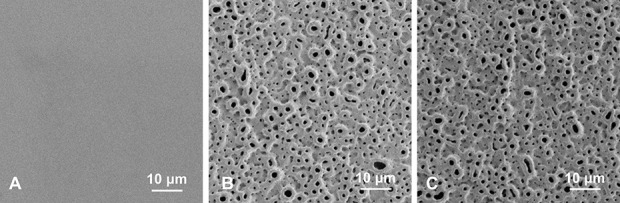
A-C These scanning electron microscopy images show the (A) untreated Ti, (B) Ti-O, and (C) Ti-I implants used in this study.
Measurement of Iodine Loss
To investigate iodine loss from the implant in vivo, we used inductively coupled plasma-mass spectrometry (Agilent7800; Agilent Technologies Japan Ltd). In preparation for using inductively coupled plasma-mass spectrometry, we placed the Ti-I samples in 10 mL of 12.5% tetramethyl ammonium hydroxide solution (Tama Chemical Co) at 60°C for 9 hours to dissolve the anodic oxide film. This treatment eliminated the multiporous structure on the surface of the Ti-I implant. The solution was sonicated for 15 minutes to further dissolve the anodic oxide film. The suspension was filtered using a 0.45-μm membrane filter, and only ionized iodine was extracted using inductively coupled plasma-mass spectrometry. This procedure was performed by the Fukushima Medical Device Development Support Centre. The extracted iodine content was measured five times at each point of time (0, 4, and 8 weeks), and the mean values are stated here.
Biofilm Formation
To investigate the antibacterial effect, MSSA (American Type Culture Collection strain 25923), MRSA (American Type Culture Collection strain 43300), and P. aeruginosa (American Type Culture Collection strain 27853) were used because they tend to form biofilms. MSSA and MRSA were incubated in 5 mL of fresh tryptic soy broth (Becton Dickinson) at 37°C for 24 hours. This culture was diluted 100-fold in tryptic soy broth and reincubated at 37°C. The reincubating medium was grown to the early exponential growth phase at an optical density of 600 = 0.2 to 0.3 in MSSA [14] and -0.4 in MRSA [23], corresponding to 1 to 5 × 107 colony-forming units/mL. P. aeruginosa was incubated in 2 mL of fresh lysogeny broth (Sigma-Aldrich) at 37°C for 24 hours, based on a previous report [25]. This culture was diluted 50-fold in lysogeny broth and reincubated at 37°C. The medium was grown to an optical density of 600 = 0.1, corresponding to 1 to 5 × 105 colony-forming units/mL. The procedure of biofilm formation was based on that used in previous reports [15, 23, 25].
Quantitative Evaluation with the Spread Plate Method
Each washer with a matured biofilm was rinsed twice with phosphate-buffered saline to remove unattached bacteria on the metal surface, as well as planktonic bacteria. Then, the washers were placed in 1.5-mL microtubes with 1 mL of phosphate-buffered saline. Based on a past report [15], we performed vortex mixing of the solution for 15 seconds, followed by sonication for 5 minutes (Bransonic Model 3800, Branson) at a frequency of 40 kHz to disrupt the biofilm on the washers. Then, the solution was vortex-mixed for another 1 minute. Subsequently, the spread plate method was applied to quantitatively evaluate the biofilm, which involved 10-fold serial dilution of the solution containing each bacterium from the biofilm with phosphate-buffered saline, followed by culturing of the bacterial suspension on an agar plate at 37°C for 24 hours. MSSA and MRSA were cultured on tryptic soy broth agar plates, and P. aeruginosa was cultured on a lysogeny broth agar plate. After 24 hours, the number of bacterial colonies was counted. This evaluation was repeated six times for each bacterial species. The assessors were not blinded to the implant type at the time of this evaluation as it was not possible to mark the implant itself.
Qualitative and Quantitative Evaluation with Fluorescence Microscopy
Each washer with a matured biofilm was rinsed in sterilized water to remove unattached and planktonic bacteria. Then, the biofilms were stained using the FilmTracer™ LIVE⁄DEAD Biofilm Viability kit (Molecular Probes, Invitrogen). The dyeing solution consists of two fluorescent dyes: SYTO-9 as a green-fluorescent nucleic acid stain and propidium iodide as a red-fluorescent nucleic acid stain. The SYTO-9 dye stains bacteria with intact cell membranes (live bacteria) in fluorescent green, while propidium iodide stains bacteria with damaged cell membranes (dead bacteria) in fluorescent red (Fig. 3). Based on the product protocol, each sample was stained with 200 mL of a mixture of SYTO-9, propidium iodide, and sterilized water and incubated for 20 minutes in darkness at room temperature. Then, the samples were gently rinsed with sterilized water to remove all excess stain. The samples were placed in a 35-mm-diameter glass-bottom dish (D11141H, Matsunami Glass Ind. Ltd.) and examined using fluorescence microscopy (BZ-X700, Keyence). Based on past reports [2, 15], we investigated the biofilm coverage percentage on the washer to measure the percentage of washer surface covered by the biofilm. The biofilm coverage percentage was investigated separately for live and dead bacteria. A lens magnification of 4 x was used when observing the entire washer, while 20 x was used when observing eight areas of each washer to calculate the biofilm coverage percentage. The biofilm coverage percentage was measured five times for each bacterial species.
Fig. 3.
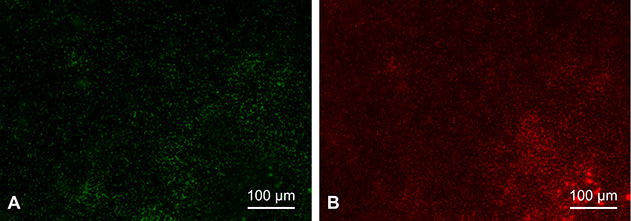
A-B These fluorescence microscopy images show the MRSA biofilm. The same area was imaged at a lens magnification of 20 x under (A) green fluorescence conditions to observe the live bacteria stained by SYTO-9 and (B) red fluorescence conditions to observe the dead bacteria stained by propidium iodide. A color image accompanies the online version of this article.
Ethical Approval
The investigational protocol and animal procedure in this study were approved by the animal ethics committee of our institution (approval number: AP-204141).
Statistical Analyses
Statistical analyses were performed using a statistical software program (SPSS version 24.0 software for Windows, SPSS Inc). Data distribution was assessed using the Shapiro-Wilk test. Quantitative data were examined using the t-test and the Mann-Whitney U test for normally (iodine content) and nonnormally (the number of bacterial colonies and the biofilm coverage percentage) distributed data, respectively. A p value < 0.05 was considered statistically significant.
Results
In Vivo (Rat Model) Reduction of Implant Iodine Content
Before using inductively coupled plasma-mass spectrometry, via scanning electron microscopy, we observed dissolution of the anodic oxide film (Fig. 4).
Fig. 4.
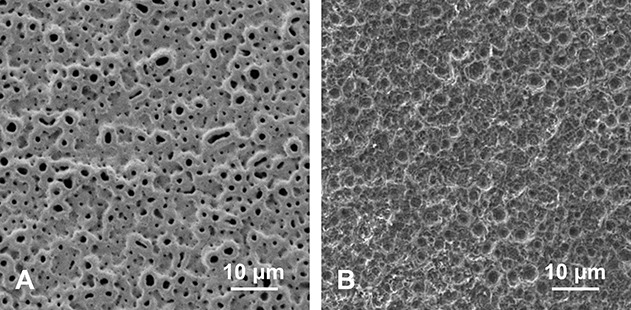
A-B These scanning electron microscopy images were taken (A) before and (B) after the multiporous structure on the surface of Ti-I was removed.
Iodine content of the Ti-I samples decreased over time in this rat model. After defining the iodine content before implant placement as 100%, we found that the iodine content decreased to 72% and 65% of the initial value after an in vivo period of 4 and 8 weeks, respectively (p = 0.001 and p < 0.001, respectively). Iodine content decreased at 4 weeks postimplantation compared with preimplantation (104 ± 7 and 146 ± 13 µg/g, respectively; mean difference 39.7 [95% CI 22.7 to 56.7]; p = 0.001), and the same was true at 8 weeks postimplantation (95 ± 7 and 146 ± 13 µg/g, respectively; mean difference 49.3 [95% CI 32.6 to 66.0]; p < 0.001). However, no difference was detected between 4 and 8 weeks postimplantation in this rat model (104 ± 7 and 95 ± 7 µg/g, respectively; mean difference 9.6 [95% CI -1.7 to 21.0]; p = 0.09) (Fig. 5).
Fig. 5.
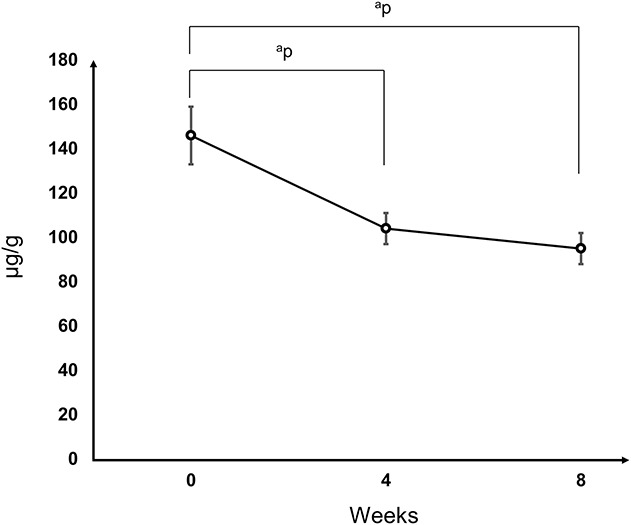
Trend of the iodine content in Ti-I samples in the rat model over time. The iodine content decreased to 72% and 65% of the initial value after a period of 4 and 8 weeks, respectively. ap<0.01.
Antimicrobial Activity In Vitro
After 4 and 8 weeks in vivo in rats, Ti-I implants inhibited the development of bacterial colonies and biofilm formation in vitro compared with Ti and Ti-O implants.
The antibacterial activity in the 4-week model had median (interquartile range) numbers of bacteria on the Ti, Ti-O, and Ti-I implants of 78 (67 to 89) × 104, 113 (86 to 173) × 104, and 12 (9 to 20) × 104 CFU of MSSA, respectively (Ti-I versus Ti, p = 0.004; Ti-I versus Ti-O, p = 0.009). Further, the antibacterial activity was 16 (9 to 24) × 104, 38 (20 to 78) × 104, and 6 (5 to 7) × 104 CFU of MRSA, respectively (Ti-I versus Ti, p = 0.009; Ti-I versus Ti-O, p = 0.002); and 14 (11 to 17) × 106, 25 (22 to 34) × 106, and 5 (5 to 7) × 106 CFU of P. aeruginosa (Ti-I versus Ti, p = 0.009; Ti-I versus Ti-O, p = 0.002), respectively (Fig. 6A). The corresponding numbers for the 8-week model were 112 (104 to 165) × 105, 147 (111 to 162) × 105, and 55 (37 to 67) × 105 of MSSA, respectively (Ti-I versus Ti, p = 0.026; Ti-I versus Ti-O, p = 0.009). Those numbers were 71 (39 to 111) × 105, 50 (44 to 62) × 105, and 26 (9 to 31)× 105 CFU of MRSA, respectively (Ti-I versus Ti, p = 0.026; Ti-I versus Ti-O, p = 0.034); and 77 (74 to 83) × 106, 111 (95 to 117) × 106, and 30 (21 to 45) × 106 CFU of P. aeruginosa, respectively (Ti-I versus Ti, p = 0.004; Ti-I versus Ti-O, p = 0.009) (Fig. 6B). After 4 and 8 weeks, the median number of bacteria on Ti-I was lower than that on the Ti or Ti-O implants for all bacterial species. However, there was no difference between the Ti and Ti-O implants.
Fig. 6.
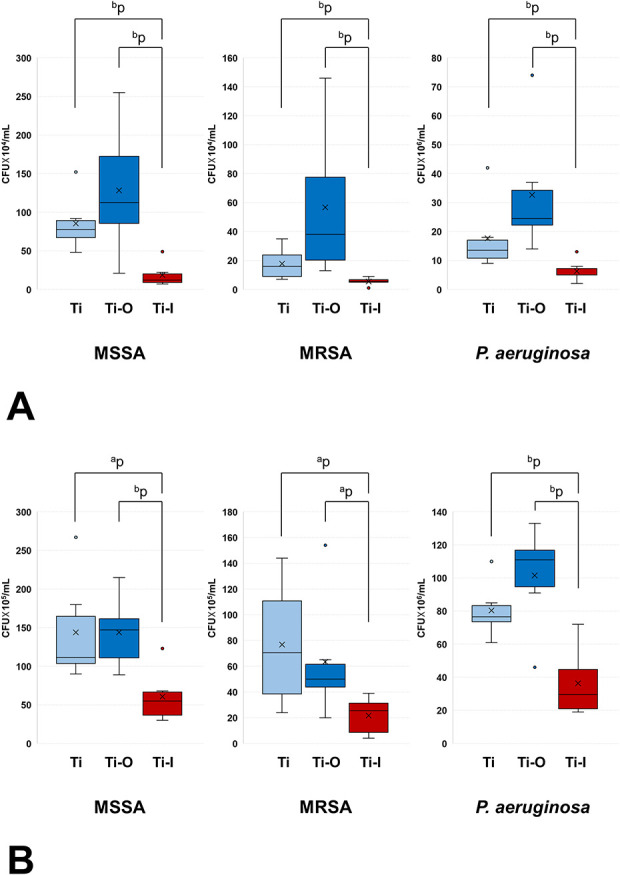
A-B Antibacterial activity was determined using the spread plate method for implants subjected to (A) 4-week and (B) 8-week indwelling in rats. ap<0.05 and bp<0.01; CFU = colony-forming unit.
Fluorescence microscopy images at 4 x lens magnification (Fig. 7) indicated that the Ti-I implants had fewer live bacteria and more dead bacteria than the Ti and Ti-O implants. In the 4-week model, the median biofilm coverage percentage (interquartile range) with live bacteria on Ti, Ti-O, and Ti-I was 32% (27% to 32%), 27% (23% to 37%), and 8% (3% to 17%) of MSSA, respectively (Ti-I versus Ti, p = 0.032; Ti-I versus Ti-O, p = 0.016). The median biofilm coverage percentage was 34% (25% to 39%), 43% (31% to 43%), and 17% (10% to 18%) of MRSA, respectively (Ti-I versus Ti, p = 0.03; Ti-I versus Ti-O, p = 0.008); and it was 25% (14% to 26%), 25% (22% to 25%), and 3% (3% to 7%) of P. aeruginosa (Ti-I versus Ti, p = 0.03; Ti-I versus Ti-O, p = 0.032), respectively (Table 1). In the 8-week model, the median biofilm coverage percentage (interquartile range) with live bacteria on Ti, Ti-O, and Ti-I was 12% (9% to 35%), 15% (14% to 16%), and 1% (1% to 2%) of MSSA, respectively (Ti-I versus Ti, p = 0.016; Ti-I versus Ti-O, p = 0.008). The median biofilm coverage percentage was 27% (22% to 42%), 59% (57% to 62%), and 6% (4% to 7%) of MRSA, respectively (Ti-I versus Ti, p = 0.008; Ti-I versus Ti-O, p = 0.008); 11% (10% to 13%), 17% (13% to 17%), and 1% (1% to 5%) of P. aeruginosa (Ti-I versus Ti, p = 0.008; Ti-I versus Ti-O, p = 0.008), respectively (Table 2). For the 4-week and 8-week models, the median biofilm coverage percentage with live bacteria on Ti-I was lower than those on Ti or Ti-O implants for each type of bacteria, and there was no difference between the Ti and Ti-O implants.
Fig. 7.
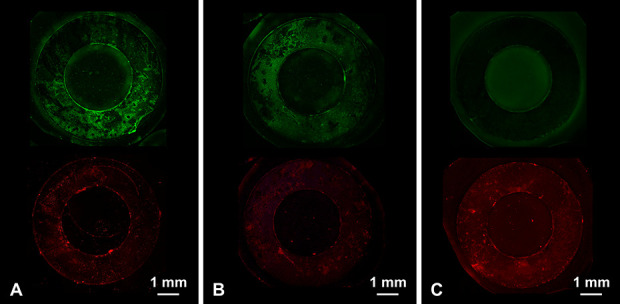
A-C These fluorescence microscopy images show the 8-week model with MSSA biofilms (4 x lens magnification) for (A) untreated Ti, (B) Ti-O, and (C) Ti-I implants. Images for each sample type were acquired from the same area of interest but under different fluorescence conditions: (top) live bacteria stained green and (bottom) dead bacteria stained red.
Table 1.
The median biofilm coverage percentage (BCP) for live and dead bacteria in the 4-week model
| Bacterial species | BCP for live bacteria, % | BCP for dead bacteria, % | ||||
| Ti | Ti-O | Ti-I | Ti | Ti-O | Ti-I | |
| MSSA, % | 32 (27-32) | 27 (23-37) | 8 (3-17) | 4 (3-5) | 9 (4-17) | 11 (5-31) |
| p value | ||||||
| Ti-I versus Ti | 0.032 | 0.222 | ||||
| Ti-I versus Ti-O | 0.016 | 0.548 | ||||
| Ti-O versus Ti | 0.841 | 0.310 | ||||
| MRSA | 34 (25-39) | 43 (31-43) | 17 (10-18) | 7 (3-20) | 20 (7-24) | 28 (22-30) |
| p value | ||||||
| Ti-I versus Ti | 0.032 | 0.222 | ||||
| Ti-I versus Ti-O | 0.008 | 0.310 | ||||
| Ti-O versus Ti | 0.548 | 0.548 | ||||
| P. aeruginosa | 25 (14-26) | 25 (22-25) | 3 (3-7) | 6 (4-7) | 13 (11-22) | 14 (12-17) |
| p value | ||||||
| Ti-I versus Ti | 0.032 | 0.056 | ||||
| Ti-I versus Ti-O | 0.032 | > 0.99 | ||||
| Ti-O versus Ti | > 0.99 | 0.056 | ||||
The data are presented as the median (interquartile range). Three types of Ti-6Al-4V titanium washer implants: untreated (Ti), surface-anodized to produce an oxide film (Ti-O), and with an iodine layer on the oxidation film (Ti-I).
Table 2.
The median biofilm coverage percentage (BCP) for live and dead bacteria in the 8-week model
| Bacterial species | BCP for live bacteria, % | BCP for dead bacteria, % | ||||
| Ti | Ti-O | Ti-I | Ti | Ti-O | Ti-I | |
| MSSA | 12 (9-35) | 15 (14-16) | 1 (1-2) | 3 (2-7) | 7 (4-8) | 8 (6-9) |
| p value | ||||||
| Ti-I versus Ti | 0.016 | 0.310 | ||||
| Ti-I versus Ti-O | 0.008 | 0.690 | ||||
| Ti-O versus Ti | 0.841 | 0.310 | ||||
| MRSA | 27 (22-42) | 59 (57-62) | 6 (4-7) | 6 (4-6) | 7 (7-11) | 8 (4-10) |
| p value | ||||||
| Ti-I versus Ti | 0.008 | 0.421 | ||||
| Ti-I versus Ti-O | 0.008 | 0.841 | ||||
| Ti-O versus Ti | 0.032 | 0.052 | ||||
| P. aeruginosa | 11 (10-13) | 17 (13-17) | 1 (1-5) | 5 (5-5) | 12 (3-13) | 7 (5-8) |
| p value | ||||||
| Ti-I versus Ti | 0.008 | 0.548 | ||||
| Ti-I versus Ti-O | 0.008 | 0.548 | ||||
| Ti-O versus Ti | 0.548 | 0.548 | ||||
The data are presented as the median (interquartile range). Three types of Ti-6Al-4V titanium washer implants: untreated (Ti), surface-anodized to produce an oxide film (Ti-O), and with an iodine layer on the oxidation film (Ti-I).
Discussion
PJI adversely affects patient quality of life [32] and mortality [11], but it remains an unresolved problem because the formation of biofilms on the implant surface inhibits effective treatment. The iodine-coated implants developed in this study may be effective in preventing postoperative infections because of the long-term antibacterial effect expected from the characteristics of iodine [37]. However, research to date has not considered the effect of reduced iodine content on the antimicrobial activity of an implant. We found that Ti-I implants have a greater antibacterial effect in vitro compared with the Ti and Ti-O implants when tested against three clinically important biofilm-forming bacteria (MSSA, MRSA, P. aeruginosa). For Ti-I, 65% of the iodine remained at 8 weeks after implantation in a rat model. Although the results show potential for iodine-coated implants preventing postoperative infections, this is only a preliminary study.
Limitations
The limitations of this study are as follows: First, although we believe our findings are relevant to PJI as well as spinal infections, the implants we placed were not in rats’ joints or spines, but rather subcutaneously on the backs of the rats. Iodine-coated processing is a highly versatile technology that can be used in a variety of applications, such as screws [7, 17], plates [30], and prostheses [16]. However, we cannot be sure that our findings from subcutaneously placed implants will generalize to other locations where orthopaedic implants are used, such as joints, against or inside of long bones, or in the spine. Second, the immunological milieu is different between humans and rats. Thus, it is unclear whether the results in this study will similarly scale in humans. Nevertheless, iodine-coated implants have shown promising results, albeit in a small number of clinical and short-term studies [7, 16, 17, 30]. Third, we only used male rats; therefore, it is unclear whether female rats will experience similar effects. Since this was an animal model study, we felt it important to use a single-sex population to control the weekly age and body size of each rat. Further, we examined the antibacterial effects in vitro. Therefore, despite the different immunological milieu between males and females in vivo, we believe the impact on the results in vitro would be small. Furthermore, several reports [15, 22, 27] have evaluated the antibacterial implants in vivo using infected rat models with an all-male population. This made it easier to compare results in this study to previous results.
In addition, three different types of implants were implanted in each rat; thus, a possibility of interaction between each implant cannot be excluded. Our experimental procedure allowed us to examine the antibacterial effect of multiple bacterial species in a single rat while maintaining a uniform setting in which the samples were implanted. Furthermore, we reduced the number of rats that needed to be euthanized. In contrast, it is worthwhile to consider the possibility that the antibacterial effect of the Ti-I implants extended over to other implants (Ti, Ti-O). If this was the case, the antimicrobial effect of the other implants would also be expected to increase. However, Ti-I showed stronger antibacterial activity than Ti and Ti-O. Thus, an extension of the antibacterial effect of the Ti-I over to the Ti and Ti-O implants would not affect the superior results obtained for Ti-I. However, verifying the validity of our findings by conducting a further study in a completely independent environment, that is, by examining the antimicrobial effect of only one bacterial species on a single animal model, will provide new insights. Finally, we did not investigate the antibacterial effect against infected animal models in vivo. Even in the 8-week model, the number of bacteria and B-cell receptors of live bacteria on Ti-I were clearly lower than those on Ti and Ti-O implants, but it was not clear whether the size of this reduction would be clinically important, that is, enough to inhibit the development of a clinical infection.
In Vivo (Rat Model) Reduction of Implant Iodine Content
Iodine content of the Ti-I samples decreased rather substantially—by more than one-third—over time in this rat model. In a review of antibacterial implants, Chouirfa et al. [4] argued that there are insufficient data regarding the residual amount of coating or antibacterial activity of implants with antibacterial coatings after implantation. Even if there are data regarding the residual amount, the biologic half-life of an antibiotic (such as gentamicin) or silver coating is only several hours or days [20, 33, 35]. Here, we showed that the iodine in the iodine-coated implants decayed slowly as per the properties of iodine [37] compared with the decay process of antimicrobial activity of conventional antimicrobial implants.
Antimicrobial Activity In Vitro
After 4 and 8 weeks in vivo in rats, Ti-I implants demonstrated a stronger antimicrobial activity in vitro compared with Ti and Ti-O implants. We believe the ideal antimicrobial implant will demonstrate a strong antimicrobial activity while being nontoxic to the body, and it will not diminish osteoconductivity. In addition, long-lasting antibacterial effect is very important for preventing late infection. Based on the present study and our previous studies [14, 15, 29, 31, 34, 36], iodine-coated Ti implants may have the above characteristics. Cytotoxicity tests using V79 cells (Chinese hamster fibroblasts) showed that iodine-coated titanium had low biologic toxicity, similar to that of ordinary stainless steel and titanium [29]. Taga et al. [31] showed that iodine-coated implants had good osteoconductivity in a rabbit femur model. Previously, we found that iodine-coated processing was feasible for a variety of titanium surfaces, including polished, blasted, and plasma-sprayed metal [36], and as such, may be highly versatile. Furthermore, the current study suggests that the iodine-coated implants have a longer-lasting antibacterial effect than conventional antibacterial implants [20, 33, 35] and are effective against PJI-causing bacteria, MSSA [38], MRSA [26], and P. aeruginosa [28].
Conclusion
Iodine-coated implants retained a substantial amount of iodine content and antibacterial activity against three important bacterial strains related to PJI for up to 8 weeks of implanting in rats. Although our findings were obtained from experiments in a rat model and do not guarantee efficacy in humans, they represent an important step toward the clinical application of iodine-coated implants. Based on the limitations of the present study, future work to evaluate the longer-lasting antibacterial efficacy of one bacterial species per animal as a completely independent environment, using larger infected animal PJI models with male and female populations, is desirable.
Acknowledgments
We thank the Fukushima Medical Device Development Support Centre for providing technical support for inductively coupled plasma-mass spectrometry.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Kanazawa University, Kanazawa, Japan (approval number: AP-204141).
This work was performed at the Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan.
Contributor Information
Ken Ueoka, Email: style8772@yahoo.co.jp.
Masaharu Tokoro, Email: para@med.kanazawa-u.ac.jp.
Yoshitomo Kajino, Email: yoshitomokajino@gmail.com.
Daisuke Inoue, Email: daisuke_i_0909@yahoo.co.jp.
Tomoharu Takagi, Email: tpuyan@gmail.com.
Takaaki Ohmori, Email: takaaki3530@gmail.com.
Junya Yoshitani, Email: junya10051005@gmail.com.
Takuro Ueno, Email: takuro1006@gmail.com.
Yuki Yamamuro, Email: y.yuuki1005@gmail.com.
Atsushi Taninaka, Email: atsushi880628@yahoo.co.jp.
Hiroyuki Tsuchiya, Email: tsuchi@med.kanazawa-u.ac.jp.
References
- 1.Aboltins CA, Antoci V, Bhattacharyya S, et al. Hip and knee section, prevention, prosthesis factors: proceedings of the international consensus on orthopedic infections. J Arthroplasty. 2019;34:S309-S320. [DOI] [PubMed] [Google Scholar]
- 2.Adachi K, Tsurumoto T, Yonekura A, et al. New quantitative image analysis of staphylococcal biofilms on the surfaces of nontranslucent metallic biomaterials. J Orthop Sci. 2007;12:178-184. [DOI] [PubMed] [Google Scholar]
- 3.Berretta JM, Jennings JA, Courtney HS, et al. Blended chitosan paste for infection prevention: preliminary and preclinical evaluations. Clin Orthop Relat Res . 2017;475:1857-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chouirfa H, Bouloussa H, Migonney V, et al. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37-54. [DOI] [PubMed] [Google Scholar]
- 5.Dale H, Hallan G, Hallan G, et al. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009;80:639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies R, Holt N, Nayagam S. The care of pin sites with external fixation. J Bone Joint Surg Br. 2005;87:716-719. [DOI] [PubMed] [Google Scholar]
- 7.Demura S, Murakami H, Shirai T, et al. Surgical treatment for pyogenic vertebral osteomyelitis using iodine-supported spinal instruments: initial case series of 14 patients. Eur J Clin Microbiol Infect Dis . 2015;34:261-266. [DOI] [PubMed] [Google Scholar]
- 8.Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine (Phila Pa 1976). 2017;43:65-71. [DOI] [PubMed] [Google Scholar]
- 9.Norambuena German A, Patel R, Karau M, et al. Antibacterial and biocompatible titanium-copper oxide coating may be a potential strategy to reduce periprosthetic infection: an in vitro study. Clin Orthop Relat Res. 2017;475:722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588. [DOI] [PubMed] [Google Scholar]
- 11.Gundtoft PH, Pedersen AB, Varnum C, et al. Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res. 2017;475:2623-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Jia S, Qiao L, et al. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surf B Biointerfaces. 2020;194:111186. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto K, Takaya M, Maejima A, et al. Antimicrobial characteristics of anodic oxidation coating of aluminum impregnated with iodine compound. Inorg Mater. 1999;6:457-62. [Google Scholar]
- 14.Inoue D, Kabata T, Kajino Y, et al. Iodine-supported titanium implants have good antimicrobial attachment effects. J Orthop Sci . 2019;24:548-551. [DOI] [PubMed] [Google Scholar]
- 15.Inoue D, Kabata T, Ohtani K, et al. Inhibition of biofilm formation on iodine-supported titanium implants. Int Orthop. 2017;41:1093-1099. [DOI] [PubMed] [Google Scholar]
- 16.Kabata T, Maeda T, Kajino Y, et al. Iodine-supported hip implants: short term clinical results. Biomed Res Int. 2015;2015:368124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato S, Murakami H, Demura S, et al. Vertebral osteomyelitis caused by mycobacterium abscessus surgically treated using antibacterial iodine-supported instrumentation. Case Rep Orthop. 2014;2014:197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazemzadeh-Narbat M, Kindrachuk J, Duan K, et al. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials . 2010;31:9519e26. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz SM, Ong KL, Lau E, et al. Prosthetic joint infection risk after TKA in the Medicare population . Clin Orthop Relat Res. 2010;468:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LL, Wang LM, Xu Y, et al. Preparation of gentamicin-loaded electrospun coating on titanium implants and a study of their properties in vitro. Arch Orthop Trauma Surg . 2012;132:897-903. [DOI] [PubMed] [Google Scholar]
- 21.Mahan J, Seligson D, Henry SL, et al. Factors in pin tract infections. Orthopedics. 1991;14:305-308. [PubMed] [Google Scholar]
- 22.Mills R, Cheng TL, Mikulec K, et al. CSA-90 promotes bone formation and mitigates methicillin resistant staphylococcus aureus infection in a rat open fracture model. Clin Orthop Relat Res . 2018;476:1311-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray J, Muruko T, Gill CIR, et al. Evaluation of bactericidal and anti-biofilm properties of a novel surface-active organosilane biocide against healthcare associated pathogens and Pseudomonas aeruginosa biolfilm. PLoS One. 2017;12:e0182624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura S, Tsurumoto T, Yonekura A, et al. Antimicrobial susceptibility of staphylococcus aureus and staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J Orthop Sci . 2006;11:46-50. [DOI] [PubMed] [Google Scholar]
- 25.Rahim MI, Rohde M, Rais B, et al. Susceptibility of metallic magnesium implants to bacterial biofilm infections. J Biomed Mater Res A. 2016;104:1489-1499. [DOI] [PubMed] [Google Scholar]
- 26.Salgado CD, Dash S, Cantey JR, et al. Higher risk of failure of methicillin resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res . 2007;461:48-53. [DOI] [PubMed] [Google Scholar]
- 27.Savvidis M, Papavasiliou K, Taitzoglou I, et al. Postoperative administration of alpha-tocopherol enhances osseointegration of stainless steel implants: an in vivo rat model. Clin Orthop Relat Res . 2020;478:406-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah NB, Osmon DR, Steckelberg JM, et al. Pseudomonas prosthetic joint infections: a review of 102 episodes. J Bone Jt Infect . 2016;1:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirai T, Shimizu T, Ohtani K, et al. Antimicrobial iodine-supported titanium implants. Acta Biomater . 2011;7:1928-1933. [DOI] [PubMed] [Google Scholar]
- 30.Shirai T, Tsuchiya H, Terauchi R, et al. The outcomes of reconstruction using frozen autograft combined with iodine-coated implants for malignant bone tumors: compared with non-coated implants. Jpn J Clin Oncol . 2016;46:735-740. [DOI] [PubMed] [Google Scholar]
- 31.Taga T, Kabata T, Kajino Y, et al. Comparison with the osteoconductivity and bone-bonding ability of the iodine supported titanium, titanium with porous oxide layer and the titanium alloy in the rabbit model. J Orthop Sci. 2018;23:585-591. [DOI] [PubMed] [Google Scholar]
- 32.Toms AD, Davidson D, Masri BA, et al. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br . 2006;88:149-155. [DOI] [PubMed] [Google Scholar]
- 33.Tsikopoulos K, Bidossi A, Drago L, et al. Is implant coating with tyrosol- and antibiotic-loaded hydrogel effective in reducing cutibacterium (propionibacterium) acnes biofilm formation? A preliminary in vitro study. Clin Orthop Relat Res . 2019;477:1736-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchiya H, Shirai T, Nishida H, et al. Innovative antimicrobial coating of titanium implants with iodine. J Orthop Sci. 2012;17:595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukamoto M, Miyamoto H, Ando Y, et al. Acute and subacute toxicity in vivo of thermal-sprayed silver containing hydroxyapatite coating in rat tibia. Biomed Res Int. 2014;902343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueoka K, Kajino Y, Kabata T, et al. The feasibility of iodine-supported processing for titanium with different surfaces. J Orthop Sci. 2020;25:1095-1100. [DOI] [PubMed] [Google Scholar]
- 37.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151:400-406. [DOI] [PubMed] [Google Scholar]
- 38.Zeller V, Kerroumi Y, Meyssonnier V, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect. 2018;76:328-334. [DOI] [PubMed] [Google Scholar]


