Abstract
Background
Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels have always been a part of the diagnostic criteria for periprosthetic joint infection (PJI), but they perform poorly anticipating the outcome of reimplantation. D-dimer has been reported in a small series as a potential marker to measure infection control after single-stage revisions to treat PJI. Nonetheless, its use to confirm infection control and decide the proper timing of reimplantation remains uncertain.
Questions/purposes
(1) What is the best diagnostic threshold and accuracy values for plasma D-dimer levels compared with other inflammatory markers (ESR and CRP) or what varying combinations of these tests are associated with persistent infection after reimplantation? (2) Do D-dimer values above this threshold, ESR, CRP, and varying test combinations at the time of reimplantation indicate an increased risk of subsequent persistent infection after reimplantation?
Methods
We retrospectively studied the electronic medical records of all 53 patients who had two-stage revisions for PJI and who underwent plasma D-dimer testing before reimplantation at one of two academic institutions from November 22, 2017 to December 5, 2020. During that period, all patients undergoing two-stage revisions also had a D-dimer test drawn. The minimum follow-up duration was 1 year. We are reporting at this early interval (rather than the more typical 2-year time point) because of the poorer-than-expected performance of this diagnostic test. Of these 53 patients, 17% (9) were lost to follow-up before 1 year and could not be analyzed; the remaining 44 patients (17 hips and 27 knees) were studied here. The mean follow-up was 503 ± 135 days. Absence or persistence of infection after reimplantation were defined according to the Delphi criteria. The conditions included in these criteria were: (1) control of infection, as characterized by a healed wound without fistula, drainage, or pain; (2) no subsequent surgical intervention owing to infection after reimplantation; and (3) no occurrence of PJI-related mortality. The absence of any of the aforementioned conditions until the final follow-up examination was deemed a persistent infection after reimplantation. Baseline patient characteristics were not different between patients with persistent infection (n = 10) and those with absence of it after reimplantation (n = 34) as per the Delphi criteria. Baseline patient characteristics evaluated were age, gender, self-reported race (white/Black/other) or ethnicity (nonHispanic/Hispanic), BMI, American Society of Anesthesiologists (ASA) status, smoking status(smoker/nonsmoker), and joint type (hip/knee). The optimal D-dimer threshold to differentiate between persistence of infection or not after reimplantation was calculated using the Youden index. A receiver operating characteristic curve analysis was performed to test the accuracy of D-dimer, ESR, CRP, and their combinations to establish associations, if any, with persistent infection after reimplantation. A Kaplan-Meier survival analysis (free of infection after reimplantation) with a log-rank test was performed to investigate if D-dimer, ESR, and CRP were associated with absence of infection after reimplantation. Survival or being free of infection after reimplantation was determined as per Delphi criteria. Alpha was set at p < 0.05.
Results
In the receiver operating characteristic curve analysis, with an area under the curve of 0.62, D-dimer showed low accuracy and did not anticipate persistent infection after reimplantation. The optimal D-dimer threshold differentiating between persistence of infection or not after reimplantation was 3070 ng/mL. When using this threshold, D-dimer demonstrated a sensitivity of 90% (95% CI 55.5% to 99.7%) and negative predictive value of 94% (95% CI 70.7% to 99.1%), but low specificity (47% [95% CI 29.8% to 64.9%]) and positive predictive value (33% [95% CI 25.5% to 42.2%]). Although D-dimer showed the highest sensitivity, the combination of D-dimer with ESR and CRP showed the highest specificity (91% [95% CI 75.6% to 98%]) defining the persistence of infection after reimplantation. Based on plasma D-dimer levels, with the numbers available, there was no difference in survival free from infection after reimplantation (Kaplan-Meier survivorship free from infection at minimum 1 year in patients with D-dimer below 3070 ng/mL versus survivorship free from infection with D-dimer above 3070 ng/mL: 749 days [95% CI 665 to 833 days] versus 615 days [95% CI 471 to 759 days]; p = 0.052). Likewise, there were no associations between high ESR and CRP levels and persistent infection after reimplantation, but the number of events was very small, and insufficient power is a concern with this analysis.
Conclusion
In this preliminary series, with the numbers available, D-dimer alone had poor accuracy and was not associated with survival free from infection after reimplantation in patients who underwent two-stage exchange arthroplasty. D-dimer alone might be used to establish that PJI is unlikely, and the combination of D-dimer, ESR, and CRP should be considered to confirm PJI diagnosis in the setting of reimplantation.
Level of Evidence Level IV, diagnostic study.
Introduction
Currently, there is no gold standard test to diagnose periprosthetic joint infection (PJI) or to anticipate the presence or absence of infection after reimplantation. The lack of such a test is a glaring shortcoming, given the tremendous harm this complication causes to patients. Two-stage exchange hip and knee arthroplasty may be the most commonly used approach in the United States for PJI treatment [7], and it also is widely used around the world for this purpose. The first stage involves implant removal, a thorough irrigation and debridement, and the insertion of either an articulating or static antibiotic-eluding cement spacer. After attempting to confirm that the infection has been eradicated (as best as can be done given shortcomings of current diagnostic modalities), the second stage involves removal of the cement spacer and reimplantation of new components. Between the two stages, antibiotics are administered up until 2 weeks before the second stage, when they are usually stopped to assess the resolution of PJI through serum and synovial tests [6, 12]. The 2011 Musculoskeletal Infection Society definition, slightly modified by the International Consensus Meeting in 2013, may be the most widely accepted and used definition for diagnosing PJI in clinical research [14, 16]. Nevertheless, this 2013 International Consensus Meeting definition has demonstrated limited value in screening for infection control before reimplantation (two-stage revision) and defining being free from infection after such procedure (0%-26% sensitivity) in previous investigations [3, 5].
Over the years, several biomarkers such as synovial alpha-defensin have been proposed to improve the accuracy of diagnosing PJI, but none of them have been proven useful before the second stage of a two-stage revision in terms of anticipating whether infection is likely to persist or recur after reimplantation [19]. Commonly, inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) have been a part of the diagnostic criteria for PJI [14, 16]. Nonetheless, ESR and CRP perform poorly in terms of anticipating the outcome of reimplantation [1, 4]. Another inflammatory marker, D-dimer, recently has been introduced as a promising diagnostic marker for PJI in the setting of reimplantation [20]. Although there have been conflicting results on the use of D-dimer for diagnosing PJI [13, 24], this test result has already been incorporated as one of the minor criteria under the most recently proposed 2018 International Consensus Meeting PJI definition [15]. Multiple serum and plasma D-dimer thresholds have been proposed for improving the diagnostic accuracy of PJI [13, 20, 24], but there are no studies to our knowledge on their use to confirm infection control and to decide the proper timing of or anticipate the fate of reimplantation in patients who undergo two-stage exchange arthroplasty.
Therefore, we sought to answer the following questions: (1) What is the best diagnostic threshold and accuracy values for plasma D-dimer levels compared with other inflammatory markers (ESR and CRP) or what varying combinations of these tests are associated with persistent infection after reimplantation? (2) Do D-dimer values above this threshold, ESR, CRP, and varying test combinations at the time of reimplantation indicate an increased risk of subsequent persistent infection after reimplantation?
Patients and Methods
Study Design and Setting
We retrospectively studied the electronic medical records of all patients who underwent two-stage exchange arthroplasties for PJI and plasma D-dimer testing before reimplantation at one of two academic institutions. In both institutions, all patients who had arthroplasty revision received a D-dimer test before each of their procedures (including reimplantations). In addition to data retrieval from the electronic medical records, all patients were contacted via telephone to collect information concerning the presence or absence of infection after reimplantation (including any reoperations related to PJI).
Table 1.
Baseline demographic and characteristics of patients undergoing the second stage of two-stage exchange arthroplasty
| Demographics | Persistent infection (n = 10) | No persistent infection (n = 34) | p value |
| Age in years, mean ± SD | 65 ± 6 | 68 ± 11 | 0.58 |
| Sex (females) | 50 (5) | 47 (16) | 0.87 |
| Racea (self-reported) | |||
| White | 60 (6) | 85 (29) | 0.20 |
| Black | 20 (2) | 9 (3) | |
| Other | 20 (2) | 6 (2) | |
| Ethnicity (self-reported) | |||
| NonHispanic | 90 (9) | 91 (31) | 0.91 |
| Hispanic | 10 (1) | 9 (3) | |
| BMI in kg/m2, mean ± SD | 29 ± 6 | 29 ± 6 | 0.98 |
| ASA grade | |||
| 1 | 0 (0) | 3 (1) | 0.79 |
| 2 | 50 (5) | 41 (14) | |
| 3 | 40 (4) | 44 (15) | |
| Smoking status (smoker) | 0 (0) | 9 (3) | 0.33 |
| Joint | |||
| Hip | 10 (1) | 47 (16) | 0.03 |
| Knee | 90 (9) | 53 (18) | |
Data are presented as the proportion or % (n), unless otherwise stated.
Available data; ASA = American Society of Anesthesiologists.
Table 2.
Procedures performed to address the persistence of infection after reimplantation in 10 cases
| Case number | Persistence of infection characteristics | Intervention at the time of infection | Time until diagnosis of infection in days |
| 1 | Recurrent infection | Removal of prosthesis and placement of antibiotic spacer | 445 |
| 2 | Recurrent infection | Above-knee amputation | 176 |
| 3 | Recurrent infection | Multiple incisions and drainages | 42 |
| 4 | Recurrent infection | Removal of prosthesis and placement of antibiotic spacer | 334 |
| 5 | Deep abscess/hematoma | Incision and drainage with debridement of skin, soft tissue, and muscle | 9 |
| 6 | Deep abscess | Incision and drainage | 6 |
| 7 | Wound abscess | Incision and drainage | 8 |
| 8 | Wound dehiscence | Incision and debridement of subcutaneous tissue | 80 |
| 9 | Recurrent infection | Removal of prosthesis and placement of antibiotic spacer | 420 |
| 10 | Recurrent infection | Removal of prosthesis and placement of antibiotic spacer | 528 |
Participants
Over the study period of approximately 3 years (November 22, 2017 to December 5, 2020), 53 patients who had two-stage hip or knee revisions for PJI underwent plasma D-dimer testing before reimplantation (each patient had only one joint involved in this study; there were no bilateral procedures). The D-dimer test was ordered in all patients at both institutions before each revision, including all reimplantation procedures. These 53 patients were considered for inclusion in this study. Of these 53 patients, 17% (9) were lost to follow-up before 1 year and could not be analyzed. The remaining 44 patients (17 hips and 27 knees) were included for statistical analyses. The mean follow-up duration for the entire cohort was 503 ± 135 days.
The baseline patient characteristics were not different between patients who had persistent infection after reimplantation (n = 10) and absence of it (n = 34), as determined by the Delphi criteria [2] (Table 1). Baseline patient characteristics evaluated were age, gender, race (white/Black/other), ethnicity (nonHispanic/Hispanic), BMI, American Society of Anesthesiologists (ASA) status, smoking status (smoker/nonsmoker), and joint type (hip/knee).
Baseline patient characteristics between those patients with and without persistent infection after reimplantation were not different (Table 1). The procedures performed in patients to address persistent infection after reimplantation included the removal of prosthesis and insertion of antibiotic spacer, incision, and drainage/debridement. (Table 2).
Description of Experiment, Treatment, or Surgery
All surgical procedures evaluated were performed from November 22, 2017 to December 5, 2020. The treatment plan involved a two-stage revision. In the study cohort, two-stage revision was an indication for confirmed PJI based on comprehensive 2013 Musculoskeletal Infection Society criteria including a clinical evaluation and blood and synovial tests before reimplantation. In the first stage, an antibiotic-loaded cement spacer was implanted after removal of the previous prosthesis, along with irrigation and debridement. All patients were given at least 6 weeks of IV antibiotics (empiric followed by specific based on culture results and antibiogram) before the second stage of the two-stage revision (interim period) and 3 months of oral antibiotics after reimplantation. After infection control was confirmed with blood and synovial tests, including negative cultures, all patients underwent reimplantation (second stage).
Variables and Outcome Measures
Baseline demographic data, including age, gender (men and women), self-reported race (white, Black, and other), self-reported ethnicity (nonHispanic or Hispanic), BMI, comorbidity status (ASA grade), and smoking status (yes or no) were collected. All inflammatory markers of PJI (plasma D-dimer [ng/mL], ESR [mm/hour], and CRP [mg/L] levels) were collected for the entire patient cohort before reimplantation (after 2 weeks of an antibiotic holiday).
Our primary goal was to ascertain whether D-dimer can anticipate the fate of reimplantation with regard to infection. The gold standard used to ascertain the absence of infection after reimplantation (second stage) at a minimum 1-year follow-up in the current investigation was the Delphi criteria developed by Diaz-Ledezma et al. [2] using the Delphi method of consensus, well-known as the Delphi criteria. The conditions listed under this criteria are as follows: (1) infection control, as characterized by a healed wound without fistula, drainage, or pain; (2) no subsequent surgical intervention owing to infection after reimplantation; and (3) no occurrence of PJI-related mortality. The absence of any of the aforementioned conditions until the final follow-up examination was deemed a persistent infection after reimplantation. It is important to note that the Delphi criteria was considered at 2 years of follow-up in the original publication, while in the current investigation, we applied these criteria at 1-year of follow-up. For our investigation, the primary goal was to ascertain the optimal threshold of D-dimer associated with persistence of infection after reimplantation and compare the performance of this test with the ability of ESR and CRP to diagnose such persistent infection. To achieve this, analytics such as sensitivity and specificity for all diagnostic tests were calculated. Our secondary study goals were to investigate whether D-dimer above this threshold, or different combinations with ESR and CRP, were associated with survival or absence of persistent infection after reimplantation, for which a detailed survival analyses was conducted. To ascertain the presence or absence of infection after reimplantation, we not only reviewed the electronic medical records in all patients but also called all patients to reconfirm the survival or absence of infection after reimplantation, or if they underwent any intervention in another medical facility. Data on the time until the most recent follow-up examination and persistence of infection after reimplantation were also collected. The persistence or not of infection after reimplantation is presented for all patients, and separately for hips and knees (Table 1). The MSIS and Delphi criteria (applied at 1 year) presented in the current investigation were deemed positive or negative after review of all involved variables by three coauthors, independently.
To estimate D-dimer levels, we processed blood as follows.
Preparation of Samples
Citrated platelet-poor plasma was the recommended type of specimen. One part sodium citrate solution (0.11 mol/L) was carefully mixed with nine parts venous blood, avoiding the formation of foam. After blood collection, the blood tube was centrifuged for 15 minutes at 1500 × g to 2500 × g. Highly lipemic plasma was further clarified using centrifugation at 15,000 × g for 10 minutes. We followed the manufacturer’s instructions regarding sample stability. To ensure sample stability, we froze plasma within 4 hours of blood collection at ≤ -18° C. When analyzing the D-dimer levels, frozen plasma was thawed for 10 minutes and homogenized by mixing it without foam formation. D-dimer levels were determined within 2 hours of thawing [18].
D-dimer Assay Procedure
Innovance D-dimer (Siemens Healthcare Diagnostics) was used to determine the plasma D-dimer levels [18]. This is a particle-enhanced immunoturbidimetric assay to quantitatively determine cross-linked fibrin degradation products (D-dimers). The Innovance D-dimer kit contains a reagent, buffer, supplement, diluent, and calibrator. Additional materials that are required include D-dimer controls, sample diluent, coagulation analyzer, distilled water, and pipettes. The coagulation analyzer automatically performs sampling, reagent delivery, mixing, and processing of the collected samples to determine D-dimer levels.
Ethical Approval
Ethical approval for this study was obtained from the Cleveland Clinic (approval number FLA 18-098).
Statistical Analysis
Continuous variables are described using means and SD, while categorical variables are presented using numbers and frequencies. We used independent t-tests, Fisher exact tests, and chi-square tests to compare the patient characteristics (age, gender, race, ethnicity, BMI, ASA, and smoking status) of patients with persistent infection and those with absence of infection after reimplantation as per the Delphi criteria. A receiver operating characteristic (ROC) curve was performed to test the accuracy of plasma D-dimer to differentiate between persistent infection or not after reimplantation. The optimal threshold of plasma D-dimer differentiating between persistence of infection or not after reimplantation was calculated using the Youden index (J-statistic = sensitivity + specificity - 1). This cutoff value was used to categorize infections as either D-dimer-positive (above the threshold) or D-dimer-negative (below the threshold). Additional ROC curve analyses were conducted for ESR (> 30 mm/hour), CRP (> 10 mg/L), and combinations of these tests to ascertain association, if any, with persistent infection after reimplantation. The diagnostic test combinations used in this study included D-dimer and ESR, D-dimer and CRP, and D-dimer, ESR, and CRP. The sensitivity, specificity, likelihood ratios, and positive and negative predictive values of the calculated plasma D-dimer threshold, ESR, CRP, and test combinations were calculated against the Delphi criteria (gold standard). A Kaplan-Meier survival analysis with a log-rank test was performed to evaluate this D-dimer threshold, ESR, CRP, or their varying combinations to establish association with persistence of infection after reimplantation. The statistical significance was set at p < 0.05. IBM SPSS Statistics, version 27.0 (IBM Corp) and MedCalc Statistical Software version 19.2.6 (MedCalc Software Ltd) were used for the statistical analyses.
Results
Diagnostic Threshold and Accuracy Values for Plasma D-dimer and Other Tests
In the ROC curve analysis, with the numbers available, and an area under the curve (AUC) of 0.62 (95% CI 0.42 to 0.81), plasma D-dimer showed low accuracy defining persistent infection after reimplantation (Fig. 1). Using the Youden index, with the numbers available, we found that the optimal plasma D-dimer threshold differentiating between persistent infection or not after reimplantation was 3070 ng/mL. With AUCs of 0.52 (95% CI 0.32 to 0.72) and 0.54 (95% CI 0.34 to 0.74), respectively, with the numbers available, ESR and CRP also performed poorly in a similar setting. Multiple combinations of plasma D-dimer with ESR and CRP generally demonstrated a decrease in AUCs (D-dimer + ESR = 0.56 [95% CI 0.31 to 0.77]; D-dimer + CRP = 0.52 [95% CI 0.31 to 0.73]; and D-dimer + ESR + CRP = 0.55 [95% CI 0.34 to 0.77]) (Fig. 2). A plasma D-dimer threshold of 3070 ng/mL demonstrated a sensitivity of 90% (95% CI 55.5% to 99.7%) and a negative predictive value of 94% (95% CI 70.7% to 99.1%), but a low specificity of 47% (95% CI 29.8% to 64.9%) and a positive predictive value of 33% (95% CI 25.5% to 42.2%) (Table 3). ESR and CRP individually demonstrated lower sensitivity (30% [95% CI 6.6% to 65.2%] [Table 4] and 20% [95% CI 2.5% to 55.6%] [Table 5]), but higher specificity (67% [95% CI 48.2% to 82%] and 73% [95% CI 55.6% to 87.1%]) than plasma D-dimer threshold. On investigating numerous test combinations of plasma D-dimer with ESR and CRP, with the numbers available, we found that although its sensitivity decreased, specificity and accuracy increased in all combinations compared with plasma D-dimer alone (sensitivity and specificity: D-dimer + ESR = 30% [95% CI 6.7% to 65.2%] and 82% [95% CI 64.5% to 93%] [Table 6], respectively; D-dimer + CRP = 20% [95% CI 2.5% to 55.6%] and 85% [95% CI 68% to 95%] [Table 7], respectively; and D-dimer + ESR + CRP = 20% [95% CI 2.5% to 55.6%] and 91% [95% CI 75.6% to 98%] [Table 8], respectively). Thus, whereas plasma D-dimer showed the highest sensitivity (90%), the combination of plasma D-dimer with ESR and CRP demonstrated the highest specificity (91%) to define persistence of infection after reimplantation.
Fig. 1.
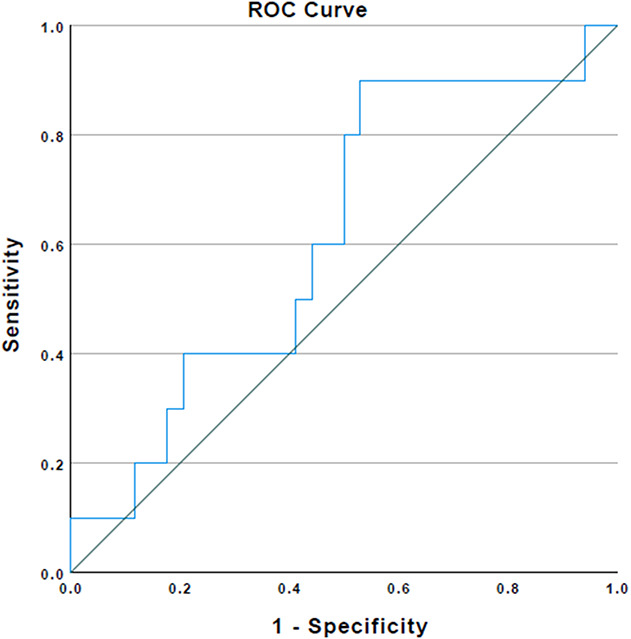
This receiver operating characteristic curve represents the association between plasma D-dimer and persistence of infection after reimplantation (area under the curve 0.62).
Fig. 2.
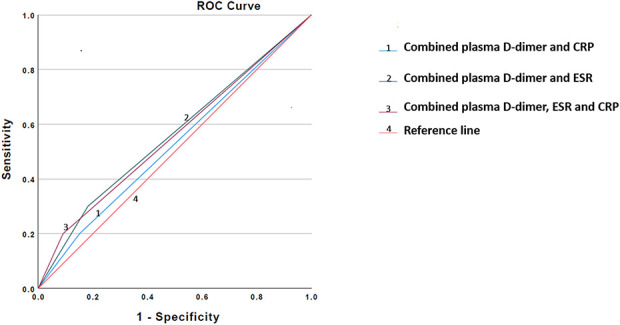
This receiver operating characteristic curve shows the association of combinations of D-dimer threshold with ESR (area under the curve 0.56), D-dimer threshold with CRP (area under the curve 0.52), and D-dimer with both ESR and CRP (area under the curve 0.55) and persistent infection after reimplantation (reference).
With the numbers available, plasma D-dimer values were not different between patients who had persistent infection after reimplantation and those without it (persistence of infection: 4375 ± 2379 ng/mL versus absence of infection: 3470 ± 2130 ng/mL; p = 0.30). Similarly, ESR and CRP values were not different between the two groups (ESR: persistence of infection: 33 ± 39 mm/hour versus absence of infection: 30 ± 31 mm/hour; p = 0.84; CRP: persistence of infection: 12.3 ± 20 mg/L versus absence of infection: 14 ± 21.2 mg/L; p = 0.83).
With regard to the organisms responsible for the infection at explantation (first stage), at the time of first-stage, 45% of patients did not grow a particular organism, while 11% of patients grew methicillin-resistant Staphylococcus aureus (MRSA) (Supplementary Appendix 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A536). There were diverse organisms at the first stage among those patients deemed having persistent infection at 1 year (Delphi criteria) (Appendix 1). Of 10 patients who were determined as having persistent infection (minimum 1 year), 30% did not grow any organism at the first stage (original infection). Not having a specific organism identified at the beginning of the treatment (first stage) seemed to be detrimental after reimplantation.
Is Using Tests at Threshold Levels More Clinically Useful?
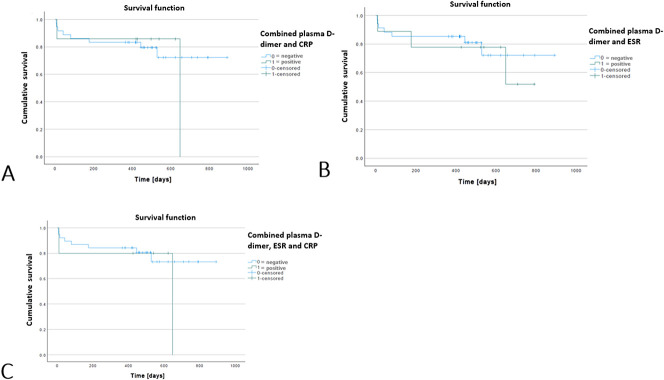
Based on plasma D-dimer levels, with the numbers available, there was no difference in survival or absence of infection after reimplantation (Kaplan-Meier survivorship free from infection at a minimum of 1 year in patients with D-dimer below 3070 ng/mL versus survivorship free from infection with D-dimer above 3070 ng/mL: 749 days [95% CI 665 to 833 days] versus 615 days [95% CI 471 to 759 days]; p = 0.052) (Fig. 3). Similar results were derived with ESR and CRP as being associated with survival or being free of infection after reimplantation (ESR: Kaplan-Meier survivorship free from infection at a minimum of 1 year in patients with D-dimer below 3070 ng/mL versus survivorship free from infection with D-dimer above 3070 ng/mL, 691 days [95% CI 562 to 821] versus 650 days [95% CI 513 to 786]; p = 0.76 and CRP: Kaplan-Meier survivorship free from infection at a minimum of 1 year in patients with D-dimer below 3070 ng/mL versus survivorship free from infection with D-dimer above 3070 ng/mL, 695 days [95% CI 575 to 814] versus 631 days [95% CI 501 to 760]; p = 0.69, respectively). For multiple combinations of plasma D-dimer with ESR and CRP, with the numbers available, there were no differences in post-reimplantation infection-free survival (Kaplan-Meier survivorship free from infection at a minimum of 1 year in patients with D-dimer below 3070 ng/mL versus survivorship free from infection with D-dimer above 3070 ng/mL: combinations of (1) D-dimer + CRP, 709 days [95% CI 597 to 821] versus 557 days [95% CI 322 to 791]; p = 0.75, (2) D-dimer + ESR, 713 days [95% CI 596 to 831] versus 598 days [95% CI 410 to 786]; p = 0.70), and (3) D-dimer + ESR + CRP, 717 days [95% CI 608 to 825] versus 520 days [95% CI 203 to 586]; p = 0.46 (Fig. 4).
Fig. 3.
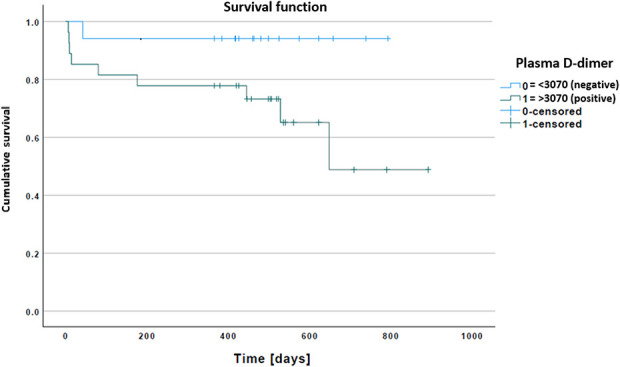
This Kaplan-Meier graph represents survival or absence of infection based on the plasma D-dimer threshold (3070 ng/mL) at reimplantation. A color image accompanies the online version of this article.
Fig. 4.
A-C These Kaplan-Meier graphs represent survival or absence of infection based on the following: (A) plasma D-dimer with CRP, (B) plasma D-dimer with ESR, and (C) plasma D-dimer with ESR and CRP at reimplantation. A color image accompanies the online version of this article.
Discussion
With time, an increasing number of total joint arthroplasties will likely be associated with an increasing number of patients with PJI [9]. Two-stage exchange hip and knee arthroplasty is one of the most common approaches for the treatment of PJI in the United States and around the world [7]. Although diagnosing PJI has generally been an active area of research in arthroplasty, a test or criterion that could specifically confirm the control of infection and so indicate a high likelihood of eventual survival or absence of infection of the prosthesis after a second-stage revision for PJI is essential. Unfortunately, the most widely used and accepted 2013 Musculoskeletal Infection Society criteria and other comparatively new PJI diagnostic tests such as alpha-defensin appear not to be able to achieve this goal [3, 5, 19]. D-dimer has gained some attention for its ability to diagnose PJI [20]. However, to the best of our knowledge, no previous study has proposed a D-dimer threshold specifically to diagnose infection or anticipate survival free of persistent infection after reimplantation. We evaluated D-dimer for this indication, but found that D-dimer had poor accuracy and it was not associated with the persistence of infection after reimplantation. Likewise, there were no associations between high ESR and CRP levels and persistent infection after reimplantation, but the number of events was very small, and insufficient power is a concern with this analysis, as we will discuss.
Table 3.
Accuracy of plasma D-dimer threshold (3070 ng/mL) in predicting persistent infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test | Infection persistence | No infection persistence | Total |
| Plasma D-dimer (-) | 1 | 16 | 17 |
| Plasma D-dimer (+) | 9 | 18 | 27 |
| Total | 10 | 34 | 44 |
| Accuracy = 100 x 9 + 16/1 + 9 + 16 + 18 = 57% Sensitivity = 100 x 9/1 + 9 = 90% Specificity = 100 x 16/16 + 18 = 47% Positive predictive value = 100 x 9/9 + 18 = 33% Negative predictive value = 100 x 16/16 + 1 = 94% Positive likelihood ratio = 0.9/1-0.47 = 1.7 Negative likelihood ratio = 1-0.9/0.47 = 0.2 | |||
Table 4.
Accuracy of ESR in predicting persistent infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test | Infection persistence | No infection persistence | Total |
| ESR (-) | 7 | 22 | 29 |
| ESR (+) | 3 | 11 | 14 |
| Total | 10 | 33 | 43 |
| Accuracy = 100 x 3 + 22/3 + 22 + 7 + 11 = 58% Sensitivity = 100 x 3/7 + 3 = 30% Specificity = 100 x 22/22 + 11 = 67% Positive predictive value = 100 x 3/3 + 11 = 21% Negative predictive value = 100 x 22/22 + 7 = 76% Positive likelihood ratio = 0.3/1-0.67 = 0.9 Negative likelihood ratio = 1-0.3/0.67 = 1 | |||
Table 5.
Accuracy of CRP in predicting the persistence of infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test | Infection persistence | No infection persistence | Total |
| CRP (-) | 8 | 25 | 33 |
| CRP (+) | 2 | 9 | 11 |
| Total | 10 | 34 | 44 |
| Accuracy = 100 x 2 + 25/2 + 25 + 8 + 9 = 61% Sensitivity = 100 x 2/2 + 8 = 20% Specificity = 100 x 25/25 + 9 = 73% Positive predictive value = 100 x 2/2 + 9 = 18% Negative predictive value = 100 x 25/25 + 8 = 76% Positive likelihood ratio = 0.2/1-0.73 = 0.7 Negative likelihood ratio = 1-0.2/0.73 = 1.1 | |||
Table 6.
Accuracy of the combination of D-dimer and ESR in predicting persistent infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test combination | Infection persistence | No infection persistence | Total |
| D-dimer and ESR (+) | 7 | 27 | 34 |
| D-dimer and ESR (-) | 3 | 6 | 9 |
| Total | 10 | 33 | 43 |
| Accuracy = 100 x 3 + 27/3 + 27 + 7 + 6 = 70% Sensitivity = 100 x 3/3 + 7 = 30% Specificity = 100 x 27/27 + 6 = 82% Positive predictive value = 100 x 3/3 + 6 = 33% Negative predictive value = 100 x 27/27 + 7 = 79% Positive likelihood ratio = 0.3/1-0.818 = 1.6 Negative likelihood ratio = 1-0.3/0.818 = 0.8 | |||
Table 7.
Accuracy of the combination of D-dimer and CRP in predicting the persistence of infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test combination | Infection persistence | No infection persistence | Total |
| D-dimer and CRP (+) | 8 | 28 | 36 |
| D-dimer and CRP (-) | 2 | 5 | 7 |
| Total | 10 | 33 | 43 |
| Accuracy = 100 x 2 + 28/2 + 28 + 8 + 5 = 70% Sensitivity = 100 x 2/2 + 8 = 20% Specificity = 100 x 28/28 + 5 = 85% Positive predictive value = 100 x 2/5 + 2 = 28% Negative predictive value = 100 x 28/28 + 8 = 78% Positive likelihood ratio = 0.2/1-0.848 = 1.3 Negative likelihood ratio = 1-0.2/0.848 = 0.9 | |||
Table 8.
Accuracy of the combination of D-dimer, ESR, and CRP in predicting the persistence of infection after reimplantation in two-stage exchange arthroplasty at a minimum 1-year follow-up
| Test combination | Infection persistence | No infection persistence | Total |
| D-dimer and ESR and CRP (+) | 8 | 30 | 38 |
| D-dimer and ESR and CRP (-) | 2 | 3 | 5 |
| Total | 10 | 33 | 43 |
| Accuracy = 100 x 2 + 30/2 + 30 + 8 + 3 = 74% Sensitivity = 100 x 2/2 + 8 = 20% Specificity = 100 x 30/30 + 3 = 91% Positive predictive value = 100 x 2/2 + 3 = 40% Negative predictive value = 100 x 30/30 + 8 = 79% Positive likelihood ratio = 0.2/1-0.909 = 2.2 Negative likelihood ratio = 1-0.2/0.909 = 0.9 | |||
Limitations
Our study has several limitations. The most important limitation here is our study’s small sample size, with relatively few events (patients with persistent infection). Since our study was retrospective, and drawn from a convenience sample, a post hoc power calculation might have been misleading, so we did not perform one. Still, there is a possibility that with a larger study and more events, D-dimer would have been shown to be more useful than appeared to be the case here. Within the relatively broad 95% CIs that we observed, there might have been a clinically meaningful finding that was missed. Consequently, although our paper is the largest that we know of on this topic, we must nonetheless consider our no-difference findings about D-dimer’s specificity to be preliminary; those findings can serve as pilot data for sample-size calculations in future large, multicenter trials on the topic. Our study was drawn from the work of two referral centers, and this conveys a sense for the size that will be required for a more-definitive study on this important topic. Related to this is the relatively short follow-up (1-year minimum); longer surveillance periods may have resulted in more events. However, we felt it important to release our data showing the lack of specificity of this test rather than waiting several more years to report; the other reason we felt that it is important to publish now was that we learned that a negative D-dimer was able to demonstrate the absence of infection (high sensitivity and negative predictive value), which is clinically useful. Again, we emphasize that our finding that a positive test was not specific enough to give the surgeon confidence that an infection is present could have been a function of insufficient power. Follow-up studies with longer surveillance periods should be performed, and we hope to continue to follow this patient group over time.
Retrospective study designs often have selection bias, but in the current study, the D-dimer test was routinely ordered in both institutions before all revisions, including reimplantations, and so we do not believe selection bias was a large issue here.
Another limitation is the nature of the D-dimer test, which might easily be affected by many conditions, such as coagulation disorders [17]. Data on the occurrence of such conditions were not collected. In our opinion, D-dimer can only be useful in clinical practice if it is studied regardless of the other diseases or conditions that are present. We believe this because many patients waiting for reimplantation have concomitant conditions or a history of many diseases, and studying this test only in patients who are nonsmokers, have no recent trauma, have no history of stroke or myocardial infarction, or do not have any coagulation or inflammatory disorders, for example, would make such studies relatively unhelpful in real-world clinical practice. It is important to note that patients with persistent infection after reimplantation who presented in the current series could have their infection due to actual persistent infection or be caused by a new infection as a direct result of the reimplantation, this is another limitation of the current investigation. Finally, with only a few patients in the group with persistent infection after reimplantation, no meaningful comparison could be performed between groups to investigate the presence of confounding factors.
Diagnostic Threshold and Accuracy Values for Plasma D-dimer and Other Tests
Our study found that plasma D-dimer had low accuracy and a positive test was not associated with persistent infection after reimplantation. ESR and CRP alone, as well as all combinations of D-dimer, ESR, and CRP, fared similarly poorly in this regard. On application of the Youden index to the coordinates of the ROC curve, we found that the best D-dimer threshold differentiating between persistence of infection or not after reimplantation was 3070 ng/mL. When the plasma D-dimer level was below this threshold, there was a substantial decrease in the probability of persistent infection. However, with a value above this threshold, there was only a slight increase in the probability of persistent infection after reimplantation. Similar D-dimer findings were reflected by the positive and negative predictive values. On one hand, the calculated plasma D-dimer threshold had maximum sensitivity (90%); on the other, combination of the three tests (D-dimer, ESR, and CRP) showed maximum specificity (91%). Regarding ESR and CRP, there was poor ability to anticipate persistent infection after reimplantation, which is in agreement with the results of some studies [6, 10, 21] but diverging from others (reported ranges: CRP: sensitivity from 11% to 65% and specificity from 40% to 94%; ESR: sensitivity from 63% to 89% and specificity from 33% to 100%) [6, 10, 11, 21]. Generally, both ESR and CRP are not recommended to decide the right time to perform reimplantation [4, 6, 10, 21, 22]. The definitions of persistent infection and absence of infection vary among these studies, limiting any direct comparisons with our results. Except for one study [23], to our knowledge, the D-dimer value has not been delineated in the setting of reimplantation. With an AUC and specificity as low as 0.565 and 42%, respectively, Xu et al. [23] concluded that D-dimer had limited benefit to confirm infection control before reimplantation. However, they demonstrated a sensitivity as high as 83%. Although plasma D-dimer showed high sensitivity and low specificity in our study as well, comparing the current analyses with that of Xu et al. [23] is not entirely valid because the previous report [23] used the modified Musculoskeletal Infection Society criteria to define persistent infection before the second stage and completely lacked follow-up data on the fate of reimplantation, which was the primary outcome of our study.
Is Using Tests at Threshold Levels More Clinically Useful?
Based on results of the survival analysis, plasma D-dimer was not associated with survival (absence of infection) after reimplantation. This was also true for ESR, CRP, and all combinations of these three tests. Current studies support the poor performance of ESR and CRP when it comes to the outcome of reimplantation [1, 4]. However, we did not find any previous investigation that analyzed plasma D-dimer on its association with the fate of reimplantation. Plasma D-dimer, one of the newest proposed tests for diagnosing PJI, showed poor overall performance in determining infection control and the eventual survival or absence of infection after reimplantation. Its diagnostic ability was comparable to that of the conventional inflammatory markers, ESR and CRP, and their combinations with D-dimer. The 2013 Musculoskeletal Infection Society criteria, which is considered the gold standard for diagnosing PJI [14], have been shown to have a very low sensitivity (0%-25%) but a high specificity (89%-96%) in diagnosing infection and anticipating infection after reimplantation [3, 5, 8]. In contrast, plasma D-dimer demonstrated very low specificity (47%) but high sensitivity (90%) in the same setting. It seems that if plasma D-dimer alone is used along with the 2013 Musculoskeletal Infection Society criteria, these two entities might compensate for each other’s shortcomings regarding diagnostic abilities. Another key takeaway from our study is that plasma D-dimer alone with high sensitivity could be used as a test to establish that PJI is unlikely, and the combination of plasma D-dimer, ESR, and CRP with high specificity could be considered to confirm the persistence of infection after reimplantation.
Conclusion
In this preliminary series, and with the numbers available, plasma D-dimer had poor accuracy and was not associated with survival free from infection after two-stage exchange arthroplasty. Plasma D-dimer alone might be used to establish that PJI is unlikely, and the combination of plasma D-dimer, ESR, and CRP might be considered to confirm PJI diagnosis in the setting of reimplantation. Even though the current investigation is the largest series of which we are aware that has examined the role of D-dimer in reimplantations, further multicenter clinical trials are needed to more definitively establish its role in this setting.
Supplementary Material
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Cleveland Clinic (approval number FLA 18-098).
This work was performed at the Cleveland Clinic Florida, Weston, FL, USA.
Contributor Information
Tejbir S. Pannu, Email: pannut@ccf.org.
Jesus M. Villa, Email: villaj2@ccf.org.
Charles Engh, III, Email: Charles_Engh@rush.edu.
Arpan Patel, Email: apatel57@luc.edu.
Brett R. Levine, Email: Brett.Levine@rushortho.com, brettlevinemd@gmail.com.
Nicolas S. Piuzzi, Email: piuzzin@ccf.org.
Carlos A. Higuera, Email: lbeadling@clinorthop.org.
Aldo M. Riesgo, Email: riesgoa@ccf.org.
References
- 1.Bian T, Shao H, Zhou Y, Huang Y, Song Y. Tests for predicting reimplantation success of two-stage revision for periprosthetic joint infection: a systematic review and meta-analysis. Orthop Traumatol Surg Res . 2018;104:1115-1123. [DOI] [PubMed] [Google Scholar]
- 2.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res . 2013;471:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frangiamore SJ, Siqueira MBP, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res . 2016;474:1630-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu J, Ni M, Li H, et al. The proper timing of second-stage revision in treating periprosthetic knee infection: reliable indicators and risk factors. J Orthop Surg Res . 2018;13:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res . 2016;474:1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res . 2009;467:1699-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087-1098. [PubMed] [Google Scholar]
- 8.Kheir MM, Ackerman CT, Tan TL, Benazzo A, Tischler EH, Parvizi J. Leukocyte esterase strip test can predict subsequent failure following reimplantation in patients with periprosthetic joint infection. J Arthroplasty. 2019;32:1976-1979. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61-65.e1. [DOI] [PubMed] [Google Scholar]
- 10.Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res . 2011;469:1002-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay CP, Olcott CW, Del Gaizo DJ. ESR and CRP are useful between stages of 2-stage revision for periprosthetic joint infection. Arthroplast Today. 2017;3:183-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller R, Higuera CA, Wu J, Klika A, Babic M, Piuzzi NS. Periprosthetic joint infection: a review of antibiotic treatment. JBJS Rev . 2020;8:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Pannu TS, Villa JM, Patel PD, Riesgo AM, Barsoum WK, Higuera CA. The utility of serum D-dimer for the diagnosis of periprosthetic joint infection in revision total hip and knee arthroplasty. J Arthroplasty. 2020;35:1692-1695. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [DOI] [PubMed] [Google Scholar]
- 15.Parvizi J, Tan T, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-1314.e2. [DOI] [PubMed] [Google Scholar]
- 16.Parvizi J, Zmistowski B, Berbari E, et al. New definition for periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res . 2011;469:2992-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson LN, Moser KA, Schmidt RL. D-dimer varies widely across instrument platforms and is not a reliable indicator of periprosthetic joint infections. Arthroplast Today. 2020;6:686-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai NA, Ollins AC. Introduction of products innovance D-dimer of new D-dimer reagent. Sysmex J Int . 2008;17:15-22. [Google Scholar]
- 19.Samuel LT, Sultan AA, Kheir M, et al. Positive alpha-defensin at reimplantation of a two-stage revision arthroplasty is not associated with infection at 1 year. Clin Orthop Relat Res . 2019;477:1615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am . 2017;99:1419-1427. [DOI] [PubMed] [Google Scholar]
- 21.Shukla SK, Ward JP, Jacofsky MC, Sporer SM, Paprosky WG, Della Valle CJ. Perioperative testing for persistent sepsis following resection arthroplasty of the hip for periprosthetic infection. J Arthroplasty. 2010;25:87-91. [DOI] [PubMed] [Google Scholar]
- 22.Stambough JB, Curtin BM, Odum SM, Cross MB, Martin JR, Fehring TK. Does change in ESR and CRP guide the timing of two-stage arthroplasty reimplantation? Clin Orthop Relat Res . 2019;477:364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C, Qu P, Chai W, Li R, Chen J. Plasma fibrinogen may predict persistent infection before reimplantation in two- stage exchange arthroplasty for periprosthetic hip infection. J Orthop Surg Res . 2019;14:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty. 2019;34:2454-2460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.