Abstract
Background
There are a variety of criteria for defining successful treatment after two-stage exchange arthroplasty for prosthetic joint infection (PJI). To accurately assess current practices and improve techniques, it is important to first establish reliable, clinically relevant, reproducible criteria for defining persistent infection and “successful” outcomes.
Question/purpose
Is the proportion of patients considered to have successful management of PJI after two-stage resection arthroplasty smaller using 2019 Musculoskeletal Infection Society Outcome Reporting Tool (MSIS ORT) criteria than when using a Delphi-based criterion?
Methods
Patients were retrospectively identified by Current Procedural Technology codes for resection arthroplasty with placement of an antibiotic spacer for infected THA or TKA between April 1, 2011 and January 1, 2018 at a tertiary academic institution. The initial review identified 180 procedures during this time period. Nine patients had documented transition of care outside the system, 16 did not meet the MSIS criteria for chronic PJI, and 34 patients were excluded for lack of documented 2-year follow-up. The mean follow-up duration of the final cohort of 121 procedures in 120 patients was approximately 3.7 ± 1.7 years. Forty percent (49 of 121) of the procedures were performed on the hip and 60% (72 of 121) were performed on the knee. The mean time from primary THA or TKA to explantation was 4.6 years. The mean age of the patients at the time of explantation was 66 years. The mean time from spacer placement to replantation was 119 days. The final 121 patient records were reviewed by a single reviewer and outcomes were subsequently assigned to “successful” and “unsuccessful” outcomes based on the MSIS ORT and Delphi-based consensus criterion, two previously published and validated multidimensional definition schemes. Chi-squared and t-test analyses were performed to identify differences between “successful” and “unsuccessful” outcomes with respect to patient baseline characteristics using each outcome-reporting criterion.
Results
Overall, the MSIS ORT classified a smaller proportion of patients as having a “successful” treatment outcome after two-stage exchange arthroplasty for PJI than the Delphi-based consensus method did (MSIS: 55% [63 of 114], Delphi: 70% [71 of 102]; relative risk 0.79 [0.65-0.98]; p = 0.03). However, there were no differences when stratified by hips (MSIS: 55% [26 of 47], Delphi: 74% [29 of 39]; relative risk 0.74 [0.54-1.02]; p = 0.07) and knees (MSIS: 55% [37 of 67], Delphi: 67% [42 of 63]; relative risk 0.83 [0.63-1.09]; p = 0.19). Notably, the disease of 16% of the patients (19 of 121) was not classifiable per the Delphi method because these patients never underwent reimplantation.
Conclusion
The present study demonstrated that the MSIS criteria detect fewer instances of “successful” infection management after two-stage resection arthroplasty for PJI than the Delphi method in this cohort. Based on these findings, researchers and surgeons should aim for standardized reporting after intervention for PJI to allow for a better comparison of outcomes across different studies and ultimately allow for improved techniques and approaches to the treatment of PJI.
Level of Evidence
Level III, diagnostic study.
Introduction
The definition of success after the treatment of prosthetic joint infection (PJI) with two-stage exchange arthroplasty is highly variable. In prior work evaluating the outcomes of such procedures, success has often been defined as a combination of laboratory evidence of infection control after replantation and treatment that does not result in subsequent orthopaedic procedures of the affected joint [1, 4, 10, 14, 20, 26]. Others report treatment failure as abnormal laboratory markers (erythrocyte sedimentation rate, C-reactive protein level, and aspiration results) or other unanticipated outcomes such as reoperation or death [26, 29]. Some studies consider long-term treatment with antibiotic suppression a treatment failure, while others consider this a success [8]. With such variation in reporting methods, it is often difficult to compare the clinical results of different treatment approaches and techniques across multiple studies.
The current use of varied outcome definitions has led to studies reporting success rates after two-stage exchange arthroplasty for PJI ranging from 66% to as high as 100% [2, 4, 6, 9, 12, 16, 17, 19-22, 27, 28, 30]. To address this lack of consistency, Diaz-Ledezma et al. [8] published a definition based on the Delphi method to outline the successful treatment of PJI. Their international multidisciplinary consensus definition considered that success was based on infection eradication without clinical evidence of wound drainage or pain, no reinfection by the same microorganisms, and no reoperations or death related to PJI. More recently, the 2019 Musculoskeletal Infection Society (MSIS) workgroup proposed the MSIS PJI treatment outcome reporting tool (MSIS ORT) to aid standardization in research [11]. The MSIS ORT uses four “tiers” in which to assign patients based on their clinical course after the initial operation for PJI (Fig. 1). To our knowledge, no prior work has sought to compare reporting methods in the same cohort of patients undergoing two-stage revision for PJI.
Fig. 1.
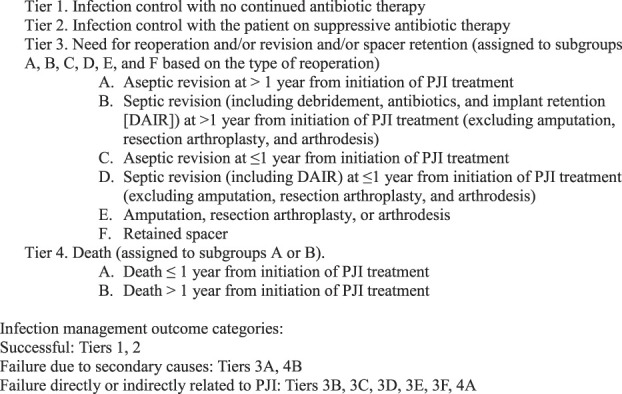
This figure shows the 2019 MSIS proposed ORT for PJI treatment [11].
To address areas for improvement and compare different treatment approaches to PJI, we must first understand how we are reporting outcomes and how the method of reporting affects perceived success rates. The current variability in reporting schemes makes comparison across studies difficult and limits our ability to draw larger, meaningful conclusions from the available evidence. Therefore, we asked: is the proportion of patients considered to have a “successful” outcome after treatment of PJI with two-stage exchange arthroplasty smaller using MSIS ORT criteria than when using the Delphi-based criterion?
Patients and Methods
Patients undergoing resection arthroplasty and antibiotic spacer placement as the first stage of an intended two-stage exchange arthroplasty for suspected PJI between April 1, 2011 and January 1, 2018 at our tertiary academic referral institution were identified and considered for inclusion in this retrospective study. The initial cohort was identified based on Current Procedural Technology codes (27091, 27090, 27122, and 27488) as well as our institutional outpatient program for parenteral antimicrobial therapy. All procedures were reviewed to confirm they were explantations as part of planned two-stage resection arthroplasty. Only patients with documented orthopaedic or infectious disease follow-up via telephone or in person at a minimum of 2 years from explantation were considered for analysis. Patients were excluded if their infection did not meet the MSIS criteria for chronic PJI [24], had a spacer placed for a native joint infection, or had documented transition of care outside our system after explantation.
The initial database search of Current Procedural Terminology codes identified 205 patients (206 procedures; one patient underwent two two-stage exchange arthroplasty procedures on separate joints during the study period) for review (Fig. 2). After removal of duplicates and improperly coded procedures (for example, the procedure was not a spacer placement or the initial surgery was not a THA, hemiarthroplasty, or TKA), 180 procedures remained for evaluation. Nine patients had documented transition of care outside the system, 16 had infections that did not meet the MSIS criteria for chronic PJI, and 22% (34 of 155) of the patients were excluded because they were lost to follow-up before 2 years. There were no differences in patient baseline characteristics including age, sex, BMI, laterality, and joint between the patients who were included and those who were excluded because of a lack of 2-year follow-up (p > 0.05). A final cohort of 121 procedures in 120 patients undergoing TKA or THA explantation for PJI as defined by the MSIS criteria were identified for review. The mean follow-up duration was approximately 3.7 ± 1.7 years. Forty percent (49 of 121) of the procedures were performed on the hip and 60% (72 of 121) were performed on the knee. The mean time from the primary THA or TKA to explantation was 4.6 years. The mean age of the patients at the time of explantation was 66 ± 10 years (Table 1). The mean time from spacer placement to replantation was 119 days.
Fig. 2.
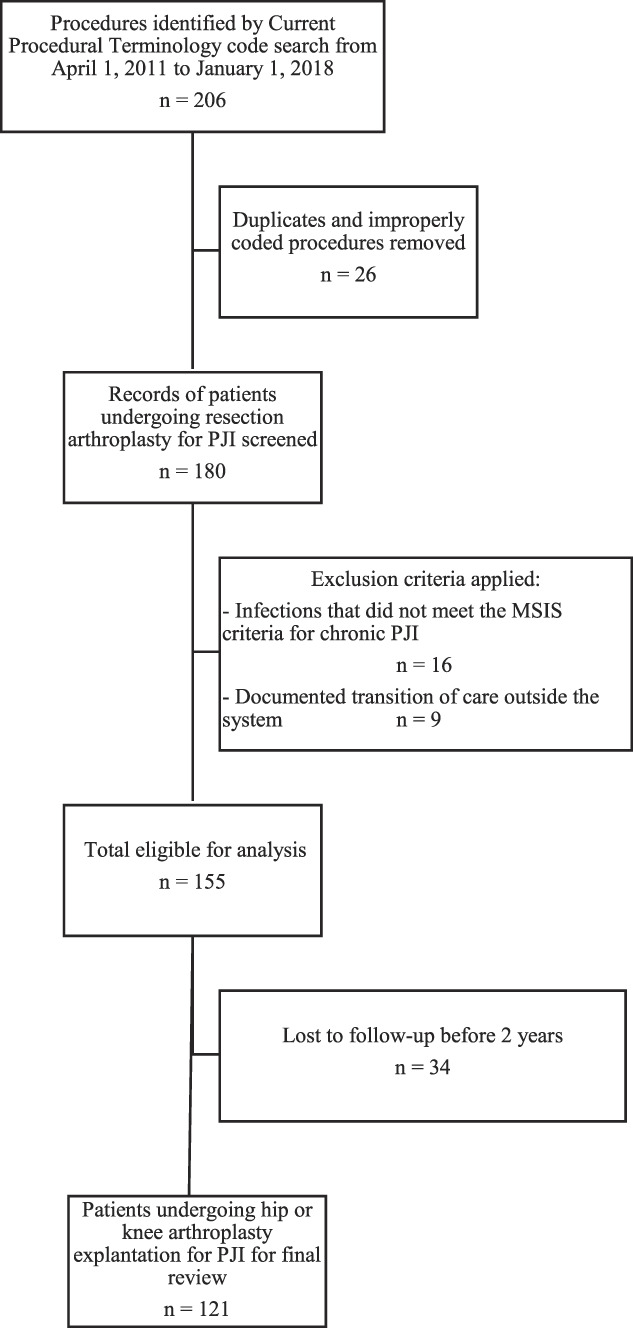
This flowchart demonstrates the retrospective study design and application of the inclusion and exclusion criteria to identify patients who were included in the final cohort.
Table 1.
Baseline demographic characteristics among the study cohort and stratified by success as defined by the Delphi and MSIS criteria
| Characteristic | All (n = 121) | Delphi | MSIS | ||||
| Unsuccessful (n = 31) | Successful (n = 71) | p value | Unsuccessful (n = 58) | Successful (n = 63) | p value | ||
| Male sex, % (n) | 47 (57) | 45 (14) | 49 (35) | 0.87 | 43 (25) | 51 (32) | 0.51 |
| Age in years at explantation, mean ± SD | 66 ± 10 | 64 ± 8 | 65 ± 10 | 0.87 | 65 ± 10 | 66 ± 10 | 0.87 |
| BMI in kg/m2, mean ± SD | 35 ± 9 | 39 ± 11 | 34 ± 8 | 0.01a | 36 ± 10 | 34 ± 8 | 0.31 |
| Right side, % (n) | 54 (65) | 58 (18) | 56 (40) | > 0.99 | 47 (27) | 60 (38) | 0.18 |
| ASA score, mean ± SD | 2.8 ± 0.6 | 2.8 ± 0.5 | 2.7 ± 0.5 | 0.37 | 3.0 ± 0.6 | 2.7 ± 0.5 | 0.01a |
| Static spacer, % (n) | 14 (17) | 16 (5) | 11 (8) | 0.74 | 14 (8) | 14 (9) | > 0.99 |
| Discharge disposition, % (n) | 0.33 | ||||||
| Deceased | 4 (4) | 0 (0) | 0 (0) | 8 (4) | 0 (0) | ||
| Home | 1 (1) | 0 (0) | 2 (1) | 2 (1) | 0 (0) | ||
| Home with VNA | 21 (21) | 28 (7) | 22 (13) | 18 (9) | 24 (12) | ||
| Rehabilitation center | 14 (14) | 20 (5) | 14 (8) | 12 (6) | 16 (8) | ||
| Skilled nursing facility | 39 (39) | 36 (9) | 37 (22) | 38 (19) | 39 (20) | ||
| Swing bed | 22 (22) | 16 (4) | 25 (15) | 22 (11) | 22 (11) | ||
| Reinfection with same organism, % (n) | 0.004a | < 0.001a | |||||
| No | 12 (14) | 23 (7) | 9 (6) | 22 (13) | 2 (1) | ||
| Yes | 82 (99) | 13 (4) | 1 (1) | 10 (6) | 3 (2) | ||
| No reinfection | 7 (8) | 65 (20) | 90 (64) | 67 (39) | 95 (60) | ||
| Revision before inplantation, % (n) | 40 (49) | 45 (14) | 38 (27) | 0.65 | 41 (24) | 40 (25) | 0.99 |
| Follow-up length in years, mean ± SD | 3.7 ± 1.67 | 4.0 ± 2.07 | 3.6 ± 1.49 | 0.26 | 3.9 ± 2.00 | 3.5 ± 1.44 | 0.23 |
Statistically significant; ASA = American Society of Anesthesiologists score; VNA = Visiting Nurse Association.
Surgical Approaches and Aftercare
The initial first-stage procedures involved removal of all implants and thorough irrigation and debridement. Multiple intraoperative cultures were obtained for microbiology review, and in select patients, intraoperative frozen sections were obtained. Spacers included prefabricated molded knee implants (StageOne, Biomet), the Prostalac hip spacer system (Depuy), handmade polymethylmethacrylate spacers, static polymethylmethacrylate spacers, and hybrid low-friction knee spacers (an antibiotic-cemented polyethylene tibial component and antibiotic-cemented femoral component). For the hip resection arthroplasty procedures, 96% (47 of 49) were articulating spacers, and 79% (57 of 72) of knee procedures were articulating spacers. Antibiotics in the cement included tobramycin, vancomycin, gentamicin, ceftazidime, or most commonly, a combination of antibiotics. In the present cohort, the most-common antibiotic combination was vancomycin and tobramycin (54 spacers), followed by vancomycin, tobramycin, and gentamicin (27 spacers). Patients were treated with a minimum of 4 weeks of antibiotics (with the exception of one patient in whom the spacer broke and who underwent replantation before 4 weeks) with co-management by our infectious disease team. The timing of reimplantation as the second stage was based on clinical improvement and laboratory markers, specifically the erythrocyte sedimentation rate and C-reactive protein level. Aspiration was also used in most patients before reimplantation to assess for residual microorganisms or an elevated cell count. Reimplantation procedures involved spacer removal and repeat debridements with subsequent insertion of revision-type THA or TKA components.
Records were retrospectively evaluated to record patient baseline characteristics including age, sex, BMI, laterality, and American Society of Anesthesiologists physical status classification score at the time of the index procedure. The electronic medical record was used to extract pertinent operative details, microbiological data, and prior or subsequent procedures.
Primary Study Endpoint
Our primary study endpoint was the comparison of the 2-year results of treatment based on the MSIS ORT tier versus Delphi-based consensus definition (Fig. 3). A single observer (TMB) applied these endpoints based on the record review and outcome definitions with respect to PJI status per the published criterion. Per the recommendation of the MSIS workgroup, Tiers 1 and 2 were defined as successful outcomes. Tiers 3B, 3C, 3D, 3E, 3F, and 4A were defined as treatment failure that was directly or indirectly related to PJI. Of note, the MSIS suggested that Tier 3B, defined as septic revision at more than 1 year from the initiation of PJI treatment (excluding amputation, resection arthroplasty, and arthrodesis), should be categorized as a failure that was not because of the PJI. In evaluating these patients, we believed these procedures for septic revision more than 1 year from the initial explantation most often represented failure because of factors related to the initial infection and were grouped as such. Failure that was not because of PJI was therefore classified as Tiers 3A and 4B.
Fig 3.
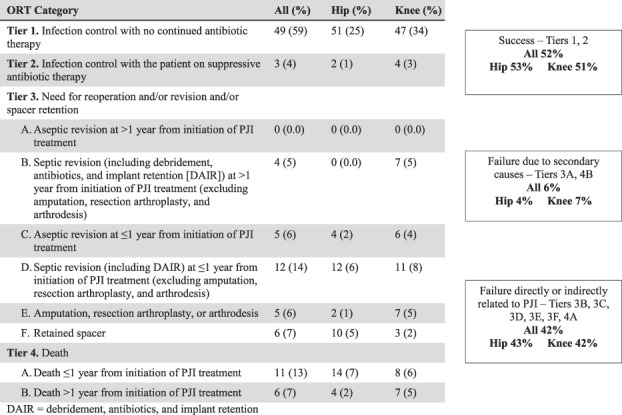
This figure shows the MSIS ORT outcomes for all patients undergoing hip and knee explantation procedures. DAIR = debridement, antibiotics, irrigation, and retention.
Finally, the Delphi-based method of reporting, which was established at the international multidisciplinary consensus meeting and published in 2013 [8], was used to categorize outcomes at 2 years in the present cohort of patients and procedures. A two-round Delphi method was used to reach a consensus definition among international experts in orthopaedic surgery, infectious disease, and clinical research with the goal of creating a standard framework for future scientific reporting. Based on this method, success was defined as infection eradication, evidenced by a healed surgical wound without drainage or a fistula, no documented infection recurrence, no mortality related to PJI (such as sepsis or necrotizing fasciitis), and no subsequent surgical intervention for infection after the second stage of the reimplantation procedure. Of note, 71% of the participants in the consensus meeting agreed that 2 years was an acceptable time period after definitive surgery for PJI when considering short-term results [8].
Ethical Approval
Ethical approval for this study was obtained from the Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA (number STUDY00030894).
Statistical Analysis
In each of these outcome-reporting methods, the initial procedure was assigned to only one final outcome category. t-test and chi-squared analyses were performed to compare patient baseline and demographic characteristics. An additional statistical analysis was performed to compare those with “successful” infection management between the two reporting criteria and calculate the relative risk, using the R environment (R Foundation for Statistical Computing). Notably, given need for dichotomous outcomes for the MSIS ORT cohort for chi-squared analysis comparison to the Delphi-based criteria, we utilized the proportion of success in relation to failure directly or indirectly related to PJI.
Results
Overall, the MSIS criteria classified a smaller proportion of patients as having a “successful” outcome after two-stage exchange arthroplasty for PJI than the Delphi-based consensus method did (MSIS: 55% [63 of 114], Delphi: 70% [71 of 102]; relative risk 0.79 [0.65-0.98]; p = 0.03)(Table 2).. However, there were no differences when stratified by hips (MSIS: 55% [26 of 47], Delphi: 74% [29 of 39]; relative risk 0.74 [0.54-1.02]; p = 0.07) and knees (MSIS: 55% [37 of 67], Delphi: 67% [42 of 63]; relative risk 0.83 [0.63-1.09]; p = 0.19). Notably, the infection of 16% of the patients (19 of 121) was not classifiable per the Delphi method because these patients never underwent reimplantation.
Table 2.
Chi-square analysis comparing the proportionof patients with success versus those with failure after PJI treatment according to the Delphi-based consensus method [8] and MSIS ORT [11]
| Procedure type | Delphi | MSISa | |
| All procedures (n = 121) | |||
| Success, % (n) | 70 (71) | 55 (63) | |
| Failure, % (n) | 30 (31) | 45 (51) | |
| Chi-squared = 4.70; p = 0.03 | |||
| Hip procedures | |||
| Success, % (n) | 74 (29) | 55 (26) | |
| Failure, % (n) | 26 (10) | 45 (21) | |
| Chi-squared = 3.35; p = 0.07 | |||
| Knee procedures | |||
| Success, % (n) | 67 (42) | 55 (37) | |
| Failure, % (n) | 33 (21) | 45 (30) | |
| Chi-squared = 1.78; p = 0.19 | |||
According to the MSIS, “failure” is defined as treatment failure that is directly or indirectly related to PJI (Tiers 3B, 3C, 3D, 3E, 3F, and 4A).
Other Findings
Forty percent (49 of 121) of the patients who underwent explantation had a documented prior revision procedure, defined as any procedure in which an implant component was removed or exchanged, including head and liner exchange and polyethylene exchange (Table 1). Sixty-seven percent (33 of 49) of the revisions before resection arthroplasty were related to infection. The BMI of patients undergoing explantation for TKA infection was higher (37 kg/m2) than that of patients undergoing THA (32 kg/m2) (p = 0.01). Of those with successful treatment per the MSIS ORT, the mean American Society of Anesthesiologists score was higher in those with an unsuccessful outcome than in those with a successful outcome (3.0 versus 2.7 points; p = 0.01). In assessing patients classified using the Delphi-based method, those with an unsuccessful outcome had a higher BMI than those with a sucessful outcome did (39.4 versus 33.9 kg/m2; p = 0.01); however there was no difference in BMI between the successful and unsuccessful groups based on the MSIS criteria (33.9 versus 35.7 kg/m2; p = 0.31). Nine of the 120 patients included in the analysis had retained a primary or revision spacer at 2 years (six hips and three knees).
Discussion
Infection is one of the most common indications for revision surgery after TKA and THA and is challenging for both patients and surgeons because of potential complications and the need to counsel patients on expected outcomes [3]. Antibiotic spacers as part of the two-stage approach to treating PJI were originally described in 1983 by Insall et al. [13] and remain the preferred treatment for patients with late chronic infections, usually defined as occurring at least 4 weeks after the primary operation [5, 7, 18]. Current studies have used a variety of reporting methods, resulting in inconsistently reported rates of “success” after two-stage exchange arthroplasty for PJI [2, 4, 6, 9, 12, 16, 17, 19-22, 27, 28, 30]. The purpose of the current study was to better understand and perform a quantitative comparison of the definition of “success” between two accepted reporting criteria in determining success after the treatment of PJI.
Limitations
The present study has a number of specific limitations, including the small sample size and reliance on a retrospective record review, which may not capture all relevant outcomes or patient characteristics. However, for the baseline characteristics and follow-up that were analyzed, all patients had 2-year clinical follow-up, limiting the potential of missing information relevant to the primary outcomes assessed (reoperations, death, or a retained spacer). Additionally, of all the patients eligible for analysis, we had 78% follow-up at 2 years and there was no difference in age, sex, BMI, or involved joint between those excluded for lack of 2-year follow-up and those who were analyzed. Despite the small sample size and assessment of rare outcomes, we were able to find differences among the groups with respect to baseline characteristics and the primary outcome of reported “success” of PJI treatment using the MSIS ORT compared with the Delphi criterion. A comprehensive record review was performed, and the primary endpoints were assessed by a single physician-reviewer. Although this does not allow for an interobserver evaluation, we believe it does not substantially limit the study’s findings, because the reporting tools have been validated through consensus among multidisciplinary groups and experts in the field with the goal of reproducible application to research [8, 11]. Another limitation is that we evaluated for persistent (or recurrent) infection at 2 years; infection could recur beyond that timepoint. Nonetheless, we believe our findings are robust in answering the question of short-term infection eradication as well as other outcomes such as reoperation, fusion, or amputation, which are of interest to patients and surgeons. Although the study population had a variety of spacer designs, we believe the findings remain pertinent with respect to the evaluation of recurrent infection, which is unlikely to be affected by the mechanical spacer’s design, although more work is needed in this area [23, 25]. All spacers in the study were impregnated with one or more antibiotics. We suspect the variation in the exact antibiotic dose and design likely represents current practice patterns, suggesting our findings are more generally applicable outside our institution [2, 6, 7, 9]. Finally, larger studies might discover differences, whereas we found none in the present study. For example, given the rarity of PJI, future work might involve multiple centers and identify differences between the TKA or THA two-stage revision cohorts, as well as between types of spacers, antibiotic administration, and timing of interventions.
MSIS Versus Delphi Criteria
The MSIS ORT criteria for successful infection management identified fewer successful outcomes than the Delphi-based method did. The purpose of this study was not to report that one method of reporting is superior to or more accurate than the other, but rather to acknowledge and quantify the potential of varied reporting of “success” after treatment for PJI based on the reporting method. The two schemes evaluated in this study have been established by consensus and experts in the field and are multidimensional in order to assess outcomes beyond simply the eradication of infection. Prior work has assessed the outcomes of two-stage procedures for PJI, but a lack of consistent or standardized definitions of success make interpretation across studies more difficult. Although no prior work, to our knowledge, has quantified the potential for varied outcome reporting in a single cohort, previous studies have demonstrated a lack of consistency in published rates of outcomes [4, 6, 9, 10, 12, 16, 17, 19-22, 27, 28, 29, 30]. For example, a retrospective review of patients undergoing two-stage exchange arthroplasty for infected TKA reported infection eradication in 91% of knees (68 of 75) [30]. However, an assessment of the initial population and methods of that study initially identified 16 knees that did not undergo reimplantation. A reevaluation of the success proportion including these patients and defining success as the eradication of infection with a “functional, stable, and painless knee joint” demonstrated a success proportion of 64% [15]. To appropriately compare outcomes such as these across studies, one must acknowledge the potential for differences in reporting schemes in order to draw meaningful conclusions regarding treatment options.
An important component of any study evaluating PJI management is the time from which to start evaluating. The Delphi-based international multidisciplinary consensus stated, “originally, we proposed the index PJI surgery as the time zero to start the follow-up” [8]. However, after receiving survey results and participant recommendations, the group chose to use the definitive surgery for PJI (replantation) as the time from which to examine outcomes. Alternatively, the MSIS multinational, multi-institutional, and multidisciplinary work group published their ORT, which defined the starting point for evaluation as the initial operation for PJI [11]. We sought to better understand outcomes after resection arthroplasty with antibiotic spacer placement as part of a planned two-stage revision for PJI; therefore, we used the explantation procedure as the time from which the outcomes were measured. The different starting point accounts for a portion of the differing success between these two methods, but not entirely. If the 19 patients who never underwent replantation are included and the Delphi-based consensus definitions are used, and assuming those who did not undergo reimplantation had failed treatment, the success proportion is 59%, which still remains higher than that obtained with the MSIS ORT (52% of the entire cohort, 55% when compared only to failure directly or indirectly related to PJI). Therefore, although the differences we found in the proportion of successful outcomes can in part be explained by the choice of the starting point from which to begin the analysis, it does not entirely account for the differences we found. Regardless, researchers must be aware of these differences when comparing the results of multiple studies and particularly when performing statistical analyses across studies (such as meta-analyses).
Conclusion
As surgeons, clinicians, and researchers, we must understand the potential for varied reporting of treatment success in current studies and use this knowledge to critically compare data assessing treatment approaches to PJI. Researchers must seek standardized definitions of treatment success in order to allow for better communication and consistency among studies, and ultimately help guide treatment. Additionally, more work is needed to understand and incorporate patient-reported outcomes and patient-perceived “success” into the evaluation of outcomes after PJI treatment. Finally, studies with larger patient populations and standardized reporting methods are needed to better understand the role patient factors play in the outcome of two-stage resection arthroplasty for PJI in order to improve outcomes and provide appropriate counseling to patients with PJI.
Acknowledgment
We thank Adriana P. Lucas MS for her contribution to the initial data review and statistical analysis.
Footnotes
Each author certifies that neither he nor she, nor any of his or her immediate family members, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA (reference: STUDY00030894).
Contributor Information
Daniel A. Pierce, Email: Daniel.A.Pierce@hitchcock.org.
Thomas M. Hanson, Email: thomas.m.hanson@hitchcock.org.
Paul M. Werth, Email: Paul.M.Werth@hitchcock.org.
Alexander R. Orem, Email: Alexander.R.Orem@hitchcock.org.
Wayne E. Moschetti, Email: Wayne.E.Moschetti@hitchcock.org.
References
- 1.Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25:1022-1027. [DOI] [PubMed] [Google Scholar]
- 2.Bejon P, Berendt A, Atkins BL, et al. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother. 2010;65:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury T, Fehring TK, Taunton M, et al. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24:101-104. [DOI] [PubMed] [Google Scholar]
- 5.Cancienne JM, Granadillo VA, Patel KJ, Werner BC, Browne JA. Risk factors for repeat debridement, spacer retention, amputation, arthrodesis, and mortality after removal of an infected total knee arthroplasty with spacer placement. J Arthroplasty. 2018;33:515-520. [DOI] [PubMed] [Google Scholar]
- 6.Castelli CC, Gotti V, Ferrari R. Two-stage treatment of infected total knee arthroplasty: two to thirteen year experience using an articulating preformed spacer. Int Orthop. 2014;38:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871-882. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768-774. [DOI] [PubMed] [Google Scholar]
- 10.Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ. A two-stage retention débridement protocol for acute periprosthetic joint infections. Clin Orthop Relat Res. 2010;468:2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillingham YA, Della Valle CJ, Suleiman LI, et al. Definition of successful infection management and guidelines for reporting of outcomes after surgical treatment of periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society (MSIS). J Bone Joint Surg Am. 2019;101:e69. [DOI] [PubMed] [Google Scholar]
- 12.Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087-1098. [PubMed] [Google Scholar]
- 14.Jämsen E, Stogiannidis I, Malmivaara A, Pajamäki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach: a systematic review of the literature. Acta Orthop. 2009;80:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenny J-Y. Re: Modern treatment of infected total knee arthroplasty with a 2-stage reimplantation protocol. J Arthroplasty. 2011;26:821-822. [DOI] [PubMed] [Google Scholar]
- 16.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res . 2002;404:116-124. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y-H, Choi Y, Kim J-S. Treatment based on the type of infected TKA improves infection control. Clin Orthop Relat Res. 2011;469:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuzyk PRT, Dhotar HS, Sternheim A, Gross AE, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: techniques, controversies, and outcomes. J Am Acad Orthop Surg. 2014;22:153-164. [DOI] [PubMed] [Google Scholar]
- 19.Leunig M, Chosa E, Speck M, Ganz R. A cement spacer for two-stage revision of infected implants of the hip joint. Int Orthop. 1998;22:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmud T, Lyons MC, Naudie DD, MacDonald SJ, McCalden RW. Assessing the gold standard: a review of 253 two-stage revisions for infected TKA. Clin Orthop Relat Res. 2012;470:2730-2736.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meek RMD, Masri BA, Dunlop D, et al. Patient satisfaction and functional status after treatment of infection at the site of total knee arthroplasty with the use of the PROSTALAC articulating spacer. J Bone Joint Surg Am . 2003;85:1888-1892. [DOI] [PubMed] [Google Scholar]
- 22.Mortazavi SMJ, Vegari D, Ho A, Zmistowski B, Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011;469:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahhas CR, Chalmers PN, Parvizi J, et al. A randomized trial of static and articulating spacers for the treatment of infection following total knee arthroplasty. J Bone Joint Surg Am. 2020;102:778-787. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-1314.e2. [DOI] [PubMed] [Google Scholar]
- 25.Pivec R, Naziri Q, Issa K, Banerjee S, Mont MA. Systematic review comparing static and articulating spacers used for revision of infected total knee arthroplasty. J Arthroplasty. 2014;29:553-557.e1. [DOI] [PubMed] [Google Scholar]
- 26.Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis. 2011;53:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherrell JC, Fehring TK, Odum S, et al. The Chitranjan Ranawat award: fate of two-stage reimplantation after failed irrigation and débridement for periprosthetic knee infection. Clin Orthop Relat Res . 2011;469:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestre A, Almeida F, Renovell P, Morante E, López R. Revision of infected total knee arthroplasty: two-stage reimplantation using an antibiotic-impregnated static spacer. Clin Orthop Surg. 2013;5:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;427:94-100. [DOI] [PubMed] [Google Scholar]
- 30.Westrich GH, Walcott-Sapp S, Bornstein LJ, Bostrom MP, Windsor RE, Brause BD. Modern treatment of infected total knee arthroplasty with a 2-stage reimplantation protocol. J Arthroplasty. 2010;25:1015-1021.e2. [DOI] [PubMed] [Google Scholar]


