Abstract
Porphyromonas gingivalis proteases are key to the virulence of this periodontitis agent and emerging Alzheimer’s disease, cancer and arthritis pathogen. Transposon sequencing has been employed to define the core essential genome of this bacterium and genes conditionally essential in multiple environments - abscess formation; epithelial colonization; and cigarette smoke toxin exposure; as well as to elucidate genes required for iron acquisition and a functional type 9 secretion system. Protein catabolism genes identified include a combination of established virulence factors and a larger set of seemingly more mundane proteolytic genes. The functions and relevance of genes that share essentiality in multiple disease-relevant conditions are examined. These common stress-related genes may represent particularly attractive therapeutic targets for the control of P. gingivalis infections.
Keywords: Essential genes, fitness, pathogenesis, periodontitis, Porphyromonas gingivalis, transposon sequencing
P. gingivalis proteases and virulence
Porphyromonas gingivalis is a causative agent of periodontitis that has also been associated with multiple systemic conditions, including pre-term birth [1, 2], arthritis [3, 4], vascular problems [5, 6] and, more recently, head and gastrointestinal tract cancers [7, 8] as well as Alzheimer’s disease [5, 9]. P. gingivalis is an asaccharolytic anaerobe that is reliant on proteolysis for metabolic function and subsequent virulence in the oral cavity and, presumably, beyond. In addition to provision of carbon [10, 11], the proteases and peptidases of this bacterium play critical roles in iron acquisition [12, 13], adhesion, colonization and invasion [14, 15], immune subversion [16–18] and tissue destruction [15, 17]. This cacophony of virulence traits, as well as other non-proteolytic mediators of pathogenesis, has led to the recognition of P. gingivalis as a key pathogen. It has been further posited that, even at low incidence, P. gingivalis is able to induce community dysbiosis, at least in mice [19, 20].
Therefore, proteinases have oft been proposed as therapeutic targets. However, while certain protein catabolism gene products of P. gingivalis are well characterized, others remain, essentially, unstudied. Transposon mutagenesis has facilitated insight into which proteolytic enzymes of P. gingivalis are essential for survival per se and which are essential in several disease-relevant environments. Of particular focus, herein, will be stress-related genes that make critical contributions to fitness in multiple niches. As delineated below, the common conditionally essential stress-related proteolytic gene set encodes a combination of established virulence factors and a larger set of seemingly more mundane and / or uncharacterized proteolytic genes that may yet prove to represent particularly attractive therapeutic targets for the control of P. gingivalis infections.
Transposon sequencing and P. gingivalis
The emergence of transposon sequencing (TnSeq) technology allows researchers to rapidly “carry out genome-wide studies in a wide range of bacterial species, under a multitude of conditions, with unprecedented depth” [21]. Initial attempts at library building used technologies that resulted in mutagenesis at non-random sites or hotspots [22–24]. Several P. gingivalis-specific TnSeq libraries, however, have now been generated by independent groups that exhibit both random insertion into the chromosome and high genome saturation [12, 14, 25–28]. Such successful libraries have been built using the Himar 1 Mariner mini-transposon system originally designed for Bacteroides thetaiotaomicron and utilizing the pSAM_Bt vector [29] or the plasmid pMI07-based system, originally designed for Bacteroides spp, in general [30]. To date, the highly penetrative libraries, containing between 54,000 and 80,000 mutant clones each, are specific to P. gingivalis ATCC 33277 [12, 25–27], the most comprehensively studied P. gingivalis strain. While we have had partial success with P. gingivalis HG66 (unpublished data), penetration into the P. gingivalis W83 genome has proven problematic, perhaps due to significant bacterial capsulation. However, Klein et al have successfully generated a smaller 12,000-transposon insertion P. gingivalis W83 library which has been employed to screen for pigmentation-related genes [12]. Therefore, unless otherwise stated, the literature discussed herein specifically refers to P. gingivalis ATCC 33277.
Absolutely essential proteases of the P. gingivalis
A comparison of the TnSeq libraries for P. gingivalis ATCC 33277 independently generated by Klein et al [26] and Hutcherson et al [25] identified 281 common genes, out of a total of 2155 (13%) in the chromosome, as essential for planktonic growth in complex media. Perhaps surprisingly, and presumably due to a lack of selective pressure, only 5 proteolytic genes, as presented in Table 1, were found to be part of this core essential genome. PGN_0202 (PG_2157; PGTDC60_1267) encodes a putative leucine aminopeptidase precursor, related to the M28 group of metalloproteinases, that likely functions to release N-terminal amino acids, preferably leucine, from polypeptides. PGN_0250 (PG_0137; PGTDC60_0414), a pepD-like peptidase gene, whose product likely targets N-terminal neutral or hydrophobic residues from Xaa-His peptide or peptide fragments [31]. PGN_0993 (PG_0956; PGTDC60_0878) encodes a M23/M27 metalloendopeptidase, also likely to have a broad specificity. The reasons for non-conditional essentiality of PGN_0202, PGN_0250 and PGN_0993 are not immediately obvious, yet they may nevertheless represent attractive, previously unheralded, therapeutic targets. On the other hand, LepB (PG_1598; PGTDC60_0703) is a single copy signal peptidase I that cleaves N-terminal signal sequences from secreted and periplasmic proteins. Lsp A (signal peptidase II, PG_0515; PG_2001; PGTDC60_0274), also encoded by a single copy gene, is responsible for the removal of signal peptides from prolipoproteins. The fundamental roles of LepB and Lsp A in protein transport likely explains core essentiality.
Table 1.
Essential proteases of the P. gingivalis ATCC 33277 core genome.
| Gene | Annotation */** | EC/ WP Number*** | |
|---|---|---|---|
| PGN_0202 |  |
Leucine aminopeptidase precursor M28 group metalloproteinase | BAG32721 WP_012457324.1 |
| PGN_0250 |  |
pepD-like aminoacyl-histidine dipeptidase | 3.4.13.- WP_012457365.1 |
| PGN_0515 |  |
IspA; signal peptidase II | 3.4.23.36 WP_004584054.1 |
| PGN_0993 | 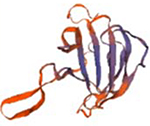 |
M23/M27 family peptidase | BAG33512 WP_012457940.1 |
| PGN_1946 | 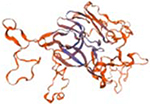 |
lepB; signal peptidase I | 3.4.21.89 WP_012458631.1 |
Cross library comparison of genes essential for planktonic growth in complex media led to the identification of 251 common genes in total [25, 26], of which 5 were related to protein catabolism, as determined by investigation of the GenBank (www.ncbi.nlm.nih.gov/genbank/) and KEGG (www.kegg.jp) databases, enhanced by MEROPS (www.ebi.ac.uk/merops).
Structural models were generated using the SWISS-MODEL homology-modelling server (https://swissmodel.expasy.org/).
Enzyme Commission and NCBI Reference Sequence accession numbers, respectively, are provided. If an Enzyme Commission number is unavailable, the GenBank accession BAG code is presented.
Conditionally essential P. gingivalis protease genes
In addition to the identification of a core genome [25, 26], TnSeq has been used to determine P. gingivalis genes that determine fitness in a number of specific environments, as presented in Figure 1. These are epithelial colonization [14], murine abscess formation [14] and survival of cigarette smoke toxins [28], as well as to pinpoint genes required for black pigmentation [12], requisite for gingipain transport and hemin acquisition, and, relatedly, for a functional type IX secretion system (T9SS) [27]. Herein, we specifically delineate and summarize those established or putative protein catabolism genes that endow P. gingivalis with fitness in such diverse niches. The specific protease-related genes essential for each condition are presented in Supplemental Table 1a–e.
Figure 1: Exploitation of transposon sequencing libraries to identify P. gingivalis genes essential for fitness in multiple environments.
Highly saturated P. gingivalis TnSeq libraries have been used to identify genes that are conditionally essential for (A) murine abscess formation [14]; (B) epithelial colonization [14]; (C) survival of cigarette smoke toxins [28]; (D) black pigmentation [12]; and (E) maintenance of a functional T9SS [27]. High throughput sequencing allows for the identification and quantification of multiple transposon insertion sites simultaneously among a large mixture of mutants. Varying library generation and analytical methods have been employed, while the technology is rapidly evolving, e.g., [21, 79–81]. Generally, however, subtraction of the sequenced output library (non-essential mutants) from the larger input library (essential and non-essential mutants) allows elucidation of those individual strains that are required for survival under a specific condition of interest and, so, unable to propagate, are absent or under-represented in the output library. Herein, we focus particularly on those P. gingivalis protease-encoding genes that are essential in several disease-related environments, assigning high potential therapeutic value to communal environmental fitness determinant(s).
P. gingivalis proteases essential under four disease-relevant conditions (n = 2)
There are two P. gingivalis proteolysis genes that are essential in 4/5 conditions in which TnSeq libraries have been examined. They are an established virulence factor, heat shock-related protein A, and an essentially uninterrogated papain-like cysteine endopeptidase, bleomycin hydrolase (PGN_1777; PG_1788; PGTDC60_0106). While bleomycin hydrolase is conserved across kingdoms, its function in bacteria is largely unknown. P. gingivalis, however, has a large number of immune defense evasion mechanisms, many protease-based [32–34]. Mammalian bleomycin hydrolase has been shown to contribute to the regulation of chemokine release by in human innate cells. More specifically, the bleomycin hydrolase signal inversely correlates with expression of CXCL8 and GROα in several inflammatory skin conditions in humans, a phenomenon reflected in keratinocytes in which bleomycin hydrolase has been knocked down [35]. While the mechanisms underlying the bleomycin hydrolase-CXCL8 axis remain to be elucidated, should the equivalent P. gingivalis enzyme function similarly, it is possible that the bacterial bleomycin hydrolase could reduce local chemokine activity. This could be in addition to the direct hydrolysis of CXCL8 by gingipains [36, 37]. The advantage, presumably, would be reduced neutrophil, or other innate cell, chemotaxis towards P. gingivalis. Bleomycin is a Streptomyces verticillus-derived antibiotic used as component of treatment for specific cancers, including several squamous cell cancers. P. gingivalis has been associated with multiple oral and gastrointestinal epithelial cancers, but the use of bleomycin in in such patients is not routine. However, while speculative, a role for PGN_1777 orthologues in other bacterial-associated cancers where bleomycin is indeed a chemotherapeutic drug of choice, may be worthy of investigation. What is clear, is that further research is required to better understand the reliance of P. gingivalis on bleomycin hydrolase for survival in multiple, highly variable environments.
Heat shock-related protein A (PGN_0637; PG_0593; PGTDC60_1718) is intimately involved in the transmembrane transport of proteins [38], helps protect against oxidative stress [39, 40] and has been shown to be important for P. gingivalis virulence in mice [40], as presented in Figure 2. More specifically, HtrA, a predominantly outer membrane protease with preference for unfolded proteins, is requisite for the degradation of T9SS cargo proteins that are not protected by the presence of an IgG-domain [38]. Consequently, in the absence of HtrA, incorrectly processed proteins, including gingipains, accumulate near the bacterial cell surface [38, 41]. Such a fundamental role in the quality control of transported proteins likely explains common conditional essentiality for PGN_0637. The ability to withstand oxidative stress is particularly relevant in murine abscess, epithelial colonization and tobacco exposure environments. It is interesting, then, that a P. gingivalis W83 htrA mutant strain exhibit increased sensitivity to hydrogen peroxide, as is the case in several other bacterial species, while gingipain activity was compromised upon heat shocking [39, 40]. LuxS, critical for the formation of autoinducer II of P. gingivalis, is a negative regulator of htrA gene activation in strain W83, suggesting a potential role for HtrA in adaptation to higher density conditions [42]. Indeed, htrA encodes a known protein which appears to be an important virulence factor. Yuan et al have shown that a heat shock-related protein A P. gingivalis W83 mutant is outcompeted by the replete strain following subcutaneous injection in mice while the mutant exhibits reduced lethality compared to the parent bacterium [40]. Therefore, the biological rationale for essentiality in multiple conditions is more obvious for htrA than it is for bleomycin hydrolase.
Figure 2: Virulence-related attributes of the commonly conditionally essential stress-related P. gingivalis ATTCC 332277 gene, htrA (PGN_0637).
Heat shock-related protease A is an essential fitness gene under four different experimental conditions (abscess formation; epithelial colonization; tobacco smoke survival; and black pigmentation), as determined using TnSeq technology [12, 14, 28]. Interestingly, HtrA has been shown to be important to several virulence-related functions of P. gingivalis, including (A) quorum sensing [42]; (B) a functional T9SS and gingipain transport [38, 41]; (C) murine abscess formation [40]; (D) oxidative stress protection [39, 40]; (E) murine lethality [40]; and (F) temperature stability [39].
P. gingivalis proteases essential under two or more disease-relevant conditions (n = 24)
In addition to PGN_0637 and PGN_1777, a number of genes are conditionally essential in multiple conditions tested, again as noted in Supplemental Table 1. Those genes essential in three conditions (n = 16) are PGN_0271 (pepO); PGN_0295 (C-terminal domain of Arg- and Lys-gingipain proteinase), recently shown to be required for appropriate gingipain transmembrane transport and maturation [43]; PGN_0303 (an M16 family zinc protease); PGN_0561 (PrtT protease); PGN_0607 (dipeptidyl peptidase 11); PGN_0754 and PGN_0771 (NLP/P60 family proteins); PGN_0780 (PrtQ protease); PGN_0788 (peptidyl-dipeptidase); PGN_0900 (thiol protease / hemagglutinin / periodontain); PGN_1335 (hypothetical peptidase); PGN_1349 (a dipeptidyl aminopeptidase / acylpeptidyl oligopeptidase [44]); PGN_1466, the classic P. gingivalis virulence-related protease, rgbB; PGN_1479 (S46 family dipeptidyl-peptidase; PGN_1694 (alanyl dipeptidyl peptidase); and PGN_2064 (Oma1, M48-family peptidase).
Those of particular interest include PGN_0271 (PG_0159; PGTDC60_0435), an established virulence-related gene encoding an endopeptidase that is common among 40 global P. gingivalis isolates [45]. Interestingly, pepO activity is upregulated during, and is requisite for, invasion of epithelial cells, as has been previously shown [46]. A mechanistic role for PepO in epithelial invasion is also implied by aberrant cytoskeleton rearrangements seen on the interaction of a pepO mutant with gingival epithelial cells [46]. Mutation of the P. gingivalis 381 orthologue similarly resulted in reduced invasion of HeLa cells [47]. PepO, which exhibits high homology to the cardiovascular disease risk factor, human endothelin-converting enzyme 1 [46–48], and can convert big endothelin-1 to functional endothelin-1 [48], a potent vasoactive peptide. PepO is also packaged into outer membrane vesicles [49] and, therefore, likely to be distributed in the periodontal tissues themselves and may circulate beyond.
PGN_0561 (PG_1427; PGTDC60_0751), prtT, encodes a secreted cysteine protease and streptopain homologue, that also possesses hemagglutinin activity, with at least two distinct interstrain catalytic domain allele variants [50, 51]. PGN_0900 encodes an extacellular cysteine protease, known as periodontain, a potent inactivator of α1-antrypsin inhibitor which exhibits high homology to PrtT [51]. Such functionalities would be expected to increase the local burden of endogenous serine proteases, particulalrly neutrophil elastase, leading to augmented host-mediated collateral tissue damage and nutrient availability to asaccharolytic bacteria. Clearly, the ability to acquire iron and peptide-based carbon sources is critical to bacterial survival. In keeping, PrtT has been shown to be an important virulence factor in vivo. Essentially, a non-human primate P. gingivalis 3079 strain lacking trypsin-like activity, unlike the parent strain, was non-lethal and unable to induce lesions following subcutaneous inoculation [52]. Further, inactivation of rgpA results in upregulation of prtA gene activity [53], suggesting proteinase T may also act as a compensatory gingipain. Despite the potential of periodontain to represent an important P. gingivalis virulence factor, PGN_0900-related pathogenesis has not yet been studied in animal models, to the best of our knowledge.
P. gingivalis produces several dipeptidyl peptidases which are considered fundamental for asaccharolytic carbon metabolism [10, 11]. Orthologues of PGN_0607 (DPP11), an Asp/Glu-specific periplasmic exopeptidase, are common among bacteria but not so in other kingdoms, increasing the attractiveness of this peptidolytic enzyme as a therapeutic target [10]. Further, aspartate and glutamate peptides are the amino acids primarily utilized by P. gingivalis [54], while disruption of PGN_0607 reduces bacterial growth even in rich medium [11], shedding light on the essentiality of this particular dipeptidase.
Finally, while gingipain-encoding rgbB (PGN_1466) did not form part of the core P. gingivalis genome, its conditional essentiality emerged upon the imposition of several environmental stresses. As the best characterized P. gingivalis proteases, there are several gingipain reviews already available, not limited to [10, 34, 55–58]. The gingipains account for ~85% of the proteolytic potential of P. gingivalis [59]. The 2 Arg-X-specific gingipains (RgpA and RgpB) contain nearly identical catalytic domains, however RgpB lacks the large C-terminal hemagglutinin domain of RgpA and the Lys-X-specific gingipain, Kgp. The absence of this adhesion-associated domain in RgpB suggests essential functions are adhesion-independent and likely specifically related to proteolytic activity. The many pathogenicity associated roles of RgpB are well characterized and includes complement evasion by degrading several complement proteins [60], degradation of host defensins [61], proteolytic destruction of numerous host receptors and effectors that dysregulates host immune responses [62].
Those genes essential in two conditions (n = 8) are PGN_0022 (porU, cysteine proteinase), PGN_1149 (prolyl tripeptidase A), PGN_1409 (a putative M20 peptidase), PGN_1416 (pepK, lysyl endopeptidase precursor), PGN_1469 (dipeptidyl peptidase IV), PGN_1728 (the classic P. gingivalis virulence-related protease, kgp), PGN_2035 (putative M16B subfamily peptidase) and PGN_2065 (Lys- and Rgp-gingipain domain protein).
PGN_0022 encodes PorU, a cysteine proteinase and integral component of the T9SS of P. gingivalis. Considering the critical role of the T9SS in Bacteroides physiology, not least the delivery of functional membrane-associated gingipains, it is perhaps not surprising that the PorU protease has been identified as conditionally essential.
PGN_1416 (pepK) encodes a cysteine proteinase, likely LPS-anchored, that appears to function as an Rgp-activated Lys-specific serine endopeptidase [63] whose translocation is reliant upon an intact T9SS [64]. The reason for the essentiality of PepK, predominantly found in the Bacteroidetes, remains elusive. However, we have shown that the specific proteolytic activity of PGN_1416, an outer membrane-associated surface protein [63], is clearly required for epithelial colonization and abscess formation [14].
Dipeptidyl peptidase IV (DppIV) activity has been shown to play an important role in P. gingivalis virulence [65–68]. The 99% identical (www.kegg.jp) dppIV serine protease encoding W83 orthologue of PGN_1469 targets a broad spectrum of X-Pro and X-Ala dipeptides at peptide N-termini and has been shown to contribute to subcutaneous murine lesion formation and lethality [65, 66, 69]. Interestingly, P. gingivalis-derived DppIV has been hypothesized to cleave RANTES into a less potent innate cell chemokine that may actually antagonize full length RANTES, and suggested to processes the CXC12 (SDF1) into a less potent lymphokine [65]. In keeping, neutrophil infiltration into P. gingivalis-induced murine lesions is reduced with dppIV mutant bacteria comparted to wild type cells [68]. Further, DppIV may contribute to periodontal connective tissue destruction through an ability to augment endogenous MMP-1,−2, −8 and −9 and subsequent degradation of resultant gelatins [67]. DppIV also contributes to the adhesion of P. gingivalis to fibronectin [66, 68], which may assist in bacterial colonization, while concomitantly inhibiting fibroblast binding to fibronectin [68], an important event in the healing process. Thus, the identification of PGN_1469 as a common conditionally essential gene is in keeping with the extant literature.
Similar to the prior discussion of gingipains and the rgpB gene, detailed physiological roles of the gingipains are extraordinarily well documented. PGN_1728-encodes the Lys-X-specific gingipain, Kgp. Kgp-mediated proteolysis of provides essential nutrients to asaccharolytic bacteria, but also functions to dysregulate immune recognition and response systems through degradation of ECM components, immunoglobulins, and cytokines [70]. Specific inhibition of Kgp activity in a mouse model significantly reduced virulence, suggesting Kgp may contribute more to P. gingivalis-associated pathogenicity than any other protease [71].
P. gingivalis proteases essential to a specific disease-relevant condition (n = 6)
It is important to consider genes that are conditionally essential to a unique condition because it is likely more straightforward to dissect their pathogenic importance, using gain and loss of function approaches, than for those genes essential in multiple conditions. P. gingivalis genes identified as critical to fitness in a single experimental environment were PGN_0335 (PG_0232; PGTDC60_0510), encoding a M14 family zinc carboxypeptidase domain protein essential for CSE survival [28]; PGN_0508 (PG_1605; PGTDC60_0696), an aminopeptdase C-encoding gene essential for epithelial colonization [14]; PGN_0952 (PG_1060; PGTDC60_1149), PGN_1914 (orthologues also annotated as PG_1060; PGTDC60_1149) and PGN_1970 (PG_2024; PGTDC60_0300) encoding, two serine peptidases and the arginine-specific cysteine proteinase A gene, respectively, and essential for black pigmentation [27]; and PGN_1434 (PG_0537; PGTDC60_1655), which encodes a PepD-like aminoacyl-histidine dipeptidase, required for murine abscess formation [14].
Other than rgpA, the mechanisms underlying conditional essentiality are not immediatelyobvious. However, in silico homology searches, primarily using www.kegg.jp, do present some clues. PGN_0335, for example, is unexamined in P. gingivalis, but orthologues of this M14-family peptidase represent T9SS C-terminal target domain-containing proteins in several Aquimarina species. PGN_0508 exhibits high homology to bleomycin hydrolase [EC:3.4.22.40], whose significance has been discussed above in the context of PGN_1777, an essential gene in multiple environments. PGN_0952 and PGN_1914 each encode a ctpA-related carboxyl-terminal processing protease. While not investigated in P. gingivalis specifically, CtpA proteins, which are not common in bacteria, are known to function in transmembrane protein transport and maturation in some Gram-negative species [72, 73]. Clearly, this may well be related to their yet be determined essential role in the sequestration of hemin at the cell surface, leading to black pigmentation, and the acquisition of iron.
The emergence of rgpA as a gene required for hemin acquisition means that all three gingipain genes have been identified as conditionally essential. RgpA and Kgp have both been shown to bind heme, porphyrins and metalloporphryins [74]. Additionally, RgpA is known to degrade transferrin, hemoglobin and hemopexin [75], demonstrating its essential role in heme acquisition. Importantly, RgpA is known to cleave pro-Kgp, leading to Kgp activation, and is involved in proteolytic processing of many cell-associated proteins. Thus RgpA plays direct roles in iron and heme recruitment but additional indirect functions in protein maturation.
Concluding Remarks and Future Perspectives
To improve our understanding of the biological relevance of TnSeq-identified essential protease genes, a number of future initiatives are envisaged, as noted in the Outstanding Questions section. Most obviously, there is a need to develop libraries in a variety of P. gingivalis strains as, currently, the data are ATCC 33277-centric. Secondly, there is a need to generate prioritized individual mutants for phenotypic characterization. Such an approach has been employed to confirm the reduced fitness of genes deemed requisite by TnSeq for epithelial colonization [14], survival of cigarette smoke-induced stress [28], murine abscess formation [14] and black pigmentation [12]. It should be noted that the deletion of single putatively essential gene can [12, 14, 27, 28], but does not necessarily, result in a definitive, growth-compromised phenotype per se. However, reduced fitness, under the relevant conditions, is often noted upon competition of the mutant of interest with the parental bacterial strain [14, 28]. When trying to gain a deeper understanding of essential genes in situ, there are emergent issues to consider. With complex datasets, it may be more efficient to test a combination of mutants in the condition of interest. Perhaps more importantly, it should be remembered that periodontal biofilms are highly intricate structures and contain spatially-oriented and temporally shifting subsets of interacting bacteria among the multitude of species comprising the overall plaque microbiome [76, 77]. Therefore, it will be important to ascertain if non-P. gingivalis bacteria may contribute compensatory biomolecules. Finally, the design of new transposon libraries is likely to be abetted by the recently launched Transposon Registry, which aims to catalogue and assign a searchable transposon number to all prokaryotic transposable elements [78]. It must be envisaged that theTransposon Registry can be utilized alongside the growing Database of Essential Genes (www.essentialgene.org).
Meantime, there are multiple obvious strengths of transposon sequencing technology, in the context of the identifying absolute and essential protease-related genes of the model periodontal pathogen P. gingivalis. They include (a) high genome saturation of the 33277 library; (b) the generation of similar libraries by several independent groups; (c) the deposition of the relevant information in the expanding Database of Essential Genes; (d) library exploitation in multiple disease-relevant conditions; and, in particular, (e) the identification of a stress-related protein catabolism gene set common to multiple environments that both confirms the significance of established virulence factors while identifying previously unheralded pathogenesis genes. Perhaps the best examples are the two genes common to 80% of the conditions tested to date, PGN_637,encoding HtrA, and PGN_1777, encoding a bleomycin hydrolase. These common stress-related genes may represent particularly attractive therapeutic targets for the control of P. gingivalis infections – an established oral pathogen with growing systemic disease relevance.
Supplementary Material
References
- 1.Miyauchi M et al. (2018) Galectin-3 Plays an Important Role in Preterm Birth Caused by Dental Infection of Porphyromonas gingivalis. Sci Rep 8 (1), 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanterpool SF et al. (2016) Porphyromonas gingivalis within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome. PLoS One 11 (1), e0146157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courbon G et al. (2019) Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann Rheum Dis 78 (5), 594–599. [DOI] [PubMed] [Google Scholar]
- 4.Perricone C et al. (2019) Porphyromonas gingivalis and rheumatoid arthritis. Curr Opin Rheumatol 31 (5), 517–524. [DOI] [PubMed] [Google Scholar]
- 5.Carter CJ et al. (2017) The Porphyromonas gingivalis/Host Interactome Shows Enrichment in GWASdb Genes Related to Alzheimer’s Disease, Diabetes and Cardiovascular Diseases. Front Aging Neurosci 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xuan Y et al. (2017) Tanshinone IIA Attenuates Atherosclerosis in Apolipoprotein E Knockout Mice Infected with Porphyromonas gingivalis. Inflammation 40 (5), 1631–1642. [DOI] [PubMed] [Google Scholar]
- 7.Gao S et al. (2016) Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafuente Ibanez de Mendoza I et al. (2019) Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: A systematic review. J Periodontal Res. [DOI] [PubMed] [Google Scholar]
- 9.Dominy SS et al. (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5 (1), eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemoto TK and Ohara-Nemoto Y (2016) Exopeptidases and gingipains in Porphyromonas gingivalis as prerequisites for its amino acid metabolism. Jpn Dent Sci Rev 52 (1), 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara-Nemoto Y et al. (2011) ASP- and GLU-specific novel dipeptidyl peptidase 11 of Porphyromonas gingivalis that ensures utilization of proteineous energy sources. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein BA et al. (2017) Using Tn-seq To Identify Pigmentation-Related Genes of Porphyromonas gingivalis: Characterization of the Role of a Putative Glycosyltransferase. J Bacteriol 199 (14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott JC et al. (2013) A two-component system regulates hemin acquisition in Porphyromonas gingivalis. PLoS One 8 (9), e73351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller DP et al. (2017) Genes Contributing to Porphyromonas gingivalis Fitness in Abscess and Epithelial Cell Colonization Environments. Front Cell Infect Microbiol 7, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi H et al. (2019) Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog 15 (11), e1008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stobernack T et al. (2018) A Secreted Bacterial Peptidylarginine Deiminase Can Neutralize Human Innate Immune Defenses. mBio 9 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potempa J et al. (2000) Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000 24, 153–92. [DOI] [PubMed] [Google Scholar]
- 18.Potempa M et al. (2009) Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog 5 (2), e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis G et al. (2012) The keystone-pathogen hypothesis. Nat Rev Microbiol 10 (10), 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamont RJ and Hajishengallis G (2015) Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med 21 (3), 172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao MC et al. (2016) The design and analysis of transposon insertion sequencing experiments. Nat Rev Microbiol 14 (2), 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T et al. (2000) Identification and cloning of genes from Porphyromonas gingivalis after mutagenesis with a modified Tn4400 transposon from Bacteroides fragilis. Infect Immun 68 (1), 420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T et al. (2000) Pleiotropic pigmentation mutants of Porphyromonas gingivalis. Microb Pathog 28 (4), 235–47. [DOI] [PubMed] [Google Scholar]
- 24.Takada K and Hirasawa M (1998) Tn4351-generated non-haemolytic and/or non-pigmented mutants of Porphyromonas gingivalis. Microbios 95 (380), 35–44. [PubMed] [Google Scholar]
- 25.Hutcherson JA et al. (2015) Comparison of inherently essential genes of Porphyromonas gingivalis identified in two transposon-sequencing libraries. Mol Oral Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein BA et al. (2012) Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC Genomics 13, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito M et al. (2019) PGN_0297 is an essential component of the type IX secretion system (T9SS) in Porphyromonas gingivalis: Tn-seq analysis for exhaustive identification of T9SS-related genes. Microbiol Immunol 63 (1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutcherson JA et al. (2020) Porphyromonas gingivalis genes conferring fitness in a tobacco-rich environment. Mol Oral Microbiol 35 (1), 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman AL et al. (2009) Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6 (3), 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichimura M et al. (2014) Mariner-based transposon mutagenesis for Bacteroides species. J Basic Microbiol 54 (6), 558–67. [DOI] [PubMed] [Google Scholar]
- 31.Aoki A et al. (2012) Transition metal ions induce carnosinase activity in PepD-homologous protein from Porphyromonas gingivalis. Microb Pathog 52 (1), 17–24. [DOI] [PubMed] [Google Scholar]
- 32.Hajishengallis G and Diaz PI (2020) Porphyromonas gingivalis: Immune Subversion Activities and Role in Periodontal Dysbiosis. Curr Oral Health Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15 (1), 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia L et al. (2019) Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors. Front Cell Infect Microbiol 9, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riise R et al. (2019) Bleomycin hydrolase regulates the release of chemokines important for inflammation and wound healing by keratinocytes. Sci Rep 9 (1), 20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayaprakash K et al. (2017) PKC, ERK/p38 MAP kinases and NF-kappaB targeted signalling play a role in the expression and release of IL-1beta and CXCL8 in Porphyromonas gingivalis-infected THP1 cells. APMIS 125 (7), 623–633. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J et al. (1999) IL-8 degradation by Porphyromonas gingivalis proteases. Microb Pathog 26 (5), 275–80. [DOI] [PubMed] [Google Scholar]
- 38.Sato K et al. (2018) Immunoglobulin-like domains of the cargo proteins are essential for protein stability during secretion by the type IX secretion system. Mol Microbiol 110 (1), 64–81. [DOI] [PubMed] [Google Scholar]
- 39.Roy F et al. (2006) HtrA in Porphyromonas gingivalis can regulate growth and gingipain activity under stressful environmental conditions. Microbiology 152 (Pt 11), 3391–8. [DOI] [PubMed] [Google Scholar]
- 40.Yuan L et al. (2008) Porphyromonas gingivalis htrA is involved in cellular invasion and in vivo survival. Microbiology 154 (Pt 4), 1161–9. [DOI] [PubMed] [Google Scholar]
- 41.Sato K et al. (2005) Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J Biol Chem 280 (10), 8668–77. [DOI] [PubMed] [Google Scholar]
- 42.Yuan L et al. (2005) Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect Immun 73 (7), 4146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono S et al. (2019) Construction and Characterization of a PGN_0297 Mutant of Porphyromonas gingivalis: Evidence of the Contribution of PGN_0297 to Gingipain Activity. Acta Med Okayama 73 (4), 315–323. [DOI] [PubMed] [Google Scholar]
- 44.Nemoto TK et al. (2016) A Porphyromonas gingivalis Periplasmic Novel Exopeptidase, Acylpeptidyl Oligopeptidase, Releases N-Acylated Di- and Tripeptides from Oligopeptides. J Biol Chem 291 (11), 5913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enersen M et al. (2006) Multilocus sequence typing of Porphyromonas gingivalis strains from different geographic origins. J Clin Microbiol 44 (1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park Y et al. (2004) Identification of Porphyromonas gingivalis genes specifically expressed in human gingival epithelial cells by using differential display reverse transcription-PCR. Infect Immun 72 (7), 3752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansai T et al. (2003) Construction of a pepO gene-deficient mutant of Porphyromonas gingivalis: potential role of endopeptidase O in the invasion of host cells. Oral Microbiol Immunol 18 (6), 398–400. [DOI] [PubMed] [Google Scholar]
- 48.Awano S et al. (1999) Sequencing, expression and biochemical characterization of the Porphyromonas gingivalis pepO gene encoding a protein homologous to human endothelin-converting enzyme. FEBS Lett 460 (1), 139–44. [DOI] [PubMed] [Google Scholar]
- 49.Bai B et al. (2016) The anti-bacterial activity of titanium-copper sintered alloy against Porphyromonas gingivalis in vitro. Dent Mater J 35 (4), 659–67. [DOI] [PubMed] [Google Scholar]
- 50.Dashper SG et al. (2017) Porphyromonas gingivalis Uses Specific Domain Rearrangements and Allelic Exchange to Generate Diversity in Surface Virulence Factors. Front Microbiol 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson D et al. (1999) Purification and characterization of a novel cysteine proteinase (periodontain) from Porphyromonas gingivalis. Evidence for a role in the inactivation of human alpha1-proteinase inhibitor. J Biol Chem 274 (18), 12245–51. [DOI] [PubMed] [Google Scholar]
- 52.Kesavalu L et al. (1996) Trypsin-like protease activity of Porphyromonas gingivalis as a potential virulence factor in a murine lesion model. Microb Pathog 20 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- 53.Tokuda M et al. (1998) Regulation of protease expression in Porphyromonas gingivalis. Infect Immun 66 (11), 5232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bezerra GA et al. (2017) Bacterial protease uses distinct thermodynamic signatures for substrate recognition. Sci Rep 7 (1), 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hocevar K et al. (2018) Host cell-surface proteins as substrates of gingipains, the main proteases of Porphyromonas gingivalis. Biol Chem 399 (12), 1353–1361. [DOI] [PubMed] [Google Scholar]
- 56.Li N and Collyer CA (2011) Gingipains from Porphyromonas gingivalis - Complex domain structures confer diverse functions. Eur J Microbiol Immunol (Bp) 1 (1), 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen I and Potempa J (2014) Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J Oral Microbiol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yongqing T et al. (2011) The lysine-specific gingipain of Porphyromonas gingivalis : importance to pathogenicity and potential strategies for inhibition. Adv Exp Med Biol 712, 15–29. [DOI] [PubMed] [Google Scholar]
- 59.Potempa J et al. (1997) Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem 378 (3–4), 223–30. [DOI] [PubMed] [Google Scholar]
- 60.Potempa M and Potempa J (2012) Protease-dependent mechanisms of complement evasion by bacterial pathogens. Biol Chem 393 (9), 873–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlisle MD et al. (2009) Degradation of human alpha- and beta-defensins by culture supernatants of Porphyromonas gingivalis strain 381. J Innate Immun 1 (2), 118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y et al. (2010) Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 54 (1), 15–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonaka M et al. (2014) Analysis of a Lys-specific serine endopeptidase secreted via the type IX secretion system in Porphyromonas gingivalis. FEMS Microbiol Lett 354 (1), 60–8. [DOI] [PubMed] [Google Scholar]
- 64.Sato K et al. (2013) Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett 338 (1), 68–76. [DOI] [PubMed] [Google Scholar]
- 65.Kumagai Y et al. (2000) Enzymatic properties of dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis and its participation in virulence. Infect Immun 68 (2), 716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumagai Y et al. (2003) Peptidase activity of dipeptidyl aminopeptidase IV produced by Porphyromonas gingivalis is important but not sufficient for virulence. Microbiol Immunol 47 (10), 735–43. [DOI] [PubMed] [Google Scholar]
- 67.Kumagai Y et al. (2005) Molecular mechanism for connective tissue destruction by dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis. Infect Immun 73 (5), 2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yagishita H et al. (2001) Histopathological studies on virulence of dipeptidyl aminopeptidase IV (DPPIV) of Porphyromonas gingivalis in a mouse abscess model: use of a DPPIV-deficient mutant. Infect Immun 69 (11), 7159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiyama M et al. (1998) Sequence analysis of the Porphyromonas gingivalis dipeptidyl peptidase IV gene. Biochim Biophys Acta 1396 (1), 39–46. [DOI] [PubMed] [Google Scholar]
- 70.Holt SC et al. (1999) Virulence factors of Porphyromonas gingivalis. Periodontol. 2000 20, 168–238. [DOI] [PubMed] [Google Scholar]
- 71.Curtis MA et al. (2002) Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun 70 (12), 6968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chueh CK et al. (2019) Structural Basis for the Differential Regulatory Roles of the PDZ Domain in C-Terminal Processing Proteases. mBio 10 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y et al. (2013) A novel carboxyl-terminal protease derived from Paenibacillus lautus CHN26 exhibiting high activities at multiple sites of substrates. BMC Biotechnol 13, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olczak T et al. (2001) Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J Bacteriol 183 (19), 5599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sroka A et al. (2001) Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J Bacteriol 183 (19), 5609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kreth J et al. (2020) Multiplex Imaging of Polymicrobial Communities-Murine Models to Study Oral Microbiome Interactions. Methods Mol Biol 2081, 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mark Welch JL et al. (2016) Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A 113 (6), E791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tansirichaiya S et al. (2019) The Transposon Registry. Mob DNA 10, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burby PE et al. (2017) Implementation and Data Analysis of Tn-seq, Whole-Genome Resequencing, and Single-Molecule Real-Time Sequencing for Bacterial Genetics. J Bacteriol 199 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thibault D et al. (2019) Droplet Tn-Seq combines microfluidics with Tn-Seq for identifying complex single-cell phenotypes. Nat Commun 10 (1), 5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shields RC and Jensen PA (2019) The bare necessities: Uncovering essential and condition-critical genes with transposon sequencing. Mol Oral Microbiol 34 (2), 39–50. [DOI] [PubMed] [Google Scholar]
- 82.Schacherl M et al. (2015) The first crystal structure of the peptidase domain of the U32 peptidase family. Acta Crystallogr D Biol Crystallogr 71 (Pt 12), 2505–12. [DOI] [PubMed] [Google Scholar]
- 83.Lasica AM et al. (2017) The Type IX Secretion System (T9SS): Highlights and Recent Insights into Its Structure and Function. Front Cell Infect Microbiol 7, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glew MD et al. (2012) PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J Biol Chem 287 (29), 24605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glew MD et al. (2014) Blue native-PAGE analysis of membrane protein complexes in Porphyromonas gingivalis. J Proteomics 110, 72–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




