ABSTRACT
Trypanosoma cruzi is the intracellular parasite of Chagas disease, a chronic condition characterized by cardiac and gastrointestinal morbidity. Protective immunity requires CD4+ T cells, and Th1 cells and gamma interferon (IFN-γ) are important players in host defense. More recently, Th17 cells and interleukin 17A (IL-17A) have been shown to exert protective functions in systemic T. cruzi infection. However, it remains unclear whether Th17 cells and IL-17A protect in the mucosa, the initial site of parasite invasion in many human cases. We found that IL-17RA knockout (KO) mice are highly susceptible to orogastric infection, indicating an important function for this cytokine in mucosal immunity to T. cruzi. To investigate the specific role of Th17 cells for mucosal immunity, we reconstituted RAG1 KO mice with T. cruzi-specific T cell receptor transgenic Th17 cells prior to orogastric T. cruzi challenges. We found that Th17 cells provided protection against gastric mucosal T. cruzi infection, indicated by significantly lower stomach parasite burdens. In vitro macrophage infection assays revealed that protection by Th17 cells is reduced with IL-17A neutralization or reversed by loss of macrophage NADPH oxidase activity. Consistently with this, mice lacking functional NADPH oxidase were not protected by Th17 cell transfer. These data are the first report that Th17 cells protect against mucosal T. cruzi infection and identify a novel protective mechanism involving the induction of NADPH oxidase activity by IL-17A. These studies provide important insights for Chagas vaccine development and, more broadly, increase our understanding of the diverse roles of Th17 cells in host defense.
KEYWORDS: Chagas disease, IL-17, IL-17A, NADPH oxidase, Th17 cells, Trypanosoma cruzi, neglected tropical diseases, parasitology, reactive oxygen species
INTRODUCTION
Chronic infection with the intracellular protozoan parasite Trypanosoma cruzi causes Chagas disease, a neglected tropical disease characterized by life-threatening cardiac and gastrointestinal pathology (1). The disease is endemic in Latin America, with geographical spread into areas of nonendemicity, and it affects at least 8 million people (2). Infected reduviid “kissing bug” insects carry T. cruzi in their gastrointestinal tracts and deposit infectious parasites in their excreta after taking a blood meal. People become infected when parasite-containing excreta are accidentally ingested or inoculated into the eye or a break in the skin. Thus, the major routes of vector-borne transmission are mucosal and percutaneous.
During chronic T. cruzi infection, the parasite load is controlled but never completely eliminated. A robust T cell response is sufficient for parasite control in some models (3, 4), and a lack of either CD4+ or CD8+ T cell responses increases susceptibility to infection (5–9). Among the CD4+ T cell subsets, Th1 cells have been demonstrated to protect against both systemic and mucosal infection (5, 10–12), while Th2 cells promote parasite persistence and mortality (12, 13). More recent studies have investigated the role of Th17 cells and interleukin 17A (IL-17A). We previously discovered using an adoptive cell transfer model that Th17 cells significantly reduce parasitemia and prevent mortality after a normally lethal challenge administered via subcutaneous injection of parasites (3). Other investigators have shown that mice deficient in IL-17A signaling due to genetic mutation of IL-17A (14) or its receptor (15) or through antibody neutralization (16) have increased susceptibility to an intraperitoneally administered T. cruzi challenge.
Despite evidence that IL-17A and Th17 cells protect against systemic parasite challenges, whether this type of response contributes to mucosal immunity is unknown. Orogastric infection has caused hundreds of outbreaks in humans (17–19). It has gained more attention in recent years and is a leading route of transmission in some areas of endemicity (19). Oral ingestion is also considered to be a major route of transmission in other mammalian hosts, such as domestic dogs, which are infected at high rates in areas of endemicity (20). Th17 cells are highly abundant at mucosal surfaces, especially in the intestinal gut, where they play a major role in homeostasis and immunity (21). Mainly through the secretion of IL-17A, these cells can recruit neutrophils, induce the expression of antimicrobial peptides, and upregulate factors that maintain epithelial integrity. These functions contribute to immunity in the intestinal mucosa (21) and may also be relevant for immunity in the gastric mucosa.
In this project, we investigated whether Th17 cells, previously demonstrated to contribute to immunity against systemic T. cruzi infection, can also promote immunity that is protective against mucosal infection. Ultimately, understanding of the full spectrum of functions of Th17 cells and IL-17A in T. cruzi infection will guide the development of vaccines inducing protective mucosal and systemic immunity.
RESULTS
IL-17A signaling is important for mucosal immunity to T. cruzi infection.
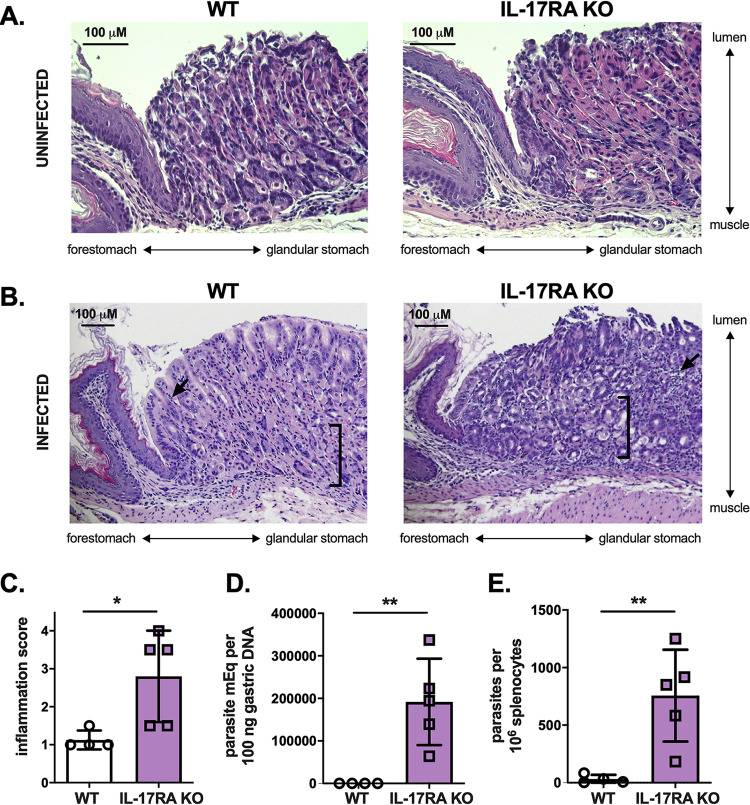
IL-17A is critical in systemic immunity against T. cruzi infection (14–16). To investigate how IL-17A functions in T. cruzi mucosal immunity, we performed orogastric infections in wild-type (WT) and IL-17RA knockout (KO) BALB/c mice, which lack a subunit of the heterodimeric receptor for IL-17A (21). Twelve days later, we performed hematoxylin and eosin staining of tissue sections taken where T. cruzi preferentially infects at the margo plicatus (22), the region of transition between the glandular corpus of the stomach and the nonglandular forestomach. At baseline, the stomachs of WT and IL-17RA KO mice were histologically similar, with the presence of well-differentiated glands (Fig. 1A). After infection, only mild histological abnormalities were observed in WT mice (Fig. 1B). However, infected IL-17RA KO mice exhibited pronounced inflammatory infiltrate and loss of specialized glandular cells, representing atrophy (23), indicating increased susceptibility to gastric mucosal parasite challenge (Fig. 1B and C). IL-17RA KO mice also had higher parasite burdens in the stomach and a greater proportion of infected spleen cells than WT mice (Fig. 1D and E), indicating poorer immune control. These data are the first evidence that IL-17A signaling plays a critical role in protective gastric mucosal immunity to T. cruzi infection.
FIG 1.
IL-17A signaling is required for mucosal immunity against gastric T. cruzi infection. WT and IL-17RA KO BALB/c mice were orogastrically infected with T. cruzi parasites and then sacrificed for tissue studies 12 days later. (A) Hematoxylin and eosin staining of stomach tissue sections taken near the margo plicatus revealed comparable findings at baseline between the two strains of mice, including well-differentiated glands. (B) After T. cruzi infection, greater histological disturbances and inflammation were observed in IL-17RA KO mice. Arrows indicate areas of immune cell infiltrate, and brackets capture areas with loss of specialized cells (e.g., chief cells) at the bases of the glands. (C) IL-17RA KO mice had higher inflammation scores in the gastric mucosa, based on the extent of mononuclear cell infiltrate. (D, E) IL-17RA KO mice had higher parasite DNA levels in the stomach as measured by qPCR (D) and greater frequencies of infected splenocytes (E), reflecting decreased control of infection. **, P < 0.01; *, P < 0.05 (by Student's two-tailed t test in a comparison with WT mice).
Th17 cells can protect against gastric mucosal infection.
Th17 cells are major CD4+ T cell producers of IL-17A. To investigate the role of CD4+ T cell subsets in gastric mucosal T. cruzi infection, we generated Th1 and Th17 cells specific for T. cruzi. This was done via in vitro Th1 or Th17 differentiation of CD4+ T cells with transgenic T cell receptors (TCR Tg) recognizing an immunodominant epitope of the T. cruzi trans-sialidase antigen (3). We have previously characterized the phenotype and persistence of these TCR Tg Th17 cells in adoptive transfer models (3), and cells were confirmed to express canonical Th1 (T-box expressed in T cells [T-bet] and gamma interferon [IFN-γ]) and Th17 (retinoic acid receptor [RAR]-related orphan receptor gamma t [RORγt] and IL-17A) cell markers prior to every transfer.
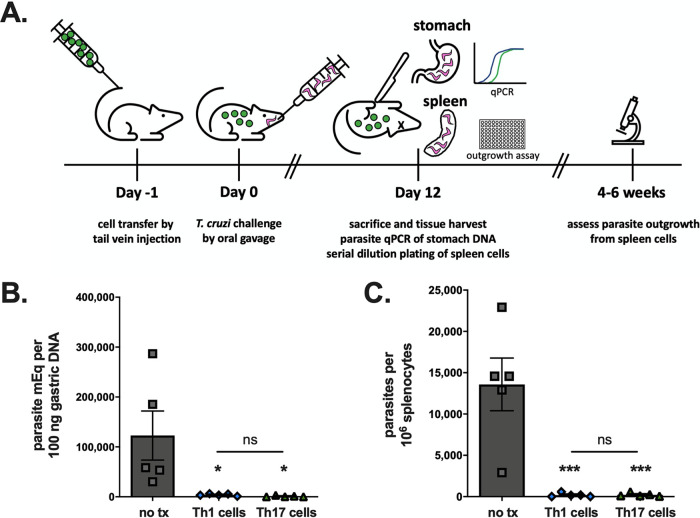
We transferred these TCR Tg Th1 or Th17 cells into RAG1 KO BALB/c mice lacking endogenous T cells. Control animals received no T cell transfer. The following day, we induced stomach infection via oral gavage of parasites. On day 12 postinfection, previously identified as the time of peak parasite burden, we sacrificed the mice for quantification of parasite burden in the stomach using quantitative PCR (qPCR) and in the spleen using parasite outgrowth assays (Fig. 2A). Mice receiving either Th1 or Th17 cells had significantly reduced T. cruzi DNA in their stomachs compared to that in control mice (Fig. 2B). In addition, these mice had a smaller proportion of infected spleen cells than control mice, indicating significantly improved overall control of the infection (Fig. 2C). No differences in protection were detected between Th1 and Th17 cell transfer (Fig. 2B and C).
FIG 2.
Th17 cells can provide direct protection against T. cruzi gastric mucosal infection. (A) RAG1 KO BALB/c mice were given TCR Tg and T. cruzi-specific Th1 or Th17 cells by adoptive transfer, orogastrically infected with T. cruzi parasites the following day, and then sacrificed 12 days postinfection for studies of tissue parasite burden. (B) Both Th1 and Th17 cells significantly lowered stomach parasite burdens arising after mucosal infection, as measured by parasite qPCR. tx, transfer; ns, not significant. (C) Th1 cells and Th17 cells comparably decreased the burden of infection in the spleen, measured by limiting dilution parasite outgrowth assay. ***, P < 0.001; *, P < 0.05 (by ANOVA). Results are representative of two separate experiments.
We previously demonstrated that in a normally lethal systemic infection induced by subcutaneous challenge, cotransfer of CD8+ T cells is required for Th17-mediated protection against mortality (3). Mechanistically, parasite-specific Th17 cells protected by providing help to CD8+ T cells via IL-21 signaling (3). However, in this gastric infection model, Th1 or Th17 cells given alone were sufficient to provide significant protection against initial infection (Fig. 2B and C). These data indicate that Th17-mediated mucosal protection operates via a different mechanism, including potentially direct protective functions rather than helper effects on CD8+ T cells.
Th17 cells provide direct protective effects in vitro via IL-17A.
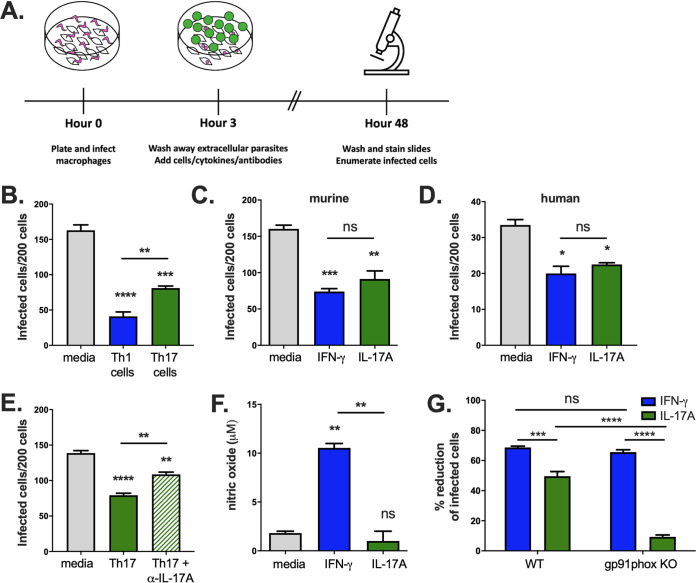
To investigate direct protective effects of Th17 cells, we infected WT BALB/c macrophages with T. cruzi in vitro and then cocultured them with either parasite-specific Th1 or Th17 cells before enumerating the number of infected macrophages arising after 2 days (Fig. 3A). Both Th1 and Th17 cells resulted in a significantly reduced number of infected cells (Fig. 3B). Treatment with IFN-γ or IL-17A, representing the major cytokines produced by Th1 and Th17 cells, respectively, also significantly reduced the number of infected cells in both murine (Fig. 3C) and human (Fig. 3D) macrophages. Adding an anti-IL-17A neutralizing antibody partially reversed the protective effects of Th17 cells (Fig. 3E). Some protection was still seen, likely due to the incomplete activity of the IL-17A blocking antibody, which was also observed with in vivo administration. These data suggest that the protective effects of Th17 cells are mediated by IL-17A.
FIG 3.
Th17 cells and IL-17A alone can inhibit T. cruzi intracellular growth in macrophages in vitro by inducing NADPH oxidase activity. (A) Macrophages were infected in vitro with T. cruzi parasites and cocultured with T cells or cytokines. The number of infected macrophages was counted after 2 days. (B) The addition of either parasite-specific Th1 or Th17 cells reduced the number of infected cells. (C, D) IFN-γ or IL-17A treatment also decreased the number of infected cells among both murine (C) and human (D) macrophages. (E) The protective action of Th17 cells was reduced in the presence of an anti-IL-17A neutralizing antibody, supporting a cytokine-mediated effect. (F) Treatment of infected macrophages with IFN-γ, but not IL-17A, induced expression of nitric oxide. (G) IFN-γ treatment could protect both WT and gp91phox KO macrophages, while IL-17A was able to significantly reduce the number of infected cells only among WT macrophages, indicating that functional NADPH oxidase is required for IL-17A-mediated protection. Experiments were performed using BALB/c mice, except for the experiment shown in panel G, which used WT and gp91phox KO C57BL/6 mice. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05 (by ANOVA). Results are representative of two or more separate experiments.
IL-17A protects via NADPH oxidase activity in infected macrophages.
Th1 cells prime macrophage activation for the killing of intracellular microorganisms through IFN-γ-mediated induction of inducible nitric oxide synthase (iNOS), which results in the generation of microbicidal nitric oxide (NO) (10, 24). We confirmed that treatment of infected bone marrow-derived macrophages (BMDMs) with IFN-γ resulted in an increase in NO concentration (Fig. 3F). In contrast, treatment with IL-17A had no effect on increasing NO levels over those in control cells (Fig. 3F). Reactive oxygen species (ROS) are generated by NADPH oxidase during the phagocyte respiratory burst response, and as with NO, they can inhibit microbial growth. To evaluate for the involvement of ROS, we treated infected WT or gp91phox KO C57BL/6 macrophages lacking a critical subunit of the NADPH oxidase with IL-17A or IFN-γ. Although the deficiency of functional NADPH oxidase had no effect on IFN-γ-mediated protection, it reversed IL-17A-mediated protection (Fig. 3G). These data indicate that direct protection by Th17 cells requires IL-17A signaling and NADPH oxidase activity.
Th17 cells induce oxidation in mucosal immune cells in vivo.
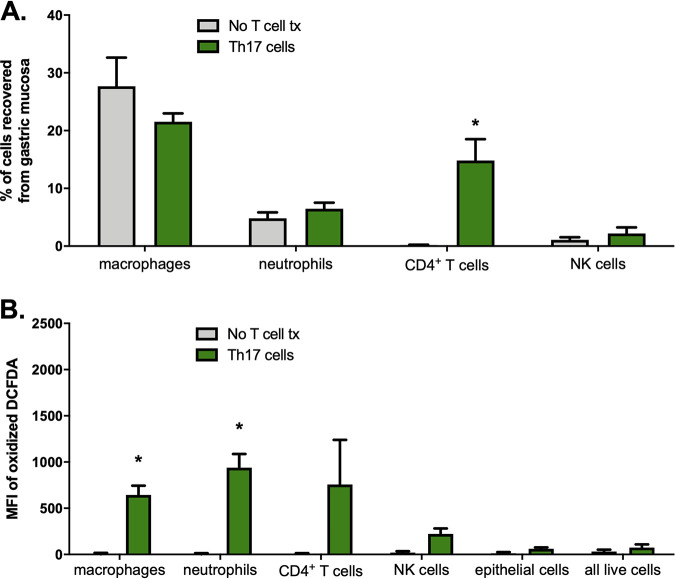
To determine whether parasite-specific Th17 cells given by adoptive transfer could be detected in the stomach, we recovered cells from the gastric mucosas of RAG1 KO BALB/c mice given no T cells or Th17 cells after orogastric infection with T. cruzi. We confirmed that parasite-specific Th17 cells were present in the gastric mucosa postinfection (Fig. 4A). Proportions of other immune cell subsets, including neutrophils, which can be recruited by IL-17A, were not significantly altered compared to those in control mice receiving no T cell transfer (Fig. 4A).
FIG 4.
Th17 cells traffic to the gastric mucosa and induce increased expression of intracellular ROS in macrophages. RAG1 KO BALB/c mice were reconstituted with Th17 cells and orogastrically infected with T. cruzi the next day. Twelve days after infection, mice were sacrificed and immune cells were isolated from the gastric mucosa for flow cytometric analysis. (A) CD4+ T cells were recovered from the gastric mucosa of mice reconstituted with Th17 cells, demonstrating that these cells migrated to the site of infection. No significant differences in proportions of other immune cell subsets were detected between mice given Th17 cells or not. (B) A significantly higher oxidation state was observed in gastric mucosal macrophages and neutrophils recovered from mice given Th17 cell adoptive transfer than in controls. n = 3/group. *, P < 0.05 (by Student's two-tailed t test in a comparison with mice given no T cell transfer). Cells were defined by the following surface markers: F4/80+ (macrophages), CD11b+ Ly6G+ (neutrophils), CD3+ (CD4+ T cells), CD3− NKp46+ (NK cells), LIVE/DEAD aqua (all live cells), EpCAM+ (epithelial cells).
We next asked whether parasite-specific Th17 cells induced NADPH oxidase activity in the gastric mucosa during T. cruzi infection in vivo. We adoptively transferred Th17 cells into mice and then orogastrically infected them; we later recovered gastric mucosal cells, which were stained with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), a probe that is oxidized to a fluorescent substrate. We determined that macrophages and neutrophils recovered from the gastric mucosas of mice given Th17 cells had significantly higher oxidation states than control mice (Fig. 4B), indicating an increase in ROS. These results are consistent with phagocytic cells being the primary cell types undergoing the respiratory burst response and support the hypothesis that Th17 cells protect against orogastric infection by inducing increased expression of ROS in certain cells.
NADPH oxidase activity is required for Th17-mediated mucosal protection.
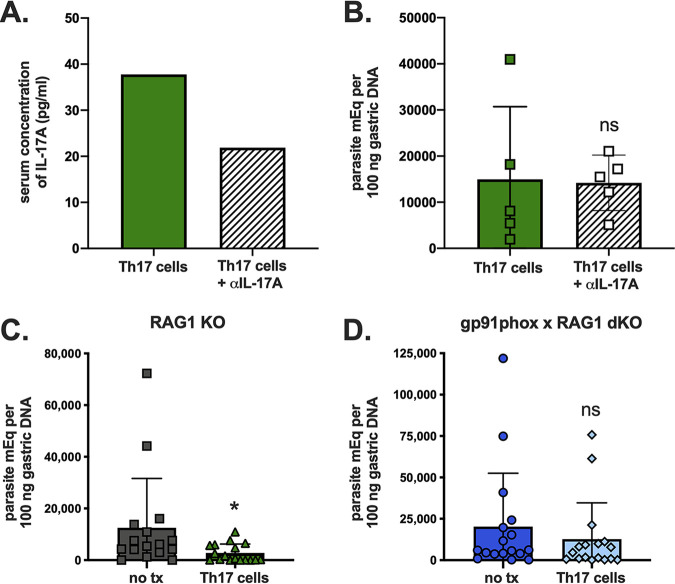
Based on the in vitro infection assays demonstrating that protection by Th17 cells operates via secretion of IL-17A and induction of NADPH oxidase in target cells, we asked if these are also required for in vivo protection. We administered an anti-IL-17A neutralizing antibody every other day in Th17-reconstituted and orogastrically infected RAG1 KO BALB/c mice. This partially reduced serum levels of IL-17A (Fig. 5A), but it did not reverse Th17-mediated protection (Fig. 5B), likely due to the incomplete abrogation of IL-17A activity (Fig. 5A).
FIG 5.
Th17 cells cannot protect in the absence of functional NADPH oxidase. RAG1 KO BALB/c mice were given Th17 cells and treated with an anti-IL-17A antibody or not following T. cruzi challenge. (A) Treatment with an anti-IL-17A antibody resulted in reduced levels of this cytokine in pooled sera; (B) however, this did not have an effect on protection, indicated by no significant changes in stomach parasite burdens at day 12 postinfection. To investigate a potential role for NADPH oxidase, we also evaluated the effects of Th17 cell transfers on orogastric infection in RAG1 KO C57BL/6 mice versus gp91phox × RAG1 dKO C57BL/6 mice lacking functional NADPH oxidase. Only RAG1 KO mice (C), not gp91phox × RAG1 dKO mice (D), were protected from orogastric T. cruzi infection by parasite-specific Th17 cells, as indicated by no significant decrease in stomach parasite DNA load in the latter group. *, P < 0.05 (by the Mann-Whitney U test). Results depict pooled data from three independent experiments.
To further study the role of NADPH oxidase in vivo, we transferred parasite-specific Th17 cells into RAG1 KO C57BL/6 and gp91phox × RAG1 double-KO (dKO) C57BL/6 mice prior to orogastric challenge. While RAG1 KO mice had significantly improved resistance to orogastric T. cruzi infection following Th17 cell transfer (Fig. 5C) (median, 634 parasite meq per 100 ng gastric DNA with Th17 cell transfer versus 5,768 without transfer; P < 0.05), Th17 cells did not confer measurable protection in mice lacking functional NADPH oxidase (Fig. 5D) (median, 4,633 parasite meq per 100 ng gastric DNA with Th17 cell transfer versus 5,992 without transfer; P = 0.3394). We cannot exclude the possibility of alternative or additional mechanisms being involved in the observed protection conferred by Th17 cells. However, taken in context with other experiments, these in vivo challenge data confirm a role for NADPH oxidase in IL-17A-mediated immunity against gastric T. cruzi infection.
DISCUSSION
Although Chagas disease is an important illness causing significant morbidity and mortality in the Western Hemisphere, few truly effective treatments exist. Drugs are limited by side effects and poor efficacy during the chronic stage of infection (25), and no vaccines are being tested in humans. The development of an effective vaccine would be an economically sound approach (26) and requires a thorough understanding of the protective immune response to this parasite, including mucosal immunity.
Th17 cells are well established as important players against various fungi and extracellular bacteria (21) but only recently have gained recognition as effectors in the immune response to intracellular pathogens like T. cruzi. Several studies over the past decade have described a role for IL-17A in the protective response against systemic T. cruzi parasite infection in mice (14, 15, 16), and more recent work suggests a protective role for this cytokine in human Chagas disease as well (27). However, none of these studies has specifically examined the role of IL-17A in immunity against T. cruzi at mucosal surfaces.
In this study, we demonstrate for the first time that Th17 cells and IL-17A contribute to immunity against gastric mucosal T. cruzi infection via induction of NADPH oxidase activity within infected phagocytes. Systemic T. cruzi infection involves multiple tissues and requires CD8+ T cell responses (3). However, CD4+ T cells alone can suppress parasitism in the gastric mucosa, and local protective effects of IL-17A may be sufficient against initial mucosal invasion. In addition, in systemic infection models, Th17 cells provided more protection against mortality than Th1 cells due to improved helper effects for CD8+ T cells. However, levels of protection by Th1 and Th17 cells were comparable in the gastric mucosa, where these cells may act directly or indirectly. In addition to IL-17A-mediated induction of NADPH oxidase, other potential mechanisms of protection by Th17 cells may include the activation of other innate immune cells or effects on the epithelium, which should be explored in future work.
In summary, we identify a role for Th17 cells and IL-17A in mucosal immunity against Trypanosoma cruzi, and we describe a novel protective mechanism of IL-17A against an intracellular pathogen. Th17 cells may have more broad protective functions than previously believed, and these studies of T. cruzi may also provide insights into infections with similar mucosally transmitted intracellular protozoa. Ultimately, these findings should be assessed for translational potential through studies targeting the induction of Th17 cells through mucosal vaccination, with the goal of reducing the significant morbidity and mortality associated with Chagas disease.
MATERIALS AND METHODS
Mice.
T. cruzi-specific BALB/c mice containing a transgenic CD4+ TCR specific for p7, an immunodominant CD4 epitope of the trans-sialidase antigen, were generated in the Hoft laboratory as previously described (3). WT BALB/c mice (NCI Charles River Laboratories), RAG1 KO BALB/c mice (The Jackson Laboratory), IL-17RA KO BALB/c mice (Amgen), WT C57BL/6 (NCI Charles River), RAG1 KO C57BL/6 (The Jackson Laboratory), and B6.129S6-Cybbtm1Din/J mice lacking the gp91phox catalytic subunit of NADPH oxidase (gp91phox KO; The Jackson Laboratory) were obtained directly from the vendor or maintained as breeding colonies within the Hoft laboratory. Gp91phox KO mice were also bred to RAG1 KO C57BL/6 mice to generate RAG1 × gp91phox dKO C57BL/6 mice. All studies were approved by the Saint Louis University Institutional Animal Care and Use Committee (IACUC) under protocol 1106 and conducted in an AAALAC-accredited facility at Saint Louis University. Euthanasia was performed using CO2 narcosis according to the American Veterinary Medical Association guidelines on euthanasia.
Parasites and challenges.
Tulahuèn strain parasites were maintained by in vivo passage through Dipetalogaster maximus insects and WT BALB/c mice and in vitro passage in an H2O-based medium containing liver digest-neutralized tryptose broth (LDNT, Oxford). Culture-derived metacyclic trypomastigotes (CMT) were generated by in vitro differentiation in supplemented Grace’s insect medium (Sigma) and maintained in a 26°C parasite incubator. For orogastric infections, mice were fasted for 3 h and then fed 500 μl of a 1.5% sodium bicarbonate solution using a 22-gauge animal feeding needle, followed by 1 × 107 T. cruzi CMT parasites in 100 μl of 1% glucose in phosphate-buffered saline (PBS). The extent of infection was assessed via quantification of parasite load in the stomach and spleen. Because orogastrically infected mice do not typically mount high blood parasite burdens compared to those of systemically infected mice or die due to orogastric infection, these parameters were not routinely measured in our study.
Parasite DNA qPCR.
Twelve days after orogastric infection, stomachs were dissected, cut along the greater curvature, rinsed in PBS to remove food contents, and minced with scissors. DNA was extracted from stomach tissue using DNeasy blood and tissue kits according to the manufacturer’s instructions (Qiagen). Primers and TaqMan probes specific for T. cruzi genomic DNA were used to measure parasite load in the stomach as previously described (22). When histological analyses were performed in the same experiment, the stomachs were cut along both curvatures, with ventral portions used for histology and dorsal portions used for qPCR.
Histology.
Longitudinal strips 2 to 3 mm in width were cut from the esophageal to the pyloric ends of the ventral stomach, placed between prewetted foam pads in biopsy cassettes (Leica), and fixed by submersion in 10% neutral buffered formalin. Several hours later, the tissue was embedded, sectioned, affixed to slides, and stained with hematoxylin and eosin at the Saint Louis University Research and Histology Microscopy Core. Images were acquired on an Olympus BX41 or Leica epifluorescence microscope. Inflammation scores were assigned according to a qualitative 0 to 4 scale by a blinded evaluator based on a scoring system adapted from Rogers et al. (28). Briefly, inflammation was scored based upon the presence of leukocytes and the extent of their infiltration into the gastric mucosa. A score of 0 indicates only the number of leukocytes present in the tissue at baseline (i.e., in a normal uninfected mouse with no inflammation), while a score of 4 indicates severe transmural inflammation with the presence of numerous infiltrating leukocytes.
Generation of Th1 and Th17 cells.
T. cruzi-specific Th1 and Th17 cells were generated in vitro as previously described (3). Briefly, CD4+ T cells were purified from total spleen cells of TCR Tg mice using CD4+ T cell isolation kits (Miltenyi) and then stimulated with irradiated, cognate peptide-pulsed dendritic cells in the presence of anti-IL-4 (α-IL-4) and IL-12 (to generate Th1 cells) or α-IL-4, α-IFN-γ, IL-6, transforming growth factor β (TGF-β), and IL-23 (to generate Th17 cells). Medium was refreshed on days 3 and 6 with IL-2 for Th1 cells or IL-23 for Th17 cells. The cells underwent two rounds of this differentiation (restimulation with peptide-pulsed antigen-presenting cells [APCs] on day 7, followed by maintenance cytokines on days 10 and 13). Cells were used on day 14. Differentiation was confirmed by intracellular cytokine staining (ICS), and the phenotypes and stability of these Th1 and Th17 cells were previously documented (3).
Flow cytometry and ICS.
To obtain gastric mucosal immune cells, stomachs were dissected open along the greater curvature. A small-gauge syringe needle was introduced into the mucosal layers and the region flushed with large volumes of medium. The recovered cells were cultured with 1 μl/ml of GolgiPlug containing monensin (BD Pharmingen) and 0.67 μl/ml of GolgiStop containing brefeldin A (BD Pharmingen) for 3 h at 37°C. Cells were then stained with LIVE/DEAD fixable aqua, followed by surface antibodies directed against CD3, CD4, CD11b, CD11c, Ly6G, F4/80, and NKp46 (all from BD or eBioscience). Cells were washed with 1% fetal bovine serum (FBS) in Dulbecco’s PBS between all steps. All incubations were performed for 30 min at 4°C. For oxidation staining, cells were incubated for 20 min at 37°C with 100 nM H2DCFDA (Invitrogen) after surface staining. All samples were acquired on a BD LSRII flow cytometer at the Saint Louis University flow cytometry core facility and analyzed on FlowJo software (TreeStar, Inc.).
Splenic parasite outgrowth assay.
After T. cruzi infection, parasites can be detected in muscle, liver, spleen, and blood, among other sites. We measured parasite burden in the spleen as a general index of dissemination from the initial site of infection with the well-established assays for spleen infection in our lab. Total spleen cells were mechanically isolated from dissected spleens 12 days after orogastric infection. Spleen cells were resuspended in LDNT+ parasite medium, plated in a limiting dilution fashion in 96-well plates, and incubated in a 26°C parasite incubator. One month later, all wells were examined for live parasites. The last well with parasite outgrowth was used to estimate the number of parasites per million cells (e.g., live parasites emerging from 100 plated cells equals at least 1 parasite per 100 cells) (22). Because a single-cell suspension can be obtained from the spleen, this method was selected over qPCR for its ability to quantify the presence of live parasites, though it may underestimate the true burden due to the detection of only parasites that can differentiate into trypomastigotes.
Generation of PEMs, BMDMs, and human monocyte-derived macrophages.
Peritoneal exudate macrophages (PEMs) were generated from BALB/c strain mice, and bone marrow-derived macrophages (BMDMs) were generated from C57BL/6 strain mice. For PEMs, mice were injected intraperitoneally with 100 μg of concanavalin A (Sigma). PEMs were recovered 3 to 4 days later via peritoneal lavage using a syringe and needle. To generate BMDMs, bone marrow cells harvested from mouse femurs were plated in supplemented RPMI medium with 20 ng/ml macrophage colony-stimulating factor (M-CSF; eBioscience), refreshed on day 3. BMDMs were harvested for use on day 7. We opted to generate peritoneal exudate macrophages from BALB/c mice due to the relative ease and rapidity of this method. Some evidence suggests that C57BL/6 macrophages are more inflammatory, with a tendency to exhibit an M1 phenotype (29). In our hands, C57BL/6 PEMs had poor survival, possibly as a result of a more activated and inflammatory state of PEMs in general (30), so BMDMs were substituted for experiments using mice in this background. For human monocyte-derived macrophages, peripheral blood mononuclear cells were cultured in 96-well plates for 7 days to induce maturation. Adherent cells were removed and replated in 8-well chamber slides (Lab-Tek) for infection assays.
In vitro infection assay.
Macrophages were plated at 200,000 cells/well of 8-well chamber slides and infected with CMT (multiplicity of infection [MOI] = 10). After 3 h, extracellular parasites were removed by repeated washing. In some experiments, macrophages were cocultured with purified recombinant murine or human IFN-γ (Genentech) at 1,000 U/ml or IL-17A (R&D) at 100 ng/ml, or an anti-IL-17A neutralizing antibody (TC11-18H10; BD Pharmingen) was added at a concentration of 10 μg/ml. Nitric oxide concentrations in supernatants were measured using the Griess reagent system (Promega). Slides were stained with Diff-Quik (IMEB, Inc.) after 2 days, and infected cells were enumerated microscopically.
In vivo IL-17A neutralization.
Mice were given 100 μg of an anti-IL-17A neutralizing antibody (17F3; Bio X Cell) or an IgG1 isotype control antibody (MOPC-21; Bio X Cell) every 2 days by intraperitoneal injection. Serum IL-17A levels were analyzed via mouse IL-17A enzyme-linked immunosorbent assay (ELISA) MAX standard kits (BD).
Statistics.
Data tables with two groups were compared using Student's t test for parametric data or Mann-Whitney’s test for nonparametric data. Data tables with three or more groups were compared using one-way analysis of variance (ANOVA) with post hoc analyses by Tukey’s multiple-comparison test after passing Shapiro-Wilk normality testing. All analyses were performed in Prism version 8 (GraphPad Software) using a significance level of 5%. P values are indicated by * for <0.05, ** for <0.01, *** for <0.001, and *** for <0.0001. All significant findings indicated are compared to findings for the control group unless otherwise noted.
ACKNOWLEDGMENTS
We thank Jennifer Franey for her assistance with animal care and handling; Joy Eslick and Sherri Koehm for their assistance with flow cytometry; Grant Kolar, Barbara Nagel, and Caroline Murphy for their assistance with histology and microscopy; and Timothy Wiemken for discussion. We thank Amgen for the provision of IL-17RA KO mice received under a material transfer agreement.
Conceptualization: C.W.C., C.S.E., D.F.H. Formal analysis: C.W.C., C.S.E., K.A.M., J.R.B., R.E.A., D.H.C., K.A.B., D.F.H. Funding acquisition: D.F.H., C.W.C. Investigation: C.W.C., C.S.E., K.A.M., J.R.B., R.E.A., D.H.C., K.A.B. Methodology: C.W.C., C.S.E., J.R.B., K.A.B., R.J.D., D.F.H. Supervision: D.F.H. Validation: C.W.C., C.S.E., K.A.M., J.R.B., R.E.A., D.H.C. Visualization: C.W.C. Writing – original draft: C.W.C. Writing – revising & editing: all authors.
We declare that no competing interests exist.
Contributor Information
Daniel F. Hoft, Email: daniel.hoft@health.slu.edu.
Jeroen P. J. Saeij, UC Davis School of Veterinary Medicine
REFERENCES
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.Bern C, Montgomery SP. 2009. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49:e52–e54. 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 3.Cai CW, Blase JR, Zhang X, Eickhoff CS, Hoft DF. 2016. Th17 cells are more protective than Th1 cells against the intracellular parasite Trypanosoma cruzi. PLoS Pathog 12:e1005902. 10.1371/journal.ppat.1005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan NL, Eickhoff CS, Sagartz J, Hoft DF. 2015. Deficiency of antigen-specific B cells results in decreased Trypanosoma cruzi systemic but not mucosal immunity due to CD8 T cell exhaustion. J Immunol 194:1806–1818. 10.4049/jimmunol.1303163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoft DF, Eickhoff CS. 2005. Type 1 immunity provides both optimal mucosal, and systemic protection against a mucosally invasive, intracellular pathogen. Infect Immun 73:4934–4940. 10.1128/IAI.73.8.4934-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarleton RL, Sun J, Zhang L, Postan M. 1994. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect Immun 62:1820–1829. 10.1128/IAI.62.5.1820-1829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarleton RL, Koller BH, Latour A, Postan M. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338–340. 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 8.Tarleton RL, Grusby MJ, Postan M, Glimcher LH. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol 8:13–22. 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Tarleton RL. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol 144:717–724. [PubMed] [Google Scholar]
- 10.Hoft DF, Schnapp AR, Eickhoff CS, Roodman ST. 2000. Involvement of CD4(+) Th1 cells in systemic immunity protective against primary and secondary challenges with Trypanosoma cruzi. Infect Immun 68:197–204. 10.1128/iai.68.1.197-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoft DF, Eickhoff CS. 2002. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect Immun 70:6715–6725. 10.1128/IAI.70.12.6715-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Tarleton RL. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J Immunol 166:4596–4603. 10.4049/jimmunol.166.7.4596. [DOI] [PubMed] [Google Scholar]
- 13.Lopes MF, Nunes MP, Henriques-Pons A, Giese N, Morse HC, III, Davidson WF, Araujo-Jorge TC, DosReis GA. 1999. Increased susceptibility of Fas ligand-deficient gld mice to Trypanosoma cruzi infection due to a Th2-biased host immune response. Eur J Immunol 29:81–89. . [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki Y, Hamano S, Wang S, Shimanoe Y, Iwakura Y, Yoshida H. 2010. IL-17 is necessary for host protection against acute-phase Trypanosoma cruzi infection. J Immunol 185:1150–1157. 10.4049/jimmunol.0900047. [DOI] [PubMed] [Google Scholar]
- 15.Tosello Boari J, Amezcua Vesely MC, Bermejo DA, Ramello MC, Montes CL, Cejas H, Gruppi A, Acosta Rodriguez EV. 2012. IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils. PLoS Pathog 8:e1002658. 10.1371/journal.ppat.1002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Matta Guedes PM, Gutierrez FR, Maia FL, Milanezi CM, Silva GK, Pavanelli WR, Silva JS. 2010. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis 4:e604. 10.1371/journal.pntd.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobrega AA, Garcia MH, Tatto E, Obara MT, Costa E, Sobel J, Araujo WN. 2009. Oral transmission of Chagas disease by consumption of acai palm fruit, Brazil. Emerg Infect Dis 15:653–655. 10.3201/eid1504.081450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alarcon de Noya B, Diaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, Suarez JA, Abate T, Naranjo L, Paiva M, Rivas L, Castro J, Marques J, Mendoza I, Acquatella H, Torres J, Noya O. 2010. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis 201:1308–1315. 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 19.Shikanai-Yasuda MA, Carvalho NB. 2012. Oral transmission of Chagas disease. Clin Infect Dis 54:845–852. 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 20.Montenegro VM, Jimenez M, Dias JC, Zeledon R. 2002. Chagas disease in dogs from endemic areas of Costa Rica. Mem Inst Oswaldo Cruz 97:491–494. 10.1590/s0074-02762002000400006. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 cells. Annu Rev Immunol 27:485–517. 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 22.Hoft DF, Farrar PL, Kratz-Owens K, Shaffer D. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect Immun 64:3800–3810. 10.1128/IAI.64.9.3800-3810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox JG, Wang TC. 2007. Inflammation, atrophy, and gastric cancer. J Clin Invest 117:60–69. 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues MM, Ribeirao M, Boscardin SB. 2000. CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol Lett 73:43–50. 10.1016/S0165-2478(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 25.Pinazo MJ, Munoz J, Posada E, Lopez-Chejade P, Gallego M, Ayala E, del Cacho E, Soy D, Gascon J. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob Agents Chemother 54:4896–4899. 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. 2010. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS Negl Trop Dis 4:e916. 10.1371/journal.pntd.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magalhaes LM, Villani FN, Nunes MC, Gollob KJ, Rocha MO, Dutra WO. 2013. High interleukin 17 expression is correlated with better cardiac function in human Chagas disease. J Infect Dis 207:661–665. 10.1093/infdis/jis724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. 2005. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res 65:10709–10715. 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 29.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173. 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Yu X, Cao Q, Wang Y, Zheng G, Tan TK, Zhao H, Zhao Y, Wang Y, Harris D. 2013. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol 14:6. 10.1186/1471-2172-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]