ABSTRACT
The spirochetal bacterium Borrelia recurrentis causes louse-borne relapsing fever (LBRF). B. recurrentis is unique because, as opposed to other Borrelia spirochetes, this strictly human pathogen is transmitted by lice. Despite the high mortality and historically proven epidemic potential and current outbreaks in African countries and Western Europe, research on LBRF has been obstructed by the lack of suitable animal models. The previously used grivet monkey model is associated with ethical concerns, among other issues. An existing immunodeficient mouse model does not limit bacteremia due to its impaired immune system. In this study, we used genetically diverse Collaborative Cross (CC) lines to develop the first LBRF immunocompetent mouse model. Out of 12 CC lines tested, CC046 mice consistently developed B. recurrentis-induced spirochetemia during the first 3 days postchallenge as concordantly detected by dark-field microscopy, culture, and quantitative PCR. However, spirochetemia was not detected from day 4 through day 10 postchallenge. The high-level spirochetemia (>107 cells/ml of blood) observed in CC046 mice was similar to that recorded in LBRF patients as well as immunocompetent mouse strains experimentally infected by tick-borne relapsing fever (RF) spirochetes, Borrelia hermsii and Borrelia persica. In contrast to the Old World and New World RF spirochetes, which develop multiple relapses (n = 3 to 9), B. recurrentis produced only single culture-detectable spirochetemia in CC046 mice. The lack of relapses may not be surprising, as LBRF patients and the grivet monkey model usually develop no or only 1 to 2 spirochetemic relapses. The novel model will now allow scientists to study B. recurrentis in the context of intact immunity.
KEYWORDS: louse-borne relapsing fever, Borrelia recurrentis, animal model, immunocompetent mice, Collaborative Cross, louse-borne relapsing fever
INTRODUCTION
Relapsing fever (RF) is caused by several species of Borrelia, which are transmitted from reservoir animals to humans by ticks (1). Borrelia recurrentis is the only exception, because this spirochete is transmitted by the human body louse, Pediculus humanus, causing louse-borne relapsing fever (LBRF) (2). Moreover, B. recurrentis has no known animal reservoir and, therefore, is considered a strictly human pathogen (3, 4). The human lice, whose life span is only several weeks, reside in clothing and acquire spirochetes of B. recurrentis by feeding on infected humans (5). Lice do not act as a reservoir, as they cannot infect their progeny (3). Humans become infected via contamination of abraded skin by coelomic fluid or feces released from lice crushed by scratching (6).
LBRF reached epidemic proportions during the first half of the 20th century. During World War II, 400,000 cases were reported in Algeria, 400,000 in Tunisia, 180,000 in Morocco, and 1,300,000 in Egypt (7). In the past, LBRF, which accounted for up to 27% of hospital admissions, was among the top 10 reasons for hospital admission and was associated with significant morbidity and mortality (8–12). To date, LBRF still remains a burden in several African nations (e.g., Ethiopia, Eritrea, Somalia, and Libya), as evidenced by frequent detection of LBRF in African refugees arriving in the European Union (EU) countries (13–17). The “humanitarian crisis,” with thousands of migrants without proper hygienic conditions, represents a risk factor for explosive LBRF outbreaks (15). Western physicians face communication challenges due to language barriers, which hamper the collection of accurate clinical and travel history (17). LBRF patients identified in the EU travel from the Horn of Africa through Sudan and Libya, which suggests that B. recurrentis circulates among East African refugees (13, 15). Because of a short incubation period (2 to 18 days), the epidemic chain is thought to begin in Somalia and then become fueled by high rates of body louse infestation, crowding, and general poor hygienic conditions that refugees or migrants endure during their long journeys (17).
LBRF is nonspecifically presented with two or more episodes of high fever and chills lasting for 5 to 7 days. It is often accompanied by malaise, nausea, general aches, nonproductive cough, and enlargement of liver and spleen (18). The mortality can be as high as 40%, if left untreated, and as low as 2 to 6% when treated with antimicrobials (3, 8, 19). Treatment is problematic, because a very high proportion of patients (e.g., 68 to 87%) develop the Jarisch-Herxeimer reaction (JHR) (8, 17). JHR and delayed onset of antimicrobial therapy are associated with elevated mortality (9, 17, 20). There is no vaccine available to date.
Despite LBRF’s epidemic potential, research on host-pathogen interactions and vaccine development has been hampered by the lack of suitable mouse models. Recently, immunocompromised mice deficient in B and T cells were shown to sustain a stable, persistent infection of B. recurrentis (21). Although the immunodeficient mouse model was a significant advancement in the field of LBRF research, these mice do not allow researchers to study B. recurrentis pathogenesis in the context of an intact immune system. Consequently, this mouse model cannot be utilized in development of anti-B. recurrentis vaccine and antibody-based diagnostics for LBRF.
The Collaborative Cross (CC) concept, proposed in 2002, aimed at generating a platform for mammalian complex trait genetics to overcome limitations of commercially available mouse strains (22). The CC resource is a genetically diverse panel of recombinant inbred CC mice that were obtained through a systemic cross of eight inbred founder mouse strains, five of which were classic laboratory strains (C57BL/6J, A/J, 129S1/SvImJ, NOD/ShiLtJ, and NZO/H1LtJ) and three were wild-derived inbred strains (CAST/EiJ, PWK/PhJ, and WSB/EiJ) (22). The founder strains represented 90% of the common genetic variation across the three major Mus musculus subspecies: M. musculus musculus, M. musculus domesticus, and M. musculus castaneus. The CC lines were produced through a funnel-breeding scheme, which resulted in random distribution of captured genetic variation among CC lines. Consequently, CC lines are independent, as they do not share recombination events: every recombination site in CC lines is unique (23). The resolution and power of using CC mice for mapping studies and development of infectious disease models have been previously demonstrated (24–27). As an example, the use of the CC panel allowed researchers to develop mouse models that reproduced hallmark symptoms of Ebola hemorrhagic fever (EHF): delayed blood coagulation, disseminated intravascular coagulation, and death from shock (28). Phenotypes ranged from complete resistance to lethal disease with 100% mortality, indicating that host genetic background determined susceptibility to EHF virus. Given the repeatedly proven potential of CC mice for identification of novel infectious disease models, we set a goal of developing an immunocompetent mouse model for the LBRF pathogen by using the CC resource.
RESULTS
Detection of B. recurrentis spirochetemia by a 5-day screen.
To explore the possibility of using the CC resource for development of immunocompetent mouse models of LBRF, a total of 11 CC lines were obtained for infectivity study. Upon challenge with in vitro-grown B. recurrentis strain A17 (referred to here as A17), blood taken daily from each mouse during the 5-day period was examined for the presence of spirochetes under dark-field microscopy (referred to here as DFM). To increase the sensitivity of the DFM assay, a previously developed approach, low-speed centrifugation of blood samples, was adapted to remove erythrocytes (29). As a result of this screen, DFM-detectable spirochetemia was consistently detected during the first 2 days postchallenge in all mice of the 11 lines, with one exception (see Table S1 in the supplemental material). Only 50% of CC004 mice (1 out of 2 mice) had DFM-detectable spirochetemia despite the two mice being spirochetemic at one of the 2 days. At days 3, 4, and 5 postchallenge, spirochetemia was not detected in any lines except for CC046 mice, whose day 3 blood samples were DFM positive. Thus, out of the 11 CC lines tested, only CC046 mice (3 out of 3 animals) had DFM-detectable spirochetemia for the first three consecutive days postchallenge.
Detection of spirochetemia.
To confirm the screen findings, a similar (follow-up) experiment was extended to 10 days and included 5 CC lines, four of which had exhibited spirochetemia upon the initial screen (CC001, CC019, CC042, and CC046), and the fifth line (CC058) had not been tested yet. All mice were challenged with in vitro-grown A17, and blood samples were collected daily, alternating mouse groups each day to ensure that each animal was bled only once every 3 days. This time, blood samples were analyzed for the presence of spirochetes by three independent (and complementary) methods, DFM, culture, and quantitative PCR (qPCR).
The DFM results demonstrated that all mice of the five lines (3 out of 3 animals per CC line/time point) developed DFM-detectable spirochetemia at days 1 and 2 postchallenge, except for CC019 mice, for which 2 out of 3 day 1 plasma samples were DFM negative (Table S2). The blood culture results confirmed that all CC mice, including the two DFM-negative animals, were positive for A17 at days 1 and 2 (Table S2). Consistent with the screen findings, the DFM results also verified the absence of spirochetemia in CC001, CC019, and CC042 mice from day 3 through day 5. Unexpectedly, in CC046 mice, day 3 spirochetemia was only detected by DFM in 1 out of 3 animals, as opposed to the respective positive data of the initial screen (Table S1). Likewise, in addition to day 1 and day 2 spirochetemia, newly tested CC058 mice showed similar results: day 3 spirochetemia was detected by DFM in 1 out of 3 animals (Table S2). In contrast, the blood culture data demonstrated that 3 out of 3 CC046 mice and 2 out of 3 CC058 animals were positive for A17. Lastly, spirochetemia was not detected by DFM or culture in mice of the five lines throughout days 4 to 10 (Table S2).
High-level and highly variable spirochetemia.
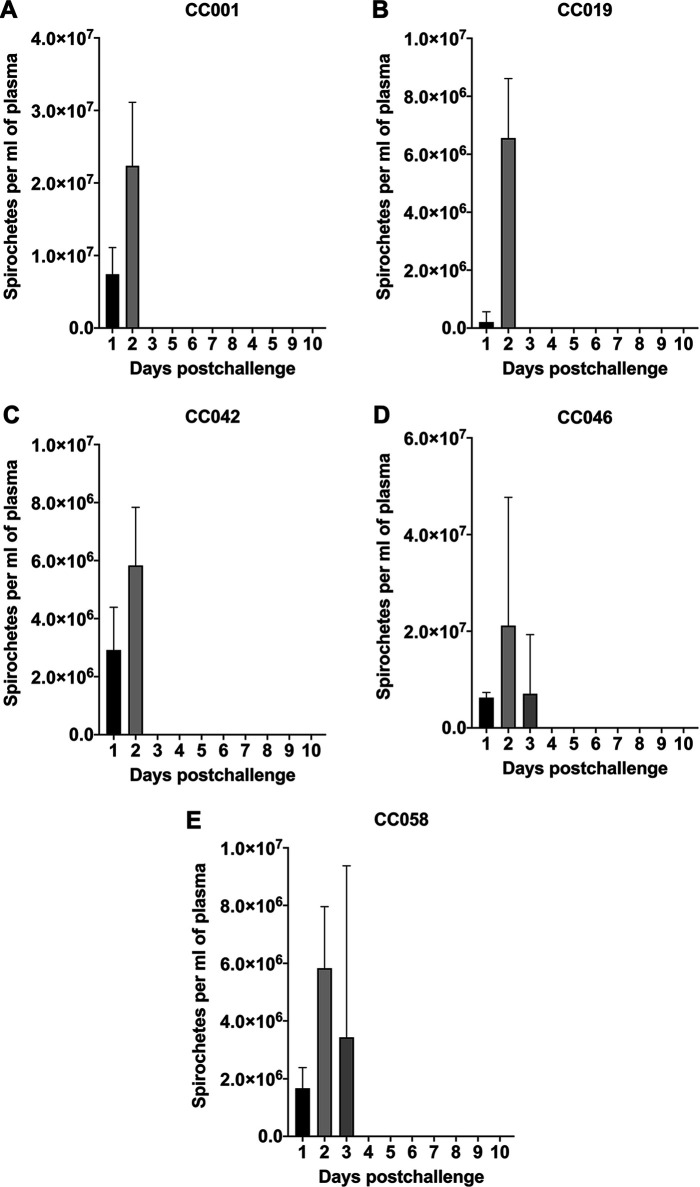
Given that the limit of DFM detection is 2.50 × 105 cells per ml of plasma, the enumeration assay only allowed us to quantify spirochetemia when mice had relatively high levels of spirochetes (Fig. 1 and Table S2). The DFM results showed that there was no statistical difference between day 1 levels of the five lines. Blood samples of CC001, CC019, CC042, CC046, and CC058 mice had, on average, 7.41 × 106, 2.07 × 105, 2.92 × 106, 6.25 × 106, and 1.67 × 106 cells per ml of plasma, respectively (P > 0.05; analysis of variance [ANOVA]). Compared to day 1 levels, noticeably higher levels of spirochetemia were observed for day 2 within each line, with the only statistical difference detected for CC019 mice (P = 0.0326; Fig. 1). When day 2 levels were compared across the five lines, the mean spirochetemic level of CC001 mice (2.24 × 107) was significantly higher than those of CC0019 (6.56 × 106; P = 0.0048; ANOVA), CC0042 (5.83 × 106; P = 0.0028), and CC058 (5.83 × 106; P = 0.0028) mice. Similar to CC001 mice, the highest day 2 spirochetemia was observed for CC046 mice (2.12 × 107), whose level was significantly higher than those of CC019 (P = 0.0111; ANOVA), CC0042 (P = 0.0067), and CC058 (P = 0.0067) (Fig. 1 and Table S2). For convenience, the counts of B. recurrentis spirochetes per milliliter of plasma were converted to the respective counts per milliliter of blood by multiplying each number by a factor of 1.5 and are provided in Table S3 (30).
FIG 1.
Detection of B. recurrentis spirochetemia in selected CC mouse lines by dark-field microscopy. Plasma samples collected daily from B. recurrentis A17-infected CC001 (A), CC019 (B), CC042 (C), CC046 (D), and CC058 (E) mice were examined by dark-field microscopy for 10 days postchallenge. Spirochete counts were determined by using a Petroff–Hauser counting chamber. The limit of detection of this approach is 2.50 × 105 spirochetes per ml of plasma. When means of day 1 and day 2 spirochetemic levels were compared within each mouse line, the significant difference was only detected for CC019 mice (P < 0.005; paired t test). When spirochetemic levels were compared between the five CC lines for each time point, the mean levels of CC001 and CC046 mice were significantly higher than those of CC0019, CC0042, and CC058 mice (P < 0.05; ANOVA).
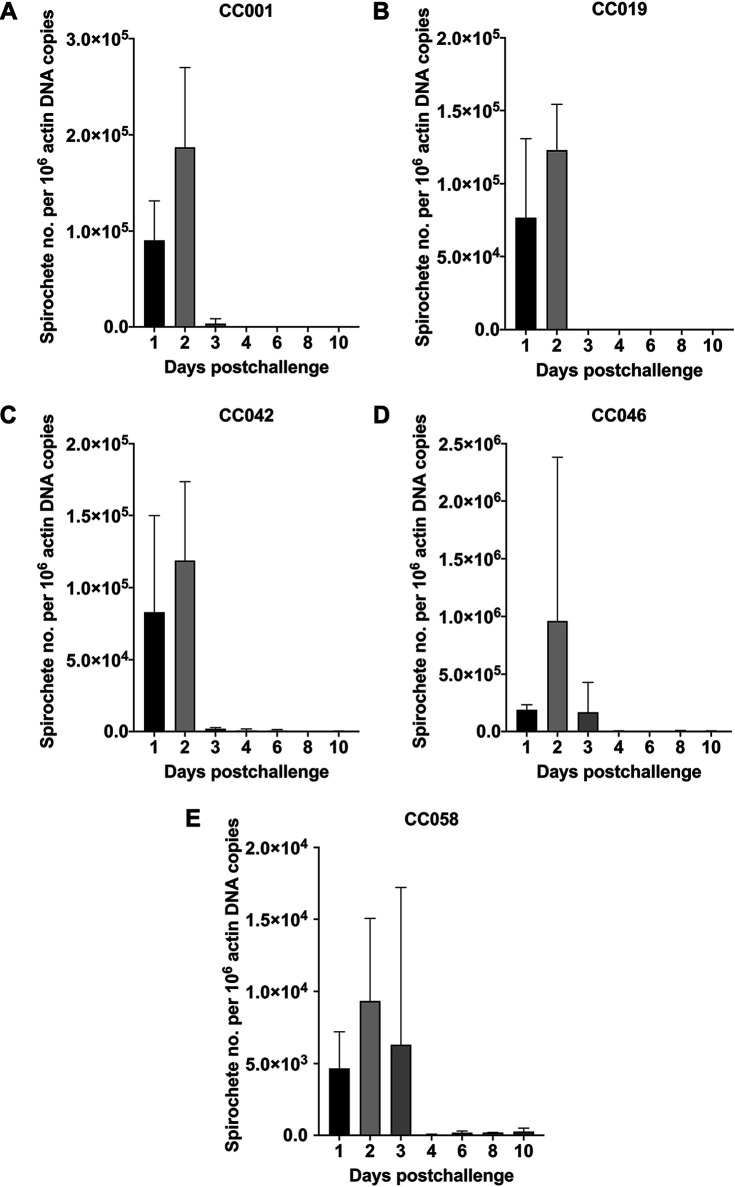
In addition to DFM and culture, spirochetemia was also examined by qPCR at days 1, 2, 3, 4, 6, 8, and 10 (Fig. 2). Overall, the qPCR results demonstrated the presence of spirochetal DNA in all day 1 and day 2 blood samples of the five lines. The mean numbers of flaB copies per 106 actin DNA in day 1 and day 2 blood samples were, respectively, 9.04 × 104 and 1.87 × 105 for CC001, 7.67 × 104 and 1.23 × 105 for CC019, 8.29 × 104 and 1.19 × 105 for CC042, 1.89 × 105 and 9.61 × 105 for CC046, and 4.67 × 103 and 9.34 × 103 for CC058. There was no significant difference in spirochetal DNA numbers when the respective means were compared between the first 2 days for each CC line (P > 0.05; paired t test; Fig. 2). Compared to day 3 DNA levels detected in CC046 mice (1.67 × 105 flaB copies per 106 actin DNA, n = 1.67 × 105), the mean spirochetal numbers (P > 0.05; ANOVA) were lower in blood samples of CC001 (3.38 × 103), CC042 (1.93 × 103), and CC058 (6.31 × 103) mice, suggesting a low-grade spirochetemia. In contrast, CC019 mice had a very low number of spirochetal DNA (n = 48) in their day 3 blood samples. No or very low numbers (n = 56 to 261) of flaB copies were detected in CC001, CC019, and CC058 mice at days 4, 6, 8, and 10 postchallenge. At days 4, 6, 8, and 10, CC042 mice were also qPCR negative (n = 0-432), with only a few outliers representing one day 4 (n = 1.94 × 103) and two day 6 (n = 1.28 × 103 and 7.65 × 102) samples. In contrast, the spirochetal DNA was consistently detected in all tested CC046 mice at days 4, 6, 8, and 10 postchallenge (n = 4.56 × 103, 2.45 × 103, 4.78 × 103, and 3.85 × 103, respectively), suggesting a persistent low-level spirochetemia. Lastly, when qPCR-detectable spirochetemias were compared between the five lines for each time point, the significant difference was only detected for day 2 between CC001 and CC046 (P = 0.0008; ANOVA), CC019 and CC046 (P = 0.0002), CC042 and CC046 (P = 0.0002), and CC046 and CC058 (P < 0.0001) mice.
FIG 2.
Detection of B. recurrentis spirochetemia in selected CC mouse lines by qPCR. DNA samples of blood collected from B. recurrentis A17-infected CC001 (A), CC019 (B), CC042 (C), CC046 (D), and CC058 (E) mice at days 1, 2, 3, 4, 6, 8, and 10 postchallenge were subjected to quantification of flaB and actB genes by qPCR. Standard dilutions and DNA samples were amplified in triplicate. The numbers of spirochetes were calculated as the ratio of flaB copies per 106 actin DNA. When day 1 and day 2 data were compared within each mouse line, the significant difference was only detected for CC001 mice (P < 0.05; paired t test). When means were compared between the five CC lines for each time point, the significant difference was only detected for day 2 between mice of CC001 and CC046, CC019 and CC046, CC042 and CC046, and CC046 and CC058 (P < 0.005; ANOVA).
Cross-bred mice.
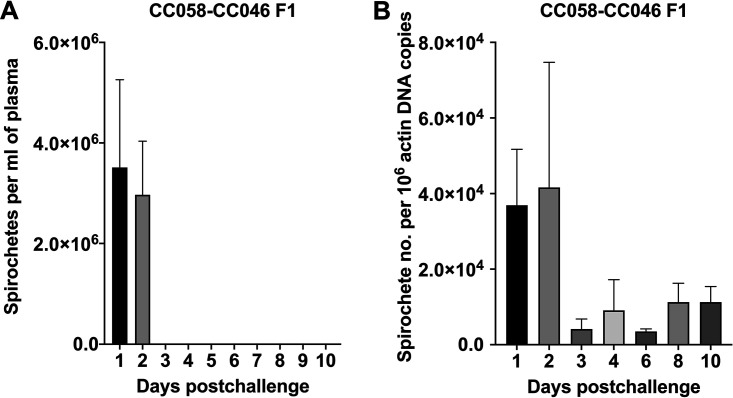
To examine whether the progeny of CC058 and CC046 lines, which exhibited the 3-day-long culture-detectable spirochetemia, would retain or enhance this infection phenotype, CC058-CC048 F1 mice were produced at the Texas A&M Institute for Genome Sciences and Society (TAGSS; College Station, TX, USA). Similarly, F1 hybrids (4 mice per group) were challenged with in vitro-grown A17, and blood samples were collected over the 10-day period for DFM, culture, qPCR, and serology. The DFM and culture results concordantly demonstrated the lack of spirochetemia in CC058-CC048 F1 mice at day 3 postchallenge (Fig. 3). Overall, the average numbers of spirochetes detected in day 1 plasma of CC058-CC048 F1 mice (3.75 × 106 cells/ml) did not significantly differ from the respective levels of the other five lines (P > 0.05; ANOVA). Day 1 and day 2 spirochetemic levels of CC058-CC048 F1 mice also were similar (P > 0.05; paired t test). Lastly, when day 2 levels of spirochetemia were compared between F1 hybrids and the other five lines, the mean spirochetemic level of the cross-breeds was significantly lower than those of CC001 (P = 0.0001; ANOVA) and CC046 (P = 0.0003).
FIG 3.
Detection of B. recurrentis spirochetemia in cross-bred CC058-CC046 F1 mice by dark-field microscopy and qPCR. (A) Daily plasma samples were examined by dark-field microscopy for 10 days postchallenge. There was no significant difference in spirochetemic levels between days 1 and 2 (P > 0.05; paired t test). (B) DNA samples of blood obtained at days 1, 2, 3, 4, 6, 8, and 10 postchallenge were subjected to qPCR. The numbers of spirochetes were calculated as the ratio of flaB copies per 106 actin DNA. The significant difference was only detected between days 2 and 3 and days 2 and 6 (P < 0.05; ANOVA).
The qPCR data showed that spirochetal DNA levels did not significantly vary between the time points, with the exception of day 2. The mean day 2 DNA level (n = 4.16 × 104) was significantly higher than day 3 (n = 4.18 × 103; P = 0.0287; ANOVA) and day 6 (n = 3.54 × 103; P = 0.0258; Fig. 3) levels. When the levels of qPCR-detectable spirochetemia were compared between F1 hybrids and the other 5 CC lines for each time point, the significance difference was only detected for day 2 with the level of CC058-CC046 F1 mice being about 2.5-fold lower (n = 4.16 × 103; P < 0.0001; ANOVA) than that of CC046 mice (Fig. 3). In CC058-CC046 F1 mice, the spirochetal DNA was detected in daily blood samples throughout the 10-day period, and its levels were relatively high: 3.70 × 104 (day 1), 4.16 × 104 (day 2), 4.18 × 103 (day 3), 9.11 × 103 (day 4), 3.54 × 103 (day 6), 1.13 × 104 (day 8), and 1.13 × 104 (day 10). Thus, the qPCR data suggested that, similar to the parental CC046 line, CC058-CC046 F1 mice also had a persistent low-grade spirochetemia.
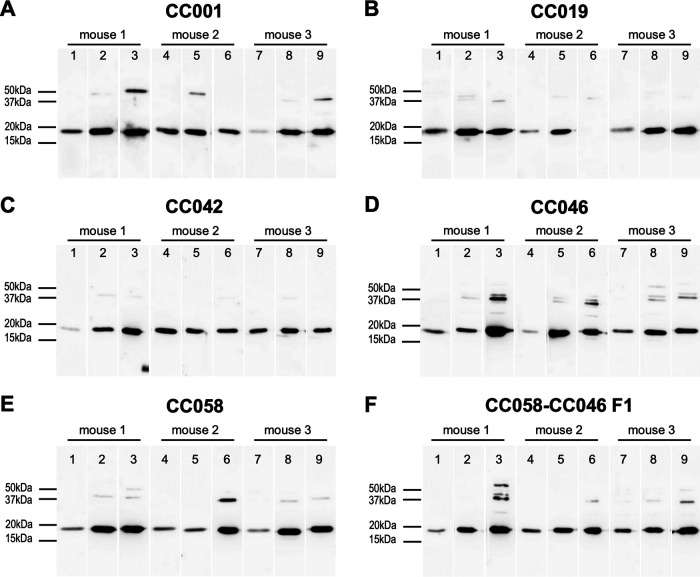
B. recurrentis-specific and highly variable IgM response.
To examine whether the CC mice had immunologically responded to A17 infection, sera sampled at days 4, 7, and 10 postchallenge were subjected to B. recurrentis-specific IgM Western blotting (WB) and total IgM enzyme-linked immunosorbent assay (ELISA). The WB results showed that all mice developed anti-B. recurrentis IgM antibodies, with more prominent levels being noted for the later time points (days 7 and 10) (Fig. 4). The total IgM levels measured by ELISA were variable between the time points for each CC line (Fig. S1). The mean day 7 IgM levels in CC001 (388 μg/ml) and CC058 (442 μg/ml) mice were significantly higher than their day 4 (205 and 276 μg/ml, respectively; P < 0.0001) and day 10 (186 and 278 μg/ml, respectively; P < 0.0001) levels. Unexpectedly, CC019 mice had the highest IgM level for day 4 (346 μg/ml; P < 0.0001; ANOVA) compared to their day 7 (223 μg/ml) and day 10 (247 μg/ml) levels. In CC042 mice, the IgM levels did not significantly differ between the three time points: 194 (day 4), 186 (day 7), and 212 (day 10) μg/ml (P > 0.05; ANOVA). In contrast, the IgM levels significantly increased over the course of infection in CC046 mice: 282 (day 4), 350 (day 7), and 460 (day 10) μg/ml (P < 0.0001; ANOVA). For CC058-CC046 F1 mice, significantly higher IgM levels were detected for days 7 (212 μg/ml) and 10 (207 μg/ml) compared to their day 4 level (164 μg/ml; P < 0.005; ANOVA).
FIG 4.
IgM seroconversion of CC mice upon infection with B. recurrentis. Western blotting was performed by using whole-cell lysates of B. recurrentis A17 (1 × 106 cells per lane) and individual plasma samples collected from B. recurrentis A17-infected mice of lines CC001 (A), CC019 (B), CC042 (C), CC046 (D), CC058 (E), and CC058-CC046 F1 (F) at days 4 (lanes 1, 4, and 7), 7 (lanes 2, 5, and 8), and 10 (lanes 3, 6, and 9) postchallenge. For each panel, lanes 1 to 3, 4 to 6, and 7 to 10, respectively, represent plasma samples from mouse 1, mouse 2, and mouse 3 of the respective line. Preimmune plasma had no signal and is not shown.
The total IgM response also significantly varied between the CC lines for each time point (Fig. S1). Compared to the other lines, CC019 mice had the highest IgM level for day 4 (P < 0.0005; ANOVA), whereas the IgM levels were highest in CC058 mice for day 7 (P < 0.0005). For day 10, the highest IgM levels were observed in CC046 mice (P < 0.0001; ANOVA).
Lack of B. recurrentis dissemination.
To examine whether B. recurrentis A17 spirochetes disseminated to different mouse tissues, brain, heart, spleen, kidneys, urinary bladder, ear pinnae, and tibiotarsal joints were collected at day 10 postchallenge and subjected to culture and histopathology (3 mice per line for each analysis). The histopathology evaluation was performed in a blind manner by two pathologists. The tissue culture results were consistently negative across all mice of the tested lines, including the cross-bred animals. Similarly, no significant histopathological findings (e.g., inflammation or the presence of spirochetes) were detected in the examined tissues.
DISCUSSION
Until this study, there had been only one experimental mouse model available to study B. recurrentis. This model was developed over a decade ago and was based on B- and T-cell-deficient mice (21). To identify it, four mouse strains (6-week-old males), BALB/c (BALB/cAnNTac), BALB/c nude (C.Cg/AnNTac-Foxn1nu NE9), SCID (C.B-Igh-1b/IcrTac-Prkdcscid), and SCID beige (C.B-Igh-1b/GbmsTac-Prkdcscid-Lystbg N7) were subcutaneously inoculated with B. recurrentis strain A17 or A11 (1 × 106 cells per mouse). As a comparison control, the tick-borne RF spirochete, Borrelia duttonii, whose genomic makeup is very similar to the B. recurrentis genome, was used (21, 31, 32). Phase contrast microscopy on blood collected daily for the first 20 days postchallenge demonstrated the lack of spirochetemia in both fully immunocompetent BALB/c and T-cell-deficient nude mice (33), indicating the minor importance of functional T cells for anti-B. recurrentis defense. In contrast, B. recurrentis-inoculated SCID and SCID beige mice, which were housed for 150 days postchallenge, developed and sustained microscopy-detectable spirochetemia during the entire period (21). Expectedly, these immunodeficient mice did not exhibit any relapsing patterns due to the missing humoral immune system (34). The levels of 150-day-long spirochetemia induced by A11 or A17 also were relatively low (∼2 × 105 cells/ml of blood), as opposed to about 200-fold higher levels observed in B. duttonii-infected SCID and SCID beige mice. Surprisingly, the highest levels of spirochetemia in some immunocompetent CC lines (CC001 and CC046) tested in this study were 1 order of magnitude higher than that observed in the previously developed immunodeficient mouse model. In addition to inherent discrepancy in mouse genetic traits, the high-grade spirochetemia demonstrated in the CC mice could also be potentially explained by a different enumeration approach taken in the present study. To quantify B. recurrentis spirochetemia, plasma samples were deliberately chosen over blood as the analysis of plasma, having the ability to detect fewer than 10 cells per ml of blood, was demonstrated to be more sensitive (29).
The highest spirochetemic levels (>107 cells/ml of blood) observed in CC001 and CC046 mice were well comparable to those induced in immunocompetent mouse strains (C57/BL6NHsd and C3H/HeOuJ) by more distantly related tick-borne RF spirochetes, Borrelia hermsii and Borrelia persica (35, 36). However, in contrast to these Old World and New World RF spirochetes, which developed multiple relapses, B. recurrentis had the capacity to produce only single culture-detectable spirochetemia. Of note, the high levels of spirochetemia observed in the CC lines also matched those of LBRF human patients, whose levels varied from 1 × 106 to 3.86 × 108 spirochetes per ml of blood (mean, 8.5 × 107 cells/ml) (37). Interestingly, as opposed to the negative microscopy and culture results, the qPCR data on CC046 and CC058-046 F1 blood samples suggested a persistent low-grade spirochetemia beyond day 3 postchallenge. The failure to detect spirochetes in the qPCR-positive blood samples by culture suggests that the molecular approach was more sensitive, a possibility that warrants future investigation. Overall, the lack of any detectable relapse may not be surprising. It is known that, compared to tick-borne RF spirochetes that cause numerous relapses (n = 3 to 9), the disease of LBRF patients is characterized by either no or only 1 to 2 spirochetemic relapses (37). Very rarely do LBRF patients develop 3 to 4 relapses (9, 20). Similar to human infection, no or a very limited number of spirochetemic relapses was also observed in the grivet monkey model, where out of 15 initially spirochetemic B. recurrentis-infected monkeys, only 8 and 1 animals developed 1 and 2 relapses, respectively (38).
Antigenic variation is an important aspect of RF spirochetal pathogenesis (39). There are two variable major proteins (Vmp), the variable large proteins (Vlp) and variable small proteins (Vsp), which constitute the antigenic variation system of RF spirochetes. These two proteins, which are surface localized and antigenically variable, allow RF spirochetes to evade host antibodies, causing multiple spirochetemic relapses (39–45). The genome of B. hermsii, whose antigenic system is well characterized, contains at least 59 silent vmp gene copies (43). During mammalian infection, only one locus is transcriptionally active, and antigenic variation occurs through nonreciprocal gene conversions that duplicate nonexpressable vmp genes (46–49). In contrast, B. recurrentis is thought to have a much more limited repertoire of vmp genes, which would account for a much lower number of relapses or their absence. As an example, B. recurrentis A11 possesses only 17 and 10 intact vlp and vsp genes, respectively (31). To date, full composition of the vmp system for other B. recurrentis isolates, including the A17 strain, which was used in the present study, remains unknown despite a recent effort to whole-genome sequence more B. recurrentis isolates (50). The study that recently sequenced the genomes of several archived B. recurrentis strains, A17, PBeK, PAbN, PAbJ, PMaC, and PUfA, failed to determine the exact makeup of their vmp systems mainly due to very high sequence similarity of vmp genes (50). However, the sequencing data of this previous study, highly discrepant coverage of vmp genes compared to the surrounding regions (10 to 20 times higher), suggested that the number of vmp copies was highly variable between the sequenced strains (50). Therefore, the hypothesis that the lack of any spirochetemic relapse, which was consistently recorded in the tested CC lines, was due to a very limited vmp repertoire of the A17 strain warrants testing.
By using various mouse models, it has been repeatedly demonstrated that T-cell-independent antibodies of mainly IgM isotype, which are produced by B1b and marginal-zone B cells, are the predominant component of the immune system that fights off the RF infection (51–56). The present study demonstrated that B. recurrentis-specific IgM antibodies were detectable at least as early as day 4 postchallenge, and this IgM response became more intensified by day 10 in all six CC lines tested. The levels of total IgM were highly variable at the chosen time points, with no unifying trend identified for the tested lines. Similarly, the total IgM response significantly varied between the six CC lines for each time point. Regardless of the extent and variability of IgM response, however, all CC mice were able to effectively clear the B. recurrentis infection, as concordantly determined by qPCR (except for CC046 and CC058-CC046 F1 mice), microscopy, and culture. The clearance of B. recurrentis infection was, therefore, most likely due to developed T-cell-independent IgM antibodies. The development of sterilizing immune response was also supported by the negative culture results of different mouse tissues tested. In addition to tissue sterility, the absence of histopathological changes in all B. recurrentis-infected CC mice suggested that the extravascular dissemination of B. recurrentis spirochetes had never occurred. The lack of dissemination is not surprising given the demonstrated short-term nature of culture-detectable spirochetemia.
One of the study limitations is that blood of the infected mice was only examined at daily intervals. It is well possible, however, that spirochetemic relapses were occurring on a more frequent (e.g., hourly) basis. The latter may explain the discrepancy observed between the results of the screen, where day 3 spirochetemia was detected via DFM in 3 out of 3 CC046 mice, and those of the follow-up experiment, where 2 out of 3 CC046 animals were DFM negative despite their blood testing positive by culture. The other limitation is that this study did not examine the CC mice for febrile episodes, the presence of which would suggest spirochetemia. To address the above-described limitations, in a future study, mouse body temperatures should be monitored in real time (e.g., via subcutaneous transponders). This monitoring would allow blood samples to be directly collected during febrile episodes, which should increase the overall sensitivity of detecting potentially short-term spirochetemic relapses.
Summary.
Together, the present study has identified the first immunocompetent mouse model of a strictly human pathogen (B. recurrentis), CC046 mice. This is a significant breakthrough in the field of LBRF research, as the novel model will allow the scientific community to finally study LBRF pathogenesis in the face of intact immune response. The novel model could also serve as the primary platform for development of efficacious vaccine, antibody-based therapeutics, and diagnostic tools for this highly understudied disease.
MATERIALS AND METHODS
Ethics statement.
All mice were maintained at Texas A&M University in an animal facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The experimental practices, which were approved by the Institutional Animal Care and Use Committee of Texas A&M University (IACUC 2017-0390), were carried out according to the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals (57), Guide for the Care and Use of Agricultural Animals in Research and Teaching (58), and Guide for the Care and Use of Laboratory Animals (59).
Bacterial strains.
Borrelia recurrentis strain A17 (arbitrarily assigned here as passage 0) was kindly provided by Sally Cutler (University of East London) by way of Sven Bergstrom (Umea University, Sweden). Originally, A17 was isolated in Ethiopia (21). A17 cells were grown in home-made liquid Barbour-Stoenner-Kelly II medium supplemented with 10% (vol/vol) rabbit serum (Sigma-Aldrich, MO, USA) and 1.4% (wt/vol) gelatin (Sigma-Aldrich; referred to here as BSK-II) at 35°C under 2.5% CO2 (21, 60, 61). Upon propagation, to avoid unnecessary subculturing, sufficient amounts of working A17 stocks (passage 1) were prepared and stored at −80°C. For each infectivity experiment, the fresh working stock was propagated in 5 ml of BSK-II (passage 2) at 35°C under 2.5% CO2 until the level of ∼6 × 107 cells per ml was reached. The inoculum was prepared by replacing BSK-II medium with sterile phosphate-buffered saline (PBS). Specifically, A17 cells were centrifuged at 6,000 × g for 10 min at room temperature (RT) and then gently resuspended with a volume of PBS (∼25°C) required to reach the final inoculum concentration of 1 × 107 cells per ml.
Infectivity studies.
Based on animal availability, a total of 11 of Collaborative Cross (CC) mouse lines were included in the initial screen. The CC mice were obtained from the Texas A&M Institute for Genome Sciences and Society (TAGSS) (College Station, TX, USA). The CC lines were represented by a group of 3 male mice with the exception of lines CC004 and CC005, which had only 2 males each (the 2 lines were of limited availability at TAGSS). All CC lines used in the screen and mouse age at which the animals were inoculated are provided in Table S1 in the supplemental material.
The CC mice were challenged with ∼1 × 106 A17 spirochetes per animal via subcutaneous inoculation of a 100-μl inoculum in the shoulder area. The outcome of infection was monitored by examining daily blood samples via dark-field microscopy (DFM). Prior to challenge (day 0) and at days 1, 2, 3, 4, and 5 postchallenge, blood (30 μl) was collected from each mouse via cheek bleed into heparin-coated tubes (BD, NJ, USA). At day 5 postchallenge, the animals were humanely sacrificed. Within approximately 1 h after sample collection, blood samples (30 μl) were centrifuged at 500 × g for 5 min at RT, and obtained plasma samples (6 μl) were examined by DFM.
Based on the DFM results, the follow-up study, where mice of selected CC lines were monitored daily for 10 days, was performed. The experiments involved five lines, CC001, CC019, CC042, CC046, and CC058, and a cross-bred progeny of CC058 females and CC046 males (referred to here as CC058-CC046 F1). Because of limited mouse availability, the five lines (9 males per line) were purchased from the Systems Genetics Core Facility at the University of North Carolina (Chapel Hill, NC, USA), and CC058-CC046 F1 mice (8 females and 4 males) were produced at the TAGSS facility. The age of the animals is provided in Table S2. All 6 lines were divided into 3 groups, A, B, and C (4 animals per group for line CC058-CC046 F1 and 3 mice per group for the other five lines). Each mouse was inoculated with ∼1 × 106 A17 spirochetes per animal as described above.
Sample collection, blood culture, and spirochete counts.
After challenge with A17, group A mice of each CC line were bled at days 1, 4, 7, and 10. Group B animals were bled at days 2, 5, and 8, and group C mice were bled at days 3, 6, and 9 postchallenge. This experimental timeline allowed sufficient amounts of blood to be collected from each mouse for multiple analyses. Specifically, during each bleed, blood was collected for culture (20 μl), qPCR (40 μl), and DFM (50 μl). Additionally, a week prechallenge (day −7) and at days 4, 7, and 10 postchallenge, 20 μl of blood was also collected from group A mice (3 mice per line) for antibody tests. After bleeding, each animal was subcutaneously injected with approximately 200 μl of sterile saline to compensate for blood loss.
To detect spirochetes in mouse blood via culture, each sample was directly transferred into 1 ml of BSK-II medium supplemented with 0.02 mg ml−1 phosphomycin, 0.05 mg ml−1 rifampin, and 2.5 mg ml−1 amphotericin B (referred to here as the antibiotic cocktail). The blood cultures were placed at 35°C under 2% CO2 and then examined weekly (or when there was noticeable color change) via DFM for 4 weeks. The cultures were called negative if no motile spirochetes were observed during this 4-week period. For qPCR and DFM, blood was collected into heparin-coated tubes (BD, NJ). The qPCR samples were placed at −80°C until further analysis.
The DFM samples were centrifuged at 500 × g for 5 min at RT to obtain at least 24 μl of plasma from each mouse per time point. Separation of erythrocytes from plasma was shown to increase the sensitivity of spirochetal detection and counts (29). Spirochetes were counted per 6 μl of plasma under dark-field illumination by using a Petroff–Hauser counting chamber. Spirochete counts were independently performed 4 times per plasma sample of each mouse (6 μl of plasma for each count; 24 μl of plasma total). Spirochetes were counted per 5 squares of a Petroff–Hauser counting chamber, and spirochetal counts were determined by multiplying an averaged number of motile spirochetes by a factor of 1.25 × 106. The limit of detection of this enumeration is 2.50 × 105 spirochetes per ml of plasma (1/5 [1 cell per 5 squares] × 1.25 × 106).
qPCR.
In addition to DFM, blood samples (40 μl) were subjected to qPCR to quantify levels of B. recurrentis A17 spirochetemia. First, DNA was obtained from each sample by using a QIAamp DNA micro kit (Qiagen, MD, USA) as described in the manufacturer’s protocols. The DNA samples were stored at −80°C until use. qPCR assays were performed with SsoAdvanced universal probe supermix using a CFX 96 Touch real-time PCR detection system (Bio-Rad Laboratories, CA, USA). To target the B. recurrentis A17 flaB, newly developed probe and primers were used: 6-carboxyfluorescein (FAM)-AGC AGC TCC AGC TCC AGC AGC AGC T-6-carboxytetramethylrhodamine (TAMRA), 5′-AGG GTG CAC AGC AAG AAG GA-3′, and 5′-GCA CCA AGA TTT GCT CTT TGA TCA GT-3′. To target the mouse actB, previously developed probe and primers were utilized: FAM-CAC TGC CGC ATC CTC TTC CTC CC-TAMRA, 5′-AGA GGG AAA TCG TGC GTG AC-3′, and 5′-CAA TAG TGA TGA CCT GGC CGT-3′ (62). The probes and primers were synthesized by Sigma-Aldrich Inc. (MilliporeSigma, MO). Each 20-μl reaction mixture contained 200 nM the probe, 300 nM each primer, and 100 ng of DNA. The cycling parameters were 95°C for 5 min followed by 39 cycles of 95°C for 15 s and 60°C for 1 min, and the melt curve was from 65°C for 5 s to 95°C with a 0.5°C increment. Standard dilutions and DNA samples were amplified in triplicate. The number of spirochetes was calculated as the ratio of flaB copies per 106 actin DNA.
Western blotting.
To detect A17-specific antibody response, the plasma samples collected at days 0, 4, 7, and 10 postchallenge from group A animals (3 mice per group) were subjected to to Western blotting (WB). WB was performed on individual mouse immune sera and pooled preimmune sera as described previously, with some modifications (63). Specifically, B. recurrentis A17 spirochetes (the working A17 stocks [passage 1]), which had been grown in BSK-II to ∼5 × 107 cells per ml, were harvested by centrifugation, washed twice with ice-cold PBS, and resuspended with SDS-PAGE sample buffer. Approximately 1 × 106 A17 cells were loaded into each lane of a 15% acrylamide gel. One slab gel was stained with Coomassie blue R-250 (Bio-Rad Laboratories, CA) (Fig. S2). After A17 proteins were resolved over 90 min, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (pore size of 0.45 μm; Bio-Rad Laboratories, CA). The PVDF membranes were cut into strips and incubated overnight at 4°C with 5% nonfat dry milk supplemented with the respective 1:500-diluted mouse serum. After washing with Tris-buffered saline with Tween 20, mouse IgM immunoglobulins were detected by using goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, CA). Each WB strip was developed by enhanced chemiluminescence.
ELISA.
To quantify the total level of IgM in A17-infected CC mice, plasma samples collected at days −7 and 4, 7, and 10 postchallenge from group A mice (3 animals per group) were individually analyzed by an IgM mouse uncoated ELISA kit according to the manufacturer's instructions (ThermoFisher Scientific, MA, USA). Each sample was diluted 1:200,000 and analyzed in triplicate. Pooled preimmune sera were used as a negative control. The standards provided by the kit served as positive controls.
Histopathology.
Brain, heart, spleen, the left kidney, urinary bladder, the left ear pinnae, and the left tibiotarsal joint from each CC mouse were fixed in 10% neutral buffered formalin immediately upon tissue harvest. The joints were decalcified for 2 h prior to trimming and then sagitally dissected. The brain and joints were processed each in separate cassettes. All tissue sections were embedded in paraffin, cut as 4-μm sections, and stained with hematoxylin and eosin using standard procedures. All tissue sections were then evaluated for pathological changes by two pathologists. The slides were read blinded.
Statistical analyses.
The statistical analyses were performed by using Prism 8 for macOS Software version 8.3.0 (328) (GraphPad Software, LLC, CA, USA). One-way and two-way (Tukey's multiple-comparison test) analysis of variance (ANOVA) was applied to analyze DFM, qPCR, and ELISA data. Paired t test was used to compare levels of DFM-detectable spirochetemia between days 1 and 2 for each mouse line. Differences were considered statistically significant at a P value of <0.05.
ACKNOWLEDGMENTS
We thank Sven Bergstrom for providing us with Borrelia recurrentis. We also thank Maliha Batool, David C. Gillis, Ingela Nilsson, and Gustavo Geraldo Medina Snel for their technical assistance. The work at Texas A&M University was supported by the Department of Veterinary Pathobiology, Texas A&M College of Veterinary Medicine & Biomedical Sciences, and Texas A&M AgriLife.
Experimental Design, A.S.R. and D.W.T.; Methodology, A.S.R., Y.V.R., B.M.T., and D.J.W; Investigation, A.S.R., Y.V.R., B.M.T., and D.J.W; Validation, A.S.R.; Data Analysis, A.S.R.; Writing, Original Draft, A.S.R.; Writing, Review and Editing, A.S.R., Y.V.R., B.M.T., and D.J.W; Visualization, A.S.R; Funding Acquisition, A.S.R. and D.W.T.; Resources, A.S.R. and D.W.T.; Supervision, A.S.R.
We have no competing interests to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Artem S. Rogovskyy, Email: arogovskyy@tamu.edu.
De'Broski R. Herbert, University of Pennsylvania
REFERENCES
- 1.Talagrand-Reboul E, Boyer PH, Bergstrom S, Vial L, Boulanger N. 2018. Relapsing fevers: neglected tick-borne diseases. Front Cell Infect Microbiol 8:98. doi: 10.3389/fcimb.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler SJ. 2010. Relapsing fever–a forgotten disease revealed. J Appl Microbiol 108:1115–1122. doi: 10.1111/j.1365-2672.2009.04598.x. [DOI] [PubMed] [Google Scholar]
- 3.Warrell DA. 2019. Louse-borne relapsing fever (Borrelia recurrentis infection). Epidemiol Infect 147:e106. doi: 10.1017/S0950268819000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snowden J, Yarrarapu SNS, Oliver TI. 2021. Relapsing fever. StatPearls, Treasure Island, FL. [PubMed] [Google Scholar]
- 5.Boutellis A, Mediannikov O, Bilcha KD, Ali J, Campelo D, Barker SC, Raoult D. 2013. Borrelia recurrentis in head lice, Ethiopia. Emerg Infect Dis 19:796–798. doi: 10.3201/eid1905.121480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houhamdi L, Raoult D. 2005. Excretion of living Borrelia recurrentis in feces of infected human body lice. J Infect Dis 191:1898–1906. doi: 10.1086/429920. [DOI] [PubMed] [Google Scholar]
- 7.Cutler SJ, Abdissa A, Trape JF. 2009. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect 15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 8.Eguale T, Abate G, Balcha F. 2002. Relapsing fever in Hossana, Ethiopia: a clinical and epidemiological study. Ethiop J Health Sci 12:103–108. [Google Scholar]
- 9.Felsenfeld O. 1971. Borrelia: strains, vectors, human and animal borreliosis. W. H. Green, St. Louis, MO. [Google Scholar]
- 10.Abdalla RE. 1969. Some studies on relapsing fever in the Sudan. J Trop Med Hyg 72:125–128. [PubMed] [Google Scholar]
- 11.de Jong J, Wilkinson RJ, Schaeffers P, Sondorp HE, Davidson RN. 1995. Louse-borne relapsing fever in southern Sudan. Trans R Soc Trop Med Hyg 89:621. doi: 10.1016/0035-9203(95)90414-X. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JM, Malmierca E, Reyes F, Tesfamariam A. 2008. Results of a 10-year survey of louse-borne relapsing fever in southern Ethiopia: a decline in endemicity. Ann Trop Med Parasitol 102:467–469. doi: 10.1179/136485908X300887. [DOI] [PubMed] [Google Scholar]
- 13.Hoch M, Wieser A, Loscher T, Margos G, Purner F, Zuhl J, Seilmaier M, Balzer L, Guggemos W, Rack-Hoch A, von Both U, Hauptvogel K, Schonberger K, Hautmann W, Sing A, Fingerle V. 2015. Louse-borne relapsing fever (Borrelia recurrentis) diagnosed in 15 refugees from northeast Africa: epidemiology and preventive control measures, Bavaria, Germany, July to October 2015. Euro Surveill 20. doi: 10.2807/1560-7917.ES.2015.20.42.30046. [DOI] [PubMed] [Google Scholar]
- 14.Grecchi C, Zanotti P, Pontarelli A, Chiari E, Tomasoni LR, Gulletta M, Barbui A, Caligaris S, Matteelli A, Castelli F. 2017. Louse-borne relapsing fever in a refugee from Mali. Infection 45:373–376. doi: 10.1007/s15010-017-0987-2. [DOI] [PubMed] [Google Scholar]
- 15.Cutler SJ. 2016. Refugee crisis and re-emergence of forgotten infections in Europe. Clin Microbiol Infect 22:8–9. doi: 10.1016/j.cmi.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Antinori S, Mediannikov O, Corbellino M, Grande R, Parravicini C, Bestetti G, Longhi E, Ricaboni D, Ehounoud CB, Fenollar F, Raoult D, Rimoldi SG. 2016. Louse-borne relapsing fever (Borrelia recurrentis) in a Somali refugee arriving in Italy: a re-emerging infection in Europe? PLoS Negl Trop Dis 10:e0004522. doi: 10.1371/journal.pntd.0004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antinori S, Mediannikov O, Corbellino M, Raoult D. 2016. Louse-borne relapsing fever among East African refugees in Europe. Travel Med Infect Dis 14:110–114. doi: 10.1016/j.tmaid.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Larsson C, Andersson M, Bergstrom S. 2009. Current issues in relapsing fever. Curr Opin Infect Dis 22:443–449. doi: 10.1097/QCO.0b013e32832fb22b. [DOI] [PubMed] [Google Scholar]
- 19.Legesse W, Gebre-Selassie S. 2005. Louse-borne relapsing fever profile at Jimma hospital, Ethiopia: a retrospective study. Ethiop J Edu Sci 1:59–64. [Google Scholar]
- 20.Bryceson AD, Parry EH, Perine PL, Warrell DA, Vukotich D, Leithead CS. 1970. Louse-borne relapsing fever. Q J Med 39:129–170. [PubMed] [Google Scholar]
- 21.Larsson C, Lundqvist J, van Rooijen N, Bergstrom S. 2009. A novel animal model of Borrelia recurrentis louse-borne relapsing fever borreliosis using immunodeficient mice. PLoS Negl Trop Dis 3:e522. doi: 10.1371/journal.pntd.0000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Threadgill DW, Hunter KW, Williams RW. 2002. Genetic dissection of complex and quantitative traits: from fantasy to reality via a community effort. Mamm Genome 13:175–178. doi: 10.1007/s00335-001-4001-y. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Cross Consortium. 2012. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 190:389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottomly D, Ferris MT, Aicher LD, Rosenzweig E, Whitmore A, Aylor DL, Haagmans BL, Gralinski LE, Bradel-Tretheway BG, Bryan JT, Threadgill DW, de Villena FP, Baric RS, Katze MG, Heise M, McWeeney SK. 2012. Expression quantitative trait loci for extreme host response to influenza A. G3 2:213–221. doi: 10.1534/g3.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris MT, Aylor DL, Bottomly D, Whitmore AC, Aicher LD, Bell TA, Bradel-Tretheway B, Bryan JT, Buus RJ, Gralinski LE, Haagmans BL, McMillan L, Miller DR, Rosenzweig E, Valdar W, Wang J, Churchill GA, Threadgill DW, McWeeney SK, Katze MG, Pardo-Manuel de Villena F, Baric RS, Heise MT. 2013. Modeling host genetic regulation of influenza pathogenesis in the collaborative cross. PLoS Pathog 9:e1003196. doi: 10.1371/journal.ppat.1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrant C, Tayem H, Yalcin B, Cleak J, Goodstadt L, de Villena FP, Mott R, Iraqi FA. 2011. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res 21:1239–1248. doi: 10.1101/gr.118786.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen AL, Okumura A, Ferris MT, Green R, Feldmann F, Kelly SM, Scott DP, Safronetz D, Haddock E, LaCasse R, Thomas MJ, Sova P, Carter VS, Weiss JM, Miller DR, Shaw GD, Korth MJ, Heise MT, Baric RS, de Villena FP, Feldmann H, Katze MG. 2014. Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science 346:987–991. doi: 10.1126/science.1259595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen BB, Andersson M, Christensen IJ, Moller S. 2008. Evaluation of and quality assurance in HER2 analysis in breast carcinomas from patients registered in Danish Breast Cancer Group (DBCG) in the period of 2002-2006. A nationwide study including correlation between HER-2 status and other prognostic variables. Acta Oncol 47:784–788. doi: 10.1080/02841860801989779. [DOI] [PubMed] [Google Scholar]
- 29.Larsson C, Bergstrom S. 2008. A novel and simple method for laboratory diagnosis of relapsing fever borreliosis. Open Microbiol J 2:10–12. doi: 10.2174/1874285800802010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riches AC, Sharp JG, Thomas DB, Smith SV. 1973. Blood volume determination in the mouse. J Physiol 228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, Drancourt M. 2008. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet 4:e1000185. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbir H, Abi-Rached L, Pontarotti P, Yoosuf N, Drancourt M. 2014. African relapsing fever borreliae genomospecies revealed by comparative genomics. Front Public Health 2:43. doi: 10.3389/fpubh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanagan SP. 1966. “Nude,” a new hairless gene with pleiotropic effects in the mouse. Genet Res 8:295–309. doi: 10.1017/S0016672300010168. [DOI] [PubMed] [Google Scholar]
- 34.Bosma GC, Custer RP, Bosma MJ. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzer S, Overzier E, Hermanns W, Baneth G, Straubinger RK. 2016. Borrelia persica infection in immunocompetent mice–a new tool to study the infection kinetics in vivo. PLoS Negl Trop Dis 10:e0004404. doi: 10.1371/journal.pntd.0004404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James AE, Rogovskyy AS, Crowley MA, Bankhead T. 2016. Characterization of a DNA adenine methyltransferase gene of Borrelia hermsii and its dispensability for murine infection and persistence. PLoS One 11:e0155798. doi: 10.1371/journal.pone.0155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler T, Hazen P, Wallace CK, Awoke S, Habte-Michael A. 1979. Infection with Borrelia recurrentis: pathogenesis of fever and petechiae. J Infect Dis 140:665–675. doi: 10.1093/infdis/140.5.665. [DOI] [PubMed] [Google Scholar]
- 38.Judge DM, La Croix JT, Perine PL. 1974. Experimental louse-borne relapsing fever in the grivet monkey, Cercopithecus aethiops. Am J Trop Med Hyg 23:969–973. doi: 10.4269/ajtmh.1974.23.969. [DOI] [PubMed] [Google Scholar]
- 39.Raffel SJ, Battisti JM, Fischer RJ, Schwan TG. 2014. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog 10:e1004056. doi: 10.1371/journal.ppat.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbour AG, Restrepo BI. 2000. Antigenic variation in vector-borne pathogens. Emerg Infect Dis 6:449–457. doi: 10.3201/eid0605.000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barstad PA, Coligan JE, Raum MG, Barbour AG. 1985. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med 161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbour AG, Tessier SL, Stoenner HG. 1982. Variable major proteins of Borrellia hermsii. J Exp Med 156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. 2006. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol Microbiol 60:1329–1343. doi: 10.1111/j.1365-2958.2006.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinnebusch BJ, Barbour AG, Restrepo BI, Schwan TG. 1998. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun 66:432–440. doi: 10.1128/IAI.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Restrepo BI, Kitten T, Carter CJ, Infante D, Barbour AG. 1992. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol 6:3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 46.Barbour AG. 2016. Chromosome and plasmids of the tick-borne relapsing fever agent Borrelia hermsii. Genome Announc 4:e00528-16. doi: 10.1128/genomeA.00528-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbour AG, Burman N, Carter CJ, Kitten T, Bergstrom S. 1991. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol 5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 48.Kitten T, Barbour AG. 1990. Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci U S A 87:6077–6081. doi: 10.1073/pnas.87.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plasterk RH, Simon MI, Barbour AG. 1985. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature 318:257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- 50.Marosevic D, Margos G, Wallich R, Wieser A, Sing A, Fingerle V. 2017. First insights in the variability of Borrelia recurrentis genomes. PLoS Negl Trop Dis 11:e0005865. doi: 10.1371/journal.pntd.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman K, Jr, Johnson RC. 1981. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun 31:465–469. doi: 10.1128/IAI.31.1.465-469.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbour AG, Bundoc V. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect Immun 69:1009–1015. doi: 10.1128/IAI.69.2.1009-1015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connolly SE, Benach JL. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol 167:3029–3032. doi: 10.4049/jimmunol.167.6.3029. [DOI] [PubMed] [Google Scholar]
- 54.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 55.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Belperron AA, Dailey CM, Bockenstedt LK. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol 174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 57.National Institutes of Health. 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 58.American Society of Animal Science. 2010. Guide for the Care and Use of Agricultural Animals in Research and Teaching. American Society of Animal Science, Champaign, IL. [Google Scholar]
- 59.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 60.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 61.Cutler SJ, Akintunde CO, Moss J, Fukunaga M, Kurtenbach K, Talbert A, Zhang H, Wright DJ, Warrell DA. 1999. Successful in vitro cultivation of Borrelia duttonii and its comparison with Borrelia recurrentis. Int J Syst Bacteriol 49:1793–1799. doi: 10.1099/00207713-49-4-1793. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Liu X, Beck DS, Kantor FS, Fikrig E. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun 74:3305–3313. doi: 10.1128/IAI.02035-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogovskyy AS, Gillis DC, Ionov Y, Gerasimov E, Zelikovsky A. 2017. Antibody response to Lyme disease spirochetes in the context of VlsE-mediated immune evasion. Infect Immun 85:e00890-16. doi: 10.1128/IAI.00890-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2; Tables S1 to S3. Download IAI.00048-21-s0001.pdf, PDF file, 1.25 MB (1.3MB, pdf)