ABSTRACT
There are no vaccines licensed for enterotoxigenic Escherichia coli (ETEC), a leading cause of diarrhea for children in developing countries and international travelers. Virulence heterogeneity among strains and difficulties identifying safe antigens for protective antibodies against STa, a potent but poorly immunogenic heat-stable toxin which plays a key role in ETEC diarrhea, are challenges in ETEC vaccine development. To overcome these challenges, we applied a toxoid fusion strategy and a novel epitope- and structure-based multiepitope fusion antigen (MEFA) vaccinology platform to construct two chimeric multivalent proteins, toxoid fusion 3xSTaN12S-mnLTR192G/L211A and adhesin CFA/I/II/IV MEFA, and demonstrated that the proteins induced protective antibodies against STa and heat-labile toxin (LT) produced by all ETEC strains or the seven most important ETEC adhesins (CFA/I and CS1 to CS6) expressed by the ETEC strains causing 60 to 70% of diarrheal cases and moderate to severe cases. Combining two proteins, we prepared a protein-based multivalent ETEC vaccine, MecVax. MecVax was broadly immunogenic; mice and pigs intramuscularly immunized with MecVax developed no apparent adverse effects but had robust antibody responses to the target toxins and adhesins. Importantly, MecVax-induced antibodies were broadly protective, demonstrated by significant adherence inhibition against E. coli bacteria producing any of the seven adhesins and neutralization of STa and cholera toxin (CT) enterotoxicity. Moreover, MecVax protected against watery diarrhea and provided over 70% and 90% protection against any diarrhea from an STa-positive or an LT-positive ETEC strain in a pig challenge model. These results indicated that MecVax induces broadly protective antibodies and prevents diarrhea preclinically, signifying that MecVax is potentially an effective injectable vaccine for ETEC.
IMPORTANCE Enterotoxigenic Escherichia coli (ETEC) bacteria are a top cause of children’s diarrhea and travelers’ diarrhea and are responsible for over 220 million diarrheal cases and more than 100,000 deaths annually. A safe and effective ETEC vaccine can significantly improve public health, particularly in developing countries. Data from this preclinical study showed that MecVax induces broadly protective antiadhesin and antitoxin antibodies, becoming the first ETEC vaccine candidate to induce protective antibodies inhibiting adherence of the seven most important ETEC adhesins and neutralizing the enterotoxicity of not only LT but also STa toxin. More importantly, MecVax is shown to protect against clinical diarrhea from STa-positive or LT-positive ETEC infection in a pig challenge model, recording protection from antibodies induced by the protein-based, injectable, subunit vaccine MecVax against ETEC diarrhea and perhaps the possibility of intramuscularly administered protein vaccines for protection against intestinal mucosal infection.
KEYWORDS: diarrhea, ETEC, enterotoxigenic Escherichia coli, MecVax, injectable multivalent vaccine, toxoid fusion, MEFA, multiepitope fusion antigen, pig model
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC), a group of heterogeneous E. coli strains producing heat-stable (STa) and/or heat-labile (LT) enterotoxins, is one of the top causes of diarrhea for children living in developing countries (children’s diarrhea) (1, 2) and international travelers, including civilian and military personnel deployed in regions of ETEC endemicity (travelers’ diarrhea) (3, 4). ETEC bacteria initiate infection by adhering to host intestinal epithelia and colonizing small intestines, mediated by fimbrial or nonfimbrial adhesins called colonization factor antigens (CFA) or coli surface antigens (CS) (5, 6), and then they effectively deliver STa and/or LT enterotoxins to host epithelial cells to disrupt homeostasis. This leads to hypersecretion of water and electrolyte-rich fluid into the gut lumen and causes watery diarrhea. Some ETEC diarrheal episodes can become severe and, without medical intervention, progress to dehydration and death (7).
Currently, there are no effective countermeasures against ETEC diarrhea (8, 9). Improved sanitation and clean drinking water (or WASH, water, sanitation, and hygiene) that could reduce diarrheal incidence are not achieved in many resource-limited countries. Antibiotic drugs treating ETEC travelers’ diarrhea are becoming less effective because ETEC strains are acquiring antimicrobial resistance at an alarmingly increasing rate (10–14). Vaccines have been considered a cost-effective countermeasure to prevent ETEC diarrhea (3, 15, 16). However, despite the development of ETEC vaccines becoming a top priority for the World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and many other public health institutions (3, 16, 17), there are no vaccines licensed for ETEC.
The key challenge for ETEC vaccine development is the genetic and immunologic heterogeneity of ETEC bacteria. ETEC strains produce various virulence factors, namely, over 25 immunologically different CFA and CS adhesins and two distinctive enterotoxins (STa and LT); an ETEC strain producing any one of these adhesins and either enterotoxin can cause diarrhea in children or travelers. Another challenge is the difficulty in identifying a nontoxic antigen(s) that is able to induce neutralizing antibodies against the potent 19-amino-acid STa toxin, which plays a key role in causing children’s diarrhea (1, 2) and travelers’ diarrhea (18). Additionally, STa and STa-derived nontoxic peptides (STa toxoids, used as safe antigens for STa toxin) are poorly immunogenic. Unlike the strongly immunogenic LT, to which children and adults develop antibodies after exposure to ETEC bacteria, and the fact that anti-LT antibodies provide some protection against subsequent LT-producing ETEC infection (19), STa (or STa peptide) does not induce anti-STa antibodies naturally.
An ideal ETEC vaccine should prevent all ETEC strains from colonizing host small intestines and should also neutralize the enterotoxicity not only of LT but, more importantly, of STa toxin (9, 15, 20). While including antigens for all CFA adhesins seems impossible, a practical and feasible approach is to develop a vaccine that is cross-protective against the ETEC strains that cause a majority of clinical cases, as well as moderate to severe cases (20, 21). ETEC strains producing adhesins CFA/I, CFA/II (CS1 to CS3), and CFA/IV (CS4 to CS6) (together with STa and/or LT toxin), are estimated to cause over 60 to 70% of diarrheal cases in children and travelers (20, 22, 23). Thus, these seven adhesins became the primary targets historically in ETEC vaccine development (9, 15). This approach has been used to develop whole-cell oral vaccines in which several live attenuated or killed ETEC strains are mixed together (cocktail vaccines) in hopes of achieving broad protection against a majority of these seven adhesins (24–26).
A broad-spectrum vaccinology platform can overcome the heterogeneity challenge and enable the development of a safe and cross-protective ETEC vaccine. The novel epitope- and structure-based multiepitope fusion antigen (MEFA) vaccinology platform empowers the development of safe and broadly protective multivalent vaccines (27–29). Using a combined structural vaccinology and epitope vaccinology concept and with assistance from computational biology and protein modeling, the MEFA platform allows multiple protective epitopes from heterogeneous pathotypes or virulence factors to be presented on a backbone immunogen and to mimic epitope native antigenicity, thus allowing the construction of a safe chimeric protein to induce broadly protective antibodies against heterogeneous strains, yielding a safe and cross-protective multivalent vaccine (27). By applying the MEFA platform, we constructed two multivalent proteins for ETEC, CFA/I/II/IV MEFA and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A. CFA/I/II/IV MEFA, which uses adhesin CFA/I major subunit CfaB as the backbone and incorporates the protective epitopes of the other adhesins to create a MEFA protein with epitopes of seven adhesins (CFA/I and CS1 to CS6), induces broad antiadhesin antibodies to protect against adherence from the seven adhesins (30–32). The toxoid fusion protein, 3xSTaN12S-mnLTR192G/L211A, which has three copies of STa toxoid STaN12S (STa with a mutation of the 12th asparagine to serine) genetically fused to LT toxoid monomer mnLTR192G/L211A (the LTA subunit mutated at the 192nd and the 211th residues is genetically fused to the LTB subunit to form a single peptide) at the N terminus, the C terminus, and between the A1 and A2 domains, shows no enterotoxic activities, induces antibodies able to neutralize both ETEC toxins (31, 33, 34), and prevents ETEC diarrhea in a pig challenge model (35, 36). When two proteins were mixed and administered intraperitoneally (i.p.), mice developed protective antibodies against the seven ETEC adhesins and the two toxins in vitro (31).
By combining the CFA/I/II/IV MEFA protein and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A, we constructed MecVax, a multivalent enterotoxigenic E. coli vaccine, and expected this protein-based injectable vaccine to provide broad protection against heterogeneous ETEC strains. To characterize MecVax’s immunogenicity and efficacy preclinically, in this study, we administered MecVax to mice and pigs intramuscularly (i.m.), bearing in mind that the i.m. route is preferred for MecVax administration for humans, and then examined vaccine-induced antibody responses to the target ETEC adhesins and toxins, measured vaccine-induced antibodies for in vitro protection against ETEC bacterial adherence and toxin enterotoxicity, and evaluated vaccine protection against ETEC diarrhea in a pig challenge model.
RESULTS
MecVax induced broad antiadhesin and antitoxin antibodies in mice.
Mice i.m. immunized with MecVax showed no physical differences from the control mice and developed robust antibody responses to seven ETEC adhesins and two toxins (Fig. 1). Anti-CFA/I, -CS1, -CS2, -CS3, -CS4, -CS5, -CS6, -STa, and anti-LT IgGs were detected at titers of 3.74 ± 0.26 (mean ± standard deviation), 3.34 ± 0.62, 3.38 ± 0.40, 3.40 ± 0.27, 3.32 ± 0.39, 3.90 ± 0.13, 3.53 ± 0.39, 5.16 ± 0.56, and 3.81 ± 0.32 (log10), respectively, in the serum samples of the immunized mice. No IgGs specific to the adhesins or toxins were detected in the control mouse serum samples.
FIG 1.
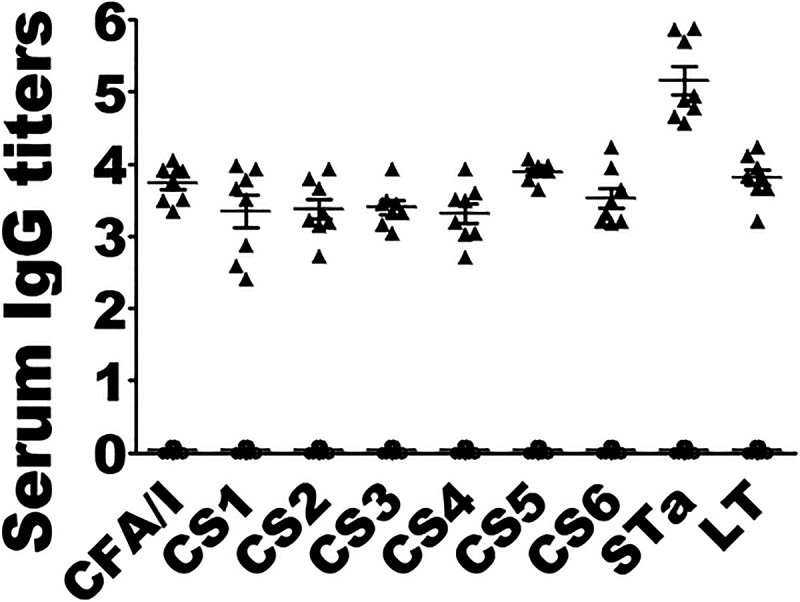
IgG antibody titers (log10) detected in serum of mice i.m. immunized with MecVax or control mice. Serum samples from each mouse in two groups, one i.m. immunized with MecVax (black triangles; n = 8) and the other with PBS as the control (open circles; n = 8), were titrated in ELISAs using fimbrial adhesin CFA/I, CS1, CS2, CS3, CS4, or CS5, recombinant CS6 subunit protein CssA, CT, or STa-ovalbumin conjugate as the ELISA coating antigen. Each triangle or circle represents an IgG titer from an individual mouse, and error bars indicate mean titers and standard deviations in the group.
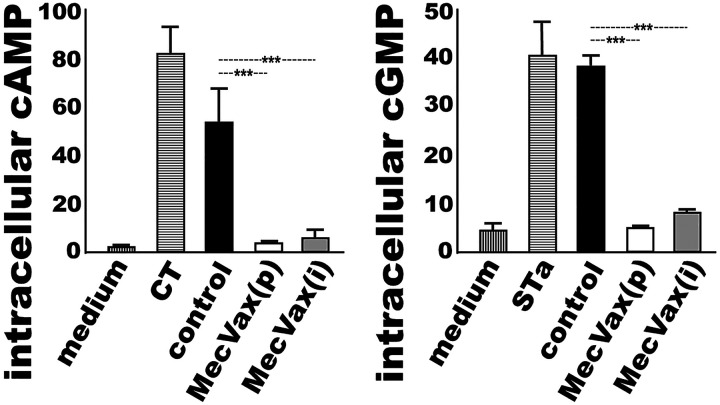
MecVax-induced mouse antibodies neutralized STa and CT enterotoxicity.
Serum samples from the mice i.m. immunized with MecVax neutralized cholera toxin (CT; an LT homologue produced by Vibrio cholerae) and STa enterotoxicity. Neither STa toxin nor CT was able to elevate intracellular cyclic GMP (cGMP) or AMP (cAMP) levels in T-84 cells after being incubated with the serum samples from the immunized mice (Fig. 2). Since STa toxin stimulates cGMP and CT elevates cAMP in T-84 cells, MecVax-induced anti-STa and anti-LT antibodies, if they are neutralizing, can prevent STa or CT from stimulating increased cGMP or cAMP levels. STa toxin, after being treated with the heat-inactivated serum samples of the immunized mice (pooled from the entire group or individual mouse samples randomly selected from five mice in the group), did not elevate cGMP in T-84 cells (Fig. 2, right). The intracellular cGMP levels were 4.9 ± 0.62 (nM, or picomole per ml) in the T-84 cells treated with STa exposed to the pooled serum sample and 7.9 ± 1.52 (nM) in the cells treated with STa exposed to the individual mouse serum sample. These cGMP levels were significantly lower than those in T-84 cells incubated with STa incubated with the control mouse serum (38.3 ± 3.09 nM with the pooled control serum and 37.5 ± 5.5 nM with the individual control mouse serum samples; P < 0.001).
FIG 2.
Mouse serum antibody neutralization activities against CT or STa enterotoxicity, measured with cAMP or cGMP EIA kit. Intracellular cAMP (left) or cGMP (right) concentrations (nM; picomole per ml) in the T84 cells were measured after incubation with CT or STa toxin preexposed to serum samples of the control mice or the immunized mice, either pooled from the group [MecVax(p)] or obtained from five individual mice [MecVax(i); n = 5]. Two additional treatments, T84 cells incubated with cell culture medium alone to establish cAMP or cGMP baseline level and cells incubated with CT alone or STa alone as the positive control to show elevation of cAMP or cGMP by CT or STa toxin, were also included. Bars and error bars represent the mean cAMP or cGMP values and standard deviations. ***, P < 0.001.
CT, after being incubated with the serum samples of the immunized mice (pooled or individual mouse serum), showed no elevation of cAMP in T-84 cells (Fig. 2, left). The cAMP levels in T-84 cells were 3.0 ± 0.28 (nM) and 5.3 ± 4.4 (nM) after treatment of CT with the pooled serum sample or the individual mouse serum samples from the immunized group. These cAMP levels were significantly lower than 53.1 ± 20.52 nM or 48.3 ± 25.1 nM (P < 0.001), the cAMP levels in cells incubated with CT exposed to the pooled or individual mouse serum samples of the control group.
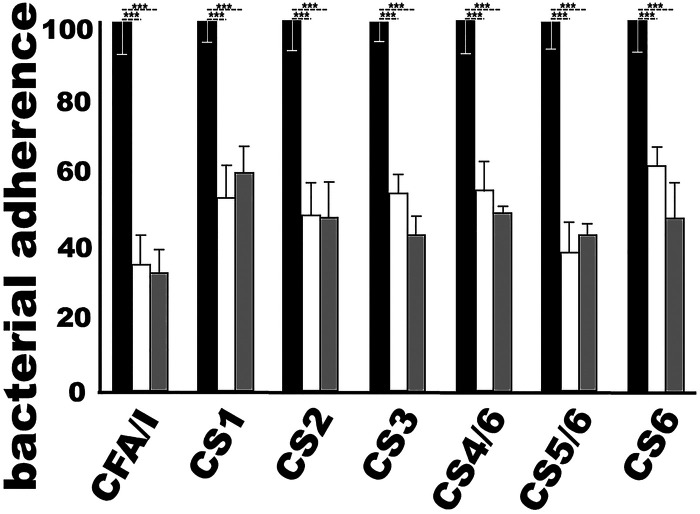
MecVax-induced mouse antibodies inhibited adherence of E. coli expressing CFA/I and CS1 to CS6 adhesins in vitro.
The serum samples of the immunized mice inhibited adherence of E. coli or ETEC strains expressing the seven adhesins (CFA/I and CS1 to CS6) to Caco-2 cells (Fig. 3). Bacterial strains H10407 (CFA/I), THK38/pEU405 (CS1), DH5α/pEU588 (CS2), E116 (CS3), E106 (CS4/CS6), UM75688 (CS5/CS6), and 2423 ETP98066 (CS6) incubated with the immunized mouse serum samples (heat inactivated), either pooled from the group or individual serum samples of five randomly selected mice, showed significant reductions (P < 0.001) in adherence to Caco-2 cells compared to bacteria incubated with the control mouse serum samples. Bacterial adherence was reduced by 40 to 66% or 52 to 68% (subtraction of an adherent percentage from 100%) when treated with the pooled or individual mouse serum samples from the immunized group.
FIG 3.
Mouse serum antibody adherence inhibition against ETEC expressing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 or recombinant E. coli bacteria producing CS1 or CS2 adhesin. Bacterial adherence (%, with CFU adherent to Caco-2 cells after treatment with control serum as 100%) was measured following three treatments: incubation with serum from the control mice (black bars), with serum pooled from the immunized group (i.m. with MecVax) (white bars), or with serum from five individual immunized mice in the MecVax-immunized group (gray bars). Bars indicate the mean percentages of adherent bacterial CFU, and error bars show the standard deviations in the groups. ***, P < 0.001.
MecVax proteins were compatible antigenically and induced levels of antitoxin and antiadhesin antibodies similar to those induced by the toxoid fusion or CFA/I/II/IV MEFA protein individually in mice.
CFA/I/II/IV MEFA protein and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A, two antigens of MecVax, were shown to be compatible antigenically in i.m. administration. Mice i.m. immunized with MecVax or CFA/I/II/IV MEFA protein developed the same IgG titers to CFA/I, CS1, CS2, CS3, CS4, and CS6, with only anti-CS5 IgG being detected at higher titers in the mice immunized with MecVax (P < 0.05). Similarly, mice i.m. immunized with MecVax or toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A developed the same levels of IgGs to STa and LT (Fig. 4).
FIG 4.
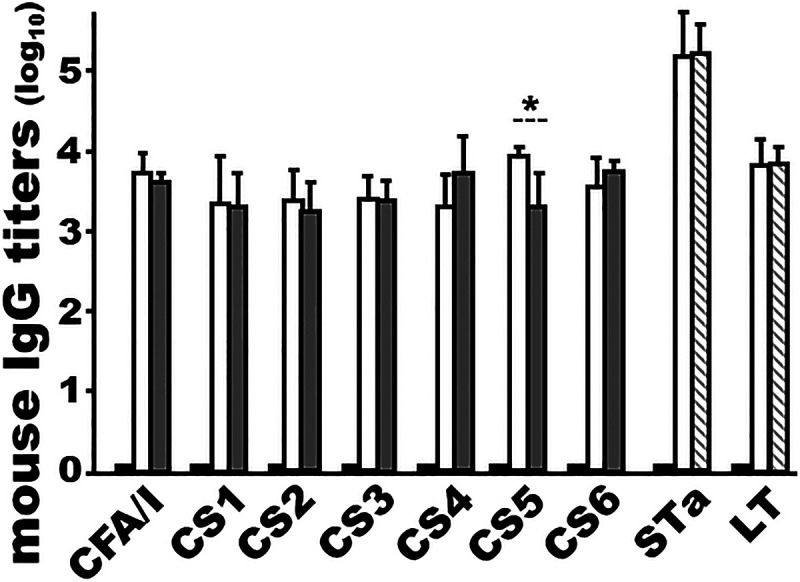
Mouse serum antiadhesin and antitoxin IgG titers (log10) from three groups i.m. immunized with MecVax, CFA/I/II/IV MEFA protein, or toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A to show antigenic compatibility of the two proteins in MecVax. Mice i.m. immunized with MecVax (white bars) or CFA/I/II/IV MEFA protein (gray bars) developed similar titers of IgG to CFA/I, CS1, CS2, CS3, CS4, and CS6 (but not CS5, to which mice immunized with MecVax developed higher IgG titers; P < 0.05). Mice i.m. immunized with MecVax (white bars) and mice i.m. immunized with the toxoid fusion protein (hatched bars) developed the same titers of anti-STa and anti-LT IgGs. The fourth group, mice i.m. immunized with PBS (black bars) as the control, is also included. Bars and error bars represent the mean IgG titers and standard deviations. *, P < 0.05.
MecVax- and CFA/I/II/IV MEFA-induced antibodies were equivalent in protecting from ETEC adherence, and antibodies induced by MecVax or toxoid fusion protein 3xSTaN12S-mnLTR192G/L211R were equally effective at neutralizing toxin enterotoxicity.
Importantly, antibodies induced by MecVax showed levels of adherence inhibition activities similar to those of antibodies derived from CFA/I/II/IV MEFA protein and also displayed the same neutralization activities against STa and CT enterotoxicity as antibodies elicited by toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A. Serum samples from the mice i.m. immunized with MecVax and from the mice immunized with CFA/I/II/IV MEFA protein exhibited the same levels of antibody adherence inhibition activities against adhesins CFA/I, CS2, CS3, CS4, and CS6; MecVax-induced antibodies had greater adherence inhibition activity against CS1 and CS5, as shown by fewer bacteria adhering to Caco-2 cells (Fig. 5).
FIG 5.
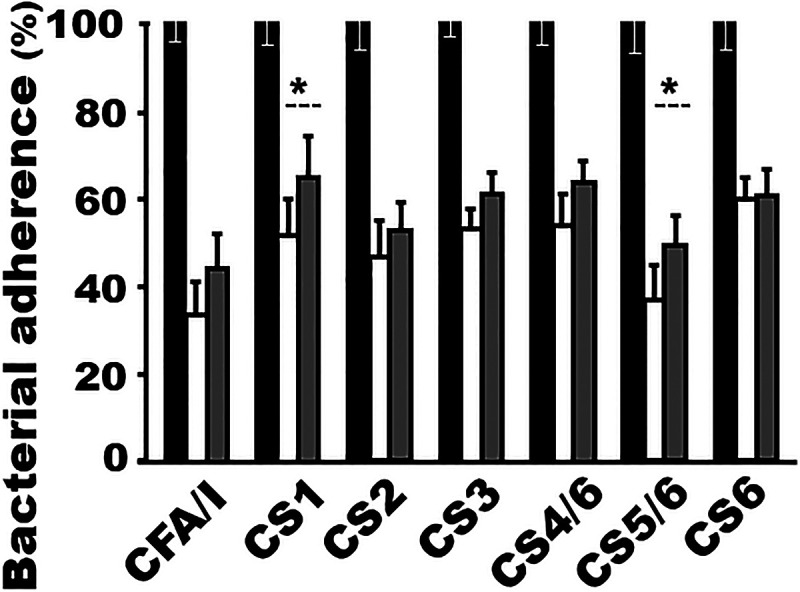
Mouse serum antibody adherence inhibition assays show similar levels of in vitro protection by MecVax- or CFA/I/II/IV MEFA-induced antibodies against adherence of CFA/I and CS1 to CS6 adhesins. Percentages of ETEC or E. coli bacteria (CFU) expressing CFA/I, CS1, CS2, CS3, CS4/CS6, CS5/CS6, or CS6 that were adherent to Caco-2 cells were compared after incubation with serum samples from three groups of mice: i.m. immunized with MecVax (white bars), with CFA/I/II/IV MEFA protein (gray bars), or with PBS (black bars) as the control. Bars represent the mean percentages of adherent bacterial CFU, and error bars the standard deviations. *, P < 0.05.
Data from cGMP and cAMP ELISAs showed that serum samples from the mice i.m. immunized with MecVax or from the mice immunized with toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A had the same levels of neutralization activities against STa or CT enterotoxicity (Fig. 6).
FIG 6.
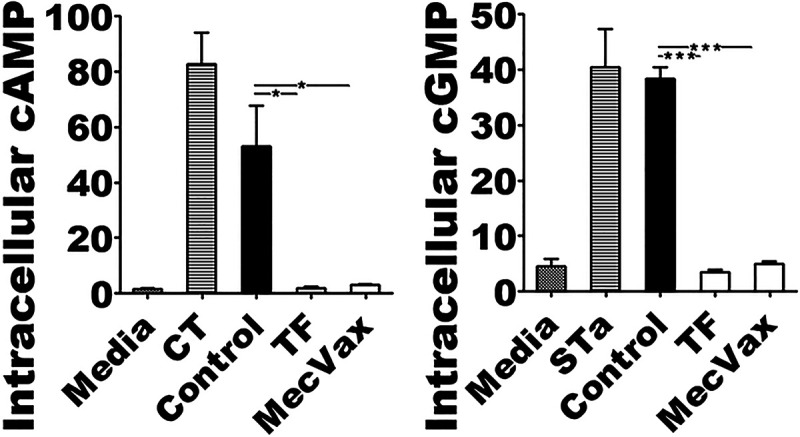
cAMP and cGMP EIA ELISAs show the same levels of in vitro protection against CT or STa enterotoxicity by serum antibodies of mice immunized with MecVax or toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A. Intracellular cAMP or cGMP levels (nM) were measured in T-84 cells exposed to CT or STa that was pretreated with serum samples from mice i.m. immunized with MecVax (MecVax), toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A (TF), or PBS (Control). Two more treatment groups were included, T-84 cells exposed to culture medium alone (Media) to establish the baseline cAMP and cGMP levels and T-84 cells exposed to CT alone (CT) or STa toxin alone (STa) as the positive control to show elevation of intracellular cAMP or cGMP by CT or STa enterotoxicity. Bars and error bars represent the mean cAMP and cGMP values and standard deviations. *, P < 0.05; ***, P < 0.001.
MecVax induced antiadhesin and antitoxin IgGs and IgAs in the i.m. immunized pigs.
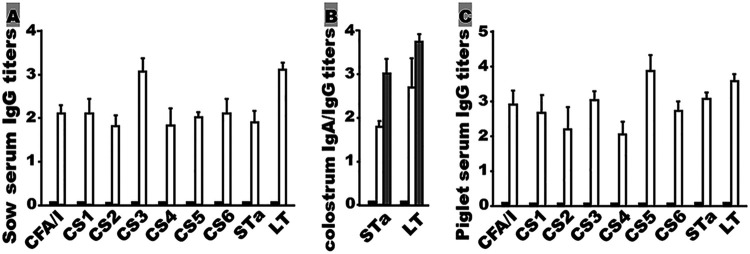
Pregnant gilts i.m. immunized with MecVax appeared healthy and developed antibodies to each target ETEC adhesin and toxin (Fig. 7A and B). IgGs to CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, STa, and LT were detected at titers of 2.09 ± 0.17, 2.08 ± 0.34, 1.81 ± 0.24, 3.04 ± 0.31, 1.81 ± 0.40, 2.00 ± 0.13, 2.08 ± 0.35, 1.88 ± 0.26, and 3.11 ± 0.16 (log10), respectively, in the serum samples of the immunized pigs (Fig. 7A). IgGs and IgAs were detected in the colostrum samples of the immunized sows (antiadhesin IgGs and IgAs were not examined due to the limited amount of colostrum samples collected). Anti-STa and anti-LT IgG titers of 3.01 ± 0.35 and 3.76 ± 0.1 (log10), respectively, and anti-STa and anti-LT IgA titers of 1.78 ± 0.14 and 2.70 ± 0.68 (log10), respectively, were detected in the colostrum samples of the immunized sows (Fig. 7B). No antiadhesin or antitoxin IgG or IgA responses were detected from the serum or colostrum samples of the control gilts.
FIG 7.
Antigen-specific IgG or IgA antibody titers (log10) from sow serum, sow colostrum, and piglet serum samples. (A) Anti-CFA/I, -CS1, -CS2, -CS3, -CS4, -CS5, -CS6, -STa, and -LT IgG titers detected in the serum samples of two groups: control gilts (black bars) or gilts i.m. immunized with MecVax (white bars). (B) Antitoxin IgG (gray bars) and IgA (white bars) titers detected in the colostrum samples of immunized gilts or control gilts (black bars). (C) Anti-CFA/I, -CS1, -CS2, -CS3, -CS4, -CS5, -CS6, -STa, and -LT IgG titers from the sera of the suckling piglets born to control mothers (black bars) or to mothers i.m. immunized with MecVax (white bars). Bars indicate the mean IgG or IgA titers, and error bars the standard deviations.
Antigen-specific IgGs were detected only from the serum samples of the suckling piglets born to the immunized mothers (Fig. 7C). Anti-CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, STa, and LT IgGs were detected at titers of 2.90 ± 0.42, 2.65 ± 0.55, 2.18 ± 0.69, 3.01 ± 0.27, 2.01 ± 0.37, 3.87 ± 0.48, 2.71 ± 0.27, 3.07 ± 0.20, and 3.55 ± 0.20 (log10), respectively. No antigen-specific antibody responses were detected from the serum of the piglets born to the control mothers.
MecVax-induced pig antibodies inhibited in vitro adherence of E. coli expressing CFA/I and CS1 to CS6 adhesins and neutralized STa and CT enterotoxicity.
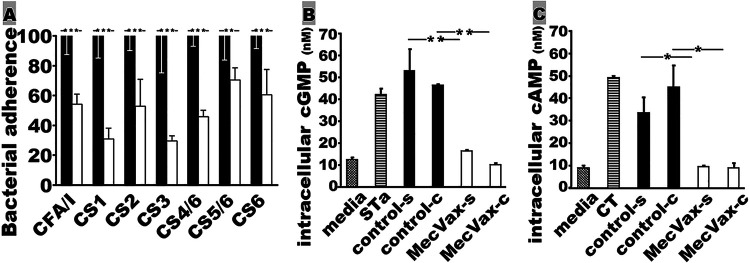
Serum samples from the gilts i.m. immunized with MecVax showed in vitro protection against ETEC adherence and enterotoxicity of STa and CT (Fig. 8). After incubation with the serum samples of the immunized sows, E. coli or ETEC bacteria expressing CFA/I, CS1, CS2, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin had significant reductions in adherence to Caco-2 cells (Fig. 8A). Bacterial adherence was reduced by 30% to 70% (against strains producing different adhesins) by the serum samples of the immunized pigs compared to the adherence after incubation with the control pig serum samples.
FIG 8.
MecVax-induced antibody in pigs for in vitro protection against ETEC or E. coli adherence and CT or STa enterotoxicity. (A) Percentages of ETEC field isolates (CFU) expressing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 or of recombinant E. coli bacteria producing CS1 or CS2 that were adherent to Caco-2 cells after treatment with serum samples from control gilts (black bars) or gilts i.m. immunized with MecVax (white bars). (B) Intracellular cGMP levels (nM; picomole/ml) in T-84 cells exposed to cell culture medium (media) as the baseline control, STa toxin alone (2 ng) (STa) as the positive control, STa toxin pretreated with control gilt serum (control-s) or colostrum (control-c) samples, or STa toxin pretreated with MecVax-immunized-gilt serum (MecVax-s) or colostrum (MecVax-c) samples. (C) Intracellular cAMP levels (nM) in T-84 cells exposed to cell culture medium only (media) as the baseline control, CT toxin alone (10 ng) (CT) as the positive control, CT toxin pretreated with control gilt serum (control-s) or colostrum (control-c) samples, or CT toxin pretreated with MecVax-immunized-gilt serum (MecVax-s) or colostrum (MecVax-c) samples. Bars and error bars represent the mean values and standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The serum or colostrum samples from the immunized pigs neutralized STa and CT enterotoxicity. The intracellular cGMP levels in T-84 cells incubated with STa which was pretreated with the serum or colostrum samples of the immunized gilts were 16.3 ± 0.51 and 10.0 ± 1.52 (nM), respectively. These cGMP levels were significantly lower than the cGMP levels in T-84 cells incubated with STa pretreated with the control serum (52.8 ± 14.4 nM; P < 0.01) or the control colostrum (45.9 ± 1.20 nM; P < 0.01) (Fig. 8B). The intracellular cAMP levels in T-84 cells incubated with CT pretreated with the serum or colostrum samples of the immunized gilts were 9.8 ± 1.36 and 8.7 ± 3.31 (nM), respectively. These cAMP levels were not different from the baseline (9.2 ± 1.68 nM) but were significantly lower than the cAMP levels in cells incubated with CT which was pretreated with the control serum (33.7 ± 10.7 nM; P < 0.05) or the control colostrum (44.1 ± 14.2 nM; P < 0.05) samples (Fig. 8C).
MecVax-induced antibodies protected piglets from STa+ or LT+ ETEC diarrhea.
Thirty-four piglets born to three immunized mothers and 33 piglets born to three control mothers were randomly divided into two groups and challenged with an STa-positive ETEC strain and an LT-positive ETEC strain, respectively. After orogastric inoculation with STa-positive (STa+) ETEC strain 8823 (987P/STa), 17 of 20 (85%) piglets born to the control mothers developed watery diarrhea within 24 h postinoculation. In contrast, none of the 20 piglets born to the immunized mothers developed watery diarrhea and only 5 piglets (25%) showed mild diarrhea after inoculation with the STa strain. The efficacy of MecVax-induced antibodies against STa+ ETEC diarrhea was 100% against watery diarrhea and 70.5% against any diarrhea (P < 0.001) (Table 1). When challenged with LT+ ETEC strain 8819 (987P/LT), 11 of 13 piglets (84.6%) born to the control mothers developed mild to watery diarrhea. In contrast, only one of 14 piglets (7.1%) born to the immunized gilts showed mild diarrhea during 24 h postinoculation. The efficacy against LT+ ETEC diarrhea was 100% against watery diarrhea and 91.6% against any diarrhea (P < 0.001). Diarrheal piglets had clear to light-yellowish fluid accumulated in the small intestines at the time of necropsy.
TABLE 1.
A pig passive protection model to show clinical outcomes and MecVax vaccine preclinical efficacy against ST+ or LT+ ETEC diarrheaa
| Type of challenge, treatment group | No. of piglets with outcome/total no. in group (%)b |
% efficacy againstb,c: |
Daily wt gain (%)d | |||
|---|---|---|---|---|---|---|
| Normal | Mild diarrhea | Watery diarrhea | Any diarrhea | Watery diarrhea | ||
| STa+ ETEC | ||||||
| Control | 3/20 (15) | 7/20 (35) | 10/20 (50) | 3.0 ± 5.5 | ||
| MecVax | 15/20 (75) | 5/20 (25) | 0/20 (0) | 70.6 | 100 | 9.6 ± 3.0* |
| LT+ ETEC | ||||||
| Control | 2/13 (15.4) | 4/13 (30.8) | 7/13 (53.8) | 4.96 ± 4.42 | ||
| MecVax | 13/14 (92.9) | 1/14 (7.1) | 0/14 (0) | 91.6 | 100 | 12.1 ± 6.0** |
aPregnant gilts were i.m. immunized with MecVax adjuvanted with dmLT; piglets born to the control or to the immunized mothers were orally challenged with an STa+ 8823 (987P/STa) or an LT+ 8819 (987P/LT) ETEC strain.
bMild diarrhea is defined as yellow-stained butt or pasty feces; watery diarrhea is defined as liquid stool, wet butt, and noticeable dehydration.
cEfficacy = [(% with diarrhea in control group − % with diarrhea in immunized group)/% with diarrhea in control group] × 100.
dAsterisks indicate significant differences between the immunized groups and the control group: *, P < 0.05; **, P < 0.01.
The daily rates of weight gain for the piglets born to immunized mothers during 24 h postinoculation with the STa+ or the LT+ ETEC strain were 9.6% ± 3.0% and 12.1% ± 6.0%, respectively. These rates were significantly higher than those in the piglets born to the control gilts after STa+ ETEC (3.0% ± 5.5%; P < 0.05) or LT+ ETEC (4.96% ± 4.42%; P < 0.01) challenge.
DISCUSSION
The results from the current preclinical study show that MecVax, an injectable vaccine carrying multivalent proteins CFA/I/II/IV MEFA and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A, is broadly immunogenic and cross protective. Because MecVax-induced antibodies inhibited the adherence of the seven most important ETEC adhesins (CFA/I and CS1 to CS6), neutralized the enterotoxicity of both toxins (LT, STa), and more importantly, prevented ETEC diarrhea in a pig challenge model, MecVax is expected to protect against diarrhea caused by ETEC strains producing any of these seven adhesins (CFA/I and CS1 to CS6) by inducing antiadhesin antibodies and antitoxin antibodies and, likely, to also protect against ETEC strains that produce STa and/or LT toxin but adhesins other than CFA/I and CS1 to CS6 by inducing antitoxin antibodies. Since ETEC strains producing adhesins CFA/I and CS1 to CS6 are responsible for over 60% of clinical cases of ETEC diarrhea (and the moderate to severe cases) and ETEC strains delivering STa and/or LT toxin cause all ETEC diarrheal cases, this marks MecVax as potentially a truly cross-protective vaccine against ETEC diarrhea.
Currently, no other ETEC vaccine candidates have been demonstrated to induce protective antibodies against these seven adhesins (CFA/I and CS1 to CS6) and, particularly, STa toxin. Protecting against STa toxin becomes essential since ETEC strains producing STa toxin play a more important role in ETEC-associated children’s diarrhea and travelers’ diarrhea. Other leading ETEC vaccine candidates under investigation include whole-cell products and are given orally with the hope of inducing intestinal mucosal immunity against ETEC diarrhea. These candidates consist of a mixture of live attenuated or killed whole-cell bacteria expressing up to six of the seven adhesins and LTB subunit or an LTB-derived protein (24, 26, 37). While whole-cell vaccines that induce intestinal mucosal immunity are desirable for enteric diseases, including ETEC, they often provide limited protection to children aged less than 5 years in countries of endemicity. Whole-cell products often carry excessive somatic antigens, including endotoxins; they tend to elicit lower immune responses to the target virulence factors and are linked to reactogenicity in young children. Indeed, protection by ETEC whole-cell vaccine candidates in young children in regions of endemicity is yet to be demonstrated. In contrast, protein-based subunit vaccines are defined and precisely target virulence determinants and pathogens, and thus, they are safer and, with an adjuvant, induce robust antigen-specific host immunity (systemic IgG mainly, and mild secretory IgA), not only in adults but also in children, even very young children living in developing countries. Moreover, a parenteral vaccine can stimulate mucosal immunity (38–41). It is expected that MecVax can potentially work in concert with local intestinal immunity resulting from natural exposure to ETEC bacteria. Children in regions of ETEC endemicity are exposed to ETEC early in life (42, 43). If immunized prior to natural exposure, MecVax protects from severe ETEC infection, and if immunized after natural exposure, MecVax will boost the local immune system. Surely, the efficacy of MecVax against ETEC diarrhea in children can only be evaluated by future clinical studies.
Importantly, this study demonstrated that the two proteins carried by MecVax are compatible antigenically. Mixing CFA/I/II/IV MEFA protein with toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A did not compromise the antigenicity of either antigen. MecVax and the CFA/I/II/IV MEFA protein, when i.m. administered, mounted similar levels of antiadhesin antibody responses in mice. Similarly, MecVax and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A elicited the same levels of antitoxin antibody responses. Moreover, the antibodies induced (by MecVax or an individual protein) showed similar levels of in vitro protection against bacterial adherence and against enterotoxicity of STa or CT. We also noted that MecVax and the toxoid fusion protein induced similar levels of anti-LT and anti-STa IgGs and IgAs in the i.m. immunized pigs and protected piglets equally from diarrhea caused by STa+ or LT+ ETEC infection. It was shown that piglets born to the mothers i.m. immunized with the toxoid fusion had levels of protection of 93% to 100% against STa+ ETEC-associated watery diarrhea, 65% against any STa+ ETEC-associated diarrhea, and 86% against any LT+ ETEC diarrhea (35, 36), mirroring MecVax’s efficacy from the current pig challenge study, which was 100% against STa+ ETEC watery diarrhea, 70.5% against any STa+ ETEC diarrhea, and 91.6% against any LT+ ETEC diarrhea.
This pig passive protection model used in this study, by immunizing pregnant sows with a vaccine candidate and challenging the born suckling piglets with ETEC strains, allows us to evaluate MecVax-induced antitoxin (but not antiadhesin) antibodies against ETEC diarrhea. ETEC adhesins are species specific, as pigs and humans express different receptors for different ETEC adhesins. The STa+ and LT+ ETEC challenge strains used in this study express porcine-type adhesin 987P and a human-type toxin (STa or LT; structurally and functionally highly homologous to porcine-type STa or LT) and, thus, colonize pig small intestines and cause diarrhea. This pig model, however, is unable to evaluate MecVax-induced antiadhesin (CFA/I and CS1 to CS6) antibodies for protection against colonization by the 987P fimbria challenge strains in pig small intestines (nor colonization by strains expressing CFA/I and CS1 to CS6, since ETEC strains expressing these adhesins cannot colonize pig small intestines). By combining the pig passive protection model with a rabbit colonization model, in which we i.m. immunize adult rabbits with MecVax and then orogastrically challenge rabbits with ETEC strains to assess the efficacy of vaccine-induced antibodies against ETEC colonization in the small intestines, we should be able to evaluate MecVax’s efficacy against ETEC colonization (the rabbit colonization model) and diarrhea (the pig passive protection model) in future studies. Additionally, future studies to directly immunize young pigs with MecVax and challenge the immunized pigs with an LT-positive ETEC strain will allow us to evaluate vaccine-induced active immunity against LT+ ETEC diarrhea. Unfortunately, because pigs develop age-associated resistance to STa toxin (but not to LT toxin), active immunity protection from STa-mediated diarrhea will not be assessed. Also, since over a quarter to a third of ETEC strains associated with clinical diarrhea produce two toxins, future challenge studies with an STa+ and LT+ ETEC strain should be informative.
The current data show that MecVax-induced antiadhesin and antitoxin IgGs in mouse or pig serum samples are protective against ETEC adherence and enterotoxicity. Serum IgGs were demonstrated to correlate to protection against shigellosis, diarrhea caused by Shigella spp. (44). Whether MecVax-induced antiadhesin and antitoxin IgGs are correlates to protection against ETEC diarrhea needs to be further investigated. Additionally, future studies to titrate IgG subtypes may be helpful to better understand the potential correlation between antibody responses and protection against ETEC infection. Though it is also unclear whether MecVax-derived IgGs or IgAs in colostrum played a more important role in protecting piglets from ETEC diarrhea, it may suggest that immunization of mothers could provide another countermeasure against ETEC diarrhea in nursing infants and very young children in particular.
It needs to be pointed out that the adjuvant dmLT (double mutant LTR192G/L211A) was included in MecVax used for i.m. immunization in mice and pigs. The adjuvant dmLT, which is intended as the adjuvant for MecVax in future clinical studies, induces anti-LT antibodies when administered with an ETEC antigen (30). Anti-LT antibodies derived from dmLT adjuvant can contribute additively to MecVax-induced antibodies in in vitro protection against CT enterotoxicity and in vivo protection against diarrhea in piglets when challenged with the LT+ ETEC. However, it was demonstrated that CFA/I/II/IV MEFA protein, with or without dmLT adjuvant, induced antiadhesin antibodies that were equally protective against CFA/I and CS1 to CS6 adherence (30) and that toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A, adjuvanted with dmLT or Freund’s adjuvant, induced the same levels of protective antitoxin antibodies against STa and CT enterotoxicity (34); furthermore, passive antibodies from an LT-STa toxoid fusion protein (adjuvanted with Freund’s adjuvant) protected piglets against STa+ ETEC diarrhea (45). Therefore, we believe that not only the in vitro protection from CFA/I and CS1 to CS6 adherence and STa enterotoxicity but also the in vivo protection from STa ETEC diarrhea in piglets was primarily the result of MecVax-induced antibodies. Further studies, including using a control group immunized with dmLT adjuvant instead of PBS, will allow us to better evaluate the efficacy of MecVax-induced anti-LT antibodies against CT (or LT) enterotoxicity and LT+ ETEC diarrhea.
In conclusion, data from the current study showed that MecVax induced broadly protective antibodies against adherence from ETEC CFA/I and CS1 to CS6 adhesins and enterotoxicity of LT and STa toxins. Moreover, MecVax-induced antibodies were shown to protect against STa+ or LT+ ETEC diarrhea in a pig challenge model. These results suggest that MecVax, a multivalent protein-based injectable product, is a promising ETEC vaccine candidate and should be further investigated with additional preclinical studies and, more importantly, human studies to determine its safety, immunogenicity, and efficacy against ETEC-associated children’s diarrhea and travelers’ diarrhea.
MATERIALS AND METHODS
E. coli strains used for in vitro and in vivo studies.
ETEC and other E. coli strains used in this study are listed in Table 2. ETEC field isolates expressing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin (46) and recombinant E. coli strains expressing CS1 or CS2 adhesin (47, 48) were used for in vitro antibody adherence inhibition assays. Recombinant ETEC strains 8823 and 8819 that produce porcine-type ETEC fimbrial adhesin 987P and STa or LT toxin were used in the pig challenge study (35, 36, 45, 49).
TABLE 2.
Escherichia coli strains used in this study
| Straina | Relevant property(ies) | Source or reference |
|---|---|---|
| H10407 | O78:H11; CFA/I, LT, STa | Johns Hopkins University |
| THK38/pEU405 | CS1 | 47 |
| DH5α/pEU588 | CS2 | 48 |
| E116 (E19446) | CS3, LT, STa | University of Gothenburg |
| E106 (E11881/9) | CS4/CS6, LT, STa | University of Gothenburg |
| UM 75688 | CS5/CS6, LT, STa | Johns Hopkins University |
| 2423 ETP98066 | CS6, LT, STa | 46 |
| 8823 (STa+ ETEC) | 987P/STa | 35 |
| 8819 (LT+ ETEC) | 987P/LT | 35 |
aETEC field isolates expressing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin and recombinant E. coli strains producing CS1 or CS2 adhesin were used in antibody adherence inhibition assays, and recombinant STa+ or LT+ ETEC strains were used in pig challenge studies.
MecVax vaccine and adjuvant.
MecVax consists of two tagless recombinant proteins, CFA/I/II/IV MEFA (15.4 kDa) and toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A (47.6 kDa). CFA/I/II/IV MEFA is an epitope- and structure-based chimeric protein, with the CFA/I major subunit as the backbone to present epitopes from the major units of CFA/II (CS1, CS2, and CS3) and CFA/IV (CS4, CS5, and CS6) (31, 32). Toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A is a peptide carrying an LT mutant monomer (one LTA subunit peptide with mutations at the 192nd and 211th residues, one LTB subunit peptide, and three copies of STa toxoid STaN12S, forming a single peptide) (31, 33). The two proteins were expressed in vector pET28a and E. coli strain BL21 CodonPlus (DE3) and extracted as inclusion body proteins with bacterial protein extraction reagent (B-PER; Thermo Fisher Scientific, Rochester, NY) following the manufacturer’s protocol. The extracted proteins were subsequently solubilized, refolded, and dialyzed by using a one-step protein refolding kit (Novagen, Madison, WI) and then stored in a −20°C freezer. Proteins were thawed on ice and mixed before immunization.
Double mutant LT (dmLT; LTR192G/L211A in the AB5 holotoxin structure), produced by Walter Reed Army Institute of Research (Silver Spring, MD) and supplied by PATH (Washington, DC), was used as the adjuvant in mouse and pig immunization.
Mouse i.m. immunization with MecVax.
A total of 32 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) in four groups with eight mice per group were included for immunization. One group was intramuscularly (i.m.) immunized with MecVax containing 25 μg CFA/I/II/IV MEFA protein and 25 μg toxoid fusion protein 3xSTaN12S-mnLTR192G/L211A in a total volume of 50 μl under a regimen of one primary immunization followed by two boosters at intervals of 2 weeks. The second group and the third group were i.m. immunized with 25 μg CFA/I/II/IV MEFA protein alone or 25 μg toxoid fusion 3xSTaN12S-mnLTR192G/L211A protein alone. By comparatively analyzing antigen-specific antibody responses and antibody protective activities in the first group and the second or the third group, we evaluated MecVax protein antigenic compatibility. The adjuvant dmLT (0.2 μg) was used for each immunization group. The fourth group was i.m. immunized with 50 μl phosphate-buffered saline (PBS) and served as the control group.
Mouse serum antigen-specific antibody titration.
Mouse IgG responses to ETEC adhesins CFA/I, CS1, CS2, CS3, CS4, CS5, and CS6, as well as to STa and LT toxins, were titrated in enzyme-linked immunosorbent assays (ELISAs) as described previously (31, 32, 50). In brief, 2HB microplates (ThermoFisher Scientific) coated overnight with heat-extracted CFA/I, CS1, CS2, CS3, CS4, or CS5 fimbriae, recombinant protein of CS6 subunit CssA, or LT homologue cholera toxin (CT; Sigma, St. Louis, MO), 100 ng per well (in 100 μl), and Costar plates (Corning, Inc., Corning, NY) coated overnight with STa-ovalbumin conjugates, 10 ng per well (in 100 μl), were washed with PBS with 0.05% Tween 20 (PBST), blocked with 5% nonfat milk, and then incubated with serum samples collected from each mouse 2 weeks after the final booster, using 2-fold serial dilutions (1:200 to 1:25,600, in triplicates). Wells were washed with PBST, incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibodies (1:5,000; Bethyl Laboratories, Montgomery, TX) for 1 h at 37°C, washed with PBST, and incubated with TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase substrate (KPL, Gaithersburg, MD). The optical density at 650 nm (OD650) was measured, and antibody titers were calculated with the highest dilution that gave an OD reading above 0.3 after subtraction of the background OD (adjusted OD times the dilution factor) and were expressed in log10 values as described previously (31, 32, 50).
Mouse serum antibody neutralization against toxin enterotoxicity and inhibition against bacterial adherence.
Mouse serum samples were examined for neutralization activities against LT or STa enterotoxicity and adherence inhibition activities against the seven target ETEC adhesins (CFA/I and CS1 to CS6). As described previously (31, 33, 36, 45), antibody neutralization activities against CT (commercially available CT is an LT homologue and is found to be consistent and more effective in stimulating cAMP in T-84 cells) or STa toxin enterotoxicity were measured using T-84 cells (CCL-248; ATCC, Fairfax, VA) with a cyclic AMP (cAMP) kit or a cyclic GMP (cGMP) enzyme immunoassay (EIA) kit (Enzo Life Sciences, Farmingdale, NY). T-84 cells were incubated with 10 ng CT or 2 ng STa that was premixed with 30 μl heat-inactivated (56°C for 30 min) pooled or individual mouse serum samples from the immunized or control group for 3 h (cAMP) or 1 h (cGMP), washed thoroughly (with PBS) to remove extracellular cAMP or cGMP, and lysed with 0.1 M HCl containing 0.5% Triton X-100 to release intracellular cAMP or cGMP. Because a total of nine in vitro antibody protection assays (two for toxins and seven for adhesins, in duplicate and repeated at least once) were carried out, we included a pooled sample and five individual serum samples from each group for in vitro antibody assays in this study to avoid the risk of running out of serum samples. The pooled sample was collected equally from each immunized or control mouse in the group (∼3.8 μl per mouse), and the individual serum samples were from each of the five randomly selected mice (30 μl for each mouse) from each group. T-84 cell intracellular cAMP or cGMP levels were measured using the EIA kit, following the manufacturer’s instructions (Enzo Life). T-84 cells incubated with CT or STa toxin alone (without serum) served as a positive control; cells incubated with cell culture medium (without toxin or serum) were used as the control for baseline cAMP or cGMP.
Antibody adherence inhibition activities against seven ETEC adhesins (CFA/I and CS1 to CS6) were used to measure in vitro protection against ETEC adherence by MecVax-induced antibodies. As described previously (30–32, 45), ETEC or other E. coli strains expressing CFA/I, CS1, CS2, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin (Table 2) were pretreated with the pooled or individual mouse serum samples (15 μl; heat inactivated), added to Caco-2 cells (ATCC HTB-37; multiplicity of infection [MOI] of 10 bacteria per cell), and incubated for 1 h at 37°C in a 5% CO2 incubator. Cells, after gentle but thorough washes with PBS (3 times) to remove nonadherent bacteria, were dislodged and lysed; the adherent E. coli bacteria were serially diluted (in PBS), plated on LB agar plates, and cultured overnight at 37°C to count colonies (CFU). Bacterial adherence was converted to percentage (%) with reference to the CFU counts from Caco-2 cells incubated with the control serum sample, set as 100%. Adherence inhibition or reduction was the subtraction of 100% by the adherence percentage.
MecVax immunization and challenge in a pig passive protection model to evaluate vaccine efficacy against ETEC diarrhea.
A sow immunization and piglet challenge model was used in this study to assess the efficacy of MecVax-induced antibodies against ETEC diarrhea. As described previously (35, 36, 51, 52), six pregnant gilts that had no preexisting antitoxin (LT and STa) antibodies were included in the study. Three gilts were intramuscularly immunized with MecVax at a dose of 250 μg CFA/I/II/IV MEFA mixed with 250 μg 3xSTaN12S-mnLTR192G/L211A protein, adjuvanted with 5 μg dmLT. The primary immunization was administered 6 to 8 weeks before farrowing, and a booster injection followed 4 weeks later. The other three gilts were i.m. immunized with PBS as the control. Serum and colostrum samples were collected from each pregnant gilt just before farrowing, titrated for anti-LT and anti-STa IgGs and IgAs in ELISAs (with secondary antibody goat anti-pig IgG or IgA; Bethyl Laboratories), and examined for antibody neutralization activities against STa or CT enterotoxicity, as described above for mouse studies.
Piglets born to the immunized mothers or the control mothers were randomly divided into two groups, and after 24 h, suckling piglets were challenged with STa-producing strain 8823 (987P/STa) or LT-producing strain 8819 (987P/LT). As described previously (35, 36, 45, 49), each piglet was inoculated by gavage with 5 × 109 CFU (in 1 ml PBS) of an STa- or LT-positive strain and observed every 2 to 4 h during the 24 h postinoculation for clinical signs including vomiting, diarrhea, dehydration, and lethargy. Diarrhea was recorded as mild if yellow-stained butt or pasty feces was observed or as watery diarrhea when liquid stool, wet butt, or noticeable dehydration were shown. Protective efficacy (%) against watery diarrhea or any diarrhea was calculated as follows: % protective efficacy = [(% with diarrhea in control group − % with diarrhea in immunized group)/% with diarrhea in control group] × 100. Piglets were euthanized 24 h postinoculation, and serum samples were collected from each piglet to be examined for antibodies to each target antigen. At necropsy, piglets were examined for fluid accumulation and inflammation in small intestines. All piglets were weighed prior to challenge and 24 h postinoculation to monitor daily weight change.
Animal immunization (mouse and pig) and challenge (pig) studies complied with the Animal Welfare Act (1996 National Research Council Guidelines) and the USDA Animal Welfare Act Regulations, and protocols were approved by the Institutional Animal Care and Use Committee of Kansas State University and supervised by an institutional attending veterinarian.
Statistical analysis.
Data are presented as mean values and standard deviations. The statistical differences between groups were analyzed using one-way analysis of variance (ANOVA). A post hoc Tukey’s test was used, and a calculated P value of less than 0.05 was set to indicate a significant difference between groups. Significant differences in clinical signs between control and immunized pig groups or levels of protective efficacy were determined with Fisher’s exact test.
ACKNOWLEDGMENTS
We are grateful to James Fleckenstein (Washington University at St. Louis), June Scott (Emory University), and Ann-Mari Svennerholm (Gothenburg University, Sweden) for sharing the ETEC or recombinant E. coli strains used in this study and to Walter Reed Army Institute of Research (via PATH) for providing dmLT adjuvant.
Research described in this article is supported financially by NIH grant number R01AI121067-01A1 and USDA NIFA Agriculture and Food Research Initiative competitive grant no. 2017-67015-31471.
Contributor Information
Weiping Zhang, Email: wpzhang@illinois.edu.
Guy H. Palmer, Washington State University
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosangadi D, Smith PG, Kaslow DC, Giersing BK, WHO ETEC and Shigella Vaccine Consultation Expert Group . 2019. WHO consultation on ETEC and Shigella burden of disease, Geneva, 6 to 7 April 2017: meeting report. Vaccine 37:7381–7390. doi: 10.1016/j.vaccine.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Jiang ZD, DuPont HL. 2017. Etiology of travellers’ diarrhea. J Travel Med 24:S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 5.Knutton S, Lloyd DR, McNeish AS. 1987. Identification of a new fimbrial structure in enterotoxigenic Escherichia coli (ETEC) serotype O148:H28 which adheres to human intestinal mucosa: a potentially new human ETEC colonization factor. Infect Immun 55:86–92. doi: 10.1128/IAI.55.1.86-92.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaastra W, de Graaf FK. 1982. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev 46:129–161. doi: 10.1128/MR.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. doi: 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res 133:188–196. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Sack DA. 2015. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli (ETEC) associated diarrhea. Clin Vaccine Immunol 22:983–991. doi: 10.1128/CVI.00224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laaveri T, Vilkman K, Pakkanen S, Kirveskari J, Kantele A. 2018. Despite antibiotic treatment of travellers’ diarrhoea, pathogens are found in stools from half of travellers at return. Travel Med Infect Dis 23:49–55. doi: 10.1016/j.tmaid.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Tribble DR. 2017. Resistant pathogens as causes of traveller’s diarrhea globally and impact(s) on treatment failure and recommendations. J Travel Med 24:S6–S12. doi: 10.1093/jtm/taw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries RM, Schuetz AN. 2015. Antimicrobial susceptibility testing of bacteria that cause gastroenteritis. Clin Lab Med 35:313–331. doi: 10.1016/j.cll.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang-Latimer J, Jafri S, VanTassel A, Jiang ZD, Gurleen K, Rodriguez S, Nandy RK, Ramamurthy T, Chatterjee S, McKenzie R, Steffen R, DuPont HL. 2011. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrob Agents Chemother 55:874–878. doi: 10.1128/AAC.00739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomi H, Jiang ZD, Adachi JA, Ashley D, Lowe B, Verenkar MP, Steffen R, Dupont HL. 2001. In vitro antimicrobial susceptibility testing of bacterial enteropathogens causing traveler’s diarrhea in four geographic regions. Antimicrob Agents Chemother 45:212–216. doi: 10.1128/AAC.45.1.212-216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker R, Dull P. 2017. Combination vaccine strategies to prevent enteric infections. Vaccine 35:6790–6792. doi: 10.1016/j.vaccine.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 16.WHO. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec 81:97–107. [PubMed] [Google Scholar]
- 17.PATH. 2011. The case for investment in enterotoxigenic Escherichia coli vaccines. PATH, Seattle, WA. [Google Scholar]
- 18.Turunen K, Antikainen J, Laaveri T, Kirveskari J, Svennerholm AM, Kantele A. 2020. Clinical aspects of heat-labile and heat-stable toxin-producing enterotoxigenic Escherichia coli: a prospective study among Finnish travellers. Travel Med Infect Dis 38:101855. doi: 10.1016/j.tmaid.2020.101855. [DOI] [PubMed] [Google Scholar]
- 19.Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, Okhuysen PC, Guerrero NH, Martinez-Sandoval FG, Melendez-Romero JH, Jiang ZD, Asturias EJ, Halpern J, Torres OR, Hoffman AS, Villar CP, Kassem RN, Flyer DC, Andersen BH, Kazempour K, Breisch SA, Glenn GM. 2008. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet 371:2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 20.Svennerholm AM, Tobias J. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vaccines 7:795–804. doi: 10.1586/14760584.7.6.795. [DOI] [PubMed] [Google Scholar]
- 21.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 22.Qadri F, Svennerholm AM, Faruque AS, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 24.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, Wiklund G, Kaim J, Lofstrand M, Carlin N, Bourgeois AL, Maier N, Fix A, Wierzba T, Walker RI, Svennerholm AM. 2020. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis 20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhtar M, Chowdhury MI, Bhuiyan TR, Kaim J, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Begum YA, Sharif MZ, Islam LN, Carlin N, Maier N, Fix A, Wierzba TF, Walker RI, Bourgeois AL, Svennerholm AM, Qadri F, Lundgren A. 2019. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 37:5645–5656. doi: 10.1016/j.vaccine.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner AK, Stephens JC, Beavis JC, Greenwood J, Gewert C, Randall R, Freeman D, Darsley MJ. 2011. Generation and characterization of a live attenuated enterotoxigenic Escherichia coli combination vaccine expressing six colonization factors and heat-labile toxin subunit B. Clin Vaccine Immunol 18:2128–2135. doi: 10.1128/CVI.05345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo H, Duan Q, Zhang W. 2020. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes 11:1486–1517. doi: 10.1080/19490976.2020.1770666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu T, Moxley RA, Zhang W. 2020. Application of a novel epitope and structure vaccinology-assisted fimbria-toxin multiepitope fusion antigen of enterotoxigenic Escherichia coli for multivalent vaccine development against porcine post-weaning diarrhea. Appl Environ Microbiol 86:e00274-20. doi: 10.1128/AEM.00274-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Q, Lee KH, Nandre RM, Garcia C, Chen J, Zhang W. 2017. MEFA (multiepitope fusion antigen)-novel technology for structural vaccinology, proof from computational and empirical immunogenicity characterization of an enterotoxigenic Escherichia coli (ETEC) adhesin MEFA. J Vaccines Vaccin 8:367. doi: 10.4172/2157-7560.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo H, Lu T, Mani S, Bourgeois AL, Walker R, Sack DA, Zhang W. 2020. Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6). Hum Vaccin Immunother 16:419–425. doi: 10.1080/21645515.2019.1649555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Q, Lu T, Garcia C, Yanez C, Nandre RM, Sack DA, Zhang W. 2018. Co-administered tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (multiepitope fusion antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1–CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front Microbiol 9:e1198. doi: 10.3389/fmicb.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan X, Knudsen DE, Wollenberg KM, Sack DA, Zhang W. 2014. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin Vaccine Immunol 21:243–249. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, the STa Toxoid Vaccine Consortium Group . 2014. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun 82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandre R, Ruan X, Duan Q, Zhang W. 2016. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett 363:fnw246. doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandre RM, Duan Q, Wang Y, Zhang W. 2017. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine 35:552–556. doi: 10.1016/j.vaccine.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo H, Lu T, Nandre RM, Duan Q, Zhang W. 2019. Immunogenicity characterization of genetically fused or chemically conjugated heat-stable toxin toxoids of enterotoxigenic Escherichia coli in mice and pigs. FEMS Microbiol Lett 366:fnz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Clements JD, Freytag LC. 2016. Parenteral vaccination can be an effective means of inducing protective mucosal responses. Clin Vaccine Immunol 23:438–441. doi: 10.1128/CVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maciel M, S T, Jr, Kim A, Ward E, Villar Z, Lee TK, Jaep K, Porter C, Poole S, Prouty MG. 2019. Serological and α4β7+ antibody-secreting cell responses after intramuscular immunization with CssBA, a CS6-subunit based of enterotoxigenic E. coli vaccine candidate, and LT(R192G/L211A) as adjuvant. Abstr Vaccines Enteric Dis VED 2019, Lausanne, Switzerland, 16 to 18 October 2019.
- 40.Sundararajan A, Sangster MY, Frey S, Atmar RL, Chen WH, Ferreira J, Bargatze R, Mendelman PM, Treanor JJ, Topham DJ. 2015. Robust mucosal-homing antibody-secreting B cell responses induced by intramuscular administration of adjuvanted bivalent human norovirus-like particle vaccine. Vaccine 33:568–576. doi: 10.1016/j.vaccine.2014.09.073. [DOI] [PubMed] [Google Scholar]
- 41.Su F, Patel GB, Hu S, Chen W. 2016. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccin Immunother 12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, McMurry TL, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, McCormick BJJ, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators. 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm A-M. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen D, Meron-Sudai S, Bialik A, Asato V, Goren S, Ariel-Cohen O, Reizis A, Hochberg A, Ashkenazi S. 2019. Serum IgG antibodies to Shigella lipopolysaccharide antigens—a correlate of protection against shigellosis. Hum Vaccin Immunother 15:1401–1408. doi: 10.1080/21645515.2019.1606971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Q, Qadri F, Kansal R, Rasko DA, Sheikh A, Fleckenstein JM. 2015. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 9:e0003446. doi: 10.1371/journal.pntd.0003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Casal J, Swartley JS, Scott JR. 1990. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect Immun 58:3594–3600. doi: 10.1128/IAI.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froehlich BJ, Karakashian A, Sakellaris H, Scott JR. 1995. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun 63:4849–4856. doi: 10.1128/IAI.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Robertson DC, Zhang C, Bai W, Zhao M, Francis DH. 2008. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl Environ Microbiol 74:5832–5837. doi: 10.1128/AEM.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan X, Sack DA, Zhang W. 2015. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS One 10:e0121623. doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nandre R, Ruan X, Lu T, Duan Q, Sack D, Zhang W. 2018. Enterotoxigenic Escherichia coli adhesin-toxoid multiepitope fusion antigen CFA/I/II/IV-3xSTaN12S-mnLTR192G/L211A-derived antibodies inhibit adherence of seven adhesins, neutralize enterotoxicity of LT and STa toxins, and protect piglets against diarrhea. Infect Immun 86:e00550-17. doi: 10.1128/IAI.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, Francis DH. 2010. Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin b (STb) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STb antibodies. Clin Vaccine Immunol 17:1223–1231. doi: 10.1128/CVI.00095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]