Abstract
Organoid technologies enable the creation of in vitro physiologic systems that model tissues of origin more accurately than classical culture approaches. Seminal characteristics, including three-dimensional structure and recapitulation of self-renewal, differentiation, and disease pathology, render organoids eminently suited as hybrids that combine the experimental tractability of traditional 2D cell lines with cellular attributes of in vivo model systems. Here, we describe recent advances in this rapidly evolving field and their applications in cancer biology, clinical translation and precision medicine.
Introduction
Over the past decades, preclinical cancer biology studies have commonly relied on immortalized 2D cancer cell lines in vitro, as well as xenografted or transgenic animal models1. Although these approaches have contributed enormous insights, extensively passaged cell lines may not accurately represent the biology and pathophysiology of the original parent tumor, and animal models are costly and time consuming.
Alternatively, newly developed 3D organoid methods now facilitate the robust culture of healthy human tissues and their cognate tumors, thus representing an independent in vitro approach to studying cancer. To be classified as an organoid, the culture should retain the identity of the modeled organ, contain multiple cell types, preserve some physiological aspects of the organ and self-organize according to the same principles as the organ2,3. Furthermore, organoids maintain several properties of primary tissues, such as self-renewal, multilineage differentiation, signaling nodes and histology. Once established, organoids can often be cultured long-term, expanded, cryopreserved, and genetically manipulated similar to traditional 2D cell lines. As such, organoids combine the tractability of in vitro systems with the 3D architecture and differentiation of in vivo model organisms. Accordingly, recent years have witnessed an exponential growth in the use of organoids as a replacement for immortalized cell lines, allowing the use of primary human cells for the in vitro modeling of biological processes, including normal physiology, stem cell biology and diverse pathophysiologic states. As relevant to this review, the singular attributes of organoids have led to their rapid adoption as powerfully exploitable cancer models.
Organoid methodologies
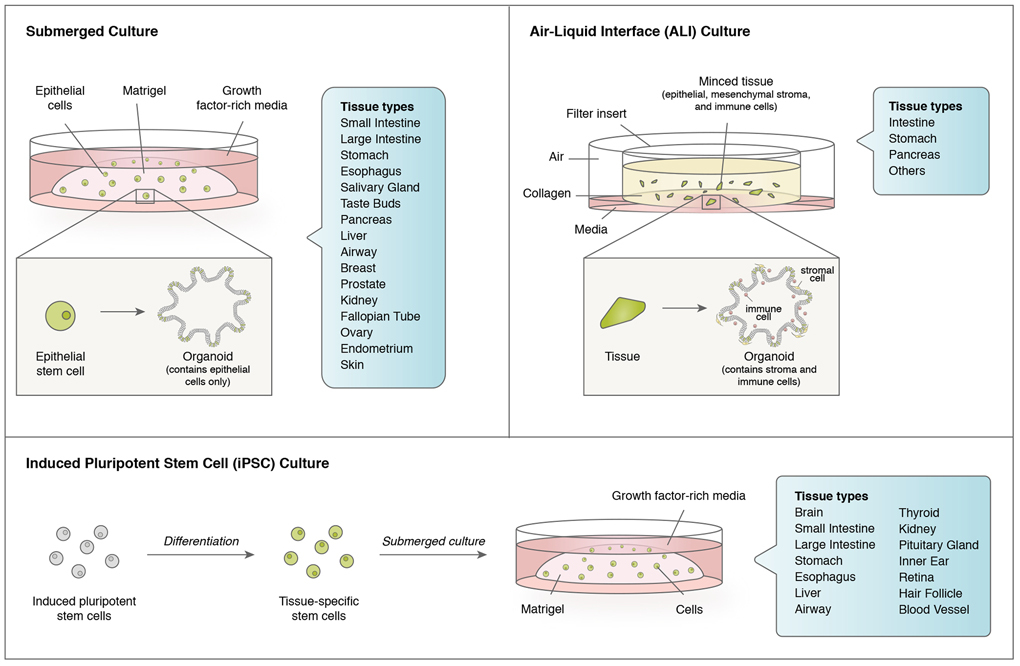
In general, organoids can be classified not only by their tissue of origin, but also by their generation from adult tissues4,5 versus embryonic stem cells6. Organoids can be further distinguished by whether they solely contain epithelium4, or also possess stromal cells5. These distinct classes of organoids can be reproducibly generated by correspondingly diverse techniques (Figure 1).
Figure 1. Organoid methodologies.
Currently employed organoid methodologies include submerged culture, which typically solely includes epithelial cells, and air-liquid interface (ALI) culture, which is a more organotypic method including epithelial cells alongside integrated stroma and immune cells. As opposed to submerged and ALI methods which utilize adult tissues as starting material, organoids can be generated from uncommitted induced pluripotent stem cells (iPSCs) by a series of differentiation steps resulting in generation of the desired tissue type, often with accompanying stroma. Organoids can be additionally classified by their tissue of origin for all three methods.
Submerged culture.
Many seminal organoid studies utilized intestine as starting material with growth within solid gels of extracellular matrix (laminin-rich Matrigel or equivalents, such as BME-2), submerged beneath tissue culture media. Intestinal organoids can be established from either purified adult small intestinal crypts or sorted single LGR5+ intestinal stem cells (ISCs) in an elegant growth factor-defined culture condition that mimics the stem cell niche4. This culture condition generally includes Wnt pathway ligands such as Wnt3a and/or R-spondin, epidermal growth factor (EGF), and the bone morphogenetic (BMP) inhibitor Noggin that allow ISCs to undergo long-term self-renewal and differentiation into all cell lineages4. As a result, the ISCs form highly polarized 3D epithelial structures that represent intestinal crypt-villus compartments. Variations of this submerged organoid culture system use specialized growth factor cocktails adapted for many different organs, including small and large intestine4,7-9, stomach10,11, esophagus9,12, salivary gland13,14, taste buds15, pancreas16-19, liver20-23, airway24-27, breast28, prostate29,30, kidney31, fallopian tube32, ovary33,34, endometrium35 and skin36. As discussed below, this method has also been used extensively for cancer modeling and to grow tumor biopsies. Because of the necessity for cellular disaggregation during processing, engendering deleterious Rho kinase (ROCK)-dependent anoikis as well as stress37 and injury responses38, ROCK inhibitors have greatly increased the efficiency of organoid generation4. Typically, submerged organoids are polarized with the apical surface facing a central lumen, although this polarity can be deliberately inverted39. A defining characteristic of submerged organoids is their possession of exclusively epithelial cells, and absence of stroma.
Air-liquid interface (ALI).
A parallel method utilizes air-liquid interface (ALI) culture to grow organoids that contain both epithelial cells and surrounding stroma as a cohesive unit directly from tissue fragments. In ALI, mechanically dissociated tissues are grown into a type I collagen matrix on top of an inner transwell insert with culture medium provided through a permeable membrane5,40,41. The top of the transwell directly contacts air, thereby facilitating oxygen diffusion42, which may underlie the ability of ALI to grow large multicellular organoids that preserve native tissue architecture, such as epithelium, en bloc with endogenous stromal cells without reconstitution. In ALI, the stromal cells are sufficient to support organoid growth without growth factor supplementation, likely by producing essential endogenous niche factors5. The ALI method grows intestine, stomach and pancreas organoids as cystic structures containing both epithelium and mesenchymal stroma, displaying expansile growth, stem cell populations, multilineage differentiation and even intestinal peristalsis5,41,42. These features render ALI a complementary method to submerged organoids, particularly for inclusion of stromal populations, which has been exploited for cancer oncogene engineering41 and tumor microenvironment modeling28 as detailed below.
Induced pluripotent stem cells (iPSC) or embryonic stem cells (ESC).
In contrast to submerged and ALI methods, the generation of iPSC-derived organoids obligately entails directed differentiation to the target tissue of interest. Diverse iPSC-derived organoids have been described, such as brain43-46, small and large intestine6,47, stomach48,49, esophagus50, liver51, airway52-55, thyroid56,57, kidney58,59, pituitary gland60,61, inner ear62-64 , retina65, hair follicle66 and blood vessels67. These efforts have been greatly aided by bespoke tissue-specific differentiation protocols, typically requiring weeks to months, to reprogram iPSCs to the desired organ type. Of note, some tissues, like brain, cannot be grown in submerged or ALI culture, making iPSC organoids a unique platform for studying the timing of development of neural tissues. A salient feature of endodermal iPSC models is the frequent co-development of epithelium alongside mesenchymal stroma6,47-49. Compared with adult cell types, molecular characteristics of iPSCs-derived organoids are more similar to immature fetal cells and in vivo transplantation can enhance maturation68-72. iPSC organoids have been broadly deployed for disease modeling, including cancer, as reviewed elsewhere73,74.
Bottom-up organoid approaches
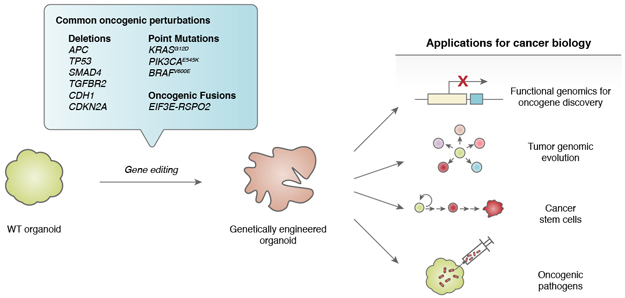
The genetic manipulation of primary wild-type organoids provides a unique opportunity to model human tumor initiation and progression in a tissue-specific fashion that closely recapitulates the oncogenic process (Figure 2). Conventionally, interrogating the function of a candidate oncogene or tumor suppressor in vitro has utilized 2D transformed cell lines, whose underlying genetic complexity both precludes true de novo cancer initiation and may contain significant modifier loci that could mask oncogenic effects. Analogously, in vivo examination of oncogenes and tumor suppressors has required transgenic manipulation, typically in a mouse context. In contrast, systematic introduction of oncogenic loci into wild-type tissue organoids provides an attractive method for “bottom-up” tumor initiation into normal cells possessing a non-mutated genetic background, yielding a hybrid combining the tissue architecture, stem cells and differentiation of in vivo mouse models with the facile genetic manipulation of transformed human cancer cell lines.
Figure 2. Bottom-up cancer modeling in wild-type organoids.
Wild-type tissue organoids can be engineered with tumorigenic alterations to model cancer initiation, evolution, initiating cells and oncogenic pathogens.
This tractability in turn renders wild-type organoids amenable to forward genetic approaches such as oncogene engineering and functional genomics.
Modeling oncogenic loci in wild-type organoids
A first proof of concept for bottom-up organoid modeling of cancer exploited ALI organoids for robust in vitro transformation of adult wild-type intestinal organoids to adenocarcinoma41 Organoids were generated from adult transgenic mice containing floxed alleles, thus allowing combinatorial CreER-mediated deletion of Apc, Tp53 and/or CreER activation of KrasG12D expression, in concert with Smad4 shRNA knockdown. The addition of tamoxifen induced CreER-dependent progressive transformation depending on whether 1, 2, 3 or 4 genetic lesions were present. In vivo growth as tumors required 4 mutations41, recapitulating the classical multihit “Vogelgram” model of colon tumorigenesis75. Thereafter, two independent studies using submerged Matrigel organoids successfully transformed normal human colonic organoids to adenocarcinoma by CRISPR-based sequential engineering of common alterations in colon cancers, including APC−/−, KRASG12V/D, SMAD4−/−, PIK3CAE545K, and TP53−/− 76,77. Importantly, colon organoids with quadruple mutations grew in vivo when implanted subcutaneously into mice, confirming oncogenic transformation76,77. To compensate for the inefficiency of CRISPR, these human organoid studies used selective media to enrich for mutants. For example, APC mutations can be selected for by growing organoids in medium without Wnt/R-spondin, whereas selection for TP53 mutations is possible using medium with the MDM2-P53 complex inhibitor Nutlin-376,77. In addition, SMAD4-mutated organoids can survive in medium lacking Noggin and the transforming growth factor-β (TGFβ) inhibitor A83-01, but containing TGFβ, whereas oncogenic KRASG12V/D and PI3KCAE545K CRISPR knock-in allows organoid growth without EGF76,77.
Engineered human colon organoids can also be used to study premalignant colorectal polyposis78. CRISPR/Cas9-engineered BRAFV600E human colon organoids in combination with TGFβ signaling manipulation induce a mesenchymal phenotype consistent with sessile serrated adenomas (SSA)79, supporting prior hypotheses of BRAFV600E function80. Furthermore, CRISPR/Cas9 introduction of an EIF3E-RSPO2 fusion gene in human colon organoids modeled progression of traditional serrated adenomas (TSA) in vitro with histologic recapitulation of SSA following in vivo orthotopic xenotransplantation81. Superimposed expression of the mesenchymal bone morphogenetic protein antagonist GREM1 in EIF3E-RSPO2; BRAFV600E organoids induced TAS-like morphological aberrations81, consistent with the phenotype of Grem1 transgenic mice82.
The paradigm of oncogenic conversion of colon organoids has now been expanded to additonal studies in microsatellite instability83.
Oncogene engineering studies have rapidly progressed from early work in colorectal cancer (CRC) to many prevalent solid tumor types. In vitro conversion of normal mouse gastric and pancreatic organoids to adenocarcinoma has been achieved by deletion of Tp53 and KrasG12D activation in ALI organoids, thereby allowing in vitro dysplasia and in vivo tumorigenicity upon transplantation and recapitulating features such as desmoplasia and epithelial-mesenchymal transition (EMT)41. Further, Tgfbr2 deletion enhances tumorigenicity of Cdh1−/−; Tp53−/− ALI gastric organoids84. Similar mouse transformation of colon85 and pancreatic16 studies have been performed using submerged Matrigel models. CRISPR/Cas9 oncogene editing of wild-type human organoids has allowed oncogenic conversion of human gastric86, pancreatic 87,88, esophagus89, breast90 and liver91 tissue. In human stomach organoids, deletion of TP53 or CDH1 confers transformation with a diffuse gastric cancer phenotype in the latter86. Normal pancreatic organoids harboring four oncogenic driver mutations, including KRASG12V, CDKN2A, TP53, and SMAD4, acquired niche factor independency and histologically resembled PanIN-like lesions and invasive pancreatic ductal adenocarcinomas (PDACs)87,88. Normal esophageal organoids lacking APC quickly developed Barrett esophagus-associated neoplasia89. Similarly, engineered breast organoids carrying mutations in four tumor suppressor genes, TP53, PTEN, RB1 and NF1, recapitulate breast tumorigenesis90.
Additionally, iPSC-derived organoids model neoplastic progression. Generation of iPSC from PDAC tumors, followed by redifferentiation to pancreatic lineages, results in rare lines recapitulating early PanIN lesions92. Early neoplastic changes are observed upon colonic differentiation of iPSC, followed by expression of mutant APC93, or upon mutant KRAS and TP53 expression in iPSC induced to pancreatic lineages94. Engineered iPSC-derived cerebral organoids carrying oncogenic mutations exhibit many features of tumor malignancy95. iPSC-derived organoids may find particular utility in modeling germline cancer predisposition syndromes, as with APC mutation in Familial Adenomatous Polyposis93 or TP53 mutation in Li-Fraumeni syndrome96. However, potential drawbacks to cancer modeling using iPSCs include their fetal context compromising the study of adult cancers.
Organoids as functional genomics platforms for new cancer gene discovery
A significant potential application of the bottom-up organoid strategy is the functional validation of novel oncogenic alterations. Numerous examples exist, including confirmation of miR-483 as a driver oncogene amplified at 11p15 in colon cancer through overexpression in colon organoids41, as well as demonstration of the oncogenicity of EIF3E-RSPO2 fusions81,97. Expression of Rhoa mutant alleles in gastric organoids showed that RhoaY42C acts as an oncogene through the activation of focal adhesion kinase in Cdh1-mediated diffuse gastric cancer98. CRISPR/Cas9-engineered liver organoids have validated the function of a previously uncharacterized tumor suppressor gene, BAP1, in hepatic carcinogenesis91. In these various cases, mutation of the test oncogene or tumor suppressor generally elicits in vitro and in vivo tumorigenic phenotypes.
Engineered human organoids also provide tractable platforms for functionally studying interactions between cancer genotypes and phenotypes. For example, recent landmark human cancer genomic projects, such as The Cancer Genome Atlas (TCGA), have molecularly characterized four distinct subtypes of primary gastric cancer, including Epstein-Barr virus (EBV)-positive, microsatellite instability (MSI), chromosomal instability (CIN), and genomically stable (GS) tumors99. Cre-lox-mediated deletion of Cdh1, recurrently mediated in the GS subtype of gastric cancers, along with Tp53, induced signet ring cells characteristic of diffuse gastric cancers and enhanced in vivo tumorigenicity in mouse ALI gastric organoids84. CRISPR/Cas9 deletion of CDH1 and RHOA in normal human gastric organoids elicited striking morphological changes and vigorous migratory features characteristic of diffuse-like gastric cancers. Interestingly, RHOA inhibition reverses these phenotypes and sustains CDH1 knockout organoid cells, supporting the concurrency of CDH1 and RHOA mutations in gastric cancer86.
Thus, many comprehensive tumor models with specific mutations have been established using organoid-based “bottom up” genetic approaches, allowing functional assessment of small numbers of genetic alterations commonly seen in patient tumor samples. The 3D properties of organoids may be especially beneficial in modeling tumor suppressor loci. For instance, lung cancer cell lines display stronger growth phenotypes in response to CRISPR-mediated knockout of tumor suppressor loci when grown as 3D spheroids versus 2D monolayer100. Such benefits may also extend to 3D organoid systems. Moving forward, substantial potential exists for exploiting organoids for broader-based unbiased oncogene screens leveraging substantial genetic and epigenetic data from genome-scale sequencing studies, for instance using sgRNA or shRNA barcoded approaches.
Studying tumor genomic evolution
Previous studies have identified mutational signatures and driver mutations by whole genome sequencing in seemingly normal colonic glands101, and clonal evolution in breast cancer has been assessed by single cell DNA sequencing102. The success in establishing organoids from normal and tumor tissue allows biomass expansion from single cell-derived clones, thus increasing fidelity of whole genome sequencing103 and extension to multi-omics sequencing104, to examine mutational processes in normal103,105,106 or tumor tissue104. Whole genome sequencing of mouse clonal organoids from single stomach glands, colon crypts or prostate cells revealed distinct mutational signatures amongst individual clones105. A similar approach demonstrated that human adult stem cells (ASCs) accumulated approximately 36 mutations per year but exhibited different turnover rates and tissue-specific mutational processes103.
Analogous studies of forward genetic oncogenic transformation of human wild-type organoids can similarly elaborate the evolutionary dynamics and driving forces underlying tumor initiation and progression. Loss of APC and TP53 in normal colon organoids stimulates abnormal chromosome segregation and extensive aneuploidy, suggesting a key driving force of chromosome instability, a hallmark of tumor progression77. Accumulation of total mutational burden was quickly observed after depletion of MLH1, a key DNA repair gene, in normal colonic organoids83. These studies indicated the potential for longitudinal tumor evolution studies in human organoids. For example, the chemotherapeutic drug 5-Fluorouracil (5-FU) was shown to accelerate tumor evolution with rapid T>G transversions after treatment107. Additionally, ongoing chromosomal instability (CIN) in colorectal tumor organoids has been documented by single-cell karyotype sequencing, further demonstrating the prevalence of genomic heterogeneity in human cancer108.
Organoid modeling of cancer stem cells
In vivo lineage tracing in engineered mouse models is a powerful tool to study stem cell properties in adult mammalian tissues109, as evidenced by inducible Cre recombinase lineage tracing of long-lived adult Lgr5+ ISCs110. Similarly, lineage tracing and clonal analysis in organoids represents a parallel strategy to study human cancer stem cells111,112. Following Apc loss in mice, Lgr5+ ISCs, but not other cell types, give rise to intestinal adenomas113 reminiscent of the cell hierarchy of normal intestine114 and consistent with Lgr5+ ISCs representing a cancer stem cell (CSC) population115. Analogously, this mouse lineage tracing strategy was subsequently applied to primary human organoids to demonstrate cancer stem cell activity112,116. Human LGR5+ cells exhibited multipotency and self-renewal in colon organoids harboring inducible CRISPR/Cas9 knock-in lineage tracing alleles of LGR5116, which following transplantation self-renewed and differentiated to generate heterogeneous progeny in tumors112. Remarkably, specific ablation of LGR5+ CSCs within tumors did not induce long-term regression of primary tumors, which instead exhibited dramatic plasticity where other cell types replenished the LGR5+ CSC population112. Surprisingly, in a parallel transgenic mouse study117, ablating Lgr5+ CSCs did not induce primary tumor regression, but formation and maintenance of colon cancer-derived liver metastases was reduced, suggesting that targeting Lgr5+ CSCs could selectively eradicate colon cancer metastases. Upon mouse transplantation of oncogene-engineered colon organoids, the majority of metastases were seeded by circulating Lgr5− colon cancer cells which could regenerate functional Lgr5+ CSCs, indicating potential prevention of metastases by targeting cellular plasticity118. In humans, it remains unclear whether LGR5+ cells serve as functional CSCs in human colon cancer metastases, where LGR5 expression is downregulated38,119. Similar cellular plasticity of Lgr5+ ISCs has also been described previously in normal mouse intestinal epithelium120,121. Implicit to cancer stem cell analysis in organoids are cell of origin questions for particular cancers. Indeed, successful oncogenic transformation of a particular lineage in organoids may represent prima facie evidence that a given population serves as a cell of origin for the cognate cancer.
Studying oncogenic pathogens
A lack of appropriate primary culture systems has previously constrained the exploration of numerous human pathogens. Organoid culture technology has filled numerous emergent needs in the field of infectious diseases pathogenesis. Co-cultures of organoids with pathogens, such as parasites122, bacteria11,49,123-129, and viruses130-132, have modeled host-pathogen interactions and pathogenic-induced oncogenesis. Microinjection of gastric cancer-associated Helicobacter pylori into the lumen of normal gastric organoids stimulates a robust inflammatory response11 and epithelial hyperproliferation49,128,129. A causal relationship between chronic Salmonella enterica infection and gallbladder cancer was revealed in a gallbladder organoid model124. The oncogenic Salmonella-infected organoids acquired growth factor independency and exhibited histological characteristics of cellular transformation, including loss of cellular polarity and increased nuclear pleomorphism. Various colorectal cancer-associated bacterial species are enriched in patients, and therefore the intestinal microbiome has been suggested to facilitate tumorigenesis133. Accordingly, colibactin-producing pks+ Escherichia coli directly induced DNA mutagenesis in a long-term organoid co-culture system. Whole exome sequencing data indicated that organoids exposed to pks+ Escherichia coli exhibited a characteristic single thymine (T) deletion signature, as well as increased numbers of single base substitution, preferably T>N substitutions. Interestingly, these genotoxic Escherichia coli-induced mutational signatures were identified in a subset of CRC patients, suggesting a possible cause of tumorigenesis127.
Top-down organoid approaches
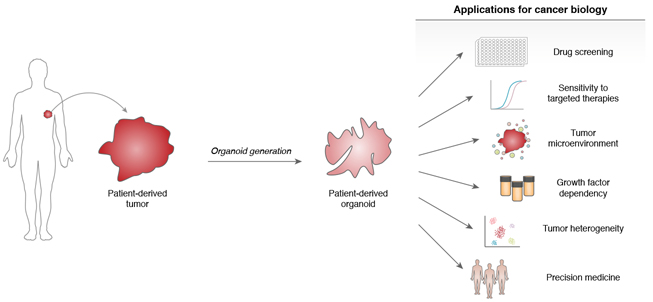
Human patient-derived tumor organoids (PDOs) can be directly established from clinical cancer biopsies in a tissue-specific fashion. As opposed to the “bottom-up” approach of engineering oncogenic alterations into wild-type tissue organoids, the direct organoid culture of fresh tumor tissue is a “top-down” strategy of studying pre-established malignancies (Figure 3). Attempts to propagate fresh tumor specimens as in vitro conventional 2D cancer cell lines or in vivo patient-derived xenografts (PDX)134,135, while successful, have been historically fraught with inefficiency. However, success rates for tumor-derived organoids and PDOs have been typically higher than conventional 2D culture and PDX and while varying for different tissues, can be robust for commonly used tumor organoid systems such as pancreatic and colon cancer (Table 1). PDOs have been established from surgical resections, tissue biopsies, circulating tumor cells, and ascitic fluid34,136-138. General features of PDO cultures, and indeed essential quality control metrics, are their accurate preservation of histologic and genetic features of the parent tumor, the latter extending to truncal alterations, such as copy number variations (CNV) and single-nucleotide variations (SNV). Furthermore, PDOs are extremely tractable from an experimental standpoint, undergoing facile lentiviral transduction, CRISPR-based gene editing and in vivo xenografting. These properties are significantly abetted by their ready expansion which renders PDOs potentially useful as preclinical models for drug screening or functional genomics with throughput greater than PDX studies. On the other hand, PDO cultures have until recently generally lacked important in vivo attributes such as stroma or immune components40. As such, tumor organoids containing exclusively epithelium generally exhibit transcriptional concordance with the original cancers86,139-141, except for downregulation of blood-, immune- and extracellular matrix-related genes139,140.
Figure 3. Top-down cancer modeling in patient-derived tumor organoids.
Human patient-derived tumor organoids possess numerous applications for translational cancer research. Potential uses include novel therapeutic drug screening, the study of the tumor microenvironment and tumor heterogeneity, as well as personalized medicine and response prediction.
Table 1.
Overview of Human Patient-derived Tumor Organoid (PDO) Biobanks
| Tumor Site | N | Histological Subtype | Sampling | Success Rate | Drug Testing | Correlation to Patient Outcome |
Ref |
|---|---|---|---|---|---|---|---|
| Colorectum | 22 | Colorectal carcinoma | Surgical resection | 90% | Yes (83 drugs) | No | 136 |
| Colorectum | 55 | Neuroendocrine carcinoma Premalignant lesions Adenocarcinoma Metastases of adenocarcinoma |
Surgical resection Endoscopic biopsy |
100% | No | No | 144 |
| Colorectum | 10 | Colorectal adenocarcinoma Metastatic colorectal adenocarcinoma |
Tissue biopsies | 71% | No | No | 137 |
| Colorectum | 91 | Colorectal adenocarcinoma (primary and metastatic specimens) | Surgical resection Synchronous multi-regional sampling PDX-derived |
58% | Yes (10 drugs) | No | 143 |
| Colorectum | 46 | Colorectal adenocarcinoma (sibling pairs of PDOs versus PDXs) | Surgical resection | 60% | Yes (16 drugs) | No | 141 |
| Colorectum | 40 | Metastatic colorectal adenocarcinoma | Tissue biopsies | 63% | Yes | Yes (N=29) | 145 |
| Rectum | 65 | Rectal adenocarcinoma | Tissue biopsies | 77% | Yes | Yes (N=13) | 146 |
| Rectum | 96 | Rectal adenocarcinoma Rectal mucinous adenocarcinoma |
Tissue biopsies | 86% | Yes | Yes (N=21) | 147 |
| Pancreas | 8 | Ductal adenocarcinoma | Surgical resection | 75%-80% normal 75%-83% cancer |
No | No | 16 |
| Pancreas | 39 | Ductal adenocarcinoma | Surgical resection EUS-FNA* Ascites puncture |
80% | No | No | 88 |
| Pancreas | 114 | Ductal adenocarcinoma (primary and metastatic specimens) | Surgical resection Tissue biopsies |
75% | Yes | Yes (N=8) | 150 |
| Pancreas | 17 | Ductal adenocarcinoma | Surgical resection | 85% | Yes | No | 94 |
| Pancreas | 52 | Ductal adenocarcinoma | Surgical resection Tissue biopsies |
62% | Yes (76 drugs) | Yes (N=4) | 151 |
| Stomach | 37 | Gastric adenocarcinoma | Surgical resection Endoscopic biopsy Ascites puncture |
55%-75% | No | No | 86 |
| Stomach | 63 | Normal, dysplastic, and cancer Lymph node metastases |
Surgical resection | >90% normal 50% cancer |
Yes (37 drugs) | Yes (N=3) | 140 |
| Stomach | 20 | Gastric adenocarcinoma | Surgical resection | N/A | Yes | No | 149 |
| Stomach | 7 | Gastric adenocarcinoma | Sleeve gastrectomies | N/A | Yes | No | 148 |
| Liver | 7 | Hepatocellular carcinoma Cholangiocarcinoma |
Surgical resection | 100% | Yes (29 drugs) | No | 162 |
| Liver | 10 | Hepatocellular carcinoma | Tissue biopsies | 26% | Yes (only Sorafenib) | No | 161 |
| Liver | 27 | Hepatocellular carcinoma Cholangiocarcinoma |
Surgical resection | N/A | Yes (129 drugs) | No | 160 |
| Bladder | 20 | Urothelial carcinoma Squamous cell carcinoma |
Cystectomy Endoscopic biopsy Cold loop resection |
70% | Yes (40 drugs) | No | 153 |
| Bladder | 77 | Urothelial carcinoma (both basal and luminal subtypes) | Radical cystectomies Transurethral resection (TUR) |
N/A | Yes (6 drugs) | No | 152 |
| Prostate | 7 | Adenocarcinoma metastases Circulating tumor cells |
Tissue biopsies | 15%-20% | Yes (2 drugs) | No | 138 |
| Prostate | 4 | Neuroendocrine prostate cancer | Tissue biopsies | 16% | Yes (129 drugs) | No | 154 |
| Ovary | 33 | High-grade serous carcinoma | Ascitic or pleural fluid | 80%-90% | Yes (4 drugs) | Yes (N=2) | 34 |
| Ovary | 56 | Borderline tumors Clear cell carcinoma Endometrioid carcinoma Mucinous carcinoma Serous carcinoma |
Surgical resection Ascitic or pleural fluid |
85% | Yes (7 drugs) | No | 33 |
| Breast | 95 | Ductal adenocarcinoma Lobular adenocarcinoma |
Surgical resection | >80% | Yes (6 drugs) | Yes (N=2) | 28 |
| Esophagus | 15 | Esophageal squamous cell carcinoma Oropharyngeal squamous cell carcinoma |
Tissue biopsies | 71% | Yes (only 5-FU) | No | 156 |
| Esophagus | 10 | Esophageal adenocarcinoma | Surgical resection | 31% | Yes (24 drugs) | No | 157 |
| Oral Mucosa | 31 | Head and neck squamous cell carcinoma | Surgical resection | 65% | Yes (8 drugs) | Yes (N=7) | 158 |
| Endometrium | 72 | Normal, endometriosis, hyperplasia, low- and high-grade carcinomas | Tissue biopsies | N/A | Yes (5 drugs) | No | 159 |
| Mixed | 56 | Tumors from prostate, breast, colorectal, esophagus, brain, pancreas, lung, small intestine, ovary, uterus, soft tissue, bladder, ureter, and kidney | Surgical resection Tissue biopsies |
39% | Yes | No | 177 |
| Mixed | 23 | Metastatic colorectal cancer Gastroesophageal cancer |
Tissue biopsies | 70% | Yes (55 drugs) | Yes (N=21) | 142 |
Endoscopic ultrasound guided-fine needle aspiration (EUS-FNA)
Recently, many organoid biobanks have emerged that contain large numbers of PDOs from different cancer types, including colon136,137,141-145, rectum146,147, stomach86,140,148,149, pancreas16,88,94,150,151, bladder152,153, prostate138,154, ovary33,34,155, esophagus156,157, breast28, oral mucosa158, endometrium159, liver160-162 , kidney163 , airway164 and brain139 typically utilizing the submerged Matrigel culture method, although ALI PDOs have also been described40. The Human Cancer Models Initiative (HCMI) seeks to biobank thousands of new PDO models, replete with genetic sequencing and clinical annotations165. Overall, the breadth of successful PDO generation provides an exciting new bridge between basic and translational cancer research.
Recapitulation of sensitivity to targeted therapies
PDOs are now used to identify new therapeutic strategies via discovery of gene-drug interactions, correlating between drug sensitivity to genomic alterations. One proof-of-concept for small molecular compound screening of PDOs was performed in an early biobank of colon cancer organoids136. By connecting drug sensitivity data and genetic information of PDOs, a positive correlation between oncogenic KRAS-mutated organoids and resistance to anti-EGFR inhibitor cetuximab was identified, recapitulating previous clinical observations166. Similarly, androgen receptor (AR) amplification in prostate cancer organoids conferred sensitivity to the AR antagonist enzalutamide. In agreement with previously identified synthetic lethality167, a recent biobank revealed poly (ADP-ribose) polymerase (PARP) inhibition as a therapeutic vulnerability of BRCA1/2-deficient breast cancer organoids28. A bladder cancer organoid biobank indicated that organoids harboring gain-of-function FGFR3 mutations exhibited significant sensitivity to the MEK inhibitor trametinib and the ERK inhibitor SCH772984153. These results illustrate the ability of PDOs to accurately recapitulate clinically observed therapeutic vulnerabilities to archetypal genetic alterations.
Dissecting tumor growth factor dependencies
Organoid cultures can dissect the growth factor “niche” dependency of tumor cells within their microenvironment, examining if cancers exhibit altered or reduced growth factor requirements. Conventionally, tumor organoid cultures have been grown in rich conditions replete with signals for Wnt, R-spondin, EGF, BMP and other pathways, while conversely, omission of such growth factors has enabled functional selection of CRISPR-induced oncogenic mutations76,77. However, alternation of niche requirements can be crucial for the selective growth advantage of certain subtypes of cancers and for establishing cognate PDOs. The use of diverse conditions allowed successful PDO generation from many rare colon cancer subtypes previously refractory to PDO culture, such as sessile serrated adenoma, tubulovillous adenoma, mucinous adenocarcinoma and neuroendocrine carcinoma, preserving pathological features of the original tumors both in vitro and following in vivo kidney capsule xenotransplantation144. Despite differences in genetic background often translating to specific growth dependencies of PDOs, the functional classification of tumor subtypes based on distinct niche dependencies instead of genetic signatures may lead to identification of new molecular mechanisms underlying inter-tumor heterogeneity144. Previously unappreciated GATA6-mediated Wnt signaling dependencies identified in organoids have defined three functional subtypes of PDACs88. Similarly, gastric cancer organoids harboring simultaneous TP53 and CDH1 mutations gained R-spondin independency whereas combined CRISPR/Cas9 double knockout of TP53 and CDH1 in wild-type gastric organoids was sufficient to allow culture without R-spondin86.
Organoid modeling of the tumor microenvironment
Cancers grow within a complex microenvironment where tumor epithelial cells are invested by diverse stromal cellular components, including fibroblasts, endothelial and immune cells168-170. The crucial roles of endothelial and immune components during tumorigenesis is particularly illustrated by the clinical efficacy of anti-angiogenic and immunotherapies, respectively171,172. Nowhere has the need for holistic tumor microenvironment culture been more acute than for tumor immunology and the accompanying need to study interactions between cancer cells and their veritable ecosystem of co-habitating immune cells. However, conventional organoid models of cancer have typically only represented tumor epithelium, and holistic culture of cancer cells alongside endogenous stromal elements has been elusive. In the absence of culture systems allowing tumor cells and stroma to be preserved alongside each other, numerous studies have reconstituted heterologous cell types along with organoids containing solely tumor epithelium. Examples include “tumor-only” submerged organoids with addition of distinct cancer-associated fibroblast (CAF) subtypes173,174, or supplementation with chimeric antigen receptor (CAR) natural killer (NK) cells to study tumor cytotoxicity175. Of note, multiple rounds of co-culture of PDOs with matched autologous peripheral blood lymphocytes were sufficient to select for tumor-reactive T cells, which performed specific killing of tumor cells but not matched normal organoids176.
As typical PDOs only contain tumor epithelium, there has been a singular lack of robust in vitro culture systems that retain cancer cells alongside infiltrating endogenous immune stroma and recapitulate in vivo checkpoint inhibition responses. However, recent ALI tumor organoid studies have robustly preserved the complex cellular diversity and physical architecture of both endogenous tumor and stroma compartments. PDOs in ALI preserved cancer-associated fibroblasts, numerous infiltrating immune cells, such as T cells (cytotoxic, helper, regulatory and exhausted), B cells, NK cells and macrophages over 1-2 months40. Importantly, subsets of ALI PDOs from non-small cell lung cancer, melanoma, renal cell carcinoma and bladder contained functional tumor-infiltrating lymphocytes (TILs) that rapidly exhibited clonal expansion, activation and/or cytotoxic responses upon short-term PD-1/PD-L1 checkpoint blockade treatment. Additionally, mouse tumors from syngeneic immunocompetent hosts could be similarly cultured in ALI with anti-PD-1/PD-L1-dependent T cell responses40. Although this type of holistic tumor microenvironment culture is in its infancy, potential applications include mechanistic immunooncology studies, screening of novel immunomodulatory agents and precision medicine.
Studying tumor heterogeneity
Cancer organoid cultures present an opportunity to capture the intratumoral heterogeneity (ITH) of human cancer for in vitro investigation. Generally, tumor organoids retain mutations from the tissue of origin16,34,142,150,162, as well as copy number status 108,140. However, PDOs can undergo dynamic changes in culture. Many studies only compare early passage organoids with the tumor tissue but following serial passaging subclonal mutations can be gained or lost, although truncal mutations tend to be retained153,164. Long-term culture of CRC organoids for >6 months revealed that MSI lines with mismatch repair deficiency developed 75 and 82 de novo non-synonymous mutations respectively, whereas a microsatellite stable (MSS) was comparatively unchanged144. Liver tumor organoids retained around 92% of the tissue mutations after <2 months and >80% of mutations after >4 months in culture162. Thus, organoid cultures show strong correlation with tissue mutational status at early passages, but genetic drift and selection can occur. In addition, any dynamic PDO genetic or epigenetic changes in culture could be biased by culture media that may not fully reflect in vivo conditions.
Since organoids can only represent their originating tumor biopsy, faithful recapitulation of tumor heterogeneity may require culture from multiple sites of a single tumor or from distinct metastatic sites. Several examples of organoid cultures from multiple tumor regions indicate that these “sibling” cultures can manifest distinct drug sensitivity profiles. For example, organoids from different metastases from a PDAC patient showed similar response to three chemotherapy drugs, but different responses to 5-FU150. In CRC, five sibling PDO cultures from multiple regions of the same tumor exhibited up to 30 fold differences in drug response, suggesting that a single tumor biopsy may not be enough to capture all ITH143. A study of 27 liver cancer organoids from 5 patients indicated that most tested drugs possessed low intra-patient variation, but several drugs, including targeted therapies such as tyrosine kinase inhibitors, displayed high intra-patient variability160. The most extensive ITH study performed genomic, transcriptomic and epigenetic analysis of 78 clonally derived CRC PDOs across multiple tumor regions from 3 previously untreated patients104. Clonal organoids from the same region shared common driver mutations but could still exhibit substantial differences in overall mutational content (40%) and sensitivity to chemotherapeutics and targeted drugs (3 log variation in IC50). In this study, principal component analysis of methylation and transcriptional state showed that while clones from the same patient clustered together, subclones, (for example TP53 WT vs TP53 mutant) clustered separately. Overall, organoid ITH studies indicate that although concordance exists, even clonal organoids derived from the same tumor region can exhibit highly variable mutations, transcriptomes, epigenomes and drug responses. Additionally, PDOs can model tumor mutational evolution with shared truncal mutations leading to subclonal divergence.
Organoid-based functional precision medicine
Precision Medicine is a treatment approach that seeks to exploit patient-specific individualized therapeutic strategies177. Given their tractability and potential to capture patient and tumor type diversity, organoids are well-suited for the development of personalized therapeutic approaches.
Drug screening against organoid biobanks
The diversity of cancers captured by organoid biobanks has created numerous applications for therapeutics screening. Such drug sensitivity profiling can be particularly powerful when combined with genomic and/or transcriptomic organoid characterization, identifying specific drug response profiles for different tumor subtypes, or alternatively, defining sensitive and resistant PDOs. PDOs can also be used for synthetic lethality analysis, identifying drugs with selective efficacy against organoids with specific mutation(s).
Numerous examples include screening a liver cancer organoid biobank of 6 liver PDOs against 29 anti-cancer drugs, which nominated ERK inhibitors as a potential therapeutic agent for a subset of liver cancers162. Similarly, exposure of 27 liver cancer organoid lines from multiple tumor regions of 5 patients to 129 FDA-approved anti-cancer drugs revealed 13 drugs with broad cytotoxicity including inhibitors of histone deacetylase (HDAC), proteasome, DNA topoisomerase II, protein translation and RNA synthesis, as well as several novel agents. Comparison of organoid drug response with publicly available drug response databases demonstrated separate clustering of organoids versus traditional liver cancer cell lines, indicating that primary 3D PDOs may respond to drugs differently than conventional 2D-cultured cells160. Systematic exposure of a genetically characterized 66 patient pancreatic cancer PDO biobank to five clinically used chemotherapeutic agents such as gemcitabine, revealed that 33% of PDAC samples were resistance to all five drugs, but half of the multi-resistant lines were sensitive to a targeted therapy when tested against a panel of 21 such agents, suggesting alternative treatment strategies. In this case, correlations between PDO gene expression and drug sensitivity revealed a gemcitabine drug sensitivity gene signature that significantly correlated with improved progression-free survival (PFS) upon post-surgical adjuvant gemcitabine treatment in a 55 patient cohort150. Large-scale drug screening of PDOs may also identify unexpected treatments or combinations thereof. Evaluation of gastric cancer PDOs against a 37 compound library has indicated genotype-specific vulnerabilities140. Further, generation of matched tumor and normal PDOs from the same patient was exploited to discover the gemcitabine as a potentiator of the EZH2 inhibitor UNC1999 combination in PDAC PDOs94.
Drug sensitivities between sibling pairs of PDOs and PDXs have been compared in CRC models141. In a panel of 46 PDOs and 59 PDXs, the 19 tumors captured in both systems displayed fairly concordant responses to 16 tested drugs. However, different patterns were observed for individual drug sensitivity and RNA signatures for hypoxia, EMT, G2/M checkpoints, proliferation, stemness and metabolism. Since both PDOs and PDXs recapitulate many key genetic and phenotypic features of their parental tumors but possess distinct drawbacks135, future studies combining the strengths from these preclinical models could provide clinically relevant metrics in precision medicine.
The large number of developing organoid biobanks provides a unique opportunity for targeted drug development178. Compound screening against large, well defined organoid repositories has the potential to identify drugs efficient against a subsets of tumors with a specific genetic composition. While such screens have been perfomed extensively for cells grown in 2D monolayers179,180, emerging evidence suggests that cells grown in 3D often respond differently to drugs160, including increased chemoresistance181. For example, 2D versus 3D drug responses in ovarian cancer found correlation for cytotoxic but divergence for cytostatic drugs182. Conceivably, 3D organoid biobanks could lower false positives in large compound screens, which in turn could reduce the extent of expensive confirmatory preclinical mouse studies. In addition, organoids can be grown from corresponding healthy tissue which would give an early indication of compound toxicity, as well inform underlying biology94,183. Organoid-based models to fully replace mouse experiments for drug discovery have yet to emerge, but there are encouraging developments including en bloc organoid culture with tumor and infiltrating immune cells40 and integrated multi-organoid body- on-a-chip systems to study drug metabolism and toxicity184. Positive compound hits will still need to be verified in xenograft experiments in the foreseeable future.
Overall, current nascent efforts with PDO biobanks represent a promising approach to screening therapeutics against different molecular subtypes within distinct solid tumors. The rigorous genetic characterization of such PDOs, combined with small molecule screening, has potential to identify new patient-relevant gene-drug interactions and synthetic lethal vulnerabilities, yielding novel insights versus traditional cancer cell lines and PDX models. Additionally, the biobank paradigm has broad application beyond cancer, such as with recently established cystic fibrosis organoid resources185.
Precision medicine and personalized therapy design
Precision Oncology efforts have traditionally exploited predictive mutational biomarkers for targeted therapies. However, such biomarkers are often lacking145; for instance, whole exome sequencing on a mixed cohort of tumors only identified actionable mutations with cognate FDA-approved drugs for 3 of 737 patients (0.4%)177. Even when targetable biomarkers are discovered, patients do not always respond to targeted therapy, raising a pressing need for strategies to predict efficacy or identify alternative options177.
PDO technologies represent a tantalizing opportunity for functional therapeutic response prediction in living cells. Several studies have reported that PDOs can forecast patient anticancer drug responses for a limited number of cases (n<5)28,34,140,151,186. A relatively few number of studies have more cases (n>5 and <10)146,150,158. Generally, published tumor organoid drug responses correlate with patient outcome. For example, in an organoid biobank of well-characterized pancreatic cancer PDOs eight out of nine organoids responded similarly to the cognate patients when exposed to chemotherapy, giving a match rate of 89%150. To date, only a handful of studies have measured patient and organoid drug correlations for more than 10 patients. For example, patient-organoid drug response across different gastrointestinal tumor types and drug regimens indicated the matched organoids could correctly predict responders and non-responders in 20 of 21 patients142. Similarly, comparison of metastatic colorectal patient versus PDO responses indicated relative success for irinotecan monotherapy (n=10), and the combination of irinotecan and 5-FU (n=12), but not for a combination of 5-FU and oxaliplatin (n=10). The authors speculated that the failure of PDOs to predict oxaliplatin response may be due to the lack of an immune system and stroma in organoids145.
Two recent publications have concluded that organoids can accurately portend responses to neoadjuvant chemoradiation (NACR) in rectal cancer. A large study established 80 PDOs from biopsies of treatment-naive patients with locally advanced rectal cancer (LARC) from a phase III clinical trial of neoadjuvant radiation combined with 5-FU with or without irinotecan147. Organoids were treated with radiation, 5-FU or irinotecan separately but not in combination. Considering the organoids as sensitive to NACR when sensitive to at least one of the three treatments (radiation, 5-FU, irinotecan), the study correctly predicted outcome in 85% of patients. Since many organoids were only sensitive to single or double agents, the authors hypothesized that not all three therapies are necessary in combination, and that organoid screening can identify ineffective treatments and thus avoid overtreatment. Another study, also studying NACR in rectal cancer, indicated varying sensitivity of rectal PDOs to ex vivo radiation, which correlated with clincal response in a cohort of 19 patients146.
Taken together, functional precision medicine, where individualized predictions are made based on a cellular drug response test instead of identification of a biomarker, is an intriguing prospect for PDOs. The success rate of establishing cultures in current biobanking efforts varies substantially between tumor types, but can be high (>80%), suggesting the feasibility of organoid-based functional precision medicine. From these few initial studies, some preliminary observations can be made. Despite tumor heterogeneity being a significant potential confounding variable for extrapolating therapeutic response from a single biopsy region, functional prediction appears to be feasible at least in the specific tumor types and drugs tested. However, the current lack of stromal and immune compartments in most organoid-based therapy prediction systems may preclude universal applicability. Negative results have not been described, raising the issue of publication bias, and perhaps most significantly, studies to date have trained predictive metrics based on foreknowledge of clinical outcome. Although current studies depict high (80-95%) rates of concurrency, it will be necessary to observe if initial successes persist in larger prospective independent validation cohorts of patient-organoid pairs where response metrics are fixed in advance.
Challenges and Perspectives
Organoid culture technologies have rapidly exerted a transformative impact on cancer research, with widespread adoption for both basic and translational applications. However, despite promising studies to date, numerous challenges remain. Although tumor PDOs often preserve the genetic composition of the original tumor at early passages, the extent of genetic drift, clonal selection and continued tumor evolution at later passage and after freeze-thaw cycles has not been well documented and may be tumor-specific144. Similar caveats apply to forward oncogene engineering of normal tissue as well. Differences in seemingly normal cells with regard to telomere length, genetic and epigenetic state could potentially influence phenotypic outcome. In addition, the ordering of mutations in serial genetic engineering, the exact location, alteration and duration of CRISPR knock-out modifications, genetic bottlenecks introduced by selection of rare transformed clones, and presumed ongoing evolution may all produce variable results. This may account for differences in invasive behavior between two recent organoid models of colorectal cancer that engineered the inclusion of the same set of mutations76,77.
Another key limitation of conventional organoid methods is a demonstrated lack of endogenous tumor-associated stromal components, particularly immune cells and fibroblasts. Thus, the continued development of organoid systems that more holistically represent the tumor microenvironment is an urgent need. Ultimately, PDOs incorporating immune and other stromal components may open new research directions such as immuno-oncology and help actualize the promise of precision cancer therapies.
Although essential culture components for growing organoids from different normal tissues and cancers have been widely investigated, costs are substantial and reagents can vary between laboratories, abetted by use of conditioned medium containing factors such as Wnt3a, R-spondin, and Noggin. Further, the presence of serum in conditioned media may disadvantage long-term culture88. Recently developed artificial water-soluble Wnt agonists187 or afamin-stabilized Wnt3a188 may facilitate serum-free alternatives to conventional Wnt conditioned media4 and enable economical, fully defined and recombinant culture reagents. A practical challenge is reproducing the potency of organoid media across different batches and laboratories, which may provide a selection pressure for a subset of cells that might not be representative of original tumors. At the same time, the evaluation of numerous different culture conditions can improve success rates of PDO generation144.
Current CRISPR/Cas9-mediated oncogene engineering in primary human organoids has been inefficient and largely restricted to selected genes in which mutational pathway activation allows bespoke media-based functional selection strategies to enrich for gene knockouts. Future approaches may bypass such screening requirements, perhaps by allowing phenotypic screening, increasing efficiency or using selectable knock-in cassettes. Opportunities also exist for large-scale genetic and chemical perturbations of organoids. Improved, highly efficient editing methods185,189,190 could facilitate screening of a broader range of target loci, extending to high-throughput genome-scale functional screens191,192. Similarly, small molecule screening in organoids has been restricted to modest panels of therapeutics, which could be enlarged to more extensive compound libraries by ultra-high throughput miniaturized cultures193. The scale of both genetic and small molecule screens could be substantially augmented by methods to expand organoid biomass to much larger degrees than currently practiced.
Perhaps the most revolutionary potential applications of organoids for oncology reside in therapeutic prediction. Numerous studies suggest the feasibility of PDO-based functional testing of patient drug responses, which now require prospective confirmation in validation cohorts. Optimally, biologic organoid testing will require rapid turnaround in clinically actionable timeframes, evidence of enhanced survival advantage and conceivably, extension to modalities such as immunotherapy. Additionally, the creation of ever-enlarging PDO biobanks, for example with the HCMI initiative165, may establish large collections representing diverse cancer subtypes and genotypes, whose systematic drug screening could establish ready-made therapeutic response patterns that are possibly relevant to patients with similar tumor genotypes. Such transformation of prior descriptive metrics into organoid-based biological predictive assays using living cells would be a capstone of the rapid progress with cancer organoids to date, portending additional advances in both basic biology and clinical translation.
Acknowledgments
We thank members of the Kuo lab for helpful discussions and Amanda Mah for figure artwork. This was also supported by NIH fellowship K00CA212433 (Y-H.L.), a Swedish Research Council International Postdoctoral Fellowship (K.K.), and grants from the NIH (U01CA217851, U54CA224081, U01CA199241 and U19AI116484), Emerson Collective and Ludwig Cancer Research to C.J.K.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Begley CG & Ellis LM Drug development: Raise standards for preclinical cancer research. Nature (2012). doi: 10.1038/483531a [DOI] [PubMed] [Google Scholar]
- 2.Lancaster MA & Knoblich JA Organogenesisin a dish: Modeling development and disease using organoid technologies. Science (2014). doi: 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- 3.Lancaster MA & Huch M Disease modelling in human organoids. Dis. Model. Mech (2019). doi: 10.1242/dmm.039347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato T et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature (2009). doi: 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 5.Ootani A et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med (2009). doi: 10.1038/nm.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spence JR et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature (2011). doi: 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii M et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell (2018). doi: 10.1016/j.stem.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 8.Jung P et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med (2011). doi: 10.1038/nm.2470 [DOI] [PubMed] [Google Scholar]
- 9.Sato T et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology (2011). doi: 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- 10.Barker N et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units In vitro. Cell Stem Cell (2010). doi: 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 11.Bartfeld S et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology (2015). doi: 10.1053/j.gastro.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWard AD, Cramer J & Lagasse E Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. (2014). doi: 10.1016/j.celrep.2014.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maimets M et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Reports (2016). doi: 10.1016/j.stemcr.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanduri LSY et al. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Reports (2014). doi: 10.1016/j.stemcr.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren W et al. Single Lgr5− or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl. Acad. Sci. U. S. A (2014). doi: 10.1073/pnas.1409064111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boj SF et al. Organoid models of human and mouse ductal pancreatic cancer. Cell (2015). doi: 10.1016/j.cell.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgakopoulos N et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev. Biol (2020). doi: 10.1186/s12861-020-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D et al. Long-term expansion of pancreatic islet organoids from resident Procr+ progenitors. Cell (2020). doi: 10.1016/j.cell.2020.02.048 [DOI] [PubMed] [Google Scholar]
- 19.Loomans CJM et al. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Reports (2018). doi: 10.1016/j.stemcr.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huch M et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature (2013). doi: 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell (2018). doi: 10.1016/j.cell.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 22.Huch M et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell (2015). doi: 10.1016/j.cell.2014.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng WC et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell (2018). doi: 10.1016/j.cell.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs N et al. Long-term expanding human airway organoids for disease modeling. EMBO J. (2019). doi: 10.15252/embj.2018100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danahay H et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. (2015). doi: 10.1016/j.celrep.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 26.Tata PR et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature (2013). doi: 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock JR et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U. S. A (2009). doi: 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachs N et al. A Living biobank of breast cancer organoids captures disease heterogeneity. Cell (2018). doi: 10.1016/j.cell.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 29.Chua CW et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol (2014). doi: 10.1038/ncb3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karthaus WR et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell (2014). doi: 10.1016/j.cell.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutgens F et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol (2019). doi: 10.1038/s41587-019-0048-8 [DOI] [PubMed] [Google Scholar]
- 32.Kessler M et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun (2015). doi: 10.1038/ncomms9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopper O et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med (2019). doi: 10.1038/s41591-019-0422-6 [DOI] [PubMed] [Google Scholar]
- 34.Hill SJ et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. (2018). doi: 10.1158/2159-8290.CD-18-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turco MY et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol (2017). doi: 10.1038/ncb3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boonekamp KE et al. Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc. Natl. Acad. Sci. U. S. A (2019). doi: 10.1073/pnas.1715272116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhaduri A et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature (2020). doi: 10.1038/s41586-020-1962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganesh K et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer (2020). doi: 10.1038/s43018-019-0006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Co JY et al. Controlling epithelial polarity: A human enteroid model for host-pathogen interactions. Cell Rep. (2019). doi: 10.1016/j.celrep.2019.01.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal JT et al. Organoid modeling of the tumor immune microenvironment. Cell (2018). doi: 10.1016/j.cell.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med (2014). doi: 10.1038/nm.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimarco RL et al. Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr. Biol. (Camb) (2014). doi: 10.1039/c3ib40188j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velasco S et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature (2019). doi: 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quadrato G et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature (2017). doi: 10.1038/nature22047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasca AM et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods (2015). doi: 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature (2013). doi: 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Múnera JO et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell (2017). doi: 10.1016/j.stem.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCracken KW et al. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature (2017). doi: 10.1038/nature21021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCracken KW et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature (2014). doi: 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trisno SL et al. Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell (2018). doi: 10.1016/j.stem.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takebe T et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature (2013). doi: 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- 52.Wong AP et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol (2012). doi: 10.1038/nbt.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang SXL et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol (2014). doi: 10.1038/nbt.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dye BR et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife (2015). doi: 10.7554/eLife.05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YW et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol (2017). doi: 10.1038/ncb3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurmann AA et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell (2015). doi: 10.1016/j.stem.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longmire TA et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell (2012). doi: 10.1016/j.stem.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morizane R et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol (2015). doi: 10.1038/nbt.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takasato M et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature (2015). doi: 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- 60.Ozone C et al. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun (2016). doi: 10.1038/ncomms10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suga H et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature (2011). doi: 10.1038/nature10637 [DOI] [PubMed] [Google Scholar]
- 62.Koehler KR, Mikosz AM, Molosh AI, Patel D & Hashino E Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature (2013). doi: 10.1038/nature12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koehler KR et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol (2017). doi: 10.1038/nbt.3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu XP, Koehler KR, Mikosz AM, Hashino E & Holt JR Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat. Commun (2016). doi: 10.1038/ncomms11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano T et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell (2012). doi: 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 66.Lee J et al. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. (2018). doi: 10.1016/j.celrep.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wimmer RA et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature (2019). doi: 10.1038/s41586-018-0858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson CL et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med (2014). doi: 10.1038/nm.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phipson B et al. Evaluation of variability in human kidney organoids. Nat. Methods (2019). doi: 10.1038/s41592-018-0253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu H et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell (2018). doi: 10.1016/j.stem.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van den Berg CW et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports (2018). doi: 10.1016/j.stemcr.2018.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dye BR et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife (2016). doi: 10.7554/eLife.19732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clevers H Modeling development and disease with organoids. Cell (2016). doi: 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 74.McCauley HA & Wells JM Pluripotent stem cell-derived organoids: Using principles of developmental biology to grow human tissues in a dish. Dev. (2017). doi: 10.1242/dev.140731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fearon ER & Vogelstein B A genetic model for colorectal tumorigenesis. Cell (1990). doi: 10.1016/0092-8674(90)90186-I [DOI] [PubMed] [Google Scholar]
- 76.Matano M et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med (2015). doi: 10.1038/nm.3802 [DOI] [PubMed] [Google Scholar]
- 77.Drost J et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature (2015). doi: 10.1038/nature14415 [DOI] [PubMed] [Google Scholar]
- 78.Ijspeert JEG, Vermeulen L, Meijer GA & Dekker E Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nature Reviews Gastroenterology and Hepatology (2015). doi: 10.1038/nrgastro.2015.73 [DOI] [PubMed] [Google Scholar]
- 79.Fessler E et al. TGFβ signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol. Med (2016). doi: 10.15252/emmm.201606184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lannagan TRM et al. Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut (2019). doi: 10.1136/gutjnl-2017-315920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawasaki K et al. Chromosome engineering of human colon-derived organoids to develop a model of traditional serrated adenoma. Gastroenterology (2019). doi: 10.1053/j.gastro.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 82.Davis H et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat. Med (2015). doi: 10.1038/nm.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drost J et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science (2017). doi: 10.1126/science.aao3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nadauld LD et al. Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer. Genome Biol. (2014). doi: 10.1186/s13059-014-0428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Es JH & Clevers H Generation and analysis of mouse intestinal tumors and organoids harboring APC and K-ras mutations. Methods Mol Biol (2015). doi: 10.1007/978-1-4939-2297-0_6 [DOI] [PubMed] [Google Scholar]
- 86.Nanki K et al. Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis. Cell (2018). doi: 10.1016/j.cell.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 87.Lee J et al. Reconstituting development of pancreatic intraepithelial neoplasia from primary human pancreas duct cells. Nat. Commun (2017). doi: 10.1038/ncomms14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seino T et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell (2018). doi: 10.1016/j.stem.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 89.Liu X et al. Modeling Wnt signaling by CRISPR-Cas9 genome editing recapitulates neoplasia in human Barrett epithelial organoids. Cancer Lett. (2018). doi: 10.1016/j.canlet.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dekkers JF et al. Modeling breast cancer using CRISPR/Cas9-mediated engineering of human breast organoids. J. Natl. Cancer Inst (2019). doi: 10.1093/jnci/djz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Artegiani B et al. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell (2019). doi: 10.1016/j.stem.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 92.Kim J et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep. (2013). doi: 10.1016/j.celrep.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crespo M et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med (2017). doi: 10.1038/nm.4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang L et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med (2015). doi: 10.1038/nm.3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bian S et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods (2018). doi: 10.1038/s41592-018-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee DF et al. Modeling familial cancer with induced pluripotent stem cells. Cell (2015). doi: 10.1016/j.cell.2015.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han T et al. R-Spondin chromosome rearrangements drive Wnt-dependent tumour initiation and maintenance in the intestine. Nat. Commun (2017). doi: 10.1038/ncomms15945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. (2019). doi: 10.1158/2159-8290.CD-19-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bass AJ et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature (2014). doi: 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han K et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature (2020). doi: 10.1038/s41586-020-2099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee-Six H et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature (2019). doi: 10.1038/s41586-019-1672-7 [DOI] [PubMed] [Google Scholar]
- 102.Wang Y et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature (2014). doi: 10.1038/nature13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blokzijl F et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature (2016). doi: 10.1038/nature19768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roerink SF et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature (2018). doi: 10.1038/s41586-018-0024-3 [DOI] [PubMed] [Google Scholar]
- 105.Behjati S et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature (2014). doi: 10.1038/nature13448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pollen AA et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell (2019). doi: 10.1016/j.cell.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christensen S et al. 5-Fluorouracil treatment induces characteristic T>G mutations in human cancer. Nat. Commun (2019). doi: 10.1038/s41467-019-12594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bolhaqueiro ACF et al. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet (2019). doi: 10.1038/s41588-019-0399-6 [DOI] [PubMed] [Google Scholar]
- 109.Kretzschmar K & Watt FM Lineage tracing. Cell (2012). doi: 10.1016/j.cell.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 110.Barker N et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature (2007). doi: 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 111.Cortina C et al. A genome editing approach to study cancer stem cells in human tumors. EMBO Mol. Med (2017). doi: 10.15252/emmm.201707550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shimokawa M et al. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature (2017). doi: 10.1038/nature22081 [DOI] [PubMed] [Google Scholar]
- 113.Barker N et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature (2009). doi: 10.1038/nature07602 [DOI] [PubMed] [Google Scholar]
- 114.Schepers AG et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science (2012). doi: 10.1126/science.1224676 [DOI] [PubMed] [Google Scholar]
- 115.Reya T, Morrison SJ, Clarke MF & Weissman IL Stem cells, cancer, and cancer stem cells. insight review articles (2001). [DOI] [PubMed] [Google Scholar]
- 116.Sugimoto S et al. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell (2018). doi: 10.1016/j.stem.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 117.De Sousa E Melo F et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature (2017). doi: 10.1038/nature21713 [DOI] [PubMed] [Google Scholar]
- 118.Fumagalli A et al. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell (2020). doi: 10.1016/j.stem.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Sousa E Melo F et al. Methylation of cancer-stem-cell-associated wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell (2011). doi: 10.1016/j.stem.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 120.Tian H et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature (2011). doi: 10.1038/nature10408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan KS et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U. S. A (2012). doi: 10.1073/pnas.1118857109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heo I et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol (2018). doi: 10.1038/s41564-018-0177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forbester JL et al. Interaction of salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun (2015). doi: 10.1128/IAI.00161-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scanu T et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe (2015). doi: 10.1016/j.chom.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 125.Wilson SS, Tocchi A, Holly MK, Parks WC & Smith JG A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. (2015). doi: 10.1038/mi.2014.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang YG, Wu S, Xia Y & Sun J Salmonella-infected crypt-derived intestinal organoid culture system for host–bacterial interactions. Physiol. Rep (2014). doi: 10.14814/phy2.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pleguezuelos-Manzano C et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature (2020). doi: 10.1038/s41586-020-2080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wroblewski LE et al. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut (2015). doi: 10.1136/gutjnl-2014-307650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bertaux-Skeirik N et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. (2015). doi: 10.1371/journal.ppat.1004663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finkbeiner SR et al. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio (2012). doi: 10.1128/mBio.00159-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ettayebi K et al. Replication of human noroviruses in stem cell-derived human enteroids. Science (2016). doi: 10.1126/science.aaf5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hui KPY et al. Tropism, replication competence, and innate immune responses of influenza virus: an analysis of human airway organoids and ex-vivo bronchus cultures. Lancet Respir. Med (2018). doi: 10.1016/S2213-2600(18)30236-4 [DOI] [PubMed] [Google Scholar]
- 133.Wirbel J et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med (2019). doi: 10.1038/s41591-019-0406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Byrne AT et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nature Reviews Cancer (2017). doi: 10.1038/nrc.2016.140 [DOI] [PubMed] [Google Scholar]
- 135.Bleijs M, Wetering M, Clevers H & Drost J Xenograft and organoid model systems in cancer research. EMBO J. (2019). doi: 10.15252/embj.2019101654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van De Wetering M et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell (2015). doi: 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weeber F et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl. Acad. Sci. U. S. A (2015). doi: 10.1073/pnas.1516689112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao D et al. Organoid cultures derived from patients with advanced prostate cancer. Cell (2014). doi: 10.1016/j.cell.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jacob F et al. A Patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell (2020). doi: 10.1016/j.cell.2019.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan HHN et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell (2018). doi: 10.1016/j.stem.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 141.Schütte M et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat. Commun (2017). doi: 10.1038/ncomms14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vlachogiannis G et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science (2018). doi: 10.1126/science.aao2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schumacher D et al. Heterogeneous pathway activation and drug response modelled in colorectal-tumor-derived 3D cultures. PLoS Genet. (2019). doi: 10.1371/journal.pgen.1008076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujii M et al. A Colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell (2016). doi: 10.1016/j.stem.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 145.Ooft SN et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med 11, eaay2574 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Ganesh K et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med (2019). doi: 10.1038/s41591-019-0584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yao Y et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell (2019). doi: 10.1016/j.stem.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 148.Steele NG et al. An organoid-based preclinical model of human gastric cancer. CMGH (2019). doi: 10.1016/j.jcmgh.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seidlitz T et al. Human gastric cancer modelling using organoids. Gut (2019). doi: 10.1136/gutjnl-2017-314549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tiriac H et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. (2018). doi: 10.1158/2159-8290.CD-18-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Driehuis E et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. U. S. A (2019). doi: 10.1073/pnas.1911273116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mullenders J et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. U. S. A (2019). doi: 10.1073/pnas.1803595116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee SH et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell (2018). doi: 10.1016/j.cell.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Puca L et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun (2018). doi: 10.1038/s41467-018-04495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Witte CJ et al. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. (2020). doi: 10.1016/j.celrep.2020.107762 [DOI] [PubMed] [Google Scholar]
- 156.Kijima T et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. CMGH (2019). doi: 10.1016/j.jcmgh.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li X et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun (2018). doi: 10.1038/s41467-018-05190-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Driehuis E et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. (2019). doi: 10.1158/2159-8290.cd-18-1522 [DOI] [PubMed] [Google Scholar]
- 159.Boretto M et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol (2019). doi: 10.1038/s41556-019-0360-z [DOI] [PubMed] [Google Scholar]
- 160.Li L et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight (2019). doi: 10.1172/jci.insight.121490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nuciforo S et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. (2018). doi: 10.1016/j.celrep.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Broutier L et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med (2017). doi: 10.1038/nm.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Calandrini C et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat. Commun (2020). doi: 10.1038/s41467-020-15155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim M et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun (2019). doi: 10.1038/s41467-019-11867-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.HCMI: Human Cancer Models Initiative (https://ocg.cancer.gov/programs/HCMI).
- 166.De Roock W et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. (2010). doi: 10.1016/S1470-2045(10)70130-3 [DOI] [PubMed] [Google Scholar]
- 167.Sonnenblick A, De Azambuja E, Azim HA & Piccart M An update on PARP inhibitors - Moving to the adjuvant setting. Nature Reviews Clinical Oncology (2015). doi: 10.1038/nrclinonc.2014.163 [DOI] [PubMed] [Google Scholar]
- 168.Vitale I, Manic G, Coussens LM, Kroemer G & Galluzzi L Macrophages and metabolism in the tumor microenvironment. Cell Metabolism (2019). doi: 10.1016/j.cmet.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 169.Joyce JA & Fearon DT T cell exclusion, immune privilege, and the tumor microenvironment. Science (2015). doi: 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 170.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nature Medicine (2013). doi: 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kalbasi A & Ribas A Tumour-intrinsic resistance to immune checkpoint blockade. Nature Reviews Immunology (2019). doi: 10.1038/s41577-019-0218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wei SC, Duffy CR & Allison JP Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discovery (2018). doi: 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 173.Biffi G et al. Il1-induced Jak/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. (2019). doi: 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Öhlund D et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med (2017). doi: 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schnalzger TE et al. 3D model for CAR -mediated cytotoxicity using patient-derived colorectal cancer organoids . EMBO J. (2019). doi: 10.15252/embj.2018100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dijkstra KK et al. Generation of tumor-reactive T Cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell(2018). doi: 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pauli C et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7, 462–477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weeber F, Ooft SN, Dijkstra KK & Voest EE Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chemical Biology (2017). doi: 10.1016/j.chembiol.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 179.Barretina J et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature (2012). doi: 10.1038/nature11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Garnett MJ et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature (2012). doi: 10.1038/nature11005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Edmondson R, Broglie JJ, Adcock AF & Yang L Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay and Drug Development Technologies (2014). doi: 10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jabs J et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol. Syst. Biol (2017). doi: 10.15252/msb.20177697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Verissimo CS et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife (2016). doi: 10.7554/eLife.18489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skardal A et al. Drug compound screening in single and integrated multi-organoid body- on-a-chip systems. Biofabrication (2020). doi: 10.1088/1758-5090/ab6d36 [DOI] [PubMed] [Google Scholar]
- 185.Geurts MH et al. CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell (2020). doi: 10.1016/j.stem.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 186.Shenoy TR et al. CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol (2017). doi: 10.1093/annonc/mdx165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Janda CY et al. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature (2017). doi: 10.1038/nature22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mihara E et al. Active and water-soluble form of lipidated wnt protein is maintained by a serum glycoprotein afamin/α-albumin. Elife (2016). doi: 10.7554/eLife.11621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Artegiani B et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat. Cell Biol (2020). doi: 10.1038/s41556-020-0472-5 [DOI] [PubMed] [Google Scholar]
- 190.Vaidyanathan S et al. High-efficiency, selection-free gene repair in airway stem cells from cystic fibrosis patients rescues CFTR function in differentiated epithelia. Cell Stem Cell (2019). doi: 10.1016/j.stem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Michels BE et al. Pooled in vitro and in vivo CRISPR-Cas9 screening identifies tumor suppressors in human colon organoids. Cell Stem Cell (2020). doi: 10.1016/j.stem.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 192.Ringel T et al. Genome-scale CRISPR screening in human intestinal organoids identifies drivers of TGF-β resistance. Cell Stem Cell (2020). doi: 10.1016/j.stem.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 193.Du Y et al. Development of a miniaturized 3D organoid culture platform for ultra-high throughput screening. J. Mol. Cell Biol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]