Abstract
Context
Atrial fibrillation (AF), cardiac arrhythmias, and related risk factors are common in patients with Cushing’s syndrome, or clinical chronic hypercortisolism. While hypercortisolism may be associated with AF, this association has not yet been ascertained causally.
Objective
To determine whether plasma cortisol is causally associated with AF using a 2-sample Mendelian randomization (MR) design.
Methods
Three genetic variants in the SERPINA1/SERPINA6 locus and functionally associated with plasma cortisol were identified in the CORtisol NETwork consortium (12 597 participants). Summary-level genome-wide association study (GWAS) data for the associations between the cortisol-associated variants and AF were obtained from a GWAS meta-analysis of 6 studies (60 620 AF cases and 970 216 noncases) and the FinnGen consortium (17 325 AF cases and 97 214 noncases). The fixed-effects inverse-variance weighted approach accounting for genetic correlations between variants was used for analysis. Multivariable MR analyses were conducted to assess potential mediating effects of systolic blood pressure (SBP) and waist circumference (WC). Summary-level GWAS data for SBP and WC were obtained respectively from the International Consortium of Blood Pressure (757 601 participants) and the Genetic Investigation of ANthropometric Traits consortium (232 101 participants).
Results
One standard deviation increase in genetically predicted plasma cortisol was associated with greater risk of AF (odds ratio [OR] 1.20, 95% CI 1.06-1.35). The association attenuated when adjusting for genetically predicted SBP and WC (OR 0.99, 95% CI 0.72-1.38).
Conclusion
Evidence derived from the MR study suggests a positive association between plasma cortisol and risk of AF, likely mediated through SBP and WC.
Keywords: Atrial fibrillation, Cushing’s syndrome, cortisol, Mendelian randomization
Cortisol is a steroid hormone that is vital in the response to stress and regulates a wide range of homeostatic functions in the human body. Unregulated cortisol levels can contribute to metabolic pathophysiology (1). Abnormally high levels of cortisol for a prolonged period of time can lead to Cushing’s syndrome (CS). Common clinical manifestations of CS include hypertension, obesity, and hypokalemia (2-4), all of which are metabolic risk factors for atrial fibrillation (AF), the most common sustained arrhythmia. Observational evidence also suggest that AF is likely in patients with CS (5).
Since patients with CS have cortisol levels at the extreme end of the population distribution, common features of CS, also risk factors for AF, can approximate potential outcomes of prolonged exposure to high levels of cortisol in otherwise healthy populations. While the connection between stress and cortisol secretion is well known (6) and the association between stress-induced hypercortisolemia and AF has been investigated in animal tissue studies (7), human observational studies have not yet investigated the association between cortisol and AF in the general population. However, large cohort studies have provided evidence supporting potential association between exposure to external stressors and AF (8, 9).
Importantly, limitations inherent to observational designs, such as confounding and reverse causality, preclude causal interpretation of the potential association between hypercortisolemia and AF both in patients with CS and in the general population. To clarify the potential causal association between physiologically high cortisol levels and risk of AF, we conducted a Mendelian randomization (MR) study using data from large-scale genetic consortia. We assessed potential mediating effects by systolic blood pressure and central obesity (measured as waist circumference). Understanding whether cortisol is causally associated with AF can provide insight into treatment and monitoring of cardiovascular-related CS symptoms and reduce CS morbidity. Additionally, this analysis may provide indirect evidence as to whether prolonged hypercortisolemia can impact AF incidence in the general population.
Materials and Methods
Data sources
Morning plasma cortisol was proxied by 3 single nucleotide polymorphisms (SNPs), rs2749527, rs12589136, and rs11621961, in SERPINA6 (encoding corticosteroid binding globulin) and SERPINA1 (encoding α1-antitrypsin which inhibits cleavage of the reactive center loop that releases cortisol from corticosteroid binding globulin). SERPINA1 and SERPINA6 genes are both highly expressed in tissues associated with cortisol physiology, such as the liver and pancreas, supporting association between SNPs and plasma cortisol levels (10). These 3 partially correlated SNPs (linkage disequilibrium R2 ranged from 0.074 to 0.265 in European populations) were identified in a genome-wide association study (GWAS) meta-analysis for morning plasma cortisol levels in 12 597 participants of European ancestries and replicated in 2795 participants (11).
Summary-level GWAS data for the associations between the cortisol-associated SNPs and AF were obtained from a GWAS meta-analysis of 6 studies (The Nord-Trøndelag Health Study, deCODE, the Michigan Genomics Initiative, DiscovEHR, UK Biobank, and the AFGen consortium) with a total of 60 620 AF cases and 970 216 noncases of European ancestries (12) and the FinnGen consortium with 17 325 AF cases and 97 214 noncases of European ancestries (13). Summary-level GWAS data for systolic blood pressure and waist circumference were obtained respectively from the International Consortium of Blood Pressure (n = 757 601 participants) (14) and Genetic Investigation of ANthropometric Traits consortium (n = 232 101 participants) (15). Ethical approval and informed consent from participants had previously been obtained in individual studies included in the GWAS meta-analyses.
Power Calculations
A power calculation was performed using the mRnD software (16). The significance level was set at .05 and the proportion of variation in cortisol explained by SNPs (R2) was 0.54% (11). Due to the lack of previous evidence explicitly assessing the association of hypercortisolemia with AF risk, we used the average estimates from 2 large-scale investigations into the effect of chronic psychological stress on AF, which resulted in an average odds ratio (OR) of 1.25 (8, 9). Under these assumptions, 99% power was estimated for the GWAS meta-analysis and 58% power for FinnGen alone (100% in the pooled sample).
Statistical Analysis
The MendelianRandomization package for the R software was used for the statistical analyses (17). The fixed-effects inverse-variance weighted model with adjustment for the correlations among SNPs was used as statistical method. The correlation matrix was obtained in 367 643 unrelated participants of European ancestries in UK Biobank. Multivariable MR analysis was conducted to assess potential mediating effect of systolic blood pressure and waist circumference. The MR estimates derived from analysis of the 2 data sources were combined using fixed-effects meta-analysis.
Results
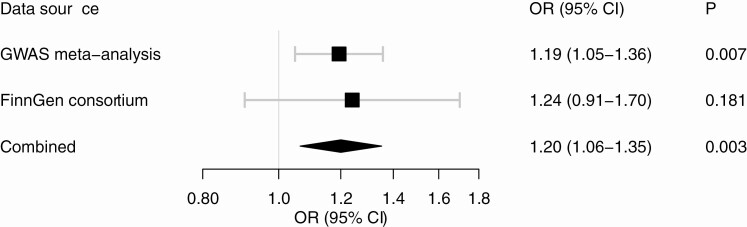
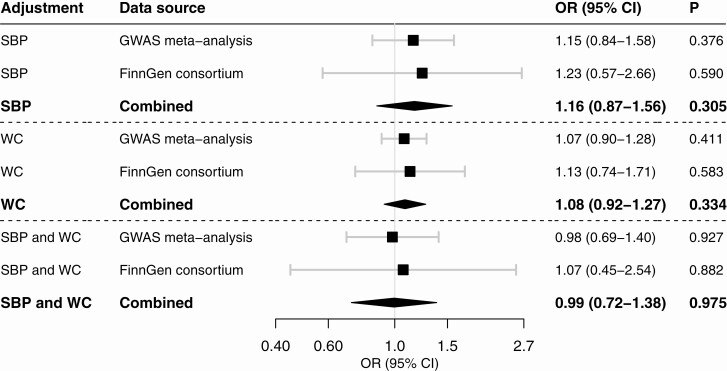
The 3 SNPs used to proxy plasma cortisol and their associations with AF in the 2 data sources are shown in Table 1. Higher genetically proxied plasma cortisol levels were associated with a statistically significant increased risk of AF in the GWAS meta-analysis, and the MR estimate was of similar magnitude but nonsignificant in the FinnGen consortium (Fig. 1). In meta-analysis of results from the 2 data sources, the OR of AF per 1 SD increase of plasma cortisol was 1.20 (95% CI 1.06-1.35). The association between genetically proxied plasma cortisol and AF was attenuated in multivariable MR analysis with adjustment for genetically predicted systolic blood pressure or waist circumference, and did not persist after adjustment for both mediators (OR 0.99, 95% CI 0.72-1.38) (Fig. 2).
Table 1.
Characteristics of the single nucleotide polymorphisms used to proxy plasma cortisol levels and their associations with AF
| Plasma cortisol | AF in GWAS meta-analysisa | AF in FinnGen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Gene | EA | Beta (SE)b | P | Beta | SE | P | Beta | SE | P |
| rs12589136 | 14 | SERPINA6 | T | 0.10 (0.015) | 3.3 × 10–12 | 0.011 | 0.008 | .175 | 0.016 | 0.020 | .418 |
| rs11621961 | 14 | SERPINA6 | T | –0.08 (0.013) | 4.0 × 10–8 | –0.017 | 0.007 | .018 | –0.019 | 0.017 | .272 |
| rs2749527 | 14 | SERPINA1 | T | –0.08 (0.013) | 5.2 × 10–11 | –0.021 | 0.007 | .002 | –0.024 | 0.016 | .143 |
Abbreviations: AF, atrial fibrillation; Chr, chromosome; EA, effect allele; GWAS, genome-wide association study; SE, standard error; SNP, single nucleotide polymorphism.
a Includes data from 6 studies, including The Nord-Trøndelag Health Study, deCODE, the Michigan Genomics Initiative, DiscovEHR, UK Biobank, and the AFGen Consortium.
b The beta coefficients and corresponding standard errors represent the age- and sex-adjusted cortisol z-score change in morning plasma cortisol per additional effect allele in 12 597 participants of European ancestries.
Figure 1.
Association between genetically proxied plasma cortisol and risk of atrial fibrillation. The estimates are scaled per 1 SD increase in plasma cortisol and were derived from the fixed-effects inverse-variance weighted method with adjustment for the correlations between genetic variants.
Figure 2.
Association between genetically proxied plasma cortisol and risk of atrial fibrillation in multivariable Mendelian randomization analysis adjusted for genetically predicted systolic blood pressure (SBP), waist circumference (WC), or both. The estimates are scaled per 1 SD increase in plasma cortisol and were derived from the fixed-effects inverse-variance weighted method with adjustment for the correlations between genetic variants and for SBP, WC, or both.
Discussion
This MR study is the first to utilize genetic variants associated with cortisol to examine causal association with AF. Results showed that genetic predisposition to higher plasma cortisol is associated with an increased risk of AF. Elevated cortisol levels in CS are associated with hypertension and central obesity (2-4), both of which result in left ventricular hypertrophy and structural changes (18, 19) that increase the risk for AF (20-23). In this study, the association between genetically predicted cortisol levels and AF appeared to be mediated by systolic blood pressure and waist circumference, as the association attenuated upon adjustment for these factors.
This study has several strengths. Compared with observational studies, MR is less prone to biases such as residual confounding and reverse causality. In an observational context, cortisol could increase AF susceptibility, but AF could also lead to increased internal stress that heightens circulating cortisol levels. This is unlikely when using genetic instruments, as these are fixed at conception. Secondly, it is worth noting that the availability of genetic variants in SERPINA1 and SERPINA6, implicated in cortisol transport via corticosteroid binding globulin, enabled us to use highly specific MR instruments. Approximately 80% to 90% of circulating cortisol is bound to corticosteroid binding globulin, which plays a critical role in regulating plasma cortisol levels and modulating tissue availability of biologically active free cortisol (24, 25). Thirdly, the slowly progressive, nonspecific set of symptoms for CS that converge and coexist with common chronic diseases contributes to profound underreporting and underdiagnosis of this disease in the general population. Finally, because MR approximates long-term, cumulative cortisol exposure over a lifetime, the findings of this study can not only encourage AF monitoring in patients with CS, but also provide insight into potential risk of AF caused by sustained hypercortisolemia in general populations.
This investigation has however a number of limitations. Firstly, the SNPs selected for the present study only explain 0.54% variation in morning plasma cortisol. In theory, our results may be susceptible to the weak instrument bias, distorting estimates towards the null. However, our preference for SNPs located in a biologically relevant locus reduces this possibility. Secondly, results of the MR study could also be confounded if any our selected SNPs were in linkage disequilibrium with other correlated SNPs causally associated to AF via a noncortisol-mediated pathway (26, 27). Since linkage disequilibrium (LD) typically affects only nearby genetic variants, our analyses mitigate the risk of LD by modelling genetic effect on cortisol via multiple variants (27). Additionally, if multiple variants in LD are used as independent genetic exposures, genetic effects on cortisol will be fundamentally overestimated. Though there may be a small degree of LD between the 3 SNPs used in this study, they also show different associations with corticosteroid binding globulin biochemistry (11), suggesting that they exert independent effects on cortisol. Thirdly, the genetic effects of cortisol might be buffered by a physiological compensatory mechanism regardless of the underlying genotype, a phenomenon termed canalization that can lead to bias in the genetic associations with cortisol (28). Since cortisol is highly regulated, compensatory processes can turn on in response to perturbations of cortisol homeostasis, resulting in underestimated genetic effects. Finally, our datasets primarily include only European cohorts which limits applicability of study results to non-European populations.
Despite these limitations, our findings highlight the potential relevance of hypercortisolemia to AF and provide mechanistic insight into this association. Overall, these results are supportive of screening for AF in patients with CS, and suggest that the impact of prolonged hypercortisolemia on AF in general populations should be investigated more closely.
Acknowledgments
The authors would like to thank the investigators of the FinnGen consortium, International Consortium of Blood Pressure, and Genetic Investigation of ANthropometric Traits consortium for sharing summary-level GWAS data.
Financial Support: S.C.L. acknowledges research support from the Swedish Research Council (Vetenskapsrådet, 2016-01042 and 2019-00977), the Swedish Research Council for Health, Working Life and Welfare (Forte, 2018-00123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden, 20190247). S.B. is supported by Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z). During the conduction of this study E.A. was supported by the EU/EFPIA Innovative Medicines Initiative Joint Undertaking BigData@Heart grant no. 116074 and is currently funded by the British Heart Foundation Programme Grant RG/18/13/33946. This work was supported by core funding from: the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194; RG/18/13/33946) and the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). This work was also supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Contributions: S.C.L., S.B., and E.A. designed the study. S.C.L. performed the statistical analyses and created the figure. S.C.L., W-H.L., and E.A. drafted the manuscript. S.C.L., W-H.L., S.B., and E.A. interpreted the data and edited the manuscript. All authors have given final approval of the version to be published.
Glossary
Abbreviations
- AF
atrial fibrillation
- CS
Cushing’s syndrome
- GWAS
genome-wide association study
- LD
linkage disequilibrium
- MR
Mendelian randomization
- OR
odds ratio
- SBP
systolic blood pressure
- SNP
single nucleotide polymorphism
- WC
waist circumference
Additional Information
Disclosures: The authors have no conflicts of interest to declare.
Data Availability
All data used in this study are publicly available summary-level data, with the relevant studies cited. Data needed for the primary analyses are available in Table 1.
References
- 1. Katsu Y, Iguchi T.In: Takei Y, Ando H, Tsutsui K, eds. Handbook of Hormones: Subchapter 95D—Cortisol. 1st ed. Academic Press; 2016:533-534, e595D-532. [Google Scholar]
- 2. Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1(4):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci. 2002;970:134-144. [DOI] [PubMed] [Google Scholar]
- 5. Koracevic G, Mićić S, Stojanović M, et al. High likelihood for atrial fibrillation in Cushing’s syndrome. Eur Rev Med Pharmacol Sci. 2020;24(3):1391-1397. [DOI] [PubMed] [Google Scholar]
- 6. Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83(6):1853-1859. [DOI] [PubMed] [Google Scholar]
- 7. Embi AA, Scherlag BJ. An endocrine hypothesis for the genesis of atrial fibrillation: the hypothalamic-pituitary-adrenal axis response to stress and glycogen accumulation in atrial tissues. N Am J Med Sci. 2014;6(11):586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosman L, Lampert R, Ramsey CM, et al. Posttraumatic stress disorder and risk for early incident atrial fibrillation: a prospective cohort study of 1.1 million young adults. J Am Heart Assoc. 2019;8(19):e013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fransson EI, Nordin M, Magnusson Hanson LL, Westerlund H. Job strain and atrial fibrillation—results from the Swedish Longitudinal Occupational Survey of Health and meta-analysis of three studies. Eur J Prev Cardiol. 2018;25(11):1142-1149. [DOI] [PubMed] [Google Scholar]
- 10. The Genotype-Tissue Expression (GTEx) portal. Broad Institute of MIT and Harvard. https://gtexportal.org/home/. Accessed April 29, 2021.
- 11. Bolton JL, Hayward C, Direk N, et al. ; CORtisol NETwork (CORNET) Consortium . Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10(7):e1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen JB, Thorolfsdottir RB, Fritsche LG, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50(9):1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FinnGen consortium. FinnGen Documentation of R4 release, 2020. Accessed December 10, 2020. https://finngen.gitbook.io/documentation/
- 14. Evangelou E, Warren HR, Mosen-Ansorena D, et al. ; Million Veteran Program . Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50(10):1412-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10A):9G-14G. [DOI] [PubMed] [Google Scholar]
- 19. Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aune D, Sen A, Schlesinger S, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(3):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YG, Han KD, Choi JI, et al. Impact of the duration and degree of hypertension and body weight on new-onset atrial fibrillation: a nationwide population-based study. Hypertension. 2019;74(5):e45-e51. [DOI] [PubMed] [Google Scholar]
- 22. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nazarzadeh M, Pinho-Gomes AC, Bidel Z, et al. Genetic susceptibility, elevated blood pressure, and risk of atrial fibrillation: a Mendelian randomization study. Genome Med. 2021;13(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardill BR, Vogl MR, Lin HY, Hammond GL, Muller YA. Corticosteroid-binding globulin: structure-function implications from species differences. PLoS One. 2012;7(12):e52759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. J Biol Chem. 2007;282(40):29594-29603. [DOI] [PubMed] [Google Scholar]
- 26. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. [DOI] [PubMed] [Google Scholar]
- 28. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are publicly available summary-level data, with the relevant studies cited. Data needed for the primary analyses are available in Table 1.