We have used a novel non-invasive doubly labelled water technique and detailed behaviour observations to show that birds might abandon incubation during hot periods to avoid dehydration as a consequence of incubating at high temperatures; potentially exposing their eggs to the risk of overheating and becoming unviable.
Keywords: Climate change, cooperative breeding, high temperatures, incubation, parental care, southern pied babbler
Abstract
High air temperatures have measurable negative impacts on reproduction in wild animal populations, including during incubation in birds. Understanding the mechanisms driving these impacts requires comprehensive knowledge of animal physiology and behaviour under natural conditions. We used a novel combination of a non-invasive doubly labelled water (DLW) technique, nest temperature data and field-based behaviour observations to test effects of temperature, rainfall and group size on physiology and behaviour during incubation in southern pied babblers Turdoides bicolor, a cooperatively breeding passerine endemic to the arid savanna regions of southern Africa. The proportion of time that clutches were incubated declined as air temperatures increased, a behavioural pattern traditionally interpreted as a benefit of ambient incubation. However, we show that (i) clutches had a <50% chance of hatching when exposed to daily maximum air temperatures of >35.3°C; (ii) pied babbler groups incubated their nests almost constantly (99% of daylight hours) except on hot days; (iii) operative temperatures in unattended nests frequently exceeded 40.5°C, above which bird embryos are at risk of death; (iv) pied babblers incubating for long periods of time failed to maintain water balance on hot days; and (v) pied babblers from incubating groups lost mass on hot days. These results suggest that pied babblers might leave their nests during hot periods to lower the risk of dehydration associated with prolonged incubation at high operative temperatures. As mean air temperatures increase and extreme heat events become more frequent under climate change, birds will likely incur ever greater thermoregulatory costs of incubation, leading to compromised nest attendance and increased potential for eggs to overheat, with implications for nest success and, ultimately, population persistence.
Introduction
Anthropogenic climate change is driving population declines in birds globally (Iknayan and Beissinger, 2018; Rosenberg et al., 2019; Saino et al., 2011), often via negative impacts on reproduction (Cahill et al., 2013; Cunningham et al., 2013; Stevenson and Bryant, 2000). Many studies have considered the impacts of climate variability and change on birds (Dunn and Møller, 2019; McKechnie, 2019; Pearce-Higgins and Green, 2014). Impacts directly attributable to adverse weather and changing climate regimes include higher risk of mortality (Bourne et al., 2020b; McKechnie and Wolf, 2010; Sharpe et al., 2019), reduced breeding success (Bourne et al., 2020a; Conrey et al., 2016; Cruz-McDonnell and Wolf, 2016; Cunningham et al., 2013; Skagen and Yackel Adams, 2012), compromised body condition and immunocompetence (du Plessis et al., 2012; Edwards et al., 2015; Gardner et al., 2018; Wingfield et al., 2017; Xie et al., 2017), declining populations (Riddell et al., 2019; Saino et al., 2011), range changes (Hockey et al., 2011; Huntley, 2019) and potentially maladaptive behavioural adjustments to foraging (Bladon et al., 2019; Cooper et al., 2019; Cunningham et al., 2015, 2021; Funghi et al., 2019; Pattinson and Smit, 2017), parental care (Bourne et al., 2021; Carroll et al., 2018; Clauser and McRae, 2017; van de Ven, 2017; Wiley and Ridley, 2016) and migration (Dunn et al., 2010; Samplonius et al., 2018).
Hatching failure in birds is particularly common during hot weather (Bourne et al., 2020a; Clauser and McRae, 2017; Wada et al., 2015) and droughts (Conrey et al., 2016), both of which are becoming more frequent under climate warming (Ripple et al., 2019). Eggs of most birds are incubated at temperatures averaging ~ 35.5°C (Drent, 1975) and egg temperatures higher than this are likely to be lethal (Walsberg and Voss-Roberts, 1983; Webb, 1987).
Incubation is energetically costly in temperate environments where eggs need to be kept warm (Ardia et al., 2010; Nord et al., 2010; Nord and Cooper, 2020), but also extremely challenging in warm environments (Amat and Masero, 2004; Coe et al., 2015; Nwaogu et al., 2017), where incubating birds must prevent eggs from overheating (Carroll et al., 2015a; Grant, 1982; McDonald and Schwanz, 2018) while also thermoregulating themselves (DuRant et al., 2019; McKechnie, 2019; O’Connor et al., 2018). Behaviourally, birds initially respond to high temperatures by increasing incubation constancy (AlRashidi et al., 2011; Cones, 2017; Conway and Martin, 2000; Mortensen and Reed, 2018; Mougeot et al., 2014) or engaging in shading behaviour (Brown and Downs, 2003; Clauser and McRae, 2017; Downs and Ward, 1997; Grant, 1982) in order to regulate nest temperatures. Physiologically, the capacity of small endotherms such as birds to tolerate heat exposure is governed by their ability to dissipate heat (McKechnie and Wolf, 2019). In free-living birds, high air temperatures are associated with lower metabolic rates (Bourne et al., 2019; Smit and McKechnie, 2015), dehydration (Bourne, 2020; Sharpe et al., 2019), higher glucocorticid levels (Moagi et al., 2021), impaired cognitive function (Soravia et al., 2021) and even death (Conradie et al., 2020; McKechnie et al., 2012). As incubating birds reach limits in their ability to tolerate high temperatures over long periods, they undertake more frequent or longer incubation recesses (Bourne, 2020; Clauser and McRae, 2017) and may ultimately abandon their nests (Clauser and McRae, 2017; Sharpe et al., 2019). Understanding the behavioural and physiological mechanisms driving hatching failure at high temperatures in situ in wild populations is critical to our ability to predict species-specific responses to climate change (Conradie et al., 2019; Stillman, 2019).
Here we present the first study of avian reproduction combining both direct observations of incubation behaviour under natural conditions and non-invasive physiological measurements from the same individuals at the same time. We investigate climate effects on the behaviour and physiology of incubating adults in southern pied babblers Turdoides bicolor (hereafter ‘pied babblers’), a cooperatively breeding bird. Pied babblers live in groups ranging in size from 3 to 12 adults (Ridley 2016). Adults are defined as individuals aged ≥12 months (Raihani and Ridley, 2007a) and groups consist of a dominant pair and one or more subordinate adults of either sex (Nelson-Flower et al., 2011). Air temperatures between 35°C and 38°C are known to correlate with negative impacts in pied babblers. At air temperatures above ~35.5°C, pied babbler eggs are half as likely to hatch (Bourne et al., 2020a), adult birds typically do not gain enough body mass during the day to offset overnight mass loss (du Plessis et al., 2012) and provisioning to nestlings declines (Wiley and Ridley, 2016). No breeding attempts produce surviving young at air temperatures exceeding 38°C (Bourne et al., 2020a). High average air temperatures during summer are associated with dramatically reduced survival probabilities in adult pied babblers, particularly when these occur in combination with drought (Bourne et al., 2020b; Ridley et al., 2021). Additionally, faecal glucocorticoid levels are elevated in pied babblers at air temperatures above 38°C (Moagi et al., 2021), indicative of an acute physiological response to high temperatures.
Cooperative species may respond differently to environmental variability compared to pair-breeding or solitary species, because reproductive investment and nest outcomes can be influenced by the presence of helpers (van de Ven et al., 2020; Wiley and Ridley, 2016), and so we also considered the influence of the number of adults present in each group and checked for interactions between group size and climate variables (Rubenstein and Lovette, 2007). We hypothesized that high Tair would reduce hatching rates via reduced nest attendance as a result of thermoregulatory costs on incubating adults. This would increase risk of lethal heat exposure for developing embryos. We addressed this hypothesis by testing predictions related to (i) nest outcomes (lower probability of hatching at high Tair); (ii) incubation behaviour (reduction in the proportion of time nests are attended at high Tair); (iii) the temperatures reached in unattended nests at high Tair (exceeding lethal limits for avian embryos, explaining why hot nests are less likely to hatch); and (iv) physiological costs of incubation for adults (higher costs of incubation at higher Tair evident in patterns of energy expenditure, water balance and body mass maintenance). We tested part of the latter prediction using a novel, non-invasive DLW technique (Anava et al., 2000; Bourne et al., 2019). We further expected that larger group sizes would be associated with reduced costs of incubation at higher Tair and improved nest outcomes in our semi-arid study system.
Materials and Methods
Unless otherwise indicated, summary statistics are presented as mean ± one standard deviation.
Study site and system
Fieldwork took place at the 33km2 Kuruman River Reserve (26°58’S, 21°49′E) in the southern African Kalahari. Mean summer daily maximum temperatures in the region averaged 34.7 ± 9.7°C and mean annual precipitation averaged 186 ± 88 mm (1995–2015; van de Ven, McKechnie and Cunningham 2019). Rainfall has been declining and high temperature extremes increasing in both frequency and severity over the past 20 years (Bourne et al., 2020a; Kruger and Sekele, 2013; van Wilgen et al., 2016).
Pied babblers are medium-sized (60–90 g), cooperatively breeding passerines that live in groups ranging in size from 3 to 15 adults (Raihani and Ridley, 2007b) and are endemic to the Kalahari (Ridley, 2016). Resident, territorial groups consist of a single breeding pair (one dominant male and female) with subordinate helpers of both sexes (Nelson-Flower et al., 2011) and can be reliably located by visits to each territory (Ridley, 2016). Individuals in the study population are habituated to observation by humans at distances of 1–5 m (Ridley and Raihani, 2007) and are individually identifiable by a unique combination of metal and colour leg rings.
Pied babblers build open cup nests, usually in camelthorn Vachellia erioloba trees and usually breed during spring and summer (Bourne et al., 2020a; Ridley, 2016). During each breeding attempt, a clutch of ~3 eggs is laid and incubated for 13–15 days (Bourne et al., 2020a; Ridley and Raihani, 2008). While only the dominant female incubates overnight (Ridley, 2016), during the day all adult group members (individuals, >1 year old), including subordinates, take turns to incubate and the nest is rarely left unattended for more than a few minutes at a time (Ridley and Raihani, 2007; Ridley and van den Heuvel, 2012). Pied babblers will drink water when it is available, but can obtain all of their water from their food, and at least two of the groups in the study population do not have access to water in their territories.
Data collection
Data were collected during each austral summer breeding season between September 2016 and February 2019 (three breeding seasons). We recorded air temperature (°C), solar radiation (W·m−2), wind speed (m·s−1), relative humidity (%) and rainfall (mm) using an on-site weather station (Vantage Pro2, Davis Instruments, Hayward, USA; factory calibration with accuracy = 0.3°C). For our analyses, we caculated daily maximum air temperature (Tmax), daily maximum solar radiation (Solmax) and daily maximum wind speed (Windmax) for each observation day and total rainfall in the two months prior to each observation day (mm). We calculated absolute humidity (g·m−3) for each pair of air temperature and relative humidity values (Campbell and Norman, 1988) and calculated the absolute humidity value coinciding with Tmax (AbsHumTmax). For analyses of nest outcomes, we additionally calculated average Tmax, Solmax, Windmax and AbsHumTmax between initiation of incubation and hatching (MeanTmaxInc, MeanSolmaxInc, MeanWindmaxInc and MeanAbsHumTmaxInc). We recorded group size (number of adults) during each breeding attempt in each group. Tmax ranged from 20.7°C to 40.8°C (mean = 34.1 ± 4.5), Solmax from 186 to 1383 W·m−2 (mean = 999 ± 150), Windmax from 0 to 8.9 m·s−1 (mean = 4.2 ± 1.4), AbsHumTmax from 0.8 to 14.4 g·m−3 (mean = 5.2 ± 3), rainfall from 0.2 to 140.2 mm (median = 15) and group size from 3 to 6 adults (mean = 4 ± 1).
Nest outcomes
Monitoring of nest outcomes (99 breeding attempts by 23 pied babbler groups, with mean = 4, range of 1–10 nests per group, over 3 breeding seasons) followed Ridley and van den Heuvel (2012). Breeding attempts were defined as discrete clutches laid and incubated. Nests were located by observing nest building during weekly monitoring visits. Once located, the nests were checked approximately every 2 days to identify incubation start and hatch dates: nests were categorized as hatched when adult group members were observed carrying food items to the nest and as failed when nests were left unattended for longer than 90 minutes on two consecutive monitoring visits or the group was observed building a new nest. Incubation starts when all eggs are laid and the dominant female begins to incubate overnight.
Incubation behaviour
Incubation bout and recess data were collected by waiting near the nest at dawn, observing the first bird to replace the dominant female in the morning (05 h00–06 h48) and remaining with the group all day until 19 h00 (46 observation days at 35 nests). Observations were collected once during the incubation period for most nests and on two or more days (up to a maximum of 4 days) for 8 nests. We recorded the start and end time of each incubation bout and the duration of any time periods during which the nest was left unattended (recesses). These data were used to calculate the proportion of time per day that clutches were incubated (sum of all incubation bout durations per day/total observation time). Both members of the dominant pair incubated on every observation day, with the help of at least one subordinate group member on most (91%) days. In over 90% of cases, the incubating bird did not leave the nest until it was replaced by another, therefore making it unlikely that many incubation recesses were missed.
Nest temperatures
To quantify variation in the thermal properties of pied babbler nests, we measured operative temperature [Te: a measure of thermal load experienced by the bird (Bakken et al., 1985)] using black bulb thermometers (Bakken et al., 1985, 2001; Carroll et al., 2015; Cunningham et al., 2015; Howell and Bartholomew, 1961; Pattinson and Smit, 2017) placed in 23 nests within 5 days of fledge/fail (Griffith et al., 2016), recording constantly for ~2 weeks (12.5 ± 3 days; range, 9–20 days; n = 21 872 records of daytime Te in total). Weather conditions were not significantly different between the active next period and the time periods during which Te was recorded in the nests (paired t-tests, all P > 0.05; see Fig. S1, Table S1). Black bulb thermometers comprised two copper half spheres (which approximates pied babbler thoracic cavity dimensions; diameter, 42 mm; thickness, 0.8 mm), sealed together using cryanoacrylate adhesive, painted matte black (Carroll et al., 2015a; van de Ven et al., 2019) and containing internally mounted temperature loggers (Thermocron iButton, DS1923, Maxim, Sunnyvale, CA, USA; resolution, 0.0625°C) logging at 10-minute intervals (Cunningham et al., 2015; van de Ven et al., 2019) synchronized with Tair records from the onsite weather station. The iButton loggers were calibrated in a circulating water bath against a factory-calibrated NiCr-NiAl thermocouple (Thermocouple HH21A, Omega Engineering, Stamford, USA; van de Ven, 2017).
Black bulb thermometers do not provide a complete representation of thermal conditions experienced by incubating pied babblers because they mimic neither feather arrangement nor colour (Carroll et al., 2017) and do not account for humidity or evaporative heat loss (Bakken et al., 1985). Nonetheless, they provide a relative measure of differences in temperature across nest microsites, which cannot be quantified by measuring Tair alone (Cunningham et al., 2015). We also acknowledge that using Tair as a predictor variable for babbler behaviour, physiology and nest outcomes (below), instead of estimated Te experienced by incubating adults, imposes limitations on the interpretation of our data. We opted for this approach for two reasons. First, accurate measurements of species-specific Te experienced by incubating adults would require the use of taxidermic mounts calibrated against measurements of evaporative water loss and metabolic rate in babblers exposed to a range of wind speeds and solar irradiance levels under laboratory conditions (Walsberg and Wolf, 1996), an undertaking beyond the scope of this study. Second, although Tair is a crude index of pied babbler’s thermal environments, this approach ensures our data are comparable to those reported in previous studies evaluating the fitness costs of periods of hot weather (Cooper et al., 2019; du Plessis et al., 2012; Edwards et al., 2015; Sharpe et al., 2019; van de Ven et al., 2019; van de Ven et al., 2020) and allows our data to be useful in the context of models of future climate, which inevitably use Tair rather than species- or site-specific Te. Synchronized recording intervals enabled comparisons between nest-specific Te and simultaneously occurring Tair. However, the differences between nest-site Te recorded by the black bulb thermometers and Tair recorded by the weather station reiterate that the latter is a crude approximation of the thermal environments experienced by pied babblers. Future studies investigating hot weather effects could benefit from including measures of Te instead of or as well as Tair as a predictor.
Energy expenditure and water balance
During observation days on which incubation bout and recess data were recorded, we also obtained detailed physiology [daily energy expenditure (DEE) and water balance] and behaviour (incubation effort) data for a subset of adult birds from the incubating groups (up to four individuals per observation day; mean = 1.6 ± 0.9; n = 70 individuals in total). We obtained physiology data from individuals across a range of Tmax values [35 measured on ‘hot’ days, Tmax ≥ 35.5°C, identified as a critical temperature threshold in pied babblers (Bourne et al., 2020a; du Plessis et al., 2012; Wiley and Ridley, 2016); 35 on ‘cool’ days, Tmax < 35.5°C] and group sizes (3–6 adults), as well as both sexes (38 females, 31 males, 1 unknown sex) and ranks (40 dominant birds, 30 subordinate birds). Data on DEE (kJ g−1 day−1) and water balance were collected using a non-invasive DLW technique (Anava et al., 2000; Scantlebury et al., 2014), recently validated and described in detail for pied babblers (Bourne et al., 2019).
In brief, selected individuals were dosed with ~50 μL of DLW—a non-toxic isotopic solution enriched with oxygen-18 (measured as δ18O) and deuterium (measured as δ2H)—injected into beetle larvae Zophobias morio and fed to the birds between 06 h00 and 09 h00 on the observation day. Body water samples were then obtained during all daylight hours over a 24-hour observation period by collecting droppings from dosed individuals as they were excreted naturally onto the ground. Water samples were extracted from droppings by cryogenic distillation, using a technique adapted from Priyadarshini et al. (2016) and analysed in a PAL autosampler and DLT-100 liquid water isotope analyser (Los Gatos Research, Mountain View, CA, USA) following the procedures described by Smit and McKechnie (2015) and Bourne et al. (2019). We calculated CO2 production (rCO2) from the body water pool and the rate of decline of the natural log of the ratio of δ18O/δ2H (Nagy and Costa, 1980; Speakman, 1997). We used Speakman’s (1997, Equation 17.7; see Equation (1) below) for calculations of rCO2 in mol d−1 because empirical testing has shown this equation to be the most accurate (Visser et al., 2000) and based on the most realistic assumptions of fractionation during evaporation (Butler et al., 2004; Speakman and Hambly, 2016):
 |
(1) |
where N is moles of body water and values of k represent turnover of an isotope identified by the subscript. The divisor of N (2.078) accounts for the fact that each molecule of CO2 expired removes two molecules of oxygen from the pool and, with the inclusion of the last term (0.0062 • kH • N), reflects a correction for fractionation. We calculated kH in the final term of Equation (1) based on change in ln(δ2H) between maximally enriched samples collected at early time points and final samples, where t is time (in days) elapsed between early and final samples:
 |
(2) |
Values of 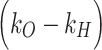 can be calculated from the rate of decline of ln(
can be calculated from the rate of decline of ln( ) (Nagy and Costa, 1980; Speakman, 1997):
) (Nagy and Costa, 1980; Speakman, 1997):
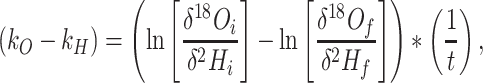 |
(3) |
where δ18Oi and δ2Hi are the initial δ18O and δ2H values in faeces or blood and δ18Of and δ2Hf are the final δ18O and δ2H values. rCO2 was converted from mol d−1 to L d−1 using the conversion factor 22.4 l of ideal gas per mol at standard temperature and pressure, and L CO2 d−1 was converted to kJ d−1 using the relationship 27.42 kJ/l CO2 for an insectivorous bird (Gessaman and Nagy, 1988) and used to estimate DEE (otherwise known as field metabolic rate, in kJ g−1 d−1).
Water balance was calculated by dividing water influx by water efflux, where values >1 indicate positive water balance (a hydrated status) and values <1 indicate negative water balance (a dehydrated status). We used Nagy and Costa’s (1980) Equation 4 (see Equation (4) below) and Equation 6 (see Equation (5) below) to calculate water efflux and water influx (ml H2O kg−1d−1), respectively:
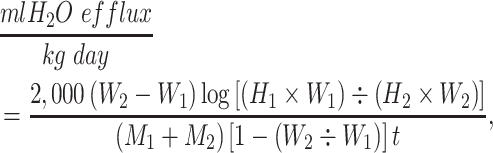 |
(4) |
 |
(5) |
where the subscripts 1 and 2 represent initial and final values respectively, H = measured deuterium enrichment levels, M = body mass in grams, W = the body water pool and t = time in days between initial and final sampling of deuterium enrichment levels. The body water pool was estimated as 69.3% of body mass, based on measured total body water in 6 pied babblers at a nearby site in a similar habitat (Bourne et al., 2019). If mean body water were 3% lower or higher in the individuals in this study than the average we used, then mean DEE would have been about 3% lower or higher than we calculated (Bourne et al., 2019). This is an acceptable consequence that follows standard practice in the single-sample DLW method, where percentage body water by mass is typically measured in a sample of other individuals (Niizuma and Shirai, 2015; Speakman, 1997) and applied as a constant to a study population.
In order to estimate the number of extra prey items pied babblers would need to eat to make up any water deficit and maintain water balance at high temperatures, we converted DEE to metabolic water production (g d−1, Equation (6)) and averaged these values for extreme temperatures in the dataset (≥39°C, n = 6; <26°C, n = 4):
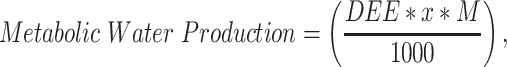 |
(6) |
where x is the average value of 27 mg kJ-1 for the rate of metabolic water production from fuel oxidation (Schmidt-Nielson, 1990), based on typical macronutrient composition of mealworm Tenebrio molitor larvae (Liu et al., 2020; Siemianowska et al., 2013), and M is the body mass of the bird in grams. Beetle larvae are a common prey consumed by pied babblers and, in terms of preformed water, mealworms are similar to species regularly consumed by pied babblers. Mealworms weigh ~0.2 g (Raihani and Ridley, 2007a) and are 56% water (Siemianowska et al., 2013).
Because continuous attention is required to collect faecal samples from wild, free-living birds, it was generally only possible to collect detailed behaviour data from one bird per observation day. To identify the proportion of time adult birds dosed with DLW allocated to incubation, we used data collected during ~4 × 20-minute continuous time-activity focal behaviour observations (‘focals’; Altmann, 1974) within each of 6 focal sessions per day (mean = 23 focals per bird per day; range, 15–27; n = 48 focal days; data were collected from 2 birds on the same day on 5 occasions, i.e. 10 of the focal days). Focal sessions lasted 2 hours each, with the first starting at 07 h00 and the last at 17 h00, and the data were captured on an Android smartphone (Mobicel Trendy), using Prim8 software (McDonald and Johnson, 2014) in which the duration of each observed behaviour is recorded to the nearest second.
Body mass
To determine effects of weather and social factors on body mass maintenance of adults from groups incubating clutches, body mass data were collected from as many adult group members as possible on observation days (mean = 2.6 ± 1.4 measurements per observation day; range, 1–5). These data were obtained by enticing individuals to stand on a top pan balance in exchange for a small food reward (Ridley, 2016), and were collected at dawn on the morning of each observation day (Mass1) and again at dawn the following morning (Mass2). Body mass change (∆Mb) was calculated in grams as Mass2 − Mass1 [n = 129; pied babblers are size monomorphic (Bourne et al., 2018; Ridley, 2016) and individuals in the study had similar starting weights (mean = 75.8 g, coefficient of variation = 0.07; Table S2), so using a relative measure (Mass2 − Mass1/Mass1) did not change interpretation of the models].
Statistical analyses
Statistical analyses were conducted in the R statistical environment, v 3.6.0 (R Core Team, 2017), primarily using mixed-effects models in the package lme4 (Bates et al., 2015). Model checking and model selection followed Harrison et al. (2018). All continuous explanatory variables were centred by subtracting the mean and scaled by dividing by the standard deviation. Additive models were built from significant terms tested in univariate models. All explanatory variables were tested for correlation with one another and correlated variables (VIF > 2) were not included in the same additive models. Akaike’s information criterion corrected for small sample size (AICc) with maximum likelihood estimation was used to determine which models best explained patterns of variation in the data; model estimates with confidence intervals that did not intersect zero were considered to explain significant patterns within our data, and model fits were evaluated using Normal Q-Q plots, histograms of residuals and dispersion parameters as appropriate (Bates et al., 2015). Rainfall in the two months prior to initiation of incubation was correlated with breeding season (F2,67 = 10.994; P < 0.001). We chose the categorical variable ‘breeding season’ for all analyses due to the fact that high rainfall only occurred during one breeding season (2016/2017). Quadratic terms for continuous predictors were included when there was no significant linear effect and visualization of the data suggested a non-linear relationship. Where several models were within 2 AICc of the top model, top model sets were averaged (Burnham and Anderson, 2002; Symonds and Moussalli, 2011) using the package MuMin (Barton, 2015) and model-averaged coefficients were presented. Sensitivity power analysis (Champely et al., 2018; Greenland et al., 2016) suggested sufficient sample size to detect all main effects, but limited power to detect interactions given our data (Table S3).
To determine which variables predicted (i) nest outcomes (hatched = 1, failed = 0) and (ii) the overall proportion of time clutches were incubated per day (time incubated/time observed), we used generalized linear mixed-effects models with binomial error structure and logit link function. We considered the influence of breeding season, weather [for (i) MeanTmaxInc, MeanSolmaxInc, MeanWindmaxInc and MeanAbsHumTmaxInc; for (ii) Tmax, Solmax, Windmax and AbsHumTmax on observation day], group size, group size^2 and the interactions between breeding season and group size and Tmax and group size. To account for repeated measures and thus for nonindependence of data, we included nest identity as a random factor. For (ii), we further included an observation level random factor to address overdispersion in the data (Harrison, 2014). The inclusion of group identity as a random term in addition to nest identity resulted in unstable models and, of the two random terms, nest identity explained the greatest proportion of variation while avoiding destabilizing the models (Grueber et al., 2011; Harrison et al., 2018).
To determine which variables predicted DEE (n = 68) and water balance (n = 69), we used maximum likelihood linear mixed-effects models (LMMs) to test the following predictors: breeding season, Tmax, Solmax, Windmax, AbsHumTmax, group size, sex, rank and the interactions between breeding season and group size and Tmax and group size. For a subset of individuals for which we collected both behaviour and physiology data from the same birds on the same day (26 different individuals), we further considered the influence of proportion of time spent incubating on DEE (38 observation days) and water balance (39 observation days), fitting separate linear regressions for hot (≥35.5°C) and cool (<35.5°C) days. Individual identity was included as a random factor for all DEE and water balance analyses. The inclusion of nest or group identity as a random term in addition to individual identity resulted in unstable models and, of the two random terms, individual identity explained the greatest proportion of variation while avoiding destabilizing the models (Grueber et al., 2011; Harrison et al., 2018).
To determine which variables predicted ∆Mb, we used the package segmented (Muggeo, 2008) to identify the temperature threshold (‘breakpoint’) above which ability to maintain body mass between days was compromised, followed by separate LMMs for the data above and below the breakpoint. For each model segment, we considered the influence of breeding season, Tmax, Solmax, Windmax, AbsHumTmax, group size, sex, rank and the interactions between breeding season and group size and Tmax and group size, with nest identity included as a random factor.
Results
Nest outcomes
Of 99 nests monitored over 3 breeding seasons, 61 hatched and 38 failed. Mean TmaxInc was the most parsimonious predictor of variation in hatching success in pied babblers (the single best-fit model had a model weight of 0.794), and pied babbler nests were less likely to hatch as Mean TmaxInc experienced during incubation increased (Est = −0.949 ± 0.254, 95% CI: −1.479 to −0.477, z = −3.744, conditional R2 = 0.215; Fig. 1; see Supporting Information Table S4 for full model outputs). When Mean TmaxInc exceeded 35.3°C during incubation, the probability of pied babbler nests hatching dropped below 50%.
Figure 1.

Nest outcomes as a function of mean daily maximum temperatures during incubation with the line showing the model fit and grey shaded area the 95% confidence interval (data from 99 nests by 23 southern pied babbler T. bicolor groups over 3 breeding seasons).
Nest attendance
The percentage of time between dawn and 19 h00 that clutches were incubated ranged from 57.3 to 100% (median = 99%). Only 3 nests were incubated for <80% of daylight hours, all of which were observed on days with Tmax > 37°C and all of which ultimately failed. Tmax was the most parsimonious predictor of variation in the proportion of time that clutches were incubated. The single best-fit model (Tmax) had a model weight of 0.898 and the percentage of time clutches were incubated declined as temperatures increased (Est = −1.650 ± 0.492, 95% CI: −2.780 to −0.754, z = −3.355, conditional R2 = 0.998; Fig. 2; see Supporting Information Table S5 for full model ouputs). Additionally, the number of times clutches were left unattended per day (Fig. S2; Table S6), the proportion of time clutches were left unattended per day (Fig. S3; Table S7) and the probability of observing clutches that were left unattended at all (Fig. S4; Table S8) all increased as Tmax increased.
Figure 2.

Proportion of time that the clutch was incubated as a function of maximum air temperature on the observation day with the line showing the model fit and grey shaded area the 95% confidence Interval, grey circles showing data collected one during the incubation period and black circles showing data collected on multiple days with a single incubation period. (data from 46 observation days at 35 southern pied babbler T. bicolor nests by 15 groups over 3 breeding seasons).
Nest temperatures
Diurnal nest Te always exceeded Tair (06 h00–19 h00; mean difference = 7.9 ± 11.2°C; range, 0.0–31.8°C; Fig. 3a). At the coolest Tair recorded during the day (~8°C, n = 2 days), nest Te averaged 10.1 ± 0.7°C (range, 8.8–11.6°C; n = 5 nests), and at the warmest Tair recorded during the day (~41°C, n = 1 day), nest Te averaged 44.4 ± 2.8°C (range, 40.9–49.1°C; n = 1 nest). Individual nests could be up to 25°C hotter than other nests for the same Tair of ~ 35.5°C, identified as a critical temperature threshold for body mass maintenance, hatching success and parental care behaviour in pied babblers (Bourne et al., 2020a; du Plessis et al., 2012; Wiley and Ridley, 2016).
Figure 3.

Comparison of (a) black bulb thermometers placed in vacated southern pied babbler T. bicolor nests (red circles) and average temperatures station (blue triangles) and solar radiation (grey squares) recorded per hour (mean±sd) by an onsite weather station, and (b) wind speed (black circles) and relative humidity (yellow crosses) recorded by the on-site weather station.
Nest Te increased significantly with Tair (linear regression; Est = 1.207 ± 0.005, 95% CI: 1.196 to 1.217, t = 229.2, Adj R2 = 0.83; Fig. 4). The highest nest Te recorded was 65°C and operative temperatures >60°C were recorded at 2 nests for Tair between ~30°C and ~37°C. We recorded 2379 instances of Te in unattended nests >41°C (10.8% of all Te records, 22 of 23 nests; mean = 108 ± 85 instances per nest; range, 30–295), identified as a potentially lethal temperature for avian embryos (DuRant et al., 2013; Webb, 1987). We further recorded 487 instances of Te in unattended nests >50°C (2.2% of all Te records, 14 of 23 nests; median = 3 instances per nest; range, 1–163), known to be lethal for the embryos of many arid-zone species (Grant, 1982; Griffith et al., 2016; Reyna and Burggren, 2012).
Figure 4.

Black bulb thermometer temperature as a function of air temperature with boxplots showing the median and interquartile range (IQR) of operative temperature for each air temperature value rounded to the nearest digit; whiskers indicate the lowest and highest value data points within 1.5*IQR; points plotted beyond the whiskers represent a relatively small number of extreme values in this large dataset of 21 872 temperature records; the optimal temperature range for avian embryo development (36°C–40°C, shaded area), the lowest potentially lethal temperature for avian embryos given prolonged exposure (40.5°C, black dotted line) and the average upper critical limit for thermoneutrality in passerines (41°C, grey dashed line) are indicated.
Energy expenditure and water balance
We quantified DEE (n = 68; mean = 1.6 ± 0.5 kJ−1g−1d; range, 0.6 to 2.9 kJ−1g−1d) and water balance (n = 69; mean = 1.0 ± 0.1; range, 0.9 to 1.7; where 1 = neutral water balance) in 45 different birds from 17 groups incubating 34 different clutches. Tmax was the most parsimonious predictor of variation in DEE (of two competing top models, the best-fit model had Tmax as the only predictor and a model weight of 0.549), and DEE declined with increasing temperature (Est = −0.222 ± 0.046, 95% CI: −0.315 to −0.129, z = 4.694, conditional R2 = 0.557; Fig. 5; see Supporting Information Table S9 for full model ouput). Variation in water balance was not predicted by any of the variables included in our models (Table S10). Our within-individual physiology and behaviour data showed no evidence that DEE was predicted by the proportion of time spent incubating on either hot or cool days (n = 38; Fig. 6a; Table 1). However, these data showed that pied babblers’ ability to maintain neutral or positive water balance declined with an increasing proportion of time spent incubating on hot days, but not on cool days (n = 39; Fig. 6b; Table 1). Average metabolic water production declined from 4.7 g d−1 for Tmax < 26°C to 2.8 g d−1 for Tmax > 39°C. To make up that deficit from pre-formed water in order to maintain water balance, which they failed to do under the highest temperatures, pied babblers would have had to eat the equivalent of an extra 17 beetle larvae during the course of the day.
Figure 5.

Variation in DEE by maximum air temperature (°C) on the measurement day in southern pied babblers T. bicolor, with the line showing the model fit and the dotted lines are the 95% confidence interval (data from 68 measurements of 45 different birds from 17 groups incubating 34 different clutches).
Figure 6.

Relationship between proportion of time southern pied babblers T. bicolor spent incubating on cool (Tmax < 35.5°C, open circles, dashed lines, dotted 95% CIs) and hot (Tmax ≥ 35.5°C, open triangles, solid lines, dashed 95% CIs) days on the (a) daily energy expenditure and (b) water balance of incubating birds with the line showing the model fit and dashed lines representing 95% confidence interval; model fit lines for non-significant relationships are faded to grey.
Table 1.
DEE and water balance as a function of proportion of time spent incubating, overall and analysed separately for cool (Tmax < 35.5°C) and hot (Tmax ≥ 35.5°C) days
| Response | n | Temperature | Estimate | Std error | 95% CI | t value | P-value |
|---|---|---|---|---|---|---|---|
| DEE | 38 | Overall | 0.564 | 0.578 | −0.607/1.736 | 0.977 | 0.335 |
| 22 | Cool | 0.360 | 0.871 | −1.456/2.177 | 0.414 | 0.684 | |
| 16 | Hot | 0.258 | 0.662 | −1.162/1.678 | 0.390 | 0.703 | |
| Water balance | 39 | Overall | −0.188 | 0.099 | −0.388/0.012 | −1.901 | 0.065 |
| 22 | Cool | 0.089 | 0.117 | −0.155/0.332 | 0.758 | 0.457 | |
| 17 | Hot | −0.369 | 0.149 | −0.687/−0.052 | −2.480 | 0.026 |
Significant relationships are shown in boldface.
Body mass
Mass change over 24 hours averaged 0.3 ± 2.2 g (range, −4.3 to 6.3 g; n = 119 individuals). Depending on starting body mass and mass change in grams, mass change ranged from −5.8 to 8.5% of body mass. We detected a threshold Tmax at 36.1°C (95% CI: 33.9 to 38.3°C). At Tmax < 36.1°C (n = 59), ∆Mb was not influenced by any of the predictor terms (Table S11). At Tmax ≥ 36.1°C (n = 60), Tmax was the only predictor that significantly influenced ∆Mb (model weight = 0.647), with mass loss becoming more likely as temperatures increased (Est = −1.016 ± 0.301, 95% CI: −1.605 to −0.427, t = −3.379, conditional R2 = 0.162; Fig. 7; see Supporting Information Table S12 for full model outputs).
Figure 7.

Change in southern pied babbler T. bicolor body mass (g) from one morning to the next as a function of maximum air temperature (°C) on the observation day with the line showing the segmented linear regressions for the relationship between mass change and temperature above and below the detected temperature threshold (36.1°C), i.e. no relationship below the threshold temperature and a significant negative relationship above the temperature threshold.
Discussion
Pied babblers exhibit poor hatching success at high temperatures (Bourne et al., 2020a). Employing a novel combination of non-invasive DLW, nest temperature data and field-based behaviour observations, we demonstrated that pied babblers generally incubated their nests almost constantly (99% of daylight hours), but the proportion of time that nests were attended declined with increasing Tair (as has also been observed in blue Cyanistes caeruleus and great tits Parus majorBueno-Enciso et al., 2017 and king rails (Rallus elegans) Clauser and McRae, 2017). Second, we found that operative temperatures in unattended nests frequently exceeded widely reported lethal limits for avian embryos (Birkhead et al., 2008; Conway and Martin, 2000; DuRant et al., 2013; Wada et al., 2015; Webb, 1987) and the inflection air temperature values above which passerine birds rapidly increase rates of evaporative water loss via panting (McKechnie et al., 2017; Smith et al., 2017). Third, we found that pied babblers incurred water costs associated with incubation at high temperatures but energy expenditure did not increase with an increase in proportion of time spent incubating at high temperature (similar to recent studies of zebra finches Taeniopygia guttataCooper et al., 2019 and white-browed sparrow-weavers Plocepasser mahaliSmit and McKechnie, 2015). Finally, we found that pied babblers from incubating groups lost mass during very hot weather [known to occur in pied babblers (du Plessis et al., 2012) and other arid-zone bird species (Sharpe et al., 2019; van de Ven et al., 2019)]. In this study, mass loss occurred at Tmax > 36.2°C, which is very similar to the threshold temperature for mass loss in pied babblers of 35.5°C previously identified in subordinate individuals in non-breeding groups at the same study site (du Plessis et al., 2012). With Te in unattended nests regularly exceeding lethal limits for avian embryos, reduced nest attendance at high Tair may contribute to reduced hatching success during hot incubation periods.
Our finding that pied babblers showed significant declines in DEE at high Tair is consistent with results from other studies (Cooper et al. 2019; Smit and McKechnie 2015) and likely reflects a decrease in activity as birds rest or seek shade at high air temperatures (Pattinson et al., 2020; Pattinson and Smit, 2017; van de Ven et al., 2019). An inflection point in metabolic rate would only be expected at environmental temperatures above the thermoneutral zone (McKechnie et al., 2016, 2017; McKechnie and Wolf, 2019). However, our finding that incubating pied babblers failed, on average, to maintain water balance when incubating for long periods of time on hot days, but not on cool days, is novel and strongly suggests that birds incubating at high temperatures might leave the nest because of the water costs incurred from incubating in the heat (Bourne, 2020). In hot and dry environments such as the Kalahari Desert, incubating birds cannot fully engage in normal behavioural thermoregulation, such as retreating to the shade or adjusting foraging and drinking behaviours (Abdu et al., 2018; Cooper et al., 2019; Smit et al., 2016). Incubating pied babblers do not eat while on the nest, instead alternating foraging bouts with incubation as all adult group members contributing to incubation throughout the day (Ridley and Raihani, 2007; Ridley and van den Heuvel, 2012), and thus are unlikely to gain additional water from food while on the nest. Using evaporative cooling to maintain body temperature below lethal levels (Brown and Downs, 2003; Grant, 1982; O’Connor et al., 2018) presumably comes at high water cost to themselves given the high nest Te we observed in pied babblers. Metabolic water production is generally too low to maintain water balance in hot environments (e.g. MacMillen 1990) and may be the reason that lethal dehydration has resulted in mass mortality of birds (Gardner et al., 2019; McKechnie and Wolf, 2010) and mammals (Ratnayake et al., 2019; Welbergen et al., 2008) during heatwaves. The water turnover rates of birds in arid environments tend to be frugal (Cooper et al., 2019; Williams and Tieleman, 2005). Those individuals that did maintain water balance when incubating for long periods at high temperatures may have been more successful while foraging during off bouts or had more food in their crops at the start of incubation (Conradie et al., 2020).
The Te we recorded in unattended pied babbler nests provided an index of the thermal environment likely experienced by incubating pied babblers in their nests and regularly exceeded (i) temperatures at which evaporative water loss increases rapidly in passerine birds (41°C; McKechnie et al., 2017; Smith et al., 2017), (ii) optimal temperatures for embryo development in passerines (36–40°C; DuRant et al., 2013) and (iii) lethal temperature limits for developing avian embryos (40.5°C–51°C; DuRant et al., 2013; Grant, 1982; Griffith et al., 2016; Stoleson and Beissinger, 1999; Webb, 1987). Such high nest temperatures have been recorded in several bird species nesting in exposed sites and some arid-zone species exhibit quite high heat tolerance in developing embryos. For example, northern bobwhite Colinus virginianus eggs can survive exposure to temperatures of 46°C for about an hour (Reyna and Burggren, 2012). Nonetheless, leaving nests unattended for long periods of time during the heat of the day risks exposing developing avian embryos to high temperatures (Carroll et al., 2015a; DuRant et al., 2019; Mayer et al., 2009), potentially exceeding lethal limits (Webb, 1987) and risking embryo death (Birkhead et al., 2008; Clauser and McRae, 2017; Wada et al., 2015) or leading to other problems such as an increased risk of nest predation (DeGregorio et al., 2015). It is therefore likely that near-constant incubation and/or shading is both highly desirable (Grant, 1982), in order to limit exposure of embryos to excessive heat, and also difficult to sustain at high temperatures, because birds prevent body temperature exceeding lethal limits by evaporative cooling (Albright et al., 2017; McKechnie and Wolf, 2019; O’Connor et al., 2017). The reduced nest attendance we observed at high temperatures is consistent with a constraint on parental investment in incubation associated with the water costs of heat exposure (Amat and Masero, 2004; Coe et al., 2015) and may suggest progress towards eventual nest abandonment (Bourne, 2020; Sharpe et al., 2019; Stoleson and Beissinger, 1999). The Te data we collected in nests clearly show that the Tair measurements used in analyses underestimated both the degree and the variability of heat exposure for incubating pied babblers on their nests. While we used Tair for analyses to increase comparability with other studies and improve the potential for our study to contribute towards climate impacts models, our findings suggest that future studies could benefit from recording Te alongside Tair. We were unable to test for a relationship between water balance and hatching success directly. We have anecdotal evidence of extended incubation recesses and signs of apparent dehydration in several birds after they had incubated for long periods of time on hot afternoons. In addition, we found at least one clutch that was definitely abandoned during the incubation phase following 5 consecutive days at >35.5°C (Bourne, 2020, reproduced in the supplementary materials). Reduced nest attendance on hot afternoons may suggest progress towards eventual nest abandonment (Clauser and McRae, 2017; Sharpe et al., 2019; Stoleson and Beissinger, 1999). However, in most cases we were (i) unable to see the incubating bird clearly to enough to record detailed data on panting or shading behaviour (nests are often >5 m high); (ii) not able to consistently record behaviour data from the incubating bird because we had to follow and record behaviour observations from birds that were dosed with DLW in order to collect their faeces for the DLW analyses; (iii) not able to visit nests repeatedly during the incubation period due to other data collection commitments (breeding was often synchronous with other groups and we prioritized data from different individuals and nests over detailed data from within a smaller number of breeding attempts) and limitations of the DLW technique (e.g. the same individuals cannot be dosed again within ~2 weeks); and (iv) we were not always able to identify the precise cause of nest failure because, in most cases, we could not be sure if the nest had been abandoned or predated. Observed mass loss may well be associated with evaporative water loss, but to provide a comprehensive explanation of the underlying processes is beyond the scope of the current study. Future research could usefully explore the relationships among temperature, incubation effort, thermoregulatory behaviour and hydration status in birds in more detail.
Conclusions
Given that (i) pied babblers incubate their eggs almost constantly during the day, (ii) lower incubation rates occurred on hot days and (iii) unusually low incubation constancy was often followed by nest abandonment or failure, we suggest that reduced incubation at high temperatures might contribute to hatching failure by increasing the risk of embryo exposure to lethal temperatures. We cannot directly test for causal relationships between effects of temperature on the behaviour and physiology of incubating pied babblers and hatching success, which would require an experimental approach or at least observations over multiple days within the same breeding attempts. However, we present multiple lines of evidence suggesting that pied babbler nests are more likely to hatch when incubated consistently. Ambient incubation at high operative temperatures may be detrimental to developing embryos, potentially exposing them to a greater risk of overheating (Cones, 2017). Incubating adults may be constrained from consistent incubation at high temperatures once thermoregulatory thresholds are approached or exceeded. We suggest that pied babblers may leave their nests on hot afternoons because incubating for prolonged periods at high temperatures may increase water costs. Considering both behaviour and physiology simultaneously in the same individuals, at the same time, under natural conditions, provides invaluable insights into the thermal constraints under which incubating birds operate. As we found no relationship between group size and any of the responses we measured, either alone or in interaction with environmental factors, we further suggest that cooperative breeding may not confer an advantage over non-cooperative breeding strategies in buffering against hot weather during the incubation phase. Future studies may usefully consider variation in the number of individuals that are actively involved in incubation rather than total group size.
Although parental care strategies are flexible in response to both climate and social conditions (Clutton-Brock et al., 2004; Langmore et al., 2016), these strategies have limits (Bourne et al., 2021; Clauser and McRae, 2017; Sharpe et al., 2019; van de Ven et al., 2020). Given that both mean temperatures and hot extremes are increasing in frequency under global climate change (IPCC, 2013), the incubation period could become a major bottleneck for reproduction across species with different reproductive strategies. Birds will likely incur ever greater thermoregulatory costs of incubation as temperatures rise, leading to reduced nest attendance, potential overheating of eggs, and ultimately, compromised population replacement and persistence.
Funding
This work was supported by the Australian Research Council (FT110100188 to A.R.R.), the BBSRC David Phillips Fellowship (BB/J014109/1 to C.N.S.), the British Ornithologists’ Union, the DST-NRF Centre of Excellence at the FitzPatrick Institute for African Ornithology, the Oppenheimer Memorial Trust (20747/01 to A.R.B.), the University of Cape Town and the National Research Foundation of South Africa (grant no. 110506 to A.E.M. and grant nos. 99050 and 118627 to S.J.C.).
Author contributions
All authors conceived the study and secured funding. A.R.R. started habituation of the study animals in 2003 and has maintained it ever since, this was central to making the study possible; A.R.B. undertook all fieldwork with paid assistants; A.R.B. analysed the data and drafted the manuscript; all authors contributed substantially to revisions and gave final approval for publication.
Data availability statement
The data underlying all analyses presented in this study have been archived at the University of Cape Town’s open access institutional data repository, ZivaHub (a figshare platform), where they are publicly available at doi:10.25375/uct.14499939.
Supplementary Material
Acknowledgements
We thank the management teams at the Kuruman River Reserve (KRR) and surrounding farms, Van Zylsrus, South Africa, for making the work possible. We also thank Sello Matjee, Paige Ezzey and Lesedi Moagi for fieldwork assistance. The KRR was financed by the Universities of Cambridge and Zurich, the MAVA Foundation and the European Research Council (grant no. 294494 to Tim Clutton-Brock) and received logistical support from the Mammal Research Institute of the University of Pretoria. The opinions, findings and conclusions are those of the authors alone, and the National Research Foundation accepts no liability whatsoever in this regard. The team also wishes to thank Dr Ben Smit and two anonymous reviewers for their valuable feedback that helped to improve this work considerably.
Supplementary material
Supplementary material is available at Conservation Physiology online.
REFERENCES
- Abdu S, McKechnie AE, Lee ATK, Cunningham SJ (2018) Can providing shade at water points help Kalahari birds beat the heat? J Arid Environ 152: 21–27. [Google Scholar]
- Albright, TP, Mutiibwa, D., Gerson, AR, Smith, EK, Talbot, WA, O’Neill, JJ, McKechnie, AE, Wolf, BO (2017) Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc Natl Acad Sci, 114(9), 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlRashidi M, Kosztolányi A, Shobrak M, Küpper C, Székely T (2011) Parental cooperation in an extreme hot environment: natural behaviour and experimental evidence. Anim Behav 82: 235–243. [Google Scholar]
- Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49: 227–266. [DOI] [PubMed] [Google Scholar]
- Amat JA, Masero JA (2004) How Kentish plovers, Charadrius alexandrinus, cope with heat stress during incubation. Behav Ecol Sociobiol 56: 26–33. [Google Scholar]
- Anava A, Kam M, Shkolnik A, Degen AA (2000) Seasonal field metabolic rate and dietary intake in Arabian Babblers (Turdoides squamiceps) inhabiting extreme deserts. Funct Ecol 14: 607–613. [Google Scholar]
- Ardia DR, Pérez JH, Clotfelter ED (2010) Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc R Soc B Biol Sci 277: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken GS, Boysen AF, Korschgen CE, Kenow KP, Lima SL (2001) Design and performance of a rugged standard operative temperature thermometer for avian studies. J Therm Biol 26: 595–604. doi: 10.1016/S0306-4565(01)00006-7. [DOI] [Google Scholar]
- Bakken GS, Santee WR, Erskine DJ (1985) Operative and standard operative temperature: tools for thermal energetics studies. Am Zool 25: 933–943. [Google Scholar]
- Barton, K (2015) MuMIn: Multi-model inference. https://cran.r-project.org/package=MuMin
- Bates, D, Maechler, M, Bolker, B, Walker, S (2015) Fitting linear mixed effects models using lme4. J Stat Softw, 67(1), 1–48. https://cran.r-project.org/web/packages/lme4/index.html [Google Scholar]
- Birkhead TR, Hall J, Schut E, Hemmings N (2008) Unhatched eggs: methods for discriminating between infertility and early embryo mortality. Ibis 150: 508–517. [Google Scholar]
- Bladon AJ, Donald PF, Jones SEI, Collar NJ, Deng J, Dadacha G, Abebe YD, Green RE (2019) Behavioural thermoregulation and climatic range restriction in the globally threatened Ethiopian Bush-crow Zavattariornis stresemanni. Ibis 161: 546–558. doi: 10.1111/ibi.12660. [DOI] [Google Scholar]
- Bourne AR (2020) Apparent dehydration in incubating southern pied babblers Turdoides bicolor. Promerops 12–14. [Google Scholar]
- Bourne, AR, Cunningham, SJ, Nupen, LJ, McKechnie, AE, Ridley, AR (2018) Male and female Southern Pied Babbler Turdoides bicolor nestlings respond similarly to heat stress. https://www.bou.org.uk/wp-content/uploads/2019/08/bou-funded-project-report-bourne.pdf.
- Bourne AR, Cunningham SJ, Spottiswoode CN, Ridley AR (2020a) High temperatures drive offspring mortality in a cooperatively breeding bird. Proc R Soc B 287: 20201140. doi: 10.1101/2020.05.31.126862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne AR, Cunningham SJ, Spottiswoode CN, Ridley AR (2020b) Hot droughts compromise interannual survival across all group sizes in a cooperatively breeding bird. Ecol Lett. doi: 10.1111/ele.13604. [DOI] [PubMed] [Google Scholar]
- Bourne AR, McKechnie AE, Cunningham SJ, Ridley AR, Woodborne SM, Karasov WH (2019) Non-invasive measurement of metabolic rates in wild, free-living birds using doubly labelled water. Funct Ecol 33: 162–174. [Google Scholar]
- Bourne AR, Ridley AR, Spottiswoode CN, Cunningham SJ (2021) Direct and indirect effects of temperatures on fledging success in a cooperatively breeding bird. BioRxiv. doi: 10.1101/2021.01.24.427934. [DOI] [Google Scholar]
- Brown M, Downs CT (2003) The role of shading behaviour in the thermoregulation of breeding crowned plovers (Vanellus coronatus). J Therm Biol 28: 51–58. [Google Scholar]
- Bueno-Enciso, J, Barrientos, R, Ferrer, ES, Sanz, JJ (2017) Do extended incubation recesses carry fitness costs in two cavity-nesting birds? J Field Ornithol, 88(2), 146–155. [Google Scholar]
- Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer. [Google Scholar]
- Butler PJ, Green JA, Boyd IL, Speakman JR (2004) Measuring meatabolic rate in the field: the pros and cons of the doubly labeled water and heart rate methods. Funct Ecol 18: 168–183. [Google Scholar]
- Cahill, AE, Aiello-Lammens, ME, Fisher-Reid, MC, Hua, X, Karanewsky, CJ, Ryu, HY, Sbeglia, GC, Spagnolo, F, Waldron, JB, Warsi, Oet al. (2013) How does climate change cause extinction? Proc R Soc B Biol Sci, 280, 20121890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GS, Norman J (1988) An Introduction to Environmental Biophysics. Springer. [Google Scholar]
- Carroll JM, Davis CA, Elmore RD, Fuhlendorf SD (2015a) A ground-nesting galliform’s response to thermal heterogeneity: implications for ground-dwelling Birds. PLoS One 10: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, JM, Davis, CA, Elmore, RD, Fuhlendorf, SD (2017) Using a historic drought and high-heat event to validate thermal exposure predictions for ground-dwelling birds. Ecol Evol, 7(16): 6413–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, JM, Davis, CA, Elmore, RD, Fuhlendorf, SD, Thacker, ET (2015b) Thermal patterns constrain diurnal behavior of a ground-dwelling bird. Ecosphere, 6: 1–15. 10.1890/ES15-00163.1 [DOI] [Google Scholar]
- Carroll RL, Davis CA, Fuhlendorf SD, Elmore RD, DuRant SE, Carroll JM (2018) Avian parental behavior and nest success influenced by temperature fluctuations. J Therm Biol 74: 140–148. doi: 10.1016/j.jtherbio.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Champely, S, Ekstrom, C, Dalgaard, P, Gill, J, Weibelzahl, S, Anandkumar, A, Ford, C, Volcic, R, De Rosario, H (2018) pwr: Basic functions for power analysis (R package version 1.2-2). https://github.com/heliosdrm/pwr.
- Clauser AJ, McRae SB (2017) Plasticity in incubation behaviour and shading by king rails (Rallus elegans) in response to temperature. J Avian Biol 48: 479–488. [Google Scholar]
- Clutton-Brock TH, Russell AF, Sharpe LL (2004) Behavioural tactics of breeders in cooperative meerkats. Anim Behav 68: 1029–1040. [Google Scholar]
- Coe BH, Beck ML, Chin SY, Jachowski CMB, Hopkins WA (2015) Local variation in weather conditions influences incubation behavior and temperature in a passerine bird. J Avian Biol 46: 385–394. [Google Scholar]
- Cones, AG (2017) Causes and consequences of individual variation in the plasticity of incubation and embryonic heart rate in the cooperatively-breeding chestnut-crowned babbler (Pomatostomus ruficeps). MSc Thesis, University of Exeter.
- Conradie SR, Woodborne SM, Cunningham SJ, McKechnie AE (2019) Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid-zone birds during the 21st century. Proc Natl Acad Sci U S A 116: 14065–14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie SR, Woodborne SM, Wolf BO, Pessato A, Mariette MM, McKechnie AE (2020) Avian mortality risk during heat waves will increase greatly in arid Australia during the 21st century. Conserv Physiol 8: coaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrey RY, Skagen SK, Yackel Adams AA, Panjabi AO (2016) Extremes of heat, drought and precipitation depress reproductive performance in shortgrass prairie passerines. Ibis 158: 614–629. [Google Scholar]
- Conway CJ, Martin TE (2000) Effects of ambient temperature on avian incubation behavior. Behav Ecol 11: 178–188. [Google Scholar]
- Cooper CE, Withers PC, Hurley LL, Griffith SC (2019) The field metabolic rate, water turnover, and feeding and drinking behavior of a small avian desert granivore during a summer heatwave. Front Physiol 10: 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-McDonnell KK, Wolf BO (2016) Rapid warming and drought negatively impact population size and reproductive dynamics of an avian predator in the arid southwest. Glob Chang Biol 22: 237–253. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Gardner JL, Martin RO (2021) Opportunity costs and the response of birds and mammals to climate warming. Front Ecol Environ 1–8. doi: 10.1002/fee.2324. [DOI] [Google Scholar]
- Cunningham SJ, Martin RO, Hockey PAR (2015) Can behaviour buffer the impacts of climate change on an arid-zone bird? Ostrich 86: 119–126. [Google Scholar]
- Cunningham SJ, Martin RO, Hojem CL, Hockey PAR (2013) Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS One 8: e74613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregorio BA, Westervelt JD, Weatherhead PJ, Sperry JH (2015) Indirect effect of climate change: shifts in ratsnake behaviour alter intensity and timing of avian nest predation. Ecol Model 312: 239–246. [Google Scholar]
- Downs CT, Ward D (1997) Does shading behavior of incubating shorebirds in hot environments cool the eggs or the adults? Auk 114: 717–724. [Google Scholar]
- Drent R (1975) Incubation. In Farner DS, King JR, eds, Avian Biology vol. 5. Academic Press, pp. 333–420. [Google Scholar]
- Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR (2012) The costs of keeping cool in a warming world: Implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob Chang Biol 18: 3063–3070. [DOI] [PubMed] [Google Scholar]
- Dunn, PO, Møller, AP (2019) Effects of Climate Change on Birds, Ed 2. Oxford University Press. [Google Scholar]
- Dunn PO, Robertson RJ, Winkler DW, Whittingham LA, Hannon SJ (2010) A test of the mismatch hypothesis: how is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92: 450–461. doi: 10.1890/10-0478.1. [DOI] [PubMed] [Google Scholar]
- DuRant SE, Hopkins WA, Hepp GR, Walters JR (2013) Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol Rev 88: 499–509. [DOI] [PubMed] [Google Scholar]
- DuRant SE, Willson JD, Carroll RB (2019) Parental effects and climate change: will avian incubation behavior shield embryos from increasing environmental temperatures? Integr Comp Biol 59: 1068–1080. [DOI] [PubMed] [Google Scholar]
- Edwards EK, Mitchell NJ, Ridley AR (2015) The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich 86: 137–144. [Google Scholar]
- Funghi C, McCowan LSC, Schuett W, Griffith SC (2019) High air temperatures induce temporal, spatial and social changes in the foraging behaviour of wild zebra finches. Anim Behav 149: 33–43. doi: 10.1016/j.anbehav.2019.01.004. [DOI] [Google Scholar]
- Gardner JL, Amano T, Peters A, Sutherland WJ, Mackey B, Joseph L, Stein J, Ikin K, Little R, Smith Jet al. (2019) Australian songbird body size tracks climate variation: 82 species over 50 years. Proc R Soc B Biol Sci 286: 20192258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Rowley E, Rebeira P, Rebeira A, Brouwer L (2018) Associations between changing climate and body condition over decades in two southern hemisphere passerine birds. Clim Change Responses 5: 1–14. [Google Scholar]
- Gessaman JA, Nagy KA (1988) Energy metabolism: errors in gas exchange conversion factors. Physiol Zool 61: 507–513. [Google Scholar]
- Grant GS (1982) Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithol Monogr 30: 1–75. [Google Scholar]
- Greenland, S, Senn, SJ, Rothman, KJ, Carlin, JB, Poole, C, Goodman, SN, Altman, DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol, 31(4), 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith SC, Mainwaring MC, Sorato E, Beckmann C (2016) High atmospheric temperatures and ‘ambient incubation’ drive embryonic development and lead to earlier hatching in a passerine bird. R Soc Open Sci 3: 150371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber CE, Nakagawa S, Laws RS, Jamieson IG (2011) Multimodal inference in ecology and evolution: challenges and solution. J Evol Biol 24: 699–711. [DOI] [PubMed] [Google Scholar]
- Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. Peer J 2: e616. doi: 10.7717/peerj.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison XA, Donaldson L, Correa-cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J 6: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey PAR, Sirami C, Ridley AR, Midgley GF, Babiker HA (2011) Interrogating recent range changes in South African birds: confounding signals from land use and climate change present a challenge for attribution. Divers Distrib 17: 254–261. doi: 10.1111/j.1472-4642.2010.00741.x. [DOI] [Google Scholar]
- Howell TR, Bartholomew GA (1961) Temperature regulation in nesting Bonin Island petrels, wedgetailed shearwaters, and Christmas Island shearwaters. Auk 78: 343–354. [Google Scholar]
- Huntley, B (2019) Consequences of climatic change for distributions. In Dunn P. O., Møller A. P. (eds.), Effects of Climate Change on Birds, Ed 2. Oxford University Press, pp. 165–184. [Google Scholar]
- Iknayan KJ, Beissinger SR (2018) Collapse of a desert bird community over the past century driven by climate change. Proc Natl Acad Sci 115: 8597–8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . (2013) Climate Change 2013: The Intergovernmental Panel on Climate Change Fifth assessment report. Cambridge University Press. [Google Scholar]
- Kruger AC, Sekele SS (2013) Trends in extreme temperature indices in South Africa: 1962–2009. Int J Climatol 33: 661–676. [Google Scholar]
- Langmore NE, Bailey LD, Heinsohn RG, Russell AF, Kilner RM (2016) Egg size investment in superb fairy-wrens: helper effects are modulated by climate. Proc R Soc B 283: 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Masri J, Perez V, Maya C, Zhao J (2020) Growth performance and nutrient composition of Mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 9: foods9020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillen RE (1990) Water economy of granivorous birds: a predictive model. Condor 92: 379–392. [Google Scholar]
- Mayer PM, Smith LM, Ford RG, Watterson DC, Mccutchen MD, Ryan MR (2009) Nest construction by a ground-nesting bird represents a potential trade-off between egg crypticity and thermoregulation. Oecologia 159: 893–901. [DOI] [PubMed] [Google Scholar]
- McDonald M, Johnson S (2014) There’ an app for that’’: a new program for the collection of behavioural field data. Anim Behav 95: 81–87. [Google Scholar]
- McDonald S, Schwanz LE (2018) Thermal parental effects on offspring behaviour and their fitness consequences. Anim Behav 135: 45–55. [Google Scholar]
- McKechnie AE (2019) Physiological and morphological effects of climate change. In Dunn PO, Møller AP, eds, Effects of Climate Change on Birds (pp. 120–133). Oxford University Press [Google Scholar]
- McKechnie AE, Gerson AR, McWhorter TJ, Smith EK, Talbot WA, Wolf BO (2017) Avian thermoregulation in the heat: evaporative cooling in five Australian passerines reveals within-order biogeographic variation in heat tolerance. J Exp Biol 220: 2436–2444. [DOI] [PubMed] [Google Scholar]
- McKechnie, AE, Hockey, PAR, Wolf, BO (2012) Feeling the heat: Australian landbirds and climate change. Emu, 112(2), i–vii.
- McKechnie AE, Smit B, Whitfield MC, Noakes MJ, Talbot WA, Garcia M, Gerson AR, Wolf BO (2016) Avian thermoregulation in the heat: evaporative cooling capacity in an archetypal desert specialist, Burchell’s sandgrouse (Pterocles burchelli). J Exp Biol 219: 2137–2144. doi: 10.1242/jeb.146563. [DOI] [PubMed] [Google Scholar]
- McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie AE, Wolf BO (2019) The physiology of heat tolerance in small endotherms. Phys Ther 34: 302–313. [DOI] [PubMed] [Google Scholar]
- Moagi LL, Bourne AR, Cunningham SJ, Jansen R, Ngcamphalala CA, Ridley AR, McKechnie AE (2021) Hot days associated with short-term adrenocortical responses in a southern African arid-zone passerine bird. J Exp Biol in press. [DOI] [PubMed] [Google Scholar]
- Mortensen JL, Reed JM (2018) Parental incubation patterns and the effect of group size in a Neotropical cooperative breeder. Auk 135: 669–692. [Google Scholar]
- Mougeot F, Benítez-López A, Casas F, Garcia JT, Viñuela J (2014) A temperature-based monitoring of nest attendance patterns and disturbance effects during incubation by ground-nesting sandgrouse. J Arid Environ 102: 89–97. doi: 10.1016/j.jaridenv.2013.11.010. [DOI] [Google Scholar]
- Muggeo VM (2008) Segmented: an R package to fit regression models with broken-line relationships. R News 8: 20–25. [Google Scholar]
- Nagy KA, Costa DP (1980) Water flux in animals: analysis of potential errors in the tritiated water method. Am J Physiol 238: 454–465. [DOI] [PubMed] [Google Scholar]
- Nelson-Flower MJ, Hockey PAR, O’Ryan C, Raihani NJ, Du Plessis MA, Ridley AR (2011) Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behav Ecol 22: 559–565. [Google Scholar]
- Niizuma Y, Shirai M (2015) Applicability of a single sample approach for the doubly labelled water method to the Streaked Shearwater Calonectris leucomelas. Ornithol Sci 14: 21–28. [Google Scholar]
- Nord A, Cooper CB (2020) Night conditions affect morning incubation behaviour differently across a latitudinal gradient. Ibis In press . doi: 10.1111/ibi.12804. [DOI] [Google Scholar]
- Nord A, Sandell MI, Nilsson JÅ (2010) Female zebra finches compromise clutch temperature in energetically demanding incubation conditions. Funct Ecol 24: 1031–1036. [Google Scholar]
- Nwaogu CJ, Dietz MW, Tieleman BI, Cresswell W (2017) Breeding limits foraging time: evidence of interrupted foraging response from body mass variation in a tropical environment. J Avian Biol 48: 563–569. [Google Scholar]
- O’Connor RS, Brigham RM, McKechnie AE (2018) Extreme operative temperatures in exposed microsites used by roosting rufous-cheeked nightjar (Caprimulgus rufigena): implications for water balance under current and future climate conditions. Can J Zool 96: 1122–1129. [Google Scholar]
- O’Connor RS, Wolf BO, Brigham RM, McKechnie AE (2017) Avian thermoregulation in the heat: efficient evaporative cooling in two southern African nightjars. J Comp Physiol B Biochem Syst Environ Phys Ther 187: 477–491. [DOI] [PubMed] [Google Scholar]
- Pattinson NB, Smit B (2017) Seasonal behavioral responses of an arid-zone passerine in a hot environment. Physiol Behav 179: 268–275. doi: 10.1016/j.physbeh.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Pattinson NB, Thompson ML, Griego M, Russell G, Mitchell NJ, Martin RO, Wolf BO, Smit B, Cunningham SJ, McKechnie AEet al. (2020) Heat dissipation behaviour of birds in seasonally hot, arid-zones: are there global patterns. J Avian Biol, jav.02350 . doi: 10.1111/jav.02350. [DOI] [Google Scholar]
- Pearce-Higgins JW, Green RE (2014) Birds and Climate Change. Oxford University Press. [Google Scholar]
- Priyadarshini KVR, Prins HHT, Bie S, Heitkönig IMA, Woodborne S, Gort G, Kirkman K, Ludwig F, Dawson TE, Kroon H (2016) Seasonality of hydraulic redistribution by trees to grasses and changes in their water-source use that change tree-grass interactions. Ecohydrology 9: 218–228. [Google Scholar]
- R Core Team . (2017) R: a language and environment for statistical computing. In R Core Team (Ed.), R Foundation for Statistical Computing (Vol. 1, Issue 2.11.1). R Foundation for Statistical Computing. http://www.r-project.org
- Raihani NJ, Ridley AR (2007a) Adult vocalizations during provisioning: offspring response and postfledging benefits in wild pied babblers. Anim Behav 74: 1303–1309. doi: 10.1016/j.anbehav.2007.02.025. [DOI] [Google Scholar]
- Raihani NJ, Ridley AR (2007b) Variable fledging age according to group size: trade-offs in a cooperatively breeding bird. Biol Lett 3: 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake HU, Kearney MR, Govekar P, Karoly D, Welbergen JA (2019) Forecasting wildlife die-offs from extreme heat events. Animal Conserv 22: 386–395. [Google Scholar]
- Reyna KS, Burggren WW (2012) Upper lethal temperatures of northern bobwhite embryos and the thermal properties of their eggs. Poult Sci 91: 41–46. [DOI] [PubMed] [Google Scholar]
- Riddell EA, Iknayan KJ, Wolf BO, Sinervo B, Beissinger SR (2019) Cooling requirements fueled the collapse of a desert bird community from climate change. Proc Natl Acad Sci 116: 21609–21615. doi: 10.1073/pnas.1908791116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AR (2016) Southern pied babblers: The dynamics of conflict and cooperation in a group-living society. In Dickinson JL, Koenig W, eds, Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution, and Behavior. Cambridge University Press, pp. 115–132 [Google Scholar]
- Ridley AR, Raihani NJ (2007) Variable postfledging care in a cooperative bird: causes and consequences. Behav Ecol 18: 994–1000. [Google Scholar]
- Ridley AR, Raihani NJ (2008) Task partitioning increases reproductive output in a cooperative bird. Behav Ecol 19: 1136–1142. [Google Scholar]
- Ridley AR, van den I (2012) Is there a difference in reproductive performance between cooperative and non-cooperative species? A southern African comparison. Behaviour 8: 821–848. [Google Scholar]
- Ridley AR, Wiley EM, Bourne AR, Cunningham SJ, Nelson-Flower MJ (2021) Understanding the potential impact of climate change on the behavior and demography of social species: The pied babbler (Turdoides bicolor) as a case study. Adv Study Behav 53: 225–266. doi: 10.1016/bs.asb.2021.03.005. [DOI] [Google Scholar]
- Ripple WJ, Wolf C, Newsome TM, Barnard P, Moomaw WR (2019) World scientists’ warning of a climate emergency: a second notice. Bioscience 67: 1026–1028. [Google Scholar]
- Rosenberg, KV, Dokter, AM, Blancher, PJ, Sauer, JR, Smith, AC, Smith, PA, Stanton, JC, Panjabi, A, Helft, L, Parr, M, Marra, PP (2019) Decline of the North American avifauna. Science, 336(6461), 120–124. [DOI] [PubMed] [Google Scholar]
- Rubenstein DR, Lovette IJ (2007) Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr Biol 17: 1414–1419. [DOI] [PubMed] [Google Scholar]
- Saino N, Ambrosini R, Rubolini D, Von J, Provenzale A, Hüppop K, Hüppop O, Lehikoinen A, Lehikoinen E, Rainio Ket al. (2011) Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc R Soc B Biol Sci 278: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samplonius JM, Bartošová L, Burgess MD, Bushuev AV, Eeva T, Ivankina EV, Kerimov AB, Krams I, Laaksonen T, Mägi Met al. (2018) Phenological sensitivity to climate change is higher in resident than in migrant bird populations among European cavity breeders. Glob Chang Biol 24: 3780–3790. doi: 10.1111/gcb.14160. [DOI] [PubMed] [Google Scholar]
- Scantlebury DM, Mills MGL, Wilson RP, Wilson JW, Mills MEJ, Durant SM, Bennett NC, Bradford P, Marks NJ, Speakman JR (2014) Flexible energetics of cheetah hunting strategies provide resistance against kleptoparasitism. Science 346: 79–81. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielson K (1990) Animal Physiology: Adaptation and Environment. Cambridge University Press [Google Scholar]
- Sharpe L, Cale B, Gardner JL (2019) Weighing the cost: the impact of serial heatwaves on body mass in a small Australian passerine. J Avian Biol 50. doi: 10.1111/jav.02355. [DOI] [Google Scholar]
- Siemianowska E, Kosewska A, Aljewicz M, Skibniewska KA, Polak-Juszczak L, Jarocki A, Jędras M (2013) Larvae of mealworm (Tenebrio molitor) as European novel food. Agric Sci 04: 287–291. [Google Scholar]
- Skagen SK, Yackel Adams AA (2012) Weather effects on avian breeding performance and implications of climate change. Ecol Appl 22: 1131–1145. http://www.ncbi.nlm.nih.gov/pubmed/22827123. [DOI] [PubMed] [Google Scholar]
- Smit B, McKechnie AE (2015) Water and energy fluxes during summer in an arid-zone passerine bird. Ibis 157: 774–786. [Google Scholar]
- Smit B, Zietsman G, Martin RO, Cunningham SJ, Mckechnie AE, Hockey PAR (2016) Behavioural responses to heat in desert birds: implications for predicting vulnerability to climate warming. Clim Change Responses 3: 1–14. [Google Scholar]
- Smith EK, O’Neill JJ, Gerson AR, McKechnie AE, Wolf BO (2017) Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. J Exp Biol 220: 3290–3300. [DOI] [PubMed] [Google Scholar]
- Soravia C, Ashton BJ, Thornton A, Ridley AR (2021) The impacts of heat stress on animal cognition: implications for adaptation to a changing climate. WIREs Climate Change . doi: 10.1016/j.optmat.2011.11.002. [DOI] [Google Scholar]
- Speakman JR (1997) Doubly Labelled Water: Theory and Practice. Springer Science Business Media [Google Scholar]
- Speakman JR, Hambly C (2016) Using doubly-labelled water to measure free-living energy expenditure: some old things to remember and some new things to consider. Comp Biochem Phys Part A 202: 3–9. [DOI] [PubMed] [Google Scholar]
- Stevenson IR, Bryant DM (2000) Avian phenology: climate change and constraints on breeding. Nature 406: 366–367. [DOI] [PubMed] [Google Scholar]
- Stillman JH (2019) Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Phys Ther 34: 86–100. [DOI] [PubMed] [Google Scholar]
- Stoleson SH, Beissinger SR (1999) Egg viability as a constraint on hatching synchrony at high ambient temperatures. J Anim Ecol 68: 951–962. [Google Scholar]
- Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65: 13–21. doi: 10.1007/s00265-010-1037-6. [DOI] [Google Scholar]
- van de Ven, TMFN (2017) Implications of climate change on the reproductive success of the southern yellow-billed hornbill Tockus leucomelas (Issue March). PhD thesis, University of Cape Town. [Google Scholar]
- Ven TMFN, Fuller A, Clutton-Brock TH (2020) Effects of climate change on pup growth and survival in a cooperative mammal, the meerkat. Funct Ecol 34: 194–202. [Google Scholar]
- van de TMFN, McKechnie AE, Cunningham SJ (2019) The costs of keeping cool: behavioural trade-offs between foraging and thermoregulation are associated with significant mass losses in an arid-zone bird. Oecologia 191: 205–215. [DOI] [PubMed] [Google Scholar]
- Ven TMFN, McKechnie AE, Er S, Cunningham SJ (2020) High temperatures are associated with substantial reductions in breeding success and offspring quality in an arid-zone bird. Oecologia 193: 225–235. [DOI] [PubMed] [Google Scholar]
- van Wilgen, N. J., Goodall, V., Holness, S., Chown, S. L., McGeoch, M. A. (2016) Rising temperatures and changing rainfall patterns in South Africa’s national parks. Int J Climatol, 36(2), 706–721. [Google Scholar]
- Visser GH, Boon PE, Meijer HAJ (2000) Validation of the doubly labeled water method in Japanese Quail Coturnix c. japonica chicks: is there an effect of growth rate? Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental. Phys Ther 170: 365–372. [DOI] [PubMed] [Google Scholar]
- Wada H, Kriengwatana B, Allen N, Schmidt KL, Soma KK, MacDougall-Shackleton SA (2015) Transient and permanent effects of suboptimal incubation temperatures on growth, metabolic rate, immune function and adrenocortical responses in zebra finches. J Exp Biol 218: 2847–2855. doi: 10.1242/jeb.114108. [DOI] [PubMed] [Google Scholar]
- Walsberg GE, Voss-Roberts KA (1983) Incubation in desert-nesting doves: mechanisms for egg cooling. Physiol Zool 56: 88–93. doi: 10.1086/physzool.56.1.30159969. [DOI] [Google Scholar]
- Walsberg GE, Wolf BO (1996) An appraisal of operative temperature mounts as tools for studies of ecological energetics. Physiol Zool 69: 658–681. [Google Scholar]
- Webb DR (1987) Thermal tolerance of avian embryos: a review. The Condor 89: 874–898. [Google Scholar]
- Welbergen JA, Klose SM, Markus N, Eby P (2008) Climate change and the effects of temperature extremes on Australian flying-foxes. Proc R Soc B 275: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley EM, Ridley AR (2016) The effects of temperature on offspring provisioning in a cooperative breeder. Anim Behav 117: 187–195. [Google Scholar]
- Williams JB, Tieleman BI (2005) Physiological adaptation in desert birds. Bioscience 55: 416–425. [Google Scholar]
- Wingfield JC, Pérez JH, Krause JS, Word KR, González-Gómez PL, Lisovski S, Chmura HE (2017) How birds cope physiologically and behaviourally with extreme climatic events. Philos Trans R Soc B Biol Sci 372: 20160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Romero LM, Htut ZW, McWhorter TJ (2017) Stress responses to heat exposure in three species of Australian desert birds. Physiol Biochem Zool 90: 348–358. doi: 10.1086/690484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying all analyses presented in this study have been archived at the University of Cape Town’s open access institutional data repository, ZivaHub (a figshare platform), where they are publicly available at doi:10.25375/uct.14499939.


