Abstract
Context
A genetic predisposition to lower thyrotropin (TSH) levels is associated with increased atrial fibrillation (AF) risk through undefined mechanisms.
Objective
Defining the genetic mediating mechanisms could lead to improved targeted therapies to mitigate AF risk.
Methods
We used 2-sample mendelian randomization (MR) to test associations between TSH-associated single-nucleotide variations and 16 candidate mediators. We then performed multivariable mendelian randomization (MVMR) to test for a significant attenuation of the genetic association between TSH and AF, after adjusting for each mediator significantly associated with TSH.
Results
Four candidate mediators (free thyroxine, systolic blood pressure, heart rate, and height) were significantly inversely associated with genetically predicted TSH after adjusting for multiple testing. In MVMR analyses, adjusting for height significantly decreased the magnitude of the association between TSH and AF from –0.12 (SE 0.02) occurrences of AF per SD change in height to –0.06 (0.02) (P = .005). Adjusting for the other candidate mediators did not significantly attenuate the association.
Conclusion
The genetic association between TSH and increased AF risk is mediated, in part, by taller stature. Thus, some genetic mechanisms underlying TSH variability may contribute to AF risk through mechanisms determining height occurring early in life that differ from those driven by thyroid hormone–level elevations in later life.
Keywords: thyrotropin, atrial fibrillation, mendelian randomization, height
Thyroid hormones (free thyroxine [FT4] and free 3,5,3′-triiodothyronine [FT3]) are global regulators of metabolism and essential contributors to multiple physiological processes (1, 2). Epidemiological and genetic association studies have both shown an inverse association between thyrotropin (TSH) level, a regulator of thyroid hormones that is measured clinically, and atrial fibrillation (AF) risk (3-5). AF is the most common abnormal heart rhythm and can lead to heart failure, stroke, and death (6). The postulated mechanisms underlying this association include direct effects of TSH or thyroid hormones on the heart; alterations to cardiac structure; altered sympathetic tone; altered electrophysiological impulse generation and conduction; and modulation of known AF risk factors including systolic blood pressure (SBP), height, and body mass index (BMI) (7, 8). Defining mediating factors underlying the association could lead to improved targeted therapies to treat hyperthyroidism and also identify important mechanisms contributing to AF risk (9).
Mendelian randomization (MR) (10) is an approach that uses single-nucleotide variations (SNVs, formerly single-nucleotide polymorphisms [SNPs]) associated with an exposure as instrument variables to identify and characterize associations between a risk factor and an outcome. Multivariable MR (MVMR) (11) can simultaneously estimate the effect of 2 or more genetic exposures to define the independent contributions of the exposures to an outcome. Thus, this approach can be used to probe how genetically mediated mechanisms that modulate an exposure, such as TSH, exert their effects on an outcome (12).
We hypothesized that MVMR could be used to identify the mechanisms by which genetically determined TSH levels contribute to elevated AF risk. We used SNVs identified by large-scale genome-wide association studies (GWAS) as instrumental variables for TSH and other AF risk factors and employed 2-sample MR to first identify AF risk factors associated with TSH levels. We then ascertained the extent to which adjusting for these risk factors attenuated the TSH-AF association. These analyses implicate a nonmodifiable genetic risk mechanism related to development, and that likely differs from mechanisms associated with thyroid hormone fluctuations in later life.
Materials and Methods
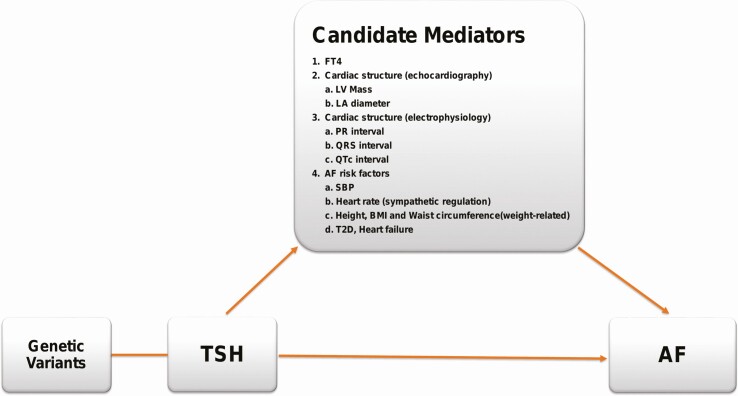
An overview of the analytic approach to identify risk mechanisms underlying the association between genetically regulated TSH and AF is presented in Fig. 1.
Figure 1.
Overview of the study design. Single-nucleotide variations associated with thyrotropin (TSH) were used as instrumental variables to identify candidate mediators for the TSH risk on atrial fibrillation (AF). Two-sample mendelian randomization (MR) was used to identify mediators associated with AF. Multivariable MR was then used to determine the effect that adjusting for the mediators had on the association between TSH and AF.
Genome-wide Association Study Summary Statistics
Summary statistics were obtained from existing large-scale GWAS of people with European ancestry. The TSH GWAS was performed on 54 288 individuals with TSH levels falling within the clinical reference range (13). The TSH GWAS did not include samples from BioVU, Vanderbilt University Medical Center’s DNA Biobank. Additional summary statistics were collected for AF (14), and 16 candidate AF risk factors including FT4 (1); heart rate (HR) (15); left ventricular mass (16); electrophysiological parameters (PR [17], QRS [18], and QTc [19] intervals) and known AF risk factors (BMI [20], height [21], waist circumference [22], SBP [23], diastolic blood pressure [11], fasting glucose [24], fasting insulin [12], type 2 diabetes [25], and heart failure [26]).
Phenotype and Genotype Data From BioVU
Individual-level data were obtained from BioVU, which is linked to a deidentified electronic health record (27). Approval for the present study was obtained from the Vanderbilt University Medical Center Institutional Review Board. SNV genotyping was acquired on the Illumina Infinium MEGAEX platform. Quality control analyses used the HRC-1000G-check tool v4.2.5 (http://www.well.ox.ac.uk/~wrayner/tools/) and prephased using Eagle v2.4.1 (28). Data were imputed using the Michigan Imputation Server in conjunction with the October 2014 release of the 1000 Genomes cosmopolitan reference haplotypes. Imputed data were filtered for a sample missingness rate of less than 2%, an SNV missingness rate of less than 4%, and SNV deviation from Hardy-Weinberg P less than 10–6. Principal components (PCs) were calculated using the SNPRelate package (29). TSH levels, measured during routine clinic care, were extracted for 22 922 individuals without a history of thyroid disease, as previously described (3). Left atrial (LA) diameter was obtained from clinically acquired transthoracic echocardiography. There were 13 978 individuals with LA diameter measurements after removing outliers (LA diameter < 1 or > 10 cm).
Validation of the Thyrotropin Genetic Instrument in BioVU Samples
To compute a weighted polygenic score (PRS) for TSH using summary statistics, an independent set of SNVs (r2 < 0.05) significantly associated with TSH (P < 5 × 10–8) were selected using a clumping algorithm that selected a linkage disequilibrium–reduced set of SNVs with a minor allele frequency greater than 1%. In the initial analysis to identify AF risk factors associated with TSH, SNVs were selected from a subset that were available among all of the GWAS summary statistics sets.
To validate the TSH instrumental variable, genetically predicted TSH levels were computed among BioVU individuals with measured TSH levels using weighted genetic risk scores according to the following formula:
where the allele dosage is a value ranging from 0 and 2 and wi is the change in TSH levels (β coefficient) for each copy of the effect allele (30). The partial correlation between the TSH PRS and measured TSH, adjusting for sex, age, and the 5 PCs, was calculated.
Genome-wide Association Study Summary Statistics for Left Atrial Diameter in BioVU
Summary statistics for LA diameter were generated among BioVU individuals of European-ancestry by running a multivariable linear regression model on log-transformed LA diameter, assuming an additive genetic model and adjusting for age, sex, and 10 PCs.
Mendelian Randomization
The association between genetically determined TSH and 16 candidate risk factors was tested using the inverse-variance weighted average meta-analysis (IVWA) method. All analyses employed a 2-sample approach. An association between TSH and risk factor was considered significant if it had a Bonferroni-adjusted P less than .05/16 = 0.003. Association measures represent the change in risk factor level per SD change in TSH level. To ensure that significant associations were not due to pleiotropy, the pleiotropy-robust MR-Egger and weighted median methods were used to confirm the magnitude and direction of associations. Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) (31) was also used to ascertain whether an inconsistency in the results among IVWA, MR-Egger, and weighted median for one phenotype may be due to horizontal pleiotropy.
All MR methods were calculated using the Mendelian Randomization R package (10). To ensure that the TSH associations were not due to reverse causality, the bidirectional Generalized Summary-data-based Mendelian Randomization (BIGSMR) (32) test was also performed.
To further probe the association between TSH levels and height, IVWA analyses were performed using previously identified subsets of SNVs associated with TSH levels related to either (1) autoimmune or (2) nonautoimmune mechanisms (33). These subsets were used to test associations with height and AF.
Multivariable Mendelian Randomization
MVMR is a technique that estimates the effect of multiple exposure variables on an outcome, after adjusting for additional risk factors. For each risk factor significantly associated with TSH, MVMR (34) analysis was performed to test the association between TSH and AF after adjusting for the risk factor. To create genetic instruments for each risk factor, a clumping algorithm in conjunction with GWAS summary statistics was used, as described earlier. We identified those risk factors that decreased the TSH coefficient by greater than 1.96 SEs (P < .05), as compared to the original TSH coefficient after adjustment.
Results
We identified 48 independent SNVs associated with TSH levels, and these were used as instrument variables to test for associations between TSH levels and candidate mediators. A TSH PRS based on these SNVs was strongly associated with measured TSH levels (partial correlation: 0.26, P < 2 × 10–16, Supplementary Fig. 1 [35]), confirming the validity of the genetic instrument. Consistent with prior reports, MR analyses showed a strong inverse association between the TSH instrumental variable and AF risk (β = –.12 [95% CI, –0.17 to –0.07] change in log-odds per SD increase in TSH).
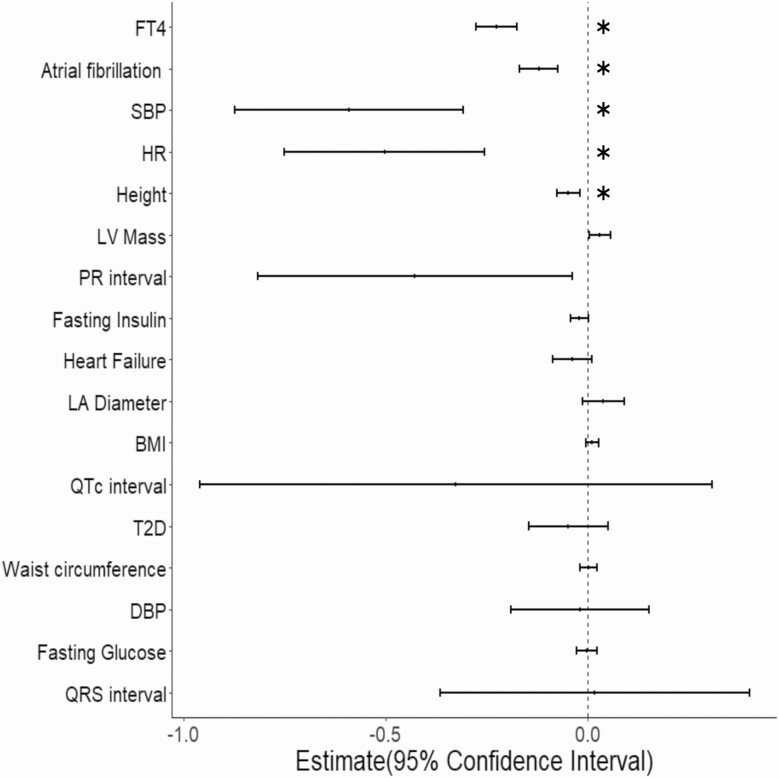
We identified candidate mediators of the TSH-AF association by testing for associations between the TSH instrumental variable and 16 candidate AF risk factors. MR analyses showed that TSH was significantly (P < .003) inversely associated with FT4 (β = –0.23 [95% CI, –0.28 to –0.18] per SD increase in TSH); SBP (β = –0.59 [–0.87 to –0.31]); HR (β = –0.50 [–0.75 to –0.256]) and height (β = –0.05 [–0.08 to –0.02]) (Fig. 2 and Supplementary Table 1 [35]). The directions of associations were consistent for each phenotype when pleiotropy-robust MR methods (MR-Egger and Median methods) were used except for heart rate, where the MR-Egger estimate differed from the other methods, suggesting the association may be due to pleiotropy (see Fig. 2). Association analyses using the GSMR method showed a significant inverse association similar to the IVW and Median methods (β = –0.39 [SE = 0.10]), supporting the observation that higher TSH is associated with lower heart rates. Bidirectional analyses using GSMR also demonstrated that genetically predicted levels of these 4 candidate mediators were not significantly associated with TSH, suggesting that associations were not due to reverse causality (Supplementary Table 2 [35]).
Figure 2.
Forest plot of summarizing 2-sample mendelian randomization analyses between thyrotropin (TSH) and candidate-mediating factors. Estimates of the association between TSH and 16 candidate-mediating factors using the inverse-variance weighted meta-analysis approach. Associations with a P value less than .003 (0.05/16) are indicated with an asterisk. SBP, systolic blood pressure.
MR analyses demonstrated that each of the 4 candidate mediators was significantly associated with AF (P < .05 for each mediator); the association was positive for height and SBP and negative (inverse) for FT4 and HR (Supplementary Table 3 [35]).
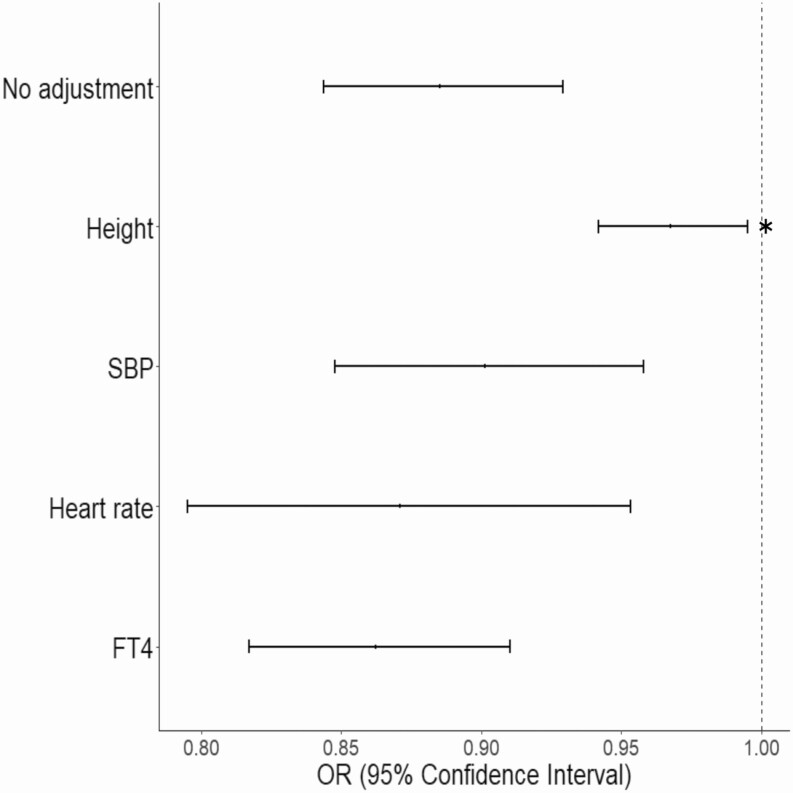
We used MVMR to determine whether adjusting for any of the TSH-associated candidate mediators significantly attenuated the association between AF and TSH. Only adjusting for height significantly decreased the magnitude of the association between TSH and AF from –0.12 (SE = 0.02) to –0.06 (SE = 0.02) (P = .005) by IVWA MVMR (Fig. 3 and Supplementary Table 4 [35]). These data demonstrate that the genetic risk for AF associated with TSH is mediated in part by changes in height (Fig. 4).
Figure 3.
Forest plot summarizing multivariable, 2-sample mendelian randomization analyses of thyrotropin (TSH) on atrial fibrillation (AF) after adjusting for candidate mediators. Shown is the odds ratio (OR) for the association between genetically determined TSH and AF risk after adjusting for a candidate mediator, as indicated in the legend. An asterisk denotes that adjustment for the mediator significantly decreased the risk estimate (P < .05). FT4, free thyroxine; SBP, systolic blood pressure.
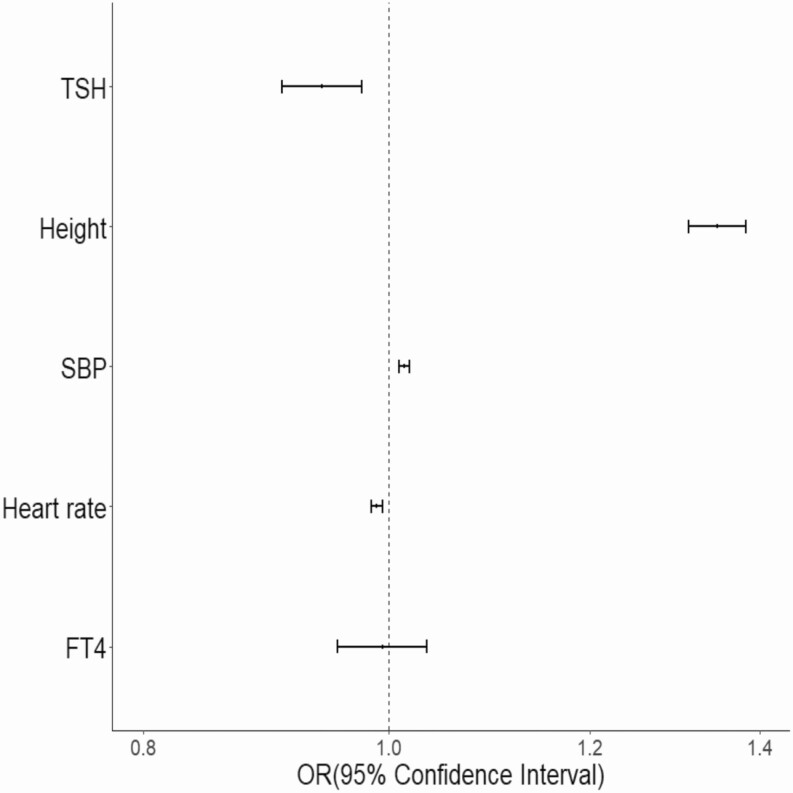
Figure 4.
Forest plot summarizing multivariable 2-sample mendelian randomization analyses of all candidate mediators on atrial fibrillation risk. An asterisk denotes an association with a nominal P value less than .05. FT4, free thyroxine; OR, odds ratio; SBP, systolic blood pressure; TSH, thyrotropin.
We examined whether there was a different pattern in the TSH-height association using subsets of TSH-associated SNVs with and without associations with autoimmune thyroid disease. Both subsets were associated with AF, but only the nonautoimmune SNVs were associated with height (Supplementary Table 5 [35]).
Discussion
The inverse association between plasma TSH levels and AF risk is well established and has been observed in epidemiological studies and, more recently, recapitulated using genetic association approaches (3, 4, 36, 37). While this could suggest that the risk mechanisms underlying the association between measured TSH and genetically determined TSH levels are similar, this has not been evaluated. We used MVMR methods to probe mediating mechanisms that might account for the genetic association between TSH and AF risk. A TSH genetic predictor was associated with 4 AF risk factors. However, adjusting only for genetically predicted height, not downstream products of TSH or hemodynamic traits, significantly attenuated the TSH-AF association. This suggests that a portion of the AF risk associated with genetically determined thyroid hormone levels is attributable to its impact on height.
We observed that genetically mediated lower levels of TSH (which would be expected to correlate with increased thyroid hormone levels) were associated with increased genetic height. We further observed that only those TSH-associated SNVs not related to autoimmune mechanisms were associated with height. This is consistent with the fact that autoimmune thyroid disease is typically not penetrant until adulthood, after the completion of growth (38). This observation is consistent with the known critical role of thyroid hormones in promoting normal childhood growth (39). Untreated hypothyroidism in childhood leads to growth retardation, and evaluation of thyroid hormone levels is an essential element of the evaluation for short stature in children (40). Growth abnormalities associated with many systemic diseases are also mediated in part by alterations in thyroid hormone physiology. Exogenous replacement of thyroid hormones can normalize growth among children with deficiency (41).
Increased height is associated with an increased risk for AF (42). Again, this association has been observed using both traditional epidemiological and genetic association approaches (43-45). In MVMR, adjusting for height was the only TSH-associated factor that significantly attenuated the association with AF risk, suggesting that a portion of the TSH association is mediated through its effects on height. Importantly, all the directions of association reported here are consistent with prior observations. Collectively, our findings suggest that the AF association is due in part to the effects of genetically modulated TSH signaling on growth during childhood and final adult height attainment.
One postulated mechanism linking height to AF risk is an enlarged LA diameter (46). We did not observe a significant inverse association between TSH and LA diameter, as would be expected if this were the mediating mechanism. Similarly, Levin et al did not observe an attenuated association between genetically predicted height and AF after adjusting for LA diameter (45). For these analyses, LA diameter measurements used for the GWAS were derived from a clinical population with multiple comorbidities including heart failure, which could attenuate an association with TSH. Another proposed mechanism is higher cardiac output and stroke volumes associated with elevated height. We observed an inverse association between TSH and the PR interval (at nominal significance), consistent with observations of longer PR intervals being associated with directly measured height (47). A prolonged PR interval is associated with increased AF risk (48). Thus, PR prolongation may reflect atrial remodeling or alterations in cardiac electrophysiology associated with height that may represent predisposing mechanisms to AF.
Multivariable adjustment for height and other candidate mediators did not completely attenuate the genetic association between TSH and AF risk, suggesting that there are additional mediating risk mechanisms. The association was modestly (though nonsignificantly) attenuated when adjusting for SBP, suggesting that part of the risk could be modulated by the chronic effects of BP elevations. Part of the association may also be attributable to the direct action of thyroid hormones on cardiomyocytes. While it is not clear that TSH directly affects cardiomyocyte signaling, thyroid hormones shorten action potential duration and increase automaticity in pulmonary vein myocytes, which predisposes to AF (49, 50). In support of these physiological observations, we observed an inverse association between TSH levels and HRs (ie, higher thyroid hormone levels associated with increased HRs). However, the overall association between HR and AF risk was in the opposite direction than would be expected: Genetically lower HRs were associated with AF risk. However, the association between HR and AF is “J”-shaped, with both high and low rates associated with increased AF risk (51). Thus, an SNV-based instrumental variable approach, as used here, may not be able to model the complex HR association, and adjusting for genetically determined HR levels may not appropriately model the direct effects of thyroid hormones on cardiomyocytes.
MR approaches are often used to provide causal effect estimates for a risk factor on an outcome. These estimates would be expected to be accurate only if genetically mediated variability in a risk factor is comparable to environmentally mediated variation. This study highlights an instance in which causal effect estimates based on genetic associations may not be valid estimates for the risks associated with thyroid hormones in later life. Genetic variation is a lifelong exposure to a risk factor. For TSH, part of the genetic AF risk appears to be related to the effects of thyroid hormones on height, a risk mechanism not relevant to thyroid hormone alterations in adulthood. Thus, causal effect estimate based on genetic TSH could be inaccurate or inflated, as compared to effect estimates based on the associations between measured TSH and AF risk in adults. Consistently, a large systematic review of epidemiological studies found the opposite findings of those reported here. Namely that measured FT4, but not TSH, was significantly associated with incident AF risk (52). Thus, risk estimates from MR must be interpreted with careful consideration of the risk mechanisms being measured by genetic vs epidemiological approaches.
The TSH genetic instrument was derived from a GWAS based on TSH measurements within the clinical reference range (13). Clinical thresholds are constructs created for the purpose of standardizing clinical practices. However, the underlying biology of common genetic variants and their influences on disease mediators is typically additive and linear throughout the extended range of the mediator (53). Thus, the associations observed here would be expected to be relevant for TSH levels within and outside the reference ranges. Collectively, these and other data (46) support the idea that individuals with higher thyroid levels values carry a higher liability for AF, as compared to those with lower thyroid values across the entire biological range.
Many of the effects of the thyroid system are due to the direct effects of free 3,5,3′-triiodothyronine (FT3). While TSH is strongly inversely associated with the T3, this study cannot confirm that the FT3 underlies the associations reported here. To date, an insufficient number SNVs associated with FT3 have been identified (54). For AF risk factors such as SBP or structural traits, SNVs identified by GWAS account for only a small portion of the genetic risk. This decreases the power to identify mediating effects of these risk factors and can lead to false-negative associations. Finally, the instrumental variables were derived from GWAS conducted on predominantly European ancestry populations, and generalizability to other ancestries is not known.
In summary, we found that genetically modulated TSH levels are inversely associated with AF risk, and this association is mediated in part by the effects of genetically determined thyroid hormone levels on height. We did not find that the mediating effects were through modifiable risk factors such as SBP. Thus, a contribution of genetic variation associated with TSH levels to AF risk may be due to its effects on development during early life.
Acknowledgments
Financial Support: This work was supported by the American Heart Association (grant Nos. 16FTF30130005 and 18SFRN34230089) and the National Institutes of Health (NIH; grant Nos. R01-GM13079, R01-HL142856, and T32-GM007569 to A Manouchehri; and K12-HD04348 to J Hellwege). Some samples and/or data set(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by institutional funding, private agencies, and federal grants. These include the NIH-funded Shared Instrumentation Grants S10OD017985 and S10RR025141; and Clinical and Translational Science Award grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include grant numbers U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, and R01HD074711; and additional funding sources listed at https://victr.vumc.org/biovu-funding/.
Glossary
Abbreviations
- AF
atrial fibrillation
- BMI
body mass index
- FT3
free 3,5,3′-triiodothyronine
- FT4
free thyroxine
- GWAS
genome-wide association studies
- IVWA
inverse-variance weighted average meta-analysis
- LA
left atrial
- MR
mendelian randomization
- MVMR
multivariable mendelian randomization
- PC
principal component
- PRS
polygenic score
- SBP
systolic blood pressure
- SNV
single-nucleotide variation
- TSH
thyrotropin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data on AF have been contributed by AFGen Consortium investigators and are available in the GWAS catalog. Data on BMI, height, and waist circumference have been contributed by the GIANT consortium and are available at https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files. Data on glycemic traits have been contributed by MAGIC investigators and have been downloaded from www.magicinvestigators.org. Data for type 2 diabetes have been contribute by the DIAGRAM Consortium and are available at http://diagram-consortium.org/downloads.html.
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bianco AC, Dumitrescu A, Gereben B, et al. . Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019;40(4):1000-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salem JE, Shoemaker MB, Bastarache L, et al. . Association of thyroid function genetic predictors with atrial fibrillation: a phenome-wide association study and inverse-variance weighted average meta-analysis. JAMA Cardiol. 2019;4(2):136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellervik C, Roselli C, Christophersen IE, et al. . Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a mendelian randomization study. JAMA Cardiol. 2019;4(2):144-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JL, Jacobs V, May HT, et al. . Free thyroxine within the normal reference range predicts risk of atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31(1):18-29. [DOI] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. [DOI] [PubMed] [Google Scholar]
- 7. Waldmann V, Jouven X, Narayanan K, et al. . Association between atrial fibrillation and sudden cardiac death. Circ Res. 2020;127(2):301-309. [DOI] [PubMed] [Google Scholar]
- 8. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease, addressing knowledge and clinical practice gaps: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(5):539-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marouli E, Kus A, Del Greco MF, et al. . Thyroid function affects the risk of stroke via atrial fibrillation: a mendelian randomization study. J Clin Endocrinol Metab. 2020;105(8):2634-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teumer A, Chaker L, Groeneweg S, et al. ; Lifelines Cohort Study . Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018;9(1):4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christophersen IE, Rienstra M, Roselli C, et al. ; METASTROKE Consortium of the ISGC; Neurology Working Group of the CHARGE Consortium; AFGen Consortium . Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eppinga RN, Hagemeijer Y, Burgess S, et al. . Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat Genet. 2016;48(12):1557-1563. [DOI] [PubMed] [Google Scholar]
- 16. Mosley JD, Levinson RT, Farber-Eger E, et al. . The polygenic architecture of left ventricular mass mirrors the clinical epidemiology. Sci Rep. 2020;10(1):7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ntalla I, Weng LC, Cartwright JH, et al. . Multi-ancestry GWAS of the electrocardiographic PR interval identifies 202 loci underlying cardiac conduction. Nat Commun. 2020;11(1):2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Harst P, van Setten J, Verweij N, et al. . 52 genetic loci influencing myocardial mass. J Am Coll Cardiol. 2016;68(13):1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arking DE, Pulit SL, Crotti L, et al. ; CARe Consortium; COGENT Consortium; DCCT/EDIC; eMERGE Consortium; HRGEN Consortium . Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46(8):826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turcot V, Lu Y, Highland HM, et al. ; CHD Exome+ Consortium; EPIC-CVD Consortium; ExomeBP Consortium; Global Lipids Genetic Consortium; GoT2D Genes Consortium; EPIC InterAct Consortium; INTERVAL Study; ReproGen Consortium; T2D-Genes Consortium; MAGIC Investigators; Understanding Society Scientific Group . Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet. 2018;50(1):26-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yengo L, Sidorenko J, Kemper KE, et al. ; GIANT Consortium . Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shungin D, Winkler TW, Croteau-Chonka DC, et al. ; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giri A, Hellwege JN, Keaton JM, et al. ; Understanding Society Scientific Group; International Consortium for Blood Pressure; Blood Pressure-International Consortium of Exome Chip Studies; Million Veteran Program . Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51(1):51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dupuis J, Langenberg C, Prokopenko I, et al. ; DIAGRAM Consortium, GIANT Consortium, Global BPgen Consortium, Anders Hamsten on behalf of Procardis Consortium, the MAGIC investigators. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahajan A, Taliun D, Thurner M, et al. . Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah S, Henry A, Roselli C, et al. ; Regeneron Genetics Center . Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roden DM, Pulley JM, Basford MA, et al. . Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loh PR, Danecek P, Palamara PF, et al. . Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28(24):3326-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatterjee N, Shi J, García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17(7):392-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu Z, Zheng Z, Zhang F, et al. . Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kjaergaard AD, Marouli E, Papadopoulou A, et al. . Thyroid function, sex hormones and sexual function: a mendelian randomization study. Eur J Epidemiol. 2021;36(3):335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi M, Mosley JD. TSH_AF_MVMR Supplementary Fig. & Table Revision. Uploaded March 31, 2021. doi: 10.17605/OSF.IO/P8FU3. Accessed March 31, 2021. [DOI]
- 36. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501-509. [DOI] [PubMed] [Google Scholar]
- 37. Biondi B, Cooper DS. Subclinical hyperthyroidism. N Engl J Med. 2018;378(25):2411-2419. [DOI] [PubMed] [Google Scholar]
- 38. De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018;6(7):575-586. [DOI] [PubMed] [Google Scholar]
- 39. Bauer AJ, Wassner AJ. Thyroid hormone therapy in congenital hypothyroidism and pediatric hypothyroidism. Endocrine. 2019;66(1):51-62. [DOI] [PubMed] [Google Scholar]
- 40. Wassner AJ. Pediatric hypothyroidism: diagnosis and treatment. Paediatr Drugs. 2017;19(4):291-301. [DOI] [PubMed] [Google Scholar]
- 41. Tarım Ö. Thyroid hormones and growth in health and disease. J Clin Res Pediatr Endocrinol. 2011;3(2):51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park YM, Moon J, Hwang IC, Lim H, Cho B. Height is associated with incident atrial fibrillation in a large Asian cohort. Int J Cardiol. 2020;304:82-84. [DOI] [PubMed] [Google Scholar]
- 43. Rosenberg MA, Patton KK, Sotoodehnia N, et al. . The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33(21):2709-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenberg MA, Kaplan RC, Siscovick DS, et al. . Genetic variants related to height and risk of atrial fibrillation: the cardiovascular health study. Am J Epidemiol. 2014;180(2):215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levin MG, Judy R, Gill D, et al. ; Regeneron Genetics Center . Genetics of height and risk of atrial fibrillation: a mendelian randomization study. PloS Med. 2020;17(10):e1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mont L, Tamborero D, Elosua R, et al. ; GIRAFA (Grup Integrat de Recerca en Fibril-lació Auricular) Investigators . Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10(1):15-20. [DOI] [PubMed] [Google Scholar]
- 47. Kofler T, Thériault S, Bossard M, et al. . Relationships of measured and genetically determined height with the cardiac conduction system in healthy adults. Circ Arrhythm Electrophysiol. 2017;10(1):e004735. [DOI] [PubMed] [Google Scholar]
- 48. Cheng S, Keyes MJ, Larson MG, et al. . Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301(24):2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Lloyd A, Bursell J, Gregory JW, Rees DA, Ludgate M. TSH receptor activation and body composition. J Endocrinol. 2010;204(1):13-20. [DOI] [PubMed] [Google Scholar]
- 50. Chen YC, Chen SA, Chen YJ, Chang MS, Chan P, Lin CI. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol. 2002;39(2):366-372. [DOI] [PubMed] [Google Scholar]
- 51. Habibi M, Chahal H, Greenland P, et al. . Resting heart rate, short-term heart rate variability and incident atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis (MESA)). Am J Cardiol. 2019;124(11):1684-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baumgartner C, da Costa BR, Collet TH, et al. ; Thyroid Studies Collaboration . Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136(22):2100-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arvanitis M, Qi G, Bhatt DL, et al. . Linear and nonlinear mendelian randomization analyses of the association between diastolic blood pressure and cardiovascular events: the J-curve revisited. Circulation. 2021;143(9):895-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Popović M, Matana A, Torlak V, et al. . Genome-wide meta-analysis identifies novel loci associated with free triiodothyronine and thyroid-stimulating hormone. J Endocrinol Invest. 2019;42(10):1171-1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on AF have been contributed by AFGen Consortium investigators and are available in the GWAS catalog. Data on BMI, height, and waist circumference have been contributed by the GIANT consortium and are available at https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files. Data on glycemic traits have been contributed by MAGIC investigators and have been downloaded from www.magicinvestigators.org. Data for type 2 diabetes have been contribute by the DIAGRAM Consortium and are available at http://diagram-consortium.org/downloads.html.
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.