Abstract
Purpose:
Reducing chemical pressure on human and environmental health is an integral part of the global sustainability agenda. Guidelines for deriving globally applicable, life cycle based indicators are required to consistently quantify toxicity impacts from chemical emissions as well as from chemicals in consumer products. In response, we elaborate the methodological framework and present recommendations for advancing near-field/far-field exposure and toxicity characterization, and for implementing these recommendations in the scientific consensus model USEtox.
Methods:
An expert taskforce was convened by the Life Cycle Initiative hosted by UN Environment to expand existing guidance for evaluating human toxicity impacts from exposure to chemical substances. This taskforce evaluated advances since the original release of USEtox. Based on these advances, the taskforce identified two major aspects that required refinement, namely integrating near-field and far-field exposure and improving human dose-response modeling. Dedicated efforts have led to a set of recommendations to address these aspects in an update of USEtox, while ensuring consistency with the boundary conditions for characterizing life cycle toxicity impacts and being aligned with recommendations from agencies that regulate chemical exposure. The proposed framework was finally tested in an illustrative rice production and consumption case study.
Results and discussion:
On the exposure side, a matrix system is proposed and recommended to integrate far-field exposure from environmental emissions with near-field exposure from chemicals in various consumer product types. Consumer exposure is addressed via submodels for each product type to account for product characteristics and exposure settings. Case study results illustrate that product-use related exposure dominates overall life cycle exposure. On the effect side, a probabilistic dose-response approach combined with a decision tree for identifying reliable points of departure is proposed for non-cancer effects, following recent guidance from the World Health Organization. This approach allows for explicitly considering both uncertainty and human variability in effect factors. Factors reflecting disease severity are proposed to distinguish cancer from non-cancer effects, and within the latter discriminate reproductive/developmental and other non-cancer effects. All proposed aspects have been consistently implemented into the original USEtox framework.
Conclusions:
The recommended methodological advancements address several key limitations in earlier approaches. Next steps are to test the new characterization framework in additional case studies and to close remaining research gaps. Our framework is applicable for evaluating chemical emissions and product-related exposure in life cycle assessment, chemical alternatives assessment and chemical substitution, consumer exposure and risk screening, and high-throughput chemical prioritization.
Keywords: Life cycle impact assessment, near-field exposure, chemical toxicity, dose-response modelling, human toxicity impacts, characterization factors, global guidance
1. Introduction
Reducing chemical pressure on human and ecological health is an integral part of the global sustainability agenda. This includes the reduction of chemical emissions into the natural environment along product life cycles as well as the reduction of human exposure to chemicals used in consumer goods, as laid out in the United Nations (UN) Sustainable Development Goals (UN 2020) and in the UN Environment Programme’s Strategic Approach to International Chemicals Management (UNEP 2015). Harmonized guidelines are required to consistently quantify life cycle toxicity impacts from chemical emissions as well as from exposure to chemicals in products or articles (hereafter referred to as ‘products’). Such guidelines should focus especially on providing recommendations for globally applicable and life-cycle-based indicators and underlying methods that are most suitable for the quantitative characterization of human and ecological toxicity impacts associated with chemical emissions and exposure. Therefore, it is important that related recommendations are consistent with the boundary conditions of characterizing toxicity impacts in life cycle impact assessment (LCIA) (Fantke et al. 2018a), while being aligned with chemicals-management recommendations from regulatory entities (Saouter et al. 2017a,b).
In response to these needs, the Life Cycle Initiative, which is hosted at the UN Environment Programme, developed and endorsed the scientific consensus model USEtox (Rosenbaum et al. 2008, Westh et al. 2015, Fantke et al. 2020b). Current practice for characterizing human toxicity and freshwater ecotoxicity impacts in LCIA is implemented in this model. It includes inhalation and ingestion exposure, and related health effects from emissions into far-field compartments (air, water, soil) or into a generic indoor compartment. However, despite reflecting—as a scientific consensus model—mature science (Hauschild et al. 2008), the original toxicity characterization framework has limitations, calling for further improvement based on scientific progress. Such improvements are mainly related to increasing the spatiotemporal and population-level resolution of impact estimates and extending the coverage and quality of substance, exposure, and dose-response data and models (Fantke et al. 2018a,b, Kirchhübel & Fantke 2019, Crenna et al. 2020, Gentil et al. 2020, Holmquist et al. 2020). Furthermore, a series of recent studies has demonstrated that environmentally-mediated exposures from chemical emissions are less important for overall exposure than consumer exposure to chemical constituents in products (Shin et al. 2015, Ernstoff et al. 2016, Fantke & Jolliet 2016, Csiszar et al. 2017, Ring et al. 2019, Fantke et al. 2020b, Jolliet et al. 2021). Hence, including pathways related to chemicals in consumer products into the original LCIA framework is crucial for considering all relevant life cycle toxicity impacts. Another specific need is the improvement of dose-response assessment for non-cancer toxicity that would benefit from latest progress in stochastic dose-response modelling (WHO 2014, 2017, Chiu & Slob 2015, WHO 2017, Chiu et al. 2018). To address these issues, the Life Cycle Initiative has established taskforces for advancing the original consensus for human toxicity and ecotoxicity characterization, in the frame of a flagship project on global guidance on environmental LCIA indicators (Jolliet et al. 2014, Frischknecht et al. 2016, Verones et al. 2017, Jolliet et al. 2018). The work conducted by these taskforces has led to a set of recommendations for refining and expanding the original LCIA toxicity characterization framework, and for implementing these recommendations into USEtox. In the present paper, we detail the methodological aspects underlying these recommendations and their implementation into the original USEtox modeling framework. We present recommendations for environmental fate, human exposure and toxicity effects with focus on including exposure to chemicals in consumer products and non-cancer dose-response modeling, while recommendations for ecotoxicity effects are presented elsewhere. The proposed updated USEtox near-field/far-field exposure and human toxicity modelling framework is finally evaluated in an illustrative rice production and consumption case study.
2. Consensus-building process
In 2016, the Life Cycle Initiative convened a Human Toxicity taskforce, consisting of leading experts from academia, industry, and government public-health institutions, to expand its guidance for evaluating human toxicity impacts from exposure to chemical substances. The taskforce followed the process of an earlier taskforce providing guidance on assessing impacts from exposure to fine particulate matter (Fantke et al. 2015, Hodas et al. 2016, Fantke et al. 2017, Fantke et al. 2019b). An initial scoping phase identified the need for advancing two major aspects, namely integrating near-field and far-field exposure and improving human dose-response modeling. To address these aspects, scientific advances were discussed and initial recommendations formulated through three expert workshops held in 2016 and 2017 (Fantke et al. 2018b). Further refinement of these initial recommendations, along with alignment with efforts to update ecotoxicity impact assessment (Fantke et al. 2018a, Owsianiak et al. 2019), led to a set of final recommendations discussed and agreed upon in a scientific expert workshop held in Valencia (Spain) in 2018. While the general recommendations of advancing USEtox have been summarized in the related workshop report (Frischknecht & Jolliet 2019), the present paper details the underlying methodological framework for human near-field exposure and toxicity characterization.
3. Proposed methodological framework
In USEtox, human toxicity impacts are characterized in terms of population-level lifetime loss associated with cumulative health risk for different health effects (aggregated into cancer and non-cancer effects, each having different average effect severity). Such health effects are associated with exposure to chemicals that distribute among far-field compartments (e.g. outdoor air), finally reaching humans via different exposure pathways (e.g. ingestion of food) (Rosenbaum et al. 2011). Combining these aspects for environmental emissions in a matrix-based framework yields characterization factors expressed in disability-adjusted life years (DALY) lost per unit mass emitted into the environment.
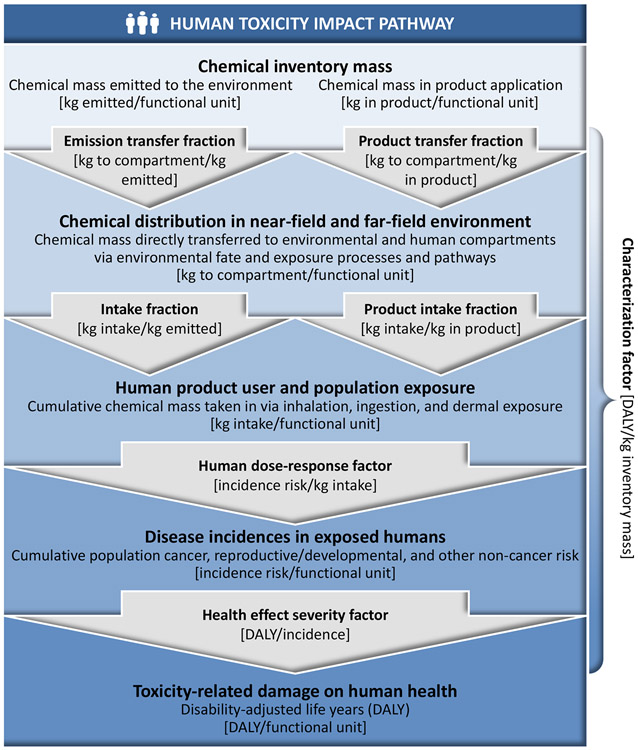
To facilitate the integration of near-field (consumer) exposure environments into the existing framework of far-field (emission-related outdoor) exposure environments, we reviewed available approaches that model near-field fate and exposure pathways for chemicals in consumer products (Huang et al. 2017). The integrated framework needed to address human exposure pathways during and after product use on a consistent mass-balance basis. Following recent recommendations for human toxicity characterization, we build on the original rate-constant-based USEtox framework to determine transfer fractions. We extend these recommendations to the near-field environment to obtain by matrix inversion cumulative transfer fractions and human-exposure-related product intake fractions (PiF). The latter combine near-field consumer household environments with far-field environments into a metric that incorporates the interactions of humans with both types of environments via dermal, inhalation, and oral exposure pathways, and potential feedback via e.g. exhalation (Jolliet et al. 2015, Fantke et al. 2016). This allows linking human intake via all exposure routes directly to chemical mass in products. The overall structure of the new recommended integrated near-field/far-field human exposure and toxicity characterization framework is illustrated in Figure 1.
Figure 1.
Recommended human toxicity characterization framework. Emission-based far-field exposure is integrated with product-related near-field consumer-exposure pathways, and linked to human toxicity dose-response and effect severity information. Unit mass emitted from or contained in a product can be expressed as unit inventory mass.
In line with the original USEtox matrix framework, we arrange in a single matrix all cumulative chemical mass transfer fractions. We start from products and emissions in the different environments along with intake fractions (relating intake to emissions) and product intake fractions (relating intake to mass in products) (Fantke et al. 2016). We obtain a matrix of characterization factors per kg inventory mass [DALY/kginventory mass], relating impacts on humans due to health effects e per unit mass of a chemical in emission or product compartment of entry c:
| (1) |
where the diagonal matrix of severity factors [DALY/incidence] multiplies matrix of dose-response factors [incidence risk/kgintake] associated with exposure routes r (i.e. inhalation, ingestion and dermal exposure, aggregated over all contributing exposure pathways x ∈ r, such as ingestion of drinking water and ingestion of food). Matrices SF and DRF are combined into matrix EF of toxicity-related health effect factors [DALY/kgintake], multiplying matrix TFcum. The dimensions of the matrix of dose-response factors is thereby matched to TFcum, i.e. , with zeroes for all non-human compartments. From this set of matrices, we derive the following recommendations for characterizing human toxicity and ecotoxicity impacts for chemical emissions and for chemicals used in products.
Impact scores ISH [DALY/functional unit] representing toxicity-related damages on human health are derived from the chemical inventory mass per functional unit, mc [kginventory/functional unit]. Chemicals are either emitted to an environmental or available in a product compartment c, multiplied by the sum of corresponding characterization factors for human toxicity (H) impacts, [DALY/kginventory], across considered exposure routes r and effect types e, aggregating over all compartments c:
| (2) |
where the chemical inventory mass corresponds directly to the emitted mass for environmental emissions, and to the mass of chemical in a given product application for chemicals in consumer products. This chemical mass in products is derived from the chemical weight fraction in a product application [kg/kgproduct] and the related product mass Mc [kgproduct]:
| (3) |
Characterization factors are derived from human-intake-related cumulative chemical mass transfer fractions, [kgintake/kginventory], summed over population groups u, and over exposure pathways x belonging to the same route r. These cumulative transfer fractions are multiplied by toxicity-related health effect factors, [DALY/kgintake], calculated as the product of the dose-response factor, [incidence risk/kgintake], for exposure route r and effect type e, and the effect-type-specific severity factor, [DALY/incidence]:
| (4) |
Human toxicity indicators are most commonly extrapolated from animals to humans (as human data are often unavailable). They express dose-response factors for carcinogenic and noncarcinogenic toxicity effect endpoints that represent a change in human population response per unit change of chemical exposure. To allow for considering the various health endpoints obtained from (human and animal) toxicity studies, results are generally aggregated into cancer and non-cancer effects in LCIA toxicity characterization. Based on the latest work in dose-response modelling (WHO 2014, Chiu & Slob 2015, Chiu et al. 2018), we refine the approaches for the selection of toxicity input data and their extrapolation to derive effect factors for non-cancer effects, as described further below. Severity factors translate an estimated human response into potential lifetime loss due to death or disease, expressed in DALY. Statistically-averaged severity factors for cancer and non-cancer health endpoints were originally based on incidence-weighted DALY from the Global Burden of Disease (GBD) study series for 1990 (Huijbregts et al. 2005), which we update specifically associated with non-cancer responses as detailed further below.
Chemicals emitted into a given environmental compartment or used in a given product application can also reach ecosystems (e.g. via landfilling the product after use or via product use-related emissions into indoor air and subsequent transfer to the outdoor environment). Hence, we also characterize ecotoxicity impacts associated with either the chemical mass emitted to a compartment or the chemical mass in a product. Impact scores [PDF m3 d/functional unit] for a given ecosystem type s (e.g. freshwater ecosystems) represent ecotoxicity-related damages on ecosystem quality. They are derived as chemical inventory mass, mc [kginventory/functional unit] multiplied by the corresponding characterization factors for ecotoxicity (E) impacts, [PDF m3 d/kginventory], aggregated over all compartments c:
| (5) |
Characterization factors are derived from the cumulative chemical mass transfer fractions from emission compartment c to the receiving exposure compartment associated with ecosystem type s, [kgto compartment/kginventory]. are multiplied by the first-order residence time in the receiving compartment, which is the inverse of the direct overall removal rate constant of chemical mass from that compartment, [(kg/d)/kg]. These are further multiplied by the ecosystem exposure factor, XFS [kgbioavailable/kgto compartment], representing the bioavailable mass fraction in the compartment of exposed ecosystem type s, and by the ecosystem-type-specific, ecotoxicological effect factor, [PDF m3/kgbioavailable]. are calculated as the product of the concentration-response factor, CRFS [PAF m3/kgbioavailable], and the severity factor, [PDF/PAF]:
| (6) |
Since further multimedia transfers are already considered in , we do not use the diagonal of the fate factor matrix as in earlier versions of USEtox, but the first-order chemical residence time (inverse of ), to avoid double counting of feedback into the original emission or product compartment (Margni et al. 2004).
4. Integrated near-field and far-field fate and exposure assessment
4.1. Extended matrix framework
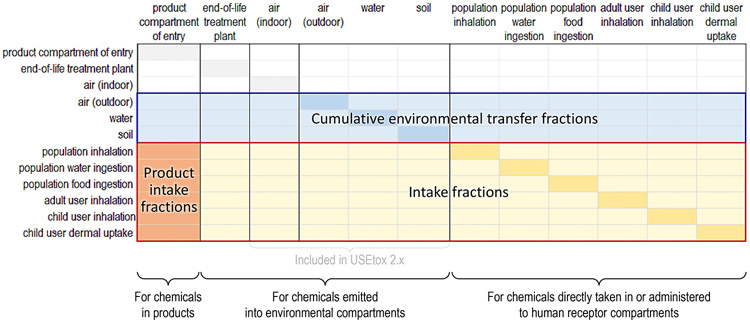
For consistency with the original USEtox far-field model, we structure the product compartment as an entry point for chemicals directly emitted to this compartment or for chemicals in products. We then link near-field and far-field emission compartments and human receptor compartments in a single matrix of cumulative chemical mass transfer fractions, TFcum (see eq. 1), as illustrated in Figure 2. Matrix TFcum is derived from inverting the matrix of direct transfer fractions, where each entry represents a direct chemical mass transfer fraction that is defined between two adjacent or nested emission sources and receiving compartments (Fantke et al. 2016). Matrix columns representing human receptor compartments are only relevant for feedback of chemical mass fractions taken in by humans that are released back to the environment, such as from exhaling a fraction of an inhaled chemical back into indoor air. Blue highlighted cumulative transfer fractions in Figure 2 divided by feedback factors are used for obtaining the matrix of fate factors as input for modeling ecotoxicity impacts, consistent with the original USEtox approach. Exposure-related matrix elements in Figure 2 are highlighted in orange. For all rows referring to human receptor compartments i, the first (i.e. product or direct emission source-related) column j of TFcum represents the vector of product intake fractions [kgintake/kginventory mass]. elements represent cumulative transfer from a given product or direct emission compartment to humans, and all other (i.e. emission source-related) columns represent the sub-matrix iF = TFcum[αi, βj≠1] of intake fractions [kgintake/kgemitted] representing cumulative transfer from different emission compartments to humans. Each element is the sum of direct and indirect transfer fractions contributing to an exposure pathway from a compartment of entry to a specific human receptor compartment. Different scenarios can be compared across exposure pathways for different compartments of entry (i.e. for chemicals in different product types), since all other TFcum columns are product-independent. The compartment of entry can refer to any near- or far-field emission compartment (for direct emissions) or to any product representing a semi-permeable boundary between product and near-field environment, which chemicals enter when installing products or bringing them into the near-field environment.
Figure 2.
Representation of the cumulative chemical mass transfer fractions matrix. It combines a product-related compartment of entry (left-most column) with near-field, far-field and human receptor compartments. Product intake fractions (PiF) start from the product compartment of entry and intake fractions (iF) start from environmental emissions, both linking to pathway- and population-specific human exposure.
4.2. Direct chemical mass transfer fractions
The first column in direct transfer fractions matrix TF is specified to be a product-type-specific compartment of entry, whose entries vary as function of transfer pathways relevant for different product types or emission scenarios. To calculate transfer fractions for chemicals in an initial emission or product compartment of entry for a variety of exposure pathways, we incorporated five main underlying models for ‘direct emission’, and for ‘article interior’, ‘skin surface layer’, ‘object surface’, and ‘food contact material’ products. Table 1 summarizes the direct transfer fractions that are determined by each underlying model.
Table 1.
Underlying models for calculating direct chemical mass transfer fractions, TF [−], from chemicals in products, including exposure pathways and example products covered.
| Model | Transfers & compartments | Direct exposure pathways | Example products | ||
|---|---|---|---|---|---|
| Direct emission | Transfers from compartment of emissions to various near-field, far-field and human compartments | Inhalation, ingestion of drinking water, and dermal uptake | All chemical emissions to environmental compartments | ||
|
|||||
| with ki→j defined in Rosenbaum et al. (2008) for environmental compartments, and with ki→jx for human compartments j via exposure pathways x: | |||||
|
|||||
| with IRi→jx defined in Rosenbaum et al. (2008) for inhalation and ingestion pathways. | |||||
| For gaseous dermal uptake (not included in earlier USEtox versions), with i ∈ {any air compartment}: | |||||
|
|||||
| For aqueous dermal uptake (not included in earlier USEtox versions), with i ∈ {any water compartment}: | |||||
|
|||||
| Article interior | Transfers from chemicals in articles to near-person or indoor air; accounting for long-term absorption on walls | Direct dermal contact, dust ingestion, (inhalation and gaseous dermal uptake are mediated via air) | Chemicals encapsulated in articles, building materials, toys, arts and crafts | ||
|
|||||
|
|||||
| with the following criterion for applying these two models: | |||||
| If log10 Kma + 0.61 × log10 Dm + 0.4 < 0, eq. 11 is applied, else eq. 12 is applied | |||||
|
|||||
|
|||||
| Skin surface layer* | Transfers from chemicals applied to skin surface to near-person air, epidermis, and wastewater treatment plant | Dermal aqueous uptake, hand-to-mouth, (inhalation and gaseous dermal uptake are mediated via air) | Personal care and hand dishwashing products | ||
|
|||||
|
|||||
|
|||||
|
|||||
| For skin area without hand-to-mouth contact, fhm = 0, khm = 0. | |||||
| Object surface* | Transfers from object surfaces to near-person and indoor air | Direct dermal contact, (inhalation and gaseous dermal uptake are mediated via air) | Surface cleaning products | ||
|
|||||
|
|||||
|
|||||
| with ktotal = kNS→NA + kNS→skin + kNS→FS + kNS,deg | |||||
| Food contact material | Transfers from food packaging to food | Food ingestion | Food packaging products | ||
|
|||||
|
|||||
Rate constants for the “skin surface layer” and “object surface” models are detailed in ESM, Sections S-2b and S-2c.
Symbols: a, b, c, α, β, τ, ω, γ: coefficients in respective regression models; A: area (m2); C: chemical concentration (μg/m3); d: product thickness (m); D: diffusion coefficient (m2/s); f: fraction of time unless indicated otherwise via indices (s/s); FQ: frequency of an activity (s/s); IR: human intake rate (m3/s for inhalation, μg/s for ingestion); K: partition coefficient (various units); k: process rate constant for processes indicated in indices (s−1); m: chemical mass (μg); Npersons: number of persons (capita); ρ: density (μg/m3); t: time (s); TF: chemical mass transfer fraction; V: volume (m3); v: arbitrary vector; λ: eigenvalue; XF: exposure rate constant (s−1); yt*: food migration transfer fraction at deviation time t*. Indices: deg: degradation; FS: far-person surface; GI tract: gastrointestinal tract; hm: hand-to-mouth; NA: near-person air; NS: near-person surface; pa: product-to-air; ps: product-to-skin.
The ‘direct emission’ model contains a set of fixed direct transfer fractions to environmental compartments. For emissions, direct transfer fractions are derived from original USEtox rate constants describing chemical transfers or losses from compartments describing outdoor environments (Rosenbaum et al. 2008), indoor environments (Rosenbaum et al. 2015), and waste treatment (e.g. Kjeldsen & Christensen 2001). Related direct human exposure fractions include inhalation of indoor air, ingestion of drinking water and dermal uptake. The ‘article interior’ model considers direct transfer fractions from articles (e.g. building materials, furniture, toys) to indoor air, to skin via dermal contact, and to the gastrointestinal tract via dust ingestion. Depending on chemical-material properties, different models are used for transfer to indoor air, namely diffusion-limited models (accounting for chemical diffusion within articles via Fick’s 2nd Law) (Huang & Jolliet 2016) and partition-limited models (assuming that chemicals are evenly distributed inside articles and accounting for indoor sorption) (see Electronic Supplementary Material, ESM, Section S-2a). Direct transfers for dermal contact and dust ingestion assume that chemical concentrations on article surfaces are in equilibrium with those in skin surface lipids and in dust, respectively. The ‘skin surface layer’ model applies to chemicals in personal care and other products (e.g. hand sanitizers) applied directly on human skin. This model uses a three-compartment mass-balance model that includes skin, indoor air, and product applied, and assumes that volatilization and skin permeation are competing loss processes (Ernstoff et al. 2016). The ‘object surface’ model is used for chemicals applied on object surfaces (e.g. surface cleaners). This model uses a four-compartment mass-balance approach that includes near-person, far-person (in the same indoor environment), surfaces, and air (Wang et al. 2016), with transfers between near-person and far-person surfaces to simulate the movement of a person when cleaning surfaces. The ‘food contact material’ model estimates the fraction of organic chemicals migrating from polymeric packaging materials into food matrices as a function of diffusion coefficients within packaging materials and packaging-food partition coefficients (Ernstoff et al. 2017). Indoor sorption is considered across product-specific models and detailed in ESM, Section S-2d.
5. Human toxicity dose-response framework
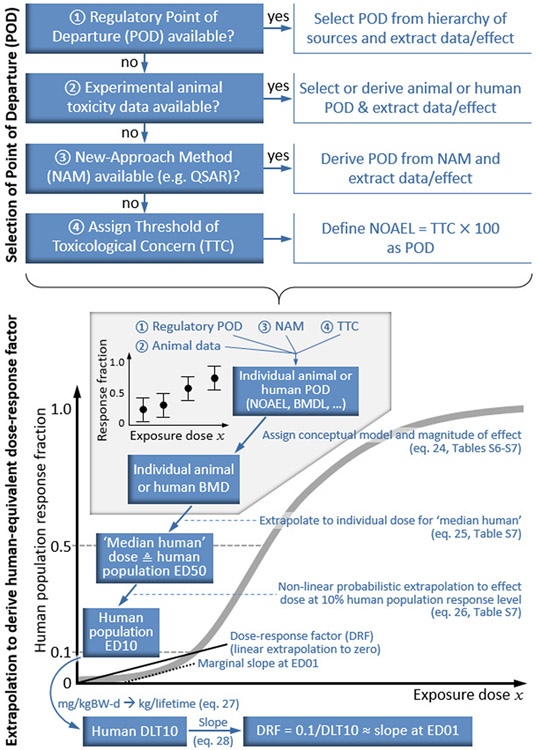
We updated the approach for deriving human toxicity dose-response factors (see eq. 1) for non-cancer endpoints. The updated approach is illustrated in Figure 3 and consists of two main steps for each chemical: (1) identifying a point of departure (POD) using a decision-tree based on data availability and reliability, and (2) deriving human lifetime equivalent dose-response factors from the selected PODs using a probabilistic framework. This framework addresses a number of key limitations of the original approach. First, it significantly broadens the coverage of chemicals for which dose-response factors can be derived by utilizing a wider range of toxicity data sources beyond regulatory human-health-hazard assessments. Second, it incorporates recent guidance from the WHO (2014, 2017) on more explicitly considering both uncertainty and human variability. Although dose-response factors are currently still fixed slopes based on point estimates, the values themselves are derived from a statistical distribution, rather than being the result of deterministic calculations. Finally, the value of the dose-response factor was chosen to better represent the marginal slope of the dose-response relationship in a dose range that is environmentally relevant.
Figure 3.
Recommended approach for deriving human toxicity dose-response information based on a hierarchy of different toxicity input data to identify a suitable individual point of departure (POD), and a probabilistic extrapolation toward human population lifetime toxicity dose-response factors.
5.1. Identifying points of departure (POD)
As shown in Figure 3 (top panel), our updated framework substantially expands the acceptable data sources for PODs. Animal or human toxicity values (e.g. benchmark dose lower confidence limit (BMDL), no-observed adverse effect level (NOAEL), or lowest observed adverse effect level (LOAEL)) used in regulatory human health hazard assessments are the preferred source. If such data are not available, other experimental animal data, New Approach Methods (NAMs), such as in vitro and in silico models, or Thresholds of Toxicological Concern (TTC) are used to derive an individual animal or human POD, as detailed in ESM, Section S-3a. However, even with regulatory assessments, it is often the case that different countries or organizations come to different conclusions and select different PODs, rendering it necessary to have an explicit hierarchy of sources. As an example, for U.S.-based regulatory values, we apply the hierarchy developed by Wignall et al. (2014), (2018) (see ESM, Section S-3b).
5.2. Deriving human lifetime equivalent dose-response factors
Once PODs are identified along with accompanying meta-data, we use the automated workflow developed by Chiu et al. (2018) to implement the WHO (2014, 2017) probabilistic framework and derive population-level dose-response functions. This workflow involves the following steps for each POD. First, we assign a ‘conceptual model’ based on the type of effect (continuous or dichotomous, reflecting a stochastic process or not) to each animal or human POD, using criteria listed in ESM, Table S6. Next, uncertainty distributions are generated for each POD using the default distributions derived by WHO (2014, 2017) and informed by the POD meta-data, as outlined in ESM, Table S7. With that, a median individual benchmark dose (BMD) is obtained from POD-specific extrapolations:
| (24) |
where efBMD is a probabilistic factor for NOAEL, LOAEL or BMDL to BMD extrapolation (for POD = BMD, the central estimate of efBMD = 1). From the BMD, we derive a human-equivalent individual dose-response factor for a given effect magnitude (e.g. % change in blood pressure) expressed in mg/kgBW/d for oral exposure and mg/m3 for inhalation exposure, which we define as ED50, which is the effect dose for the median (i.e. 50th %-ile) individual of the human population, thus inducing a 50% human population response:
| (25) |
where efBW, efTKTD and eft are probabilistic factors for, respectively, interspecies body weight (BW) scaling, interspecies toxicokinetic (TK)/toxicodynamic (TD) differences, and exposure duration extrapolation (ESM, Table S7). In case of human-based POD, central estimates for efBW = efTKTD = 1, but their uncertainty remains. These distributions are applied using the approach illustrated in Figure 3 (bottom panel), based on the WHO model. In this model, the human-population incidence-response level I (e.g. 10%) can be expressed as a cumulative lognormal distribution reflecting intra-species variability around the exposure dose level X, namely I(X) = Φ[z(X)],z(X) = (ln X – In ED50)/σH. In this notation, the ED50 is defined as in eq. 25, σH is the log-scale standard deviation that reflects human variability in sensitivity, and the dose level X = ED10 corresponds to the population incidence response level I = 10%, which is then used to derive a human-equivalent population lifetime dose by following the steps described below. This model is implemented, using the approach proposed by Chiu and Slob (2015), by dividing the ED50 by a probabilistic factor efH,I, calculated for a incidence level of I = 10%:
| (26) |
Specifically, efH,I=0.1 = exp(1.282 × σH), where 1.282 corresponds to the absolute value of the z-score for a 10% human population response level. Not only the POD but also all extrapolation factors in eqs. 24 to 26 are assigned distributions, from which ED10 is then derived probabilistically.
Next, to derive an effect metric compatible with the USEtox framework, the central tendency (median estimate) of the ED10 is converted into a human-equivalent lifetime dose, DLT10 (kg/lifetime):
| (27) |
where for oral exposure F{BW,BR} = 70 kg is the average human body weight and for inhalation exposure F{BW,BR} = 16 m3/d is the average human individual breathing rate, LT = 70 years is the average human lifetime, and cfd/yr = 365.25 d/yr and cfmg/kg = 106 mg/kg are conversion factors for, respectively, days per year and mg per kg. Finally, the human-equivalent lifetime dose-response factor, DRF (incidence risk/kg), is determined as linear slope to DLT10:
| (28) |
This central-tendency linearly-extrapolated slope from ED10 is also approximately equal to that of the marginal slope at 1% human population response level as illustrated in Figure 3, bottom panel (Fantke et al. 2019a), a response level that corresponds to a dose range that is environmentally relevant, especially when considering near-field exposure to chemicals in products.
In LCIA, the derived DRF is meant to represent the incremental (or marginal) increase in cumulative population risk for each unit of additional exposure, which is the slope of the dose-response relationship. The original LCIA approach uses the line between the point at 50% response and zero to represent this slope—using the above notation, this slope is simply 0.5/ED50. A key limitation of this previous approach is that it does not incorporate any information about population variability, which can be substantial, and can vary widely across chemicals (Zeise et al. 2013, Chiu et al. 2018). Indeed, the true slope of the dose-response relationship is non-linear, with a value that varies continuously depending on dose.
Ideally, each LCIA-related study would establish a ‘working point’ exposure at which this slope is evaluated, and the distribution to calculate the DRF in each case. However, there are several difficulties with implementing such a case-specific DRF. First, due to lack of exposure data, it is very difficult to establish the current ‘background’ exposure level for any given compound and spatially-varying emission, even at the population level, let alone its population distribution. Second, as pointed out by Zeise et al. (2013), chemicals are additive to background disease, rather than background exposure to a specific compound. Indeed, Huijbregts et al. (2005) and Fantke et al. (2018b) have suggested that the ‘background exposure’ could be set as an ‘effective’ exposure at the equivalent background incidence rate. In sum, there is substantial uncertainty in the appropriate exposure dose at which to evaluate the slope of the dose-response relationship. Given these uncertainties, the linear slope from the 10% response level was found to be adequately representative of a reasonable range of possible marginal slopes at 1% response as further detailed in ESM, Section S-3d.
6. Human health effect severity
To allow comparisons of human toxicity impacts with other impacts contributing to damages on human health, levels of severity are assigned to the predicted population response levels, yielding DALY estimates associated with incremental exposure. Huijbregts et al. (2005) provided estimates of the incidence-weighted-average DALY associated with a range of both cancer and non-cancer health endpoints. They proposed that impacts could be assessed using these average DALY values (albeit with high uncertainty, particularly for non-cancer endpoints), even though responses estimated from animal toxicity data can rarely be mapped to specific human diseases.
We propose that the previous approach be refined to address at least one additional subset of non-cancer responses separately from other non-cancer responses. Substances that cause birth defects may be of special interest, because of the clear dichotomous nature of the response, the presence of directly analogous disease states in humans, and the severity and duration of the outcome (US-EPA 1991). This includes a group of disease categories designated in the Global Burden of Disease (GBD) databases as congenital anomalies (birth defects). However, inclusion of these endpoints in the broader non-cancer category can severely underestimate such outcomes. Separation of this category of effects is potentially justifiable from both a mechanistic and a statistical point of view, given the heterogeneity in DALY between this category and other non-cancer effect categories (Hay et al. 2017). The term congenital anomalies as used in the GBD refers, in the context of toxicity data, to “reproductive/developmental toxicity” effects. Developmental outcomes are effects that manifest in the offspring, while reproductive effects are those that affect the fertility or function of a parent for reproduction. This entire category of reproductive/developmental toxicity draws special attention in the regulatory world (along with cancer and mutagenesis). An example is the CMR (carcinogenicity, mutagenicity, reproductive/developmental toxicity) designation used in Europe (EC 2008). Developmental outcomes thereby range in their severity from mild to extreme. However, the important point for all reproductive or developmental effects is that they have the potential to adversely affect humans for their entire lifetime, either because they were never born (effects on reproduction) or because they were born with either functional or morphological deficits (effects on development).
We recommend using incidence-weighted DALY values for all cancer endpoints from Huijbregts et al. (2005), as well as separate values for the reproductive or developmental effects and other non-cancer diseases as presented in ESM, Table S8. Separation of the reproductive/developmental category from other non-cancer responses results in a substantial decrease in the uncertainty associated with the average non-cancer (other than reproductive or developmental effects) DALY values. The reproductive/developmental DALY should be applied for any substance for which the effect factor is derived from reproductive or developmental outcomes. In addition to this separation, we recommend that DALY values per incidence for both non-cancer categories be updated with the most recent GBD statistics (Salomon et al. 2015, Stanaway et al. 2018).
7. Case study on rice production and consumption
The proposed updated USEtox near-field/far-field exposure and human toxicity modelling framework was demonstrated in an illustrative rice production and consumption case study based on three scenarios, using 1 kg of cooked, white rice as functional unit (FU). In the first scenario, rice is produced/processed in rural areas and distributed/cooked in urban China (CN); in the second scenario, rice production-to-cooking takes place in rural India (IN); and in the third scenario, rice is produced/processed in rural U.S. and distributed/cooked in urban Switzerland (US/CH). Further case study details are provided in Frischknecht et al. (2016). For human toxicity impacts, we considered 115 organic chemicals for which we could determine non-zero emissions along the rice production life cycle for at least one scenario. To specifically evaluate our near-field component, we additionally considered consumer exposure to six chemicals found in rice packaging (see ESM, Table S9), namely a single recycled cardboard package (CN), a single polyethylene rice package (IN), and multiple polyethylene rice cooking bags stored in a single recycled cardboard package (US/CH).
We applied the ‘article interior’ model to calculate chemical mass released from rice packaging to indoor air, and the ‘food contact material’ model to obtain the chemical mass migrated from rice packaging into rice during storage and boiling (see Table 1 for model descriptions). On the toxicity side, we derived non-cancer DRF values according to the approach presented in Figure 3 for 71 chemicals based on regulatory toxicity values, 47 based on in vivo experimental animal toxicity studies, and 3 utilizing QSAR (see ESM, Figure S5 for ingestion exposure and Figure S6 for inhalation exposure). Furthermore, we obtained information on carcinogenicity for 58 chemicals. Among those, we derived cancer DRF values for 37 chemicals, while the data for 21 chemicals showed no carcinogenic effect indication in the underlying experimental studies.
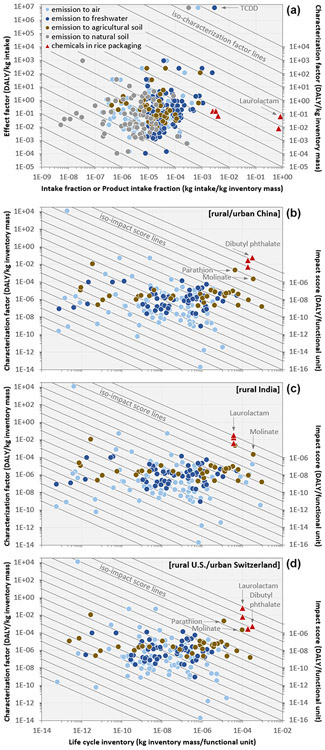
All individual chemicals across inventory, intake, effect, and impact-score levels were compared and ranked by decreasing impact scores for the US/CH scenario (ESM, Figure S7). Packaging-related manufacturing and disposal emissions are for most chemicals one to three orders of magnitude lower than total cradle-to-gate emissions. Overall, inventory results span over 10 orders of magnitude, with varying contributions of emission compartments across chemicals and scenarios. Intake fractions, aggregated over all contributing exposure pathways, span over 8 orders of magnitude, ranging from 5 × 10−9 to almost 1. For individual pathways, emission-based intake fractions range from < 10−15, i.e. 1 pg inhaled per kg halosulfuron-methyl, up to 3 × 10−3, i.e. 3 g ingested per kg 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), for both being emitted to freshwater, spanning 16 orders of magnitude. Product intake fractions for chemicals in rice packaging range from 2 ppt of laurolactam inhaled by household members after volatilization during storage to >90% ingested by users after migration to rice during boiling, spanning 12 orders of magnitude across exposed sub-populations and pathways for the same chemical. Human-toxicity effect factors combining dose-response and effect severity span over more than 10 orders of magnitude, from 10−4 DALY per kg propene inhaled to 7 million DALY per kg TCDD ingested. Inventory data, (product) intake fractions, and effect factors for all case study chemicals are provided in ESM, Table S10.
Combining emission-based intake fractions and product intake fractions with effect factors yields characterization factors across the rice-case-study chemicals, aggregated over all pathways per exposure route (Figure 4a). Significant characterization factors are found for all six chemicals in rice packaging (ranging from 0.0003 to 0.07 DALY/kg in packaging) and for some of the 115 emitted chemicals (highest being TCDD with 21000 DALY/kg emitted to freshwater). Characterization factors span 18 orders of magnitude, with varying contributions of effect types (cancer, reproductive/developmental, and other non-cancer effects) and exposure routes (inhalation, ingestion, and dermal exposure) across chemicals.
Figure 4.
Human toxicity characterization factors (a) and impact scores (b-d) for the rice case study. Characterization factors (right-side y-axis) are plotted as function of human (product) intake fractions combining fate and exposure (x-axis) and corresponding human toxicity-related effect factors combining dose-response and effect severity (left-side y-axis). Impact scores (right-side y-axis) are plotted as function of life cycle inventory mass (x-axis) and characterization factors (left-side y-axis). Industrial chemicals are emitted to rural air, freshwater and natural soil, and pesticides are emitted to agricultural soil. TCDD: 2,3,7,8-Tetrachlorodibenzo-p-dioxin.
Combining characterization factors with chemical mass in packaging and cradle-to-gate emissions yields the final impact scores expressed as DALY per functional unit (Figure 4b-d). In this cumulative score, results from near-field exposure to chemicals in consumer products (in our case rice packaging) are directly comparable with results from far-field population exposure to chemical emissions. Chemicals in packaging all show high impact scores, mostly via ingestion following migration from rice packaging into rice, and to a lesser extent via inhalation after volatilization from packaging into indoor air. This emphasizes the importance of including exposure to chemicals in consumer products and an adequate indoor environment into toxicity characterization methods used in LCA. While this primarily illustrates how chemicals in food packaging and near-field exposure are relevant, it would also be interesting in future studies to compare the impacts of packaging, accounting for the entire supply chain of the packaging and a larger number of contained chemicals. Impact scores range from 10−23 DALY for trichlorofluoromethane to 2 × 10−5 DALY for dibutyl phthalate, spanning 18 orders of magnitude. Aggregating impact scores yields between 5 × 10−8 DALY for US/CH and 2 × 10−7 DALY for IN, dominated by pesticide emissions to soil across scenarios. Rice packaging manufacturing and disposal contributes with <1% to overall impact scores across scenarios. Impacts from direct exposure to packaging chemicals exceed cradle-to-gate impacts by up to >2 orders of magnitude, ranging from 2 × 10−6 DALY for IN to 3 × 10−5 DALY for CN, dominated by exposure of consumers via ingestion of packaging chemicals migrating into rice. When translating the functional unit into daily use, we would arrive at impacts per individual usage per person. With that, our framework can also be applied in chemical exposure assessment and risk prioritization frameworks (Fantke and Illner 2019, Fantke et al. 2020a, Jolliet et al. 2021).
Based on the application of our framework in the rice case study, we developed additional recommendations. First, we recommend expressing human toxicity characterization factors as DALY per unit mass emitted (for environmentally mediated exposure) or as DALY per unit mass in a product application (for product use-related exposure), which can be interpreted as relative capacity to cause harm. We further recommend presenting impact scores separately for organic substances and metals/metalloids (the latter have not been discussed in the present document), to acknowledge important differences in characterizing fate, exposure and effects for these substance groups. Finally, we recommend presenting human toxicity impact results on log10-scale and allow for no more than two significant digits (e.g. 3.4 × 10−5) due to the substantial variability across and uncertainty in characterization factors.
8. Conclusions and next steps
Following up on recommendations from an expert task force for refining and expanding the original LCIA toxicity characterization approach, we present the methodological framework underlying these recommendations, and describe their implementation in USEtox. We considered recommendations for environmental fate, human exposure and toxicity effects, with a focus on expanding the exposure assessment to consider consumer exposure to chemicals in products and refining the non-cancer dose-response assessment by adapting a probabilistic framework. These adaptations enable future studies to (a) go beyond life cycle emissions by also considering exposure to chemicals related to various product use settings, (b) obtain inhalation, ingestion, and dermal exposure estimates for various chemical-product combinations, and delineating between product users, other relevant sub-populations, and the rest of the human population, (c) derive dose-response information for a much broader range of chemicals, (d) improve the reliability of human effect factors by applying a non-linear extrapolation from systematically selected points of departure, and (e) discriminate severity for three indicators, namely cancer, developmental/reproductive non-cancer, and other non-cancer effects. In all, the presented methodological advancements of the USEtox toxicity characterization model address several key limitations in earlier approaches. This includes considering both emission-based and product-based exposure along with their respective health effects in a consistent matrix framework. This is supported by our case study results, illustrating that product-use related exposure dominates overall life cycle exposure. Sub-models for product-related exposure already allow for considering dynamics in fate and exposure settings. To consider such dynamics also for environmental far-field emissions, we would need to define an exposure duration and introduce a dynamic solution for the entire multi-compartment transfer fractions system.
Applying a single overarching framework for considering various exposure and toxicity effects for a wide range of chemicals found in multiple products and their life cycles in a comparable way is very beneficial. However, it requires some necessary simplifications and assumptions (especially with respect to low-dose exposure and the consideration of differences in exposure and toxicity), which all contribute to a substantial level of uncertainty. In a comparative context with results ranging over many orders of magnitude and with a limited number of chemicals often dominating overall impact profiles, such uncertainties are acceptable, while guiding further research efforts for refining our framework.
For impact of food contact substances, useful insights on maximal levels and potential material matrices for intended conditions of use can be found in regulatory databases (e.g. the U.S. FDA ‘Inventory of Environmental Impact Decisions for Food Contact Substance Notifications’ with more than 2000 substance-material combinations). To study specific types of packaging, foreground data from manufacturing companies might be necessary to determine the specific food contact substances used in packaging, also considering the life cycle of the packaging. In the longer term, LCI databases might add data on typical chemical masses in products for a range of household products, such as food packaging, cosmetics, and paints. However, these are likely to be customized to each specific product and usage type, thus the importance of making the USEtox tool and its near-field component available to practitioners to determine exposure and impacts for the specific product studied.
Next steps are to test the recommended characterization framework in various case studies and to close remaining research gaps related to, for example, missing chemical content in product information, introducing additional exposure pathways and product types, as well as including the latest effect test data and effect severity information. The new toxicity data that have recently been made available also open the possibility to substantially extend the training set for in silico extrapolation and improve the quality of route-of-exposure-specific dose-response extrapolations. Our framework is applicable not only for evaluating chemical emissions and product-related exposure in LCIA, but also in chemical alternatives assessment (CAA) and chemical substitution, consumer exposure and risk screening, and high-throughput chemical prioritization (see Text box 1). The formal USEtox near-field/far-field model is freely available at https://usetox.org.
Textbox 1. Primary application areas of the recommended near-field/far-field USEtox modelling framework.
| The presented, recommended near-field/far-field USEtox modelling framework is suitable for comparative evaluations of chemicals emitted along product life cycles and chemicals in various types of product applications. Primary application areas, where the framework is applicable and where it has already been tested are: | ||
|---|---|---|
| Application area | Product types covered (emissions are always directly or indirectly included) |
References |
| Product life cycle assessment (LCA) | Food contact materials | Ernstoff et al. (2019), Figure 4 (present study) |
| High-throughput screening (HTS) of chemical exposure | Personal care products; food contact materials | Csiszar et al. (2016), Ernstoff et al. (2016), Ernstoff et al. (2017) |
| High-throughput screening (HTS) of chemical risk | Children toys; building materials | Huang et al. (2019), Aurisano et al. (2021) |
| Chemical exposure and risk prioritization | Household products (cleaning, personal care, and home maintenance products) | Jolliet et al. (2021), Figure 4 (present study) |
| Chemical alternatives assessment (CAA) and chemical substitution | Building materials; personal care products; pesticides | Fantke et al. (2016), Steingrímsdóttir et al. (2018), Fantke et al. (2020b) |
Supplementary Material
Acknowledgements
The authors would like to thank all members of the UNEP GLAM Human Toxicity taskforce for their input into the consensus-building process. W.A. Chiu and S. Jang were partly supported by the U.S. National Institute of Environmental Health Sciences (grant no. P42 ES027704). The present work was supported by the ‘Global Best Practices on Emerging Chemical Policy Issues of Concern under UN Environment’s Strategic Approach to International Chemicals Management (SAICM)’ (GEF project ID 9771, grant no. S1-32GFL-000632), and by the ‘Safe and Efficient Chemistry by Design (SafeChem)’ project funded by the Swedish Foundation for Strategic Environmental Research, MISTRA (grant no. DIA 2018/11).
References
- Aurisano N, Huang L, Mila i Canals L, Jolliet O, Fantke P (2021) Chemicals of concern in plastic toys. Environ. Int 146:106194. doi: 10.1016/j.envint.2020.106194 [DOI] [PubMed] [Google Scholar]
- Chiu WA, Slob W (2015) A unified probabilistic framework for dose-response assessment of human health effects. Environ. Health Perspect 123:1241–1254. doi: 10.1289/ehp.1409385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Axelrad DA, Dalaijamts C, Dockins C, Shao K, Shapiro AJ, Paoli G (2018) Beyond the RfD: Broad application of a probabilistic approach to improve chemical dose-response assessments for noncancer effects. Environ. Health Perspect 126:1–14. doi: 10.1289/EHP3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenna E, Jolliet O, Collina E, Sala S, Fantke P (2020) Characterizing honey bee exposure and effects from pesticides for chemical prioritization and life cycle assessment. Environ. Int 138:105642. doi: 10.1016/j.envint.2020.105642 [DOI] [PubMed] [Google Scholar]
- Csiszar SA, Ernstoff AS, Fantke P, Meyer DE, Jolliet O (2016) High-throughput exposure modeling to support prioritization of chemicals in personal care products. Chemosphere 163:490–498. doi: 10.1016/j.chemosphere.2016.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar SA, Ernstoff AS, Fantke P, Jolliet O (2017) Stochastic modeling of near-field exposure to parabens in personal care products. J. Expos. Sci. Environ. Epidemiol 27:152–159. doi: 10.1038/jes.2015.85 [DOI] [PubMed] [Google Scholar]
- EC (2008) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Commission of the European Communities, Brussels, pp. 1355 [Google Scholar]
- Ernstoff A, Niero M, Muncke J, Trier X, Rosenbaum RK, Hauschild M, Fantke P (2019) Challenges of including human exposure to chemicals in food packaging as a new exposure pathway in life cycle impact assessment Int. J. Life Cycle Assess 24:543–552. doi: 10.1007/s11367-018-1569-y [DOI] [Google Scholar]
- Ernstoff AS, Fantke P, Csiszar SA, Henderson AD, Chung S, Jolliet O (2016) Multi-pathway exposure modelling of chemicals in cosmetics with application to shampoo. Environ. Int 92-93:87–96. doi: 10.1016/j.envint.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Ernstoff AS, Fantke P, Huang L, Jolliet O (2017) High-throughput migration modelling for estimating exposure to chemicals in food packaging in screening and prioritization tools. Food Chem. Toxicol 109:428–438. doi: 10.1016/j.fct.2017.09.024 [DOI] [PubMed] [Google Scholar]
- Fantke P, Jolliet O, Apte JS, Cohen AJ, Evans JS, Hänninen OO, Hurley F, Jantunen MJ, Jerrett M, Levy JI, Loh MM, Marshall JD, Miller BG, Preiss P, Spadaro JV, Tainio M, Tuomisto JT, Weschler CJ, McKone TE (2015) Health effects of fine particulate matter in life cycle impact assessment: Conclusions from the Basel guidance workshop. Int. J. Life Cycle Assess 20:276–288. doi: 10.1007/s11367-014-0822-2 [DOI] [Google Scholar]
- Fantke P, Ernstoff AS, Huang L, Csiszar SA, Jolliet O (2016) Coupled near-field and far-field exposure assessment framework for chemicals in consumer products. Environ. Int 94:508–518. doi: 10.1016/j.envint.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Fantke P, Jolliet O (2016) Life cycle human health impacts of 875 pesticides. Int. J. Life Cycle Assess 21:722–733. doi: 10.1007/s11367-015-0910-y [DOI] [Google Scholar]
- Fantke P, Jolliet O, Apte JS, Hodas N, Evans J, Weschler CJ, Stylianou KS, Jantunen M, McKone TE (2017) Characterizing aggregated exposure to primary particulate matter: Recommended intake fractions for indoor and outdoor sources. Environ. Sci. Technol 51:9089–9100. doi: 10.1021/acs.est.7b02589 [DOI] [PubMed] [Google Scholar]
- Fantke P, Aurisano N, Backhaus T, Bulle C, Chapman PM, Cooper CA, De Zwart D, Dwyer R, Ernstoff A, Golsteijn L, Henderson A, Holmquist H, Jolliet O, Kirchhübel N, Nordheim E, Otte N, Owsianiak M, Peijnenburg W, Posthuma L, Roos S, Saouter E, Schowanek D, van Straalen N, Vijver M, Hauschild M (2018a) Toward harmonizing ecotoxicity characterization in life cycle impact assessment. Environ. Toxicol. Chem 37:2955–2971. doi: 10.1002/etc.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantke P, Aylward L, Bare J, Chiu WA, Dodson R, Dwyer R, Ernstoff A, Howard B, Jantunen M, Jolliet O, Judson R, Kirchhübel N, Li D, Miller A, Paoli G, Price P, Rhomberg L, Shen B, Shin H-M, Teeguarden J, Vallero D, Wambaugh J, Wetmore BA, Zaleski R, McKone TE (2018b) Advancements in life cycle human exposure and toxicity characterization. Environ. Health Perspect 126:125001. doi: 10.1289/EHP3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantke P, Aylward L, Chiu W, Gouin T, Jolliet O, Judson R, Rhomberg L, McKone TE (2019a) Human toxicity. In: Frischknecht R , Jolliet O (Editors), Global Guidance on Environmental Life Cycle Impact Assessment Indicators: Volume 2. UNEP/SETAC Life Cycle Initiative, Paris, France, pp. 80–103 [Google Scholar]
- Fantke P, Illner N (2019) Goods that are good enough: Introducing an absolute sustainability perspective for managing chemicals in consumer products. Curr. Opin. Green Sustain. Chem 15:91–97. doi: 10.1016/j.cogsc.2018.12.001 [DOI] [Google Scholar]
- Fantke P, McKone TE, Tainio M, Jolliet O, Apte JS, Stylianou KS, Illner N, Marshall JD, Choma EF, Evans JS (2019b) Global effect factors for exposure to fine particulate matter. Environ. Sci. Technol 53:6855–6868. doi: 10.1021/acs.est.9b01800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantke P, Aurisano N, Provoost J, Karamertzanis PG, Hauschild M (2020a) Toward effective use of REACH data for science and policy. Environ. Int 135:105336. doi: 10.1016/j.envint.2019.105336 [DOI] [PubMed] [Google Scholar]
- Fantke P, Huang L, Overcash M, Griffing E, Jolliet O (2020b) Life cycle based alternatives assessment (LCAA) for chemical substitution. Green Chem. 22:6008–6024. doi: 10.1039/D0GC01544J [DOI] [Google Scholar]
- Frischknecht R, Fantke P, Tschümperlin L, Niero M, Antón A, Bare J, Boulay A-M, Cherubini F, Hauschild MZ, Henderson A, Levasseur A, McKone TE, Michelsen O, Mila y Canals L, Pfister S, Ridoutt B, Rosenbaum RK, Verones F, Vigon B, Jolliet O (2016) Global guidance on environmental life cycle impact assessment indicators: Progress and case study. Int. J. Life Cycle Assess 21:429–442. doi: 10.1007/s11367-015-1025-1 [DOI] [Google Scholar]
- Frischknecht R, Jolliet O (2019) Global Guidance on Environmental Life Cycle Impact Assessment Indicators: Volume 2. UNEP/SETAC Life Cycle Initiative, Paris, France [Google Scholar]
- Gentil C, Fantke P, Mottes C, Basset-Mens C (2020) Challenges and ways forward in pesticide emission and toxicity characterization modeling for tropical conditions. Challenges and ways forward in pesticide emission and toxicity characterization modeling for tropical conditions 25:1290–1306. doi: 10.1007/s11367-019-01685-9 [DOI] [Google Scholar]
- Hauschild MZ, Huijbregts MAJ, Jolliet O, MacLeod M, Margni MD, van de Meent D, Rosenbaum RK, McKone TE (2008) Building a model based on scientific consensus for life cycle impact assessment of chemicals: The search for harmony and parsimony. Environ. Sci. Technol 42:7032–7037. doi: 10.1021/es703145t [DOI] [PubMed] [Google Scholar]
- Hay SI, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF et al. (2017) Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodas N, Loh M, Shin H-M, Li D, Bennett D, McKone TE, Jolliet O, Weschler CJ, Jantunen M, Lioy P, Fantke P (2016) Indoor inhalation intake fractions of fine particulate matter: Review of influencing factors. Indoor Air 26:836–856. doi: 10.1111/ina.12268 [DOI] [PubMed] [Google Scholar]
- Holmquist H, Fantke P, Cousins I, Owsianiak M, Liagkouridis I, Peters G (2020) An (eco)toxicity life cycle impact assessment framework for per- and polyfluoroalkyl substances. Environ. Sci. Technol 54:6224–6234. doi: 10.1021/acs.est.9b07774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Jolliet O (2016) A parsimonious model for the release of chemicals encapsulated in products. Atmos. Environ 127:223–235. doi: 10.1016/j.atmosenv.2015.12.001 [DOI] [Google Scholar]
- Huang L, Ernstoff A, Fantke P, Csiszar S, Jolliet O (2017) A review of models for near-field exposure pathways of chemicals in consumer products. Sci. Total Environ 574:1182–1208. doi: 10.1016/j.scitotenv.2016.06.118 [DOI] [PubMed] [Google Scholar]
- Huang L, Anastas N, Egeghy P, Vallero DA, Jolliet O, Bare J (2019) Integrating exposure to chemicals in building materials during use stage. Int. J. Life Cycle Assess. 24:1009–1026. doi: 10.1007/s11367-018-1551-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts MAJ, Rombouts LJA, Ragas AMJ, van de Meent D (2005) Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic chemicals for life cycle impact assessment. Integr. Environ. Assess. Manage 1:181–244. doi: 10.1897/2004-007R.1 [DOI] [PubMed] [Google Scholar]
- Jolliet O, Frischknecht R, Bare J, Boulay A-M, Bulle C, Fantke P, Gheewala S, Hauschild M, Itsubo N, Margni M, McKone T, Mila y Canals L, Postuma L, Prado V, Ridoutt B, Sonneman G, Rosenbaum R, Seager T, Struis J, van Zelm R, Vigon B, Weisbrod A (2014) Global guidance on environmental life cycle impact assessment indicators: Findings of the scoping phase. Int. J. Life Cycle Assess 19:962–967. doi: 10.1007/s11367-014-0703-8 [DOI] [Google Scholar]
- Jolliet O, Ernstoff AS, Csiszar SA, Fantke P (2015) Defining product intake fraction to quantify and compare exposure to consumer products. Environ. Sci. Technol 49:8924–8931. doi: 10.1021/acs.est.5b01083 [DOI] [PubMed] [Google Scholar]
- Jolliet O, Antón A, Boulay A-M, Cherubini F, Fantke P, Levasseur A, McKone TE, Michelsen O, Milà i Canals L, Motoshita M, Pfister S, Verones F, Vigon B, Frischknecht R (2018) Global guidance on environmental life cycle impact assessment indicators: Impacts of climate change, fine particulate matter formation, water consumption and land use. Int. J. Life Cycle Assess 23:2189–2207. doi: 10.1007/s11367-018-1443-y [DOI] [Google Scholar]
- Jolliet O, Huang L, Hou P, Fantke P (2021) High throughput risk and impact screening of chemicals in consumer products. Risk Anal.:(in press). doi: 10.1111/risa.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhübel N, Fantke P (2019) Getting the chemicals right: Toward characterizing toxicity and ecotoxicity impacts of inorganic substances. J. Cleaner Prod 227:554–565. doi: 10.1016/j.jclepro.2019.04.204 [DOI] [Google Scholar]
- Kjeldsen P, Christensen TH (2001) A simple model for the distribution and fate of organic chemicals in a landfill: MOCLA. Waste Manage. Res 19:201–216. doi: 10.1177/0734242x0101900303 [DOI] [PubMed] [Google Scholar]
- Margni MD, Pennington DW, Bennett DH, Jolliet O (2004) Cyclic exchanges and level of coupling between environmental media: Intermedia feedback in multimedia fate models. Environ. Sci. Technol 38:5450–5457. doi: 10.1021/es049716a [DOI] [PubMed] [Google Scholar]
- Owsianiak M, Fantke P, Posthuma L, Saouter E, Vijver M, Backhaus T, Schlekat T, Hauschild M (2019) Ecotoxicity. In: Frischknecht R , Jolliet O (Editors), Global Guidance on Environmental Life Cycle Impact Assessment Indicators: Volume 2. UNEP/SETAC Life Cycle Initiative, Paris, France, pp. 138–172 [Google Scholar]
- Ring CL, Arnot J, Bennett DH, Egeghy P, Fantke P, Huang L, Isaacs KK, Jolliet O, Phillips K, Price PS, Shin H-M, Westgate JN, Setzer RW, Wambaugh JF (2019) Consensus modeling of median chemical intake for the U.S. population based on predictions of exposure pathways. Environ. Sci. Technol 53:719–732. doi: 10.1021/acs.est.8b04056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RK, Bachmann TM, Gold LS, Huijbregts MAJ, Jolliet O, Juraske R, Koehler A, Larsen HF, MacLeod M, Margni MD, McKone TE, Payet J, Schuhmacher M, van de Meent D, Hauschild MZ (2008) USEtox - The UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess 13:532–546. doi: 10.1007/s11367-008-0038-4 [DOI] [Google Scholar]
- Rosenbaum RK, Huijbregts MAJ, Henderson AD, Margni M, McKone TE, van de Meent D, Hauschild MZ, Shaked S, Li DS, Gold LS, Jolliet O (2011) USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: Sensitivity to key chemical properties. Int. J. Life Cycle Assess 16:710–727. doi: 10.1007/s11367-011-0316-4 [DOI] [Google Scholar]
- Rosenbaum RK, Meijer A, Demou E, Hellweg S, Jolliet O, Lam NL, Margni M, McKone TE (2015) Indoor air pollutant exposure for life cycle assessment: Regional health impact factors for households. Environ. Sci. Technol 49:12823–12831. doi: 10.1021/acs.est.5b00890 [DOI] [PubMed] [Google Scholar]
- Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, Cassini A, Devleesschauwer B, Kretzschmar M, Speybroeck N, Murray CJL, Vos T (2015) Disability weights for the Global Burden of Disease 2013 study. Lancet Global Health 3:e712–e723. doi: 10.1016/S2214-109X(15)00069-8 [DOI] [PubMed] [Google Scholar]
- Saouter E, Aschberger K, Fantke P, Hauschild MZ, Bopp SK, Kienzler A, Paini A, Pant R, Secchi M, Sala S (2017a) Improving substance information in USEtox®, Part 1: Discussion on data and approaches for estimating freshwater ecotoxicity effect factors. Environ. Toxicol. Chem 36:3450–3462. doi: 10.1002/etc.3889 [DOI] [PubMed] [Google Scholar]
- Saouter E, Aschberger K, Fantke P, Hauschild MZ, Kienzler A, Paini A, Pant R, Radovnikovic A, Secchi M, Sala S (2017b) Improving substance information in USEtox®, Part 2: Data for estimating fate and ecosystem exposure factors. Environ. Toxicol. Chem 36:3463–3470. doi: 10.1002/etc.3903 [DOI] [PubMed] [Google Scholar]
- Shin H-M, Ernstoff AS, Arnot JA, Wetmore B, Csiszar SA, Fantke P, Zhang X, McKone TE, Jolliet O, Bennett DH (2015) Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ. Sci. Technol 49:6760–6771. doi: 10.1021/acs.est.5b00498 [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F et al. (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrimsdottir MM, Petersen A, Fantke P (2018) A screening framework for pesticide substitution in agriculture. J. Cleaner Prod 192:306–315. doi: 10.1016/j.jclepro.2018.04.266 [DOI] [Google Scholar]
- UN (2020) United Nations Sustainable Development Goals
- UNEP (2015) Strategic Approach to International Chemicals Management, United Nations Environment Programme, Geneva, Switzerland [Google Scholar]
- US-EPA (1991) Guidelines for Developmental Toxicity Risk Assessment, United States - Environmental Protection Agency, Washington, D.C. [Google Scholar]
- Verones F, Bare J, Bulle C, Frischknecht R, Hauschild M, Hellweg S, Henderson A, Jolliet O, Laurent A, Liao X, Lindner JP, de Souza DM, Michelsen O, Patouillard L, Pfister S, Posthuma L, Prado V, Ridoutt B, Rosenbaum RK, Sala S, Ugaya C, Vieira M, Fantke P (2017) LCIA framework and cross-cutting issues guidance within the UNEP-SETAC Life Cycle Initiative. J. Cleaner Prod 161:957–967. doi: 10.1016/j.jclepro.2017.05.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Huang L, Nguyen V, Jolliet O (2016) Human exposure to household cleaning products: Application of a two-field model, The International Society of Exposure Science 26th Annual Meeting, 9-13 October, 2016, Utrecht, The Netherlands, pp. 825–826 [Google Scholar]
- Westh TB, Hauschild MZ, Birkved M, Jørgensen MS, Rosenbaum RK, Fantke P (2015) The USEtox story: A survey of model developer visions and user requirements. Int. J. Life Cycle Assess. 20:299–310. doi: 10.1007/s11367-014-0829-8 [DOI] [Google Scholar]
- WHO (2014) Guidance document on evaluating and expressing uncertainty in hazard characterization, World Health Organization, Geneva, Switzerland [Google Scholar]
- WHO (2017) Guidance document on evaluating and expressing uncertainty in hazard characterization. Second Edition, World Health Organization, Geneva, Switzerland [Google Scholar]
- Wignall JA, Shapiro AJ, Wright FA, Woodruff TJ, Chiu WA, Guyton KZ, Rusyn I (2014) Standardizing benchmark dose calculations to improve science-based decisions in human health assessments. Environ. Health Perspect 122:499–505. doi: 10.1289/ehp.1307539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall JA, Muratov E, Sedykh A, Guyton KZ, Tropsha A, Rusyn I, Chiu WA (2018) Conditional toxicity value (CTV) predictor: An in silico approach for generating quantitative risk estimates for chemicals. Eviron. Health Perspect 126:057008. doi: 10.1289/EHP2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeise L, Bois FY, Chiu WA, Hattis D, Rusyn I, Guyton KZ (2013) Addressing human variability in next-generation human health risk assessments of environmental chemicals. Environ. Health Perspect 121:23–31. doi: 10.1289/ehp.1205687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.