In Drosophila males, the changes that give rise to reproductive behaviors are activational rather than organizational.
Abstract
Newborns and hatchlings can perform incredibly sophisticated behaviors, but many animals abstain from sexual activity at the beginning of life. Hormonal changes have long been known to drive both physical and behavioral changes during adolescence, leading to the largely untested assumption that sexuality emerges from organizational changes to neuronal circuitry. We show that the transition to sexuality in male Drosophila is controlled by hormonal changes, but this regulation is functional rather than structural. In very young males, a broadly acting hormone directly inhibits the activity of three courtship-motivating circuit elements, ensuring the complete suppression of sexual motivation and behavior. Blocking or overriding these inhibitory mechanisms evokes immediate and robust sexual behavior from very young and otherwise asexual males. Similarities to mammalian adolescence suggest a general principle in which hormonal changes gate the transition to sexuality not by constructing new circuitry but by permitting activity in otherwise latent motivational circuit elements.
INTRODUCTION
The drives to eat, drink, sleep, and avoid danger vary with time and circumstance, but they are with us from birth until death. Mating drive stands apart from these and other core drives by its apparent nonexistence in early life. In mammals, the emergence of sexual behavior is associated with changes in circulating hormones, but it is not clear whether these hormones awaken a latent drive or instruct the brain to build the foundation for a new one. This question has been most commonly considered under the organizational-activational hypothesis, first articulated in 1959 (1): Organizational functions are long-lasting and structural, such as the prenatal establishment of sexual identity and the maturation of brain circuitry during adolescence (2), while activational functions use previously organized neuronal circuitry to promote sexually dimorphic behaviors and tendencies (3). Despite this longstanding framework, the extent to which the motivational component of the transition to sexuality results from either organizational or activational hormonal functions is not clear (4).
Indirect evidence for an organizational role of gonadal hormones in the onset of sexual behavior comes from the long time scales over which their effects become apparent (5). For example, administration of testosterone to juvenile or castrated adult male rodents induces sexual behavior, but only after several days or weeks (6, 7), in accord with the delay between testosterone elevation and the onset of sexuality at puberty (5). Similarly, while castration immediately causes a large reduction in circulating testosterone, the resulting progression toward asexuality is gradual (8, 9). To our knowledge, no experimental evocation of juvenile sexual behavior has been reported in any animal without manipulation of hormonal signaling, which might be taken as further evidence for organizational gating of mating drive. However, the circuitry underlying sexual motivation and behavior is poorly understood in almost all animals, preventing a thorough analysis of its potential functionality during asexual stages of life.
Recently, we and others have characterized much of the circuitry underlying the decision of male Drosophila to court females (10–12) and how this decision is weighted by the male’s recent mating history (12–14). Since newly eclosed male flies never engage in courtship (15), this system presents a unique opportunity to examine the molecular- and circuit-level mechanisms underlying the emergence of sexual motivation. Though, in the fly, each cell’s sexual identity is independently determined by cell-intrinsic mechanisms (16), organism-wide developmental transitions (e.g., metamorphosis) are organized by hormonal signaling (17). Here, we show that hormonal changes also control the transition to sexuality and that sexual behavior emerges from the relaxation of stringent inhibition imposed on developed but functionally dormant motivational circuitry.
The identification and evaluation of a potential mating partner require multisensory integration, but, in our experimental paradigm, the decision to court is ultimately triggered by a male tapping a female with his pheromone receptor–bearing leg (18–21). The tap delivers parallel excitatory and inhibitory inputs to the male’s P1 courtship command neurons, which initiate and maintain courtship when sufficiently stimulated (10–12). The sensitivity of P1 neurons to the inhibitory input from a tap is decreased by a local dopamine signal, which, in mature males, is tuned to reflect recent mating history (13). If a male has not mated for several days, the dopamine tone is high, increasing the probability that a tap will lead to courtship. Once courtship has commenced, the same dopamine signal maintains courtship bouts for the tens of seconds to several minutes required before mating starts. This motivating dopaminergic activity is maintained for days in the absence of females, is decremented by each mating, and slowly recovers over 3 to 4 days.
Matings promote satiety by activating a set of copulation reporting neurons [CRNs; (14)]. These neurons, as a population, project dendrites to the external genitalia and send axons to the brain, where they reduce the activity of fruitless-positive neuropeptide F (NPF) producing neurons. This decrease is relayed through the NPF receptor to the motivation-promoting dopamine neurons, reducing their activity. After a few matings, substantial satiety is induced, and reproductive motivation remains low for several days. Mating drive has an intrinsic tendency to recover because of recurrent excitation: NPF neurons excite and are excited by Doublesex-positive pCd neurons (14), forming a loop that holds and gradually accumulates activity during periods of abstinence. Loop activity is prevented from immediately rebounding by the activity-dependent transcription factor cyclic adenosine 3′,5′-monophosphate (cAMP) response element–binding protein 2 (CREB2), which, during the period of high motivation that precedes mating, transcribes inhibitory genes (e.g., potassium channels) that will sustain the decremented activity state, forcing recovery to proceed on a biochemical, rather than electrical, time scale (14).
This circuit architecture suggests several hypotheses for the prevention of courtship in newly eclosed males. For example, a juvenile male might not recognize or tap females; the CRNs may be constantly active, inducing overwhelming satiety; or, as in the organizational hypothesis, some or all of the courtship circuitry may not be fully developed. We present evidence refuting all these hypotheses. Instead, we find that a spike in juvenile hormone levels at eclosion directly and selectively imposes long-lasting activity suppression on all known motivation-promoting circuit elements: both populations of loop neurons and the downstream dopaminergic neurons. Overriding any of these repressive mechanisms evokes robust courtship from extremely young males, providing clear evidence that much of the reproductive circuitry is developed and functional but lays dormant in juveniles. The multitiered suppression of motivational circuitry appears to be the key difference between the complete inactivation of mating drive in early life and the fluctuations experienced in adulthood.
RESULTS
A latent capacity to court in juvenile males
Male flies show no courtship behavior during the first 6 hours after eclosion (15), which we refer to as the juvenile stage (Fig. 1, A and B). Sexual behavior then gradually increases such that ~90% of males will show some courtship toward virgin females by 24 hours after eclosion (fig. S1A). Courtship bouts occurring in the first few days terminate frequently, but, as males mature, their courtship becomes increasingly sustained such that a near-peak courtship index (fraction of time spent courting over 5 min) is achieved by 72 hours (Fig. 1B and fig. S1A). This increasing courtship behavior mirrors gains in reproductive potency, as measured by the reserves of sperm and seminal fluid stored in the ejaculatory bulb (Fig. 1, A and C), and resembles the gradual and initially fragmented recovery from satiety seen in mature males (fig. S1, B and C) (12). In mature males, the reproductive organs themselves have no apparent impact on mating drive (13), and, as expected, their removal from juveniles did not disinhibit courtship (fig. S1D). There is also no obligate inhibitory impact of olfactory cues in juveniles, as removing their antennae had no effect on courtship (fig. S1E).
Fig. 1. Male flies gradually accumulate mating drive and reproductive capacity after eclosion.
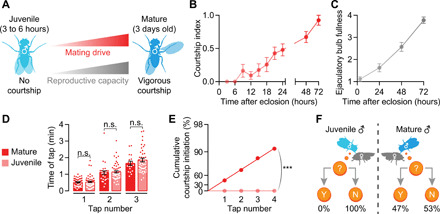
(A) Schematic showing accumulating mating drive an reproductive capacity from juvenile (3 to 6 hours after eclosion) to mature (3 days) male flies. (B and C) Both courtship and ejaculatory bulb fullness increase steadily over the first 3 days after eclosion [(B) n = 12 to 32 males; (C) †n = 7 to 9)]. (D) Mature and juvenile males tap females with similar frequency [one-way analysis of variance (ANOVA), = 48 to 57 males]. (E and F) Each tap by a mature male has a ~47% chance of initiating courtship, whereas taps by juveniles never trigger courtship (bootstrap, n = 48 to 57 males). Throughout the paper, error bars represent SEM, unless otherwise stated, but are left out of tap-induced courtship plots because of log scaling. One way ANOVA, ***P < 0.001, **P < 0.01, and *P < 0.05; n.s., not significant for all figures.
In our experiments [e.g., in (12)], each courtship bout is initiated by the male tapping the female with a pheromone receptor–bearing leg, and, in mature males, each tap triggers courtship with a probability that reflects reproductive capacity. We found no difference in the frequency with which juvenile males tapped mature females (Fig. 1D), but juvenile taps never led to courtship (Fig. 1, E and F). The suppression of sexual behavior in juveniles is therefore independent of the decision to collect sensory information by tapping and, instead, must occur somewhere in the circuitry that translates this information into courtship behavior.
A set of ~20 P1 neurons per brain hemisphere (Fig. 2A) is the primary decision center for courtship initiation and maintenance: Their inhibition or feminization prevents courtship (12, 22, 23), and their stimulation evokes courtship regardless of the male’s satiety state or the quality of the courtship target (13, 23, 24). We found no obvious anatomical differences between the P1 neurons of mature and juvenile males (Fig. 2A). Strikingly, the thermogenetic stimulation of juvenile P1 neurons using the warmth-sensitive cation channel TrpA1 (transient receptor potential cation channel A1) evoked robust courtship, including orienting toward the female, following, and singing to her (Fig. 2B and movies S1 and S2). Juvenile courtship did not escalate to the abdomen bending required for the initiation of copulation, potentially due to incomplete development of abdominal muscles (25) or hardening of the abdominal cuticle. These results show that the neural circuitry responsible for executing most courtship behaviors is developed and capable of functioning in juvenile males, implying that the prevention of courtship must occur somewhere downstream of a tap and upstream of P1 activation.
Fig. 2. Mating-drive circuitry is functional but quiescent in juveniles.
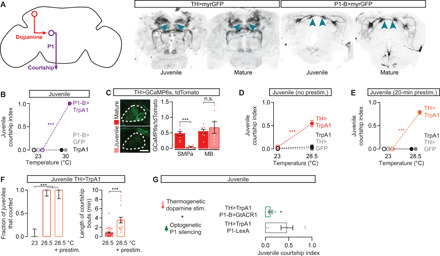
(A) Left: Schematic depicting dopamine-to-P1 circuitry. Right: Dopaminergic and P1 neurons are morphologically similar in juvenile and mature males. Blue arrowheads point to the SMPa. (B) Thermogenetic stimulation of courtship commanding P1 neurons evokes courtship from juvenile males (two-way ANOVA, n = 5 to 10 males). (C) In juvenile males, calcium activity is low in dopaminergic projections to the SMPa but not in the neighboring vertical lobe of the mushroom body (one-way ANOVA, n = 7 to 9 male brains). (D and E) Thermogenetic activation of juvenile dopaminergic neurons drives courtship (D), which becomes more vigorous in males that have been prestimulated for 20 min before the assay [two-way ANOVA, (D) n = 8 to 31 males; (E) n = 12 to 15]. (F) Acute dopaminergic stimulation causes frequent courtship initiation (left), but consolidated courtship bouts require prolonged stimulation (right) (left: Fisher’s exact test, n = 12 to 17 males; right: t test, n = 14 to 43 bouts from 12 to 17 males). (G) Optogenetically silencing P1 neurons with GtACR1 (see Methods) blocks the juvenile courtship that is induced by dopamine stimulation (t test, n = 6 to 7 males).
Stimulating motivational circuitry evokes courtship from juveniles
Dopaminergic activity in the anterior of the superior medial protocerebrum (SMPa) is a functional neural correlate of mating drive: It is high in motivated males and decreases with satiety to reduce courtship behavior (13). This dopaminergic signal is received by P1 where it sets the probability of courtship initiation following a tap (12). In the absence of tap-driven excitation, the baseline activity of P1 appears low, potentially preventing spurious courtship even in mature and highly motivated males (11). In juveniles, dopaminergic projections to the SMPa are evident (Fig. 2A), but the baseline activity of these projections is essentially undetectable, although nearby mushroom body projecting dopaminergic neurons showed high levels of activity (Fig. 2C). When we thermogenetically stimulated either all dopaminergic neurons (Fig. 2D and movie S3) or only the courtship-motivating subset (fig. S2, A and B) (13), we induced robust courtship from juvenile males. This courtship was qualitatively similar to that evoked by P1 stimulation, although the males spent only ~50% of their time courting compared to ~100% with P1 stimulation. In previous work on this circuitry (12), we found that while acute dopaminergic stimulation evokes frequent courtship initiation from satiated mature males, P1 must receive prolonged dopaminergic signaling before courtship bouts become sustained. Similarly, stimulation of juvenile dopaminergic neurons that began 20 min before presentation of a female resulted in longer courtship bouts and a near-maximal courtship index (Fig. 2, E and F, and fig. S2, A and B). This artificially induced juvenile courtship can be blocked by optogenetically silencing P1 neurons (Fig. 2G), indicating that, similar to satiated mature males, decreased dopaminergic activity in the SMPa of juveniles prevents the activation of P1 that would otherwise drive courtship behavior. As dopaminergic activity increases with age or through synthetic stimulation, males first increase their propensity to initiate courtship and then gradually consolidate these bouts for the length of time often required to achieve copulation (fig. S1, A and C).
The motivating dopamine tone in mature males is set by the activity held and adjusted within a recurrent excitation loop. The loop contains two known populations of neurons: fruitless-positive NPF neurons that directly stimulate the dopaminergic neurons and Doublesex-positive pCd neurons that are stimulated by, and feed back to stimulate, the NPF neurons (Fig. 3A) (14). In juveniles, the morphologies of NPF and pCd neurons appear normal, including their projections to the SMPa (Fig. 3A). As with the dopaminergic neurons, the activities of both NPF and pCd populations are high in mature, sexually naïve males (14) but extremely low in juvenile males (Fig. 3, B and C). Stimulation of the NPF neurons drives juvenile courtship to a similar extent as direct activation of the dopaminergic neurons (Fig. 3D and fig. S2C). Stimulation of the pCd neurons also induced juvenile courtship (Fig. 3E), although less effectively (fig. S2D), presumably due to their indirect access to the dopaminergic neurons (14). In summary, these results reveal a qualitative similarity between the motivational circuitries of highly satiated mature males and juvenile males (Fig. 3F), although the quantitatively stronger suppression in juveniles implies additional mechanisms.
Fig. 3. Upstream inputs to the motivating dopamine neurons are quiescent in juvenile males.
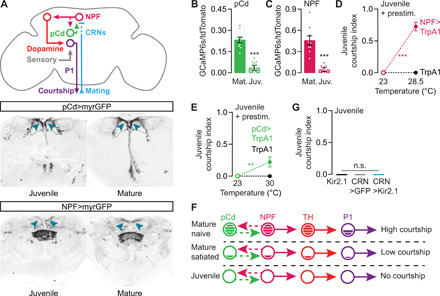
(A) Top: Schematic depicting mating-drive circuitry. Solid lines between circuit elements indicate connections that are known to be direct. Middle and bottom: Expression patterns of 41A01-Gal4 (pCd) and NPF-Gal4. (B and C) Baseline calcium activities in the SMPa for both pCd (B) and NPF neurons (C) are low in juvenile males [t test, (B) n = 10 brains each; (C) n = 9 each]. (D and E) Thermogenetic stimulation of NPF (D) or pCd (E) neurons beginning 20 min before a courtship assay induces courtship from juvenile males [two-way ANOVA, (D) n = 14 to 16 males; (E) n = 13 to 17 males; see Methods for explanation of stimulation temperatures]. (F) Mating-drive circuitry is quiescent in juveniles. (G) Silencing the satiety-inducing CRNs does not promote courtship in juveniles (one-way ANOVA, n = 8 to 9 males).
During mating, satiety is incrementally induced in the male through the activity of the CRNs (Fig. 3A), which, as a population, project dendrites to the bristles surrounding the external genitalia and report copulations by recruiting inhibition onto NPF neurons in the brain (14). In mature males, silencing the CRNs during mating prevents satiety, while stimulating them in males that have never mated induces lasting satiety (14). Similar to the motivational circuitry examined above, the CRNs were capable of functioning in early life: CRN stimulation produced a long-lasting delay in the gradual increase of courtship behavior (fig. S2, E and F). However, CRN activity is dispensable for the suppression of juvenile sexual behavior, as their tonic silencing via the potassium leak channel Kir2.1 did not provoke courtship from juveniles (Fig. 3G). This result rules out the hypothesis that the CRNs suppress sexual behavior in juveniles, pointing to the existence of an alternative mechanism for preventing the accrual of mating drive in young males.
A decreasing juvenile hormone titer gradually releases mating drive
Juvenile hormone (JH) is a candidate signal that acts just after eclosion, although not for reasons that its name implies. It was first hypothesized (26) and then shown [reviewed by Riddiford (27) and Wigglesworth (28)] to prevent insect larvae from undergoing metamorphosis, and it is largely this larval “juvenile” phase and other developmental functions that have been studied to date (27, 29). In Drosophila, the predominant juvenile hormone species is methyl 6,7;10,11-bisepoxyfarnesoate (JHB3) (Fig. 4A) (30), and its titer has been reported to increase around the time of eclosion of the imago (31). We verified this earlier report using liquid chromatography–mass spectrometry, finding a ~500-fold spike of JHB3 titer at eclosion that decays over ~2 days (Fig. 4A).
Fig. 4. High juvenile hormone titer suppresses mating drive in juvenile males.
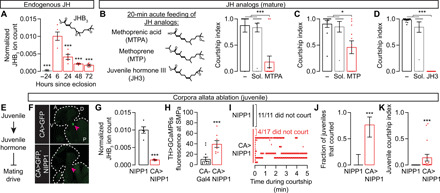
(A) JHB3 titer increases 500-fold around eclosion and decreases over 2 to 3 days (one-way ANOVA, only comparisons to the 6-hour group are indicated; n = 5 groups of five males each). JH, juvenile hormone. See Methods for quantification details. (B to D) Acutely feeding 3-day-old males with three JHB3 homologs decreases mating drive [one-way ANOVA, (B) n = 8 to 19 males; (C) n = 11 to 12; (D) n = 12 to 22)]. The minus sign indicates flies that were fed neither juvenile hormone analogs nor solvent (Sol). (E) Model: High juvenile hormone titer suppresses mating drive in juveniles. (F) Sagittal cryosections of flies (two examples per condition) with or without corpora allata ablation. Arrowheads point to where the corpora allata are or would be. Outlines trace the fly’s head and upper thorax. Letters denote dorsal-ventral and anterior-posterior axes. (G) Corpora allata ablation decreases JHB3 titer in juvenile males (t test, n = 5 groups of five males each). (H) Corpora allata ablation increases GCaMP6s fluorescence in the dopamine axons at the SMPa (t test, n = 8 to 13 males). (I to K) Corpora allata ablation increases both the fraction of juveniles that court (J) and their courtship index (K) [(J) Fisher’s exact test; (K) t test; n = 11 to 17 males)].
Despite this striking titer profile, the few studies of the effects of juvenile hormone on male courtship behavior have focused on older males and have suggested a modest courtship-promoting role (32–35). These previous studies either topically applied juvenile hormone analogs (32, 33) or fed the analogs ad libitum (34, 35). Instead, we starved mature males for 24 to 48 hours before transferring them to food containing juvenile hormone analogs for 20 min, producing a much higher titer (fig. S3A; see Methods). Acute feeding of three different analogs markedly lowered mating drive (Fig. 4, B to D, and fig. S3B). This demotivating effect was not due to either the fasting or the solvents (Fig. 4, B to D) and lasted long after the males were returned to normal food (fig. S3C). These results suggest a function for juvenile hormone in suppressing juvenile sexual behaviors (Fig. 4E).
Juvenile hormone is synthesized in an endocrine organ called the corpus allatum (30). Examining the effects of ablating the two corpora allata of early adults is complicated by the fact that changes in juvenile hormone levels are required for larval molting and metamorphosis. Whereas expressing proapoptotic factors in the corpora allata results in larval death [(36) and our observations], the Tatar laboratory found that targeted expression of nuclear inhibitor of protein phosphatase type 1 (NIPP1) allowed for normal metamorphosis but ablated the corpora allata in newly eclosed flies (37, 38). We confirmed this method (Fig. 4F) and found that ablating the corpora allata caused a marked reduction in juvenile hormone titer (Fig. 4G) and an increase in calcium activity in the dopamine projections at the SMPa (Fig. 4H). Although control males almost never court within the first 6 hours after eclosion, ~75% of males lacking corpora allata exhibited courtship behaviors in a standard courtship assay (Fig. 4, I and J). The courtship from the allatectomized males was sporadic and fragmented (Fig. 4, I and K), consistent with the higher motivational requirement to maintain courtship bouts (Fig. 1B and fig. S1A) (12). Our results below argue that this fragmentation is likely due to the long-lasting effects of earlier juvenile hormone signaling.
Juvenile hormone directly inhibits multiple motivation-promoting circuit elements
Recent work has argued that juvenile hormone increases mating drive in older males by up-regulating the response of an olfactory receptor (Or47b) in primary sensory neurons, thereby enhancing pheromone detection (34, 35). These conclusions were drawn largely from competition assays between 2- and 7-day-old males and were carried out in the dark. When they can see, males that are mutant for Or47b courted well (39), a result that we reproduce using trans-heterozygous null mutations (fig. S4A). Similarly, in our standard, well-lit, one-male and one-female courtship assays, we see little or no reduction in mature males’ courtship when the neurons expressing Or47b are silenced (fig. S4, B and C). We do not find any increase in courtship when these sensory neurons are stimulated in juveniles (fig. S4D) or any influence on recovery from satiety in mature males (fig. S4E). These results join those above and below in arguing that the suppression of sexual behavior in juveniles cannot be explained by adjustments to primary sensory neurons.
Acutely feeding mature males, the juvenile hormone analog methoprenic acid (MTPA) caused severe activity decreases in all three sets of courtship-motivating neurons that normally sustain high calcium levels in the SMPa: the pCd/NPF loop neurons and the downstream dopaminergic neurons (Fig. 5, A to C). In contrast, MTPA feeding caused no change in the baseline activity of the circuit elements that are activated by sensory stimuli: The P1 courtship command neurons and the CRNs that are activated by copulation (Fig. 5, D and E). Together with the suppression of courtship behavior (Fig. 4, B to D) and the endogenous temporal profile of its titer (Fig. 4A), these results indicate that juvenile hormone imposes a juvenile-like state on courtship motivation circuitry (Fig. 5F). Also, similar to a natural juvenile state (and arguing against drug-induced poisoning), stimulating the dopaminergic neurons, the upstream NPF neurons, or the downstream P1 neurons restored high courtship levels to males fed with the juvenile hormone analog (Fig. 5, G to J). As in juvenile males, prolonged, but not acute, stimulation of the pCd neurons rescued courtship behavior, and no rescue was seen when the CRNs were silenced (Fig. 5K and fig. S5A).
Fig. 5. Juvenile hormone signaling suppresses activity in mating-drive circuitry.
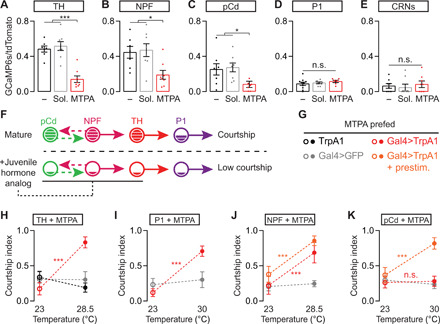
(A to E) Feeding mature males the juvenile hormone analog MTPA decreases baseline calcium activity in dopaminergic (A), NPF (B), and pCd neurons (C) [one-way ANOVA, (A) n = 7 to 8 male heads; (B) n = 7 to 8; (C) n = 7 to 8; (D) n = 6 to 10; (E) n = 8 each]. The minus sign indicates flies that were fed neither juvenile hormone analogs nor solvent. (F) Model summarizing the results in (A) to (E). (G to J) Acute thermogenetic stimulation of dopaminergic (H), P1 (I), and NPF neurons (J) recovers mating drive after MTPA feeding [two-way ANOVA, (H): n = 15 to 32 males; (I): n = 12 to 21; (J): n = 12 to 16; see Methods for an explanation of stimulation temperatures)]. (K) Prolonged, but not acute, thermogenetic stimulation of the pCd neurons rescues mating drive following MTPA feeding (two-way ANOVA, n = 9 to 22 males).
There are two known juvenile hormone receptors in Drosophila, the closely related bHLH-PAS transcription factors methoprene-tolerant (Met) and germ cell expressed (Gce). Met and Gce have redundant functions during larval development (40) but separable roles after metamorphosis (41). As previously reported (42), mature males mutant for Met courted poorly, but we found that Gce mutants showed no courtship defects (Fig. 6A and fig. S5B). When we used a tap-induced courtship assay (12), which measures the probability that each tap will lead to courtship and therefore removes the ceiling effect of standard courtship assays, we found that Gce mutants initiated courtship on a higher fraction of taps, indicating hypersexuality (Fig. 6, A and B). Feeding juvenile hormone analogs had no effect on either the hypo- or hypersexual phenotypes of mutations in the two receptors (Fig. 6, A and B, and fig. S5C), providing further evidence against poisoning by the drugs and demonstrating that both known receptors are required to receive the juvenile hormone signal. Previous work has shown that juvenile hormone disrupts Met-Gce dimerization (43), suggesting a plausible model in which juvenile hormone releases Gce from binding to Met to suppress courtship (Fig. 6C). However, since we only rarely observed courtship from Gce mutant juveniles (and never from Met mutant juveniles; Fig. 6D), juvenile hormone likely has courtship-suppressing effects that are independent of Gce, possibly requiring another, currently unidentified receptor. Regardless of the inadequately understood details of its signal transduction, these results show that juvenile hormone works through its known receptors to instruct the mating drive of the male.
Fig. 6. Juvenile hormone directly suppresses multiple components of mating-drive circuitry.
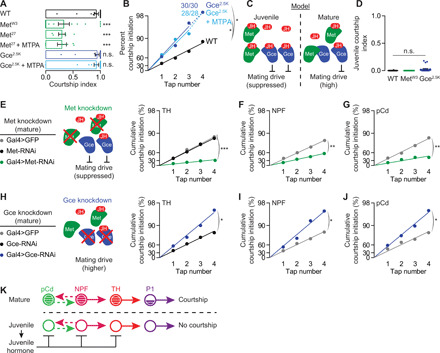
(A) Mutation of the juvenile hormone receptor Met decreases mating drive in mature males, while mutation of a second juvenile hormone receptor, Gce, does not decrease mating drive in a standard courtship assay. Mutations in both receptors render courtship insensitive to MTPA feeding (one-way ANOVA, only comparisons to the wild-type flies are indicated = 7 to 26). WT, wild type. (B) Using a tap-induced courtship assay to remove the ceiling effect of standard assays shows that mutating Gce increases mating drive and makes courtship impervious to MTPA feeding (bootstrap, n = 28 to 32 males) (C) Model: Juvenile hormone may disrupt Met-Gce dimerization (43), allowing Gce to suppress mating drive. (D) Little or no juvenile courtship is seen in males that are mutant for Met or Gce (n = 12 to 22). (E to G) RNAi knockdown of Met in neurons that store mating-drive information; dopaminergic (E), NPF (F), and pCd neurons (G); decreases courtship behavior [bootstrap, (E): n = 30 to 39 males; (F) n = 28 to 47; (G) n = 29 to 32)]. (H to J) Knocking down Gce in dopaminergic (H), NPF (I), and pCd neurons (J) increases mating drive [bootstrap, (H) n = 30 males each; (I) n = 28 to 33; (J) n = 29 to 30)]. (K) Model.
The clear involvement of the two known juvenile hormone receptors allowed us to ask whether the hormone acts on previously identified motivational circuitry. We found reductions in courtship when Met was knocked down in the dopaminergic neurons or in either of the populations of loop neurons (Fig. 6, E to G, and fig. S5D). These effects were relatively modest, consistent with the idea that inhibition at one level of the circuit can be overcome by activity in other motivating neurons. Inversely, courtship probability was increased if any of these courtship-motivating populations were targeted with Gce–RNA interference (RNAi) (Fig. 6, H to J). No effect was seen when Met or Gce levels were decreased in P1 courtship command neurons or in the CRNs (fig. S5, D to F). The direct suppression of all known circuit elements that sustain motivational signals by juvenile hormone is in contrast to the action of the CRNs, which, as far as we can tell, rapidly inhibit only the NPF neurons, with consequent and indirect effects on the pCd and dopaminergic neurons (14). The multitiered suppression of motivational circuitry seen in juvenile males (Fig. 6K) likely explains their complete lack of mating drive, whereas even highly satiated mature males still display occasional courtship behavior (13).
Juvenile hormone activates CREB2 for long-term suppression of mating drive
The juvenile hormone titer that spikes at eclosion decays away by 48 hours (Fig. 4A), but mating drive continues to increase for several days (31), suggestive of an enduring courtship-suppressing mechanism. Recently, we found that the satiety state in mature males is maintained and slowly released by CREB-controlled transcription of inhibitory effectors such as the potassium channel subunit TASK7 (TWIK-related acid-sensitive potassium channel 7) in the pCd/NPF loop (14). Using a luciferase-based reporter of CREB activity targeted to either the pCd or NPF loop neurons, we found a large spike in CREB activity at eclosion that declined over 48 hours (Fig. 7, A and B). Disrupting CREB activity in the loop by expressing a suppressive isoform of the fly version of CREB (CREB2SUP) elicited courtship from juveniles (Fig. 7, C and D), demonstrating CREB2’s critical role in the inactivation of courtship circuitry.
Fig. 7. Juvenile hormone activates CREB2 to suppress mating drive.
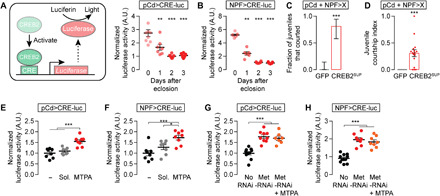
(A and B) A CREB2 activity assay, which uses an FLP-dependent luciferase construct under the control of the cAMP-response element (CRE), shows that CREB activity in male pCd (A) and NPF (B) neurons is high just after eclosion and decreases over the ensuing 24 to 48 hours (one-way ANOVA, only comparisons to the day zero groups are indicated; (A) n = 7 to 9 groups of three brains; (B) n = 6 to 7 groups). A.U., arbitrary units. (C and D) Decreasing CREB2 activity in pCd and NPF loop neurons disinhibits juvenile courtship [(C) Fisher’s exact test; (D) t test; n = 16 males each). (E and F) MTPA feeding increases CREB activity in pCd (E) and NPF (F) loop neurons [one-way ANOVA, (E) n = 8 groups of three brains each; (F) n = 8 groups each]. The minus sign indicates flies that were fed neither juvenile hormone analogs nor solvent. (G and H) RNAi knockdown of Met in pCd (G) and NPF neurons (H) both mimics and precludes the CREB-activating effect of juvenile hormone [one-way ANOVA, (G) n = 7 to 9 groups of three; (H) n = 8 to 11 groups].
In mature males, CREB2 is activated in the pCd/NPF loop by the electrical activity that motivates courtship (14). In juveniles, there is no prior motivation, and the NPF and pCd neurons are inactive (Fig. 3, B and C), suggesting a neuronal activity–independent potentiation of CREB2 transcription. When we fed mature males the juvenile hormone analog MTPA, we found increased CREB activity specifically in the pCd and NPF loop neurons (Fig. 7, E and F, and fig. S5, G to I). RNAi-mediated knockdown of the juvenile hormone receptor Met in either pCd or NPF neurons elevated CREB activity, and these effects were not enhanced by feeding juvenile hormone (Fig. 7, G and H). These results argue that the early spike in juvenile hormone activates CREB2 to impose a long-lasting suppression of loop activity (see below).
Late into this study, we were able to obtain juvenile hormone 3 (JH3), structurally the closest and, we find, the most potently demotivating analog of endogenous JHB3 (Fig. 4D). Feeding mature males JH3 for 20 min led to a suppression of mating behaviors that was still evident 24 hours later (Fig. 8A), an effect that required the Gce receptor in loop neurons (Fig. 8, A and B). When CREB2 activity was suppressed in the loop, courtship was inhibited immediately after JH3 feeding (Fig. 8C), but the long-lasting reduction in courtship was reverted (Fig. 8D). We previously found that CREB2 sustains satiety in loop neurons by transcribing the leak potassium channel subunit Task7 (14). Similarly, we find that the transcription of Task7 [quantified using single-molecule fluorescent in situ hybridization (smFISH)] is higher in the pCd neurons (which are identifiable by their location and the expression of doublesex) of juveniles than of mature males (Fig. 8E). As expected for a gene product that must be transcribed, translated, and trafficked to the membrane to be effective, knocking down TASK7 in the loop had no effect on the immediate inhibition of courtship in mature males but, similar to CREB2 activity suppression, prevented the enduring effects of JH3 feeding (Fig. 8, F and G). These results underscore the multitiered nature of juvenile hormone’s suppression of motivation: at the circuit level (direct action on dopaminergic, pCd, and NPF neurons), over time (immediate and long-lasting), and at the molecular level (CREB2 and TASK7 for long-lasting suppression in the loop but other unidentified mechanisms for immediate suppression and in the dopaminergic neurons) (Fig. 8H). These mechanisms ensure the complete suppression of sexual motivation, which, propelled by the recurrent excitation loop, has a natural tendency to increase over time.
Fig. 8. Juvenile hormone causes an enduring suppression of mating drive through CREB2-mediated transcription of a potassium channel subunit.
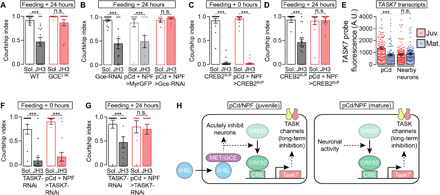
(A and B) Suppression of courtship lasts at least 24 hours after feeding the juvenile hormone analog juvenile hormone 3 (JH3), and this effect is blocked by Gce mutation (A) or RNAi knockdown in the pCd/NPF loop (B) [one-way ANOVA, (A) n = 13 to 21 males; (B) n = 6 to 16)]. (C and D) CREB2 suppression in the pCd/NPF loop does not affect the acute inhibition of courtship by JH3 feeding (C) but prevents the long-term reduction of courtship behavior (D) [one-way ANOVA, (C) n = 14 to 17 males; (D) n = 12 to 20)]. (E) In pCd neurons (which are identifiable with smFISH by their expression of doublesex transcripts), TASK7 transcripts are expressed at a higher level in juveniles than in adults (one-way ANOVA, n = 105 to 142 cells from 12 to 14 brains). (F and G) TASK7 knockdown in the pCd/NPF loop does not affect the acute inhibition of courtship by JH3 feeding (F) but eliminates the long-term reduction in courtship behavior (G) [one-way ANOVA, (F) n = 15 to 18 males; (G) n = 8 to 16]. (H) Model for the fast and slow mechanism through which juvenile hormone suppresses mating drive compared to satiety-inducing mechanisms in the mature animal.
DISCUSSION
Ancient literature (e.g., the story of Eden) indicates that the diverse behavioral changes coinciding with reproductive maturity had been fascinating and frustrating humans thousands of years before they were known to involve the brain. The hormonal signaling pathways that trigger these changes in mammals are now understood, but little is known about their mechanistic impact on behavioral and motivational circuitry. Teleological explanations for delays in the onset of sexual behavior likely vary across species, but the pervasiveness of this phenomenon suggests the possibility of a core mechanistic conservation that transcends idiosyncrasies of duration, purpose, and even the molecular nature of the hormonal trigger. The system we develop here for studying the transition to sexuality allows rapid insight, with principles that suggest molecular and circuit hypotheses in other animals and other late-emerging brain functions.
One obvious but likely superficial difference in the control of behavioral maturation between Drosophila and mammals is the sign of the regulation by circulating hormones. Although some gonadal hormones can suppress mating drive (44), in juvenile male rodents, experimental elevation of testosterone causes precocious mating behavior (7), whereas we find that juvenile hormone suppresses mating behavior. This discordance is reconciled early in signal transduction, as loss of the Met receptor for juvenile hormone decreases sexual behavior, similar to the effects of androgen (testosterone) receptor antagonists in humans (45). The receptors for testosterone, estrogen, and progesterone are all transcription factors, as are the Met and Gce receptors for juvenile hormone. Unlike ablating the source of juvenile hormone or overriding its action at the level of neuronal activity or CREB activation, removing the suppressive receptor, Gce, does not cause substantial juvenile courtship. This points to the likely existence of yet another juvenile hormone receptor, the identification of which will provide a deeper understanding of the suppressive mechanisms used to completely, selectively, and transiently inactivate this fundamental drive.
In mature animals, the recurrent loop that drives the male to court also primes itself for satiety through activity-induced, CREB-mediated production of suppressive TASK7-containing potassium channel complexes (14). This motivation-suppressing module is also used in juveniles, with hormonal signaling causing CREB2 activation. Although we have not yet identified additional suppressive effectors in juveniles, we see evidence of fast-acting suppression by juvenile hormone and direct suppression of the dopaminergic neurons, neither of which can be accounted for by CREB activity in the pCd/NPF loop. Parallel fast and slow responses have also been found in the response to mammalian gonadal hormones [e.g., (46)]. Multiple repressive mechanisms are likely necessary to completely inactivate mating drive, since individually removing or overriding individual suppressive effectors allows juvenile courtship. This is again similar to findings in mammals, where administering testosterone to either the preoptic area of the hypothalamus or to the medial amygdala suffices to restore mating behaviors in castrated males (6, 47).
A second clear difference between Drosophila and vertebrate juvenile stages is the time scale. The fly system requires suppression of sexual behavior for days, not months or years [even 150 years as suggested in Greenland sharks; (48)]. However, several nonneuronal structures are still maturing in juvenile flies, such as the abdominal musculature, the cuticle, and the ejaculatory bulb, indicating that the neuronal circuitry that will eventually animate these features may require structural development as well. Although we have not undertaken the kind of detailed anatomical analysis required to argue for or against fine-scale structural rearrangements, courtship circuitry appears grossly normal in juveniles, and the stimulation of several circuit elements (P1, dopaminergic, NPF, and pCd) can rapidly drive coherent courtship. While we do not know yet whether the courtship performed by juvenile males is identical to that of mature males (e.g., in song production), our findings serve as strong evidence against a strict developmental ontogeny for the posteclosion emergence of reproductive motivation and behavior.
A recent report found structural changes in a key hypothalamic population during estrus and showed that these neurons could not drive sexual behaviors when activated in ovariectomized mice (49). We note that we are not able to restore sexual behavior in male flies if we stimulate only subpopulations of individually necessary dopaminergic neurons (fig. S2B) (13), a consideration that, we argue, leaves open the possibility that the motivational and behavioral circuitry may be at least partially functional, but suppressed, in nonestrus female mice. The most parsimonious explanation may be that both structural and activational changes take place between asexual and sexual periods of life. Increased connectivity in motivational circuitry could be a result, rather than a cause, of increased activity, as has been reported in a growing number of systems (50–52). We can find no reports of neuronal stimulation eliciting juvenile sexual behavior in the mammalian literature, but stimulation of hypothalamic neurons can produce coherent and robust parenting behaviors from otherwise nonparental mice (53), demonstrating latent but functional circuitry for these late-emerging behaviors.
Recently, new functions for pCd and NPF neurons have been reported in male-specific behaviors. In the Anderson laboratory, pCd neurons were found to sustain sexual and aggressive behaviors after P1 activation (54). This sustained excitation is remarkably similar to the role of pCd in the accrual and maintenance of sexual motivation in mature male flies (14). Since P1 activity is constantly required to sustain courtship in our experiments (12) and in those from the Anderson laboratory (54), we suggest that subsets of the eight pCd neurons may be specialized for maintaining recurrent activity over minutes (together with P1 neurons in sustaining courtship and aggression) and over days (together with NPF neurons in sustaining reproductive motivation). In work from the Montell laboratory, male-specific NPF neurons were reported to decrease sexual motivation generally and to prevent male-male courtship in particular (55). These conclusions appear to be in direct contrast with the courtship-promoting roles for these same NPF neurons we described here and previously (14), as well as with earlier findings from the Park laboratory (56), although they are in line with recent results from the Pan laboratory (57). The results obtained across these four laboratories are derived from a variety of assays and genetic manipulations, complicating direct comparisons, although they clearly point to multiple roles for NPF in courtship target selection and motivation. While several experiments have argued for various forms of hypersexuality resulting from decreased NPF signaling (55, 57), the lone NPF neuronal stimulation experiment to yield a reduction in courtship behavior assessed courtship toward decapitated females and the effect was modest [figure 4B in (55)]. The robust effects we see [Fig. 3D, fig. S2C, and (14)] make us confident that increasing the output of NPF neurons promotes a net increase in sexual motivation in otherwise asexual juveniles and in satiated males recovering from ad libitum access to virgin females.
Our results provide an alternative explanation for what has been the strongest evidence in favor of the organizational hypothesis for the maturation of reproductive behaviors in mammals: the delay between hormonal changes and the behaviors they induce (4). In the fly, the long-lasting, CREB-imposed effects of an earlier suppressive hormonal state must decay away to allow loop activity to ramp up and promote sexual behavior. Enduring and distributed suppressive effects may explain why the awakening of sexuality triggered by hormonal changes is gradual and halting in both flies and mammals.
METHODS
Fly stocks
Fly husbandry was performed as previously described (13). Flies were maintained on conventional cornmeal agar medium under a 12-hour light/12-hour dark cycle at 25°C and ambient humidity. Because of the focus of this study, all tested flies were males. Unless otherwise noted, juvenile males were collected 3 hours after clearing the food vials and were tested 3 hours later (3 to 6 hours old). Mature males were collected on the day of eclosion and group-housed away from females for 3 to 4 days before testing. Virgin females were generated using an hs-hid transgene integrated on the Y chromosome (58, 59). All behavioral experiments were carried out within the first 10 hours of each light-day phase. Detailed genotypes of all strains used in the paper are listed in table S1.
Courtship assays
Courtship assays were carried out in the same setting as previously described (13, 58). A male fly and a w1118 virgin female fly were videotaped in cylindrical courtship chambers (10 mm in diameter and 3 mm in height) at 23°C and ambient humidity. In some experiments, we analyzed tap-induced courtship initiations, a metric that is less likely to reach ceiling than the standard courtship index (12). These experiments are conducted in the same way as the regular courtship assays but recorded with a more zoomed-in camera and analyzed differently (see below). In fig. S1 (B and C), we measured courtship by mature males that are sexually satiated. In those experiments, each male was paired with 15 virgin females for 4.5 hours to ensure satiety (13) and then tested after 0 to 3 days of being away from females (recovery).
For thermogenetic experiments using TrpA1, half of the males were assayed at 23°C and the other half at a higher temperature (28.5° or 30°C). We typically started testing with 30°C, and if the flies show abnormal behaviors (e.g., seizure in TH>TrpA1 flies), then we lower the temperature to 28.5°C. We previously showed that, in mature-satiated males, acute dopaminergic stimulation instantly restores robust courtship initiations, but it requires several minutes of dopaminergic stimulation for the flies to sustain courtship bouts (12). Similar observations are also seen here in juvenile males (Fig. 2F). To ensure the consistency of stimulation, unless otherwise stated, stimulation of the dopaminergic and its upstream neurons are preceded by a 20-min prestimulation at the same temperature.
For conditional silencing experiments involving TubGal80ts (e.g., Fig. 5L), male flies were moved to 30°C after eclosion and kept there (isolated from females) until the assay, which took place at 23°C. For the abdomen removal experiment (fig. S1D), males were anesthetized on ice, and their abdomens were surgically removed as described in (13). For the large chamber experiment (fig. S5B), a male fly and a w1118 virgin female fly were videotaped in cylindrical courtship chambers (~2.75 cm in diameter and 3 mm in height) at 23°C and ambient humidity.
For the antennectomy experiment (fig. S1E), male flies were anesthetized on ice, and their antennae were bilaterally removed while in a dry dissecting tray over ice. The flies were then allowed to recover in vials with food at room temperature for 3 hours before testing. Control males went through the same anesthetization and recovery procedures, but no antenna was removed.
For conditional silencing using GtACR1, flies were collected within 3 hours of eclosion and immediately transferred to vials containing rehydrated potato flakes (Carolina Biological Supply, 173200) and 100 μl of all-trans retinal stock solution (Sigma-Aldrich, R2500; 35 mM in ethanol) for 3 hours before testing. All assays were conducted at 28.5°C with a 20-min thermogenetic prestimulation period before the addition of a w1118 female. Flashing green light (30 s on, 30 s off) was used to activate the channel during the prestimulation and testing period. Courtship was scored for a continuous 5-min period.
Analysis of courtship behavior
The courtship index was manually scored as previously described (13, 58). It is defined as the fraction of time during which the male fly is engaged in mating behaviors (courtship and copulation) over 5 min after courtship initiation. A bout of courtship was scored as initiated when the male oriented toward the female, began tracking her, and unilaterally extended his wing to sing to her. A bout was scored as terminated if the male stopped tracking the female or turned away from her. Once courtship was terminated, it was almost never reinitiated within 10 s. Whenever possible, terminations were verified by confirming that the male did not resume singing when the female subsequently passed in front of him.
For experiments that measure tap-induced courtship probabilities, the experimenter who scored the videos was blind to the genotypes and experimental conditions. Taps are defined as a male foreleg touching any part of the female body. In our assays, courtship is always initiated by a tap, always within a few seconds, and most often within 500 ms. The details of this analysis can be found in (12). Briefly, we calculated the cumulative courtship probabilities (y) after each tap (x). We plotted –Ln(1 – y)against x fitted a straight line that is forced through the point of origin ([0, 0]). The Y-axis ticks are labeled with the same log transformation. This fitted line represents the expected cumulative courtship-initiation probability if the tap-to-courtship transitions are modeled as a perfect coin (with varying odds). A steeper line represents higher courtship probability (i.e., high odds of the coin). From the slope of the line (s), we can calculate the per-tap courtship probability as 1 – e–s.
We used bootstrapping to perform hypothesis tests on courtship probabilities, with the underlying null hypothesis that these two datasets were generated with the same unknown courtship probability. Before bootstrapping, we first pooled two datasets together. Then, we resampled two bootstrapped datasets from the pooled data with the same sample size as in the experiments. Then, using the same linearization and linear regression procedures, we calculated new bootstrapped courtship probabilities. We calculated their difference and repeated the resampling and recalculations 100,000 times. The P value was then calculated as the fraction of iterations that generate a difference in courtship probabilities at least as large as the experimental one. We used Bonferroni corrections to adjust the P values for multiple comparisons. The MATLAB code to perform these calculations is available online.
Satiety assay and reversal
Satiety assays were used to test whether a neuronal population (e.g., Or47b neurons) has an acute motivation-promoting role and were carried out as previously described, with minor modifications (13). Individual male flies were placed with ~15 virgin females in a food vial at 23°C and ambient humidity for 4.5 hours. Mating behaviors (courtship and copulation) were scored manually at the following time points: 0.5, 1, 4, and 4.5 hours. Satiety assays (4.5 hours) were used to satiate males for courtship and imaging experiments. To thermogenetically revert satiety, the temperature was raised after the last time point, and mating behaviors were scored 20 min after the incubator reached the appropriate temperature.
Analysis of satiety assays
For standard 4.5-hour satiety assays, individual males were paired with ~15 virgin females, and mating behaviors (courtship and copulation) were scored manually at the following time points: 0.5, 1, 4, and 4.5 hours. Satiety assays (4.5 hours) were used to satiate males for courtship and imaging experiments. A within-group (five to seven males per group) average was generated for each time point. The first two (generally 0.5 and 1 hour) and last two (generally 4 and 4.5 hours) time points were averaged to generate initial and end percentages, respectively. For satiety reversal, another percentage is generated from the mating and courtship behaviors during stimulation (20 min after temperature equilibration).
Quantification of endogenous JHB3
To quantify JHB3, groups of five flies were homogenized and then mixed with 100 μl of methanol to extract the hormone. The mixture was spun down at 17,000g for 20 s, and the supernatant was transferred to a new vial and diluted three times with dH2O (one part supernatant and two parts dH2O). Exogenous JH3 (μM) was added to the solution as an internal standard for quantification (controlling for the deviations of the instrument performance from experiment to experiment). If needed, samples were stored at −20°C before quantification.
We used liquid chromatography coupled with mass spectrometry to quantify JHB3. Each sample was first loaded in a standard reverse-phase C18 column (Agilent, ZORBAX SB-C18) by high-performance liquid chromatography (Agilent 1200 Series). Each sample was then eluted off the column over the course of 23 min using two solvents, ddH2O (with 0.1% formic acid) and acetonitrile (with 0.1% formic acid), and analyzed with a mass spectrometer (Agilent 6210 time-of-flight mass spectrometer) in cation mode. During each elution, acetonitrile concentration was ramped up from 5% (T = 2 min) to 100% (T = 13 min), kept at 100% for 5 min, and then ramped down from 100% (T = 18 min) to 5% again (T = 18.1 min). JHB3 and JH3 were consistently detected at T = ~10 min and T = ~12 min, respectively.
Analysis of JHB3 titer
We extracted the ion counts corresponding to the mass/charge ratios of monoprotonated JHB3 ([M + H]+ = 283.1909 ± 0.0057) and the internal control JH3 ([M + H]+ = 267.1960 ± 0.0053) from the chromatograph. Peaks corresponding to JHB3 and JH3 are consistently seen at T = ~10 min and T = ~12 min, respectively, and integrated to estimate the ion counts. The ion counts of JH3 are generally consistent between experiments (SD/mean = 11%). We reasoned that this deviation is largely due to fluctuations of instrumental performances and normalized the ion counts of JHB3 (target of quantification) by taking their ratios over the ion counts of the internal standard.
Acute feeding of juvenile hormone analogs
To ensure consistent drug feeding (and juvenile hormone titer), male flies were food-deprived (but not water-deprived) in vials containing 5 ml of 0.8% agarose gel (made with dH2O) for 48 hours before feeding. However, for less starvation-resistant genotypes (>10% lethality at the end of starvation), the time was shortened to 24 hours. The exact time of starvation was kept consistent within each genotype. For feeding, flies were transferred to vials containing 2 ml of 0.8% agarose gel (made with 50% dH2O and 50% grape juice) with a juvenile hormone analog for either 20 min (if the flies were tested right away) or 3 hours (if the flies were tested 24 hours later). For the prestimulation experiments in Fig. 5 (J and K), flies were fed juvenile hormone analog for 20 min at 23°C and moved into a different vial with a conventional cornmeal agar medium in an incubator at 28.5° to 30°C for 20 min before being placed in the courtship assays to be tested at 28.5° to 30°C. To assist feeding, the vials are placed upside down so that the flies can easily find the food antigeotactically. The concentrations of the drugs were 200 μg/ml for MTPA, 200 μg/ml for methoprene (MTP), and 168 μg/ml for JH3.
Whole-fly cryosectioning and imaging
Whole flies with green fluorescent protein (GFP)–expressing corpora allata were briefly dipped in 75% ethanol to remove cuticle hydrocarbons before being fixed in 4% paraformaldehyde dissolved in phosphate-buffered saline (PBS) with 0.3% Triton X-100 (PBST) for 60 min. After 3× 20-min washes with PBST, flies were transferred into Neg-50 medium (Thermo Fisher Scientific, 6502) and frozen in a dry ice/acetone cooling bath (−78°C). The frozen flies were sliced sagittally into 100-μm sections in a cryosection device and mounted directly on a glass slide with mounting medium (Neg-50). Confocal sections (four to seven sections at 3-μm intervals) were taken from the medial sections where corpora allata are seen (if not ablated).
Antibody staining and two-photon volume microscopy
Antibody staining was performed as described before (13). Myristoylated GFP, which was driven with different Gal4 lines, was stained with chicken anti-GFP (1:1000) and then donkey anti-chicken (1:400) antibodies. Two-photon microscopy was performed using a Neurolabware microscope and a tunable pulse laser (InSight X3, Spectra-Physics) as described before (60). Volume imaging was done with an 8-kHz resonant scanning mirror (CRS8, Cambridge Technology) along the x axis, a galvanometer scanning mirror (6215H, Cambridge Technology) along the y axis, and an electrically tunable lens (EL-10-30-TC-NIR-12D, Optotune) along the z axis. For each sample, we imaged 20 volumes (31 frames per volume and 15.5 frames per second spanning 100 μm along the z axis) at 960 nm, calculated a median volume to cancel out photomultiplier tube noise, and, lastly, acquired an SD projection of the volume to visualize the processes.
Baseline calcium imaging of dissected brains
Fly brains expressing GCaMP6s (61) and tdTomato were dissected from either juvenile or mature males in HL3.1 solution (62) and mounted anterior side down onto the base of a glass-bottom petri dish (MatTek, P35G-1.5-20-c) housing 3 ml of HL3.1 saline, as previously described (13). Confocal sections (four to seven sections at 3-μm intervals) were taken from the anterior of the SMPa. Whenever possible, we also coexpressed and imaged tdTomato in the same cells to normalize the GCaMP6s signal. Whenever possible, we also imaged from unrelated brain regions (the mushroom body) to show that the changes are specific to the SMPa.
Fluorescent in situ hybridization
Whole-mount in situ hybridization was performed as described previously (14). Brains dissected from juvenile and mature wild-type Canton-S males were used for these experiments. smFISH was performed following the published protocol (63), with the exception that we omitted (i) the overnight incubation in 100% ethanol and (ii) the bleaching steps. Fluorescence-tagged TASK7 (Quasar 570) and Dsx (Quasar 670) probes were designed and manufactured using a commercial source (LGC Biosearch Technologies). Confocal sections were acquired using an Olympus Fluoview 1000 microscope at 3-μm intervals.
Quantifying imaging data
For quantification of baseline calcium level, after one person (S.X.Z.) took the images, another person (D.R. or E.G.) selected regions of interest (ROIs) of ipsilateral SMPa, mushroom body medial lobe, and background (near the antennal lobe, where dopamine projections are sparse) using the tdTomato channel. ROI sizes were kept roughly the same between samples. The average pixel fluorescence in each ROI was calculated with a custom-written MATLAB script. Whenever possible, normalized fluorescence was calculated as (GCaMP6s_SMPa − GCaMP6s_background) / (tdTomato_SMPa − tdTomato_background). The same formulas were used for the mushroom body medial lobe as well. The MATLAB code to perform these steps is available online.
For smFISH quantification, we used a custom, semi-automated MATLAB script to segment images and measure fluorescence intensities as described previously (14). Cell bodies were first segmented using the Dsx channel and the TASK7 channel. The TASK7 puncta were then grouped into either a Dsx-positive or a Dsx-negative population, and the average fluorescence intensities were calculated separately. Background fluorescence was measured from an unrelated brain region (the antenna lobe) and subtracted from the data.
Measuring CREB2 activity
To measure CREB2 activity, we used a FLP-dependent luciferase construct driven by the cAMP-response element promoter (64), with UAS (Upstream Activating Sequence)–FLP driven in the neurons under examination. The luciferase assays were performed using a commercial kit (Promega, E1910). Brains of males were dissected in HL3.1 solution and transferred, in groups of three, into 50 μl of dissociation solution for 5 min at −20°C. Then, a 20 μl of supernatant of the dissociation solution was added to 50 μl of substrate solution, and the luciferase activity was measured in a photoluminometer (Turner Designs TD-20/20) over a 5-s window.
Pharmacology
MTPA (Sigma-Aldrich, M6682) was dissolved in dimethyl sulfoxide (DMSO) at 5 mg/ml (18.6 mM). MTP (Sigma-Aldrich, 33375) was dissolved in DMSO at 100 mg/ml (322.1 mM). JH3 (Sigma-Aldrich, J2000; lot purity = 79%) was dissolved in DMSO at 4.21 mg/ml (15.8 mM).
Additional statistical tests
One-way analysis of variance (ANOVA), two-way ANOVA, two-tailed Student’s t test, and Fisher’s exact test were performed using Prism 7. All tests are unpaired. All one-way ANOVA tests use post hoc Tukey corrections. All two-way ANOVA tests use post hoc Bonferroni corrections. Error bars represent 1 SEM for most panels and Jeffreys’ 95% confidence interval for proportions.
Data analysis tools
MATLAB scripts and functions used to (i) quantify baseline calcium activity, (ii) analyze tap-induced courtship initiation, and (iii) analyze in situ hybridization data can be found available online at https://github.com/CrickmoreRoguljaLabs.
Acknowledgments
We thank the Rogulja and Crickmore laboratories for comments on the manuscript. M. Andermann and C. Weitz provided equipment for two-photon microscopy and luciferase assays, respectively. L. Goodrich and M. Frank provided equipment and assistance for whole-fly cryosectioning. Mass spectrometry was conducted at the Harvard Center for Mass Spectrometry. M. Frank, C. Boutros, B. Gorko, A. Corredera, and B. Karavas helped with experiments. S.X.Z. is a Stuart H.Q. and Victoria Quan Fellow at Harvard Medical School. D.R. is a New York Stem Cell Foundation-Robertson Investigator. Funding: This work was supported by The New York Stem Cell Foundation and a grant from the NIH (DP1DA044358). Author contributions: S.X.Z., E.H.G., L.E.M, and M.A.C. performed the experiments. All authors designed the experiments, analyzed the data, and wrote the manuscript. Competing interests: The authors declare that they have no competing interest. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/25/eabg6926/DC1
REFERENCES AND NOTES
- 1.Phoenix C. H., Goy R. W., Gerall A. A., Young W. C., Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 (1959). [DOI] [PubMed] [Google Scholar]
- 2.Simerly R. B., Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res. Mol. Brain Res. 6, 297–310 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Wu M. V., Manoli D. S., Fraser E. J., Coats J. K., Tollkuhn J., Honda S. I., Harada N., Shah N. M., Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139, 61–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz K. M., Molenda-Figueira H. A., Sisk C. L., Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 55, 597–604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisk C. L., Zehr J. L., Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 26, 163–174 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Putnam S. K., Du J., Sato S., Hull E. M., Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Horm. Behav. 224, 216–224 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Södersten P., Damassa D. A., Smith E. R., Sexual behavior in developing male rats. Horm. Behav. 8, 320–341 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Davidson J. M., Characteristics of sex behaviour in male rats following castration. Anim. Behav. 14, 266–272 (1966). [DOI] [PubMed] [Google Scholar]
- 9.Krey L. C., Mcginnis M. Y., Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: Relationships with gonadotropin secretion. J. Steroid Biochem. 35, 403–408 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Kallman B. R., Kim H., Scott K., Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clowney E. J., Iguchi S., Bussell J. J., Scheer E., Ruta V., Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S. X., Miner L. E., Boutros C. L., Rogulja D., Crickmore M. A., Motivation, perception, and chance converge to make a binary decision. Neuron 99, 376–388.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S. X., Rogulja D., Crickmore M. A., Dopaminergic circuitry underlying mating drive. Neuron 91, 168–181 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Zhang S. X., Rogulja D., Crickmore M. A., Recurrent circuitry sustains Drosophila courtship drive while priming itself for satiety. Curr. Biol. 29, 3216–3228.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spieth H. T., Courtship behavior in Drosophila. Annu. Rev. Entomol. 19, 385–405 (1974). [DOI] [PubMed] [Google Scholar]
- 16.Baker B. S., Belote J. M., Sex determination and dosage compensation in Drosophila melanogaster. Annu. Rev. Genet. 17, 345–393 (1983). [DOI] [PubMed] [Google Scholar]
- 17.Mirth C. K., Tang H. Y., Makohon-Moore S. C., Salhadar S., Gokhale R. H., Warner R. D., Koyama T., Riddiford L. M., Shingleton A. W., Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 111, 7018–7023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan P., Manoli D. S., Ahmed O. M., Chen Y., Agarwal N., Kwong S., Cai A. G., Neitz J., Renslo A., Baker B. S., Shah N. M., Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154, 89–102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K., Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohatsu S., Yamamoto D., Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat. Commun. 6, 6457 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Kohatsu S., Koganezawa M., Yamamoto D., Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Kimura K.-I., Hachiya T., Koganezawa M., Tazawa T., Yamamoto D., Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769 (2008). [DOI] [PubMed] [Google Scholar]
- 23.von Philipsborn A. C., Liu T., Yu J. Y., Masser C., Bidaye S. S., Dickson B. J., Neuronal control of Drosophila courtship song. Neuron 69, 509–522 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Pan Y., Meissner G. W., Baker B. S., Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc. Natl. Acad. Sci. U.S.A. 109, 10065–10070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence P. A., Johnston P., The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell 45, 505–513 (1986). [DOI] [PubMed] [Google Scholar]
- 26.Wigglesworth V. B., The function of the corpus allatum in the growth and reproduction of rhodnius prolixus (Hemiptera). Quart. J. Miscr. Sci. 79, 91–119 (1936). [Google Scholar]
- 27.Riddiford L. M., Juvenile hormone action: A 2007 perspective. J. Insect Physiol. 54, 895–901 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Wigglesworth V. B., The juvenile hormone. Nature 208, 522–524 (1965). [Google Scholar]
- 29.Robinson G. E., Division of Labor in Insect Societies. Encycl. Insects 37, 637–655 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Richard D. S., Applebaum S. W., Sliter T. J., Baker F. C., Schooley D. A., Reuter C. C., Henrich V. C., Gilbert L. I., Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila melanogaster: A putative juvenile hormone in the higher Diptera. Proc. Natl. Acad. Sci. U.S.A. 86, 1421–1425 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bownes M., Rembold H., The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur. J. Biochem. 164, 709–712 (1987). [DOI] [PubMed] [Google Scholar]
- 32.Wijesekera T. P., Saurabh S., Dauwalder B., Juvenile hormone is required in adult males for Drosophila courtship. PLOS ONE 11, e0151912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argue K. J., Yun A. J., Neckameyer W. S., Early manipulation of juvenile hormone has sexually dimorphic effects on mature adult behavior in Drosophila melanogaster. Horm. Behav. 64, 589–597 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin H.-H., Cao D.-S., Sethi S., Zeng Z., Chin J. S. R., Chakraborty T. S., Shepherd A. K., Nguyen C. A., Yew J. Y., Su C.-Y., Wang J. W., Hormonal modulation of pheromone detection enhances male courtship success. Neuron 90, 1272–1285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethi S., Lin H.-H., Shepherd A. K., Volkan P. C., Su C.-Y., Wang J. W., Social context enhances hormonal modulation of pheromone detection in Drosophila. Curr. Biol. 29, 3887–3898.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riddiford L. M., Truman J. W., Mirth C. K., Shen Y.-c., A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 1126, 1117–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto R., Bai H., Dolezal A. G., Amdam G., Tatar M., Juvenile hormone regulation of Drosophila aging. BMC Biol. 11, 85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker L., Gross S., Beullens M., Bollen M., Bennett D., Alphey L., Functional interaction between nuclear inhibitor of protein phosphatase type 1 (NIPP1) and protein phosphatase type 1 (PP1) in Drosophila: Consequences of over-expression of NIPP1 in flies and suppression by co-expression of PP1. Biochem. J. 368, 789–797 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Han X., Mehren J., Hiroi M., Billeter J.-C., Miyamoto T., Amrein H., Levine J. D., Anderson D. J., Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci. 14, 757–762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdou M. A., He Q., Wen D., Zyaan O., Wang J., Xu J., Baumann A. A., Joseph J., Wilson T. G., Li S., Wang J., Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Bilen J., Atallah J., Azanchi R., Levine J. D., Riddiford L. M., Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 110, 18321–18326 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson T. G., DeMoor S., Lei J., Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant(27) mutant phenotype. Insect Biochem. Mol. Biol. 33, 1167–1175 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Godlewski J., Wang S., Wilson T. G., Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem. Biophys. Res. Commun. 342, 1305–1311 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Freund K., Therapeutic sex drive reduction. Acta Psychiatr. Scand. 62, 5–38 (1980). [DOI] [PubMed] [Google Scholar]
- 45.Boccardo F., Rubagotti A., Barichello M., Battaglia M., Carmignani G., Comeri G., Conti G., Cruciani G., Dammino S., Delliponti U., Ditonno P., Ferraris V., Lilliu S., Montefiore F., Portoghese F., Spano G., Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: Results of an italian prostate cancer project study. J. Clin. Oncol. 17, 2027–2038 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Levin E. R., Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 20, 477–482 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coolen L. M., Wood R. I., Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: Redundancy without amplification. Behav. Brain Res. 98, 143–153 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Nielsen J., Hedeholm R. B., Heinemeier J., Bushnell P. G., Christiansen J. S., Olsen J., Ramsey C. B., Brill R. W., Simon M., Steffensen K. F., Steffensen J. F., Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353, 702–704 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Inoue S., Yang R., Tantry A., Davis C.-h., Yang T., Knoedler J. R., Wei Y., Adams E. L., Thombare S., Golf S. R., Neve R. L., Tessier-Lavigne M., Ding J. B., Shah N. M., Periodic remodeling in a neural circuit governs timing of female sexual behavior. Cell 179, 1393–1408.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Q., Tabuchi M., Liu S., Kodama L., Horiuchi W., Daniels J., Chiu L., Baldoni D., Wu M. N., Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science 356, 534–539 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan H., Opachaloemphan C., Mancini G., Yang H., Gallitto M., Mlejnek J., Leibholz A., Haight K., Ghaninia M., Huo L., Perry M., Slone J., Zhou X., Traficante M., Penick C. A., Dolezal K., Gokhale K., Stevens K., Fetter-Pruneda I., Bonasio R., Zwiebel L. J., Berger S. L., Liebig J., Reinberg D., Desplan C., An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170, 736–747.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song B. J., Sharp S. J., Rogulja D., Daily rewiring of a neural circuit generates a predictive model of environmental light. Sci. Adv. 7, eabe4284 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Z., Autry A. E., Bergan J. F., Watabe-Uchida M., Dulac C. G., Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung Y., Kennedy A., Chiu H., Mohammad F., Claridge-Chang A., Anderson D. J., Neurons that function within an integrator to promote a persistent behavioral state in Drosophila. Neuron 105, 322–333.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W., Ganguly A., Huang J., Wang Y., Ni J. D., Gurav A. S., Aguilar M. A., Montell C., Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. eLife 8, e49574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee G., Bahn J. H., Park J. H., Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 103, 12580–12585 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao C., Guo C., Peng Q., Cao J., Shohat-Ophir G., Liu D., Pan Y., Sex and death: Identification of feedback neuromodulation balancing reproduction and survival. Neurosci. Bull. 36, 1429–1440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boutros C. L., Miner L. E., Mazor O., Zhang S. X., Measuring and altering mating drive in male Drosophila melanogaster. J. Vis. Exp. , 55291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., Couto A., Marra V., Keleman K., Dickson B. J., A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Burgess C. R., Ramesh R. N., Sugden A. U., Levandowski K. M., Minnig M. A., Fenselau H., Lowell B. B., Andermann M. L., Hunger-dependent enhancement of food cue responses in mouse postrhinal cortex and lateral amygdala. Neuron 91, 1154–1169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen T.-W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V., Looger L. L., Svoboda K., Kim D. S., Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Y., Ueda A., Wu C.-F., A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila Larvae. J. Neurogenet. 18, 377–402 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Long X., Colonell J., Wong A. M., Singer R. H., Lionnet T., Quantitative mRNA imaging throughout the entire Drosophila brain. Nat. Methods 14, 703–706 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Tanenhaus A. K., Zhang J., Yin J. C. P., In vivo circadian oscillation of dCREB2 and NF-κB activity in the Drosophila nervous system. PLOS ONE 7, e45130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/25/eabg6926/DC1


