Atrial fibrillation (AF) is a problem of major clinical importance, accounting for about 9 million cases in the European Union in 2010 with projections for an almost doubling by 2060.1 The worldwide economic burden of AF was estimated at 6 million disability-adjusted life-years of lost productivity in 2017.2 The average 3-year societal cost per patient in Denmark attributable to AF over the period 2001–2012 was estimated to be of the order of 25 000 Euros ($30 000), providing an overall cost burden in Europe of the order of 10 billion Euros.3 Clearly, AF is a major issue at the level of public health and economic burden, motivating the regular establishment of detailed society guidelines, including two in the last year.4,5
Not only is AF a substantial public health problem at the moment, but its impact is projected to increase in the future. The prevalence of AF increases exponentially with advancing age6 and with population-ageing projections point to an almost 90% increase in the number of elderly AF patients in the European Union by 2060.7 Modelling of UK data illustrate major projected increases in AF-related healthcare costs over the next decades, largely from hospitalizations.8 Improving the management of AF is an important goal that has motivated major investigative efforts, but substantial challenges remain.9 Improvements in understanding the basic mechanisms of AF and translating them clinically with rapidly advancing technologies are key to combating AF and its human/ societal/ economic consequences. Recognizing the importance of this area and the active efforts being made in the field, the Editorial Board of Cardiovascular Research decided to create an issue of the journal focused on the problem of effective clinical translation of advances in basic AF research.
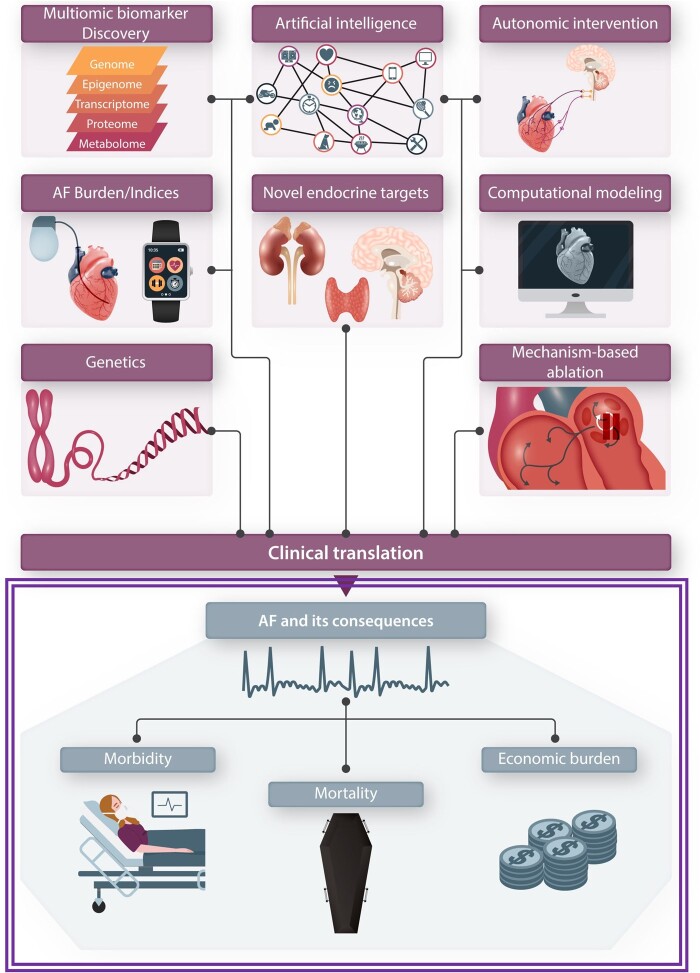
The issue includes nine invited review articles, as illustrated in Figure 1. Lin et al. discuss the use of multiomic approaches, including genomics, epigenomics, transcriptomics, metabolomics, and their integration for the discovery of novel biomarkers that can be used to stage AF, follow its course and evaluate the results of its management. Kany et al. have provided a state-of-the-art paper on AF genetics, dealing with their practical application to guiding the management of AF and priorities/prospects for future development. Lip et al. deal with the rapidly evolving area of artificial intelligence and machine-learning, discussing its enormous capacity to identify novel ways to diagnose, characterize, prevent, and treat AF. The classification and characterization of AF and its response to treatment is an important challenge; Boriani et al. analyse the use of novel non-invasive criteria, particularly the quantification of AF burden, as indices of the arrhythmia. Aguilar et al. review the highly significant but often underappreciated subject of endocrine control of AF. In addition to the fundamental mechanisms and clinical aspects of the atrial effects of well-recognized endocrine factors like diabetes, abnormalities in thyroxin production, obesity, the renin-angiotensin system, and sex hormones, they discuss recent insights into the role of natriuretic peptides, adrenal cortical and medullary products, hypothalamic-pituitary hormones, and the thyroid hormone calcitonin in AF control.
Figure 1.
A schematic illustration of the clinical importance of AF, along with the various topics dealt with in the invited review articles of the present issue. The principal consequences of AF that are illustrated include associated morbidity, mortality, and economic costs. The double-line surrounding AF and its consequences indicates the difficulty of making a significant impact by innovations in diagnosis, prevention, and therapy. Eight of the review articles deal with specific themes of AF research and one review paper discusses in a more general way the challenges and obstacles to clinical translation of novel basic research insights, as well as some potential solutions. AF, atrial fibrillation.
Ablation has emerged as the single most effective approach to maintaining normal sinus rhythm in patients who have experienced AF. Filgueiras-Rama et al. deal with this important area, reviewing in detail the prospects for materially improving the success of these important procedures by targeting the basic mechanisms underlying AF initiation and maintenance, with the exploitation of new knowledge and technologies. These authors also describe practical challenges that scientists and clinicians face when attempting to implement novel mapping methods into regular clinical practice. It has long been known that the autonomic nervous system (ANS) plays a key role in AF occurrence.10 Shivkumar et al. discuss the active research being performed to harness interventions acting via ANS modulation to suppress AF and prevent its recurrence and maintenance.
Research in AF generates a wide range of information at the molecular, subcellular, cellular, tissue, organ, organismal, human-subject, and population levels. These disparate sets of data need to be integrated in order to realistically determine their implications for arrhythmia likelihood and burden. Heijman et al. discuss a critical tool in the integration, validation, and application of research discoveries, the use of computational modelling. Rapid advances in computing technology have permitted the analysis of massive amounts of data and the creation of integrative and population models that permit the quantitative testing of important hypotheses about AF risk and its mechanisms, predictors, and modifiers.
Finally, once all of these discoveries are made, they need to be converted into effective, safe, and practical clinical tools to improve the management of AF patients, a process often called ‘clinical translation’. Nattel et al. provide an overview of the challenges to translating basic research discoveries to clinically applicable innovations in AF management, dealing with such issues as the consequences of the regulatory and commercial landscape, the need for, availability and limitations of animal models, the alternatives to animal models (like heart cells and engineered tissue derived from human induced pluripotent stem cells), and possible paths forward to overcome impasses in therapeutic development and implementation.
In addition, the issue contains a number of highly innovative original articles presenting new research findings in this area. These articles deal with topics like the role of the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) as a mediator of the AF-promoting effects of obesity, the effects of a molecule that promotes the resolution of inflammatory changes in a rat model of AF associated with right heart disease, the transcriptomic and proteomic landscape of AF progression in a chronic sheep model, and the involvement of calcium-handling abnormalities in post-operative AF.
We are very excited about this issue, which we hope will both inform readers of Cardiovascular Research interested in this area and stimulate further research and development to tackle this important problem.
Funding
This work was supported by the Canadian Institutes of Health Research and the Quebec Heart and Stroke Foundation (to S.N.), the National Institutes of Health (R01-HL131517, R01-HL136389, and R01-HL089598 to D.D.), the European Union (large-scale network project MAESTRIA, No. 965286 to D.D. and D.F-R.), the German Research Foundation (DFG, Do 769/4-1 to D.D.), the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación, and the Pro CNIC Foundation, the Severo Ochoa Center of Excellence (SEV-2015-0505 to D.F.-R.), and the Spanish Ministry of Science and Innovation (to D.F-R., PID2019-109329RB-I00).
Conflict of interest: S.N.: Consultant for LQTS Therapeutics. G.Y.H.L.: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are received personally. D.F.-R.: Co-inventor on a patent (Patent#EP3636147A1) related to a method for the identification of cardiac fibrillation drivers and the footprint of rotational activations. D.D.: Scientific advisory board member for OMEICOS Therapeutics GmbH and Acesion Pharma.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–221. [DOI] [PubMed] [Google Scholar]
- 3. Johnsen SP, Dalby LW, Täckström T, Olsen J, Fraschke A. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Serv Res 2017;17:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L; Members of the Secondary Panel. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847–1948. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 7. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M, Cattarinussi A, D'Alfonso MG, Gradia C, Sgherzi B, Pracucci G, Piccardi B, Polizzi B, Inzitari D; National Research Program: Progetto FAI. La Fibrillazione Atriale in Italia. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 2019;21:1468–1475. [DOI] [PubMed] [Google Scholar]
- 8. Burdett P, Lip GYH. Atrial fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes 2020;qcaa093. doi:10.1093/ehjqcco/qcaa093. [DOI] [PubMed] [Google Scholar]
- 9. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surtshin A, Rucknagel DL. Vagal sensitivity and the production of auricular fibrillation in experimentally hyperthyroid dogs. Am Heart J 1953;45:781–789. [DOI] [PubMed] [Google Scholar]