Abstract
Despite significant advances in its detection, understanding and management, atrial fibrillation (AF) remains a highly prevalent cardiac arrhythmia with a major impact on morbidity and mortality of millions of patients. AF results from complex, dynamic interactions between risk factors and comorbidities that induce diverse atrial remodelling processes. Atrial remodelling increases AF vulnerability and persistence, while promoting disease progression. The variability in presentation and wide range of mechanisms involved in initiation, maintenance and progression of AF, as well as its associated adverse outcomes, make the early identification of causal factors modifiable with therapeutic interventions challenging, likely contributing to suboptimal efficacy of current AF management. Computational modelling facilitates the multilevel integration of multiple datasets and offers new opportunities for mechanistic understanding, risk prediction and personalized therapy. Mathematical simulations of cardiac electrophysiology have been around for 60 years and are being increasingly used to improve our understanding of AF mechanisms and guide AF therapy. This narrative review focuses on the emerging and future applications of computational modelling in AF management. We summarize clinical challenges that may benefit from computational modelling, provide an overview of the different in silico approaches that are available together with their notable achievements, and discuss the major limitations that hinder the routine clinical application of these approaches. Finally, future perspectives are addressed. With the rapid progress in electronic technologies including computing, clinical applications of computational modelling are advancing rapidly. We expect that their application will progressively increase in prominence, especially if their added value can be demonstrated in clinical trials.
Keywords: Atrial fibrillation, Computer modelling, Electrophysiology, In silico, Personalized therapy
Graphical Abstract
1. Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, affecting >43 million individuals globally.1 In the European Union, AF currently affects >10 million adults, half of whom are ≥75 years old.2 These numbers are projected to double by 2060.1, 2 Current European guidelines recommend a holistic AF Better Care (ABC) pathway, involving anticoagulation to avoid stroke, better symptom management through rate- and rhythm control, and cardiovascular and comorbidity optimization.1 AF management according to the ABC pathway improves outcomes and reduces healthcare costs in retrospective cohort studies,3, 4 with limited data available from randomized clinical trials on the ABC pathway,5 or related components such as comprehensive nurse-led care.6 Recent data indicate that the adherence and persistence of AF patients during long-term use of mHealth-supported care based on the ABC pathway was good and was associated with a reduction in adverse clinical outcomes.7 However, despite significant advances in its detection, mechanistic understanding and management, AF continues to have a major impact on morbidity and mortality of millions of patients,1 partly because of unresolved knowledge gaps in AF pathophysiology, screening, and therapeutic strategies, including rate/rhythm control and stroke prevention.8 The development of actionable personalized approaches, which take into account patient-specific profiles and arrhythmia mechanisms, will likely be essential to overcome current challenges in AF management.9 Over the last decades, multiscale computational modelling of cardiac electrophysiology has emerged as a modality to better understand complex arrhythmia mechanisms and the multifaceted interactions between arrhythmia substrates, drivers and triggers.10–14 Employing the full control over parameters and complete observability inherent to computational models (e.g. enabling simultaneous evaluation of multiple readouts under precisely defined conditions), the key contributors to cardiac arrhythmias in various experimental settings and pathologies have been identified in realistic simulated contexts. In this narrative review, we highlight the emerging and future applications of computational modelling in AF management, providing a conceptual overview of the different in silico approaches that are available together with their notable achievements. We also discuss challenges to the routine clinical application of these approaches. For more methodological details on model development, the interested reader is referred to other reviews.13, 15–17
2. AF pathophysiology
AF is a complex multifactorial disease promoted by several dynamic predisposing factors, including genetics, age-/disease-associated remodelling, and AF-related remodelling. The occurrence of AF is modulated by several systemic regulators such as the autonomic nervous system, haemodynamic changes and inflammation.18–20 Strong interactions between AF determinants, including autonomic remodelling, calcium-handling abnormalities, ion-channel alterations, and structural remodelling are evident and have been described in detail in recent reviews.21–23 In brief, the incidence of diastolic spontaneous calcium release events (SCaEs) is increased in paroxysmal, persistent and post-operative AF (POAF).18, 24 Together with AF-associated ion-channel remodelling, SCaEs can lead to early and delayed afterdepolarizations (EADs and DADs, respectively). At the tissue and organ levels, afterdepolarizations can induce focal ectopic firing, often arising from myocardial sleeves around the pulmonary veins. Re-entrant activity initiated by ectopic activity acting on a vulnerable substrate (characterized by short effective refractory periods and slow, heterogeneous conduction) is considered a primary AF-maintaining mechanism and becomes more complex with advancing atrial remodelling.18, 21 Atrial structural remodelling is a major cause of AF-promoting conduction abnormalities. AF is generally a progressive condition, moving from paroxysmal (resolving in <7 days) to persistent (remaining for >7 days) and, if not adequately managed, may become resistant to pharmacological and interventional therapies (permanent AF). AF progression is in part due to advancing age, the effects of underlying risk factors and AF-related atrial remodelling, and is independently associated with worse outcomes.18 Of note, most patients have multiple AF-promoting factors and their interaction can produce distinct atrial remodelling patterns. For example, AF-related remodelling is distinct in dogs with and without ventricular tachycardiomyopathy (due to the absence or presence of atrioventricular block), as well as in persistent AF patients with and without left ventricular (LV) dysfunction.25, 26 Although a major part of the AF-promoting substrate develops secondary to advancing age and acquired risk factors, AF also has an important genetic component. Common and rare genetic variants in numerous genes have been associated with AF, although for many variants, including the most common ones at 4q25 near PITX2, the exact pathophysiological mechanisms remain unknown.27 Despite the growing knowledge of the arrhythmia substrates and triggers in AF, the contribution of each component in specific patient subgroups and individuals remains incompletely understood.21
3. Clinical challenges in AF management potentially benefiting from computational modelling
Important knowledge gaps and challenges in AF management have recently been summarized.8, 9 Two challenges in particular have received significant attention in computational modelling studies: identification of asymptomatic AF to enable early therapy and personalized rhythm-control therapy, including prediction of AF recurrences. This section summarizes the clinical motivation for addressing these challenges. The contributions of computational modelling are detailed in the next section.
It is estimated that 10–40% of AF patients are asymptomatic.28, 29 These patients often remain undiagnosed and therefore do not receive appropriate treatment, including early anticoagulation therapy, increasing their risk of thromboembolic strokes that may even be the first recognizable manifestation of the arrhythmia.28–30 Undetected asymptomatic AF also delays initiation of rhythm-control therapy, allowing irreversible structural remodelling to develop, reducing therapeutic efficacy. Earlier AF-detection may enable prompt treatment, preventing disease progression and AF-related complications. In agreement, the EAST-AFNET4 trial demonstrated the benefit of early rhythm-control therapy in reducing the risk of adverse cardiovascular outcomes.31 Although opportunistic screening is recommended and new technologies for recording heart rhythm might greatly facilitate AF-screening, the optimal approach for systematic screening and its potential benefit remain uncertain.29, 30 By analysing large amounts of data and quantitatively projecting the effects of different screening strategies, computational approaches may facilitate AF-risk prediction and enable more reliable, cost-effective identification of asymptomatic AF.
Rate and rhythm control strategies using antiarrhythmic drugs (AADs) and catheter ablation are the cornerstones of symptomatic AF management. Historically, rate control was shown to be equally effective as rhythm control for the prevention of mortality and morbidity from cardiovascular causes in several studies.32–35 The lack of benefit of rhythm control in earlier trials has largely been attributed to the limited efficacy and proarrhythmic side-effects of AADs.36 Pulmonary vein isolation (PVI) through catheter ablation improves sinus rhythm maintenance compared to AADs and is devoid of ventricular proarrhythmia, potentially enabling better rhythm control. Indeed, a number of recent clinical trials have suggested that modern rhythm-control strategies may improve outcomes, at least in certain subpopulations of AF patients.31, 37, 38 Nonetheless, the efficacy of catheter ablation remains suboptimal,39, 40 largely due to a one-size-fits-most approach. For example, in patients with persistent AF,41 arrhythmia-free survival rates after a single procedure were 35.3 ± 3.9%, 28.0 ± 3.7%, and 16.8 ± 3.2% at 1, 2, and 5 years, respectively.42 Time to AF recurrence is a major determinant of AF outcome, where patients with later recurrences were more likely to have sporadic episodes and respond better to AADs and repeat ablation.43 Despite the increasing prominence of catheter ablation,44–46 AADs remain a major component of AF management, because of the large number of affected individuals, as well as the costs and risks of the invasive procedure required for ablation.1, 47–50 However, drug-induced proarrhythmia and long-term toxicity often limit the choice of AADs.36, 51 It is likely that specific rate- or rhythm-control strategies respond differently to distinct fundamental molecular and cellular determinants of AF, modulating the outcome of specific treatments. The underlying molecular and cellular determinants of AF might also be associated with the likelihood and time to AF recurrence.52 Nevertheless, predicting which patients are likely to recur and may therefore require additional follow-up or more aggressive therapy is challenging. As such, a more personalized approach is needed to better stratify the benefit and risk of each treatment strategy, and select the optimal (combination of) AF therapies. Using personalized multiscale computational modelling, the outcome of both pharmacological and interventional rhythm-control strategies in an individual patient might be predicted, facilitating the identification of the best patient-specific treatment strategy for AF.
4. Key achievements of computational modelling in AF
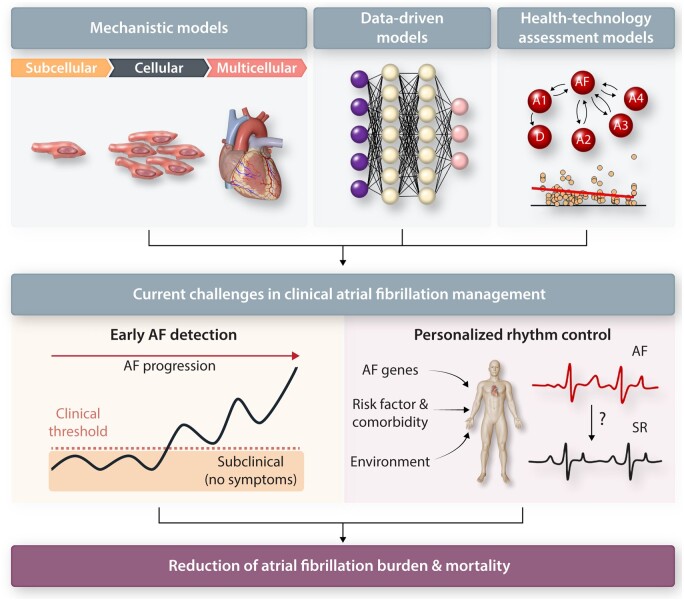
Conceptually, most of the computational models employed for studying AF can be divided into mechanistic and data-driven models (Figure 1, top). Mechanistic models integrate fundamental biophysical laws and concepts with experimental data to simulate cardiac electrophysiology. These models are usually dynamic, simulating changes in quantities of interest (membrane potential, intracellular concentrations, etc.) over time, and are therefore typically represented by systems of non-linear ordinary or partial differential equations. Their dynamic nature also means that they are inherently causal, e.g. reflecting how electrical activity spreads from a stimulus site by successively activating neighbouring cells. As such, these models are frequently employed to investigate the underlying pathophysiology of cardiac arrhythmia and to unravel the complex dynamic interactions among variables of interest, from atomic to organ levels.53 Meanwhile, data-driven models employ statistical or machine learning (ML) approaches to establish associations between predefined inputs and outputs. These models require fewer a priori assumptions than mechanistic models and can suggest hitherto unknown factors contributing to AF. Inputs of data-driven models are typically clinical data, including (semi-)quantitative clinical characteristics, as well as raw signals [e.g. electrocardiogram (ECG) or telemetry data] and imaging data. Their outputs are most often clinical outcomes or diagnoses, making their clinical application more direct than that of mechanistic models. Classically, data-driven models have employed multivariable regression to link inputs and outputs, but recent advances in ML and artificial intelligence have significantly expanded their abilities and range of applications.54 Finally, some approaches fall somewhere in between mechanistic and data-driven models. For example, health-technology assessment models used in cost-effectiveness assessment of medical therapies,55, 56 are dynamic and causal, but do not include fundamental biophysical laws and are primarily based on data from clinical trials. The following three subsections provide key examples of these computational approaches that are relevant for AF management (as summarized in Table 1 and Figure 1, blue and yellow boxes).
Figure 1.
Overview of existing computational approaches, achievements, challenges, and their potential future directions in AF management. Both mechanistic and data-driven models are available and currently being used to study the AF pathophysiology (blue box) and improve AF clinical care (yellow box). However, several limitations exist (red box) that need to be resolved, e.g. through experimental and technological advances. By overcoming these limitations, demonstrating clinical benefit in randomized clinical trials (RCTs) and improving ease of use, computational modelling can improve AF management.
Table 1.
The current contributions of computational modelling of atrial electrophysiology on AF pathophysiology and clinical care
| Clinical challenge | Model scale/type | Contribution | Example |
|---|---|---|---|
| Mechanistic models | |||
| Early AF detection | Cellular and organ | Insights on proarrhythmic electrical and structural remodelling associated with AF risk factors | 57–66 |
| Personalized rhythm-control therapy | Subcellular and cellular | Identification of the ionic mechanisms underlying atrial arrhythmias and consequences of AF-related remodelling | 12 , 24, 67–74 |
| Cellular and tissue | Evaluation of potential novel AAD targets, notably Kv1.5 and K2P3.1 | 75 , 76 | |
| Cellular and tissue | Identification of optimal pharmacodynamic characteristics of new AADs, including state-dependent and multi-channel inhibition properties | 77–79 | |
| Cellular | Evaluation of drug safety as part of the comprehensive in vitro proarrhythmia assay (CiPA) initiative | 80 | |
| Organ | Evaluating the outcome of different catheter ablation strategies in patient-specific models | 81–84 | |
| Organ | Simulation driven-targeting of AF (emergent) re-entrant drivers | 85–87 | |
| Organ | Prediction and prevention of post-ablation atrial arrhythmia and AF recurrences | 88 , 89 | |
| Data-driven models | |||
| Early AF detection | Statistical | Prediction of AF risk based on clinical and genetic information | 90–94 |
| ML | Prediction of AF based on sinus rhythm ECGs | 95–97 | |
| ML | Detection of AF based on facial pulsatile photoplethysmographic signals | 98 | |
| Statistical | Estimation of patient-specific atrial electrical remodelling patterns based on remote-monitoring technology | 99 | |
| Personalized therapy | Statistical | Predicting spontaneous conversion to sinus rhythm in symptomatic atrial fibrillation | 100 |
| Statistical | Predicting the likelihood of AF recurrence | 101 | |
| ML | Prediction of AF recurrence after the first catheter ablation procedure | 102–104 | |
| ML | Classification of intracardiac activation patterns during AF to detect regional rotational activity | 105 , 106 | |
| ML | Identification of patients who may benefit from AF cardioversion | 107 | |
| Health-technology assessment models | |||
| Early AF detection | Population | Cost-effectiveness analyses of AF screening | 56 , 108 |
| Personalized therapy | Population | Cost-effectiveness analyses of AF therapies (e.g. AADs, anticoagulants and ablation) | 55 , 109–111 |
5. The role of mechanistic models to improve understanding and management of AF
5.1 Brief overview of the historical evolution of mechanistic models of AF
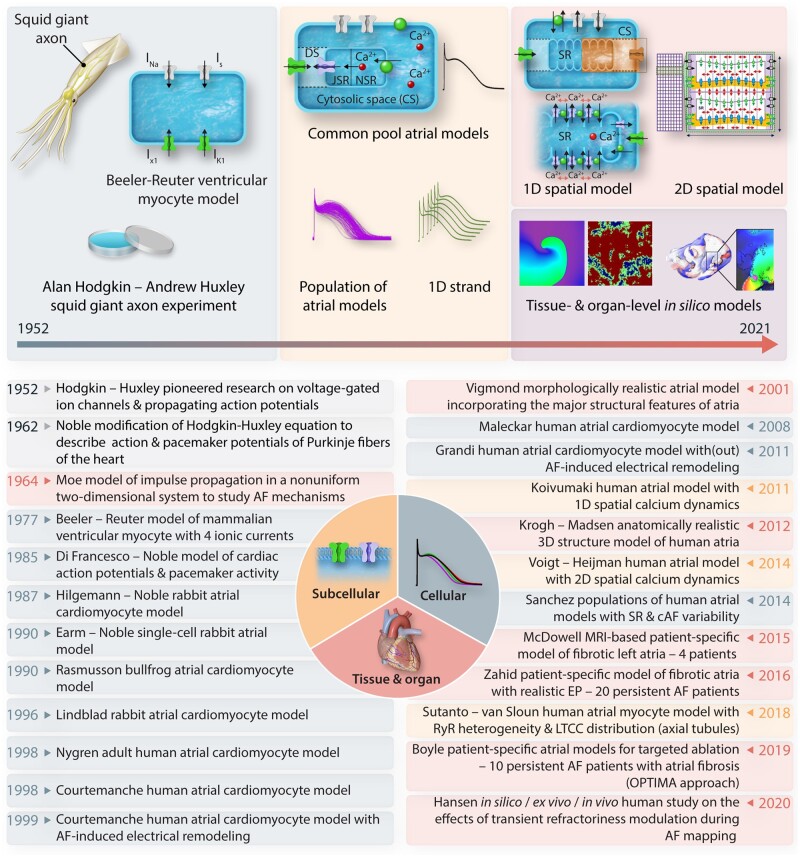
Computational modelling of electrophysiology began following the famous experimental work on cellular electrical activity in the squid giant axon by Hodgkin and Huxley in 1952 (Figure 2).112 Subsequently, various mathematical models of both neural and cardiac electrophysiology have been developed to address specific research questions and bridge knowledge gaps identified during laboratory experiments.13 In 1962, Denis Noble for the first time utilized the Hodgkin-Huxley equations to explain the action potential of cardiac Purkinje fibres.113 A similar approach was employed by Beeler and Reuter to develop the first ventricular cardiomyocyte model in 1977.114 Inspired by the detailed ionic model of cardiac electrical activity by DiFrancesco and Noble,115 the 1987 mathematical model by Hilgemann and Noble116 was among the first to explore the electrophysiological properties of mammalian atrial cardiomyocytes. In 1998, the first human atrial in silico models were formulated by Nygren et al.117 and Courtemanche et al.118 These models were employed to study the consequences of experimentally characterized AF-induced electrical remodelling.75 Subsequently, computational models of atrial cellular electrophysiology were refined to study atrial cell-cell interactions119 and calcium-handling abnormalities.120 Advances in experimental modalities such as (super-resolution) confocal microscopy revealed important differences in subcellular structure between atrial and ventricular cardiomyocytes, which motivated atrial cardiomyocyte-specific model structures. For example, atrial cardiomyocytes have a limited transverse-tubule network, which causes centripetal calcium wave propagation from the cell periphery towards the centre of the cell.13, 121 This phenomenon can only be captured by models with spatial calcium handling.67, 122 In 2014, a population modelling approach in which a large population of models is generated by varying individual parameters was first applied to atrial cardiomyocyte models to capture interindividual and intercellular variability.123
Figure 2.
Timeline of milestones in multiscale mechanistic modelling of atrial electrophysiology. The subcellular spatial models of human atria are depicted in yellow, the cellular models in blue/grey, and the tissue/organ-level (whole atria) models in red. Two of the earliest electrophysiological models are shown in grey. As displayed in the figure, the model complexity and prominence has increased over the year, from covering only four ionic currents in 1977 to advanced patient-specific whole-atria models and detailed models of subcellular calcium handling in 2020.
Already in 1964, Moe et al.124 described a mathematical model of impulse propagation in a non-uniform two-dimensional system exhibiting self-sustained turbulent activity resembling AF. This seminal work has provided insights into the determinants of AF maintenance and revealed potential therapeutic interventions to control multiple wavelets in atrial tissue, which subsequently gave rise to the Cox maze procedure. Since the early 2000s, morphologically realistic organ-level atrial models, incorporating the major atrial structures, have also been developed125 and advances in clinical imaging and computational resources have enabled the development of patient-specific models. For example, in 2015 McDowell et al.57 provided the first proof-of-concept that a modelling approach with patient-specific fibrosis patterns could non-invasively identify AF ablation targets prior to the clinical procedure. This approach was subsequently expanded to better predict, diagnose or treat atrial arrhythmias.57, 58, 85, 126, 127 Taken together, during the past 35 years, numerous atrial models ranging from subcellular to organ levels have been developed to examine atrial electrophysiology under both physiological and disease conditions.120–123, 125 The milestones that have been briefly mentioned in this section are summarized in Figure 2, and those relevant for clinical AF management are discussed in detail below.
5.2 Cellular electrophysiology modelling
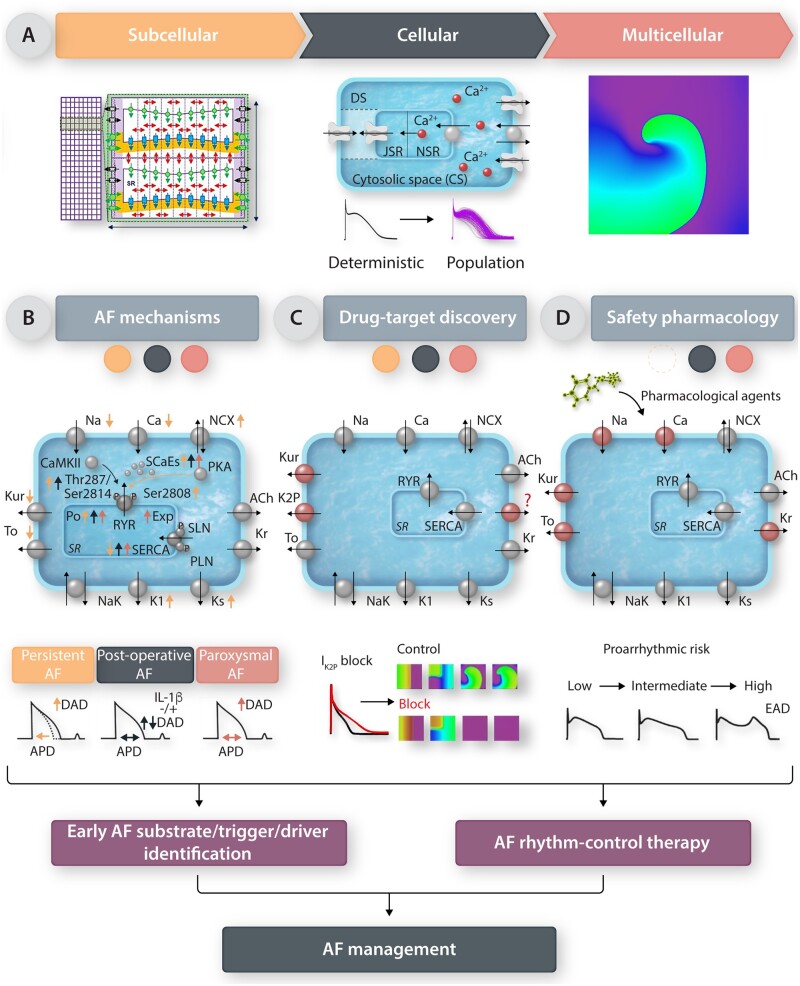
(Sub)cellular and multicellular computational models of cardiac electrophysiology (Figure 3A) can help to overcome experimental limitations by providing full control over parameters of interest. Among other things, (sub)cellular in silico modelling of atrial electrophysiology has contributed to the identification of the ionic mechanisms underlying atrial arrhythmias, including the determinants of alternans, as well as DADs and associated triggered activities in different forms of AF (Figure 3B). For example, a two-dimensional spatial calcium-handling model indicated that both ryanodine receptor (RyR) dysregulation and enhanced SERCA2a activity promoted increased sarcoplasmic reticulum calcium leak and SCaEs, causing DADs/triggered activity in paroxysmal AF.67 Meanwhile, interleukin-1β-induced RyR dysfunction exacerbated the pre-existing calcium-handling abnormalities, leading to DADs in models of POAF.24 In silico studies also demonstrated that AF-promoting atrial repolarization alternans might occur due to decreased RyR inactivation, and that calcium-driven repolarization alternans due to AF-associated electrical remodelling increased the vulnerability to ectopy-induced arrhythmia.12, 68, 69 Finally, in silico models of human atrial cardiomyocytes75, 118, 120 have been used to study the impact of AF-related ion-channel remodelling70–74 and remodelling associated with AF risk factors.59 Until recently, most computational studies employed a single deterministic model, reflecting a representative atrial cardiomyocyte, thereby disregarding experimentally observed variability. However, with growing awareness on the importance of such variability, several modelling studies have started incorporating intra- and inter-individual variability of cellular parameters. The most common method involves stochastic scaling of the cellular parameters, typically the maximal ionic conductances, reflecting variability in ion-channel expression, resulting in a population of models.123, 128, 129 Subsequently, models within this population that are too far outside of the experimental range are removed. The resulting calibrated populations reflect the natural heterogeneity within a population, encompass a wide range of cellular phenotypes and are therefore expected to yield more representative results.
Figure 3.
(Multi)cellular models of atrial electrophysiology and their roles in AF management. (A) Illustrations of currently available subcellular, cellular, and multicellular atrial electrophysiology models. (B–D) The role of cellular modelling to improve the understanding of basic AF mechanisms and determinants (B), in drug-target discovery, exemplified by IKur and IK2P block, resulting in prolongation of APD and destabilization of reentrant waves (C), and in safety pharmacology (i.e. the CiPA initiative; D).
Together, these cellular studies suggest a potential for model-based identification of therapeutic targets (Figure 3C). Moreover, by integrating experimental data on disease- and AF-associated remodelling, these computer models may provide insight into therapeutic effectiveness under different clinical conditions. For example, the atrial-selective expression of Kv1.5 ion-channels suggested that they may constitute an interesting atrial-selective ionic target for AAD therapy, but simulation of their role in atrial repolarization raised concerns75 that were subsequently confirmed in clinical trials.130 Simulations indicated that remodelling of K2P3.1 plays a major role in APD-shortening in long-standing persistent AF, but that down-regulation of K2P3.1 with LV dysfunction reduced antiarrhythmic efficacy of K2P3.1 current inhibition.76 The perfect control offered by computer models in combination with the increasing availability of detailed Markov models of cardiac ion channels may also help to identify the optimal pharmacodynamic characteristics of new AADs, including state-dependent and multi-channel inhibition properties.77–79 Thus, detailed cellular models provide information that might be relevant for future tailored rhythm-control therapy of AF.
In addition to their potential for AF drug development, computational cardiomyocyte models play an increasingly important role in cardiac safety pharmacology as part of the comprehensive in vitro proarrhythmia assay (CiPA) initiative (Figure 3D).80 Although the main focus of CiPA is predicting the risk of potentially life-threatening ventricular arrhythmias, the initiative has shown, using stringent predefined validation criteria, that a population of ventricular cell models can reliably predict the risk of drug-induced proarrhythmia. This gives credibility to the use of computational models for guiding (regulatory) decisions with major financial and healthcare implications. Furthermore, in the future the CiPA initiative may indirectly affect AF management by influencing the regulatory approval of drugs involved in risk-factor management.
5.3 Organ-level modelling
The majority of contemporary simulation research in organ-level electrophysiology of the atria and AF accurately captures the complex 3-dimensional human atrial geometry acquired from computed tomography81, 131 and magnetic resonance imaging (MRI),57, 60, 61, 132 or employs surface models reconstructed from invasively-acquired electro-anatomic maps.133 Such atrial models incorporate multi-scale representations of cell and tissue properties (Figure 4). Several of the aforementioned cellular ionic models have been used to capture atrial cell electrophysiology, including AF-promoting electrical remodelling, while properties at the tissue level account for the orthotropic conduction of electrical waves governed by atrial fibre orientation, which is commonly retrieved from atlases as atrial fibre orientation currently cannot be acquired in vivo.134, 136 Studies have modelled atrial fibrosis detected on late gadolinium enhancement (LGE) MRI by changes in cellular electrophysiology and tissue conductivities, by stochastic removal of mesh elements in fibrotic areas, or by introducing patchy areas of electrical isolation.58, 62, 137 Fibrotic remodelling has also been represented by explicitly modelling fibroblasts, which affect the electrophysiological properties of neighbouring cardiomyocytes via direct electrical coupling or paracrine mechanisms.10 The multi-scale personalized atrial models simulate, following electrical stimulation, the propagation of electrical waves, as well as emergent phenomena such as the generation and maintenance of AF. The incorporation of structural and electrical remodelling in these models has enabled investigating the links between the altered electro-anatomical substrate and the dynamics of AF re-entrant drivers, as well as to propose options for simulation-guided AF treatment.
Figure 4.
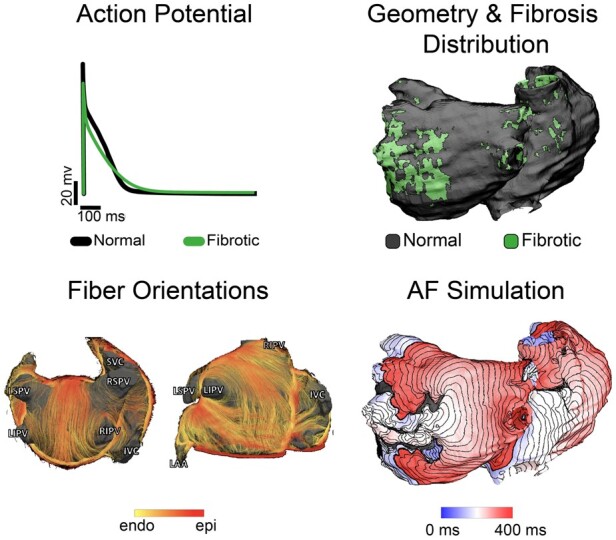
Organ-level atrial models integrate information from cell-, tissue-, and organ-level scales. For cell-level atrial electrophysiology, an example of atrial action potentials in fibrotic (green) and normal cells (black) is shown. At the tissue scale, atrial fibre architecture creates a preferential direction for wave propagation along the atrial fibres, as shown by the diffusion-tensor MRI fibre orientations acquired in explanted human atria.134 At the organ scale, personalized atrial models with patient-specific geometry and fibrotic remodelling are reconstructed from patients’ LGE-MRI scans and used in simulations of AF inducibility. Shown is an activation map of an AF episode. Modified with permission from Aronis et al.135
Organ-scale atrial computational modelling has provided insights into the fundamental mechanisms that govern propensity to AF. Notably, the extent and distribution of atrial fibrosis have been identified as critical determinants of atrial arrhythmogenicity and propagation dynamics during AF. The patient-specific distribution of fibrosis significantly affects AF dynamics in personalized atrial models. A number of studies have provided evidence that re-entrant drivers within regions of structural inhomogeneities and fibrotic remodelling have a significant role in maintenance of persistent AF.57, 58, 62–65 Re-entrant drivers persisted in fibrosis boundary zones characterized by high fibrosis density and fibrosis entropy (Figure 5), corresponding to atrial regions with a high degree of intermingling between fibrotic and non-fibrotic tissue. Patient-specific simulation studies have also explored the role of fibrosis in atrial arrhythmogenic propensity in relation to other atrial parameters. In atrial models reconstructed from LGE-MRI and histological data, re-entrant drivers persist in atrial areas of distinct structural ‘fingerprints’,66 i.e. a combination of intermediate wall thickness, intermediate fibrosis, and twisted myofibre orientation. However, removal of fibrosis rendered the atrial model non-inducible for AF, reinforcing the primary role of fibrosis.66 Furthermore, changes in action potential duration or conduction velocity changed the likelihood of a re-entrant driver being anchored in a specific fibrosis location.60 Atrial wall thickness was also found to be an important determinant of re-entrant driver behaviour but only in the right atrium, while in the left atrium AF dynamics were primarily determined by fibrosis distribution.61
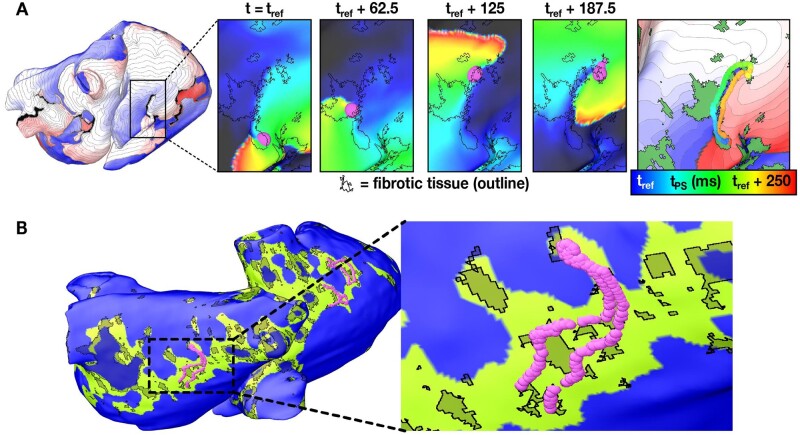
Figure 5.
Re-entrant drivers sustain AF in persistent AF patients. (A) Dynamic (i.e. time-varying) locations of the re-entrant driver organizing centre during an AF episode in a patient. An activation map (left) shows a re-entrant driver near the inferior vena cava. Inset panels show a zoomed-in view of the organizing centre (purple) at four timepoints. The fibrotic tissue boundaries are indicated by black outlines. Right-most panel shows the trajectory of the organizing centre over time superimposed on the activation map of the reentry and the fibrosis spatial pattern (green regions). (B) Location of the re-entrant driver organizing centre over time overlaid on the regions of the atria with characteristic fibrosis density and fibrosis entropy values predicted to be most likely to harbour re-entrant drivers (green). Modified with permission from Zahid et al.58
One of the most impactful applications of organ-level atrial modelling is the potential for developing personalized AF ablation strategies, tailored to target each patient’s unique atrial AF substrate. A number of simulation studies presented personalized AF ablation strategies adjuvant to standard-of-care PVI.81–84 Atrial models reconstructed from cardiac computed tomography scans of AF patients have been used to compare the outcome of different ablation strategies,81 identifying PVI with posterior box isolation and anterior line ablation as the most effective strategy. The efficacy of these ablation strategies was subsequently evaluated in a prospective clinical trial of 108 patients with persistent AF, randomized to receive either standard-of-care ablation or ablation guided by the patient-specific models.82 The study demonstrated that simulation-guided selection of the adjuvant lesion geometry is non-inferior to empirical AF ablation, but generally resulted in more extensive lesion sets.82 This groundbreaking work showed the feasibility of applying simulation-guided AF-ablation therapy, but showed that further work is needed before any added-value can be confirmed. The atrial models used in these studies were homogeneous atrial models and did not incorporate patient-specific fibrosis or fibre orientations, possibly explaining failure to show improvement.
Similar ablation approaches were tested using bilayer atrial models but incorporating patient-specific fibrosis derived from LGE-MRI83; lesions used in clinical practice such as PVI, roof, and mitral lines were compared to circle lesions, perforated circles, lines, and crosses, as well as to lines streamlining the sequence of electrical activation during sinus rhythm, with the latter found to be most effective. Using the same modelling approach, Roney et al.84 recently demonstrated that model ablation approaches based on clinical standards had limited success. To develop an optimal AF ablation approach for each patient, a random forest algorithm was trained to predict simulated ablation outcome for several input variables, including imaging metrics and simulated electrophysiological and lesion metrics. The results indicate that achieving optimal outcomes may require different AF ablation strategies in different patients.
Targeting AF-sustaining re-entrant drivers is a natural ablation strategy. However, as these drivers are difficult to localize during the procedure, simulations with personalized atrial models have offered an alternative strategy—determining the re-entrant driver locations in the models prior to the procedure. McDowell et al.57, 86 were the first to demonstrate, in a four-patient proof-of-concept study, that ablation of atrial regions encompassing the meander of persistent re-entrant drivers rendered the model non-inducible for AF. The current methods for clinical intra-procedure localization of AF re-entrant drivers include focal impulse and rotor mapping (FIRM),138 local electrogram-based parameters such as spatiotemporal electrogram dispersion139 and instantaneous frequency modulation of single atrial signals,140 as well as non-invasive electrocardiographic imaging (ECGI).141 AF patients that underwent FIRM-guided (11 patients)126 or ECGI-guided (12 patients)142 re-entrant driver ablation, in addition to PVI, had a significantly higher risk for AF recurrence if the clinical ablation sites were different from those found in the atrial models, suggesting that simulation driven-targeting of re-entrant drivers for AF can play a role in the clinical procedure. However, as personalized atrial models are reconstructed from cardiac images, and cannot prospectively incorporate invasively-acquired personalized electrophysiological information, so that predictions are rendered pre-procedurally, there is uncertainty in the predicted location of re-entrant drivers. Hakim et al.87 demonstrated that this uncertainty can be substantially mitigated by repeating the AF inducibility simulations post-simulation of the initial ablation to capture and ablate emergent re-entrant drivers. Nonetheless, because of the reliance on non-invasive pre-procedural information, simulation-guided identification of AF ablation targets can at present not be applied to patients with a primarily functional substrate.
The first prospective entirely simulation-driven ablation study85 enrolled 10 patients with persistent AF, for whom personalized atrial model with fibrosis distribution were reconstructed from LGE-MRI scans. Optimal ablation targets were determined pre-procedure non-invasively via computational modelling and utilized to steer patient treatment in an approach termed optimal target identification via modelling of arrhythmogenesis (OPTIMA). The approach was designed to completely eliminate the ability of the patient’s fibrotic substrate to sustain AF: not only the clinically manifested AF but also emergent atrial arrhythmias that could arise from the fibrotic substrate, e.g. following initial ablation. OPTIMA was thus designed not only to make ablation efficacious in patients with persistent AF but also to potentially eliminate the need for repeat ablations. An example of the OPTIMA ablation target set in one patient is shown in Figure 6A, together with the procedure to import the predicted ablation targets in the electro-anatomical mapping system (Figure 6B), so that the ablation catheter can be directly navigated to the targets. The initial feasibility study evaluating the OPTIMA approach included a limited number of patients and ablation times were not evaluated. Of the 10 patients enrolled, only one returned for re-ablation during the follow-up period, with atrial flutter rather than AF. For clinical acceptance, OPTIMA’s efficacy will need to be evaluated in larger studies. A 160-patient randomized clinical study based on the OPTIMA approach has been approved (ClinicalTrials.gov identifier NCT04101539) and will establish the potential of computer modelling for the personalized management of AF.
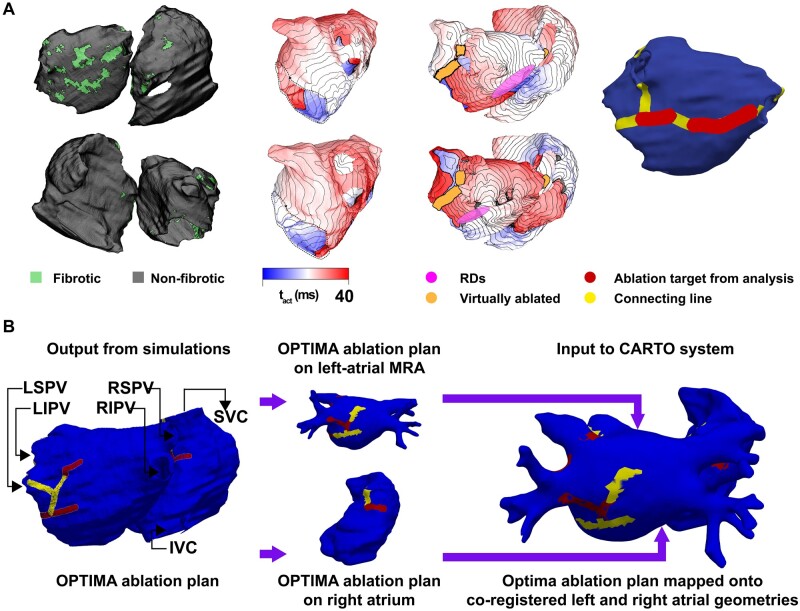
Figure 6.
Example of the process to determine the OPTIMA ablation targets in a patient with two previous failed ablations. (A) Left-most: Posterior (top) and anterior (bottom) views of the patient-specific atrial model as reconstructed from segmented LGE-MRI scans, including the distribution of fibrotic tissue. Middle-left: Two examples of AF activation sequences induced by rapid pacing, and the corresponding persistent reentrant drivers (pink). Middle-right: Two examples of activation sequences associated with arrhythmia emerging in the model following virtual ablation (lesions shown in orange), and the corresponding emergent re-entrant drivers. Right-most: Custom-tailored OPTIMA ablation treatment plan, including targets corresponding to all reentrant drivers (pre-ablation and emergent) and lesion lines connecting these target drivers to non-conductive tissue boundaries. RD, re-entrant driver. (B) Schematic summarizing the process of importing OPTIMA ablation targets into the CARTO electroanatomical navigation system. Modified with permission from Boyle et al.85
Atrial macro-reentrant tachycardias or left-atrial flutters frequently occur after AF ablation due to modifications of the substrate introduced by the ablation lesions. Patient-specific atrial modelling has been employed to determine what lesion would render atrial flutter no longer inducible in a study of patients who were successfully treated for AF via catheter ablation, but experienced post-procedure left atrial flutter.88 In a longitudinal study of 12 paroxysmal or persistent AF patients, Ali et al.89 applied LGE-MRI-based atrial computational modelling to understand the differences in AF propensity of atrial fibrotic substrates pre- and post-ablation. The study demonstrated that AF recurrence correlated with the presence of re-entrant drivers, which were either drivers unaffected by ablation, or new ones emerging post-ablation. Taken together, atrial organ-level modelling constitutes a unique tool for designing ablation strategies that optimize efficacy and minimize ablation-induced atrial pro-arrhythmia, thus providing new opportunities for improved rhythm control, as well as prediction and prevention of post-ablation recurrences.
6. Data-driven models for AF management
Traditional statistical models have significantly contributed to contemporary clinical AF management, e.g. in the prediction of stroke and bleeding risks143, 144 and are typically derived from large-scale registries and trials that investigated the relevant clinical outcomes. Statistical models have also been applied to several of the current challenges in AF management, including early AF detection,90, 91 rhythm-control management,100 and AF recurrence prediction.101 Several statistical models have combined conventional risk scores based on clinical characteristics with biomarkers to increase their predictive performance.145 Advanced statistical models have also been applied to large-scale genetic analyses, e.g. genome-wide association studies, to derive polygenic risk scores for the development of AF.92–94 Nonetheless, statistical approaches are limited in their ability to identify relevant, potentially non-linear, interactions between numerous parameters that may be required for optimal prediction of the outcome of interest in today’s large data sets. Artificial intelligence and ML may overcome this limitation.
Applications of artificial intelligence and ML in cardiac electrophysiology are emerging54, 146, 147 and are discussed in more detail in a separate review of this spotlight issue.148 In brief, the ability of ML and ‘big data’ to identify complex associations between numerous variables of interest in a data-driven, hypothesis-free approach make them attractive for identifying occult AF determinants and establishing clinical decision support systems.146 ML has been employed to predict the future AF incidence from electronic health records95, 96 and sinus rhythm ECGs,97 predict stroke risk from a daily AF burden signature,149 define AF clinical classifications based on different risks for adverse clinical outcomes,150 classify intracardiac activation patterns during AF to detect regional rotational activity,105, 106 identify patients who may benefit from AF cardioversion,107 and predict AF recurrence after the first catheter ablation procedure.102–104 These ML approaches exploited diverse pre-procedural patient characteristics as inputs, including laboratory and clinical parameters,102, 103 atrial geometry,151 and imaging data.104 Recent work has also demonstrated the feasibility of contact-free AF detection by ML-supported classification of facial pulsatile photoplethysmographic signals,98 as well as data-driven estimation of patient-specific atrial electrical remodelling patterns based on remote-monitoring technology.99 Such approaches may expand the opportunities for AF screening and subsequent monitoring in the future.
There is a growing interest in combining mechanistic and data-driven models to leverage the advantages of both approaches. In theory, data-driven approaches could directly predict the functional consequences of point mutations in ion-channel genes, which could then automatically be integrated in mechanistic models to study their impact on cardiac electrophysiology and arrhythmogenic risk. However, current experimental data sets are heavily skewed towards disease-causing variants, which limited the predictive accuracy of various ML approaches.152 Alternatively, ML may support molecular dynamics simulations to study ion-channel gating in health and disease over physiological time scales,153 thereby indirectly supporting mechanistic simulations of cardiac electrophysiology. At the organ-level, the perfect observability provided by mechanistic computer models creates unique opportunities for analysing and validating data-driven approaches to the detection of AF drivers, as has already been done for several electrogram-based biomarkers.139, 140 Such approaches may help to evaluate and optimize the specificity of driver-detection algorithms (ML-based or otherwise), potentially reducing the number of unnecessary lesions. The recurrence of AF post-PVI could be predicted pre-procedurally by conducting mechanistic simulations of AF induction in atrial models reconstructed from paroxysmal AF patients with fibrosis on LGE-MRI, and training an ML classifier on simulated AF episodes and imaging features.104 If this approach is confirmed to predict failure of PVI pre-procedurally, the patient’s ablation plan could then potentially be adjusted to include targeting of patient-specific extra-PVI areas of arrhythmogenic driver propensity using the simulation-driven OPTIMA ablation approach,85 highlighting the potential of hybrid mechanistic- and ML-based modelling approaches.
7. Health-technology assessment models
Besides mechanistic and data-driven modelling, health-technology assessment models also play an important role in AF management, particularly for cost-effectiveness analyses of AF screening56, 108 and AF therapies (e.g., AADs, anticoagulants and ablation).55, 109–111 Health-technology assessment models are typically implemented using Markov models that simulate the transition of virtual populations between different clinical states, each of which have a specific value (e.g. quality-of-life) and are associated with certain costs. The probability of individual transitions may differ for specific conditions, enabling the comparison of different therapies. For example, using a lifelong decision-analytic Markov model, cost-effectiveness of screening for asymptomatic AF was analysed and shown to be of clinical interest beyond the age of 75 years.108 Meanwhile, a Markov model to calculate the total costs and quality-adjusted life-years associated with cryoablation and AAD therapy in paroxysmal AF patients demonstrated the superiority of cryoablation over AADs.111 Some of these models have been combined with conventional statistical models (e.g., to detect asymptomatic AF154) or ML approaches. For example, a cost-effectiveness analysis of targeted screening for AF identification utilized a hybrid screening decision tree and Markov disease progression model.56 Finally, patient-level Markov models can also be used to simulate AF progression patterns in an individual patient.155
8. Major limitations hindering application of computational modelling in clinical AF management
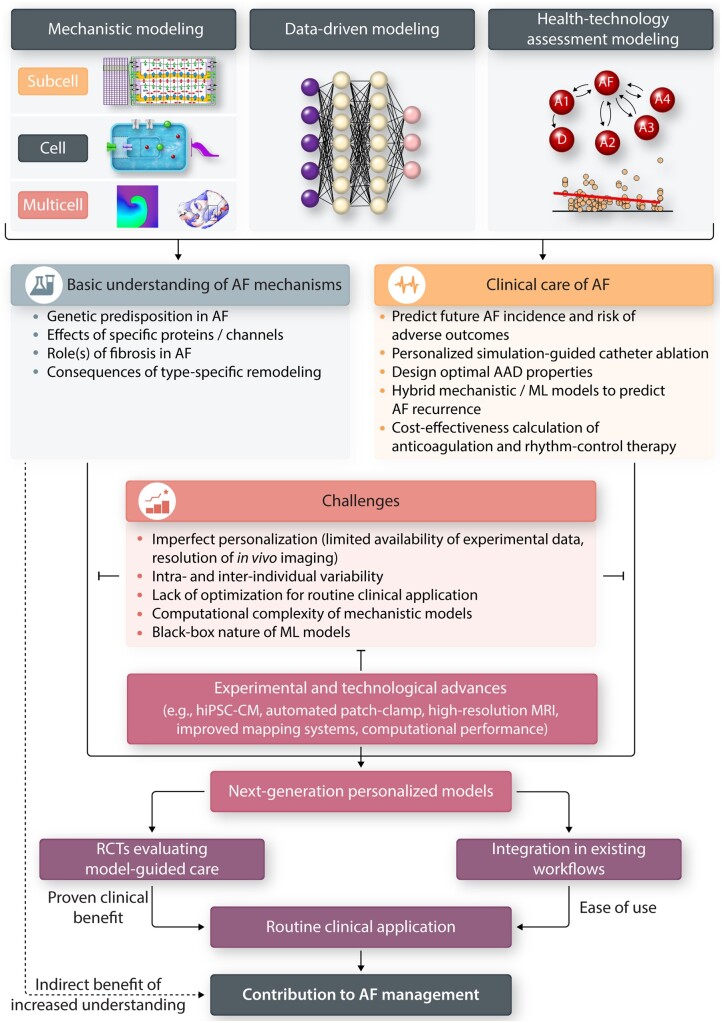
Despite the significant contributions of multiscale computational modelling of cardiac electrophysiology in improving the understanding of basic AF mechanisms, several challenges remain (summarized in Table 2 and in the red box in Figure 1) and their routine clinical application remains limited.
Table 2.
Current challenges and future perspectives of computational modelling in AF management
| No. | Challenge |
|---|---|
| 1 | Lack of personalization details (e.g. incorporation of genetic and acquired risk factors) |
| 2 | Limited availability of experimental data used to validate computational models (e.g. limited access to atrial tissues other than atrial appendage and to patient cohorts without an indication for cardiac surgery) |
| 3 | Limited pre-procedural availability of patient-specific electrophysiological information |
| 4 | Inability to image patient-specific fibre orientations |
| 5 | Limited spatial resolution of traditional MRI makes resolving the complex fibrosis patterns in the thin atrial walls challenging |
| 6 | Intra-individual heterogeneities are not fully characterized |
| 7 | Lack of cellular details in organ-level models that may be required to simulate realistic AAD effects due to high computational cost |
| 8 | Issues regarding simulation of intervening gaps, PV reconnection, focal ectopic firing and progression of the underlying substrates due to continued atrial remodelling remain unresolved |
| 9 | Complex integration with existing workflows and systems (e.g. requirement for LGE-MRI and its time-consuming segmentation, integration with electro-anatomical mapping systems) |
| 10 | ‘Black box’ characteristic of deep-learning based machine learning models |
| No. | Future perspective |
|---|---|
| 1 | Advances in experimental methodologies as well as clinical imaging modalities may provide new opportunities for model development and personalization |
| 2 | Technological innovations in combination with new approaches for model simplification are expected to provide additional computational performance, enabling simulation of more detailed mechanistic-models |
| 3 | Increased standardization and improved attention to re-usability will likely facilitate the exchange of modelling approaches and their integration into existing workflows |
| 4 | An increasing availability of large data sets and modern (explainable) machine learning models |
| 5 | Hard evidence for the clinical benefit of using computational models (e.g. RCT) will be needed to motivate their routine use |
Cellular electrophysiology models have proven useful for elucidating fundamental arrhythmia mechanisms. However, for tailoring AF therapy more detailed personalization of these models is needed. For example, accumulating evidence suggests that the antiarrhythmic efficacy of AADs is modulated by genetic and acquired risk factors.156 Although cardiomyocyte models for a few subgroups of AF patients, including paroxysmal/long-standing persistent AF and absence or presence of left-ventricular dysfunction have been developed,67, 76 more detailed personalization of cellular electrophysiology is hindered by the numerous interactions between individual risk factors producing complex atrial remodelling and the limited availability of experimental data. Cellular electrophysiological data are only available from patients undergoing open heart surgery (who may have a different risk-factor profile than typical AF patients) and are mostly restricted to the right-atrial appendage (which may not always be representative for other regions of the atria). Moreover, most experimental data were not obtained from studies with a predefined prospective design, potentially introducing a bias when post hoc defined subpopulations are used for model personalization. Due to the limited availability of human atrial samples and the challenging nature of cellular electrophysiology experiments, most currently available models still contain numerous components that have been parametrized based on data from various non-human species, which could also affect their ability to accurately reproduce arrhythmogenic mechanisms observed in patients. These and other challenges involved in translating mechanistic discoveries into clinically applicable therapies are discussed in more detail in another review in this spotlight issue.157
Similarly, although anatomical differences and patient-specific fibrosis patterns can be personalized in organ-level models based on LGE-MRI, the pre-procedural availability of electrophysiological information, which can affect model behaviour,60 is limited. As such, currently available organ-level models are also not able to predict AF ablation targets in patients with a primarily functional substrate. Furthermore, it is at present not possible to model patient-specific fibre orientations and the limited spatial resolution of traditional MRI makes resolving the complex atrial fibrosis patterns, including thin endomysial fibrosis and patches of surviving cardiomyocytes, difficult. Thus, model personalization at all spatial scales remains challenging.
Even when models can be personalized, it might be necessary to address intra-individual heterogeneities to capture uncertainty and temporal variation of electrophysiological properties. Although recent studies have integrated variability by applying population-modelling approaches, this is typically done by varying the maximum conductance of atrial ionic currents, based on in vitro experiments.128, 129 However, at present, the extent of the variability of each cellular component is unknown and co-expression of some channels, which has been recently observed,158 is not considered. Furthermore, the electrotonic coupling influencing this intrinsic variability might vary across regions of the atria (affected by cell types, number of (myo)fibroblasts and neighbouring structures). At present, it is unknown to what extent variability in atrial anatomical, structural and functional properties would influence the overall behaviour of proarrhythmic substrates, drivers and triggers.
The computational requirements associated with multiscale mechanistic modelling represent another challenge potentially hindering clinical application, particularly when variability needs to be assessed in large-scale populations of models. While early in silico models were able to simulate the cellular behaviour of cardiomyocytes by only using a limited number of parameters and state variables, these are insufficient to capture all relevant properties of cardiomyocytes. Although detailed cell models have been developed, their computational complexity precludes their incorporation in organ-level models. Recent work has proposed innovative approaches to phenomenologically reproduce the electrophysiological consequences of subcellular calcium-handling abnormalities in multiscale models, enabling investigation of the interactions between ectopic activity and re-entry.159 Nonetheless, some elements from detailed cell models may have to be sacrificed in organ-level models to retain a reasonable computational cost, which may limit their applications. For example, although simplified ionic models might be sufficient for tailored ablation therapy, evaluation of concomitant AAD effects will likely require more detailed ion-channel models that can capture state-dependent drug effects. Similarly, although organ-level computational investigations of AF recurrence following ablation therapy have provided important insight into the role of untreated and emergent re-entrant drivers,89 issues related to the simulation of potential intervening gaps, pulmonary vein reconnection, focal ectopic firing and progression of the underlying substrates due to continued atrial remodelling remain unresolved.160 Thus, more accurate representations of currently available therapies may be needed for model-guided selection of AF therapy in clinical practice.
Finally, the clinical application of personalized organ-level models requires significant multidisciplinary expertise and integration with complex existing workflows and systems, including availability of (high-resolution) LGE-MRI and integration in electro-anatomical mapping systems employed during ablation procedures. Moreover, processing of clinical data for model personalization (e.g. segmentation of atrial structures on LGE-MRI) is time consuming. Together, these challenges make routine application in the large population of AF patients across the globe challenging.
After data-driven models have been derived, their computational costs are significantly lower than those of mechanistic models and their integration in clinical workflows is more readily achieved, e.g. as clinical decision-support systems accessible through websites, apps or other digital systems. One weakness of deep learning-based ML approaches is their ‘black box’ characteristic, in which the intermediate process cannot be scrutinized. This property has traditionally hindered application of these models for clinical decision with potentially severe consequences, despite their popularity in research settings.161 However, as ML predictions are being more extensively validated in multiple independent data sets, the confidence in their clinical applicability is expected to increase.
9. Future perspectives
As summarized in the lower part of Table 2, continuing advances in experimental methodologies (e.g. induced pluripotent stem cell-derived cardiomyocytes,80 medium/high-throughput automated patch-clamping),162 as well as clinical imaging modalities (high-resolution MRI and contrast-enhanced computed tomography, advanced mapping systems)163 may provide new opportunities for model development and personalization. At the same time, technological innovations in combination with new approaches for model simplification are expected to provide additional computational performance, enabling simulation of more detailed mechanistic models.159 Increased standardization and improved attention to re-usability will likely facilitate the exchange of modelling approaches and their integration into existing workflows, in preparation for routine clinical care. The integration of ML and mechanistic modelling is expected to continue, e.g. through ML-based automated segmentation of LGE-MRI data to generate personalized models and to integrate simulation data and clinical characteristics.104 For data-driven models used directly in clinical decision-making, there is an increasing emphasis on explainable ML models.164 Explainable models may help to overcome some of the reservations towards black-box models, but, may also give a false sense of security, and inaccurate (low-fidelity) explanation models may limit trust in the explanation, and by extension, trust in the black box that it is trying to explain.165
Given the promise of recent proof-of-concept studies, but also in light of the aforementioned challenges, hard evidence for the clinical benefit of using computational models will be needed to motivate their routine use. Ideally, this evidence will come in the form of randomized controlled trials comparing model-guided care with routine clinical care. Since there are no commercial incentives to improve the use of currently available pharmacological AF therapies, and given the developmental challenges, as well as the size and costs of clinical trials required for approval of new AADs (reviewed in detail elsewhere in this spotlight issue157) it is likely that such trials will initially focus on optimizing AF ablation. In addition, AF ablation can be simulated with simpler cellular models, reducing the computational burden. Nonetheless, such trials will require personalized simulations for hundreds of patients, requiring significantly more resources than existing proof-of-concept studies. For example, assuming an AF recurrence rate of 25% with model-guided therapy instead of 35% with an alpha of 0.05 and 80% power would require a trial with 2× 328 AF patients. As such, these studies can only be initiated after the methodological and logistical challenges have been solved. However, if such studies confirm that simulation-guided AF ablation improves sinus rhythm maintenance, this may help to establish the clinical value of AF ablation in subsequent large outcome studies.
10. Conclusions
AF remains a major healthcare burden in need of better management approaches. Several challenges in AF management might benefit from computational modelling, including the detection of asymptomatic AF, improved rhythm-control management and prediction of AF recurrence. Computational modelling of atrial electrophysiology and arrhythmogenesis has advanced significantly over the last decades and initial regulatory and clinical applications are emerging (Figure 1). Nonetheless, several limitations remain, including the extent of personalization, incorporation of intra- and inter-individual variability, computational requirements, and model integration into clinical tools. These limitations have to be overcome to enable the randomized controlled trials required for routine clinical adoption of model-guided AF management (Figure 1). Finally, computational modelling has also provided important insight into AF mechanisms. The indirect contribution of this mechanistic understanding is hard to quantify, but will also facilitate improved AF management.
Conflict of interest: S.N. reports consulting fees from LQTS Therapeutics not related to this topic. The other authors have no disclosures.
Funding
This work was supported by the Netherlands Organization for Scientific Research NWO/ZonMW Vidi 09150171910029 to J.H.; by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014-9 (RACE V) to H.J.G.M.C.; by the Canadian Institutes of Health Research (148401) and the Heart and Stroke Foundation of Canada (to S.N.); by NIH grants U01HL141074, R01HL142893, and R01HL142496 to N.A.T., and grants from Leducq Foundation and the Lowenstein Foundation to N.A.T.
Data availability
This review article does not contain new original data.
This article is part of the Spotlight Issue on Atrial Fibrillation.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Sung JH, Pak HN, Lee MH, Joung B, Lip GYH. Improved population-based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (Atrial Fibrillation Better Care) pathway for integrated care management: nationwide cohort study. Thromb Haemost 2019;119:1695–1703. [DOI] [PubMed] [Google Scholar]
- 4. Pastori D, Farcomeni A, Pignatelli P, Violi F, Lip GY. ABC (Atrial fibrillation Better Care) pathway and healthcare costs in atrial fibrillation: the ATHERO-AF study. Am J Med 2019;132:856–861. [DOI] [PubMed] [Google Scholar]
- 5. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH; mAF-App II Trial Investigators. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 6. Wijtvliet E, Tieleman RG, van Gelder IC, Pluymaekers N, Rienstra M, Folkeringa RJ, Bronzwaer P, Elvan A, Elders J, Tukkie R, Luermans J, Van Asselt A, Van Kuijk SMJ, Tijssen JG, Crijns H; RACE 4 Investigators. Nurse-led vs. usual-care for atrial fibrillation. Eur Heart J 2020;41:634–641. [DOI] [PubMed] [Google Scholar]
- 7. Guo Y, Guo J, Shi X, Yao Y, Sun Y, Xia Y, Yu B, Liu T, Chen Y, Lip GYH; mAF-App II Trial investigators. Mobile health technology-supported atrial fibrillation screening and integrated care: a report from the mAFA-II trial Long-term Extension Cohort. Eur J Intern Med 2020;82:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goette A, Auricchio A, Boriani G, Braunschweig F, Terradellas JB, Burri H, Camm AJ, Crijns H, Dagres N, Deharo J-C, Dobrev D, Hatala R, Hindricks G, Hohnloser SH, Leclercq C, Lewalter T, Lip GYH, Merino JL, Mont L, Prinzen F, Proclemer A, Pürerfellner H, Savelieva I, Schilling R, Steffel J, van Gelder IC, Zeppenfeld K, Zupan I, Heidbüchel H; ESC Scientific Document Group. EHRA White Paper: knowledge gaps in arrhythmia management-status 2019. Europace 2019;21:993–994. [DOI] [PubMed] [Google Scholar]
- 9. Heijman J, Guichard JB, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ Res 2018;122:752–773. [DOI] [PubMed] [Google Scholar]
- 10. Trayanova NA. Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circ Res 2014;114:1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vagos M, van Herck IGM, Sundnes J, Arevalo HJ, Edwards AG, Koivumaki JT. Computational modeling of electrophysiology and pharmacotherapy of atrial fibrillation: recent advances and future challenges. Front Physiol 2018;9:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aronis KN, Ali RL, Liang JA, Zhou S, Trayanova NA. Understanding AF mechanisms through computational modelling and simulations. Arrhythm Electrophysiol Rev 2019;8:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutanto H, Lyon A, Lumens J, Schotten U, Dobrev D, Heijman J. Cardiomyocyte calcium handling in health and disease: insights from in vitro and in silico studies. Prog Biophys Mol Biol 2020;157:54–75. [DOI] [PubMed] [Google Scholar]
- 14. Corrado C, Avezzu A, Lee AWC, Mendoca Costa C, Roney CH, Strocchi M, Bishop M, Niederer SA. Using cardiac ionic cell models to interpret clinical data. Wiley Interdiscip Rev Syst Biol Med 2020;e1508. doi:10.1002/wsbm.1508. [DOI] [PubMed] [Google Scholar]
- 15. Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D. Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation. J Physiol 2016;594:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grandi E, Dobrev D, Heijman J. Computational modeling: What does it tell us about atrial fibrillation therapy? Int J Cardiol 2019;287:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benson AP, Stevenson-Cocks HJ, Whittaker DG, White E, Colman MA. Multi-scale approaches for the simulation of cardiac electrophysiology: II—tissue-level structure and function. Methods 2021;185:60–81. [DOI] [PubMed] [Google Scholar]
- 18. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 19. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart 2019;105:1860–1867. [DOI] [PubMed] [Google Scholar]
- 23. Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, Hendriks JM, Kalman JM, Sanders P. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J 2016;37:1573–1581. [DOI] [PubMed] [Google Scholar]
- 24. Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Kunzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial myocyte NLRP3/CaMKII Nexus forms a substrate for postoperative atrial fibrillation. Circ Res 2020;127:1036–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guichard JB, Xiong F, Qi XY, L'Heureux N, Hiram R, Xiao J, Naud P, Tardif JC, Da Costa A, Nattel S. Role of atrial arrhythmia and ventricular response in atrial fibrillation induced atrial remodelling. Cardiovasc Res 2021;117:462–471. [DOI] [PubMed] [Google Scholar]
- 26. Molina CE, Abu-Taha IH, Wang Q, Rosello-Diez E, Kamler M, Nattel S, Ravens U, Wehrens XHT, Hove-Madsen L, Heijman J, Dobrev D. Profibrotic, electrical, and calcium-handling remodeling of the atria in heart failure patients with and without atrial fibrillation. Front Physiol 2018;9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feghaly J, Zakka P, London B, MacRae CA, Refaat MM. Genetics of atrial fibrillation. J Am Heart Assoc 2018;7:e009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One 2018;13:e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones NR, Taylor CJ, Hobbs FDR, Bowman L, Casadei B. Screening for atrial fibrillation: a call for evidence. Eur Heart J 2020;41:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Force U, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:478–484. [DOI] [PubMed] [Google Scholar]
- 31. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbuchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns H, Breithardt G; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 32. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 33. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J MedMed 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 34. Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kapłon B, Kołodziej P, Achremczyk P; Investigators of the Polish How to Treat Chronic Atrial Fibrillation Study. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest 2004;126:476–486. [DOI] [PubMed] [Google Scholar]
- 35. Kumana CR, Cheung BM, Cheung GT, Ovedal T, Pederson B, Lauder IJ. Rhythm vs. rate control of atrial fibrillation meta-analysed by number needed to treat. Br J Clin Pharmacol 2005;60:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heijman J, Hohnloser SH, Camm AJ. Antiarrhythmic drugs for atrial fibrillation: Lessons from the past and opportunities for the future. Europace 2021;23:ii14–ii22. [DOI] [PubMed] [Google Scholar]
- 37. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 38. Kelly JP, DeVore AD, Wu J, Hammill BG, Sharma A, Cooper LB, Felker GM, Piccini JP, Allen LA, Heidenreich PA, Peterson ED, Yancy CW, Fonarow GC, Hernandez AF. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines-heart failure. J Am Heart Assoc 2019;8:e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, Mangat I, Khoo C, Steinberg C, Bennett MT, Tang ASL, Khairy P; For the CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–1788. [DOI] [PubMed] [Google Scholar]
- 40. Wynn GJ, El-Kadri M, Haq I, Das M, Modi S, Snowdon R, Hall M, Waktare JE, Todd DM, Gupta D. Long-term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Heart 2016;3:e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Terricabras M, Piccini JP, Verma A. Ablation of persistent atrial fibrillation: challenges and solutions. J Cardiovasc Electrophysiol 2020;31:1809–1821. [DOI] [PubMed] [Google Scholar]
- 42. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O’Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haissaguerre M, Jais P. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 43. Gaztanaga L, Frankel DS, Kohari M, Kondapalli L, Zado ES, Marchlinski FE. Time to recurrence of atrial fibrillation influences outcome following catheter ablation. Heart Rhythm 2013;10:2–9. [DOI] [PubMed] [Google Scholar]
- 44. Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, Rubulis A, Malmborg H, Raatikainen P, Lönnerholm S, Höglund N, Mörtsell D. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol 2019;12:e007414. [DOI] [PubMed] [Google Scholar]
- 46. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, Roux JF, Yung D, Skanes A, Khaykin Y, Morillo C, Jolly U, Novak P, Lockwood E, Amit G, Angaran P, Sapp J, Wardell S, Lauck S, Macle L, Verma A; EARLY-AF Investigators. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 47. Dan GA, Dobrev D. Antiarrhythmic drugs for atrial fibrillation: imminent impulses are emerging. Int J Cardiol Heart Vasc 2018;21:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang AY, Kaiser D, Ullal A, Perino AC, Heidenreich PA, Turakhia MP. Evaluating the cost-effectiveness of catheter ablation of atrial fibrillation. Arrhythm Electrophysiol Rev 2014;3:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khan AR, Khan S, Sheikh MA, Khuder S, Grubb B, Moukarbel GV. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:853–860. [DOI] [PubMed] [Google Scholar]
- 50. Markman TM, Geng Z, Epstein AE, Nazarian S, Deo R, Marchlinski FE, Groeneveld PW, Frankel DS. Trends in antiarrhythmic drug use among patients in the United States between 2004 and 2016. Circulation 2020;141:937–939. [DOI] [PubMed] [Google Scholar]
- 51. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 52. Garvanski I, Simova I, Angelkov L, Matveev M. Predictors of recurrence of AF in patients after radiofrequency ablation. Eur Cardiol 2019;14:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang PC, DeMarco KR, Aghasafari P, Jeng MT, Dawson JRD, Bekker S, Noskov SY, Yarov-Yarovoy V, Vorobyov I, Clancy CE. A computational pipeline to predict cardiotoxicity: from the atom to the rhythm. Circ Res 2020;126:947–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feeny AK, Chung MK, Madabhushi A, Attia ZI, Cikes M, Firouznia M, Friedman PA, Kalscheur MM, Kapa S, Narayan SM, Noseworthy PA, Passman RS, Perez MV, Peters NS, Piccini JP, Tarakji KG, Thomas SA, Trayanova NA, Turakhia MP, Wang PJ. Artificial intelligence and machine learning in arrhythmias and cardiac electrophysiology. Circ Arrhythm Electrophysiol 2020;13:e007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baio G. Statistical modeling for health economic evaluations. Annu Rev Stat Appl 2018;5:289–309. [Google Scholar]
- 56. Hill NR, Sandler B, Mokgokong R, Lister S, Ward T, Boyce R, Farooqui U, Gordon J. Cost-effectiveness of targeted screening for the identification of patients with atrial fibrillation: evaluation of a machine learning risk prediction algorithm. J Med Econ 2020;23:386–393. [DOI] [PubMed] [Google Scholar]
- 57. McDowell KS, Zahid S, Vadakkumpadan F, Blauer J, MacLeod RS, Trayanova NA. Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PLoS One 2015;10:e0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zahid S, Cochet H, Boyle PM, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Hocini M, Haissaguerre M, Jais P, Trayanova NA. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res 2016;110:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sutanto H, Cluitmans MJM, Dobrev D, Volders PGA, Bebarova M, Heijman J. Acute effects of alcohol on cardiac electrophysiology and arrhythmogenesis: Insights from multiscale in silico analyses. J Mol Cell Cardiol 2020;146:69–83. [DOI] [PubMed] [Google Scholar]
- 60. Deng D, Murphy MJ, Hakim JB, Franceschi WH, Zahid S, Pashakhanloo F, Trayanova NA, Boyle PM. Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate. Chaos 2017;27:093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roy A, Varela M, Aslanidi O. Image-based computational evaluation of the effects of atrial wall thickness and fibrosis on re-entrant drivers for atrial fibrillation. Front Physiol 2018;9:1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vigmond E, Pashaei A, Amraoui S, Cochet H, Hassaguerre M. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm 2016;13:1536–1543. [DOI] [PubMed] [Google Scholar]
- 63. Saha M, Roney CH, Bayer JD, Meo M, Cochet H, Dubois R, Vigmond EJ. Wavelength and fibrosis affect phase singularity locations during atrial fibrillation. Front Physiol 2018;9:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roy A, Varela M, Chubb H, MacLeod R, Hancox JC, Schaeffter T, Aslanidi O. Identifying locations of re-entrant drivers from patient-specific distribution of fibrosis in the left atrium. PLoS Comput Biol 2020;16:e1008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgan R, Colman MA, Chubb H, Seemann G, Aslanidi OV. Slow conduction in the border zones of patchy fibrosis stabilizes the drivers for atrial fibrillation: insights from multi-scale human atrial modeling. Front Physiol 2016;7:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao J, Hansen BJ, Wang Y, Csepe TA, Sul LV, Tang A, Yuan Y, Li N, Bratasz A, Powell KA, Kilic A, Mohler PJ, Janssen PML, Weiss R, Simonetti OP, Hummel JD, Fedorov VV. Three-dimensional Integrated Functional, Structural, and Computational Mapping to Define the Structural "Fingerprints" of Heart-Specific Atrial Fibrillation Drivers in Human Heart Ex Vivo. J Am Heart Assoc 2017;6:e005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chang KC, Bayer JD, Trayanova NA. Disrupted calcium release as a mechanism for atrial alternans associated with human atrial fibrillation. PLoS Comput Biol 2014;10:e1004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chang KC, Trayanova NA. Mechanisms of arrhythmogenesis related to calcium-driven alternans in a model of human atrial fibrillation. Sci Rep 2016;6:36395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sánchez C, Corrias A, Bueno-Orovio A, Davies M, Swinton J, Jacobson I, Laguna P, Pueyo E, Rodríguez B. The Na+/K+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. Am J Physiol Heart Circ Physiol 2012;302:H1146–H1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA, Narayan SM. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol 2012;5:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koivumaki JT, Seemann G, Maleckar MM, Tavi P. In silico screening of the key cellular remodeling targets in chronic atrial fibrillation. PLoS Comput Biol 2014;10:e1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabo G, Kallenbach K, Karck M, Borggrefe M, Biliczki P, Ehrlich JR, Baczko I, Lugenbiel P, Schweizer PA, Donner BC, Katus HA, Dobrev D, Thomas D. Upregulation of K3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 2015;132:82–92. [DOI] [PubMed] [Google Scholar]
- 75. Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res 1999;42:477–489. [DOI] [PubMed] [Google Scholar]
- 76. Schmidt C, Wiedmann F, Zhou XB, Heijman J, Voigt N, Ratte A, Lang S, Kallenberger SM, Campana C, Weymann A, De Simone R, Szabo G, Ruhparwar A, Kallenbach K, Karck M, Ehrlich JR, Baczko I, Borggrefe M, Ravens U, Dobrev D, Katus HA, Thomas D. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy. Eur Heart J 2017;38:1764–1774. [DOI] [PubMed] [Google Scholar]
- 77. Aguilar M, Xiong F, Qi XY, Comtois P, Nattel S. Potassium channel blockade enhances atrial fibrillation-selective antiarrhythmic effects of optimized state-dependent sodium channel blockade. Circulation 2015;132:2203–2211. [DOI] [PubMed] [Google Scholar]
- 78. Ellinwood N, Dobrev D, Morotti S, Grandi E. In silico assessment of efficacy and safety of IKur inhibitors in chronic atrial fibrillation: role of kinetics and state-dependence of drug binding. Front Pharmacol 2017;8:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Whittaker DG, Hancox JC, Zhang H. In silico assessment of pharmacotherapy for human atrial patho-electrophysiology associated with hERG-linked short QT syndrome. Front Physiol 2018;9:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li Z, Mirams GR, Yoshinaga T, Ridder BJ, Han X, Chen JE, Stockbridge NL, Wisialowski TA, Damiano B, Severi S, Morissette P, Kowey PR, Holbrook M, Smith G, Rasmusson RL, Liu M, Song Z, Qu Z, Leishman DJ, Steidl-Nichols J, Rodriguez B, Bueno-Orovio A, Zhou X, Passini E, Edwards AG, Morotti S, Ni H, Grandi E, Clancy CE, Vandenberg J, Hill A, Nakamura M, Singer T, Polonchuk L, Greiter-Wilke A, Wang K, Nave S, Fullerton A, Sobie EA, Paci M, Musuamba Tshinanu F, Strauss DG. General principles for the validation of proarrhythmia risk prediction models: an extension of the CiPA in silico strategy. Clin Pharmacol Ther 2020;107:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hwang M, Kwon SS, Wi J, Park M, Lee HS, Park JS, Lee YS, Shim EB, Pak HN. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: comparison with clinical catheter ablation. Prog Biophys Mol Biol 2014;116:40–47. [DOI] [PubMed] [Google Scholar]
- 82. Shim J, Hwang M, Song JS, Lim B, Kim TH, Joung B, Kim SH, Oh YS, Nam GB, On YK, Oh S, Kim YH, Pak HN. Virtual in-silico modeling guided catheter ablation predicts effective linear ablation lesion set for longstanding persistent atrial fibrillation: multicenter prospective randomized study. Front Physiol 2017;8:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bayer JD, Roney CH, Pashaei A, Jais P, Vigmond EJ. Novel radiofrequency ablation strategies for terminating atrial fibrillation in the left atrium: a simulation study. Front Physiol 2016;7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roney CH, Beach ML, Mehta AM, Sim I, Corrado C, Bendikas R, Solis-Lemus JA, Razeghi O, Whitaker J, O'Neill L, Plank G, Vigmond E, Williams SE, O'Neill MD, Niederer SA. In silico comparison of left atrial ablation techniques that target the anatomical, structural, and electrical substrates of atrial fibrillation. Front Physiol 2020;11:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH, Hakim JB, Murphy MJ, Prakosa A, Zimmerman SL, Ashikaga H, Marine JE, Kolandaivelu A, Nazarian S, Spragg DD, Calkins H, Trayanova NA. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, MacLeod RS, Trayanova NA. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J Electrocardiol 2012;45:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hakim JB, Murphy MJ, Trayanova NA, Boyle PM. Arrhythmia dynamics in computational models of the atria following virtual ablation of re-entrant drivers. Europace 2018;20:iii45–iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zahid S, Whyte KN, Schwarz EL, Blake RC 3rd, Boyle PM, Chrispin J, Prakosa A, Ipek EG, Pashakhanloo F, Halperin HR, Calkins H, Berger RD, Nazarian S, Trayanova NA. Feasibility of using patient-specific models and the "minimum cut" algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm 2016;13:1687–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ali RL, Hakim JB, Boyle PM, Zahid S, Sivasambu B, Marine JE, Calkins H, Trayanova NA, Spragg DD. Arrhythmogenic propensity of the fibrotic substrate after atrial fibrillation ablation: a longitudinal study using magnetic resonance imaging-based atrial models. Cardiovasc Res 2019;115:1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schmidt C, Benda S, Kraft P, Wiedmann F, Pleger S, Buscher A, Thomas D, Wachter R, Schmid C, Eils R, Katus HA, Kallenberger SM. Prospective multicentric validation of a novel prediction model for paroxysmal atrial fibrillation. Clin Res Cardiol 2020; doi:10.1007/s00392-020-01773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Himmelreich JCL, Veelers L, Lucassen WAM, Schnabel RB, Rienstra M, van Weert H, Harskamp RE. Prediction models for atrial fibrillation applicable in the community: a systematic review and meta-analysis. Europace 2020;22:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huang HD, Darbar D. Genetic risk scores for atrial fibrillation: do they improve risk estimation? Can J Cardiol 2017;33:422–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Choi SH, Jurgens SJ, Weng LC, Pirruccello JP, Roselli C, Chaffin M, Lee CJ, Hall AW, Khera AV, Lunetta KL, Lubitz SA, Ellinor PT. Monogenic and polygenic contributions to atrial fibrillation risk: results from a National Biobank. Circ Res 2020;126:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Okubo Y, Nakano Y, Ochi H, Onohara Y, Tokuyama T, Motoda C, Amioka M, Hironobe N, Okamura S, Ikeuchi Y, Miyauchi S, Chayama K, Kihara Y. Predicting atrial fibrillation using a combination of genetic risk score and clinical risk factors. Heart Rhythm 2020;17:699–705. [DOI] [PubMed] [Google Scholar]
- 95. Tiwari P, Colborn KL, Smith DE, Xing F, Ghosh D, Rosenberg MA. Assessment of a machine learning model applied to harmonized electronic health record data for the prediction of incident atrial fibrillation. JAMA Netw Open 2020;3:e1919396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hill NR, Ayoubkhani D, McEwan P, Sugrue DM, Farooqui U, Lister S, Lumley M, Bakhai A, Cohen AT, O'Neill M, Clifton D, Gordon J. Predicting atrial fibrillation in primary care using machine learning. PLoS One 2019;14:e0224582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S, Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 98. Yan BP, Lai WHS, Chan CKY, Chan SC, Chan LH, Lam KM, Lau HW, Ng CM, Tai LY, Yip KW, To OTL, Freedman B, Poh YC, Poh MZ. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lillo-Castellano JM, González-Ferrer JJ, Marina-Breysse M, Martínez-Ferrer JB, Pérez-Álvarez L, Alzueta J, Martínez JG, Rodríguez A, Rodríguez-Pérez JC, Anguera I, Viñolas X, García-Alberola A, Quintanilla JG, Alfonso-Almazán JM, García J, Borrego L, Cañadas-Godoy V, Pérez-Castellano N, Pérez-Villacastín J, Jiménez-Díaz J, Jalife J, Filgueiras-Rama D. Personalized monitoring of electrical remodelling during atrial fibrillation progression via remote transmissions from implantable devices. Europace 2020;22:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Niederdockl J, Simon A, Cacioppo F, Buchtele N, Merrelaar A, Schutz N, Schnaubelt S, Spiel AO, Roth D, Schorgenhofer C, Herkner H, Domanovits H, Schwameis M. Predicting spontaneous conversion to sinus rhythm in symptomatic atrial fibrillation: the ReSinus score. Eur J Intern Med 2021;83:45–53. [DOI] [PubMed] [Google Scholar]
- 101. Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz L, Bayliss S, Kotecha D, Deeks JJ, Kirchhof P, Takwoingi Y. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. Europace 2020;22:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Budzianowski J, Hiczkiewicz J, Burchardt P, Pieszko K, Rzeźniczak J, Budzianowski P, Korybalska K. Predictors of atrial fibrillation early recurrence following cryoballoon ablation of pulmonary veins using statistical assessment and machine learning algorithms. Heart Vessels 2019;34:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Furui K, Morishima I, Morita Y, Kanzaki Y, Takagi K, Yoshida R, Nagai H, Watanabe N, Yoshioka N, Yamauchi R, Tsuboi H, Murohara T. Predicting long-term freedom from atrial fibrillation after catheter ablation by a machine learning algorithm: validation of the CAAP-AF score. J Arrhythm 2020;36:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shade JK, Ali RL, Basile D, Popescu D, Akhtar T, Marine JE, Spragg DD, Calkins H, Trayanova NA. Preprocedure application of machine learning and mechanistic simulations predicts likelihood of paroxysmal atrial fibrillation recurrence following pulmonary vein isolation. Circ Arrhythm Electrophysiol 2020;13:e008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alhusseini MI, Abuzaid F, Rogers AJ, Zaman JAB, Baykaner T, Clopton P, Bailis P, Zaharia M, Wang PJ, Rappel WJ, Narayan SM. Machine learning to classify intracardiac electrical patterns during atrial fibrillation: machine learning of atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zolotarev AM, Hansen BJ, Ivanova EA, Helfrich KM, Li N, Janssen PML, Mohler PJ, Mokadam NA, Whitson BA, Fedorov MV, Hummel JD, Dylov DV, Fedorov VV. Optical mapping-validated machine learning improves atrial fibrillation driver detection by multi-electrode mapping. Circ Arrhythm Electrophysiol 2020;13:e008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vinter N, Frederiksen AS, Albertsen AE, Lip GYH, Fenger-Grøn M, Trinquart L, Frost L, Møller DS. Role for machine learning in sex-specific prediction of successful electrical cardioversion in atrial fibrillation? Open Heart. 2020;7:e001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, Frykman-Kull V, Levin LA. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–1029. [DOI] [PubMed] [Google Scholar]
- 109. Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLoS One 2012;7:e47473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Verhoef TI, Redekop WK, Hasrat F, de Boer A, Maitland-van der Zee AH. Cost effectiveness of new oral anticoagulants for stroke prevention in patients with atrial fibrillation in two different European healthcare settings. Am J Cardiovasc Drugs 2014;14:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reynolds MR, Lamotte M, Todd D, Khaykin Y, Eggington S, Tsintzos S, Klein G. Cost-effectiveness of cryoballoon ablation for the management of paroxysmal atrial fibrillation. Europace 2014;16:652–659. [DOI] [PubMed] [Google Scholar]
- 112. Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 1952;117:500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Noble D. A modification of the Hodgkin–Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J Physiol 1962;160:317–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol 1977;268:177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. DiFrancesco D, Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci 1985;307:353–398. [DOI] [PubMed] [Google Scholar]
- 116. Hilgemann DW, Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci 1987;230:163–205. [DOI] [PubMed] [Google Scholar]
- 117. Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res 1998;82:63–81. [DOI] [PubMed] [Google Scholar]
- 118. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol 1998;275:H301–321. [DOI] [PubMed] [Google Scholar]
- 119. Maleckar MM, Greenstein JL, Trayanova NA, Giles WR. Mathematical simulations of ligand-gated and cell-type specific effects on the action potential of human atrium. Prog Biophys Mol Biol 2008;98:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 2011;109:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sutanto H, van Sloun B, Schonleitner P, van Zandvoort M, Antoons G, Heijman J. The subcellular distribution of ryanodine receptors and L-Type Ca2+ channels modulates Ca2+-transient properties and spontaneous Ca2+-release events in atrial cardiomyocytes. Front Physiol 2018;9:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Koivumaki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS Comput Biol 2011;7:e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sanchez C, Bueno-Orovio A, Wettwer E, Loose S, Simon J, Ravens U, Pueyo E, Rodriguez B. Inter-subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation. PLoS One 2014;9:e105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–220. [DOI] [PubMed] [Google Scholar]
- 125. Vigmond EJ, Ruckdeschel R, Trayanova N. Reentry in a morphologically realistic atrial model. J Cardiovasc Electrophysiol 2001;12:1046–1054. [DOI] [PubMed] [Google Scholar]
- 126. Boyle PM, Hakim JB, Zahid S, Franceschi WH, Murphy MJ, Prakosa A, Aronis KN, Zghaib T, Balouch M, Ipek EG, Chrispin J, Berger RD, Ashikaga H, Marine JE, Calkins H, Nazarian S, Spragg DD, Trayanova NA. The fibrotic substrate in persistent atrial fibrillation patients: comparison between predictions from computational modeling and measurements from focal impulse and rotor mapping. Front Physiol 2018;9:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hansen BJ, Zhao J, Helfrich KM, Li N, Iancau A, Zolotarev AM, Zakharkin SO, Kalyanasundaram A, Subr M, Dastagir N, Sharma R, Artiga EJ, Salgia N, Houmsse MM, Kahaly O, Janssen PML, Mohler PJ, Mokadam NA, Whitson BA, Afzal MR, Simonetti OP, Hummel JD, Fedorov VV. Unmasking arrhythmogenic hubs of reentry driving persistent atrial fibrillation for patient-specific treatment. J Am Heart Assoc 2020;9:e017789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muszkiewicz A, Britton OJ, Gemmell P, Passini E, Sanchez C, Zhou X, Carusi A, Quinn TA, Burrage K, Bueno-Orovio A, Rodriguez B. Variability in cardiac electrophysiology: using experimentally-calibrated populations of models to move beyond the single virtual physiological human paradigm. Prog Biophys Mol Biol 2016;120:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lawson BAJ, Drovandi CC, Cusimano N, Burrage P, Rodriguez B, Burrage K. Unlocking data sets by calibrating populations of models to data density: a study in atrial electrophysiology. Sci Adv 2018;4:e1701676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shunmugam SR, Sugihara C, Freemantle N, Round P, Furniss S, Sulke N. A double-blind, randomised, placebo-controlled, cross-over study assessing the use of XEN-D0103 in patients with paroxysmal atrial fibrillation and implanted pacemakers allowing continuous beat-to-beat monitoring of drug efficacy. J Interv Card Electrophysiol 2018;51:191–197. [DOI] [PubMed] [Google Scholar]
- 131. Fastl TE, Tobon-Gomez C, Crozier A, Whitaker J, Rajani R, McCarthy KP, Sanchez-Quintana D, Ho SY, O'Neill MD, Plank G, Bishop MJ, Niederer SA. Personalized computational modeling of left atrial geometry and transmural myofiber architecture. Med Image Anal 2018;47:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Krueger MW, Rhode KS, O'Neill MD, Rinaldi CA, Gill J, Razavi R, Seemann G, Doessel O. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. J Electrocardiol 2014;47:324–328. [DOI] [PubMed] [Google Scholar]
- 133. Corrado C, Williams S, Karim R, Plank G, O'Neill M, Niederer S. A work flow to build and validate patient specific left atrium electrophysiology models from catheter measurements. Med Image Anal 2018;47:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pashakhanloo F, Herzka DA, Ashikaga H, Mori S, Gai N, Bluemke DA, Trayanova NA, McVeigh ER. Myofiber architecture of the human atria as revealed by submillimeter diffusion tensor imaging. Circ Arrhythm Electrophysiol 2016;9:e004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Aronis KN, Ali R, Trayanova NA. The role of personalized atrial modeling in understanding atrial fibrillation mechanisms and improving treatment. Int J Cardiol 2019;287:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roney CH, Cantwell CD, Qureshi NA, Chowdhury RA, Dupont E, Lim PB, Vigmond EJ, Tweedy JH, Ng FS, Peters NS. Rotor tracking using phase of electrograms recorded during atrial fibrillation. Ann Biomed Eng 2017;45:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Boyle PM, Zahid S, Trayanova NA. Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia. Europace 2016;18:iv136–iv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Seitz J, Bars C, Theodore G, Beurtheret S, Lellouche N, Bremondy M, Ferracci A, Faure J, Penaranda G, Yamazaki M, Avula UM, Curel L, Siame S, Berenfeld O, Pisapia A, Kalifa J. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol 2017;69:303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Quintanilla JG, Alfonso-Almazán JM, Pérez-Castellano N, Pandit SV, Jalife J, Pérez-Villacastín J, Filgueiras-Rama D. Instantaneous amplitude and frequency modulations detect the footprint of rotational activity and reveal stable driver regions as targets for persistent atrial fibrillation ablation. Circ Res 2019;125:609–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–538. [DOI] [PubMed] [Google Scholar]
- 142. Boyle PM, Hakim JB, Zahid S, Franceschi WH, Murphy MJ, Vigmond EJ, Dubois R, Haissaguerre M, Hocini M, Jais P, Trayanova NA, Cochet H. Comparing reentrant drivers predicted by image-based computational modeling and mapped by electrocardiographic imaging in persistent atrial fibrillation. Front Physiol 2018;9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 144. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 145. Rivera-Caravaca JM, Marín F, Vilchez JA, Gálvez J, Esteve-Pastor MA, Vicente V, Lip GYH, Roldán V. Refining stroke and bleeding prediction in atrial fibrillation by adding consecutive biomarkers to clinical risk scores. Stroke 2019;50:1372–1379. [DOI] [PubMed] [Google Scholar]
- 146. Wang QC, Wang ZY. Big data and atrial fibrillation: current understanding and new opportunities. J Cardiovasc Transl Res 2020;13(6):944–952. [DOI] [PubMed] [Google Scholar]
- 147. Trayanova NA, Popescu DM, Shade JK. Machine learning in arrhythmia and electrophysiology. Circ Res 2021;128:544–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lip GY. Artificial intelligence applications to improve AF management. Cardiovasc Res 2021. [Google Scholar]
- 149. Han L, Askari M, Altman RB, Schmitt SK, Fan J, Bentley JP, Narayan SM, Turakhia MP. Atrial fibrillation burden signature and near-term prediction of stroke: a machine learning analysis. Circ Cardiovasc Qual Outcomes 2019;12:e005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Inohara T, Shrader P, Pieper K, Blanco RG, Thomas L, Singer DE, Freeman JV, Allen LA, Fonarow GC, Gersh B, Ezekowitz MD, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Steinberg BA, Peterson ED, Piccini JP. Association of atrial fibrillation clinical phenotypes with treatment patterns and outcomes: a multicenter registry study. JAMA Cardiol 2018;3:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Varela M, Bisbal F, Zacur E, Berruezo A, Aslanidi OV, Mont L, Lamata P. Novel computational analysis of left atrial anatomy improves prediction of atrial fibrillation recurrence after ablation. Front Physiol 2017;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Clerx M, Heijman J, Collins P, Volders PGA. Predicting changes to INa from missense mutations in human SCN5A. Sci Rep 2018;8:12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ramasubramanian S, Rudy Y. The structural basis of IKs ion-channel activation: mechanistic insights from molecular simulations. Biophys J 2018;114:2584–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Quer G, Freedman B, Steinhubl SR. Screening for atrial fibrillation: predicted sensitivity of short, intermittent electrocardiogram recordings in an asymptomatic at-risk population. Europace 2020;22(12):1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chang ET, Lin YT, Galla T, Clayton RH, Eatock J. A stochastic individual-based model of the progression of atrial fibrillation in individuals and populations. PLoS One 2016;11:e0152349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Goyal SK, Wang L, Upender R, Darbar D, Monahan K. Severity of obstructive sleep apnea influences the effect of genotype on response to anti-arrhythmic drug therapy for atrial fibrillation. J Clin Sleep Med 2014;10:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nattel S, Sager P, Huser J, Heijman J, Dobrev D. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc Res 2021;117:1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ballouz S, Mangala MM, Perry MD, Heitmann S, Gillis JA, Hill AP, Vandenberg JI. Co-expression of calcium and hERG potassium channels reduces the incidence of proarrhythmic events. Cardiovasc Res 2020; doi:10.1093/cvr/cvaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Colman MA. Arrhythmia mechanisms and spontaneous calcium release: Bi-directional coupling between re-entrant and focal excitation. PLoS Comput Biol 2019;15:e1007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nattel S. Computational models of the atrial fibrillation substrate: can they explain post-ablation recurrences and help to prevent them. Cardiovasc Res 2019;115:1681–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Watson DS, Krutzinna J, Bruce IN, Griffiths CE, McInnes IB, Barnes MR, Floridi L. Clinical applications of machine learning algorithms: beyond the black box. BMJ 2019;364:l886. [DOI] [PubMed] [Google Scholar]
- 162. Obergrussberger A, Friis S, Bruggemann A, Fertig N. Automated patch clamp in drug discovery: major breakthroughs and innovation in the last decade. Expert Opin Drug Discov 2021;16:1–5. [DOI] [PubMed] [Google Scholar]
- 163. Hansen BJ, Zhao J, Fedorov VV. Fibrosis and atrial fibrillation: computerized and optical mapping; a view into the human atria at submillimeter resolution. JACC Clin Electrophysiol 2017;3:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zihni E, Madai VI, Livne M, Galinovic I, Khalil AA, Fiebach JB, Frey D. Opening the black box of artificial intelligence for clinical decision support: a study predicting stroke outcome. PLoS One 2020;15:e0231166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell 2019;1:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review article does not contain new original data.