Abstract
The cardiac autonomic nervous system (ANS) plays an integral role in normal cardiac physiology as well as in disease states that cause cardiac arrhythmias. The cardiac ANS, comprised of a complex neural hierarchy in a nested series of interacting feedback loops, regulates atrial electrophysiology and is itself susceptible to remodelling by atrial rhythm. In light of the challenges of treating atrial fibrillation (AF) with conventional pharmacologic and myoablative techniques, increasingly interest has begun to focus on targeting the cardiac neuraxis for AF. Strong evidence from animal models and clinical patients demonstrates that parasympathetic and sympathetic activity within this neuraxis may trigger AF, and the ANS may either induce atrial remodelling or undergo remodelling itself to serve as a substrate for AF. Multiple nexus points within the cardiac neuraxis are therapeutic targets, and neuroablative and neuromodulatory therapies for AF include ganglionated plexus ablation, epicardial botulinum toxin injection, vagal nerve (tragus) stimulation, renal denervation, stellate ganglion block/resection, baroreceptor activation therapy, and spinal cord stimulation. Pre-clinical and clinical studies on these modalities have had promising results and are reviewed here.
Keywords: Atrial fibrillation, Autonomic nervous system, Neurocardiology, Neuromodulation, Vagus nerve
1. Introduction
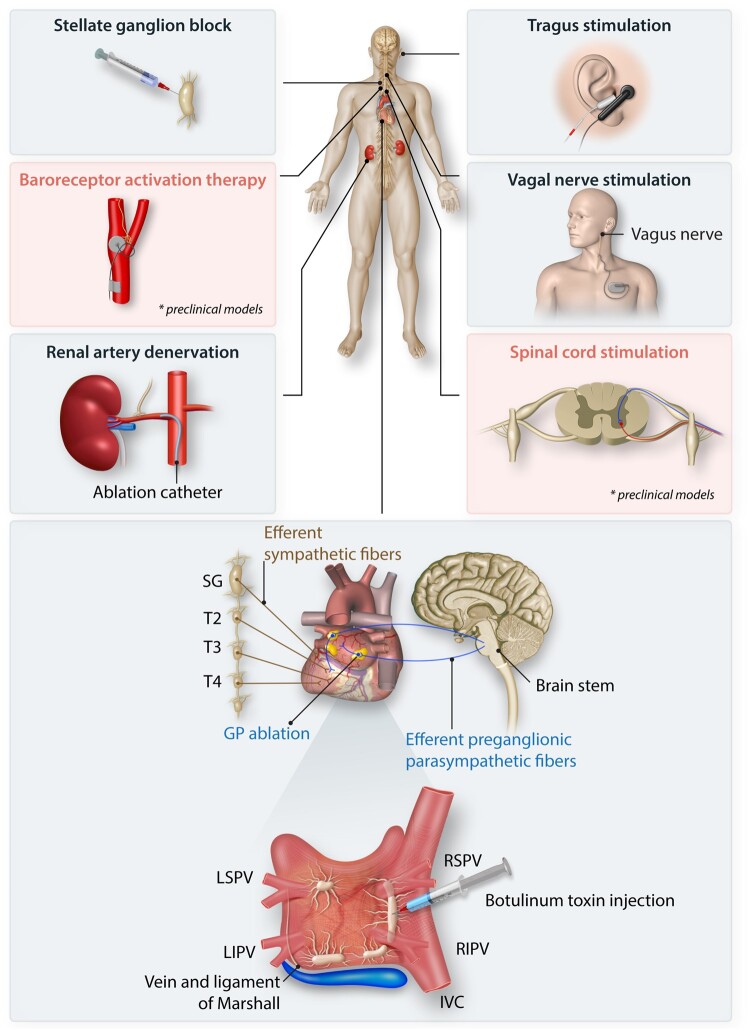
The cardiac autonomic nervous system (ANS) is integral to normal cardiac physiology to maintain electrical activity and mechanical contraction, and its dysfunction is involved in the pathogenesis of cardiovascular diseases including arrhythmias, such as atrial fibrillation (AF).1 Given the variable success of pharmacologic and catheter-based treatments for AF and the supporting evidence of the role of the cardiac ANS in AF,2–4 attention has turned to interventions, both neuroablative and neuromodulatory, targeting the ANS. These therapeutic strategies include ganglionated plexus (GP) ablation, epicardial botulinum toxin injection, vagal nerve (tragus) stimulation, renal denervation, stellate ganglion block, baroreceptor activation therapy, and spinal cord stimulation. Although some of these modalities have been assessed in heart failure and have had mixed results, several are at various stages of development and translation to the clinical setting for AF. In this review, we explore the supporting evidence for the cardiac ANS in AF and autonomic interventions that have been evaluated for AF.
2. Cardiac neuroanatomy—defining targets for neuromodulation
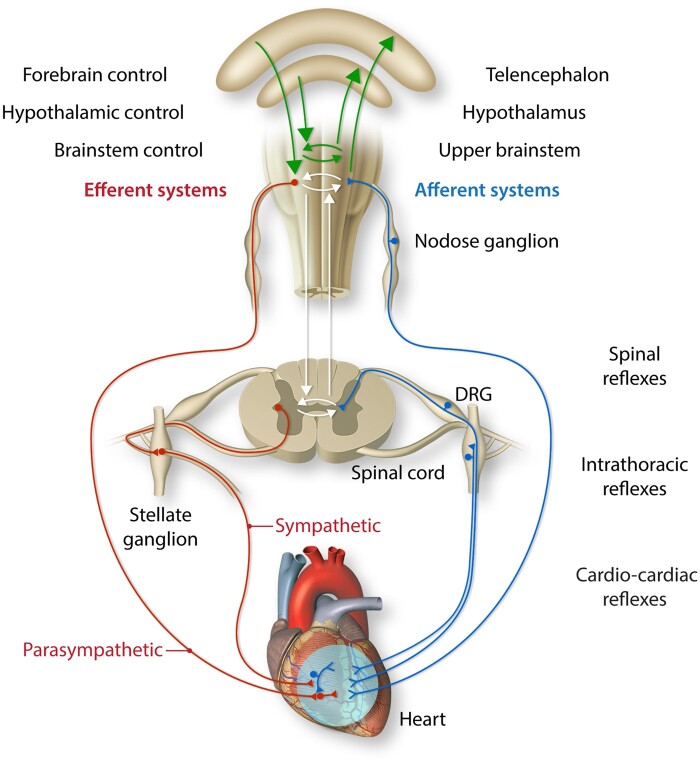
The cardiac ANS has been studied across mammals and appears quite conserved in humans.5 The cardiac neural hierarchy is composed of the central nervous system (brainstem and spinal cord); extrinsic intrathoracic ganglia (e.g. stellate ganglia); and the intrinsic cardiac nervous system (ICNS) (Figure 1).5 Beat-to-beat information is sensed by cardiac afferents expressing chemo- and mechanoreceptors and transduced to different levels of the cardiac ANS via the vagal nerve to the nodose ganglia and sympathetic fibres to the dorsal root ganglia. These afferent signals are processed across different levels within the neuraxis in a series of interacting feedback loops to modulate efferent outflow in the form of parasympathetic output via the vagus nerve and sympathetic output via the intrathoracic extracardiac ganglia. To maintain volume homeostasis, cardiopulmonary receptors in the atria, ventricles, and pulmonary vessels provide inputs to modulate sympathovagal balance. Systemic blood pressure is sensed by baroreceptors in the carotid arteries and large vessels, and activation of the baroreceptor reflex triggers changes to sympathovagal balance of the heart and vasculature. Oxygen sensing via chemoreceptors in the circulation (carotid body and aortic arch) additionally mediates autonomic circuitry controlling heart rate.
Figure 1.
Cardiac ANS. The cardiac ANS is composed of afferent and efferent (sympathetic and parasympathetic) components that interact at multiple levels within the hierarchy that spans the heart, intrathoracic extracardiac ganglia, and central nervous system (brain and spinal cord). DRG indicates dorsal root ganglion. Adapted from Shivkumar et al., 2016.
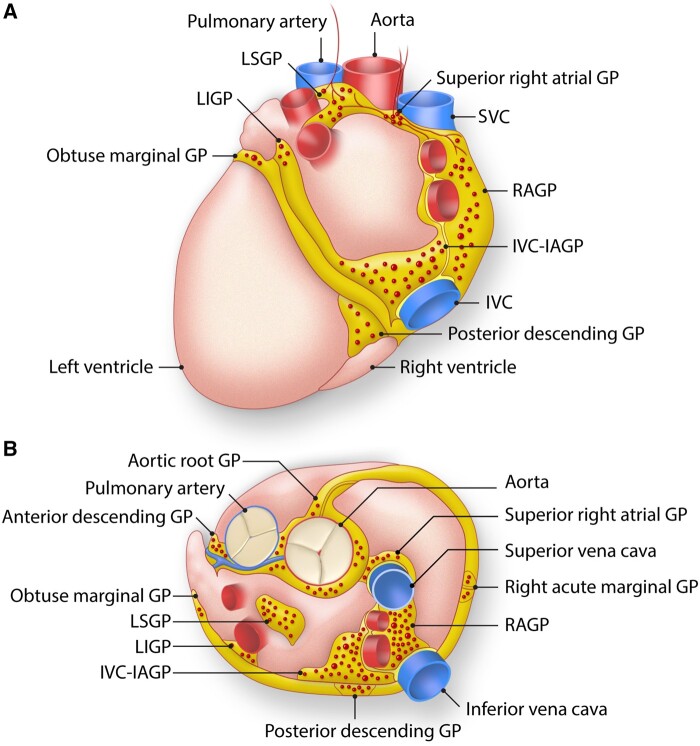
At the level of the heart, the ICNS consists of GPs embedded in epicardial fat pads and in cardiac muscle, primarily at the posterior aspect of the atria (Figure 2).6,7 Within these GPs are autonomic ganglia that contain afferent, efferent (parasympathetic and sympathetic, as well as peptidergic, neurons), and interconnecting, local circuit neurons. While the local circuit neurons compose the majority of intrinsic cardiac neurons, a significant proportion are post-ganglionic parasympathetic neurons.8 The GPs serve as integration centers for afferent information of sensed stimuli and efferent outflow to regulate cardiac electrical and contractile function and vasomotor tone via cardio-cardiac reflexes. They are mainly found circumferentially around the pulmonary veins (PVs) as they enter the posterior left atrium. The five major GPs are variably named in the literature and include the right atrial GP (RAGP), inferior vena cava-inferior atrium GP (IVC-IAGP), left inferior GP, left superior GP, and ligament of Marshall tract. PV isolation (PVI) may inadvertently cause damage to some of these GPs, particularly the left superior GP, left inferior GP, RAGP, and Marshall tract GP, that harbour neuronal cell bodies and/or their axons9 resulting in changes to cardiac autonomic control.10–12 Several studies have demonstrated an acute increase in average heart rate following PVI to suggest partial vagal denervation that generally persists at up to 1-year follow-up and is associated with decreased risk of AF recurrence.11,13–23 PVI-induced release of S100B from ablation may also modulate cardiac ganglia and result in reduction of AF recurrence.24 The functional effects of GPs based on anatomic distribution has been evaluated in patients, and the GPs may be subdivided into two groups: those that cause atrioventricular (AV) dissociation and those that cause atrial ectopy.25–27 Identification of these functional types and enrichment of ectopy-triggering GPs at the left atrial roof and around PVs further supports the notion that the benefits of PVI may be due to non-specific and unintentional destruction of GPs.
Figure 2.
ICNS. The ICNS is composed of ganglionated plexi that cluster at the hilum of the heart, close to where the PVs enter the left atrium. Anatomy of the ganglionated plexuses (GPs) that comprise the intrinsic cardiac nervous system. These GPs are typically found on the posterior (A) and superior (B) epicardial surfaces of the heart. IVC indicates inferior vena cava; IVC-IAGP, inferior vena cava-inferior atrial ganglionated plexus; LIGP, left inferior ganglionated plexus; LSGP, left superior ganglionated plexus; RAGP, right atrial ganglionated plexus; SVC, superior vena cava. Adapted from Rajendran et al., 2017.
3. The role of cardiac ANS in AF
While the aetiology of AF, the most common clinical arrhythmia, is often considered multifactorial, aberrations in autonomic tone based on heart rate variability analysis have been implicated in paroxysmal and post-operative AF (POAF).28–30 Coumel described a case series in which autonomic tone was correlated with AF.31 The Euro Heart Survey found that of 1517 patients with paroxysmal AF, 33% had autonomic triggers.32 Sympathetic and parasympathetic coactivation through the cold face test unmasked altered sinus nodal control and, in some patients, induced atrial ectopy.12 Vagally mediated AF is more common in men compared to women (30–50-year-old age group compared to older age groups), and typically occurs in the absence of structural heart disease. Paroxysms of AF occur predominantly at night, post-prandially and during relaxation, especially following physical or emotional stress. In contrast, adrenergically induced AF is most recognized in rarer endocrinopathies, such as hyperthyroidism and pheochromocytoma. More often, the role of the sympathetic nervous system is implied by clinical history, such as episodes of AF occurring during times of stress or intensive exercise. While identifying the autonomic triggers of AF has the potential for tailored treatment and lifestyle modification, limited evidence exists for this approach.33 Previous versions of the European Society of Cardiology Guidelines on management of AF recommend disopyramide for vagal AF and β blockers, sotalol and dronedarone for adrenergically mediated AF, but only the recommendation for disopyramide (Class IIb, Level of evidence B) remains in the most recent guidelines.34,35 The AHA/ACC Guidelines on the management of AF do not recommend specific anti-arrhythmic therapies based on autonomic triggers.36 Furthermore, catheter ablation targeting GPs mediating a vagal reflex only successfully mitigated AF in 2 of 7 patients in one series.37 This highlights that while the contributions of the parasympathetic and sympathetic limbs of the cardiac ANS are generally somewhat oversimplified as opposing forces, they interact in a complex and dynamic manner across multiple levels of the cardiac neuraxis in AF.38,39 To clarify the rationale for current clinical studies on autonomic modulation in AF, we first review the pre-clinical models and human data that have provided supporting evidence for these lines of investigation (Figure 3).
Figure 3.
Neuroscientific interventions for AF. Multiple approaches to neuroscientific therapies for AF have been developed at multiple levels of the cardiac neuraxis and include ganglionated plexus (GP) ablation; botulinum toxin injection in the GPs; vagal nerve and tragus stimulation; renal artery denervation; stellate ganglionic blockade; spinal cord stimulation; and baroreceptor activation therapy. Baroreceptor activation therapy and spinal cord stimulation have only been evaluated in preclinical models. IVC indicates inferior vena cava; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RSPV, right superior pulmonary vein. Adapted from Zhu et al., 2019.
3.1 Neural control of the atria and its role in electrical remodelling
The ANS is involved in the electrical remodelling that is characteristic of the initiation and maintenance of AF, as suggested following electrical or pharmacological stimulation of the cardiac ANS in pre-clinical models. In animal models, parasympathetic stimulation increases susceptibility to AF. Vagal nerve stimulation (VNS) in canines, at levels that cause significant bradycardia, increases the likelihood that a premature stimulus will initiate AF as well as the duration of AF episodes.4 Pharmacologic stimulation via injection of cholinergic agents into epicardial fat pads in a canine model causes heart rate slowing, spontaneous premature depolarization, and AF.40,41 Furthermore, in canine, application of atropine mitigated the pro-fibrillatory effect of high frequency stimulation (HFS), implicating the parasympathetic nervous system as predominating autonomic influences on AF. Acetylcholine administration and VNS have been shown to shorten the atrial effective refractory period (AERP) and action potential duration (APD) while increasing AERP dispersion, providing a substrate for AF.42,43 Of note, while moderate-high levels of VNS promote AF,44 low-level VNS (LLVNS) through stimulation of vagal preganglionics in the vicinity of the superior vena cava could mitigate the effects on atrial electrical (AERP dispersion and AF inducibility) and autonomic (RAGP) remodelling.45 An important and attractive property for autonomic modulation for arrhythmia is that short-duration interventions have long-lasting effects, a corollary to long-term potentiation/depression in cellular neurophysiology. Specifically, in a canine study of neurally induced AF, short 3 min bursts of pre-emptive VNS prior to mediastinal nerve stimulation reduced AF inducibility, and its effects persisted for an average of 26 min indicating memory.46
The sympathetic nervous system also plays a role in AF. Predominantly adrenergic nerves that course through the ligament of Marshall contribute to atrial tachycardia and AF in canine and human AF and heterogeneous atrial sympathetic denervation in a canine model was demonstrated to sustain AF.47–50 In contrast, an autopsy study of 39 human subjects suggested a higher density of adrenergic fibres in the atria of humans with history of persistent AF.51 Functionally, sympathetic stimulation in canines decreases AERP and the re-entrant wavelength.42 However, in normal hearts, VNS is more effective in promoting AF, attributed to a more pronounced effect on AERP heterogeneity.52–54 Relative contributions of neural inputs may depend on remodelling of the cardiac substrate. For example, a Cardiac Arrhythmias and Risk Stratification after Myocardial Infarction substudy of patients post-myocardial infarction demonstrated changes in heart rate variability, a measure of autonomic tone, were associated with increased risk of new-onset AF.55 In a myocardial infarction heart failure model in rats, AF was more easily induced by sympathetic stimulation than VNS.56
At the level of the heart, the ICNS is another important integrative neural network relevant to cardiac autonomic control and AF pathophysiology. In large animal models, HFS during the atrial refractory period activates cardiac ganglia and/or nerves and induces atrial ectopy and fibrillation.57 In further evaluation of the role of the ICNS in acute atrial electrical remodelling, Lu et al.58 demonstrated that rapid atrial pacing following GP ablation prolonged AERP and AF was less inducible, as compared to a control group that did not undergo GP ablation. Pharmacologic stimulation of dendrites and cell bodies of intrinsic cardiac neurons within GPs using nicotine demonstrated spatially divergent changes in atrial repolarization.59 Recordings in conscious dogs identified a rise in intrinsic cardiac neural activity prior to episodes of atrial tachyarrhythmia.60 The contribution of the ICNS was further demonstrated with autonomic blockade using atropine and propranolol suggesting that disruption of the ICNS reduces triggered activity and limits AERP dispersion as a substrate for AF.57
With the identification of PV triggers of AF, it was determined that the parasympathetic and sympathetic nervous system influence the electrophysiological properties of PV muscle.57,61 For example, HFS in the PVs shortened AERPs in the PV muscle sleeves, but not the left atrial appendage. In vitro experiments further demonstrated that autonomic nerve stimulation of both sympathetic and parasympathetic components decreases PV sleeve APD and initiated rapid firing from early afterdepolarizations.62 In a canine model of focal AF mimicking the clinical arrhythmia, atropine abolished AF in response to HFS. In humans, autonomic modulation via phenylephrine injection has been shown to suppress PV triggers of AF.63 In addition to PV triggers, atrial appendage aetiologies of AF have been modulated by autonomic influences. In a canine model, acetylcholine applied to the right atrial appendage was shown to induce AF.41 In short, pre-clinical models of AF indicate that autonomic modulation of APD, atrial refractoriness, and conduction velocity provide the triggers and substrate for AF. Notably, electrical remodelling and autonomic remodelling form a vicious cycle, one perpetuating the other to maintain AF.64
3.2 Electrically induced autonomic remodelling
While the ANS has downstream effects on cardiac electrophysiology, cardiac electrical activity can also induce changes in the cardiac ANS. Rapid atrial pacing has been shown to heterogeneously increase sympathetic innervation in the atria in rabbit and canine models.65–67 In addition to increases in sympathetic innervation, a heterogeneous increase in parasympathetic innervation was noted throughout the left atrium following right atrial pacing in a canine model.68 At the molecular level, atrial tachycardia reduces protein expression of muscarinic receptors and their downstream potassium currents in canine atrial tissue.69 As well as causing AERP dispersion and AF, 6 h of rapid atrial pacing in canine increased activity in the adjacent RAGP. Therefore, changes in atrial electrophysiology may induce remodelling of the cardiac ANS.
4. Key concepts of cardiac neuromodulation
Three main concepts in neuromodulation for cardiac disease bear specific mention (Figure 1).70 First, neural control of cardiac function integrates multi-level reflexes across the neuraxis. Centrally, neural networks involve the spinal cord, brainstem and higher, cortical centres including the insular cortex.71 Preganglionic neurons housed in the brainstem (parasympathetic) and intermediolateral cell column (sympathetic) mediate outflow that is modulated by local circuits in the brainstem and higher, cortical centres. Peripherally, neural networks involved in cardiac control involve intrinsic cardiac and extracardiac intrathoracic ganglia to coordinate reflex control of the heart.72 Cardiac sensory afferents transduce information to intrathoracic ganglia and to the CNS via sensory ganglia (dorsal root, nodose, and petrosal). The central and peripheral processing of this information results in an integrated autonomic response to precisely control cardiac function. Second, the interdependent cardiac electrical and autonomic remodelling induced by cardiac pathology facilitates progression of cardiac disease in a vicious cycle. Heightened sympathoexcitation further contributes to progression of disease as evidenced by increased risk of sudden cardiac death following infarction or reduced freedom from AF recurrence in severely hypertensive patients undergoing PVI for AF.73,74 This autonomic remodelling is a result of neural plasticity, in the form of changes in strength of interconnections, loss of neuronal subpopulations, and memory.75 Finally, neuromodulatory interventions act on axons of passage, neurons, and neural networks to impact the heart, and the effects are a product of stimulation parameters, nexus point within the neuraxis, and cardioneural pathologic substrate involved.76 Furthermore, because of autonomic remodelling and the interdependence of central and peripheral networks and remodelling, interventions may result in shifts to a new operating point in the cardiac ANS. Given the heterogeneity in aetiologies underlying AF, such as increased vagal or sympathetic tone or left atrial hypertension (systemic hypertension and heart failure), patient selection should also be factored in when considering an autonomic intervention. As a result, ‘one-size-fits-all’ approach is unlikely to be successful in neuromodulatory therapy and a therapy may require titration and further adjustments in time, even within a given patient.
5. Neuroscientific interventions for AF
5.1 GP ablation
Following the discovery that GP stimulation result in triggered activity in PVs and fractionated action potentials thought to maintain AF, targeted ablation of atrial GPs has been pursued.41,77–80 The GPs may be localized during an electrophysiology study via endocardial HFS and subsequently targeted for ablation.78,79 HFS is performed by delivering 20–50 Hz stimulation with a pulse width of 10 m at 5–24 V to elicit a vagal response, defined as induction of AV block (>2 s) and hypotension or >50% R–R interval prolongation during AF.78 During catheter ablation and/or HFS, the presence of fractionated atrial electrograms in conjunction with a vagal response indicates the location of GPs and parasympathetic innervation.78,80 If HFS does not elicit a response or the patient is unable to tolerate (conscious patients may not withstand >15 V stimulation), an anatomic approach may be used.81 Once localized, the GPs may be ablated using radiofrequency energy with the endpoint of abolition of a vagal response upon repeat HFS.77 Because of the interconnectedness of the GPs and the predominant influence of the IVC-IAGP over the AV node, it may be best to ablate the IVC-IAGP last. This is to preserve a measurable outcome of vagal response to HFS until the final GP (IVC-IAGP) is ablated. One recommended order is: Marshall tract GP, left superior GP, RAGP, left inferior GP, and, finally, the IVC-IAGP. Wide area circumferential ablation of PVs, and isolation of the left atrial posterior wall or other substrate, may inadvertently ablate GPs; autonomic responses are sometimes observed even when GPs are not specifically targeted.
How GP ablation may be incorporated into the AF therapeutic approach has been studied in several clinical studies. Patients undergoing PVI that lose the expected vagal response to HFS of GPs have a reduced rate of AF recurrence.82 Initial investigations of GP ablation were performed without PVI and had variable success with freedom from AF recurrence ranging from 26% to 77% in the first year.25,37,79,81,83,84 Our understanding of the localization of GPs in human hearts is mainly based on autopsy studies6,7,51,85 and, when compared against a functional (HFS) approach, anatomic-guided ablation had superior outcomes.79,84,86 Advances in nuclear cardiology allow in vivo imaging of smaller structures, such as the GPs with improved spatial resolution.87 A pilot study of SPECT/CT imaging using radionuclide 123I-metaiodobenzylguanindine showed that the technique may be used to identify sympathetic innervation based on correlation with response to HFS.88 In prospective studies comparing GP ablation alone directly against PVI, anatomic-guided GP ablation was shown to have significantly reduced freedom from AF recurrence 26–34% compared to 63–66% in PVI groups at up to 3-year follow-up.81,89 Adjunctive anatomic-guided GP ablation with PVI has shown improved outcomes with success rates up to 80%.90,91 A randomized controlled trial evaluating PVI alone against combination GP ablation and PVI demonstrated improved freedom from AF recurrence in the combination group at 1-year follow-up (85.3% vs. 60.6%, log-rank P=0.19).90 This was further supported by another randomized controlled trial in which freedom from arrhythmia recurrence was 48% in the GP ablation alone, 56% in the PVI alone group and 74% PVI with GP ablation group at 2-year follow-up (log-rank P=0.004).91 It should be noted that the typical AF ablation procedure, which includes PVI, interrupts the axons of at least three major GP (Marshall tract GP, left superior GP, and RAGP), which may contribute to the success of the procedure.64
For those undergoing surgical AF ablation, the epicardial fat pads containing GPs may be directly visualized and HFS applied to localize GPs for ablation.92–94 The addition of GP ablation to the Cox maze procedure yielded significantly improved success rates over the first year post-operatively in single-centre studies.95,96 Furthermore, a minimally invasive approach to perform thorascopic GP ablation as an adjunct to surgical PVI was developed.97–100 While early studies appeared promising, the randomized controlled trial of 240 patients with paroxysmal or persistent AF in the AF Ablation and Autonomic Modulation via Thorascopic Surgery (AFACT) study showed no reduction in AF recurrence at 2-year follow-up and was associated with significant adverse events of major bleeding, sinus node dysfunction, and need for pacemaker implantation.101,102 It should be noted, however, that a significant proportion of patients had persistent AF (59%) with enlarged LA (68%, mean 42.2±5.6 mm). The mixed results of GP ablation may be due, in part, to the variability in how studies are conducted with respect to patient selection and AF burden. Several ongoing clinical trials are further evaluating the role of GP ablation in AF (Table 1).
Table 1.
Ongoing clinical trials evaluating autonomic therapies for AF
| Intervention | Target | Trial design | Disease | n | ClinicalTrials.gov identifier | Description |
|---|---|---|---|---|---|---|
| GP ablation | GPs | RCT | AF | 60 | NCT03535818 | Adjunctive Ganglionated Plexus Ablation in Redo-PVI (ADD-GP); testing efficacy of GP ablation in patients with paroxysmal or persistent AF undergoing redo PVI for recurrent paroxysms of arrhythmia |
| GP ablation | GPs | RCT | POAF | 62 | NCT02035163 | AF Prevention in Post-Coronary Artery Bypass Graft Surgery with Cryoablation for Ganglionic Plexi; evaluating cryoablation of GPs during cardiac surgery to prevent POAF |
| GP ablation | GPs | RCT | AF | 180 | NCT02487654 | Ectopy-Triggering GP Ablation to Prevent AF (GANGLIA-AF); evaluating GP ablation alone vs. PVI alone |
| GP ablation | GPs | Single-arm feasibility | AF | 16 | NCT04642976 | Prospective Evaluation of CT-Guided Ablation of Cardiac GPs; evaluating feasibility and efficacy of CT-guided GP localization to guide AF ablation |
| Botulinum toxin | GPs | RCT | POAF | 330 | NCT03779841 | Botulinum Toxin Type A (AGN-151607) for the Prevention of POAF in Patients Undergoing Open-chest Cardiac Surgery (NOVA); evaluating epicardial fat pad injection of botulinum toxin A to prevent post-operative AF |
| Botulinum toxin | GPs | RCT | POAF | 220 | NCT04075981 | Prevention AF by BOTulinum Toxin Injections (BOTAF) ; evaluating botulinum toxin injection into epicardial fat pads during cardiac surgery to reduce POAF |
| LLVNS | Tragus | RCT | POAF | 266 | NCT04514757 | Transcutaneous (Tragus) Vagal Nerve Stimulation for POAF (tVNS_POAF); evaluating low-level tragus stimulation for POAF |
| LLVNS | Tragus | RCT | POAF | 80 | NCT03392649 | Tragus Stimulation to Prevent AF After Cardiac Surgery (TraP-AF); evaluating low-level tragus vagal nerve stimulation for POAF |
| LLVNS | Tragus | RCT | HRV, MAST | 90 | NCT04682704 | The Effect of Different Low-Level Tragus Stimulation Parameters On Autonomic Nervous System Function (LLT-SPANS); evaluating different stimulation parameters on the autonomic nervous system |
| RDN | Renal nerves | RCT | AF | 40 | NCT03246568 | Renal Nerve Denervation After Pulmonary Vein Isolation for Persistent AF; evaluating the effect of RDN in addition to PVI for persistent AF |
| RDN | Renal nerves | RCT | AF | 130 | NCT04182620 | Ultrasound-Based Renal Sympathetic Denervation as Adjunctive Upstream Therapy During AF Ablation (ULTRA-HFIB); evaluating RDN as adjunctive treatment for patients undergoing PVI for AF |
| RDN | Renal nerves | RCT | AF | 100 | NCT01686542 | CPVI Plus Renal Sympathetic Modification Versus CPVI Alone for AF Ablation; evaluating RDN as adjunctive treatment for patients undergoing PVI for AF |
| RDN | Renal nerves | RCT | AF | 138 | NCT02115100 | Treatment of AF in Patients by PVI in Combination With Renal Denervation or PVI Only (ASAF); evaluating RDN as adjunctive treatment for patients undergoing PVI for AF and resistant hypertension |
| RDN | Renal nerves | RCT | AF | 50 | NCT01635998 | Adjunctive Renal Denervation in the Treatment of AF (H-FIB); evaluating adjunctive RDN in patients with hypertension undergoing PVI for AF |
| RDN | Renal nerves | RCT | AF | 100 | NCT01990911 | Renal Sympathetic Denervation Prevents AF in Patients With Hypertensive Heart Disease: a Pilot Study (RDPAF); evaluating whether RDN in patients with hypertensive heart disease reduces new-onset AF |
| RDN | Renal nerves | RCT | AF | 61 | NCT01907828 | A feasibility study to evaluating the Effect of Concomitant Renal Denervation and Cardiac Ablation on AF Recurrence (RDN+AF); evaluating adjunctive RDN in patients with paroxysmal or persistent AF and uncontrolled hypertension. |
AF indicates atrial fibrillation; CPVI, circumferential pulmonary vein isolation; GP, ganglionated plexus; LLVNS, low-level vagal nerve stimulation; POAF, post-operative atrial fibrillation; RCT, randomized controlled trial; PVI, pulmonary vein isolation; RDN, renal denervation.
The need for pacemaker implantation in the AFACT study highlights the need for improved understanding cardiac morphological and functional neuroanatomy. The difficulty in localizing GPs, such as in instances in which HFS does not elicit a vagal response; incomplete endocardial approaches restricted to a unilateral atrium; or epicardial vs. endocardial targeting of epicardial fat pads in which neurons span the thickness may explain variable success of the technique and limit generalizability of findings. As ablating GPs may prove beneficial for atrial electrophysiology and AF, off-target effects on the SA and AV nodes and ventricles must be considered. For instance, the RAGP controls the sinoatrial node, and ablation of this GP may explain the increased sinus node dysfunction and need for pacemaker implantation in the GP ablation group.103 Moreover, in a canine study on GP ablation in myocardial infarction, GP ablation increased the risk of ventricular arrhythmias.104 Another study in dogs showed that ablation of another GP located between the superior vena cava and aorta (aorta-SVC GP) shortened AERP and increased AF/atrial tachycardia burden.105 Disruption of cardiac cholinergic neurons in the murine heart was also shown to shorten ventricular refractoriness and increase the risk of ventricular arrhythmia, raising the possibility that bystander autonomic denervation during PVI for human AF may explain the increased premature ventricular contraction burden often noted post-procedure.106 Interestingly, similar to PVI alone, adjunctive surgical GP ablation performed as part of the AFACT study was associated with an initial increase in mean heart rates at 6- and 9-month follow-up, but this effect dissipated by 12 and 24 months.101,102 A recent study of endocardial GP ablation for paroxysmal AF similarly did not show significant differences in mean heart rate between the GP ablation and control groups at 3, 6, 9, and 12-month follow-up, suggesting that GP ablation does not provide improved rate control in AF.107 Cases have also been reported of coronary vasospasm following GP ablation that precipitate life-threatening ventricular arrhythmias.108 Lastly, the permanence of GP ablation is as yet unclear, as reinnervation has been suggested following GP ablation in a canine model.109
5.2 Epicardial botulinum toxin injection
As opposed to the destructive approach of GP ablation, neuromodulatory approaches seek to harness neural control of myocardial tissue. Botulinum toxin is a neurotoxin that has been shown to reduce vagal-induced AERP shortening in animal models and prevent autonomic remodelling.110–112 The transient nature of the toxin is attractive as a therapeutic for the short-term increased risk of POAF following cardiac surgery. Thus far, botulinum toxin injection has been evaluated in two small clinical trials. In one study, 60 patients with paroxysmal AF undergoing coronary artery bypass graft surgery received either botulinum toxin or placebo injection in four major atrial GPs (RAGP, IVCI-IAGP, left superior GP, and left inferior GP). The results demonstrated reduced incidence of POAF in the month and up to 3 years following surgery.113–115 In a second study, 130 patients undergoing CABG or valve surgery were randomized to botulinum toxin injection in the four epicardial fat pads as well as the aorta-SVC fat pad.116 In contrast, this study demonstrated no reduction in POAF, although POAF episodes were shorter with botulinum toxin injections. Ongoing clinical trials are further evaluating the efficacy of botulinum toxin injections for POAF post-cardiac surgery (Table 1). Lastly, a recently published randomized, sham-controlled trial of 200 patients demonstrated that injection of CaCl2 into the four major atrial GPs reduces risk of POAF by 63%.117 As opposed to the purported transient effects of botulinum toxin, the CaCl2 is thought to cause intracellular calcium overload to induce neurotoxicity. This neurotoxicity increase AERP and limiting sympathetic effects to reduce AF vulnerability in the post-operative period.118,119
5.3 Low-level vagal nerve (tragus) stimulation
Although initially developed for treatment in epilepsy and refractory depression, VNS devices have been used in cardiac disease, namely heart failure.120–122 VNS is thought to increase parasympathetic tone and tamp down adrenergic outflow to the heart by way of reflex pathways. Benefits of VNS are also thought to include limiting cardiomyocyte apoptosis and remodelling as well as inflammation.123–126 VNS reduces inflammatory cytokine release via the ‘cholinergic anti-inflammatory pathway’ to mitigate myocardial injury.127 Activation of parasympathetic efferent neurons induces release of acetylcholine at the heart, which binds nicotinic acetylcholine receptors on tissue macrophages to inhibit the release of pro-inflammatory cytokines, such as tumour necrosis factor-alpha, C-reactive protein, and interleukin-6.64,128 In a rat model of ischaemia-induced arrhythmias, VNS was also shown to reduce connexin 43 loss to promote electrical stability.129
Stimulation parameters are critical in determining the effects of VNS on end-organ function, a concept previously described as the neural fulcrum.76 In the case of arrhythmia, while VNS induces shortening and dispersion of AERP to promote AF, LLVNS, at currents significantly below threshold for bradycardia, has been shown to be anti-arrhythmic. LLVNS damages the stellate ganglia; increases IVC-IAGP and RAGP neural activity; reduces AF inducibility; and ventricular rate in dogs with pacing-induced persistent AF.45,130–132 In a randomized sham-controlled trial, Stavrakis et al.128 demonstrated in 54 patients undergoing cardiac surgery that temporary LLVNS of preganglionic vagal nerve fibres at the lateral aspect of the superior vena cava in the post-operative period suppresses POAF.
Transcutaneous stimulation of the auricular branch of the VNS is an emerging non-invasive approach to VNS that acts via afferent neurotransmission to elicit changes in neural activity in the brainstem and reduces sympathetic activity and centrally mediated parasympathetic outflow.133–135 Yu et al.136 showed that low-level tragus stimulation (LLTS) increases neural activity in the RAGP and decreased risk of AF in dogs. In a pilot study of 40 patients with paroxysmal AF randomized to LLTS at 50% threshold for sinus rate slowing vs. sham stimulation, LLTS reduced pacing-induced AF duration and increased AERP.64 More recently, in the Transcutaneous Electrical Vagus Nerve Stimulation to Suppress AF study, 53 patients with paroxysmal AF were randomized to LLTS or sham in the ambulatory setting for 1 h daily for 6 months.137 LLTS was shown to reduce AF burden by 85% compared to control. As opposed to cervical VNS, tragus stimulation preferentially engages afferent vagal fibres and decreases central sympathetic outflow.138,139 Furthermore, this approach obviates the potential for incidental concomitant stimulation of sympathetic fibres of the cervical vagus.140 This is a promising, non-invasive approach to autonomic modulation that is currently being evaluated in several randomized controlled trials (Table 1).
5.4 Stellate ganglion block
The stellate ganglion may be targeted via a percutaneous approach to inhibit cardioneural signalling. Recordings of left stellate ganglion and vagal nerve activity demonstrated sympathovagal imbalance associated with tachybrady syndrome in dogs with pacing-induced heart failure, and cryoablation of the lower half of the stellate and T2-4 thoracic ganglia abolished atrial tachycardia episodes.141,142 In humans, a pilot study of 36 patients undergoing PVI demonstrated that unilateral (either left or right) stellate ganglion block prolongs atrial ERP and decreased AF inducibility and duration.143 Another pilot study of 25 patients undergoing CABG and/or aortic valve surgery showed efficacy of left-sided stellate ganglion block.144 As opposed to temporary blockade, cardiac sympathetic denervation via a stellate ganglionectomy has been shown to impact atrial rhythm.145 However, at this time, there are currently no ongoing clinical trials evaluating stellate ganglionic blockade further.
5.5 Renal denervation
Renal nerves are composed of afferent nerves that transduce mechanosensitive and chemosensitive stimuli centrally and efferent nerves. Renal denervation was initially studied for refractory hypertension and is performed by placing an ablation catheter just proximal to the origin of the second-order renal artery branch and ablating 4–6 regions in a spiral fashion along the length of each of the two arteries.146 Catheter ablation is performed by applying radiofrequency energy of 8–12 W based on anatomy or by HFS eliciting a hypertensive response, defined as 15 mmHg increase in blood pressure. The randomized controlled Symplicity HTN trials have had mixed results stemming from inadequate technique for assessing successful ablation of renal nerves.147,148 In instances in which HFS does elicit a hypertensive response, abolition of this response serves as a readily identifiable endpoint. However, in the anatomic-guided approach, there is no clear intraprocedural measure of success. Endovascular ultrasound renal denervation has emerged as an alternative technology in which short bursts of sonication are delivered in the main branches of the renal arteries in a longitudinal manner. A porcine model demonstrated that the catheter, supported centrally in the vessel lumen by a water-filled cooling balloon, allows for circumferential ablation at depths of 1–6 mm while preserving the arterial wall.149,150 The randomized sham-controlled trial RADIANCE-HTN SOLO trial showed that this approach of thermal ablation was effective in reducing blood pressure at 2-month follow-up.151
Studies of renal denervation in AF have thus far been encouraging. Renal denervation in conjunction with PVI has been used in AF and improved outcomes, as 61–69% of patients with AF undergoing renal denervation with PVI were free from AF recurrence at 12-month follow-up compared to 29–36% of patients undergoing PVI alone.152,153 A pooled analysis of five randomized controlled trials identified a more pronounced benefit in patients with a combination of AF and hypertension undergoing renal denervation and PVI, of whom 61.9% were AF-free at 12 months compared to 37.3% in the PVI only group (OR = 0.40, 95% CI 0.20–0.78).154 Patients with AF and moderate hypertension did not have significantly improved outcomes with combination renal denervation and PVI therapy compared to PVI alone (65% vs. 52%, P=0.19). Patients with paroxysmal AF and chronic kidney disease undergoing PVI alone had increased incidence of AF recurrence at 22-month follow-up compared to those patients undergoing both renal denervation PVI with HR of 1.86 (95% CI 1.14–3.03).155 A pilot study of 20 patients with 1-year follow-up demonstrated that renal denervation decreased AF burden from a median (IQR) of 1.39 (0–11) to 0.67 (0–31.6) min/day and improved quality of life.156 Ablating renal nerves may reduce systemic and cardiac catecholamine levels and reduce atrial fibrosis through attenuation of the renin–angiotensin–aldosterone system. Beneficial effects on atrial electrophysiological properties have been measured including increases in global conduction velocity and reduction in complex fractionated activity.157 In addition to electrical remodelling, renal denervation impacts structural remodelling, as it has been shown to reduce atrial nerve sprouting and AF in goats and reduces atrial fibrosis and the number and duration of AF episodes in a pacing-induced AF model in dogs.158–161 Conversely, renal nerve stimulation has been shown to be proarrhythmic by inducing AERP shortening, increasing AERP dispersion and AF inducibility.162 In the largest randomized controlled trial to date, the Effect of Renal Denervation and Catheter Ablation vs. Catheter Ablation Alone on AF Recurrence study randomized 302 patients with uncontrolled hypertension and paroxysmal AF to PVI alone or PVI plus renal denervation. At 12 months, 72.1% of patients who underwent adjunctive renal denervation had freedom from recurrence of AF and atrial flutter or tachycardia compared to 56.5% in the PVI alone group (P=0.006).163 Many clinical trials are evaluating adjunctive renal denervation to PVI (Table 1).
5.6 Pre-clinical studies of spinal cord stimulation
In a canine model of AF, spinal cord stimulation prolonged the AERP and reduces AF burden and inducibility.164 These effects are thought to be mediated through reduced activity in the ICNS.165 Autonomic remodelling of the RAGP and left stellate ganglion induced by rapid atrial pacing-induced AF was shown to be mitigated by spinal cord stimulation in a canine model.166 The invasive nature of this approach likely limits its translational potential to human patients.
5.7 Pre-clinical studies of baroreceptor stimulation
Baroreceptor activation therapy has been studied in cardiovascular diseases, such as hypertension and heart failure.122 In pigs, stimulation of carotid baroreceptors at levels typically used for hypertension increased vagal tone resulting in shortening of AERP and increased AF inducibility.167 Conversely, lowering the stimulation level below the threshold to lower blood pressure reduced RAGP neural activity, increased AERP, and, hence, reduced AF inducibility in a canine model.168 As such, similar to LLVNS, low-level baroreceptor activation therapy exerts anti-arrhythmic effects. However, as with spinal cord stimulation, the invasiveness of this approach limits its applicability in clinical AF.
6. Conclusions
Interest in intervention of the cardiac neuraxis for cardiac arrhythmias has increased in recent years. Indeed, cardiac sympathetic denervation is now accepted as guideline-based treatment for inherited arrhythmia syndromes of long QT and catecholaminergic polymorphic VT and acquired refractory ventricular arrhythmias.169 For the treatment of AF, neuroscientific interventions have spanned from destructive to modulatory and additional understanding of cardiac neuroanatomy and potential off-target effects will help tailor therapies. In addition, as a patient’s sympathovagal balance may be dynamic during the natural history of disease progression, neuromodulatory (akin to pharmacologic) therapy may need to be titrated. Although data are mixed for GP ablations and botulinum toxin injection, randomized controlled studies have been encouraging for therapies, such as LLTS and renal denervation but additional studies with larger sample sizes are necessary.
Conflict of interest: University of California, Los Angeles has patents developed by J.L.A. and K.S. relating to cardiac neural diagnostics and therapeutics. J.L.A. and K.S. are co-founders of NeuCures, Inc. E.B. has served as a consultant for Biosense Webster. P.H., S.S., C.M., and J.D.T. have no disclosures to declare.
Funding
This work was supported by the NIH through the Common Fund’s Stimulating Peripheral Activity to Relieve Conditions (SPARC) programme, Grant OT2 OD023848 (PI: K.S.), and National Heart, Lung, and Blood Institute Ruth L. Kirschstein Postdoctoral Individual National Research Service Award F32 HL152609 (PI: P.H.). P.H. is a fellow in the UCLA Specialty Training and Advanced Research (STAR) programme.
This article is part of the Spotlight Issue on Atrial Fibrillation.
References
- 1. Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen P-S, Esler M, De Ferrari GM, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M, Zipes DP. Clinical neurocardiology defining the value of neuroscience‐based cardiovascular therapeutics. J Physiol 2016;594:3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garrey WE. Auricular fibrillation. Physiol Rev 1924;4:215–250. [Google Scholar]
- 3. Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 1994;15:9–16. [DOI] [PubMed] [Google Scholar]
- 4. Andrus EC, Carter EP. The refractory period of the normally-beating dog's auricle; with a note on the occurrence of auricular fibrillation following a single stimulus. J Exp Med 1930;51:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armour JA. Potential clinical relevance of the ‘little brain’on the mammalian heart. Exp Physiol 2008;93:165–176. [DOI] [PubMed] [Google Scholar]
- 6. Armour JA, Murphy DA, Yuan BX, MacDonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289–298. [DOI] [PubMed] [Google Scholar]
- 7. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000;259:353–382. [DOI] [PubMed] [Google Scholar]
- 8. Hoover D, Isaacs E, Jacques F, Hoard J, Page P, Armour J. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 2009;164:1170–1179. [DOI] [PubMed] [Google Scholar]
- 9. Lemola K, Chartier D, Yeh Y-H, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation 2008;117:470–477. [DOI] [PubMed] [Google Scholar]
- 10. Bauer A, Deisenhofer I, Schneider R, Zrenner B, Barthel P, Karch M, Wagenpfeil S, Schmitt C, Schmidt G. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm 2006;3:1428–1435. [DOI] [PubMed] [Google Scholar]
- 11. Jungen C, Alken F-A, Eickholt C, Scherschel K, Kuklik P, Klatt N, Schwarzl J, Moser J, Jularic M, Akbulak RO. Respiratory sinus arrhythmia is reduced after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Arch Med Sci 2020;16:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eickholt C, Jungen C, Drexel T, Alken F, Kuklik P, Muehlsteff J, Makimoto H, Hoffmann B, Kelm M, Ziegler D, Kloecker N, Willems S, Meyer C. Sympathetic and parasympathetic coactivation induces perturbed heart rate dynamics in patients with paroxysmal atrial fibrillation. Med Sci Monit 2018;24:2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327–334. [DOI] [PubMed] [Google Scholar]
- 14. Nilsson B, Chen X, Pehrson S, Hilden J, Svendsen JH. Increased resting heart rate following radiofrequency catheter ablation for atrial fibrillation. Europace 2005;7:415–420. [DOI] [PubMed] [Google Scholar]
- 15. Ketels S, Houben R, Van Beeumen K, Tavernier R, Duytschaever M. Incidence, timing, and characteristics of acute changes in heart rate during ongoing circumferential pulmonary vein isolation. Europace 2008;10:1406–1414. [DOI] [PubMed] [Google Scholar]
- 16. Yamada T, Yoshida N, Murakami Y, Okada T, Yoshida Y, Muto M, Inden Y, Murohara T. The difference in autonomic denervation and its effect on atrial fibrillation recurrence between the standard segmental and circumferential pulmonary vein isolation techniques. Europace 2009;11:1612–1619. [DOI] [PubMed] [Google Scholar]
- 17. Kang KW, Pak HN, Park J, Park JG, Uhm JS, Joung B, Lee MH, Hwang C. Additional linear ablation from the superior vena cava to right atrial septum after pulmonary vein isolation improves the clinical outcome in patients with paroxysmal atrial fibrillation: prospective randomized study. Europace 2014;16:1738–1745. [DOI] [PubMed] [Google Scholar]
- 18. Nedios S, Sommer P, Dagres N, Kosiuk J, Arya A, Richter S, Gaspar T, Kanagkinis N, Dinov B, Piorkowski C, Bollmann A, Hindricks G, Rolf S. Long-term follow-up after atrial fibrillation ablation in patients with impaired left ventricular systolic function: the importance of rhythm and rate control. Heart Rhythm 2014;11:344–351. [DOI] [PubMed] [Google Scholar]
- 19. Seaborn GE, Todd K, Michael KA, Baranchuk A, Abdollah H, Simpson CS, Akl SG, Redfearn DP. Heart rate variability and procedural outcome in catheter ablation for atrial fibrillation. Ann Noninvasive Electrocardiol 2014;19:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu HT, Kim T-H, Uhm J-S, Kim J-Y, Joung B, Lee M-H, Pak H-N. Prognosis of high sinus heart rate after catheter ablation for atrial fibrillation. Europace 2017;19:1132–1139. [DOI] [PubMed] [Google Scholar]
- 21. Goff ZD, Laczay B, Yenokyan G, Sivasambu B, Sinha SK, Marine JE, Ashikaga H, Berger RD, Akhtar T, Spragg DD, Calkins H. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J Cardiovasc Electrophysiol 2019;30:2818–2822. [DOI] [PubMed] [Google Scholar]
- 22. Galloo X, Abugattas J-P, Tijskens M, Dendale P, Varnavas V, Wolf M, De Cocker J, Schwagten B, Sieira J, Ströker E, Chierchia G-B, de Asmundis C, De Greef Y. Impact of cryoballoon-guided pulmonary vein isolation on non-invasive autonomic tests in patients with paroxysmal atrial fibrillation. Indian Pacing Electrophysiol J 2019;19:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marinković M, Mujović N, Vučićević V, Steffel J, Potpara TS. A square root pattern of changes in heart rate variability during the first year after circumferential pulmonary vein isolation for paroxysmal atrial fibrillation and their relation with long-term arrhythmia recurrence. Kardiologia Polska 2020;78:209–218. [DOI] [PubMed] [Google Scholar]
- 24. Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, Klatt N, Lindner D, Westermann D, Casini S, Kuklik P, Eickholt C, Klöcker N, Shivkumar K, Christ T, Zeller T, Willems S, Meyer C. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med 2019;11:eaav7770. [DOI] [PubMed] [Google Scholar]
- 25. Kim M-Y, Lim PB, Coyle C, Sandler B, Koa-Wing M, Kanagaratnam P. Single ectopy-triggering ganglionated plexus ablation without pulmonary vein isolation prevents atrial fibrillation. Case Rep 2020;2:2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim M-Y, Sandler BC, Sikkel MB, Cantwell CD, Leong KM, Luther V, Malcolme-Lawes L, Koa-Wing M, Ng FS, Qureshi N. Anatomical distribution of ectopy-triggering plexuses in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008715. [DOI] [PubMed] [Google Scholar]
- 27. Kim MY, Sikkel MB, Hunter RJ, Haywood GA, Tomlinson DR, Tayebjee MH, Ali RL, Cantwell CD, Gonna H, Sandler BC. A novel approach to mapping the atrial ganglionated plexus network by generating a distribution probability atlas. J Cardiovasc Electrophysiol 2018;29:1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–2759. [DOI] [PubMed] [Google Scholar]
- 29. Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precedethe onset of postoperative atrial fibrillation. J Am Coll Cardiol 2003;42:1262–1268. [DOI] [PubMed] [Google Scholar]
- 30. Agarwal SK, Norby FL, Whitsel EA, Soliman EZ, Chen LY, Loehr LR, Fuster V, Heiss G, Coresh J, Alonso A. Cardiac autonomic dysfunction and incidence of atrial fibrillation: results from 20 years follow-up. J Am Coll Cardiol 2017;69:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coumel P, Attuel P, Lavallée J, Flammang D, Leclercq JF, Slama R. [The atrial arrhythmia syndrome of vagal origin]. Arch Mal Coeur Vaiss 1978;71:645–656. [PubMed] [Google Scholar]
- 32. de Vos CB, Nieuwlaat R, Crijns HJGM, Camm AJ, LeHeuzey J-Y, Kirchhof CJ, Capucci A, Breithardt G, Vardas PE, Pisters R, Tieleman RG. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur Heart J 2008;29:632–639. [DOI] [PubMed] [Google Scholar]
- 33. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 34., Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Association DwtscotEHR, Surgery EbtEAfC-T, Members ATF. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 35. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 36. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 37. Scanavacca MI, Pisani CF, Hachul D, Trombetta IC, Lara S, Hardy C, Grupi C, Darrieux F, Negrão CE, Sosa EA. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Heart Rhythm 2006;3:S283. [DOI] [PubMed] [Google Scholar]
- 38. Levy MN. Brief reviews: sympathetic-parasympathetic interactions in the heart. Circ Res 1971;29:437–445. [DOI] [PubMed] [Google Scholar]
- 39. Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol 2004;43:483–490. [DOI] [PubMed] [Google Scholar]
- 40. Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, Geng N, Lazzara R, Jackman WM. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm 2006;3:201–208. [DOI] [PubMed] [Google Scholar]
- 41. Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE). J Cardiovasc Electrophysiol 2007;18:1197–1205. [DOI] [PubMed] [Google Scholar]
- 42. Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. American Journal of Physiology-Heart and Circulatory Physiology 1997;273:H805–H816. [DOI] [PubMed] [Google Scholar]
- 43. Takei M, Furukawa Y, Narita M, Ren LM, Karasawa Y, Murakami M, Chiba S. Synergistic nonuniform shortening of atrial refractory period induced by autonomic stimulation. Am J Physiol 1991;261:H1988–H1993. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Ilsar I, Sabbah HN, David TB, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 2009;6:244–250. [DOI] [PubMed] [Google Scholar]
- 45. Yu L, Scherlag BJ, Sha Y, Li S, Sharma T, Nakagawa H, Jackman WM, Lazzara R, Jiang H, Po SS. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm 2012;9:804–809. [DOI] [PubMed] [Google Scholar]
- 46. Salavatian S, Beaumont E, Longpré J-P, Armour JA, Vinet A, Jacquemet V, Shivkumar K, Ardell JL. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. American Journal of Physiology-Heart and Circulatory Physiology 2016;311:H1311–H1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doshi RN, Wu T-J, Yashima M, Kim Y-H, Ong JJC, Cao J-M, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen P-S. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation 1999;100:876–883. [DOI] [PubMed] [Google Scholar]
- 48. Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol 1999;10:636–648. [DOI] [PubMed] [Google Scholar]
- 49. Katritsis D, Ioannidis JP, Anagnostopoulos CE, Sarris GE, Giazitzoglou E, Korovesis S, Camm AJ. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2001;12:750–758. [DOI] [PubMed] [Google Scholar]
- 50. Olgin JE, Sih HJ, Hanish S, Jayachandran JV, Wu J, Zheng QH, Winkle W, Mulholland GK, Zipes DP, Hutchins G. Heterogeneous atrial denervation creates substrate for sustained atrial fibrillation. Circulation 1998;98:2608–2614. [DOI] [PubMed] [Google Scholar]
- 51. Deneke T, Chaar H, de Groot JR, Wilde AA, Lawo T, Mundig J, Bösche L, Mügge A, Grewe PH. Shift in the pattern of autonomic atrial innervation in subjects with persistent atrial fibrillation. Heart Rhythm 2011;8:1357–1363. [DOI] [PubMed] [Google Scholar]
- 52. Alessi R, Nusynowitz M, Abildskov J, Moe G. Nonuniform distribution of vagal effects on the atrial refractory period. Am J Physiol 1958;194:406–410. [DOI] [PubMed] [Google Scholar]
- 53. Ninomiya I. Direct evidence of nonuniform distribution of vagal effects on dog atria. Circ Res 1966;19:576–583. [DOI] [PubMed] [Google Scholar]
- 54. Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation on atrial refractoriness. Cardiovasc Res 1974;8:647–655. [DOI] [PubMed] [Google Scholar]
- 55. Jons C, Raatikainen P, Gang UJ, Huikuri HV, Joergensen RM, Johannesen A, Dixen U, Messier M, McNITT S, Thomsen PEB, for the Cardiac Arrhythmias and Risk Stratification after Acute Myocardial Infarction (CARISMA) Study Group. Autonomic dysfunction and new‐onset atrial fibrillation in patients with left ventricular systolic dysfunction after acute myocardial infarction: a CARISMA substudy. J Cardiovasc Electrophysiol 2010;21:983–990. [DOI] [PubMed] [Google Scholar]
- 56. Delfiner MS, Nofi C, Li Y, Gerdes AM, Zhang Y. Failing hearts are more vulnerable to sympathetic, but not vagal stimulation–induced, atrial fibrillation—ameliorated with dantrolene treatment. J Card Fail 2018;24:460–469. [DOI] [PubMed] [Google Scholar]
- 57. Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, Lazzara R, Jackman WM. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592–599. [DOI] [PubMed] [Google Scholar]
- 58. Lu Z, Scherlag BJ, Lin J, Niu G, Fung K-M, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 2008;1:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cardinal R, Pagé P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 2009;145:55–62. [DOI] [PubMed] [Google Scholar]
- 60. Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 2010;121:2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haissaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 62. Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624–631. [DOI] [PubMed] [Google Scholar]
- 63. Tai C-T, Chiou C-W, Wen Z-C, Hsieh M-H, Tsai C-F, Lin W-S, Chen C-C, Lin Y-K, Yu W-C, Ding Y-A. Effect of phenylephrine on focal atrial fibrillation originating in the pulmonary veins and superior vena cava. J Am Coll Cardiol 2000;36:788–793. [DOI] [PubMed] [Google Scholar]
- 64. Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 2015;65:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation 2000;101:1185–1191. [DOI] [PubMed] [Google Scholar]
- 66. Chang C-M, Wu T-J, Zhou S, Doshi RN, Lee M-H, Ohara T, Fishbein MC, Karagueuzian HS, Chen P-S, Chen LS. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation 2001;103:22–25. [DOI] [PubMed] [Google Scholar]
- 67. Zhang L, Po SS, Wang H, Scherlag BJ, Li H, Sun J, Lu Y, Ma Y, Hou Y. Autonomic remodeling: how atrial fibrillation begets atrial fibrillation in the first 24 hours. J Cardiovasc Pharmacol 2015;66:307–315. [DOI] [PubMed] [Google Scholar]
- 68. Gussak G, Pfenniger A, Wren L, Gilani M, Zhang W, Yoo S, Johnson DA, Burrell A, Benefield B, Knight G, Knight BP, Passman R, Goldberger JJ, Aistrup G, Wasserstrom JA, Shiferaw Y, Arora R. Region-specific parasympathetic nerve remodeling in the left atrium contributes to creation of a vulnerable substrate for atrial fibrillation. JCI Insight 2019;4:e130532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yeh YH, Qi X, Shiroshita, Takeshita A, Liu J, Maguy A, Chartier D, Hebert T, Wang Z, Nattel S. Atrial tachycardia induces remodelling of muscarinic receptors and their coupled potassium currents in canine left atrial and pulmonary vein cardiomyocytes. Br J Pharmacol 2007;152:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ardell JL, Shivkumar K. Foundational concepts for cardiac neuromodulation. Future Med 2018;1:9–11. [Google Scholar]
- 71. Jänig W. Neurocardiology: a neurobiologist's perspective. J Physiol 2016;594:3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ardell J, Andresen M, Armour J, Billman G, Chen PS, Foreman R, Herring N, O'leary D, Sabbah H, Schultz H. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 2016;594:3877–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 2015;116:2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Bayramova S, Losik D, Baranova V, Karaskov A, Steinberg JS. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: early experience. Heart Rhythm 2014;11:1131–1138. [DOI] [PubMed] [Google Scholar]
- 75. Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 2016;594:321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ardell JL, Nier H, Hammer M, Southerland EM, Ardell CL, Beaumont E, KenKnight BH, Armour JA. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol 2017;595:6887–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009;6:S26–S34. [DOI] [PubMed] [Google Scholar]
- 78. Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006;3:387–396. [DOI] [PubMed] [Google Scholar]
- 79. Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm 2009;6:1257–1264. [DOI] [PubMed] [Google Scholar]
- 80. Lellouche N, Buch E, Celigoj A, Siegerman C, Cesario D, De Diego C, Mahajan A, Boyle NG, Wiener I, Garfinkel A. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 2007;50:1324–1331. [DOI] [PubMed] [Google Scholar]
- 81. Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol 2008;102:330–334. [DOI] [PubMed] [Google Scholar]
- 82. Kurotobi T, Shimada Y, Kino N, Ito K, Tonomura D, Yano K, Tanaka C, Yoshida M, Tsuchida T, Fukumoto H. Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical significance after catheter ablation in patients with atrial fibrillation. Heart Rhythm 2015;12:470–476. [DOI] [PubMed] [Google Scholar]
- 83. Danik S, Neuzil P, d'Avila A, Malchano ZJ, Kralovec S, Ruskin JN, Reddy VY. Evaluation of catheter ablation of periatrial ganglionic plexi in patients with atrial fibrillation. Am J Cardiol 2008;102:578–583. [DOI] [PubMed] [Google Scholar]
- 84. Pokushalov E, Romanov A, Artyomenko S, Turov A, Shirokova N, Katritsis DG. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2010;33:1231–1238. [DOI] [PubMed] [Google Scholar]
- 85. Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen P-S, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol 2006;48:132–143. [DOI] [PubMed] [Google Scholar]
- 86. Calò L, Rebecchi M, Sciarra L, De Luca L, Fagagnini A, Zuccaro LM, Pitrone P, Dottori S, Porfirio M, de Ruvo E, Lioy E. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:22–31. CIRCEP. [DOI] [PubMed] [Google Scholar]
- 87. Gambhir SS, Berman DS, Ziffer J, Nagler M, Sandler M, Patton J, Hutton B, Sharir T, Haim SB, Haim SB. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J Nucl Med 2009;50:635–643. [DOI] [PubMed] [Google Scholar]
- 88. Stirrup J, Gregg S, Baavour R, Roth N, Breault C, Agostini D, Ernst S, Underwood S. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: technique and validation in patients with atrial fibrillation. J Nucl Cardiol 2020;27:1939– 1950. [DOI] [PubMed] [Google Scholar]
- 89. Mikhaylov E, Kanidieva A, Sviridova N, Abramov M, Gureev S, Szili-Torok T, Lebedev D. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: a 3-year follow-up study. Europace 2011;13:362–370. [DOI] [PubMed] [Google Scholar]
- 90. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 2011;8:672–678. [DOI] [PubMed] [Google Scholar]
- 91. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62:2318–2325. [DOI] [PubMed] [Google Scholar]
- 92. Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol 2007;20:89–93. [DOI] [PubMed] [Google Scholar]
- 93. Mehall JR, Kohut RM Jr, Schneeberger EW, Taketani T, Merrill WH, Wolf RK. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg 2007;83:538–541. [DOI] [PubMed] [Google Scholar]
- 94. Doll N, Pritzwald-Stegmann P, Czesla M, Kempfert J, Stenzel MA, Borger MA, Mohr F-W. Ablation of ganglionic plexi during combined surgery for atrial fibrillation. Ann Thorac Surg 2008;86:1659–1663. [DOI] [PubMed] [Google Scholar]
- 95. Onorati F, Curcio A, Santarpino G, Torella D, Mastroroberto P, Tucci L, Indolfi C, Renzulli A. Routine ganglionic plexi ablation during Maze procedure improves hospital and early follow-up results of mitral surgery. J Thorac Cardiovasc Surg 2008;136:408–418. [DOI] [PubMed] [Google Scholar]
- 96. Ware AL, Suri RM, Stulak JM, Sundt IIT, Schaff HV. Left atrial ganglion ablation as an adjunct to atrial fibrillation surgery in valvular heart disease. Ann Thorac Surg 2011;91:97–102. [DOI] [PubMed] [Google Scholar]
- 97. McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:1289–1295. [DOI] [PubMed] [Google Scholar]
- 98. Han FT, Kasirajan V, Kowalski M, Kiser R, Wolfe L, Kalahasty G, Shepard RK, Wood MA, Ellenbogen KA. Results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation: single-center experience with 12-month follow-up. Circ Arrhythm Electrophysiol 2009;2:370–377. [DOI] [PubMed] [Google Scholar]
- 99. Yilmaz A, Geuzebroek GS, Van Putte BP, Boersma LV, Sonker U, De Bakker JM, Van Boven W-J. Completely thoracoscopic pulmonary vein isolation with ganglionic plexus ablation and left atrial appendage amputation for treatment of atrial fibrillation. Eur J Cardiothorac Surg 2010;38:356–360. [DOI] [PubMed] [Google Scholar]
- 100. Krul SP, Driessen AH, van Boven WJ, Linnenbank AC, Geuzebroek GS, Jackman WM, Wilde AA, de Bakker JM, de Groot JR. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:262–270. [DOI] [PubMed] [Google Scholar]
- 101. Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, Chan Pin Yin D, de Jong J, van Boven WP, de Groot JR. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol 2016;68:1155–1165. [DOI] [PubMed] [Google Scholar]
- 102. Berger WR, Neefs J, van den Berg NWE, Krul SPJ, van Praag EM, Piersma FR, de Jong J, van Boven WP, Driessen AHG, de Groot JR. Additional ganglion plexus ablation during thoracoscopic surgical ablation of advanced atrial fibrillation: intermediate follow-up of the AFACT study. JACC Clin Electrophysiol 2019;5:343–353. [DOI] [PubMed] [Google Scholar]
- 103. Hanna P, Dacey MJ, Brennan J, Moss A, Robbins S, Achanta S, Biscola NP, Swid MA, Rajendran PS, Mori S, Hadaya JE, Smith EH, Peirce SG, Chen J, Havton LA, Cheng Z(J), Vadigepalli R, Schwaber J, Lux RL, Efimov I, Tompkins JD, Hoover DB, Ardell JL, Shivkumar K. Innervation and neuronal control of the mammalian sinoatrial node: a comprehensive atlas. Circ Res 2021;128:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. He B, Lu Z, He W, Wu L, Cui B, Hu X, Yu L, Huang C, Jiang H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int J Cardiol 2013;168:86–93. [DOI] [PubMed] [Google Scholar]
- 105. Lo L-W, Scherlag BJ, Chang H-Y, Lin Y-J, Chen S-A, Po SS. Paradoxical long-term proarrhythmic effects after ablating the “head station” ganglionated plexi of the vagal innervation to the heart. Heart Rhythm 2013;10:751–757. [DOI] [PubMed] [Google Scholar]
- 106. Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, Salzbrunn T, Alken F, Angendohr S, Klene C. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat Commun 2017;8:14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sandler B, Kim MY, Sikkel MB, Malcolme-Lawes L, Koa-Wing M, Whinnett ZI, Coyle C, Linton NWF, Lim PB, Kanagaratnam P, other members of the Imperial College London, Cardiovascular Study Group/Consortium. Targeting the ectopy-triggering ganglionated plexuses without pulmonary vein isolation prevents atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sakamoto S, Kataoka T, Kanai M, Tamura K, Iguchi T, Tatsumi H, Doi A, Yoshiyama M. Multiple coronary artery spasms triggering life-threatening ventricular arrhythmia associated with the radiofrequency ablation of ganglionated plexuses of the left atrium. J Cardiol Cases 2018;17:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sakamoto S-I, Schuessler RB, Lee AM, Aziz A, Lall SC, Damiano RJ. Jr Vagal denervation and reinnervation after ablation of ganglionated plexi. J Thorac Cardiovasc Surg 2010;139:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Oh S, Choi E-K, Zhang Y, Mazgalev TN. Botulinum toxin injection in epicardial autonomic ganglia temporarily suppresses vagally mediated atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:560–565. [DOI] [PubMed] [Google Scholar]
- 111. Lo LW, Chang HY, Scherlag BJ, Lin YJ, Chou YH, Lin WL, Chen SA, Po SS. Temporary suppression of cardiac ganglionated plexi leads to long‐term suppression of atrial fibrillation: evidence of early autonomic intervention to break the vicious cycle of “AF begets AF”. J Am Heart Assoc 2016;5:e003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nazeri A, Ganapathy AV, Massumi A, Massumi M, Tuzun E, Stainback R, Segura AM, Elayda MA, Razavi M. Effect of botulinum toxin on inducibility and maintenance of atrial fibrillation in ovine myocardial tissue. Pacing Clin Electrophysiol 2017;40:693–702. [DOI] [PubMed] [Google Scholar]
- 113. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev-Prokophiev A, Zheleznev S, Shipulin V, Salakhutdinov N. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. J Am Coll Cardiol 2014;64:628–629. [DOI] [PubMed] [Google Scholar]
- 114. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev-Prokophiev A, Zheleznev S, Shipulin V, Lomivorotov VV, Karaskov A, Po SS, Steinberg JS. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one year follow up of a randomized pilot study. Circ Arrhythm Electrophysiol 2015;8:1334–1341. CIRCEP. [DOI] [PubMed] [Google Scholar]
- 115. Romanov A, Pokushalov E, Ponomarev D, Bayramova S, Shabanov V, Losik D, Stenin I, Elesin D, Mikheenko I, Strelnikov A, Sergeevichev D, Kozlov B, Po SS, Steinberg JS. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: three-year follow-up of a randomized study. Heart Rhythm 2019;16:172–177. [DOI] [PubMed] [Google Scholar]
- 116. Waldron NH, Cooter M, Haney JC, Schroder JN, Gaca JG, Lin SS, Sigurdsson MI, Fudim M, Podgoreanu MV, Stafford-Smith M. Temporary autonomic modulation with botulinum toxin type A to reduce atrial fibrillation after cardiac surgery. Heart Rhythm 2019;16:178–184. [DOI] [PubMed] [Google Scholar]
- 117. Wang H, Zhang Y, Xin F, Jiang H, Tao D, Jin Y, He Y, Wang Q, Po SS. Calcium-induced autonomic denervation in patients with post-operative atrial fibrillation. J Am Coll Cardiol 2021;77:57–67. [DOI] [PubMed] [Google Scholar]
- 118. O’Quinn MP, Dormer KJ, Huizar JF, Nguyen KT, Kaszala K, Sima A, Ellenbogen KA, Tan AY. Epicardial injection of nanoformulated calcium into cardiac ganglionic plexi suppresses autonomic nerve activity and postoperative atrial fibrillation. Heart Rhythm 2019;16:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yu L, Scherlag BS, Dormer K, Rutel I, Huang B, Zhou X, Kuriakose AE, Nguyen KK, Po S. Targeted ganglionated plexi denervation using magnetic nanoparticles carrying calcium chloride payload. JACC Clin Electrophysiol 2018;4:1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Heck C, Helmers SL, DeGiorgio CM. Vagus nerve stimulation therapy, epilepsy, and device parameters Scientific basis and recommendations for use. Neurology 2002;59:S31–S37. [DOI] [PubMed] [Google Scholar]
- 121. Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 2005;58:347–354. [DOI] [PubMed] [Google Scholar]
- 122. Hanna P, Shivkumar K, Ardell JL. Calming the nervous heart: autonomic therapies in heart failure. Card Fail Rev 2018;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007;117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van WD, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009;2:692–699. [DOI] [PubMed] [Google Scholar]
- 125. Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 2015;309:H1198–H1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Beaumont E, Wright GL, Southerland EM, Li Y, Chui R, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal remodeling and cardiac hypertrophy induced by chronic pressure overload in guinea pig. Am J Physiol Heart Circ Physiol 2016;310:H1349–H1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tracey KJ. The inflammatory reflex. Nature 2002;420:853. [DOI] [PubMed] [Google Scholar]
- 128. Stavrakis S, Humphrey MB, Scherlag B, Iftikhar O, Parwani P, Abbas M, Filiberti A, Fleming C, Hu Y, Garabelli P. Low-level vagus nerve stimulation suppresses post-operative atrial fibrillation and inflammation: a randomized study. JACC Clin Electrophysiol 2017;3:929–938. [DOI] [PubMed] [Google Scholar]
- 129. Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, Sato T. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation 2005;112:164–170. [DOI] [PubMed] [Google Scholar]
- 130. Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R, Po SS. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol 2011;57:563–571. [DOI] [PubMed] [Google Scholar]
- 131. Chinda K, Tsai W-C, Chan Y-H, Lin AY-T, Patel J, Zhao Y, Tan AY, Shen MJ, Lin H, Shen C. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm 2016;13:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jiang Z, Zhao Y, Tsai W-C, Yuan Y, Chinda K, Tan J, Onkka P, Shen C, Chen LS, Fishbein MC. Effects of vagal nerve stimulation on ganglionated plexi nerve activity and ventricular rate in ambulatory dogs with persistent atrial fibrillation. JACC Clin Electrophysiol 2018;4:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fallgatter A, Neuhauser B, Herrmann M, Ehlis A-C, Wagener A, Scheuerpflug P, Reiners K, Riederer P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm 2003;110:1437–1443. [DOI] [PubMed] [Google Scholar]
- 134. Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014;7:871–877. [DOI] [PubMed] [Google Scholar]
- 135. Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 2015;309:H1740–H1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, Varma V, Sha Y, Stavrakis S, Po SS. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm 2013;10:428–435. [DOI] [PubMed] [Google Scholar]
- 137. Stavrakis S, Stoner JA, Humphrey MB, Morris L, Filiberti A, Reynolds JC, Elkholey K, Javed I, Twidale N, Riha P, Varahan S, Scherlag BJ, Jackman WM, Dasari TW, Po SS. TREAT AF (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC Clin Electrophysiol 2020;6:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Deuchars SA, Lall VK, Clancy J, Mahadi M, Murray A, Peers L, Deuchars J. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol 2018;103:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 2015;8:624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Verlinden T, Rijkers K, Hoogland G, Herrler A. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol Scand 2016;133:173–182. [DOI] [PubMed] [Google Scholar]
- 141. Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol 2007;50:335–343. [DOI] [PubMed] [Google Scholar]
- 142. Ogawa M, Tan AY, Song J, Kobayashi K, Fishbein MC, Lin S-F, Chen LS, Chen P-S. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm 2009;6:1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Leftheriotis D, Flevari P, Kossyvakis C, Katsaras D, Batistaki C, Arvaniti C, Giannopoulos G, Deftereos S, Kostopanagiotou G, Lekakis J. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm 2016;13:2111–2117. [DOI] [PubMed] [Google Scholar]
- 144. Connors CW, Craig WY, Buchanan SA, Poltak JM, Gagnon JB, Curry CS. Efficacy and efficiency of perioperative stellate ganglion blocks in cardiac surgery: a pilot study. J Cardiothorac Vasc Anesth 2018;32:e28–e30. [DOI] [PubMed] [Google Scholar]
- 145. Dusi V, Sorg JM, Gornbein J, Gima J, Yanagawa J, Lee JM, Vecerek N, Vaseghi M, Bradfield JS, De Ferrari GM, Shivkumar K, Ajijola OA. Prognostic impact of atrial rhythm and dimension in patients with structural heart disease undergoing cardiac sympathetic denervation for ventricular arrhythmias. Heart Rhythm 2020;17:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet (London, England) 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 147. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet (London, England) 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 148. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 149. Sakakura K, Roth A, Ladich E, Shen K, Coleman L, Joner M, Virmani R. Controlled circumferential renal sympathetic denervation with preservation of the renal arterial wall using intraluminal ultrasound: a next-generation approach for treating sympathetic overactivity. EuroIntervention 2015;10:1230–1238. [DOI] [PubMed] [Google Scholar]
- 150. Pathak A, Coleman L, Roth A, Stanley J, Bailey L, Markham P, Ewen S, Morel C, Despas F, Honton B. Renal sympathetic nerve denervation using intraluminal ultrasound within a cooling balloon preserves the arterial wall and reduces sympathetic nerve activity. EuroIntervention 2015;11:477. [DOI] [PubMed] [Google Scholar]
- 151. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L, Wang Y, Jay D, Skeik N, Schwartz R, Rader F, Dohad S, Victor R, Sanghvi K, Costello J, Walsh C, Abraham J, Owan T, Abraham A, Fisher NDL, Mauri L, Sobieszczky P, Williams J, Bloch MJ, Roongsritong C, Todoran T, Basile J, Powers E, Hodskins E, Fong P, Laffer C, Gainer J, Robbins M, Reilly JP, Cash M, Goldman J, Aggarwal S, Ledley G, Hsi D, Martin S, Portnay E, Calhoun D, McElderry T, Maddox W, Oparil S, Huang P-H, Jose P, Khuddus M, Zentko S, O'Meara J, Barb I, Garasic J, Drachman D, Zusman R, Rosenfield K, Devireddy C, Lea J, Wells B, Stouffer R, Hinderliter A, Pauley E, Potluri S, Biedermann S, Bangalore S, Williams S, Zidar D, Shishehbor M, Effron B, Costa M, Kirtane AJ, Radhakrishnan J, Lobo MD, Saxena M, Mathur A, Jain A, Sayer J, Iyer SG, Robinson N, Edroos SA, Levy T, Patel A, Beckett D, Bent C, Davies J, Chapman N, Shun-Shin M, Howard J, Sharp ASP, Joseph A, D'Souza R, Gerber R, Faris M, Marshall AJ, Elorz C, Lurz P, Höllriegel R, Fengler K, Rommel K-P, Mahfoud F, Böhm M, Ewen S, Lucic J, Schmieder RE, Ott C, Schmid A, Uder M, Rump LC, Stegbauer J, Kröpil P, Azizi M, Sapoval M, Cornu E, Fouassier D, Gosse P, Cremer A, Trillaud H, Papadopoulos P, Pathak A, Honton B, Lantelme P, Berge C, Courand P-Y, Daemen J, Feyz L, Blankestijn PJ, Voskuil M, Rittersma Z, Kroon AA, van Zwam WH, Persu A, Renkin J. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 152. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–1170. [DOI] [PubMed] [Google Scholar]
- 153. Kiuchi MG, Chen S, Hoye NA, Pürerfellner H. Pulmonary vein isolation combined with spironolactone or renal sympathetic denervation in patients with chronic kidney disease, uncontrolled hypertension, paroxysmal atrial fibrillation, and a pacemaker. J Interv Card Electrophysiol 2018;51:51–59. [DOI] [PubMed] [Google Scholar]
- 154. Chen S, Kiuchi MG, Yin Y, Liu S, Schratter A, Acou W‐J, Meyer C, Pürerfellner H, Chun KRJ, Schmidt B. Synergy of pulmonary vein isolation and catheter renal denervation in atrial fibrillation complicated with uncontrolled hypertension: mapping the renal sympathetic nerve and pulmonary vein (the pulmonary vein isolation plus renal denervation strategy)? J Cardiovasc Electrophysiol 2019;30:658–667. [DOI] [PubMed] [Google Scholar]
- 155. Kiuchi MG, Chen S, e Silva GR, Paz LMR, Kiuchi T, de Paula Filho AG, Souto GLL. The addition of renal sympathetic denervation to pulmonary vein isolation reduces recurrence of paroxysmal atrial fibrillation in chronic kidney disease patients. J Interv Card Electrophysiol 2017;48:215–222. [DOI] [PubMed] [Google Scholar]
- 156. Feyz L, Theuns DA, Bhagwandien R, Strachinaru M, Kardys I, Van Mieghem NM, Daemen J. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol 2019;108:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. McLellan AJA, Schlaich MP, Taylor AJ, Prabhu S, Hering D, Hammond L, Marusic P, Duval J, Sata Y, Ellims A, Esler M, Peter K, Shaw J, Walton A, Kalman JM, Kistler PM. Reverse cardiac remodeling after renal denervation: atrial electrophysiologic and structural changes associated with blood pressure lowering. Heart Rhythm 2015;12:982–990. [DOI] [PubMed] [Google Scholar]
- 158. Zhao Q, Yu S, Zou M, Dai Z, Wang X, Xiao J, Huang C. Effect of renal sympathetic denervation on the inducibility of atrial fibrillation during rapid atrial pacing. J Interv Card Electrophysiol 2012;35:119–125. [DOI] [PubMed] [Google Scholar]
- 159. Wang X, Zhao Q, Huang H, Tang Y, Xiao J, Dai Z, Yu S, Huang C. Effect of renal sympathetic denervation on atrial substrate remodeling in ambulatory canines with prolonged atrial pacing. PLoS One 2013;8:e64611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hou Y, Hu J, Po SS, Wang H, Zhang L, Zhang F, Wang K, Zhou Q. Catheter-based renal sympathetic denervation significantly inhibits atrial fibrillation induced by electrical stimulation of the left stellate ganglion and rapid atrial pacing. PLoS One 2013;8:e78218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger H-R, Casadei B, Reilly SN, Verheule S, Böhm M. Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol 2015;8:466–474. [DOI] [PubMed] [Google Scholar]
- 162. Yu L, Huang B, Wang Z, Wang S, Wang M, Li X, Zhou L, Meng G, Yuan S, Zhou X, Jiang H. Impacts of renal sympathetic activation on atrial fibrillation: the potential role of the autonomic cross talk between kidney and heart. J Am Heart Assoc 2017;6:e004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, Polyakov K, Ptaszynski P, Keweloh B, Yao CJ, Pokushalov EA, Romanov AB. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 2020;323:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, Lader JM, Mahoney VM, Budylin T, Älvstrand M, Rakowski-Anderson T, Bharmi R, Shah R, Fowler S, Holmes D, Farazi TG, Chinitz LA, Morley GE. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm 2012;9:1426–1433.e1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yu L, Huang B, He W, Wang S, Liao K, Zhou X, He B, Lu Z, Jiang H. Spinal cord stimulation suppresses focal rapid firing–induced atrial fibrillation by inhibiting atrial ganglionated plexus activity. J Cardiovasc Pharmacol 2014;64:554–559. [DOI] [PubMed] [Google Scholar]
- 166. Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, Yu L, Jiang H. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm 2016;13:274–281. [DOI] [PubMed] [Google Scholar]
- 167. Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Boehm M. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol 2013;24:1028–1033. [DOI] [PubMed] [Google Scholar]
- 168. Liao K, Yu L, Zhou X, Saren G, Wang S, Wang Z, Huang B, Yang K, Jiang H. Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can J Cardiol 2015;31:767–774. [DOI] [PubMed] [Google Scholar]
- 169. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC. AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017;72:e91–e220. [DOI] [PubMed] [Google Scholar]