Abstract
Hyperreflexia of the peripheral chemoreceptors is a potential contributor of apnoeas of prematurity (AoP). Recently, it was shown that elevated P2X3 receptor expression was associated with elevated carotid body afferent sensitivity. Therefore, we tested whether P2X3 receptor antagonism would reduce AoP known to occur in newborn rats. Unrestrained whole-body plethysmography was used to record breathing and from this the frequency of apnoeas at baseline and following administration of either a P2X3 receptor antagonist - AF-454 (5 mg/kg or 10 mg/kg s.c.) or vehicle was derived. In a separate group, we tested the effects of AF-454 (10 mg/kg) on the hypoxic ventilatory response (10 % FiO2). Ten but not 5 mg/kg AF-454 reduced the frequency of AoP and improved breathing regularity significantly compared to vehicle. Neither AF-454 (both 5 and 10 mg/kg) nor vehicle affected baseline respiration. However, P2X3 receptor antagonism (10 mg/kg) powerfully blunted hypoxic ventilatory response to 10 % FiO2. These data suggest that P2X3 receptors contribute to AoP and the hypoxic ventilatory response in newborn rats but play no role in the drive to breathe at rest.
Keywords: Apnoea of prematurity, P2X3 receptors, Carotid body, Neonates
1. Introduction
Apnoea of prematurity (AoP) is highly prevalent in human infants born before 32 weeks of gestation, and its incidence is inversely related to gestational age and weight at birth (Henderson-Smart, 1981; Zhao et al., 2011). It is defined as an interruption of breathing for at least 20 s accompanied by both a desaturation of oxygen (SpO2) to ≤ 80 % and bradycardia (heart rate lower than 2/3 of the baseline) for at least four seconds (Eichenwald et al., 2016; Ren et al., 2015; Zhao et al., 2011).
As with sleep apnoea, AoP is classified as either central (characterized by cessation of breathing effort), or obstructive where the airflow is obstructed usually at the pharyngeal level, or mixed (Eichenwald et al., 2016). In the short-term it may lead to cerebral hypoperfusion and eventually to ischemic brain injury (Pichler et al., 2003; Zhao et al., 2011). Indeed, persistence of AoP has been associated with neurodevelopmental impairment (Horne et al., 2017; Janvier et al., 2004).
The mechanisms underlying AoP are not fully understood, which makes the condition difficult to treat. Potential mechanisms causing AoP include: an immaturity of the brainstem respiratory rhythm generator, decreased central CO2 sensitivity and increased peripheral chemoreceptor sensitivity to hypoxia (Martin and Wilson, 2012). An increase in carotid body sensitivity to hypoxia can trigger respiratory instability through an increase in chemical loop gain (Dempsey et al., 2014; Younes, 2014). In preterm infants with a high frequency of AoP, Nock et al. (2004) reported an exaggerated ventilatory response to hypoxia (15 % fraction of inspired O2, FiO2) consistent with CB hyperreflexia. Moreover, Cardot et al. (2007) found that acute hyperoxia (100 % FiO2), used to reversibly block peripheral chemoreceptors, caused the greatest reduction in minute ventilation in the preterm infants that had the highest number of apnoeic episodes. These data suggest that peripheral chemoreceptor hyperreflexia may exist in infants with AoP.
Hyper-excitability of the carotid body has been found in animals with heart failure (Schultz et al., 2013). Our recent studies also demonstrated peripheral chemoreceptor hyperreflexia in the spontaneously hypertensive rat (SHR), which is caused by upregulated expression and activation of P2X3 receptors in chemoreceptive petrosal ganglion neurones (Pijacka et al., 2016). We showed that selective P2X3 receptor antagonism abolished aberrant tonic discharge of chemoreceptive petrosal neurones, and normalised the peripheral chemoreceptor reflex sensitivity measured from reflex evoked increases in sympathetic activity in the SHR in vivo (Pijacka et al., 2016). Finally, expression of P2X3 receptors can be regulated by the stage of development in rats. They have been shown to be higher in the intrinsic neurons of the myenteric plexus during the post-natal period but decline as maturity ensues (Xiang and Burnstock, 2004). Based on these findings, we tested the hypothesis that selective P2X3 receptor antagonism would reduce the frequency of the AoP in newborn rats.
2. Materials and methods
All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986. Experiments were conducted on male Wistar rats on the day of birth (P0, total n = 60).
2.1. Experimental design
All perinatal pups exhibited apnoeas during the 10 min baseline recording within a range consistent with previous reports (Bairam et al., 2013a; Lefter et al., 2007). Two experimental protocols were performed in separate groups of animals. In the first, the effects of a selective P2X3 receptor antagonist - AF-454 on respiratory parameters and apnoea frequency were examined. In this protocol, pups were assigned randomly to one of three experimental groups: vehicle (n = 5), AF-454 (5 mg/kg, n = 5) or AF-454 (10 mg/kg, n = 7) all administered subcutaneously (s.c.). The experimenter was not blinded to these groups but data analysis was automated. In the second experimental protocol, the respiratory response to normobaric hypoxia (10 % FiO2 in N2) was assessed before and after AF- 454 (10 mg/kg s.c., n = 5).
In addition, to establish systemic exposure to AF-454, separate P0 rats (n = 34) were injected s.c. with either 5 or 10 mg/kg dose and subsequently killed by decapitation for blood and brain collection at three different time-points (10, 20 or 60 min after drug injection). The timings of collections were related to the time points at which respiratory variables were recorded. Blood was collected into lithium heparin tubes, processed in a refrigerated-centrifuge (10.000 rpm at 4 °C for 5 min) and the resultant plasma was stored at −80 °C. The brain was quickly extracted and placed into Eppendorf tubes, flash-frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. Quantification of AF-454 was carried out by Afferent Pharmaceuticals (San Mateo, CA, USA) as described previously (Pijacka et al., 2016). The number of samples analysed for 5 mg/kg dose was: n = 5 at 10 and 20 min, and n = 4 at 60 min. For 10 mg/kg: n = 5 at 10 min, n = 7 at 20 min and n = 8 at 60 min.
2.2. P2X3 receptor antagonist
A P2X3 receptor antagonist, AF-454 (Afferent Pharmaceuticals, San Mateo, CA, USA), was freshly prepared on each experimental day. The drug was dissolved in propylene glycol (50 % propylene glycol in sterile distilled water) using an ultrasonic bath. Two different AF-454 doses were used in the present study: 5 mg/kg and 10 mg/kg. The drug was administered subcutaneously in a volume of 50–60 μl. An equivalent volume of propylene glycol (50 % in sterile distilled water) was used as a vehicle control.
2.3. Plethysmography
Unrestrained whole-body plethysmography (Emka Technologies, Paris, France) was used to record respiratory frequency, tidal volume and minute ventilation (Bairam et al., 2013a; Drorbaugh and Fenn, 1955). Newborn rats were placed inside an adapted, purpose-made, 45 mL animal chamber with an adjacent reference chamber that was connected to a differential pressure transducer and individual pneumotachographs. The temperature was kept constant throughout the experiments by using a homoeothermic blanket set at 35 °C as previously described (Miller and Spear, 2010). Calibration of the plethysmography system was performed using a two-point method as recommended by the manufacturer. Humidity and temperature were recorded from the bias flow outlet. During all plethysmography experiments the bias flow was kept constant at 100 mL/min. After a 5 min period of adaptation to the plethysmograph, baseline data were acquired for 10 min followed by a dose of AF-454 (either 5 or 10 mg/kg s.c.) or vehicle, and breathing was recorded for a further 30 min.
2.4. Hypoxia exposure
After a five-minute adaptation period, restful breathing was recorded for 5 min in normoxia (21 % FiO2 balance N2; baseline). Subsequently the inlet flow was changed to a hypoxic mixture (10 % FiO2 in N2; hypoxia) for 3 min. A 10 % FiO2 was reached within approximately 30 s from the start of its infusion and from this time onwards the ventilatory responses to hypoxia were measured. Then, the inlet flow was reverted to normoxic gas for a five min recovery period. All gases were humidified prior to animal exposure and the bias flow at both normoxia and hypoxia was kept constant at 100 mL/min. These procedures – baseline normoxic, hypoxic exposure and recovery – were performed three times: before AF-454 and at 10 and 20 min after AF-454 (10 mg/kg s.c.) administration.
2.5. Immunofluorescence
A separate group of pups (n = 4) were overdosed with ketamine (60 mg/kg, Vetalar, Zoetis, London, UK). The common carotid artery bifurcations were removed and the carotid bodies (CBs) were dissected under a stereo microscope (Leica M80) and fixed in ice-cold methanol/DMSO (4:1). Immunocytochemical labelling of P2X3 receptors and tyrosine hydroxylase was carried out as described previously (Pijacka et al., 2016). Briefly the carotid bifurcations were incubated with 10 % goat serum/0.1 % Saponin (Sigma-Aldrich, UK) and then transferred to the primary antibodies (rabbit anti- P2X3 receptor 1:25; APR026AN0202, Alomone, Israel; mouse anti–tyrosine hydroxylase 1:25; F-11, sc-25269, Santa Cruz Biotechnology). Antibody selectivity was confirmed as previously described using pre-absorption in anti-genic peptide (Pijacka et al., 2016). P2X3 receptors were visualised by goat anti-rabbit Alexa Fluor 488 (Thermo Fisher Scientific, UK) whereas tyrosine hydroxylase was visualized using the goat anti-mouse Alexa Fluor 594 (Thermo Fisher Scientific, UK). CBs were whole-mounted on slides using mounting medium (Vectashield, H-1000, Vector Laboratories) and later examined under a Leica DFC365FX (Leica Microsystems, UK) widefield microscope.
2.6. Plethysmography data analysis
Plethysmography data were acquired at a sampling frequency of 1000 Hz and analysed using IOX 2.9.5.28 software (Emka technologies, Paris, France). Respiratory flow was calculated from changes in chamber pressure taking into account the humidity and temperature of the air inside the chamber as described by Drorbaugh and Fenn (1955). Respiratory flow waveforms were imported into Spike2 (CED, Cambridge, UK) for analysis of the apnoeic events. We defined apnoeas as cessation in breathing lasting at least 3 breaths as proposed previously (Nanduri et al., 2012; Rieusset et al., 2013; Sheikhbahaei et al., 2017). For automated apnoea detection, a running average of expiratory time (TE) in 1-minute bins was calculated; apnoeas were then identified as breaths having > 3-fold the average TE. Defining apnoeas based on the entire respiratory cycle based on automated detection could be tricky due to the presence of sighs. The frequency of apnoeas was counted over a 10 min epoch at baseline, 10 and 20 min following administration of either AF-454 or vehicle and expressed as number of apnoeas/10 min. Respiratory minute volume (V̇E) was calculated from respiratory frequency (fR) and tidal volume (VT); (i.e. fR x VT). In addition, Poincare plots and analysis of short-term variability (SD1) and long-term variability (SD2) were performed to assess the regularity of breathing before and after drug exposure as described previously (Brennan et al., 2001; Peng et al., 2011). Briefly, a Poincare scattergram was constructed by plotting each breath-to-breath interval (BBn) against its subsequent interval (BBn+1). The analysis was carried out by fitting an ellipse to the scattergram, where the centre of the ellipse represented the average BBn. SD1 is the standard deviation of the scattergram perpendicular to the line of identity, whereas SD2 is the standard deviation of the scattergram along the line of identity. 200 breaths were used in these analyses.
2.7. Statistical analysis
Statistical analyses were conducted using SigmaStat 3.5 software (Systat Software Inc., San Jose, CA, USA). Normality of the data distribution was tested by Kolmogorov-Smirnov test. The statistical test performed is indicated in the Results or in the Figure legends. Data are presented as mean ± SEM, with a significance level of p < 0.05.
3. Results
3.1. Plasma and brain exposure of AF-454
Plasma and brain concentrations of AF-454 were measured in separate pups (n = 34) and data are presented in Fig. 1. The timings of blood samples were related to the time points at which respiratory variables were recorded.
Fig. 1.
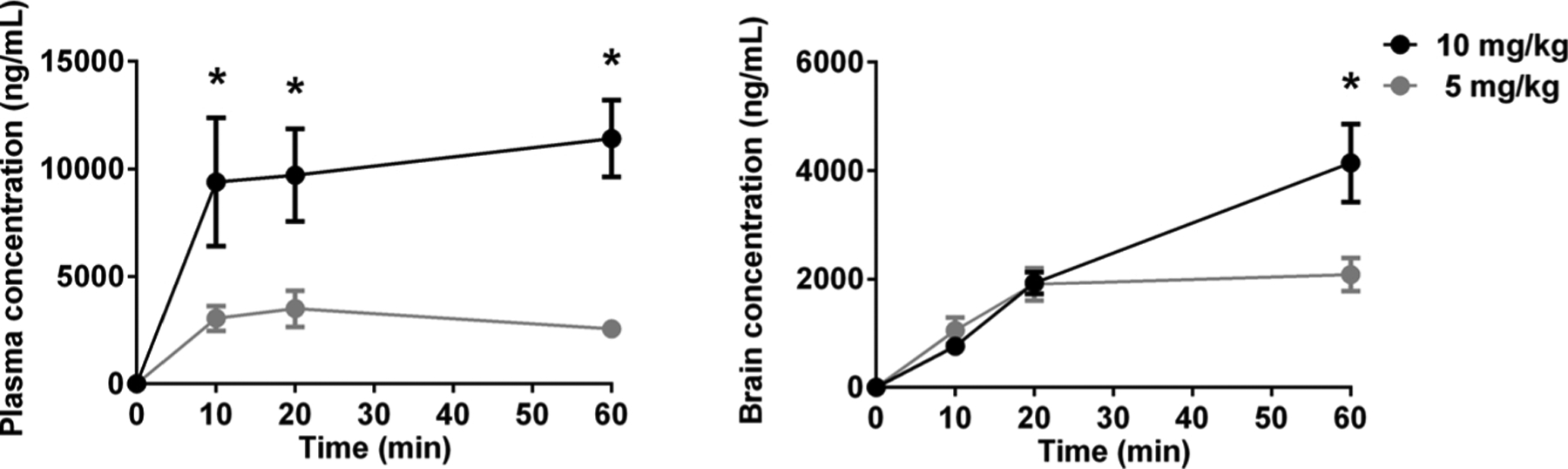
Pharmacokinetic profile of the two doses of AF-454 used in this study as detected in the plasma and brain from newborn rats at the day of birth (P0). Gray symbols, 5 mg/kg at 10 min (n = 5), 20 min (n = 5) and 60 min (n = 4); Black symbols, 10 mg/kg at 10 min (n = 5), 20 min (n = 7) and 60 min (n = 8). Data were analysed by two-way ANOVA with Tukey post hoc test. *P < 0.05 vs 5 mg/kg at the same time point.
3.2. Effect of AF-454 on baseline respiration
In normoxia, no differences in baseline respiratory frequency or tidal volume (and hence minute ventilation) were found after vehicle or either dose of AF-454 (5 or 10 mg/kg doses; Table 1).
Table 1.
Respiratory parameters before and following AF-454 or vehicle administration.
| AF-454 | AF-454 | Vehicle | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (5 mg/kg) | (10 mg/kg) | (n = 5) | |||||||
| (n = 5) | (n = 7) | ||||||||
| Baseline | 10 min | 20 min | Baseline | 10 min | 20 min | Baseline | 10 min | 20 min | |
| fR (breaths min −1) | 102 ± 3 | 101 ± 2 | 99 ± 4 | 104 ± 5 | 104 ± 5 | 101 ± 7 | 99 ± 6 | 94 ± 4 | 90 ± 6 |
| VT (ml kg −1) | 9.1 ± 0.3 | 9.2 ± 0.2 | 8.9 ± 0.4 | 8.5 ± 0.5 | 9.1 ± 0.8 | 9 ± 1 | 9.5 ± 0.9 | 10.1 ± 0.8 | 10.4 ± 1.5 |
| V̇E (ml kg−1 min −1) | 928 ± 44 | 926 ± 42 | 884 ± 53 | 892 ± 88 | 945 ± 127 | 940 ± 152 | 953 ± 112 | 984 ± 114 | 984 ± 172 |
Abbreviations: fR: Respiratory frequency, VT: Tidal volume, V̇E: Minute ventilation. For each time point, 10-minute epochs were considered for analysis. Data were analysed by repeated measures one-way ANOVA. No differences were found between the different time-points within each treatment group.
3.3. Effects of AF-454 on apnoea frequency
AF-454 (10 mg/kg s.c., n = 7) reduced the occurrence of apnoeas from 6.3 ± 1.7 apnoeas/10 min at baseline to 3.1 ± 0.9 apnoeas/10 min at 10 min post drug administration (P = 0.027). By 20 min after drug delivery this was further decreased to 2.4 ± 0.7 apnoeas/10 min (P = 0.024; Fig. 2B). We noted that the apnoeas were not preceded by any change in ventilation relative to baseline. In contrast, both the lower dose of AF-454 (5 mg/kg s.c. n = 5) and vehicle (n = 5) did not change the frequency of occurrence of apnoeas (Fig. 2B).
Fig. 2.
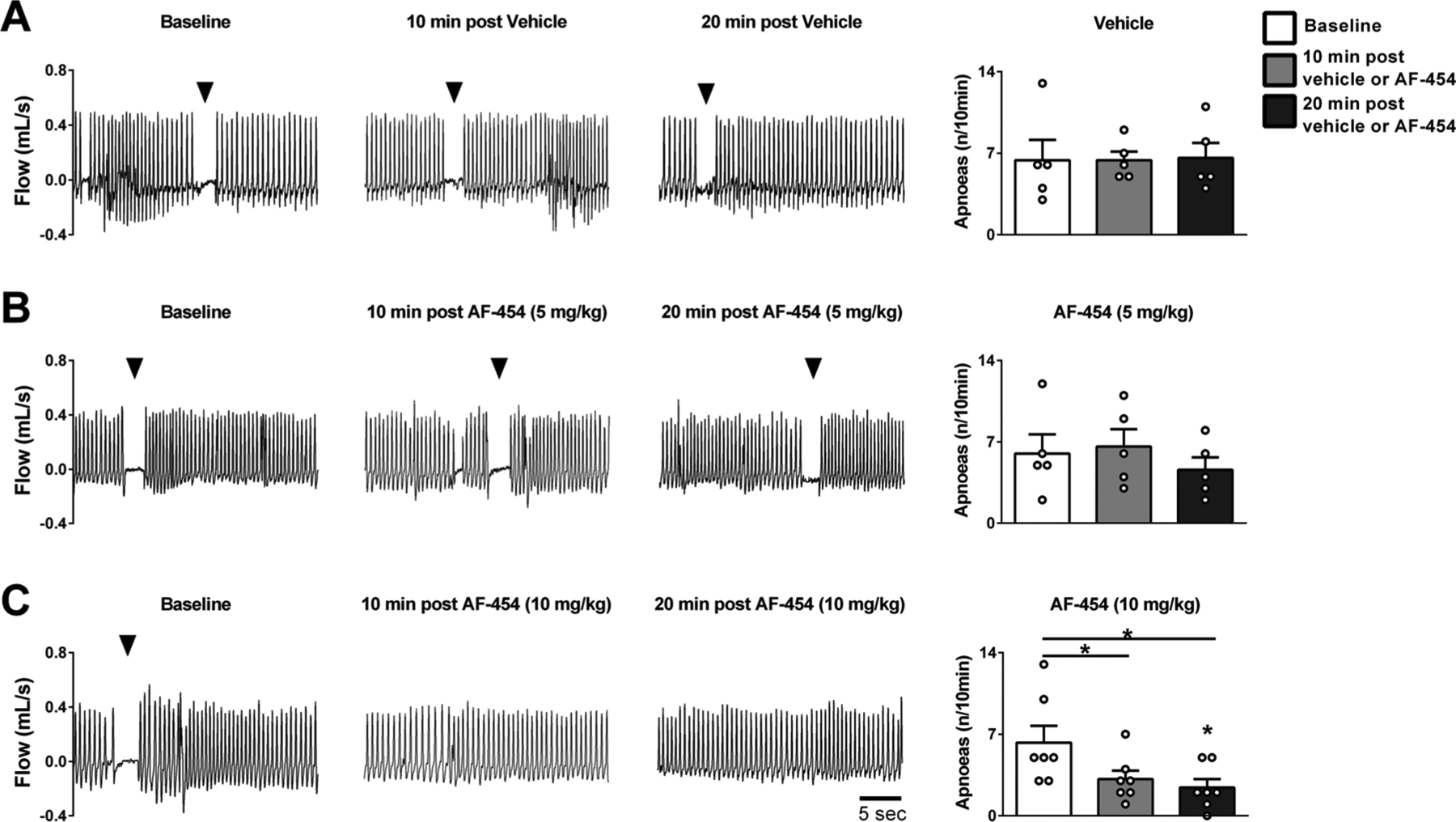
Representative plethysmographic recordings and grouped data showing the effects of vehicle or AF-454 on apnoea frequency at baseline, 10 and 20 min post injection. A. Vehicle (n = 5), B. AF-454 (5 mg/kg, n = 5) and C. AF-454 (10 mg/kg, n = 7). Arrows indicate an apnoea episode. Data were analysed by repeated measures one-way ANOVA with Tukey post hoc test. *P < 0.05 vs respective baseline values.
At the higher dose, P2X3 receptor blockade also increased the regularity of respiration in newborn rats. Fig. 3C represents the grouped data of SD1 (short term variability) and SD2 (long term variability). AF-454 (10 mg/kg, n = 7) decreased SD1 at 10 (P = 0.011) and 20 min (P = 0.041) post drug administration indicating a lower inter-breath variability. However, AF-454 did not change SD2 at either time point (NS). Both SD1 and SD2 were unaffected in pups receiving the lower dose of AF-454 (5 mg/kg, n = 5).
Fig. 3.
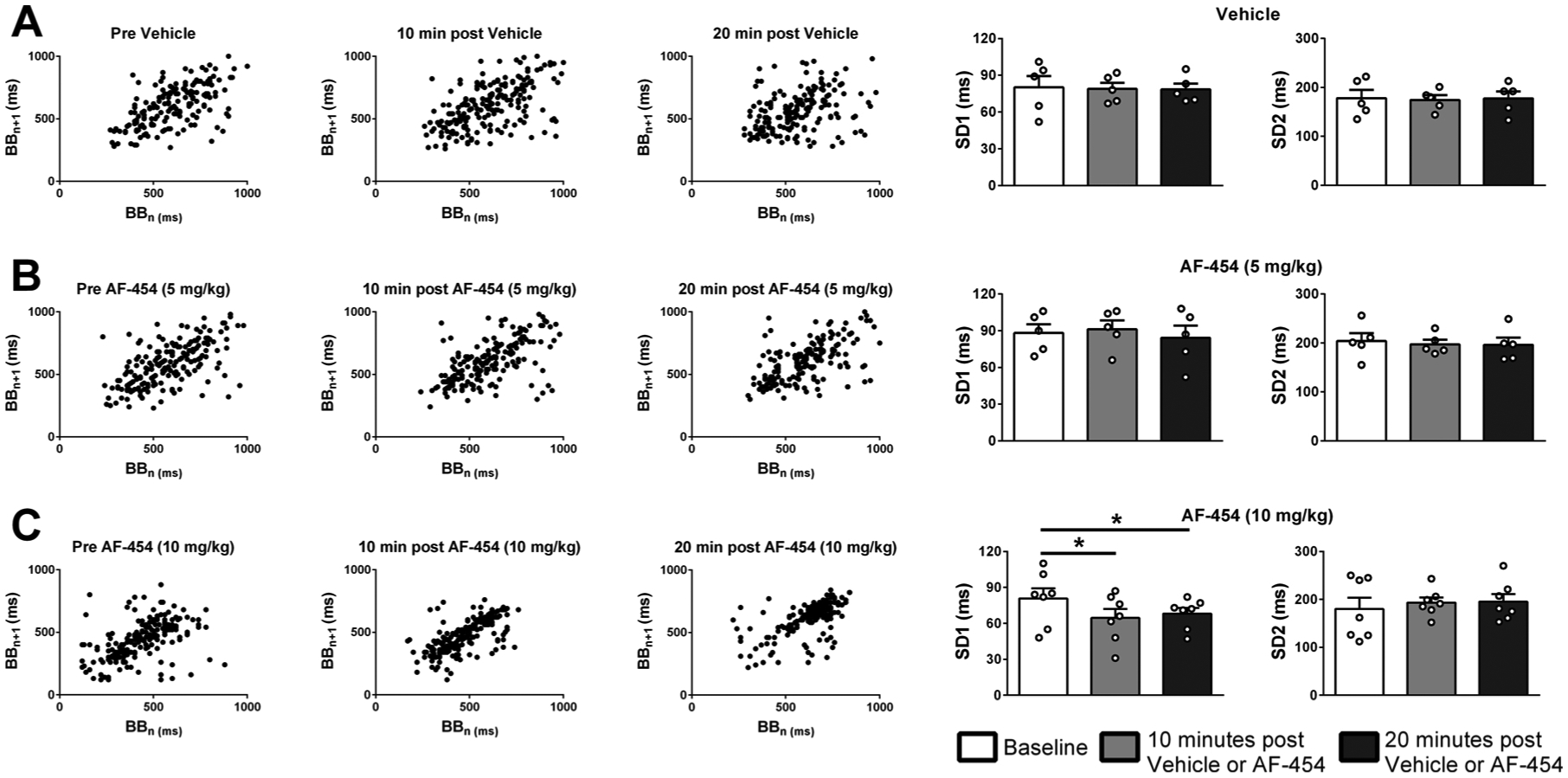
Representative Poincaré plots showing the consecutive breath-to-breath intervals and grouped data of SD1 (short term variability) and SD2 (long term variability) of breathing before, 10 and 20 min after vehicle or AF-454. A. Vehicle (n = 5), B. AF-454 (5 mg/kg, n = 5) and C. AF-454 (10 mg/kg, n = 7). Data were analysed by repeated measures one-way ANOVA with Tukey post hoc test. *P < 0.05 vs respective baseline values.
3.4. Effects of AF-454 on the response to hypoxia
Fig. 4A shows the changes in respiratory frequency, tidal volume and minute ventilation in response to hypoxia (10 % FiO2) before, 10 and 20 min after AF-454 (10 mg/kg s.c.) administration (n = 5). The reflex increases in respiratory frequency (P < 0.001) and minute ventilation (P < 0.01) in response to hypoxic exposure were blunted significantly after AF-454. Although tidal volume response to hypoxia presented a trend to a decrease, it was not significant (P = 0.055).
Fig. 4.
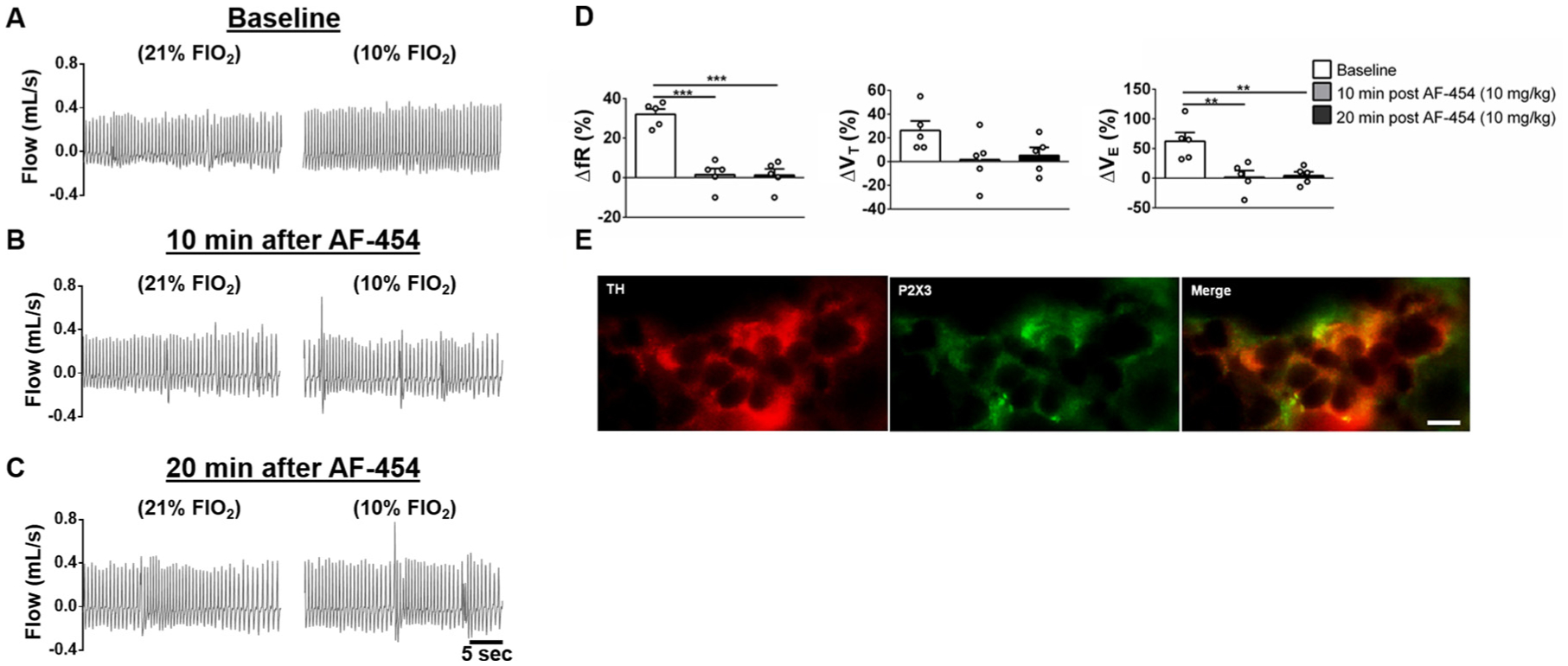
Effects of AF-454 (10 mg/kg, n = 5) on the acute ventilatory responses to hypoxia (10 % FiO2) and P2X3 receptor immunostaining in the rat carotid body. A, B and C. Representative plethysmographic recordings during normoxia (21 % FiO2) and hypoxia (10 % FiO2) at baseline (A), 10 (B) and 20 min after AF-454 (10 mg/kg) administration. D. Grouped data showing the changes in respiratory frequency, tidal volume and minute ventilation in response to 10 % FiO2 exposure at baseline, 10 and 20 min after AF-454 (10 mg/kg) administration. Data were analysed by repeated measures one-way ANOVA with Tukey post hoc test, **P < 0.01; ***P < 0.001 vs Baseline. E: P2X3 receptor and tyrosine hydroxylase (TH) immune-colocalization in the carotid body of newborn rats. Scale bar represents 10 μm.
3.5. Immunofluorescence
Fig. 4B demonstrates co-localization of P2X3 receptors and tyrosine hydroxylase immunofluorescence in the carotid body from a newborn rat. The presence of these receptors in the carotid body was observed in three additional rat pups.
4. Discussion
The data presented demonstrate that P2X3 receptor antagonism reduced the frequency of occurrence of AoP in newborn rats. After acute antagonism of P2X3 receptors (10 mg/kg AF-454), the frequency of AoP was reduced significantly by 51 % and 61 % at 10 and 20 min post-treatment, respectively. Both tested doses of AF-454 (5 and 10 mg/kg) did not alter baseline respiratory parameters evaluated (fR, VT and V̇E) indicating that P2X3 receptors are functionally inactive and do not play a role in the drive to breathe at rest under normoxic conditions. In contrast, the ventilatory response to hypoxia (fR and V̇E) was abolished after P2X3 antagonism with 10 mg/kg AF-454 suggesting that P2X3 receptors are crucial for the hypoxia-induced hyperventilation in newborn rat pups. We also confirmed that P2X3 receptors are expressed in the carotid body of P0 rats. Thus, our data suggest that P2X3-mediated endogenous signalling may contribute to AoP in this rat model. However, we cannot exclude a potential contribution from other signalling pathways within the carotid body or other afferent systems such as pulmonary/laryngeal mechanoreceptor afferents, which may also express P2X3 receptors (Cardot et al., 2007; Martin and Wilson, 2012). Given the finding of the antagonist within the brain (Fig. 1), we cannot rule out an effect within the central nervous system including an action on the central chemoreceptors, for example. However, this appears unlikely as despite numerous P2X receptor sub-types being identified in the ventrolateral medulla, which included the ventral respiratory column, P2X3 receptors were not identified (Thomas et al., 2001). Brain penetrance of this compound likely reflects a leaky blood brain barrier in these immature animals as AF-454 has not be found within the brain of mature rodents when administered systemically (A.P. Ford – personal communication). However, a caveat here is that the blood was not removed from the cerebral circulation so the brain samples did contain the drug within brain vasculature.
It has been suggested that peripheral chemoreceptor gain is increased in AoP and can trigger exaggerated ventilatory responses to hypoxia (Martin and Wilson, 2012). The resulting ventilatory overshoot would drop PaCO2 below the central apnoeic threshold thereby eliciting a subsequent apnoeic episode (Al-Matary et al., 2004; Cardot et al., 2007). Such a mechanism is comparable to the heightened chemical loop gain thought to underpin sleep disordered breathing in adult humans (Dempsey et al., 2014; Eckert, 2018; Edwards et al., 2016). In this context, the reduction of peripheral chemoreceptor sensitivity with hyperoxia has lowered the incidence of sleep apnoeas (Edwards et al., 2014) and this supports the notion of the carotid body as a potential therapeutic target. Additionally, our study confirms that pharmacological antagonism of P2X3 receptors, which may include those within the carotid body, is a viable treatment strategy for rat AoP. Barrington et al. (1986), found that the respiratory stimulant – doxapram, reduced AoP in newborn humans. Although it is well established as a stimulant of carotid bodies, doxapram also activates central chemoreceptors (Osaka et al., 2014) so the mechanism of the response described by Barrington et al. (1986) remains equivocal.
P2X3 receptors in the carotid body appear to mediate its hyper-excitability in hypertensive rats, consisting of both hyperreflexia and aberrant tone generation (Pijacka et al., 2016). However, given the lack of a change in baseline respiration after P2X3 receptor antagonism, it appears that the carotid bodies of P0 rat pups do not generate aberrant tone, at least in afferent fibres connected to brainstem circuits controlling breathing (Zera et al., 2019). In contrast, Niane et al. (2011) suggested that P2X3 receptors could contribute to both the baseline drive to breathe and chemoreflex gain during hypoxia in newborn rats. These authors found that both suramin, a non-selective P2X receptor antagonist and A-317491, a selective P2X3 antagonist, decreased baseline ventilation and the responses to hypoxia in newborn rats. However, Niane et al. (2011) studied newborn rats at P4, P7, P12 and P21 while in the present study we only used newborn rats on the day of birth and a different selective P2X3 receptor antagonist. All told, that the contribution of P2X3 receptor signalling for baseline ventilation may change with development.
An interesting aspect of our study was the robust reduction in the ventilatory response to hypoxia after P2X3 receptor blockade in P0 day rats. These findings are in agreement with the results of Niane et al. (2011), who demonstrated that P2X3 antagonism caused a significant reduction in the respiratory responses to hypoxia in P4, P7 and P21 rats. Although the role of ATP in the carotid body during development of the rat is not fully explored, some studies have shown that ATP contributes to the hypoxic ventilatory response through P2X receptors in newborn rats from four days old and later (Bairam et al., 2013b; Niane et al., 2012, 2011). We speculate that at birth there is either enhanced ATP release to hypoxia and/or the P2X3 receptors are upregulated at this time to sensitise the detection of hypoxia, which may be a protective mechanism to ensure initiation and maintenance of respiration at the onset of air breathing. Thereafter these receptors may down regulate.
Given the lack of an effect of P2X3 receptor blockade on ventilation at rest, the question prompted is how blocking these receptors attenuates the AoP in newborn rats. We speculate that the P2X3 receptor antagonist was acting, in part, at the level of the carotid body. We did not observe any hyperventilation preceding the AoP suggesting that disturbances in the partial pressure of carbon dioxide and chemical loop gain, known to trigger sleep apnoea in adults (Dempsey et al., 2014; Younes, 2014), are unlikely and therefore cannot explain the AoP recorded in the present study. However, we do not believe that this rules out a role for the carotid body in mediating AoP. As we recently reviewed (Zera et al., 2019), functionally separate sub-populations of glomus cells connected to distinct reflex pathways exist. We propose that one line of chemoreceptive petrosal neurone transmission contains P2X3 receptors that are connected to glomus cells that release episodically ATP and project to post-inspiratory neuronal circuits causing prolongation of their activity resulting in inspiratory off-switching and increasing expiratory time causing the apnoeas. Alternatively, we do not rule out mechanisms involving immaturity of other reflexes such as the Hering-Breuer deflation/inflation reflexes (Dutschmann et al., 2009, 2014; Hannam et al., 1998; Thach and Franz, 1978).
The data presented support peripheral P2X3 receptor antagonism as a potential novel approach to suppress AoP in newborn rats although penetrance into the brain may present undesirable off target effects. The treatment for AoP in human infants includes methylxanthines such as caffeine and theophylline (Henderson-Smart and De Paoli, 2013). However, methylxanthine therapy has diverse side-effects such as ta-chycardia, emesis and jitteriness (Eichenwald et al., 2016) and has been associated with necrotizing enterocolitis (Cox et al., 2016). Because of this, new therapies for the treatment of AoP are needed and P2X3 receptor antagonism could fulfil such a role.
Acknowledgements
This work was funded by grants from São Paulo Research Foundation (FAPESP; Grant #2016/02184-0) and British Heart Foundation. APA was funded by NIH (1R01AT008632-01). JFR is funded by a programme grant from the Health Research Council of New Zealand and the Royal Society of New Zealand. We thank Afferent Pharmaceuticals for the generous gift of AF-454.
Footnotes
Declaration of Competing Interest
Both A.P.F. and J.G. were employees of Afferent Pharmaceuticals. The other authors declare no competing interests.
References
- Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H, 2004. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin. Perinatol 28, 264–272. 10.1053/j.semperi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Bairam A, Lumbroso D, Joseph V, 2013a. Effect of progesterone on respiratory response to moderate hypoxia and apnea frequency in developing rats. Respir. Physiol. Neurobiol 185, 515–525. 10.1016/j.resp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Bairam A, Niane LM, Joseph V, 2013b. Role of ATP and adenosine on carotid body function during development. Respir. Physiol. Neurobiol 185, 57–66. 10.1016/j.resp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Barrington KJ, Finer NN, Peters KL, Barton J, 1986. Physiologic effects of doxapram in idiopathic apnea of prematurity. J. Pediatr 108, 125–129. 10.1016/s0022-3476(86)80786-7. [DOI] [PubMed] [Google Scholar]
- Brennan M, Palaniswami M, Kamen P, 2001. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng 48, 1342–1347. 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- Cardot V, Chardon K, Tourneux P, Micallef S, Stéphan E, Léké A, Bach V, Libert JP, Telliez F, 2007. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr. Res 62, 591–596. 10.1203/PDR.0b013e318155868e. [DOI] [PubMed] [Google Scholar]
- Cox C, Hashem NG, Tebbs J, Brandon Bookstaver P, Iskersky V, 2016. Evaluation of caffeine and the development of necrotizing enterocolitis. J. Neonatal. Med 8, 339–347. 10.3233/NPM-15814059. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Xie A, Patz DS, Wang D, 2014. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment–considerations beyond airway anatomy. J. Appl. Physiol. (Bethesda, Md. : 1985) 116, 3–12. 10.1152/japplphysiol.01054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO, 1955. A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Rybak IA, Dick TE, 2009. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J. Physiol 587, 4931–4948. 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Bautista TG, Morschel M, Dick TE, 2014. Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation. Respir. Physiol. Neurobiol 195, 44–49. 10.1016/j.resp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, 2018. Phenotypic approaches to obstructive sleep apnoea – new pathways for targeted therapy. Sleep Med. Rev 37, 45–59. 10.1016/j.smrv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, Malhotra A, Wellman A, 2014. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J. Physiol 592, 4523–4535. 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS, Wellman A, 2016. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med 194, 1413–1422. 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenwald EC, Committee on Fetus and Newborn, American Academy of Pediatrics, 2016. Apnea of prematurity. Pediatrics 137, e20153757. 10.1542/peds.2015-3757. [DOI] [Google Scholar]
- Hannam S, Ingram DM, Milner AD, 1998. A possible role for the Hering-Breuer deflation reflex in apnea of prematurity. J. Pediatr 132, 35–39. 10.1016/s0022-3476(98)70481-0. [DOI] [PubMed] [Google Scholar]
- Henderson-smart DJ, De Paoli AG, 2013. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev 2010. 10.1002/14651858.CD000140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Smart DJ, 1981. The effect of gestational-age on the incidence and duration of recurrent apnea in newborn babies. Aust. Paediatr. J 17, 273–276. [DOI] [PubMed] [Google Scholar]
- Horne RSC, Fung ACH, NcNeil S, Fyfe KL, Odoi A, Wong FY, 2017. The longitudinal effects of persistent apnea on cerebral oxygenation in infants born preterm. J. Pediatr 182, 79–84. 10.1016/j.jpeds.2016.11.081. [DOI] [PubMed] [Google Scholar]
- Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ, 2004. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J. Perinatol 24, 763–768. 10.1038/sj.jp.7211182. [DOI] [PubMed] [Google Scholar]
- Lefter R, Morency CE, Joseph V, 2007. Progesterone increases hypoxic ventilatory response and reduces apneas in newborn rats. Respir. Physiol. Neurobiol 156, 9–16. 10.1016/j.resp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Wilson CG, 2012. Apnea of prematurity. Comprehensive Physiology John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 2923–2931. 10.1002/cphy.c100021. [DOI] [PubMed] [Google Scholar]
- Miller SS, Spear NE, 2010. Mere odor exposure learning in the rat neonate immediately after birth and one day later. Dev. Psychobiol 52, 343–351. 10.1002/dev.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, et al. , 2012. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc. Natl. Acad. Sci 109, 2515–2520. 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niane LM, Donnelly DF, Joseph V, Bairam A, 2011. Ventilatory and carotid body chemoreceptor responses to purinergic P2X receptor antagonists in newborn rats. J. Appl. Physiol. (Bethesda, Md. : 1985) 110, 83–94. 10.1152/japplphysiol.00871.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niane LM, Joseph V, Bairam A, 2012. Systemic blockade of nicotinic and purinergic receptors inhibits ventilation and increases apnoea frequency in newborn rats. Exp. Physiol 97, 981–993. 10.1113/expphysiol.2012.065011. [DOI] [PubMed] [Google Scholar]
- Nock ML, DiFiore JM, Arko MK, Martin RJ, 2004. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J. Pediatr 144, 291–295. 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Osaka Y, Onimaru H, Kotani S, Kashiwagi M, Morisaki H, Takeda J, 2014. The effects of doxapram on medullary respiratory neurones in brainstem-spinal cord preparations from newborn rats. Anaesthesia 69, 468–475. 10.1111/anae.12590. [DOI] [PubMed] [Google Scholar]
- Peng Y-J, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR, 2011. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc. Natl. Acad. Sci. U. S. A 108, 3065–3070. 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler G, Urlesberger B, Müller W, 2003. Impact of bradycardia on cerebral oxygenation and cerebral blood volume during apnoea in preterm infants. Physiol. Meas 24, 671–680. 10.1088/0967-3334/24/3/304. [DOI] [PubMed] [Google Scholar]
- Pijacka W, Moraes DJA, Ratcliffe LEK, Nightingale AK, Hart EC, Da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JFR, 2016. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med 22, 1151–1159. 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ, 2015. Ampakines enhance weak endogenous respiratory drive and alleviate apnea in perinatal rats. Am. J. Respir. Crit. Care Med 191, 704–710. 10.1164/rccm.201410-1898OC. [DOI] [PubMed] [Google Scholar]
- Rieusset A, Schaller F, Unmehopa U, Matarazzo V, Watrin F, Linke M, Georges B, Bischof J, Dijkstra F, Bloemsma M, Corby S, Michel FJ, Wevrick R, Zechner U, Swaab D, Dudley K, Bezin L, Muscatelli F, 2013. Stochastic loss of silencing of the imprinted Ndn/NDN allele, in a mouse model and humans with Prader-Willi syndrome, has functional consequences. PLoS Genet 9, e1003752. 10.1371/journal.pgen.1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ, Del Rio R, 2013. Role of the carotid body in the pathophysiology of heart failure. Curr. Hypertens. Rep 15, 356–362. 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Gourine AV, Smith JC, 2017. Respiratory rhythm irregularity after carotid body denervation in rats. Respir. Physiol. Neurobiol 246, 92–97. 10.1016/j.resp.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT, Frantz ID, Adler SM, Taeusch HW Jr., 1978. Maturation of reflexes influencing inspiratory duration in human infants. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 45, 203–211. 10.1152/jappl.1978.45.2.203. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Bardini M, Burnstock G, Spyer KM, 2001. Evidence for the involvement of purinergic signalling in the control of respiration. Neuroscience 107, 481–490. 10.1016/S0306-4522(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G, 2004. Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem. Cell Biol 122, 111–119. 10.1007/s00418-004-0680-2. [DOI] [PubMed] [Google Scholar]
- Younes M, 2014. CrossTalk proposal: elevated loop gain is a consequence of obstructive sleep apnoea. J. Physiol 592, 2899–2901. 10.1113/jphysiol.2014.271833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera T, Moraes DJ, da Silva M, Fisher JP, Paton JFR, 2019. The logic of connectivity of the carotid body to the brain. Physiology 34, 264–282. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gonzalez F, Mu D, 2011. Apnea of prematurity: from cause to treatment. Eur. J. Pediatr 170, 1097–1105. 10.1007/s00431-011-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


