Abstract
Background
The World Health Organization (WHO) recommends Xpert MTB/RIF in place of smear microscopy to diagnose tuberculosis (TB), and many countries have adopted it into their diagnostic algorithms. However, it is not clear whether the greater accuracy of the test translates into improved health outcomes.
Objectives
To assess the impact of Xpert MTB/RIF on patient outcomes in people being investigated for tuberculosis.
Search methods
We searched the following databases, without language restriction, from 2007 to 24 July 2020: Cochrane Infectious Disease Group (CIDG) Specialized Register; CENTRAL; MEDLINE OVID; Embase OVID; CINAHL EBSCO; LILACS BIREME; Science Citation Index Expanded (Web of Science), Social Sciences citation index (Web of Science), and Conference Proceedings Citation Index ‐ Social Science & Humanities (Web of Science). We also searched the WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and the Pan African Clinical Trials Registry for ongoing trials.
Selection criteria
We included individual‐ and cluster‐randomized trials, and before‐after studies, in participants being investigated for tuberculosis. We analysed the randomized and non‐randomized studies separately.
Data collection and analysis
For each study, two review authors independently extracted data, using a piloted data extraction tool. We assessed the risk of bias using Cochrane and Effective Practice and Organisation of Care (EPOC) tools. We used random effects meta‐analysis to allow for heterogeneity between studies in setting and design. The certainty of the evidence in the randomized trials was assessed by GRADE.
Main results
We included 12 studies: eight were randomized controlled trials (RCTs), and four were before‐and‐after studies. Most included RCTs had a low risk of bias in most domains of the Cochrane 'Risk of bias' tool.
There was inconclusive evidence of an effect of Xpert MTB/RIF on all‐cause mortality, both overall (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.75 to 1.05; 5 RCTs, 9932 participants, moderate‐certainty evidence), and restricted to studies with six‐month follow‐up (RR 0.98, 95% CI 0.78 to 1.22; 3 RCTs, 8143 participants; moderate‐certainty evidence). There was probably a reduction in mortality in participants known to be infected with HIV (odds ratio (OR) 0.80, 95% CI 0.67 to 0.96; 5 RCTs, 5855 participants; moderate‐certainty evidence).
It is uncertain whether Xpert MTB/RIF has no or a modest effect on the proportion of participants starting tuberculosis treatment who had a successful treatment outcome (OR) 1.10, 95% CI 0.96 to 1.26; 3RCTs, 4802 participants; moderate‐certainty evidence).
There was also inconclusive evidence of an effect on the proportion of participants who were treated for tuberculosis (RR 1.10, 95% CI 0.98 to 1.23; 5 RCTs, 8793 participants; moderate‐certainty evidence).
The proportion of participants treated for tuberculosis who had bacteriological confirmation was probably higher in the Xpert MTB/RIF group (RR 1.44, 95% CI 1.29 to 1.61; 6 RCTs, 2068 participants; moderate‐certainty evidence). The proportion of participants with bacteriological confirmation who were lost to follow‐up pre‐treatment was probably reduced (RR 0.59, 95% CI 0.41 to 0.85; 3 RCTs, 1217 participants; moderate‐certainty evidence).
Authors' conclusions
We were unable to confidently rule in or rule out the effect on all‐cause mortality of using Xpert MTB/RIF rather than smear microscopy. Xpert MTB/RIF probably reduces mortality among participants known to be infected with HIV. We are uncertain whether Xpert MTB/RIF has a modest effect or not on the proportion treated or, among those treated, on the proportion with a successful outcome. It probably does not have a substantial effect on these outcomes. Xpert MTB/RIF probably increases both the proportion of treated participants who had bacteriological confirmation, and the proportion with a laboratory‐confirmed diagnosis who were treated. These findings may inform decisions about uptake alongside evidence on cost‐effectiveness and implementation.
Plain language summary
Does using the Xpert MTB/RIF diagnostic test instead of smear microscopy when evaluating people for tuberculosis reduce death and successful treatment completion?
What is the aim of the review?
Tuberculosis (TB) is a bacterial infection that is spread by inhaling tiny droplets from the coughs or sneezes of an infected person. It mainly affects the lungs, but it can affect any part of the body. Tuberculosis can usually be cured by taking anti‐tuberculosis antibiotics for six months. Some bacteria are resistant, and then need to be treated with combinations of different antibiotics. Many countries use the Xpert MTB/RIF test to diagnose tuberculosis. We wanted to find out if using this test affected health outcomes, such as death or successful treatment in people suspected of having tuberculosis.
What was studied in this review?
A rapid, accurate diagnosis of tuberculosis ensures people who are ill start taking the right antibiotics as soon as possible. This might reduce the number of people dying, but also, if rifampicin‐resistant tuberculosis is detected early, they are more likely to get appropriate treatment. It also helps ensure people who do not have tuberculosis are not treated unnecessarily.
The Xpert MTB/RIF test is an automated molecular test, commonly used to identify tuberculosis and rifampicin resistance at the same time, in less than two hours. The World Health Organization (WHO) recommends using the Xpert MTB/RIF test to diagnose tuberculosis instead of smear microscopy – using a microscope to look for bacteria in samples of sputum (a mixture of saliva and mucus, coughed up from the lungs). This review investigates whether using Xpert MTB/RIF instead of microscopy improves health outcomes.
What are the main results of the review?
We searched for studies that assessed health outcomes in people who had sought treatment and were suspected of having tuberculosis and who were diagnosed using either the Xpert MTB/RIF test or smear microscopy. We found 12 relevant studies. Eight studies included only adults; four included people of all ages. Ten studies took place in sub‐Saharan Africa, one in Brazil, and one in Indonesia. The studies followed people for between two months and two years.
An effect of using the Xpert MTB/RIF test to diagnose tuberculosis, compared with smear microscopy, could not be ruled in or out a for the numbers of people who:
· died (5 studies; 10,409 people); · were successfully treated (3 studies; 4802 people); · died within six months (3 studies; 8143 people); or · were treated for tuberculosis (5 studies; 8793 people).
Compared with smear microscopy, use of the Xpert MTB/RIF test probably:
· reduced the number of HIV‐positive people who died during follow‐up (5 studies; 1789 people); · increased the number of people with confirmed tuberculosis who started treatment (3 studies; 1217 people); and · increased the number of treated people who had a confirmed diagnosis of tuberculosis (6 studies; 2068 people).
None of the studies reported people's satisfaction, or the number of visits before tuberculosis was diagnosed. Only one study looked at the treatment of tuberculosis resistant to rifampicin.
Key messages
The results showed that there was a beneficial effect of Xpert MTB/RIF for some health outcomes, and inconclusive results (where an effect could not be ruled in or out) for others.
Together, these findings can help inform decisions about the uptake of Xpert MTB/RIF, alongside information on cost‐effectiveness and implementation.
How up to date is this review?
We included studies published to 24 July 2020.
Summary of findings
Summary of findings 1. Impact of Xpert MTB/RIF compared to smear microscopy.
| Impact of Xpert MTB/RIF compared to smear microscopy | ||||||
|
Patient or population: people being investigated for tuberculosis (TB) Setting: primary healthcare clinics, specialized clinics, hospitals Intervention: Xpert MTB/RIF Comparison: smear microscopy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with smear microscopy | Risk with Xpert MTB/RIF | |||||
| All‐cause mortality among participants | 5.9% | 5.3% (4.4 to 6.2) | RR 0.89 (0.75 to 1.05) | 9932 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | Xpert MTB/RIF compared to smear microscopy probably does not increase mortality. We are uncertain whether there is a reduction in mortality or not with Xpert MTB/RIF. |
|
All‐cause mortality at 6 months (subgroup) |
5.3% | 5.2% (4.1 to 6.5) | RR 0.98 (0.78 to 1.22) | 8143 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | It is uncertain whether there is an effect of Xpert MTB/RIF compared with smear microscopy on mortality at 6 months. |
|
All‐cause mortality in people who were HIV‐positive (subgroup) |
8.3% | 6.8% (5.7 to 8.0) | OR 0.80 (0.67 to 0.96) | 5855 (5 RCTs) | ⊕⊕⊕⊝ Moderatea |
Xpert MTB/RIF probably reduces mortality compared to smear microscopy in people who are HIV‐positive. |
| Successful treatment outcome in participants treated for tuberculosis | 70% | 72% (69 to 75) | OR 1.10 (0.95 to 1.26) | 4802 (3 RCTs) | ⊕⊕⊝⊝ Moderatea |
There are probably no fewer participants with a successful treatment outcome for Xpert MTB/RIF. We are uncertain if there is no effect or a modest increase. |
| Proportion of participants who were treated for tuberculosis | 20% | 22% (19.6 to 24.6) | RR 1.10 (0.98 to 1.23) | 8793 (5 RCTs) | ⊕⊕⊕⊝ Moderatea |
There are probably no fewer patients started on treatment with Xpert MTB/RIF. It is uncertain if there is no effect or a modest increase. |
| Pre‐treatment loss to follow‐up in participants with bacteriological confirmation | 14% | 8.3% (5.7 to 11.9) | RR 0.59 (0.41 to 0.85) | 1217 (3 RCTs) | ⊕⊕⊕⊝ Moderatea |
There are probably fewer patients lost after the test and before treatment is started with Xpert MTB/RIF than with smear microscopy. |
| Treated participants with bacteriological confirmation of tuberculosis | 50% | 72% (65 to 81) | RR 1.44 (1.29 to 1.61) | 2068 (6 RCTs) | ⊕⊕⊕⊝ Moderatea |
Of the participants started on TB treatment, probably more had a bacterial confirmation of TB with Xpert MTB/RIF than with smear microscopy |
| *The risk in the Xpert MTB/RIF group is based on the assumed risk in the comparison group and the relative effect of the intervention. The risk in the smear microscopy group was calculated from the total number of events and number of participants in the smear microscopy arms of the studies included in each analysis. CI: confidence interval; RR: risk ratio; OR: odds ratio; RCT: randomized controlled trial | ||||||
|
GRADE Working Group certainty of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by 1 for imprecision
Background
Description of the condition
Tuberculosis (TB) is caused by the Mycobacterium tuberculosis, an obligate aerobe bacilli that belongs to the Mycobacteria tuberculosis complex (MTBC; (Cook 2008)). Transmission of tuberculosis most commonly occurs through the inhalation of droplets containing bacilli, from a person with pulmonary tuberculosis who has coughed or sneezed. It is estimated that 1.7 billion people are infected by M tuberculosis globally without having disease, and that about 5% to 15% will develop disease (Houben 2016; WHO 2019). The probability of developing disease is higher in immunocompromised individuals, including those infected with HIV (WHO 2019). It can affect all age‐groups, but mostly affects adults. Tuberculosis primarily affects the lungs (pulmonary tuberculosis), however, the disease can involve virtually any extrapulmonary site in the human body. Clinically, the most common symptoms of pulmonary tuberculosis include cough with sputum and blood at times, chest pain, weakness, weight loss, fever, and night sweats.
In 2018, there were an estimated 10 million new tuberculosis cases globally and people living with HIV accounted for 9% of these (WHO 2019). In the same year, tuberculosis was associated with 1.2 million deaths, and a further 251,000 deaths from disease among people living with HIV (WHO 2019). Both the emergence and under‐reporting of drug resistance to antimicrobials used to treat tuberculosis remain major problems. In 2018, it was estimated that half a million people developed tuberculosis disease that was resistant to rifampicin (RR‐TB) or multi‐drug resistant tuberculosis (MDR‐TB), and only a third of these received appropriate treatment (WHO 2019).
Commonly used diagnostic techniques for tuberculosis have limitations. Culture, which is the gold standard for diagnosis, is normally centrally located, requires a set of biocontainment precautions, and takes up to six weeks for liquid, or eight weeks for solid culture, before results can be obtained (Corbett 2006). For many years, sputum smear microscopy has been the most common method used to diagnose tuberculosis, particularly in low‐ and middle‐income countries (LMICs), and it remains the main diagnostic technique in primary healthcare facilities in LMICs (Corbett 2006; Parsons 2011). However, the sensitivity of smear microscopy is limited, ranging from 20% to 80% (Levy 1989; Parsons 2011), and sensitivity is further reduced in people who are HIV‐seropositive (Corbett 2006). Other limitations of smear microscopy are that it is labour intensive, dependent on individual skills and experience, and unable to detect drug resistance (Parsons 2011). Without molecular tests, MDR‐TB diagnosis depends on culture infrastructure or line probe assays, which are both expensive and not widely available at point of care (WHO 2016a; WHO 2019). Consequently, there have been calls for early, accurate, and affordable diagnosis of tuberculosis, including universal drug susceptibility testing for all people who are being evaluated for tuberculosis (WHO 2014b).
Description of the intervention
In 2010, the World Health Organization (WHO) released a policy statement endorsing the Xpert MTB/RIF assay, and recommending it as the initial diagnostic test for people with suspected MDR‐TB and HIV. The WHO made conditional recommendations, based on resource availability, for its use as a follow‐up test for people with negative smears. Further recommendations for use in individuals with extrapulmonary, and paediatric tuberculosis were made in 2013, following new supporting evidence (WHO 2013; WHO 2014). By the end of 2016, a total of 6659 GeneXpert instruments, and more than 23 million Xpert MTB/RIF cartridges had been procured by the public sector among 130 LMICs, at a price concession (WHO 2016b). Among 48 high‐burden countries, only 15 reported they had used the WHO‐recommended diagnostic test in more than half of all notified cases (WHO 2019).
The Xpert MTB/RIF assay is an automated nucleic‐acid amplification test. It consists of a single‐use multi‐chambered cartridge, preloaded with the liquid buffers and lyophilized reagent beads required for sample processing, DNA extraction, and hemi‐nested, real‐time polymerase chain reaction. The assay can be used with sputum samples, as recommended by the WHO, and also, with varying sensitivity, with other specimens, including cerebrospinal fluid, lymph node tissue or aspirates, pleural fluid, ascetic fluid, urine, dialysis fluid, and pus (Denkinger 2014; Scott 2014). The assay can be performed without biosafety cabinets at peripheral laboratories or health facilities; minimal training is required for laboratory staff (Boehme 2010; Boehme 2011). Xpert MTB/RIF can detect MTBC and rifampicin resistance within two hours (Helb 2010).
An updated Cochrane Review on the accuracy of Xpert MTB/RIF for the detection of tuberculosis estimated the pooled sensitivity of the assay to be 85% (79% to 90%) and the pooled specificity 98% (97% to 99%) (Zifodya 2021). When compared with smear microscopy, Xpert MTB/RIF sensitivity in smear‐negative, culture‐positive individuals was 61% (48% to 72%) (Zifodya 2021). They estimated sensitivity to be slightly lower in people with HIV infection (75% (59% to 86%)) than in those who were HIV seronegative (89% (78% to 95%)). For the detection of rifampicin resistance, the Xpert MTB/RIF assay had an estimated pooled sensitivity of 95% (90% to 99%) and specificity of 99% (97% to 99%) (Zifodya 2021).
There have been practical problems with the introduction of Xpert MTB/RIF: high rates of modular failure linked to interrupted power supply, and infrastructure, training, and procurement challenges were observed among early Xpert MTB/RIF adopters in LMICs (Creswell 2014). The potential impact of Xpert MTB/RIF depends partly on a stable power supply, or alternative reliable sources of energy, such as batteries or solar energy (Albert 2016).
A growing number of trials have investigated whether the increased sensitivity of Xpert MTB/RIF translates into an impact on outcomes important to people seeking care, such as time to treatment, pre‐ treatment loss to follow up, treatment success and mortality. These are the focus of this review.
How the intervention might work
Xpert MTB/RIF has a higher diagnostic accuracy than smear microscopy, is able to detect resistance against rifampicin, can be used close to the people being tested, and has a fast turn‐around time. It may be that these features will lead to a positive impact on participant‐important outcomes. The use of the assay may translate into a decrease in pre‐diagnostic and pre‐treatment loss to follow‐up and, perhaps reduce time to treatment. Pre‐treatment loss to follow‐up is associated with high mortality rates, and thus, a reduction in pre‐treatment loss to follow‐up may lead to reduced mortality (MacPherson 2014). A larger proportion of people with true tuberculosis may receive effective therapy, and fewer individuals may be falsely diagnosed with the disease on clinical grounds alone, and incorrectly treated. Rapid detection of rifampicin resistance may lead to more rapid initiation of effective treatment. These factors could potentially improve important participant outcomes.
Why it is important to do this review
Recent evidence from pragmatic trials in programmatic settings yielded largely inconclusive results for the impact of Xpert MTB/RIF on different patient outcomes (Di Ruffano 2017a; Di Ruffano 2017b). A narrative review by Auld 2016 reported a limited impact on morbidity and mortality, but included studies that demonstrated that Xpert MTB/RIF increased the diagnostic yield of bacteriologically confirmed tuberculosis, and reduced the time to the initiation of treatment. A recent individual participant data meta‐analysis, including 8567 participants, found that the effect of Xpert MTB/RIF on all‐cause mortality was inconclusive (odds ratio 0.88, 95% CI 0.68 to 1.14; Di Tanna 2019). A large number of participants are needed to conclusively demonstrate the presence or absence of an effect of modest size on mortality (Di Ruffano 2017a; Di Ruffano 2017b). This review adds recent studies to estimate as precisely as possible the impact of this diagnostic test on mortality and includes further patient outcomes to inform the allocation of resources in LMICs.
Objectives
To assess the impact of Xpert MTB/RIF on patient outcomes in people being investigated for tuberculosis.
Methods
Criteria for considering studies for this review
Types of studies
We included cluster‐ and individually‐randomized controlled trials, and observational studies with before‐and‐after implementation periods. We performed meta‐analyses with data from randomized studies only. There were too few non‐randomized studies per outcome to perform separate meta‐analyses.
Types of participants
Individuals being investigated for tuberculosis. This included individuals presenting with one or more symptoms of tuberculosis, or those newly diagnosed as HIV positive, who were able to provide a sputum sample.
Types of interventions
Intervention
Diagnostic strategies that used Xpert MTB/RIF
Control
Diagnostic strategies that used smear microscopy
Types of outcome measures
Primary outcomes
All‐cause mortality from the time of the first diagnostic test to the end of trial follow‐up
Proportion of participants starting treatment for tuberculosis who had a successful treatment outcome (an unfavourable outcome was defined as death from any cause, default, failure, being transferred out, or lost to follow‐up). This outcome was added subsequently, at the request of the WHO guideline development group.
Secondary outcomes
Proportion of participants who were treated for tuberculosis
Proportion of treated participants who were microbiologically confirmed
Proportion of participants with microbiological confirmation who had pre‐treatment loss to follow‐up
Time from first contact with the health system to initiation of treatment
Proportion of participants who were diagnosed and treated for MDR‐TB
Number of visits to the same, or any other, healthcare facilities prior to diagnosis
Patient satisfaction
Search methods for identification of studies
We identified all potential trials, regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases, using the MEDLINE search terms, detailed in Appendix 1, and adapted for the other databases:
Cochrane Infectious Disease Group (CIDG) Specialized Register (searched 24 July 2020);
Cochrane Central Register of Controlled Trials (CENTRAL, 2020, Issue 7), published in the Cochrane Library (searched 24 July 2020);
MEDLINE OVID (2007 to 24 July 2020);
Embase OVID (2007 to 24 July 2020);
CINAHL EBSCO (2007 to 24 July 2020);
LILACS BIREME (Latin American and Caribbean Health Science Information database; 2007 to 24 July 2020);
Science Citation Index Expanded, Conference Proceedings Citation Index‐ Science (both Web of Science; searched 24 July 2020)
Social Sciences citation index, and Conference Proceedings Citation Index ‐ Social Science & Humanities (both Web of Science; searched 24 July 2020).
To identify ongoing trials, we searched these trials registers, using the search terms: (tuberculosis OR TB) AND (Xpert or GeneXpert or "sputum microbiology" or "sputum microscopy").
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/search/en/; searched 24 July 2020)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/; searched 24 July 2020); and the
Pan African Clinical Trials Registry (www.pactr.org/).
Searching other resources
We searched the past two years' proceedings of the International Union Against Tuberculosis and Lung disease (UNION) conference, the Conference on Retroviruses and Opportunistic Infections (CROI), and the International AIDS Conference (IAS).
We reviewed reference lists of all included studies and relevant systematic reviews.
We contacted leading researchers at the Foundation for Innovative New Diagnostics (FIND), the WHO, Centers for Disease Control and Prevention (CDC), and TB‐REACH to identify unpublished data.
Data collection and analysis
Selection of studies
Two review authors (FH and MK) independently screened the reports identified by the literature search, and coded them as either 'potentially include' or 'exclude'. Based on the screening results, we assessed full‐text reports retrieved from the 'potentially include' category for eligibility, using an eligibility assessment form. We resolved differences in opinion through discussion. We contacted study authors for clarification as needed. We reported all excluded studies, with reasons for exclusion, in the PRISMA flow diagram.
Data extraction and management
Two review authors (FH and MK) independently extracted data, using a piloted data extraction tool similar to a tool previously used by Schumacher 2016. We resolved disagreements through discussion, or by consulting a third review author (AR). We extracted the following data: study details (first author, year of publication), participant details, intervention, control, outcome measured and how it was measured, covariates, length of follow‐up, and measure of effect, with 95% confidence intervals (CIs). For binary outcomes, we extracted the relative risk and odds ratio if available. For time‐to‐event outcomes, we extracted the log hazard ratio with standard error or CI, if available.
In addition, we recorded the number of participants and clusters randomized to each diagnostic arm, the number of participants monitored for each outcome of interest, and the number of events. For cluster‐RCTs that were adjusted for clustering, we extracted the adjusted measures of effect for each outcome, and the method of adjustment. We also extracted data relevant for the assessment of the risk of bias.
Assessment of risk of bias in included studies
Two review authors (FH and MK) independently assessed the risk of bias, using categories modified from the Cochrane 'Risk of bias' assessment tool for assessing risk of bias for randomized studies (Higgins 2011), and the EPOC categories for assessing risk of bias in non‐randomized studies (EPOC 2018). Many categories applied to both types of study.
We assessed the included studies for selection bias, with categories for the method of allocation, sequence generation (we classified non‐randomized studies as having a high risk of bias), allocation concealment (adequate, inadequate, not done, or unclear (as defined by Jüni 2001)), having similar baseline characteristics. We also assessed performance bias (blinding of participants and personnel, and protection against contamination), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. We assessed the risk of bias as low, high, or unclear.
Measures of treatment effect
For the outcomes that assessed proportions, we present the impact of Xpert MTB/RIF using risk ratios, if available, with their 95% CIs. We aimed to present the impact of Xpert MTB/RIF on time to treatment, using hazard ratios, if available. We used the estimates unadjusted for covariates, except in the case of Churchyard 2015, where imbalance was reported due to a small number of clusters, and for Di Tanna 2019 where the estimates were adjusted for age and sex.
Unit of analysis issues
We carried out the analysis based on the intervention groups. All clustered studies considered the cluster design in their analysis.
Cox 2014 reported that they used design‐based F‐tests, to take into account clustering by calendar week, in a single large primary healthcare clinic. We increased the width of the CIs to match the P values. The odds ratio for the successful treatment outcome was calculated from the numbers in each arm: we inflated the standard error of log(OR) by a factor of 1.10, which corresponds to the average effect of clustering on the other outcomes.
Dealing with missing data
We used a modified intention‐to‐treat (mITT) analysis, where the analysis adhered to ITT principles, except that we excluded participants with missing outcome data (Higgins 2019).
Assessment of heterogeneity
We calculated the I² statistic (the proportion of variance in the meta‐analysis that is attributable to study heterogeneity).
Assessment of reporting biases
We did not test for heterogeneity in the intervention effect between studies, or use a funnel plot, since this is not recommended for meta‐analyses with fewer than 10 studies (Page 2019).
Data synthesis
We conducted analyses using Review Manager 5 (Review Manager 2014), and RevMan Web (RevMan Web 2020). We used a random‐effects model, as we expected the intervention effect to vary between studies, due to the participant mix, settings, aspects of study design, and health system factors.
Subgroup analysis and investigation of heterogeneity
We conducted an additional subgroup analysis, which was not in the protocol, by analysing mortality only in studies that reported mortality at six months. This analysis helped to estimate the effect of Xpert MTB/RIF at the completion of tuberculosis treatment. We conducted a subgroup analysis for mortality in HIV‐infected participants. We planned to conduct subgroup analyses for other outcomes for HIV‐positive, for HIV‐negative, and for children, adults, and people with resistant and sensitive tuberculosis, but sufficient data were not available.
Sensitivity analysis
We did not perform any planned sensitivity analyses, since the few circumstances we anticipated were not observed:
missing data that are likely to influence the outcome;
excluding studies with outliers that are suspected to influence the outcome;
excluding studies with a high risk of bias that are likely to affect the outcome.
Summary of findings and assessment of the certainty of the evidence
We summarized the studies and outcomes in a 'Summary of findings' table.
We assessed the certainty of evidence using the GRADE approach (GRADE 2014; Guyatt 2011), and GRADEpro GDT software (GRADEpro GDT). We rated each important outcome as described by Balshem 2011.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Evidence from RCTs starts as high certainty, but can be downgraded if there are valid reasons within the following five categories: risk of bias, imprecision, inconsistency, indirectness, and publication bias. Evidence also can be upgraded if there is a large effect, and if all plausible residual confounding would reduce a demonstrated effect, or would suggest a spurious effect if no effect was observed (Balshem 2011).
Results
Description of studies
Results of the search
Our search found 1577 records: 1572 from the databases search after we removed duplicates, and five from additional sources. We excluded 1544 records after screening the abstracts and fully assessed the remaining 33 records for the eligibility criteria. Of the 33 records, we excluded eight studies because they examined the accuracy of Xpert MTB/RIF, two did not have data on the outcome or population of interest, seven were observational studies, four had no comparison group; we included the remaining 12 studies (Figure 1; Table 2).
1.
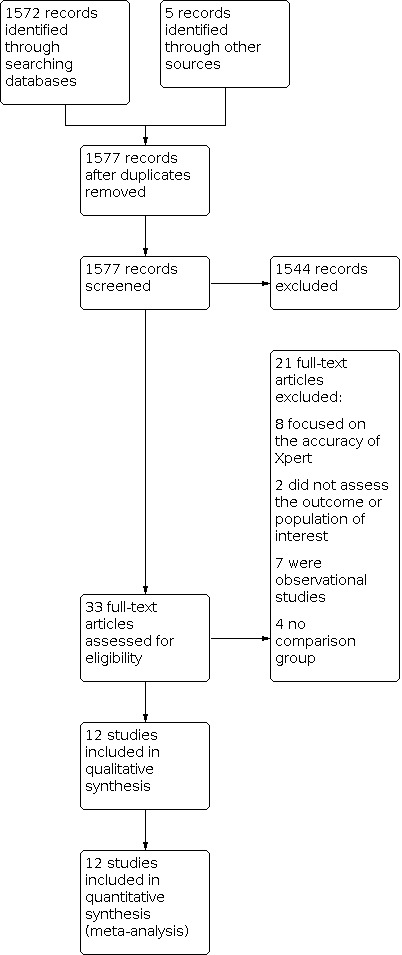
Flow diagram of included studies
1. Descriptive summary of studies.
| Study | Country | Design | Settings | Unit of randomization | Diagnostic strategies | Participant eligibility | Age group | Months of follow‐up | Number of clusters per arm | Total sample |
| Calligaro 2015 | South Africa | RCT | intensive care units | individual | smear microscopy + culture vs Xpert MTB/RIF + culture using tracheal aspirate samples | evaluated for TB | 18 years old & older | 26 | n/a | 242 |
| Mupfumi 2014 | Zimbabwe | RCT | ART initiation centre | individual | smear microscopy vs Xpert MTB/RIF | HIV positive and on ART | 18 years old & older | 3 | n/a | 424 |
| Theron 2014a | South Africa, Tanzania, Zambia, Zimbabwe | RCT | primary health facilities | individual participant | smear microscopy vs Xpert MTB/RIF on sputum samples | people evaluated for TB | 18 years old & older | 6 | n/a | 1502 |
| Churchyard 2015 | South Africa | cluster‐RCT | primary health facilities | health facility | smear microscopy vs Xpert MTB/RIF | evaluated for TB | 18 years old & older | 6 | 10 | 4656 |
| Cox 2014 | South Africa | cluster‐RCT | primary health facility | people seen within the calendar week | Xpert MTB/RIF vs routine diagnostic algorithm of smear, culture, and DST | evaluated for TB | 18 years old & older | 6 | 26 weeks (Xpert) and 25 weeks (smear microscopy) | 1985 |
| Ngwira 2019 | Malawi | Cluster ‐RCT | public HIV clinics | Clinic | smear microscopy vs Xpert MTB/RIF | newly registered people with HIV | 18 years old & older | 12 | 6 | 1842 |
| Agizew 2019a | Botswana | stepped‐wedge | public HIV clinics | clinic | smear microscopy vs Xpert MTB/RIF | newly registered people with HIV | all age groups | 6 | 22 | 6041 |
| Durovni 2014 | Brazil | stepped‐wedge | clinics using laboratory services | laboratories | smear microscopy vs Xpert MTB/RIF | evaluated for TB | all age groups | 6 | 14 | 11,705 smear tests; 12,522 Xpert tests performed |
| Schmidt 2017 | South Africa | before/after | primary healthcare facilities | n/a | participants investigated by smear microscopy vs Xpert MTB/RIF | evaluated for TB | 18 years old & older | 6 | n/a | 15,629 before; 10,741 after |
| van Kampen 2015 | Indonesia | before/after | clinics offering PMDT services | n/a | before: sputum sample underwent smear microscopy + culture + DST after: Xpert MTB/RIF + culture + DST |
at risk of MDR‐TB | all age groups | 24 | n/a | 975 before; 1442 after |
| Van den Handel 2015 | South Africa | before/after | district, sub district and primary healthcare facilities | n/a | smear microscopy vs decentralized Xpert MTB/RIF (we excluded centralized Xpert) | evaluated for TB | all age groups | 6 | n/a | 959 |
| Yoon 2012 | Uganda | before/after | national referral hospital | n/a | smear microscopy (baseline) vs Xpert MTB/RIF (implementation) | evaluated for TB | 18 years old & older | 2 | n/a | 287 baseline; 190 implementation |
ART: antiretroviral therapy DST: drug susceptibility testing MDR‐TB: multi‐drug resistant TB n/a: not applicable PMDT: programmatic management of drug‐resistant tuberculosis RCT: randomized controlled trial TB: tuberculosis vs: versus
Included studies
Designs
We included three individually‐randomized trials (Calligaro 2015; Mupfumi 2014; Theron 2014a), five cluster‐randomized trials (Agizew 2019a; Churchyard 2015; Cox 2014; Durovni 2014; Ngwira 2019), and four before‐after implementation studies (Schmidt 2017; Van den Handel 2015; van Kampen 2015; Yoon 2012). Three cluster‐randomized trials used a parallel design (Churchyard 2015; Cox 2014; Ngwira 2019), and two a stepped‐wedge design (Agizew 2019a; Durovni 2014).
The units of randomization for the cluster‐randomized trials were primary care facilities (Churchyard 2015), clinics served by single tuberculosis laboratories (Durovni 2014), clinics with participants infected with HIV (Agizew 2019a; Ngwira 2019), or calendar week in a single large primary care tuberculosis clinic (Cox 2014).
The length of follow‐up varied across the studies: two months (Yoon 2012), three months (Calligaro 2015; Mupfumi 2014), six months (Agizew 2019a; Churchyard 2015; Cox 2014; Schmidt 2017; Theron 2014a; Van den Handel 2015), eight months (Durovni 2014), 12 months (Ngwira 2019), and 24 months (van Kampen 2015).
Settings
Most included studies were conducted in sub‐Saharan Africa. There were five in South Africa (Calligaro 2015; Churchyard 2015; Cox 2014; Schmidt 2017; Van den Handel 2015), one in each of Zimbabwe (Mupfumi 2014), Uganda (Yoon 2012), Malawi (Ngwira 2019), and Botswana (Agizew 2019a), and one multi‐country study with sites in South Africa, Tanzania, Zimbabwe, and Zambia (Theron 2014a). The remaining two studies were conducted in Brazil (Durovni 2014), and Indonesia (van Kampen 2015). The settings for the studies were primary health facilities (Churchyard 2015; Cox 2014; Durovni 2014; Schmidt 2017; Theron 2014a; Ngwira 2019), a mix of district, sub‐district, and primary healthcare facilities (Van den Handel 2015), an antiretroviral therapy (ART) initiation clinic (Mupfumi 2014), intensive care units (Calligaro 2015), a national referral hospital (Yoon 2012), public HIV clinics (Agizew 2019a ) and clinics for the management of people with multi‐drug resistant tuberculosis (MDR‐TB) (van Kampen 2015).
Participants
Eight studies included participants who were 18 years and older, and four included all age groups (Agizew 2019a; Durovni 2014; Van den Handel 2015; van Kampen 2015). All but one study reported HIV co‐infection (Schmidt 2017), three studies included only HIV‐infected participants (Agizew 2019a; Mupfumi 2014; Ngwira 2019). People seeking care were eligible to be included in the studies if they were evaluated for pulmonary tuberculosis (Calligaro 2015; Churchyard 2015; Cox 2014; Durovni 2014; Schmidt 2017; Theron 2014a; Van den Handel 2015; Yoon 2012), were at risk of MDR‐TB (van Kampen 2015), were infected with HIV and on ART (Mupfumi 2014), or were newly registered as infected with HIV, and undergoing tuberculosis screening (Agizew 2019a; Ngwira 2019).
Interventions
All studies compared Xpert MTB/RIF diagnostic strategies to smear microscopy strategies and conventional drug sensitive tests (DST), if used. All studies collected expectorated sputum samples for Xpert MTB/RIF and smear tests except Calligaro 2015, which used tracheal aspirate samples. All of the randomized studies collected two sputum samples for smear microscopy and one sputum for Xpert MTB/RIF except Agizew 2019a, which collected four sputum samples (two on screening day, and two the day after). While one sample per day was tested by smear microscopy or Xpert MTB/RIF, depending on the randomized arm, the other was submitted to the national reference laboratory for liquid culture. In Theron 2014a, at least two expectorated sputum samples were collected, one was selected at random for smear microscopy or Xpert MTB/RIF, and the other for microbiological culture.
In the non‐randomized trials, Yoon 2012 collected and evaluated two sputum samples (early morning and spot,taken at the time of the clinic visit) for smear microscopy, and one extra spot sputum sample for Xpert MTB/RIF; Van den Handel 2015 collected two sputum samples during the smear period, and a single sputum sample in the Xpert MTB/RIF period; Schmidt 2017 collected electronic data from the National Health Laboratory Services (NHLS) South Africa database, which records all microbiological tests for tuberculosis in the region, including the type of test (sputum smear microscopy, Xpert MTB/RIF, or liquid culture), and the result of each test; and van Kampen 2015 evaluated one sputum sample for smear and culture pre‐intervention, and two samples during the intervention (one for Xpert MTB/RIF, the second sample for culture and drug susceptibility testing (DST)).
Only two studies had chest x‐rays available at baseline for all participants (Theron 2014a; Yoon 2012).
Outcomes
Primary outcomes
All‐cause mortality was assessed at different time points across the randomized studies: at six months by three studies (Churchyard 2015; Cox 2014; Theron 2014a); at three months by two studies (Calligaro 2015; Mupfumi 2014); and at 12 months by one study (Ngwira 2019). One before‐and‐after study assessed mortality at two months (Yoon 2012). All studies reported estimates for mortality as risk ratios (RR), with the exception of an incidence rate ratio by Ngwira 2019 and odds ratios by Di Tanna 2019. For low rates, the incidence rate ratio is similar to the RR, and for low proportions the RR is a slightly conservative estimate of the odds ratio.
Three studies reported the proportion of participants with a successful treatment outcome (Agizew 2019a; Cox 2014; Durovni 2014).
Secondary outcomes
The proportion of participants starting tuberculosis treatment was reported at different time points across the studies: at one month (Agizew 2019a; Durovni 2014; Schmidt 2017; Yoon 2012), two months (Theron 2014a; van Kampen 2015), three months (Calligaro 2015; Cox 2014), six months (Churchyard 2015), or one year (Ngwira 2019).
Six studies reported the proportion of treated participants who were microbiologically confirmed (Calligaro 2015; Churchyard 2015; Cox 2014; Mupfumi 2014; Ngwira 2019; Agizew 2019a). All studies confirmed participants bacteriologically with smear microscopy or Xpert MTB/RIF. Five studies routinely did culture (Agizew 2019a; Calligaro 2015; Theron 2014a; van Kampen 2015; Yoon 2012); six studies did them only for specific subgroups or if requested (Churchyard 2015; Cox 2014; Durovni 2014; Ngwira 2019; Schmidt 2017; Van den Handel 2015). One study did not report cultures.
Three studies reported post‐enrolment, pre‐treatment loss to follow‐up: at 28 days (Churchyard 2015), two months (Theron 2014a), and three months (Cox 2014). It is unlikely that processes for trial‐related follow‐up would have affected health system loss to follow‐up: assessment visits in Churchyard 2015 and Cox 2014 were scheduled after these time points and Theron 2014a followed routine procedures.
Durovni 2014 defined time to treatment initiation as the time between diagnosis or confirmation of tuberculosis to the start of treatment or notification date; three trials defined it as the time from sputum collection to treatment initiation (Agizew 2019a; Schmidt 2017: Van den Handel 2015); four as the time between the date of enrolment in the study and the date of treatment initiation (Churchyard 2015; Cox 2014; van Kampen 2015; Yoon 2012); Mupfumi 2014 defined it as the time from baseline visit to initiation of treatment); and Theron 2014a, as the proportion of participants initiating treatment by day one (Theron 2014a).
Five studies reported a diagnosis of drug‐resistant tuberculosis in the Xpert MTB/RIF arm only (Calligaro 2015; Churchyard 2015; Cox 2014; Durovni 2014; Theron 2014a), thus, we could not compare the health impact of Xpert MTB/RIF on MDR‐TB. Only one study reported the impact of Xpert MTB/RIF on participant outcomes in MDR‐TB (van Kampen 2015).
None of the studies reported either the number of visits prior to diagnosis, or self‐reported satisfaction, so we could not assess these outcomes.
Excluded studies
We excluded 21 studies (Characteristics of excluded studies; Figure 1). Eight studies assessed accuracy, two did not assess any of the outcomes of interest or the population of interest, four had no comparison group, and seven were observational studies.
Risk of bias in included studies
We provided the summary of our judgement of the risks of bias in Figure 2. We listed individual risks of bias in the Characteristics of included studies section. The study by Di Tanna 2019 was not assessed for risks of bias since it is a meta‐analysis of other included studies.
2.

Summary of risk of bias for all included studies
Allocation
Of the eight randomized trials, we assessed five at low risk of selection bias (Calligaro 2015; Churchyard 2015; Mupfumi 2014; Ngwira 2019; Theron 2014a); and three with unclear risk, due to unclear concealment, combined with changes of intervention arm within the same health facilities (Agizew 2019a; Cox 2014; Durovni 2014).
We assessed the four non‐randomized before‐after trials at high risk of bias (Schmidt 2017; Van den Handel 2015; van Kampen 2015; Yoon 2012).
The characteristics were similar between the Xpert MTB/RIF and smear groups for all trials except Churchyard 2015, which reported an imbalance due to a small number of large clusters but adjusted for the covariates with imbalances in their analyses. van Kampen 2015, a before‐after study, reported a higher rate of testing after Xpert MTB/RIF had been introduced.
Blinding
Blinding was not feasible for any of the trials. We considered that knowledge of the diagnostic test was part of the intervention. However, there may be other elements of lack of blinding that may lead to bias, for example staff knowing which arm was the standard and which the new diagnostic test. Therefore, we judged all of the trials to be at high risk of performance bias. There was a low or unknown risk of detection bias for all studies: they could not be blinded to the assessors but the outcomes are clearly defined and objective. We assessed there to be a low risk of contamination (performance bias) for 10 of the studies. Two studies (Cox 2014 ;van Kampen 2015) reported that the intervention was not always correctly assigned.
Incomplete outcome data
We considered six of the randomized trials to have a low risk of attrition bias; two trials were at unclear risk (Cox 2014; Calligaro 2015). Cox 2014 did not clearly state what proportion of the unfavourable outcome was linked to loss to follow‐up, since the unfavourable outcome was reported as a combined outcome for death, failure, and loss to follow‐up. Calligaro 2015 had different loss to follow‐up in the two arms. We assessed three of the non‐randomized trials to have a low risk of attrition bias; we assessed Yoon 2012 to be at high risk since loss to follow‐up was significantly different before and after implementation. Loss to follow‐up is included in two of the outcomes in this meta‐analyses: pre‐treatment loss to follow‐up and successful treatment outcome.
Selective reporting
Ten of the 12 studies had a low risk of selective bias. Two studies included some outcomes that were not pre‐specified (Cox 2014; Durovni 2014).
Other potential sources of bias
We assessed there to be a risk of confounding in the before‐after studies (Schmidt 2017; van Kampen 2015; Van den Handel 2015;Yoon 2012). We did not detect any other biases.
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
Simple totals across six studies showed recorded deaths as 4.9% (248/5013) in the Xpert MTB/RIF group compared to 5.9% (292/4919) in the smear microscopy group (Table 3). The estimated risk ratio (RR) for death using Xpert MTB/RIF compared to smear microscopy was 0.89 (95% confidence interval (CI) 0.75 to 1.05; 5 RCTs, 9932 participants; Analysis 1.1; Figure 3). We did not include Calligaro 2015 since participants in the intensive care units (ICU) had a very different risk of mortality than those in non‐ICU settings. We did not include the one non‐randomized studies reporting mortality (Yoon 2012). They found a similar risk of mortality before and after the implementation of Xpert MTB/RIF (RR 1.04, 95% CI 0.75 to 1.46; Table 3).
2. Summary of studies: all‐cause mortality.
| Study | Country | Design | Settings | Months of follow‐up | Proportion deaths | RR (95%CI) | |
| smear arm (n/N) | Xpert arm (n/N) | ||||||
| Churchyard 2015 | South Africa | cluster‐RCT | primary health clinics | 6 | 116/2332 (5.0%) | 91/2324 (3.9%) | 1.10 (0.75 to 1.62) |
| Cox 2014 | South Africa | cluster‐RCT | primary health clinic | 6 | 38/1003 (3.8%) | 33/983 (3.4%) | 0.89 (0.56 to 1.40) |
| Mupfumi 2014 | Zimbabwe | RCT | ART initiation centre | 3 | 17/172 (9.9%) | 11/182 (6.0%) | 0.61 (0.29 to 1.27) |
| Ngwira 2019 | Malawi | cluster‐RCT | primary health centres | 12 | 58/685 (8.9%) | 55/818 (7.8%) | 0.79 (0.59 to 1.06) |
| Theron 2014a | multiple | RCT | primary health clinics | 6 | 63/758 (6%) | 58/744 (8%) | 0.94 (0.67 to 1.32) |
| Yoon 2012 | Uganda | before/after | national referral hospital | 2 | 44/186 (24%) | 64/259 (25%) | 1.04 (0.75 to 1.46) |
CI: confidence interval RCT: randomized controlled trial RR: risk ratio ART: antiretroviral therapy for HIV
Ngwira 2019 reported estimates as incidence rate ratios (IRR) 0.78 (95% CI 0.58 to 1.06). We converted to RR, assuming 12 months of follow‐up.
All estimates are unadjusted, apart from Churchyard 2015, who reported imbalance due to a small number of large clusters.
1.1. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 1: All‐cause mortality
3.

1.1 All‐cause mortality
Subgroup analyses
Restricting the analysis to the three studies that assessed mortality at six months produced an estimate of RR 0.98 (95% CI 0.78 to 1.22; 3 RCTs, 8143 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 2: All‐cause mortality in the subgroup assessed at six months
In participants infected with HIV, we estimated the effect of using Xpert MTB/RIF on mortality to be odds ratio (OR) 0.80 (95% CI 0.67 to 0.96; 5 RCTs, 5855 participants; Analysis 1.3). There was no evidence of a difference in the effect of Xpert MTB/RIF on the risk of death between participants known to be infected with HIV who attended general clinics (Ngwira 2019; Di Tanna 2019 ) and who attended a specialised HIV clinic (Mupfumi 2014) (P = 0.46). A previous analysis by Di Tanna 2019 estimated the effect of Xpert MTB/RIF in participants known to be HIV negative (OR 0.83 (95% CI 0.46 to 1.50)), we did not find any further studies. There was no evidence of a difference in the effect of Xpert MTB/RIF in participants known to be HIV positive and HIV negative (P = 0.91). We do not know about those with unknown HIV status.
1.3. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 3: All‐cause mortality: subgroup analysis by HIV status
Due to lack of data, we were unable to conduct subgroup analyses for children; for adults; for participants with resistant and sensitive tuberculosis; and by HIV status other than for mortality.
Proportion of participants treated for tuberculosis who had a successful treatment outcome
We are uncertain whether there is a modest or no effect of Xpert MTB/RIF on the proportion of treated participants with a successful outcome (OR 1.10, 95% CI 0.95 to 1.26; 3 RCTs, 4802 participants; Analysis 1.4; Figure 4; Table 4). One trial dominated the meta‐analysis with a weight of 85% due to a large sample size (Durovni 2014).
1.4. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 4: Proportion of participants starting tuberculosis treatment who had a successful treatment outcome
4.

1.4 Proportion of participants starting tuberculosis treatment who had successful treatment outcomes
3. Summary of trial results: proportion of those treated for tuberculosis who had a successful outcome.
| Study | Country | Design | Setting | Smear (n/N) | Xpert (n/N) | OR (95% CI) |
| Cox 2014 | South Africa | cluster‐RCT | primary health clinic | 176/224 (79%) | 215/268 (80%) | 1.11 (0.67 to 1.84) |
| Agizew 2019a | Botswana | stepped‐wedge | public HIV clinics | 36/57 (63%) | 136/199 (68%) | 1.26 (0.64 to 2.42) |
| Durovni 2014 | Brazil | stepped‐wedge | primary health clinic | 1276/1840 (68%) | 1571/2214 (70%) | 1.09 (0.94 to 1.27) |
CI: confidence interval RCT: randomized controlled trial OR: odds ratio
Agizew 2019a defined an unsuccessful outcome as default, all‐cause death, failure, or transfer, Durovni 2014 and Cox 2014 also included a small number of participants who were not evaluated.
We calculated the OR for Cox 2014 from the number in each arm. The CI took clustering by week into account, by inflating the standard error of log(OR) by a factor in line with the other outcomes in the same study.
Subgroup analyses
We were unable to conduct subgroup analyses for this outcome due to lack of data.
Secondary outcomes
Proportion of participants who were treated for tuberculosis
We are uncertain whether there was a modest or no effect for Xpert MTB/RIF on the proportion of participants who were treated for tuberculosis (RR 1.10, 95% CI 0.98 to 1.23; 5 RCTs, 8793 participants, Analysis 1.5, Figure 5, Table 5).
1.5. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 5: Proportion of participants treated for tuberculosis
5.

1.6 Proportion of participants treated for tuberculosis who were microbiologically confirmed
4. Summary of studies: proportion of participants treated for tuberculosis.
| Study | Country | Design | Setting | Proportion participants treated | RR (95% CI) | |
| Smear arm (n/N) | Xpert arm (n/N) | |||||
| Calligaro 2015 | South Africa | RCT | intensive care units | 16/115 (14%) | 24/111 (22%) | 1.55 (0.87 to 2.77) |
| Churchyard 2015 | South Africa | cluster‐RCT | primary health clinics | 291/2332 (12%) | 250/2324 (11%) | 1.04 (0.76 to 1.43) |
| Cox 2014 | South Africa | cluster‐RCT | primary health clinic | 229/1003 (23%) | 277/982 (28%) | 1.23 (1.04 to 1.46) |
| Mupfumi 2014 | Zimbabwe | RCT | ART initiation centre | 45/210 (21%) | 43/214 (20%) | 0.94 (0.65 to 1.36) |
| Theron 2014a | multi‐country | RCT | primary health clinics | 317/758 (42%) | 324/744 (44%) | 1.03 (0.91, 1.16) |
ART: antiretroviral therapy CI: confidence interval RCT: randomized controlled trial RR: risk ratio
All estimates are unadjusted, apart from Churchyard 2015, who reported imbalance due to a small number of large clusters.
Proportion of treated participants who were microbiologically confirmed
A higher proportion of those treated in the Xpert MTB/RIF group were confirmed microbiologically than those treated in the smear microscopy group (RR 1.44, 95% CI 1.29 to 1.61; 6 RCTs, 2068 participants; Analysis 1.6; Figure 6; Table 6).
1.6. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 6: Proportion of participants treated for tuberculosis who were microbiologically confirmed
6.

1.7 Proportion of participants with microbiological confirmation who had pre‐treatment loss to follow‐up
5. Summary of studies: proportion of treated participants with microbiological confirmation.
| Study | Country | Design | Setting | Smear (n/N) | Xpert (n/N) | RR (95% CI) |
| Agizew 2019a | Botswana | stepped‐wedge | public HIV clinics | 18/57 | 102/199 | 1.62 (1.08 to 2.44) |
| Calligaro 2015 | South Africa | RCT | intensive care units | 7/16 | 20/24 | 1.90 (1.06 to 3.41) |
| Churchyard 2015 | South Africa | cluster‐RCT | primary health clinics | 189/291 | 196/250 | 1.20 (0.98 to 1.47) |
| Cox 2014 | South Africa | cluster‐RCT | primary health clinic | 131/229 | 226/277 | 1.41 (1.13 to 1.77) |
| Mupfumi 2014 | Zimbabwe | RCT | specialized hospital | 14/45 | 20/43 | 1.50 (0.87 to 2.57) |
| Theron 2014a | multiple | RCT | primary health clinics | 120/317 | 190/320 | 1.57 (1.33 to 1.86) |
CI: confidence interval RCT: randomized controlled trial RR: risk ratio
All estimates use unadjusted, apart from Churchyard 2015, who reported imbalance due to a small number of large clusters.
Proportion of patients with pre‐treatment loss to follow‐up
There was evidence that Xpert MTB/RIF reduced the risk of pre‐treatment loss to follow‐up over smear microscopy (RR 0.59, 95% CI 0.41 to 0.85; 3 RCTs, 1217 participants; Analysis 1.7; Table 7).
1.7. Analysis.

Comparison 1: Xpert MTB/RIF vs smear microscopy, Outcome 7: Proportion of participants with microbiological confirmation, who had pre‐treatment loss to follow‐up
6. Summary of studies: pre‐treatment loss to follow‐up.
| Study | Country | Design | Setting | Smear (n/N) | Xpert (n/N) | RR (95%CI) |
| Churchyard 2015 | South Africa | cluster‐RCT | primary health facilities | 26/174 (15%) | 34/200 (17%) | 0.96 (0.48 to 1.93) |
| Cox 2014 | South Africa | cluster‐RCT | primary health facility | 41/167 (25%) | 32/257 (13%) | 0.51 (0.32 to 0.82) |
| Theron 2014a | multiple | RCT | primary health facilities | 30/204 (15%) | 16/215 (7%) | 0.52 (0.29 to 0.92) |
CI: confidence interval RCT: randomized controlled trial RR: risk ratio
All estimates are unadjusted, apart from Churchyard 2015, who reported imbalance due to a small number of large clusters.
Time from first contact with the health system to initiation of treatment
Only one randomized study reported the hazard ratio (HR) for time‐to‐treatment initiation (HR 0.76, 95% CI 0.63 to 0.92; 1985 participants; Cox 2014). Table 8 reports the medians of the skewed distributions for treatment initiation reported in eight studies, and the proportion treated on the same day in one study (Theron 2014a).
7. Summary of studies: time to initiate treatment.
| Study | Country | Design | Setting |
Smear Median days (IQR) |
Xpert Median days (IQR) |
Comparison |
| Mupfumi 2014 | Zimbabwe | RCT | specialized hospital | 8 (3 to 23) | 5 (3 to 13) | P = 0.26 |
| Theron 2014a | multiple | RCT | primary healthcare facilities | 15% (115/178) | 23% (168/744) | P = 0.0002 |
| Churchyard 2015 | South Africa | cluster‐RCT | primary healthcare facility | 10 | 7 | |
| Cox 2014 | South Africa | cluster‐RCT | primary healthcare facility | 8 (2 to 27) | 4 (2 to 8) | HR 0.76 (0.63 to 0.92) P = 0.005 |
| Agizew 2019a | Botwsana | stepped‐ wedge | public HIV clinics | 22 (3 to 51) | 6 (2 to 17) | P = 0.005 |
| Durovni 2014 | Brazil | stepped‐wedge | clinics using laboratories | 11.4 (8.5 to 14.5) | 8.1 (5.4 to 9.3) | P = 0.04 |
| Van den Handel 2015 | South Africa | before/after | district, sub‐district, and primary healthcare facilities | 11.5 (6 to 24) | 1 (0 to 2) | |
| Yoon 2012 | Uganda | before/after | national referral hospital | 1 (0 to 5) | 0 (0 to 2) | P = 0.06 |
| Schmidt 2017 | South Africa | before/after | primary healthcare facilities | 5 (2 to 14) | 4 (2 to 8) | P < 0.001 |
| van Kampen 2015 | Indonesia | before/after | drug‐resistant TB clinics | 42 (25 to 55) | 15 (7 to 51) | P < 0.001 |
HR: hazard ratio IQR: interquartile range RCT: randomized controlled trial TB: tuberculosis
Theron 2014a reported the proportion of participants initiating treatment on the day of diagnosis.
Di Tanna 2019 included an individual participant data (IPD) meta‐analysis for time to treatment for Churchyard 2015; Cox 2014; Mupfumi 2014; and Theron 2014a. The HR was estimated to be 1.00 (95% CI 0.75 to 1.32) for Xpert MTB/RIF compared to smear microscopy, adjusting for age and sex.
This outcome was the subject of a recent individual participant meta‐analysis (Di Tanna 2019), which included four of the included studies, with an overall estimated hazard ratio of 1.00 (95% CI 0.75 to 1.32; Churchyard 2015; Cox 2014; Mupfumi 2014; Theron 2014a), adjusting for age and gender. We do not replicate this analysis.
Proportion of participants who were diagnosed and treated for multi‐drug resistant tuberculosis (MDR‐TB)
We were unable to compare between Xpert MTB/RIF and smear strategies for outcomes specific to drug‐resistant tuberculosis because drug‐resistant tuberculosis diagnoses were reported for the Xpert MTB/RIF arms only.
One non‐randomized study assessed participant outcomes among those diagnosed with multi‐drug resistant tuberculosis (van Kampen 2015). MDR‐TB was diagnosed with first‐line drug sensitive tests (DST). The median time to MDR‐TB treatment initiation was 88 days before, and 16 days after Xpert MTB/RIF was used as a diagnostic tool.
Number of visits to the same or any other healthcare facilities prior to diagnosis
None of the included studies measured this outcome.
Self‐reported satisfaction
None of the included studies measured this outcome.
Subgroup analyses
We were unable to conduct subgroup analyses for any of the secondary outcomes due to lack of data.
Discussion
Summary of main results
We included 12 studies that assessed the effect of Xpert MTB/RIF on health outcomes. Compared to diagnostic strategies using smear microscopy, there was evidence of a positive effect of Xpert MTB/RIF for some outcomes, and inconclusive evidence for others.
The effect on all‐cause mortality overall was uncertain (moderate‐certainty evidence; Table 1). There was probably a decrease in mortality among participants known to be infected with HIV.
There was probably no decrease in the proportion of participants initiating treatment who had a successful treatment outcome with Xpert MTB/RIF, but whether there was no or a modest increase was uncertain (moderate‐certainty evidence).
There was inconclusive evidence of a modest or no effect on the proportion of participants treated for tuberculosis. There was probably a reduction in loss to follow‐up before treatment initiation, and an increase in the proportion of treated participants who had microbiological confirmation (moderate‐certainty evidence).
Overall completeness and applicability of evidence
This review includes studies carried out in low‐ and middle‐income countries, where most tuberculosis cases and tuberculosis‐related deaths occur (Nliwasa 2018; WHO 2019). Most included studies were undertaken in sub‐Saharan Africa, in areas with a high burden of tuberculosis and HIV. The findings were generally similar in the trials set in South America and Asia, suggesting that the findings may be more widely applicable to high burden areas.
A limitation is that there are a small number of studies conducted on the impact of Xpert MTB/RIF on participant outcomes, and some of our outcomes have few studies contributing to the overall estimates. A further limitation is the variability in trial characteristics, such as the duration of follow‐up among studies, which may increase heterogeneity in the study effect estimates. We could not assess all objectives planned for this review due to limitations in data availability, highlighting an information gap. We were unable to assess participant satisfaction, the number of visits to health facilities, and drug sensitive and resistant tuberculosis. Subgroup analyses in people who were HIV positive, negative or who have unknown HIV status were limited.
Our findings should be interpreted in the context of healthcare systems. Patient‐important outcomes, such as mortality, may depend on health system factors other than diagnosis, such as treatment completion rates, co‐morbidities, and empirical treatment (Pai 2018). Studies have reported different experiences in the gaps in the healthcare cascade, for example South Africa performed generally better in terms of individuals accessing tuberculosis tests, but had poor treatment outcomes (Subbaraman 2019). The effects of diagnostic strategies may depend, in part, on the linkage between diagnosis and treatment. Most included studies were from countries where tuberculosis is primarily managed in the public sector. The impact of Xpert MTB/RIF may be different in countries where tuberculosis is managed in the private sector, with poor linkage to national programmes.
Further molecular diagnostic tests have recently been introduced or are under development, such as Xpert MTB/RIF Ultra (Cepheid) and Truenat (Molbio Diagnostics). The results here may inform the likely impact of these new tests.
Quality of the evidence
Overall, there were a small number of trials, although some had a large number of participants. We judged the certainty of the evidence for the outcomes as moderate, due to imprecision.
The trials included in the analysis could not be blinded. Clinicians' knowledge and perception of the diagnostic test could be regarded as part of the intervention, but the lack of blinding may lead to other biases, caused by knowing which new diagnostic was under evaluation. We judged that there were high risks of performance bias in all studies.
In endemic settings, empirical treatment (when there is no bacteriological confirmation, and the decision to treat is based on a chest X‐ray, or clinical judgement, or both) is common. Empirical treatment may result from a perceived lack of availability or access to confirmation of tuberculosis (Boyles 2017). Empirical treatment has long been recommended by the World Health Organization (WHO), particularly in resource‐limited settings (Walusimbi 2013). It is more frequent when there is a high tuberculosis or tuberculosis and HIV burden, a high pre‐test probability for tuberculosis, or when a delay in initiating treatment could result in severe morbidity or mortality. By acting without test results, empirical treatment may diminish any potential benefits of a more sensitive test (Di Tanna 2019; Theron 2014b). It has also been suggested that a higher quality of care during the trials may have reduced the observed impact (Ochodo 2019).
Potential biases in the review process
We carried out a comprehensive search, as far as possible without language restriction, to ensure we included all studies that met the inclusion criteria. The studies included those in two recent reviews which have some overlap in outcomes (Agizew 2019b; Di Tanna 2019).
Agreements and disagreements with other studies or reviews
The evidence for an effect of Xpert MTB/RIF on mortality was inconclusive. A similar conclusion was reached in a recent meta‐analysis using participant level data, in which an impact on mortality could be neither ruled in nor out (Di Tanna 2019). Di Tanna 2019 included three studies for six‐month mortality, giving an overall estimated odds ratio (OR) of 0.88 (95% confidence interval (CI) 0.68 to 1.14; Churchyard 2015; Cox 2014; Theron 2014a). We included five studies, including these three, with varying lengths of follow‐up in our analysis and reached a similar estimate (RR 0.89) with a slightly narrower 95% CI (0.75 to 1.05). In a narrative literature review of eight trials, which assessed the impact of Xpert MTB/RIF on participant outcomes, none of the individual trials reported a significant impact of Xpert MTB/RIF on mortality (Auld 2016). We included all eight studies in our review.
Among participants who were HIV positive, we found evidence of a reduction in all‐cause mortality in the Xpert MTB/RIF arm (OR 0.80, 95% CI 0.67 to 0.95). The findings in Di Tanna 2019 were inconclusive for the proportion dying by six months in this subgroup (OR 0.83, 95% CI 0.65 to 1.05), but there was evidence of an increase in survival (HR 0.76, 95% CI 0.60 to 0.97). Our review included the studies in Di Tanna 2019, plus two more.
Participants who were treated for tuberculosis in the Xpert MTB/RIF groups were more likely to be confirmed bacteriologically than those in the smear microscopy groups. Similar findings were reported in a recent review, in which Xpert MTB/RIF was shown to be superior for tuberculosis bacteriological confirmation, in a subset of seven of the nine studies that we included (Agizew 2019b). In South Africa, the introduction of Xpert MTB/RIF in 101 primary healthcare facilities was shown to increase the rate of bacteriological confirmation and reduce the rate of empirical treatment over a period of four years, in a large population‐based programmatic cohort (Hermans 2017). Similar observations were made in Nepal (Creswell 2015).
We found that Xpert MTB/RIF reduced the risk of pre‐treatment loss to follow‐up compared to smear microscopy (RR 0.59, 95% CI 0.41 to 0.85). A recent review of 23 studies from 14 countries reported that pre‐treatment loss to follow‐up of participants who were smear or culture positive was commonly reported in studies from Africa (6% to 38%) and Asia (4% to 28%; (MacPherson 2014)).
A meta‐analysis of a related outcome, the difference in median times to diagnosis, found substantial heterogeneity between studies (McGrath 2020). Although we did not analyse time to treatment, the medians appeared to vary greatly (Table 8). This is likely to be due to the differences in study design, participant mix, and settings.
Authors' conclusions
Implications for practice.
Compared with smear microscopy, we found evidence that using Xpert MTB/RIF had a beneficial impact on some participant outcomes, but was inconclusive for others.
The evidence for a reduction in all‐cause mortality among all participants was inconclusive, but there was evidence of a modest reduction for the subgroup of participants who were known to be infected HIV. We were unable to rule in or out, an impact on the proportion of those treated who had a successful treatment outcome, or on the proportion treated. There was evidence of a decrease in pre‐treatment loss to follow‐up, and an increase in the proportion of those treated who had been microbiologically confirmed.
The summary of the current evidence for an impact of Xpert MTB/RIF on participant outcomes can help to inform decision‐makers, alongside additional information, such as cost‐effectiveness and feasibility.
Implications for research.
Future studies on newly developed molecular point‐of‐care tests should incorporate the assessment of participant outcomes. Such studies are valuable when carried out in settings close to where people live, such as primary healthcare facilities. We identified gaps in knowledge. Future studies should include the number of visits to health facilities, participant satisfaction, participant outcomes for drug‐resistant tuberculosis, and the mechanisms of empirical treatment, as well as cost‐effectiveness and implementation.
History
Protocol first published: Issue 2, 2018 Review first published: Issue 4, 2021
Acknowledgements
The Academic Editor is Dr Nathan Ford.
The Cochrane Infectious Diseases Group (CIDG) editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies. We thank Paul Garner, Anne‐Marie Stephani, Deirdre Walshe, Philomena Hinds, and Vittoria Lutje for their support and comments.
We extend our gratitude to European Developing Countries Clinical Trial Partnership (EDCTP) for the fellowship grant to Mwaka Kakolwa. This grant supported Mwaka Kakolwa during the review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials: Issue 7 of 12, July 2020
ID Search
#1 Xpert* or GeneXpert*or Cepheid or "near* patient":ti,ab,kw (Word variations have been searched)
#2 (smear and microscop*) or (sputum and microscopy):ti,ab,kw
#3 MeSH descriptor: [Sputum] explode all trees
#4 #1 or #2 or #3
#5 tuberculosis:ti,ab,kw
#6 MeSH descriptor: [Tuberculosis] explode all trees
#7 TB:ti,ab,kw
#8 #5 or #6 or #7
Database: Embase OVID
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Xpert*.mp.
2 genexpert*.mp.
3 Cepheid.mp.
4 "near* patient".mp.
5 1 or 2 or 3 or 4
6 (smear adj3 microscopy).mp.
7 *sputum/
8 6 or 7
9 exp Tuberculosis/
10 tubercul*.ab. or tubercul*.ti.
11 TB.ab. or TB.ti.
12 Mycobacterium tuberculosis/
13 9 or 10 or 11 or 12
14 5 or 8
15 14 and 13
16 limit 15 to human
17 limit 16 to yr="2007 ‐Current"
18 (treatment or effect* or outcome* or mortality or impact*).m_titl.
19 (effect* or outcome* or mortality or impact*).ab.
20 19 or 18
21 17 and 20
22 randomized controlled trial/
23 (randomized or placebo or double‐blind* or single‐blind*).mp.
24 controlled clinical trial.tw. or controlled clinical trial/
25 follow up/ or cohort analysis/ or cohort.mp.
26 (before and after).mp.
27 22 or 23 or 24 or 25 or 26
28 21 and 27
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to present
1 (Xpert* or genexpert* or Cepheid or "near* patient").mp.
2 (smear adj3 microscop*).mp.
3 (sputum adj3 microscopy).mp.
4 *sputum/
5 2 or 3 or 4
6 exp Tuberculosis/
7 tubercul*.ab. or tubercul*.ti.
8 TB.ab. or TB.ti.
9 Mycobacterium tuberculosis/
10 6 or 7 or 8 or 9
11 1 or 5
12 10 and 11
13 (treatment or effect* or outcome* or mortality or impact*).m_titl.
14 (effect* or outcome* or mortality or impact*).ab.
15 13 or 14
16 12 and 15
17 randomized controlled trial.pt
18 (randomized or placebo or double‐blind* or single‐blind*).tw
19 controlled clinical trial.tw. or controlled clinical trial.pt
20 Follow‐Up Studies/ or cohort.mp.
21 (before and after studies).mp. (745518)
22 exp Cohort Studies/ (2012868)
23 17 or 18 or 19 or 20 or 21 or 22
24 16 and 23
Cinahl EBSCOHost
| # | Query |
| S4 | S1 AND S2 AND S3 |
| S3 | TI ( effect or impact or influence or consequences or outcome* or mortality ) OR AB ( effect or impact or influence or consequences or outcome* or mortality ) |
| S2 | TI ( Xpert mtb/rif or GeneXpert* ) OR AB ( Xpert mtb/rif or GeneXpert* ) |
| S1 | TI ( tuberculosis or tb or "mycobacterium tuberculosis" ) OR AB ( tuberculosis or tb or "mycobacterium tuberculosis" ) |
Science Citation Index – Expanded, Social Sciences citation index (Web of Science), Conference Proceedings Citation Index‐ Science, Social Sciences citation index, and Conference Proceedings Citation Index ‐ Social Science & Humanities (all Web of Science)
| # 1 | TOPIC: (tuberculosis or tb or "mycobacterium tuberculosis") AND TOPIC: (xpert mtb/rif or GeneXpert* or sputum or microscopy) AND TOPIC: (effect or impact* or influence or consequences or outcome* or mortality) AND TOPIC: (randomized or trial or "controlled trial" or cohort or "before and after") |
| Database : | LILACS |
| Search on : | tuberculosis or TB [Words] and xpert$ or GeneXpert$ [Words] |
ClinicalTrials.gov
Xpert* or GeneXpert or sputum | Tuberculosis
WHO ICTRP
Tuberculosis and (Xpert* or GeneXpert*)
Data and analyses
Comparison 1. Xpert MTB/RIF vs smear microscopy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 5 | 9932 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.05] |
| 1.2 All‐cause mortality in the subgroup assessed at six months | 3 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.78, 1.22] | |
| 1.3 All‐cause mortality: subgroup analysis by HIV status | 3 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 HIV positive | 3 | Odds Ratio (IV, Random, 95% CI) | 0.80 [0.67, 0.96] | |
| 1.3.2 HIV negative | 1 | Odds Ratio (IV, Random, 95% CI) | 0.83 [0.46, 1.50] | |
| 1.4 Proportion of participants starting tuberculosis treatment who had a successful treatment outcome | 3 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.95, 1.26] | |
| 1.5 Proportion of participants treated for tuberculosis | 5 | 8793 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.98, 1.23] |
| 1.6 Proportion of participants treated for tuberculosis who were microbiologically confirmed | 6 | 2068 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.29, 1.61] |
| 1.7 Proportion of participants with microbiological confirmation, who had pre‐treatment loss to follow‐up | 3 | 1217 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.41, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agizew 2019a.
| Study characteristics | ||
| Methods | Stepped‐wedge cluster‐randomized trial. A cluster was defined as HIV care and treatment clinic. Twenty‐two clusters, located at five district hospitals and 17 primary healthcare facilities, were purposively selected to: (1) be representative of HIV treatment clinics in Botswana, and (2) have new antiretroviral therapy (ART) initiation rates sufficient to meet sample size requirements per protocol | |
| Participants | Participants were new HIV clinic attendees, regardless of age, and who were not prisoners at
the time of the first HIV clinic visit between August 2012 and November 2014. Female: 66% in the Xpert arm, 68% in the smear arm HIV infection: all were HIV infected Settings:primary healthcare facilities Country: Botswana Sample size: 4225 Xpert arm, 1816 smear arm |
|
| Interventions | Participants who screened positive for at least one tuberculosis symptom were requested to provide four sputa samples: two were provided on the screening day (spot 1 and 2) and two on the following day. On day 2, one sputum sample was collected at home early in the morning (morning sample), and another sample was taken at the clinic (spot 3). Participants in the smear arm were enrolled in XPRES before Xpert MTB/RIF instrument implementation; therefore, spots 1 and 3 were tested only with Ziehl–Nielson smear at the peripheral laboratory. However, if participants in the smear arm screened positive for tuberculosis during a follow‐up appointment after Xpert MTB/RIF instrument implementation, spots 1 and 3 were tested by Xpert MTB/RIF at the peripheral laboratory or point of care sites. For the Xpert arm, all spot 1 and 3 samples were tested by Xpert MTB/RIF either at the peripheral laboratory or at point of care sites. Spot 2 and morning samples were submitted to the National tuberculosis Reference Laboratory for liquid culture. |
|
| Outcomes | Treatment outcome Time to treatment initiation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors reported that the statistician randomly selected one of the roll‐out permutations |
| Allocation concealment (selection bias) | Unclear risk | No details on how the allocation list was concealed |
| Baseline characteristics similar (selection bias) | Low risk | There were no substantial differences |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not done, however, blinding is not feasible for a diagnostic test, and knowledge of the test is part of the intervention |
| Protection against contamination (performance bias) | Low risk | Allocation of the intervention replaced smear microscopy at different periods in a stepped‐wedge design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not done, however, blinding was not feasible given pragmatic nature of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The rate of loss to follow‐up was similar between the Xpert MTB/RIF arm and smear microscopy arm |
| Selective reporting (reporting bias) | Low risk | All outcomes in the trial were reported |
| Other bias | Low risk | Not detected |
Calligaro 2015.
| Study characteristics | ||
| Methods | Individual randomized controlled trial in ICUs in four hospitals. | |
| Participants | Participants: people who were mechanically ventilated, and suspected of having tuberculosis, 18 years old and older, admitted between 1 Aug 2010 and 31 July 2013. with no tuberculosis treatment in the previous 60 days Female: 40% in the Xpert arm, 41% in the smear microscopy arm HIV infection: 27% Xpert, 32% smear Settings: intensive care units (ICUs) in four tertiary and secondary hospitals in Cape Town Country: South Africa Sample size: 317 participants in total |
|
| Interventions | Smear and culture (control), or Xpert MTB/RIF and culture (intervention) of tracheal aspirate samples | |
| Outcomes |
Primary outcome: proportion of culture‐positive participants started on anti‐tuberculous treatment in each trial group 48 hrs after enrolment Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of block size of 10, by computer‐generated allocation list |
| Allocation concealment (selection bias) | Low risk | Allocation list was kept centrally by the data manager, a nurse contacted the data manager each time an eligible participant was identified |
| Baseline characteristics similar (selection bias) | Low risk | No substantial differences observed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Laboratory staff were blinded to clinical and microbiological details of the participants. Participants and clinicians were not blinded to the test used. |
| Protection against contamination (performance bias) | Low risk | Allocation and assignment of the group was done centrally, by a data manager, after a nurse called following availability of eligible participants. No risk of contamination |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of the outcome was done using study staff who were not blinded, but the outcome was clear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The proportions of loss to follow‐up were 29% in smear, and 8% in the Xpert MTB/RIF groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section were reported |
| Other bias | Low risk | not detected |
Churchyard 2015.
| Study characteristics | ||
| Methods | Two‐arm, parallel, cluster‐randomized trial. A cluster was defined as a laboratory and two primary care clinics, served by but not co‐located with that laboratory. | |
| Participants | Participants had suspected tuberculosis: a systematic sample of adults giving sputum for tuberculosis investigation; 18 years old and older Female: 62% overall HIV: 62% overall, 33% of whom had ever been on antiretroviral therapy Setting: primary healthcare clinics and laboratories in medium‐burden districts in four provinces Country: South Africa Sample size: 4658 participants in total, 10 clusters in each arm |
|
| Interventions | In the Xpert group, participants had one spot sputum specimen collected for Xpert MTB/RIF testing at the associated laboratory. In the microscopy group, participants had two sputum specimens collected for fluorescence microscopy. |
|
| Outcomes |
Primary outcome: mortality, measured 6 months after enrolment Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated by a statistician using Stata statistical software |
| Allocation concealment (selection bias) | Low risk | Randomization was by laboratory, at the outset |
| Baseline characteristics similar (selection bias) | Unclear risk | Differences between groups reported, but these were adjusted for in the analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded. It is not possible to blind in pragmatic settings. |
| Protection against contamination (performance bias) | Low risk | randomization by cluster |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Deaths were recorded through participants' nominated contacts, clinic staff, and by accessing the department of home affairs vital statistics using South African's identification numbers. In case of ascertainment conflict, an endpoint small committee assigned vital status, but for small number of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of loss to follow‐up was similar in both arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Not detected |
Cox 2014.
| Study characteristics | ||
| Methods | Pragmatic prospective cluster‐randomized trial. The study took place in one large primary care facility, with randomization by week to the intervention or routine care. |
|
| Participants | Participants were presumptive pulmonary tuberculosis presenting at Ubuntu clinic in Khayelitisha Cape town; 18 years old and older Female: 44.7% in the Xpert group, and 46% in smear microscopy group HIV infection: 59% in Xpert group, 59.7% in smear microscopy group Setting: primary healthcare clinic Country: South Africa Sample size: 982 Xpert MTB/RIF arm, and 1003 smear microscopy arm |
|
| Interventions | Randomization was done on weekly basis. Each week during the study period was randomized to either Xpert MTB/RIF or smear microscopy Intervention: Xpert MTB/RIF Routine care: smear, culture and DST for high risk of drug resistance |
|
| Outcomes |
Primary outcome: proportion of bacteriologically confirmed tuberculosis cases that had not initiated appropriate treatment by 3 months after enrolment Secondary outcomes:
|
|
| Notes | Target condition: tuberculosis case definition: bacteriological confirmation of tuberculosis (smear, Xpert, or culture) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Weeks during the study period were randomized to either intervention (Xpert MTB/RIF) or control (smear microscopy). Sequence was generated using web based (Random.org) system prior to the start of the study. |
| Allocation concealment (selection bias) | Unclear risk | The principal investigator generated the schedule list however no details on how and who kept the list |
| Baseline characteristics similar (selection bias) | Low risk | Characteristics were similar in both group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded |
| Protection against contamination (performance bias) | Unclear risk | Randomization was done weekly during the study weeks; the intervention was not always correctly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were objectively assessed. It is unlikely that treatment initiation was done differently across group. Mortality was ascertained through regional and national registry, using civil identification numbers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of unfavourable outcomes was reported in combination (defaulters, death, and failure). It is not clearly stated how much of the unfavourable outcome was linked to loss to follow‐up, and whether this affected the groups differently |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes were not prespecified |
| Other bias | Low risk | No other biases were detected |
Di Tanna 2019.
| Study characteristics | ||
| Methods | Di Tanna 2019 is a meta‐analysis of three studies. The study characteristics for those three are included separately. We have included Di Tanna 2019 as a study, so it will appear in the meta‐analysis. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Durovni 2014.
| Study characteristics | ||
| Methods | Stepped‐wedge cluster‐randomized trial. All 14 laboratories started with smear microscopy. Two laboratories then switched overnight to the Xpert arm every month, so that by the eighth and final month of the trial, all clusters were in the Xpert arm. The unit of comparisons were laboratories and the clinics that used their services. | |
| Participants | Participants who had sputum samples sent to the study laboratories for the diagnosis of pulmonary tuberculosis between February and October 2012 All age groups Female: 35.6% Xpert, 35.9% smear microscopy HIV infection: 7.4% Xpert, 9.8% smear Settings: primary healthcare facilities that used the laboratories in the study Country: Brazil, the study was conducted in the cities of Manaus (three laboratories) and Rio de Janeiro (eleven laboratories) Sample size: 2610 Xpert, 2050 smear microscopy, 14 clusters |
|
| Interventions | The diagnostic test for pulmonary tuberculosis was: Intervention: Xpert Comparison arm: sputum smears |
|
| Outcomes |
Primary outcome
Secondary outcomes notification rates: for
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stepped‐wedge trial design: randomization of the order in which the intervention was implemented in the laboratories was stratified by case load and estimated HIV prevalence. No detailed information was given on how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was not concealed, but the staff were blinded to the order of entry into the intervention |
| Baseline characteristics similar (selection bias) | Low risk | Baseline characteristics were similar in the two groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded to the intervention itself. |
| Protection against contamination (performance bias) | Low risk | Laboratories and clinics serving laboratories were randomized in a stepped‐wedge design; Xpert MTB/RIF replaced smear microscopy; no risk of contamination |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not clear who assessed the outcomes, and whether there was a difference in how the outcome was assessed between the baseline and intervention periods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion lost to follow‐up were disclosed and analysed. 41% of notifications could not be linked to any test, but the proportion was similar in the two arms. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes were not prespecified. |
| Other bias | Low risk | Not detected |
Mupfumi 2014.
| Study characteristics | ||
| Methods | Single centre, pragmatic individually‐randomized controlled trial | |
| Participants | Participants included were consecutive symptomatic and asymptomatic HIV‐infected participants initiating anti‐retroviral therapy 18 years old and older Female: 55% HIV infection: 100% (HIV clinic) Setting: specialized infectious disease hospital Country: Zimbabwe Sample size: 424 |
|
| Interventions | participants provided 2 spot sputum specimens at least 1 hour apart. If participants were unable to expectorate sputum, attempts were made to induce sputum using nebulized 6% hypertonic saline. Samples in the microscopy group had a direct smear completed on each sample followed by staining with auramine O (Leica, Germany). Xpert MTB/RIF assays were completed on direct sputum. |
|
| Outcomes | Primary outcome: proportion of participants who were diagnosed with ART‐associated tuberculosis, or who died within 3 months of randomization | |
| Notes | Target condition: Tuberculosis Case definition: participants with at least 1 positive Xpert or microscopy (for 'scanty' samples, both smears needed to be recorded as 'scanty') |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation list was generated by the data manager and supplied to the laboratory manager in sealed envelope |
| Allocation concealment (selection bias) | Low risk | Allocation was given in sealed opaque envelope |
| Baseline characteristics similar (selection bias) | Low risk | Similar baseline characteristics in both groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not done. Blinding was not feasible given a pragmatic trial design, however, sample transporter to the central laboratory for quality control was blinded to participant identification |
| Protection against contamination (performance bias) | Low risk | Randomization was generated by a data manager, and codes were kept in envelopes; participants were assigned based on codes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not feasible; death occurring after tuberculosis diagnosis was considered tuberculosis‐related death, however, the authors did not clearly explain the different ways to verify death. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of loss to follow‐up was not significantly different between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods were reported |
| Other bias | Low risk | Not detected |
Ngwira 2019.
| Study characteristics | ||
| Methods | Cluster‐randomized trial in 12 primary healthcare centres in rural Thyolo district Malawi | |
| Participants | Participants included were newly diagnosed with HIV 18 years old and older Female: 55% HIV infection: 100% (HIV clinic) setting: primary healthcare facilities Country: Malawi Sample size: 1842 |
|
| Interventions | Primary healthcare clinics were randomized to either screen tuberculosis in newly HIV participants by Xpert MTB/RIF, or light emitting diode fluorescence microscopy (LED FM). Symptom screening and sputum evaluation were performed on‐site by trained study personnel, and results were provided to participants on the same day. Participants testing positive for active tuberculosis were referred for treatment. Participants with tuberculosis symptoms but negative Xpert or LED FM results were asked to return in one month, and provided with Isoniazid Preventive Therapy (IPT) at that time if asymptomatic. All participants with positive Xpert or LED FM results had sputum taken for confirmatory culture, performed at a central laboratory. All participants were asked to return to study clinics for assessment every three months (with one extra visit when on IPT). | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Target condition: tuberculosis in newly diagnosed HIV‐positive participants Case definition: positive Xpert MTB/RIF, LED FM, or positive culture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by study statistician who identified possible randomization that would result in prespecified balance, and the randomization was carried out by an official using the coin flip method |
| Allocation concealment (selection bias) | Low risk | Investigators and laboratory staffs were blinded to allocation |
| Baseline characteristics similar (selection bias) | Low risk | Baseline characteristics were reported to be similar, no detection of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinics were not blinded |
| Protection against contamination (performance bias) | Low risk | Intervention was allocated at a cluster, and recruitment done in waves based on geography |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible, mortality is an objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was similar in both arms |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported |
| Other bias | Low risk | Not detected |
Schmidt 2017.
| Study characteristics | ||
| Methods | Before and after evaluation cohort to evaluate impact of roll‐out of Xpert MTB/RIF on detection and treatment of adults with pulmonary tuberculosis | |
| Participants | 18 years old and above Adults suspected of pulmonary tuberculosis Female: 45.9% before, 44.1% after HIV infection was not reported Setting: primary health care in the Cape Winelands East in Weastern Cape. The Cape Winelands is a semi‐rural area with a very high estimated total tuberculosis case notification rate of 1400 per 100,000 population Country: South Africa Sample size: 15,629 before; 10,741 after |
|
| Interventions | Data were collected from the electronic NHLS database, which records all microbiological tests for tuberculosis in the region, including the type of test (sputum smear microscopy, Xpert MTB/RIF, or liquid culture), and the result of each test. Unique individuals tested for pulmonary tuberculosis were identified by unique laboratory identifiers. Data from the two periods were compared for the proportion of participants investigated for tuberculosis who tested positive by sputum smear microscopy, liquid culture, or Xpert MTB/RIF, and the proportion of sputum smear microscopy, liquid culture, or Xpert MRB/RIF tests that were positive. | |
| Outcomes |
Primary outcome: tuberculosis detection Secondary outcome:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A before‐after study, no randomization was done |
| Allocation concealment (selection bias) | High risk | A before‐after study, where allocation of intervention was not concealed; allocation was determined by calender period |
| Baseline characteristics similar (selection bias) | Low risk | No substantial differences observed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was done |
| Protection against contamination (performance bias) | Low risk | Data were extracted by specific test used, based on the unique laboratory identifier |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data were available prior to the evaluation; outcome assessor unlikely to be blinded in routine care |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were similar before and after intervention, and not statistically different |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods were reported |
| Other bias | High risk | Risk of confounding due to before‐after design |
Theron 2014a.
| Study characteristics | ||
| Methods | Pragmatic, randomized, parallel‐group, multi‐centre trial, Eligible participants were randomly assigned to undergo either Xpert MTB/RIF or smear microscopy |
|
| Participants | Eligible participants had one or more symptoms of tuberculosis according to WHO criteria, able to spontaneously expectorate two sputum samples, had not received anti‐tuberculosis treatment within the previous 60 days, gave informed consent, 18 years old and older Female: 43% HIV infection: 60% Setting: five peri‐urban primary healthcare tuberculosis clinics, with attached or close‐by treatment facilities and microscopy laboratories Countries: South Africa, Tanzania, Zambia, Zimbabwe Sample size: 758 participants randomized to microscopy, 744 to Xpert |
|
| Interventions | Intervention group received Xpert MTB/RIF sputum testing, and control group smear microscopy sputum testing | |
| Outcomes |
Primary outcome: tuberculosis‐related morbidity (graded using tuberculosis score and Karnofsky performance score) Secondary outcomes:
|
|
| Notes | Target condition: tuberculosis case definition: culture positive participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedule generated prior to the study, using computer‐generated allocation list |
| Allocation concealment (selection bias) | Low risk | A nurse at each site, blinded to the lists, contacted the data manager by telephone to obtain assignment once an eligible participant was identified |
| Baseline characteristics similar (selection bias) | Low risk | Selection bias was not detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Central laboratory personnel were blinded, however clinicians could not be blinded |
| Protection against contamination (performance bias) | Low risk | Participants were assigned to intervention based on computer‐generated allocation list by the data manager |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar proportions of loss to follow‐up were observed in both groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods sections were reported |
| Other bias | Low risk | Not detected |
Van den Handel 2015.
| Study characteristics | ||
| Methods | A before‐and‐after implementation study | |
| Participants | All age groups Individuals were screened for signs and symptoms of tuberculosis (cough for 2 weeks, weight loss, night sweats) Female: 46% smear microscopy period, 46% Xpert MTB/RIF decentralized period HIV: 32% smear microscopy period, 27% Xpert MTB/RIF decentralized period Settings: National Health Laboratory services (NHLS) laboratories and peripheral hospitals (district, sub‐district, and primary healthcare facilities) in a district municipality Sample size: 584 smear microscopy period, and 375 Xpert MTB/RIF decentralized period |
|
| Interventions | Tuberculosis diagnosis and treatment initiation were evaluated at six of the nine towns/communities in the Karoo, during three distinct periods. We extracted estimates from the smear microscopy and decentralized Xpert periods. Between April and October 2011 (the smear microscopy period), all sputum samples were sent for smear microscopy to the assigned NHLS laboratory. In October 2011, a single one‐module Xpert instrument was placed in a safe, secure space at hospitals located in Laingsburg, Murraysburg, and Prince Albert (the decentralized Xpert period), and the three nurses in charge of tuberculosis care were trained in performing the assay. |
|
| Outcomes | Time to treatment initiation | |
| Notes | Target condition: tuberculosis Case definition; bacteriological confirmation using smear microscopy, Xpert MTB/RIF, or culture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pre‐ and post‐study allocation was determined by calendar dates |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Baseline characteristics similar (selection bias) | Low risk | Baseline characteristics were similar |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding due to study design |
| Protection against contamination (performance bias) | Low risk | Xpert MTB/RIF allocated after smear microscopy period, and in areas which were geographically apart, minimal risk of contamination |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding was done, but outcomes were objective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was reported during smear period, no clear reports on number of participants who were lost to follow‐up during Xpert MTB/RIF |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods were reported |
| Other bias | High risk | Risk of confounding due to pre‐post design |
van Kampen 2015.
| Study characteristics | ||
| Methods | Before‐and‐after implementation study | |
| Participants | Criteria: individuals at high risk of MDR‐TB according to guidelines, March 2011 to March 2013 All age groups Female: pre 40%, post 38% HIV: pre 0.8%, post 2.9% Setting: three clinics offering programmatic management of drug resistant tuberculosis in East, Central, and West Java in Indonesia Country: Indonesia Number of eligible participants: pre 871, post 966 |
|
| Interventions | The diagnostic approach in the before period was to collect one sputum sample from each individual, and conduct smear microscopy and culture on solid or liquid media. If the culture was positive for tuberculosis, an isolate was re‐cultured for first‐line DST. During the intervention, one sputum sample was collected for Xpert testing and a second sample was used for diagnostic workup with culture and first‐line DST | |
| Outcomes | Proportion of individuals positive for tuberculosis Second‐line treatment initiation in participants with rifampicin‐resistant tuberculosis Time from client registration to diagnosis Time from diagnosis to treatment start |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A pre‐post study without randomization |
| Allocation concealment (selection bias) | High risk | A pre‐post study where allocation of intervention was not concealed, allocation was determined by calender period |
| Baseline characteristics similar (selection bias) | Unclear risk | participant characteristics were reported; no substantial differences were observed. However, a higher rate of testing occurred after the introduction of Xpert MTB/RIF. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Protection against contamination (performance bias) | High risk | Some participants were tested using conventional methods during the intervention period |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding was done, and could not have been achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were reported; there were no differences between before and after |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the methods were reported |
| Other bias | High risk | Risk of confounding due to pre‐post design |
Yoon 2012.
| Study characteristics | ||
| Methods | A multicentre implementation study of Xpert MTB/RIF with two phases: baseline and implementation phases, using a cohort of patients at the Mulago national referral hospital | |
| Participants | 18 years old and older History of cough for more than two weeks, but less than six months 48% female HIV infection: 76% setting: Mulago national referral hospital Country: Uganda Sample size: 477 |
|
| Interventions | In the baseline phase (August 2009 to March 2010), Xpert MTB/RIF results were not reported to clinicians, or used for patient management. This phase allowed for the collection of baseline data on study outcomes, and was necessary for local validation of Xpert MTB/RIF performance compared with conventional laboratory methods. In the subsequent implementation phase (March 2010 to August 2010), Xpert MTB/RIF results were provided to clinicians, and were used to inform tuberculosis treatment decisions. Each sample underwent smear microscopy, Xpert MTB/RIF, and culture. | |
| Outcomes | Primary outcome: two‐month mortality Secondary outcome: time to tuberculosis detection and treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A pre and post study design without randomization |
| Allocation concealment (selection bias) | High risk | Allocation of intervention was determined by calender dates |
| Baseline characteristics similar (selection bias) | Low risk | Baseline characteristics were reported; were not significantly different |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Protection against contamination (performance bias) | Low risk | No Xpert MTB/RIF data were used for clinical management during the baseline period |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not possible, and it is unclear whether blinding could have affected the assessment of mortality |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was significantly different between the before and after intervention periods |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods were reported |
| Other bias | High risk | Risk of confounding due to pre‐post design |
DST (PPV): drug sensitive test TB: tuberculosis hr: hour IPT: isoniazid preventive therapy MDR‐TB: multi‐drug resistant tuberculosis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boehme 2011 | Diagnostic accuracy study |
| Buchelli Ramirez 2014 | Observational retrospective study focused on diagnostic performance |
| Chilembo 2020 | Observational retrospective study based on tuberculosis registers |
| Feasey 2013 | Diagnostic performance study |
| Hanrahan 2013 | Describes a single group screened with both Xpert MTB/RIF and smear microscopy |
| Hanrahan 2015 | Reviewed retrospective records in health facilities that had implemented Xpert, and those that had not |
| Kim 2015 | Diagnostic accuracy of Xpert MTB/RIF in detecting RMP resistance against the conventional phenotypic DST |
| Kwak 2013 | Primarily a diagnostic study, allocation to group by clinical decision |
| Lawn 2011 | This study assessed the accuracy of the Xpert in diagnosing tuberculosis and drug resistance against the fluorescent smear microscopy |
| Lebina 2016 | The study included household contacts of people recently diagnosed with tuberculosis, whereas our review focused on individuals with suspected tuberculosis. |
| Lessells 2017 | The comparison arm did not use smear microscopy |
| Mboze 2016 | Xpert MTB/RIF test was used as an add‐on test |
| Metcalfe 2016 | There was no comparison group, all participants received Xpert MTB/RIF test, smear microscopy, and cultures |
| Mwansa‐Kambafwile 2016 | All PHC facilities had Xpert machines |
| O'Grady 2012 | All study participants received Xpert MTB/RIF smear microscopy tests; there was no comparison group. |
| Padayatchi 2016 | no smear microscopy strategies as comparison group |
| Rachow 2011 | Evaluated accuracy of Xpert MTB/RIF |
| Sachdeva 2015 | The study did not include our prespecified outcomes of interest |
| Scott 2010 | The study assessed the accuracy of the Xpert against the conventional methods |
| Theron 2011 | Study was designed to assess the accuracy of Xpert test against the conventional methods |
| Wang 2020 | Observational prospective study, focusing on re‐treatment cases |
RMP: rifampicin DST: drug susceptibility testing PHC: primary healthcare facilities
Differences between protocol and review
| Protocol | Review |
| Title | |
| Impact of diagnostic test Xpert MTB/RIF® on health outcomes for tuberculosis | Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis |
| Outcomes | |
Primary outcomes
Secondary outcomes
|
During review, some primary outcomes were moved to secondary outcomes following the advice of the reviewers. We also used more specific wording for the outcomes. Primary outcomes
Secondary outcomes
|
| Subgroup analysis | |
In the protocol we planned to conduct the following subgroup analysis:
|
During the review, the only subgroup analysis we were able to perform was for mortality in participants by HIV status. Other planned analyses were not feasible due to lack of data availability. We introduced one subgroup analysis that we had not planned in the protocol: mortality assessed at six months. Mortality assessed at six month would provide relevant data to understand impact of Xpert MTB/RIF during treatment. |
| Selection and review of studies | |
| In the protocol, we had indicated FH and RN to review studies | In the review, studies were reviewed by FH and MK. |
| In the protocol, the affiliation for Claudia Denkinger was FIND Geneva | In the review, the affiliation for Claudia Denkinger is both the Division of Tropical Medicine, Center of Infectious Diseases, University of Heidelberg, Germany and FIND, Geneva. Claudia Denkinger no longer works for FIND. |
Contributions of authors
FH, MK, and AR wrote the first draft of the review. KR, AR, SGS, CMD, SG, and RRN reviewed the review. FH, KR, and AR wrote the final review. All authors read and approved the final review version.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
-
Ifakara Health Institute, Tanzania
In kind (Internet access, work space)
-
Canton of Basel, Switzerland
Scholarship for PhD studentship
-
Foundation for Innovative New Diagnostics, Switzerland
In kind (institutional access to peer reviewed articles)
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
-
European and Developing Countries Clinical Trials and Partnership (EDCTP), Netherlands
EDCTP‐TDR clinical research and development fellowship awarded to Mwaka Kakolwa. Project number:TMA2015‐1172
Declarations of interest
FH has no known conflict of interest.
RRN has no known conflict of interest.
SGS is employed by the Foundation for Innovative New Diagnostics (FIND). FIND has conducted studies and published on Xpert MTB/RIF as part of a collaborative project between FIND, a Swiss non‐profit, Cepheid, a US company, and academic partners. The product developed through this partnership was developed under a contract that obligated FIND to pay for development costs and trial costs, and Cepheid to make the test available at preferential pricing to the public sector in developing countries. In addition, FIND conducted studies for the Xpert MTB/RIF Ultra assay, which have also been published.
MK has no known conflict of interest.
CMD was also employed by FIND during this review.
SG has no known conflict of interest.
KR is a board member of the Trial Safety Board, iM4TB, Lausanne, CH for a tuberculosis drug trial unrelated to the submitted work.
AR has no known conflict of interest.
New
References
References to studies included in this review
Agizew 2019a {published data only}
- Agizew T, Chihota V, Nyirenda S, Tedla Z, Auld AF, Mathebula U, et al. Tuberculosis treatment outcomes among people living with HIV diagnosed using Xpert MTB/RIF versus sputum-smear microscopy in Botswana: a stepped-wedge cluster randomised trial. BMC Infectious Diseases 2019;19:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calligaro 2015 {published data only}
- Calligaro GL, Theron G, Khalfey H, Peter J, Meldau R, Matinyenya B, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Respiratory Medicine 2015;3:621-30. [DOI] [PubMed] [Google Scholar]
Churchyard 2015 {published data only}
- Churchyard GJ, Stevens WS, Mametja KM, Chihota V, Nicol MP, Erasmus LK, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet. Global Health 2015;3:e450-7. [DOI] [PubMed] [Google Scholar]
Cox 2014 {published data only}
- Cox HS, Mbhele S, Mohess N, Whitelaw A, Muller O, Zemanay W, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Medicine 2014;11(11):e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Tanna 2019 {published data only}
- Di Tanna GL, Khaki AR, Theron G, McCarthy K, Cox H, Mupfumi L, et al. Effect of MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet. Global Health 2019;7(2):e191-9. [DOI: 10.1016/S2214-109X(18)30458-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durovni 2014 {published data only}
- Durovni B, Saraceni V, den Hof S, Trajman A, Cordeiro-Santos M, Cavalcente S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomised trial. PLoS Medicine 2014;11(12):e1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajman A, Durovni B, Saraceni V, Menezes A, Cordeiro-Santos M, Cobelens F, et al. Impact on patients' treatment outcomes of Xpert MTB/RIF implementation for the diagnosis of tuberculosis: follow-up of a stepped-wedge randomized clinical trial. PloS One 2015;10(4):e0123252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mupfumi 2014 {published data only}
- Mupfumi L, Makamure B, Chirehwa M, Sagonda T, Zinyowera S, Mason P, et al. Impact of Xpert MTB/RIF on antiretroviral therapy-associated tuberculosis and mortality: a pragmatic randomised controlled trial. Open Forum Infectious Diseases 2014;1(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngwira 2019 {published data only}
- Ngwira LG, Corbett EL, Khundi M, Barnes GL, Nkhoma A, Murowa M, et al. Screening for tuberculosis with Xpert MTB/RIF assay versus fluorescent microscopy among adults newly diagnosed with human immunodeficiency virus in rural Malawi: a cluster randomised trial (Chepetsa). Clinical Infectious Diseases 2019;68(7):1176-83. [DOI: 10.1093/cid/ciy590] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2017 {published data only}
- Schmidt B-M, Geldenhuys H, Tameris M, Luabeya A, Mulenga H, Bunyasi E, et al. Impact of Xpert MTB/RIF rollout on management of tuberculosis in a South African community. South African Medical Journal 2017;107(12):1078-81. [DOI] [PubMed] [Google Scholar]
Theron 2014a {published data only}
- Theron G, Zijenah L, Chandra D, Clowes P, Rachow A, Lesoky M, et al, for the TB-NEAT Team. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383:424-35. [DOI] [PubMed] [Google Scholar]
Van den Handel 2015 {published data only}
- Van den Handel T, Hampton K, Sanne I, Stevens W, Croux R, Van Rie A. The impact of Xpert MTB/RIF in sparsely populated rural settings. International Journal of Tuberculosis and Lung Disease 2015;19(4):392-8. [DOI] [PubMed] [Google Scholar]
van Kampen 2015 {published data only}
- Kampfen SC, Susanto NS, Simon S, Astiti SD, Chandra R, Burhan E, et al. Effects of introducing Xpert MTB/RIF on diagnosis and treatment of drug-resistant tuberculosis patients in Indonesia: a pre-post intervention study. PloS One 2015;10(6):e0123536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2012 {published data only}
- Yoon C, Cattamanchi A, Davis JL, Worodria W, den Boom S, Kalema N, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalised patients in Uganda. PloS One 2012;7(11):e48599. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Boehme 2011 {published data only}
- Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377(9776):1495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchelli Ramirez 2014 {published data only}
- Buchelli Ramirez HL, García-Clemente MM, Alvarez-Álvarez C, Palacio-Gutierrez JJ, Pando-Sandoval A, Gagatek S, et al. Impact of the Xpert(®) MTB/RIF molecular test on the late diagnosis of pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2014;18(4):435-7. [DOI: 10.5588/ijtld.13.0747] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chilembo 2020 {published data only}
- Chilembo M, Oguri S, Matsuoka Y, Ota M, Musiankuni P, Kabungo J. Pre-treatment lost to follow-up tuberculosis patients, Chongwe, Zambia, 2017: a retrospective cohort study. Public Health Action 2020;10(1):21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feasey 2013 {published data only}
- Feasey NA, Banada PP, Howson W, Sloan DJ, Mdolo A, Boehme C, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. Journal of Clinical Microbiology 2013;51(7):2311-6. [DOI: 10.1128/JCM.00330-13] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanrahan 2013 {published data only}
- Hanrahan CF, Selibas K, Deery CB, Dansey H, Clouse K, Bassett J, et al. Time to treatment and patient outcomes among TB suspects screened by a single point of care Xpert MTB/RIF at primary care clinic in Johannesburg, South Africa. PloS One 2013;8(6):e65421. [PMID: 10.1371/journal.pone.0065421] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanrahan 2015 {published data only}
- Hanrahan CF, Haguma P, Ochom E, Kinera I, Cobelens F, Cattamanchi A, et al. Implementation of Xpert MTB/RIF in Uganda: missed opportunities to improve the diagnosis of tuberculosis. Open Forum Infectious Diseases 2016;3(2):ofw068. [DOI: 10.1093/ofid/ofw068] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim YW, Seong MW, Kim TS, Yoo CG, Kim YW, Han SK, et al. Evaluation of Xpert(®) MTB/RIF assay: diagnosis and treatment outcomes in rifampicin-resistant tuberculosis. International Journal of Tuberculosis and Lung Disease 2015;19(10):1216-21. [DOI: 10.5588/ijtld.15.0183] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kwak 2013 {published data only}
- Kwak N, Choi SM, Park YS, Lee CH, Lee SM, Yoo CG, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PloS One 2013;8(10):e77456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2011 {published data only}
- Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Medicine 2011;8(7):e1001067. [DOI: 10.1371/journal.pmed.1001067] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lebina 2016 {published data only}
- Lebina L, Fuller N, Osoba T, Scott L, Motlhaoleng K, Rakgokong M, et al. The use of Xpert MTB/Rif for active case finding TB contacts in North West Province, South Africa. Tuberculosis Research and Treatment 2016;2016:4282313. [DOI: 10.1155/2016/4282313] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lessells 2017 {published data only}
- Lessells RJ, Cooke GS, McGrath N, Nicol MP, Newell ML, Godfrey-Faussett. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation: a cluster-randomized trial. American Journal of Respiratory and Critical Care Medicine 2017;196(7):901-10. [DOI: 10.1164/rccm.201702-0278OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mboze 2016 {published data only}
- Mbonze NB, Tabala M, Wenzi LK, Bakoko B, Brouwer M, Creswell J, et al. Xpert(®) MTB/RIF for smear-negative presumptive TB: impact on case notification in DR Congo. International Journal of Tuberculosis and Lung Disease 2016;20(2):240-6. [DOI: 10.5588/ijtld.15.0177] [PMID: ] [DOI] [PubMed] [Google Scholar]
Metcalfe 2016 {published data only}
- Metcalfe JZ, Makumbirofa S, Makamure B, Sandy C, Bara W, Mason P, et al. Xpert MTB/RIF detection of rifampicin resistance and time to treatment initiation in Harare, Zimbabwe. International Journal of Tuberculosis and Lung Disease 2016;20(7):882-9. [DOI: 10.5588/ijtld.15.0696] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwansa‐Kambafwile 2016 {published data only}
- Mwansa-Kambafwile J, Maitshotlo B, Black A. Microbiologically confirmed tuberculosis: factors associated with pre-treatment loss to follow-up, and time to treatment initiation. PloS One 2016;12(1):e0168659. [DOI: 10.1371/journal.pone.0168659] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Grady 2012 {published data only}
- O'Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clinical Infectious Diseases 2012;55(9):1171-8. [PMID: 10.1093/cid/cis631] [PMID: ] [DOI] [PubMed] [Google Scholar]
Padayatchi 2016 {published data only}
- Padayatchi N, Naidu N, Yende-Zuma N, Roe O’Donnell M, Naidoo K, Augustine S, et al. Implementation and Operational Research: clinical impact of the Xpert MTB/RIF assay in patients with multidrug-resistant tuberculosis. Journal of Acquired Immune Deficiency Syndromes 2016;73(1):e1-7. [DOI] [PubMed] [Google Scholar]
Rachow 2011 {published data only}
- Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Ceipheid Xpert MTB/RIF assay - a clinical validation study. PloS One 2011;6(6):e204558. [DOI: 10.1371/journal.pone.0020458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachdeva 2015 {published data only}
- Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PloS One 2015;10(5):e0126065. [DOI: 10.1371/journal.pone.0126065] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2010 {published data only}
- Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Medicine 2011;8(7):e1001061. [DOI: 10.1371/journal.pmed.1001061] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theron 2011 {published data only}
- Theron G, Peter J, Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for diagnosing of pulmonary tuberculosis in a high prevalence setting. American Journal of Respiratory and Critical Care Medicine 2011;184(1):132-40. [DOI: 10.1164/rccm.201101-0056OC] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang P, Gu J, Yang J, Yang C, Wu X, Yu F, et al. Effect of Xpert MTB/RIF on the treatment of multi-drug-resistant or rifampicin-resistant tuberculosis screened out from re-treatment pulmonary tuberculosis patients, a prospective cohort study. Annals of Palliative Medicine 2020;9(2):239-46. [DOI] [PubMed] [Google Scholar]
Additional references
Agizew 2019b
- Agizew T, Boyd R, Auld AF, Payton L, Pals SL, Lekone P, et al. Treatment outcomes, diagnostic and therapeutic impact: Xpert vs. smear. A systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease 2019;23(1):82-92. [DOI: 10.5588/ijtld.18.0203] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 2016
- Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? European Respiratory Journal 2016;48(2):516-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Auld 2016
- Auld AF, Fielding KL, Gupta-Wright A, Lawn SD. Xpert MTB/RIF - why the lack of morbidity and mortality impact in intervention trials? Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(8):432-44. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;54(4):401-6. [DOI] [PubMed] [Google Scholar]
Boehme 2010
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. New England Journal of Medicine 2010;363(11):1005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyles 2017
- Boyles TH. Why do clinical trials of Xpert® MTB/RIF fail to show an effect on patient relevant outcomes? International Journal of Tuberculosis and Lung Disease 2017;21(3):249-50. [DOI: 10.5588/ijtld.16.0801] [DOI] [PubMed] [Google Scholar]
Cook 2008
- Cook GC, Zumla A. Manson's Tropical Diseases. 22nd edition. Philadelphia: Saunders Ltd, 2008. [Google Scholar]
Corbett 2006
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367(9514):926-37. [DOI] [PubMed] [Google Scholar]
Creswell 2014
- Creswell J, Codlin AJ, Andre E, Micek MA, Bedru A, Carter EJ, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infectious Diseases 2014;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creswell 2015
- Creswell J, Rai B, Wali R, Sudrungrot S, Adhikari LM, Pant R, et al. Introducing new tuberculosis diagnostics: the impact of Xpert MTB/RIF testing on case notifications in Nepal. International Journal of Tuberculosis and Lung Disease 2015;19(5):545-51. [DOI: 10.5588/ijtld.14.0775] [PMID: ] [DOI] [PubMed] [Google Scholar]
Denkinger 2014
- Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2014;44(2):435-46. [DOI] [PubMed] [Google Scholar]
Di Ruffano 2017a
- di Ruffano LF, Dinnes J, Sitch AJ, Hyde C, Deeks JJ. Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests. BMC Medical Research Methodology 2017;17(35):35. [DOI: 10.1186/s12874-016-0287-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Ruffano 2017b
- di Ruffano LF, Dinnes J, Taylor-Phillips S, Davenport C, Hyde C, Deeks JJ. Research waste in diagnostic trials: a methods review evaluating the reporting of test-treatment interventions. BMC Medical Research Methodology 2017;17(32):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2018
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews 2018. Available at epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 30 November 2020).
GRADE 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5360. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. [DOI] [PubMed] [Google Scholar]
Helb 2010
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. Journal of Clinical Microbiology 2010;48(1):229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermans 2017
- Hermans S, Caldwell J, Kaplan R, Cobelens F, Wood R. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa. Bulletin of the World Health Organization 2017;95(8):554-63. [DOI: 10.2471/BLT.16.185314] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Houben 2016
- Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Medicine 2016;13(10):e1002152. [DOI: 10.1371/journal.pmed.1002152] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 1989
- Levy H, Feldman C, Sacho H, Meulen H, Kallenbach J, Koornhof H. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 1989;95(6):1193-7. [DOI] [PubMed] [Google Scholar]
MacPherson 2014
- MacPherson P, Houben RMGJ, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bulletin of the World Health Organization 2014;92(2):126-38. [PMID: 10.2471/BLT.13.124800] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrath 2020
- McGrath G, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biometrical Journal 2020;62:69-98. [DOI] [PubMed] [Google Scholar]
Nliwasa 2018
- Nliwasa M, MacPherson P, Gupta-Wright A. High HIV and active tuberculosis prevalence and increased mortality risk in adults with symptoms of TB: a systematic review and meta-analyses. Journal of the International AIDS Society 2018;21(7):e25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochodo 2019
- Ochodo EA, Kalema N, Schumacher S, Steingart K, Young T, Mallett S, et al. Variation in the observed effect of Xpert MTB/RIF testing for tuberculosis on mortality: a systematic review and analysis of trial design considerations. Wellcome Open Research 2019;4:173. [DOI: 10.12688/wellcomeopenres.15412.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2019
- Page MJ, HIggins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Pai 2018
- Pai M, Schumacher SG, Abimbola S. Surrogate endpoints in global health research: still searching for killer apps and silver bullets? BMJ Global Health 2018;3(2):e000755. [DOI: 10.1136/bmjgh-2018-000755] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 2011
- Parsons LM, Somoskövi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. American Society of Microbiology 2011;24(2):314-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 1.22. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Schumacher 2016
- Schumacher SG, Sohn H, Qin ZZ, Gore G, Davis JL, Denkinger CM, et al. Impact of molecular diagnostics for tuberculosis on patient-important outcomes: a systematic review of study methodologies. PloS One 2016;11(3):e0151073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2014
- Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. Journal of Clinical Microbiology 2014;52(6):1818-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subbaraman 2019
- Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Medicine 2019;16(2):e1002754. [PMID: 10.1371/journal.pmed.1002754] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theron 2014b
- Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infectious Diseases 2014;14:527-32. [DOI] [PubMed] [Google Scholar]
Walusimbi 2013
- Walusimbi S, Bwanga F, De Costa A, Haile M, Joloba M, Hoffner S. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infectious Diseases 2013;30(13):507. [DOI: 10.1186/1471-2334-13-507] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization (WHO). Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf 2013. [PubMed]
WHO 2014
- World Health Organization (WHO). Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. Available at www.who.int/tb/publications/xpert_implem_manual/en/ 2014. [PubMed]
WHO 2014b
- World Health Organization (WHO). The End TB strategy - Global strategy and targets for tuberculosis prevention, care and control after 2015. www.who.int/tb/strategy/End_TB_Strategy.pdf March 2014.
WHO 2016a
- World Health Organization (WHO). The use of molecular line probe assays for the detection of resistance to isoniazid and rifampicin. Available at www.who.int/tb/publications/molecular-test-resistance/en/ 2016.
WHO 2016b
- World Health Organization (WHO). WHO monitoring of Xpert MTB/RIF roll-out. Available at www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/ 2016.
WHO 2019
- World Health Organization (WHO). Global report for tuberculosis. Available at www.who.int/tb/publications/global_report/en/ 2019.
Zifodya 2021
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Haraka 2018
- Haraka F, Kakolwa M, Schumacher SG, Nathavitharana RR, Denkinger CM, Gagneux S, et al. Impact of diagnostic test Xpert MTB/RIF® on health outcomes for tuberculosis. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012972. [DOI: 10.1002/14651858.CD012972] [DOI] [PMC free article] [PubMed] [Google Scholar]


