Abstract
Purpose
To describe structural changes in corneal epithelium using anterior segment optical coherence tomography (AS-OCT) in two relapsed and refractory multiple myeloma (RRMM) patients with bilateral belantamab-associated superficial keratopathy (BASK).
Observations: case 1
A 56-year-old male who was diagnosed with RRMM and initiated on belantamab mafodotin, presented on day 42 (three weeks after the second infusion) with decreased pinhole visual acuity from 20/20 and 20/25 to 20/70 and 20/50 in the right eye and left eye, respectively. Slit-lamp examination revealed moderate superficial keratopathy with microcystic-like epithelial changes (MECs) in the paracentral cornea in both eyes. AS-OCT demonstrated increased bilateral heterogeneous signal intensity and hyperreflective lesions as well as increased thickness in the paracentral corneal epithelium with uninvolved central cornea. Given bilateral MECs, the third infusion was withheld, and then given on day 62 after five weeks of drug-free interval. Although MECs had improved on day 82, pinhole visual acuity remained at 20/50 and 20/40 in the right eye and the left eye. AS-OCT showed that hyperreflective lesions mostly resolved and corneal epithelial thickness returned to baseline, despite a slightly increased persisting heterogeneous signal intensity in the peripheral corneal epithelium in both eyes.
Case 2
A 77-year-old male with RRMM was started on belantamab mafodotin infusions. His pinhole visual acuity decreased from 20/40 and 20/30 at baseline to 20/60 and 20/40 on day 41 (three weeks after the second infusion) in the right eye and left eye, respectively. Slit-lamp examination showed diffuse, moderate MECs in both eyes, which was more severe in the peripheral cornea. AS-OCT demonstrated increased bilateral heterogeneous signal intensity and hyperreflective lesions in the corneal epithelium, which are more severe in the right eye along with increased corneal epithelial thickness. Therefore, belantamab mafodotin was withheld.
Conclusions and Impotance
AS-OCT objectively demonstrated structural changes such as signal intensity and thickness alterations with hyperreflective lesions in the corneal epithelium related to BASK. AS-OCT might be useful for clinicians to monitor ocular surface adverse events in RRMM patients receiving belantamab mafodotin and to adjust therapeutic plans for the patients.
Keywords: Multiple myeloma, Belantamab, Antibody-drug conjugate, Microcystic-like epithelial changes, Keratopathy, Anterior segment optical coherence tomography
1. Introduction
Multiple myeloma (MM) is a malignancy of the hematopoietic system characterized by abnormal proliferation of plasma cells in the bone marrow.1 MM constitutes 1–2% of all malignancies, with the median age at diagnosis of 69 years.2 Although MM is relatively rare, a significant number of patients suffer from relapsed or refractory MM (RRMM), management of which is still quite challenging.2 RRMM displays an extremely poor prognosis (median overall survival of 9.3 months) and is resistant to current treatment plans, demonstrating unmet needs for novel therapeutics.3,4
Recently, belantamab mafodotin, an antibody-drug conjugate (ADC) against B-cell maturation antigen (BCMA), has shown promise with fast and favorable responses in RRMM.5, 6, 7 However, it has been shown to cause significant ocular surface adverse events (AEs) in the form of “superficial keratopathy” involving multiple, bilateral, microcystic-like epithelial changes (MECs) that might necessitate temporary cessation or discontinuation of treatment.8,9 Belantamab-associated superficial keratopathy (BASK) is currently assessed according to the DREAMM-2 study protocol-specified criteria, called “keratopathy and visual acuity (KVA) scale”.9 Aside from changes in best-corrected visual acuity (BCVA), KVA scale is based on corneal changes on slit-lamp examination (SLE) which is partially subjective and requires special consideration. In the DREAMM-2 study,9 69% of patients receiving belantamab (2.5mg/kg) developed BASK by the fourth infusion. Among patients with MECs, over 70% had 2 lines BCVA decline, and/or symptoms of blurred vision or subjective dry eye. However, the majority (80%) of patients with MECs spontaneously recovered at follow-up visits, which was mainly approximately 2–6 months post-treatment.9 Despite BASK being well described using in vivo confocal microscopy (IVCM),9 its pathogenesis has not been completely elucidated.
Anterior segment optical coherence tomography (AS-OCT) is a non-invasive, objective tool which provides high-resolution details about corneal changes in ocular surface and corneal diseases.10 AS-OCT is widely used for diagnostic and follow-up purposes in clinical practice. It enables quantitative measurement of corneal epithelial, stromal, and endothelial thicknesses.11,12 Given the fact that BASK was commonly observed in patients receiving belantamab mafodotin, it is important to evaluate this novel finding objectively. Herein, we present AS-OCT findings of two RRMM patients who developed BASK during their treatment course.
2. Case presentation
2.1. Case 1
A 56-year-old male with a history of hypertension and type 2 diabetes mellitus was diagnosed with RRMM as a result of unresponsiveness to multiple prior therapies three and a half years ago. Given previous multiple treatment failures (4), belantamab mafodotin was chosen as the next therapeutic agent. Following the protocol under a Risk Evaluation and Mitigation Strategy (REMS), the patient was referred to our clinic for baseline evaluation. At that time, his pinhole visual acuity (VA) was 20/20 and 20/25 in the right eye (OD) and left eye (OS), respectively. SLE was unremarkable with clear corneas and no abnormal findings in the anterior and posterior segments in OU. The patient denied any ocular symptoms, previous ocular disease or surgery. Belantamab mafodotin infusion (2.5 mg/kg) was initiated for him (day 0), and the second infusion was given on day 21. One week following the second infusion, the patient noticed visual loss in both eyes (OU). Additional symptoms of ocular discomfort such as blurriness and dryness were also experienced two weeks after the second infusion. At the follow-up visit on day 42, his pinhole VA had decreased to 20/70 in OD, and 20/50 in OS. In SLE, a ring-shaped, moderate amount of superficial keratopathy in the form of diffuse MECs in the paracentral cornea was observed in OU. The central portion of the cornea was clear in OU (Fig. 1). At the same time, corneal topography revealed irregular astigmatism which was greater in OD than in OS. The horizontal B-scan image through central cornea on AS-OCT(OptoVue RTVue, Fremont, CA) revealed increased heterogeneous signal intensity and hyperreflective lesions in the paracentral corneal epithelium with uninvolved central cornea. Several disruptions in the line of the Bowman's layer (BL) were observed in OD (Fig. 1). In addition, the mean of the full corneal epithelial thickness (CET) increased from 49μm to 47μm at the baseline to 79μm and 73μm on day 42 in OD and OS, respectively. Although there was no change in CET at the central cornea, increased CET was demonstrated at the 1mm, 2mm, and 3mm nasally and temporally away from the central cornea in OU (Fig. 3). The indicated bilateral corneal findings were recorded as grade 2 (moderate) superficial keratopathy (KVA scale); therefore, the third infusion was withheld based on regulations under REMS. The patient was placed on bilateral topical prednisolone acetate twice daily and preservative-free artificial tears eight times daily. Despite profound changes observed in OD compared to OS, MECs clinically improved based on the KVA scale on SLE in OU on day 59, and pinhole VA was 20/40 and 20/30 in OD and OS, respectively. After discussion with the Hematology team, the patient received the third dose of belantamab mafodotin (2.5 mg/kg) on day 62. At the most recent visit on day 82, his pinhole VA was 20/50 and 20/40 in OD and OS, respectively. There was residual mild superficial keratopathy in OU, which improved further with bilateral topical prednisolone acetate once daily. On AS-OCT, the hyperreflective lesions mostly resolved despite the slightly increased persistent heterogeneous signal intensity in the peripheral corneal epithelium in OU (Fig. 1). The mean of full CET decreased to 51μm OD and 51μm in OS, which was similar to baseline measurements; no thickening of CET at any point on day 82 was detected on AS-OCT in OU compared to baseline measurements. Except for corneal epithelium and BL, no abnormality of the stromal layer or other components of the cornea in OU was observed on OCT throughout the clinical course (Fig. 3). No other AE was detected during the follow-up period.
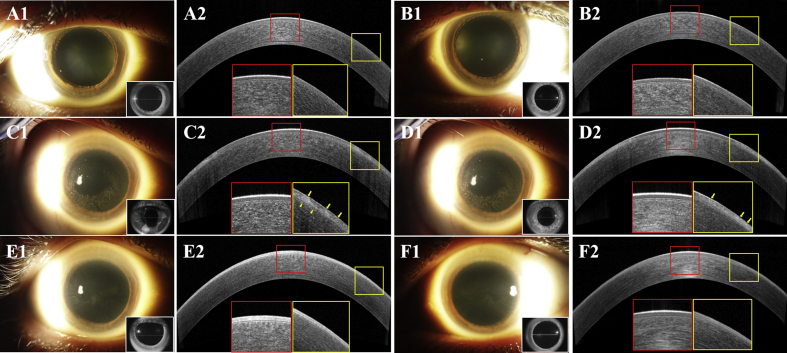
Fig. 1.
Slit lamp photographs and anterior segment coherence tomography (AS-OCT) scans of Case 1 during the follow-up in the right eye (OD) (A1, A2, C1, C2, E1 and E2) and in the left eye (OS). (B1, B2, D1, D2, F1 and F2)
At baseline, clear cornea in both eyes (OU) (A1 and B1) and homogenous signal intensity in corneal epithelium with an intact Bowman layer (BL) at the central cornea (red box) and paracentral cornea (yellow box) on AS-OCT (A2 and B2). After the second infusion of belantamab mafodotin on day 42, microcystic-like epithelial changes (MECs) with no central cornea involvement in OU were observed (C1 and D1). The presence of homogenous signal intensity and clear BL at the central cornea (red box), with increased heterogeneous signal intensity and hyperreflective lesions (yellow arrows) in the corneal epithelium in OU, BL disruptions (yellow arrow heads) in OD and increasing corneal epithelial thickness (CET) at paracentral cornea (yellow box) in OU (C2 and D2). On day 82, there were mild residual keratopathies OU (E1 and F1), which improved as compared to day 42. There were no change of epithelial intensity and CET at central cornea (red box) in OU. At paracentral cornea (yellow box), hyperreflective lesions in epithelium and epithelial intensity improved to homogeneity and CET decreased to baseline level in OU (E2 and F2). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
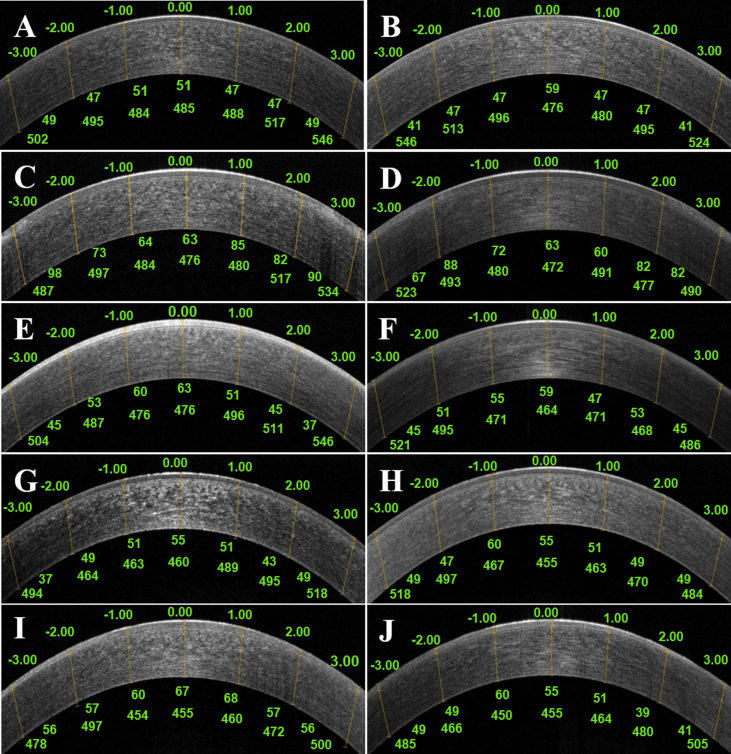
Fig. 3.
Corneal epithelial thickness (CET) and corneal thickness without CET in horizontal scan on anterior segment optical coherence tomography
Measurement of corneal epithelial thicknesses using anterior segment optical coherence tomography (AS-OCT) was performed with the software cursors at the central cornea and at 1mm, 2mm, and 3mm points nasally and temporally. Corneal epithelial thicknesses (CET), which are shown under the cornea, were measured manually as the distances (μm) between the air-tear and the epithelium-Bowman's layer interfaces. Corneal thicknesses without CET, which are shown in the bottom of CET, was also measured manually as the distances (μm) between epithelium- Bowman's layer and endothelium layer interfaces.
Case1: At baseline with pinhole visual acuity (VA) of 20/20 and 20/25 (OD/OS), CET and corneal thickness without CET at baseline in OU were shown (A and B). On Day 42 with pinhole VA of 20/70 and 20/50 (OD/OS), thicken CET except at the central cornea was shown in OU (C and D). On Day 82 with pinhole VA of 20/50 and 20/40 (OD/OS), no thickening of CET at any point on day 82 was shown in OU (E and F). No change of corneal thickness without CET were shown in the time course in OU (A,B,C,D,E, and F).
Case2: At baseline with pinhole VA of 20/40 and 20/30 (OD/OS), CET and corneal thickness without CET in OU were shown (G and H). On Day 41 with pinhole VA of 20/60 and 20/40 (OD/OS), thicken CET was shown in OD (I) but no change of CET was shown in OS (J). No change of corneal thickness without CET were shown in the time course in OU (G,H,I, and J).
2.2. Case 2
A 77-year-old male with a history of MM was referred to our clinic for ophthalmic evaluation before belantamab mafodotin therapy. He was initially diagnosed with MM about two decades ago. Relapsed myeloma was later treated with multiple therapies. Subsequently, the patient developed anaplastic large cell non-Hodgkin's lymphoma (NHL) 7 years ago, which was treated with intensive chemotherapy that led to clinical remission. During the past three years, the patient had been treated for relapsed myeloma. However, four months ago, the patient had a relapse of MM, and was diagnosed with RRMM. The Hematology team then decided to initiate belantamab mafodotin therapy due to the previous multiple treatments (≧4). At the baseline evaluation, his pinhole VA was 20/40 in OD and 20/30 in OS. SLE revealed bilateral clear corneas and no abnormality of the anterior and posterior segments except mild nuclear sclerosis. The patient denied any ocular symptoms, previous ocular disease or surgery. The patient received his first belantamab mafodotin (2.5 mg/kg) infusion (day 0). He was also given the second infusion on day 21. There was no ocular AE after the first infusion. Subsequently, one week after the second infusion, the patient noted bilateral decrease in his vision despite using preservative-free artificial tears four times daily. At the follow-up visit on day 41, his pinhole VA had decreased to 20/60 and 20/40 in OD and OS, respectively. SLE and VA assessment established grade 2 (moderate) diffuse superficial keratopathy with involvement of the entire cornea according to KVA scale in OU; the findings were more severe in the peripheral cornea. AS-OCT also showed increased heterogeneous signal intensity and hyperreflective lesions in the corneal epithelium in the paracentral region in OU, which was more severe in OD (Fig. 2). The mean of full CET demonstrated an increase to 60 μm from the baseline value of 48 μm in OD, while there was no change in OS (Fig. 3). Therefore, belantamab mafodotin infusions were held until the re-initiation criteria can be achieved. There were no abnormal findings in corneal stroma or other components of the cornea except corneal epithelium, and no changes in corneal thickness without CET was observed in OU throughout the clinical cause (Fig. 3). Given the decreased vision with moderate diffuse superficial keratopathy in both eyes, topical prednisolone acetate 1% was started bilaterally four times daily and artificial tears were increased to eight times daily to help to relieve visual symptoms due to superficial keratopathy. The patient did not experience any other AEs during his follow-up.
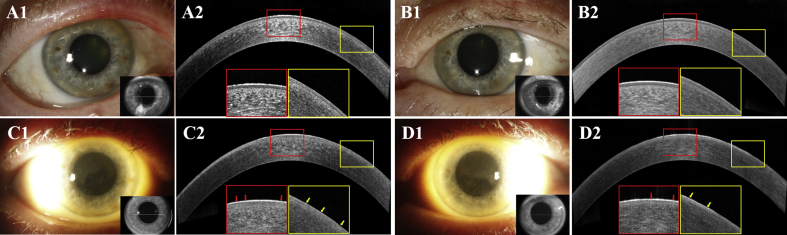
Fig. 2.
Slit lamp photographs and anterior segment coherence tomography (AS-OCT) scans of Case 2 during the follow-up in OD (A1, A2, C1 and C2) and OS (B1, B2, D1 and D2)
At the baseline, clear cornea in OU (A1 and B1) and homogenous signal intensity in corneal epithelium with an intact BL at the central cornea (red box) and paracentral cornea (yellow box) on AS-OCT (A2 and B2) were seen.
After the second infusion of belantamab mafodotin on day 41, mild diffuse microcystic-like epithelial changes (MECs) (C1 and D1) were detected. The presence of mildly increased heterogeneous signal intensity (red arrows) at the central cornea (red box), and more severe increased heterogeneous signal intensity (yellow arrows) with small hyperreflective lesions at the paracentral cornea (yellow box) in OD (C2). Mild heterogeneous signal intensity increase (red and yellow arrows) at the central cornea (red box) and at paracentral cornea (yellow box) in OS (D2) were observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Antibody-drug conjugates (ADCs) are among the newest biological therapeutics in cancer treatment, consisting of monoclonal antibodies coupled to an antitumor cytotoxin (cytotoxic payload). The ADCs bind to a targeted tumor cell surface antigen via specific receptors resulting in internalization of the cytotoxic payloads into the tumor cell. Such process eventually leads to cell death by apoptosis.13 In addition to several existing ADCs in clinical use,14, 15, 16, 17 the United States Food and Drug Administration (FDA) has recently approved belantamab mafodotin as a first-in-class ADC targeting B-cell maturation antigen (BCMA) for the treatment of RRMM in patients who failed at least four prior treatments, including anti-CD38 monoclonal antibody, proteasome inhibitor, and immunomodulatory agent.13
Although ADCs use is associated with ocular surface AEs in the form of superficial keratopathy,8 the underlying mechanism for the development of superficial keratopathy might involve either on-target or off-target mechanisms. In an on-target mechanism, non-cancerous cells express the target antigen which can bind to monoclonal antibody, and subsequently the cytotoxic payload is released into the cell. Alternatively, an off-target mechanism occurs when non-cancerous cells that do not express the target antigen are exposed to the cytotoxic payload via several mechanisms (e.g., Fc-receptor-mediated endocytosis, pinocytosis and bystander toxicity).18,19 It is believed that belantamab-associated superficial keratopathy (BASK) arises as a result of an off-target mechanism, because the protein targeted by belantamab mafodotin, BCMA, is not expressed in the cornea.
In the phase 2 trial (DREAMM-2 study), ocular surface AEs (superficial keratopathy involving MECs) were observed in 69% of the patients by the fourth treatment cycle.7, 9According to KVA scale, 8%, 17%, 45% and 1% of the study patients had grade1 (mild), grade 2 (moderate), grade 3 and grade 4 (severe) superficial keratopathy, respectively.9 Many of the patients developed superficial keratopathy within the first 2 treatment cycles, with the cumulative incidence of 69% by cycle 4.9 In addition, 72% of the patients with MECs had ocular symptoms such as blurred vision and subjective dry eye sensation with/without BCVA decline in the better-seeing eye. Bausell et al.20 also reported that 12 patients treated with belantamab mafodotin developed MECs that initially appeared in the peripheral cornea and progressed centrally with time. These changes developed after an average of 1.7 treatment cycles or 34 days after the first treatment dose. Similarly, our report demonstrated bilateral grade 2 ocular surface AEs (moderate superficial keratopathy) which were first detected after the second infusion in both cases. The keratopathy was accompanied by bilateral moderate (≧2 -line decline in pinhole VA) visual loss in Case 1, and mild (<2 lines decline in pinhole VA) visual loss in Case 2.
Other differential diagnoses which should be ruled out include dry eye syndrome, corneal dystrophy, infectious keratitis, and/or keratitis associated with autoimmune connective tissue diseases, were not found in our cases, as well as drug-induced corneal epithelial changes [e.g. amiodarone, chlorpromazine, tamoxifen, tilorone, clarithromycin, topical ciprofloxacin, gold salts, or cytarabine (Ara-C)].21 Although on rare occasions, MM patients might also display corneal epithelial keratopathy as a concurrent disease,22 given the clinical time course, the presented corneal changes were unlikely to be linked to MM. Therefore, BASK was thought to be the most probable diagnosis in the index cases.
In addition, the DREAMM-2 study investigated the corneas of patients with MECs using in vivo confocal microscopy (IVCM) which demonstrated intracellular hyperreflective lesions. Similar features in IVCM were also reported in association with other ADCs in the literature.8,9 Besides, despite the clinically detected corneal epithelial microcysts, IVCM did not reveal any evidence of true cysts within or under the affected corneal epithelium.23 AS-OCT has played an essential role in the objective assessment of cornea and other anterior segment structures since 1994,24 and can clearly distinguish the five corneal layers, including epithelium, BL, corneal stroma layer, Descemet's membrane, and endothelium.25 In contrast to IVCM, AS-OCT is also helpful in evaluating corneal epithelial thickness and morphological assessment of total cornea with B-scan images.26 Recently, a report by Rousseau A et al. showed epithelial change with hyperreflectivity and “thicken aspect”in BASK on AS-OCT.27 Similarly, all eyes of our cases showed hyperreflective lesions in the corneal epithelium on AS-OCT during the follow-up period. In both cases, there was homogeneous signal intensity throughout the cornea at baseline. However, in Case 1, increased heterogeneous signal intensity with hyperreflective lesions was detected mainly in the paracentral corneal epithelium in OU after the second belantamab mafodotin infusion. In addition, BL which is usually seen as a relatively hyporeflective layer between two hyperreflective lines dividing epithelium, BL, and stroma on AS-OCT,28 was also slightly disrupted under the area of hyperreflective lesions in the corneal epithelium in OD (Fig. 1). In addition, these AS-OCT findings were consistent with the localization of MCEs in SLE, and no structural change was observed in the central cornea in OU. In Case 2, there was also diffuse increased heterogeneous signal intensity with hyperreflective lesions in OU after the second belantamab mafodotin infusion. As revealed by the AS-OCT finding in both cases, all layers of corneal epithelium seemed to be involved with hyperreflective lesions simultaneously, in parallel to the appearance of MECs on SLE. These features are similar to the hyperreflective lesions in IVCM. Some reports also showed similar epithelial hyperreflective lesions on AS-OCT in other corneal diseases, including Meesman corneal dystrophy and microcystic keratitis related to contact lens use.26,29,30 Dembski et al.26 reported a case of bilateral acute microcystic epitheliopathy after daily soft contact lens wear, and proposed that the hyperreflective material in the corneal epithelium as well as the edema of some cells possibly represent the accumulation of apoptotic end products in the intercellular spaces of the corneal epithelium. Similarly, the hyperreflective lesions on AS-OCT might be consistent with the accumulation of pre-apoptotic cells or degenerated cells caused by internalization of belantamab mafodotin or deposited drug products itself.20
The analysis of CETs of both cases at each visit indicated that 3 of 4 eyes showed thickening of CETs on AS-OCT compared to the baseline. In Case 1, except for the central CET, a diffuse increase in CET was observed in OU after the second belantamab mafodotin infusion. After the discontinuation of belantamab mafodotin infusion, CETs decreased back to baseline values in concordance with the improvement of SLE findings. On the other hand, Case 2 demonstrated mild diffuse increase in CETs with involvement of central cornea in OD, whereas no change of CETs in OS after the second belantamab mafodotin infusion. In the three eyes, the thickening of CETs was detected not only in the area of heterogeneous signal intensity changes and/or hyperreflective lesions, but also in the other regions of corneal epithelium. Although these areas appeared normal with homogeneous signal intensity on AS-OCT, they might result in irregular astigmatism which could cause visual loss as Rousseau A et al.27 has reported. The increased CET in these cases might be caused by the other subclinical epithelial conditions such as chronic inflammation or desiccating stress,28,31 as well as the accumulation of pre-apoptotic cells or degenerated cells assumed to be manifested as hyperreflective lesions on AS-OCT. Therefore, AS-OCT may show the subclinical epithelial change of BASK even in cornea which appears normal on SLE.
The corneal epithelium is constantly renewed by the stem cells in the vascularized limbal region. Limbal stem cells migrate toward the central and superficial region of the cornea as they become more differentiated.32,33 In addition, old and dead epithelial cells are washed out from the surface of the cornea by a tear film. In humans, it takes approximately 20 days for the total corneal epithelium renewal's completion.34,35 Regarding the mechanism of BASK, Farooq et al.9 proposed that belantamab mafodotin reaches the cornea through the tear film or the limbus. Afterward, it is internalized by the basal layer cells of corneal epithelium, such as transient amplifying cells through macropinocytosis, and activates apoptotic cascade. Belantamab-containing cells in various stages of apoptosis continue to migrate centrally and anteriorly, while the cells that have completed apoptosis are extruded. Over time, new epithelial cells are regenerated by the limbus replacing belantamab-containing cells that have undergone apoptosis. In Case 1, after the discontinuation of belantamab mafodotin, increased heterogeneous signal intensity with hyperreflective lesions started to resolve in parallel with the improvement of SLE findings. It seems appropriate that AS-OCT findings improved during the drug-free interval of five weeks, after the initial detection of MECs. However, possibly due to the third belantamab infusion, mild changes were still present in the corneal epithelium on SLE and AS-OCT, as well as the decrease in pinhole VA in OU.
In conclusion, AS-OCT can objectively demonstrate structural epithelial changes such as heterogeneous signal intensity, thickness alterations, and hyperreflective lesions related to BASK in patients with RRMM. These findings were also consistent with MECs detected on SLE, and closely linked to belantamab mafodotin infusion schedule and drug-free interval period. Therefore, AS-OCT may be a useful, complementary, objective imaging modality to evaluate BASK. Future prospective studies will be of importance to elaborate further and confirm these findings.
Patient consent
Consent to publish this case report has been obtained from the patients.
Funding
Research to Prevent Blindness Department Challenge Award and National Eye Institute of the National Institutes of Health P30 Award (EY026877) have been awarded to the Byers Eye Institute at Stanford University.
WM is supported by a grant from the Japan Eye Bank Association Oversea Research Program and Kobe University Long Term Oversea Visit Program for Young Researchers.
IK is supported by a grant from the International Council of Ophthalmology (ICO) Retina Research Foundation (RRF) Helmerich Fellowship Program as the recipient of 2020 ICO-RRF Helmerich One-Year Fellowship Award.
Authorship
All authors attest that they met the current ICMJE criteria.
Declaration of competing interest
ML reports personal fees from GSK. The other following authors declare that there are no conflicts of interest related to this manuscript: WM, IK, HG, AA, AM, MH, JR, CY, KK, and QDN.
Acknowledgements
None.
References
- 1.Palumbo A., Anderson K. Multiple Myeloma. New England journal of medicine. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Mikhael J., Ismaila N., Cheung M.C. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. J Clin Oncol. 2019;37:1228–1263. doi: 10.1200/JCO.18.02096. [DOI] [PubMed] [Google Scholar]
- 3.Nooka A.K., Kastritis E., Dimopoulos M.A., Lonial S. Treatment options for relapsed and refractory multiple myeloma. Blood. J Am Soc Hematol. 2015;125:3085–3099. doi: 10.1182/blood-2014-11-568923. [DOI] [PubMed] [Google Scholar]
- 4.Bazarbachi A.H., Al Hamed R., Malard F., Harousseau J.-L., Mohty M. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33:2343–2357. doi: 10.1038/s41375-019-0561-2. [DOI] [PubMed] [Google Scholar]
- 5.Trudel S., Lendvai N., Popat R. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–1653. doi: 10.1016/S1470-2045(18)30576-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trudel S., Lendvai N., Popat R. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Canc J. 2019;9:1–10. doi: 10.1038/s41408-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonial S., Lee H.C., Badros A. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–221. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 8.Eaton J.S., Miller P.E., Mannis M.J., Murphy C.J. Ocular adverse events associated with antibody–drug conjugates in human clinical trials. J Ocul Pharmacol Therapeut. 2015;31:589–604. doi: 10.1089/jop.2015.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farooq A.V., Degli Esposti S., Popat R. Corneal epithelial findings in patients with multiple myeloma treated with antibody–drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9:889–911. doi: 10.1007/s40123-020-00280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkateswaran N., Galor A., Wang J., Karp C.L. Optical coherence tomography for ocular surface and corneal diseases: a review. Eye Vis. 2018;5:1–11. doi: 10.1186/s40662-018-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Q., Le Q., Cordova D.W., Tseng C.-H., Deng S.X. Corneal epithelial thickness measured using anterior segment optical coherence tomography as a diagnostic parameter for limbal stem cell deficiency. Am J Ophthalmol. 2020;216:132–139. doi: 10.1016/j.ajo.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher D., Collins M.J., Vincent S.J. Anterior segment optical coherence tomography scanning protocols and corneal thickness repeatability. Contact Lens Anterior Eye. 2020;43:433–440. doi: 10.1016/j.clae.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Nejadmoghaddam M.-R., Minai-Tehrani A., Ghahremanzadeh R., Mahmoudi M., Dinarvand R., Zarnani A.-H. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol (AJMB) 2019;11:3. [PMC free article] [PubMed] [Google Scholar]
- 14.Katz J., Janik J.E., Younes A. Brentuximab vedotin (SGN-35) Clin Canc Res. 2011;17:6428–6436. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 15.Erickson H.K., Park P.U., Widdison W.C. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Canc Res. 2006;66:4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- 16.García-Alonso S., Ocaña A., Pandiella A. Resistance to antibody–drug conjugates. Canc Res. 2018;78:2159–2165. doi: 10.1158/0008-5472.CAN-17-3671. [DOI] [PubMed] [Google Scholar]
- 17.Jen E.Y., Ko C.-W., Lee J.E. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin Canc Res. 2018;24:3242–3246. doi: 10.1158/1078-0432.CCR-17-3179. [DOI] [PubMed] [Google Scholar]
- 18.Williams M., Spreafico A., Vashisht K., Hinrichs M.J. Patient selection strategies to maximize therapeutic index of antibody–drug conjugates: prior approaches and future directions. Mol Canc Therapeut. 2020;19:1770–1783. doi: 10.1158/1535-7163.MCT-19-0993. [DOI] [PubMed] [Google Scholar]
- 19.Mahalingaiah P.K., Ciurlionis R., Durbin K.R. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–125. doi: 10.1016/j.pharmthera.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Bausell R.B., Soleimani A., Vinnett A. Corneal changes after belantamab mafodotin in multiple myeloma patients. Eye Contact Lens. 2021;47:362–365. doi: 10.1097/ICL.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 21.Raizman M.B., Hamrah P., Holland E.J. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017;62:286–301. doi: 10.1016/j.survophthal.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Kleta R., Blair S.C., Bernardini I., Kaiser-Kupfer M.I., Gahl W.A. Keratopathy of multiple myeloma masquerading as corneal crystals of ocular cystinosis. Mayo Clin Proc: Elsevier. 2004:410–412. doi: 10.4065/79.3.410. [DOI] [PubMed] [Google Scholar]
- 23.Lambert J.M. Drug‐conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol. 2013;76:248–262. doi: 10.1111/bcp.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izatt J.A., Hee M.R., Swanson E.A. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–1589. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 25.Lim S.-H. Clinical applications of anterior segment optical coherence tomography. J Ophthalmol. 2015;2015 doi: 10.1155/2015/605729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dembski M., Nowińska A., Ulfik K., Teper S., Wylęgała E. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the microcystic keratitis. J Ophthalmol. 2020;2020 doi: 10.1155/2020/8871904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousseau A., Michot J.M., Labetoulle M. Belantamab mafotodin-induced epithelial keratopathy masquerading myopic surgery. Ophthalmology. 2020;127:1626. doi: 10.1016/j.ophtha.2020.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Tao A., Wang J., Chen Q. Topographic thickness of Bowman's layer determined by ultra-high resolution spectral domain–optical coherence tomography. Investig Ophthalmol Vis Sci. 2011;52:3901–3907. doi: 10.1167/iovs.09-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino T., Kobayashi A., Mori N. In vivo histology and p. L132V mutation in KRT12 gene in Japanese patients with Meesmann corneal dystrophy. Jpn J Ophthalmol. 2019;63:46–55. doi: 10.1007/s10384-018-00643-6. [DOI] [PubMed] [Google Scholar]
- 30.Thanathanee O., Laohapitakvorn S., Anutarapongpan O., Suwan-Apichon O., Bhoomibunchoo C. Anterior segment optical coherence tomography images in Microsporidial keratoconjunctivitis. Cornea. 2019;38:943–947. doi: 10.1097/ICO.0000000000001994. [DOI] [PubMed] [Google Scholar]
- 31.Fabiani C., Barabino S., Rashid S., Dana M.R. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89:166–171. doi: 10.1016/j.exer.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schermer A., Galvin S., Sun T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secker G., Daniels J. StemBook.; Cambridge, MA: 2008. Limbal Epithelial Stem Cells of the Cornea. [PubMed] [Google Scholar]
- 34.Joe A.W., Yeung S.N. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med. 2014;3:318–322. doi: 10.5966/sctm.2013-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H., Atkinson J., Gulesserian S. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody–drug conjugates. Canc Res. 2018;78:2115–2126. doi: 10.1158/0008-5472.CAN-17-3202. [DOI] [PubMed] [Google Scholar]