Abstract
Both the noradrenergic and galaninergic systems have been implicated in stress-related neuropsychiatric disorders, and these two neuromodulators are co-released from the stress-responsive locus coeruleus (LC); however, the individual contributions of LC-derived norepinephrine (NE) and galanin to behavioral stress responses are unclear. Here we aimed to disentangle the functional roles of co-released NE and galanin in stress-induced behavior. We used foot shock, optogenetics, and behavioral pharmacology in wild-type (WT) mice and mice lacking either NE (Dbh−/−) or galanin (GalcKO-Dbh) specifically in noradrenergic neurons to isolate the roles of these co-transmitters in regulating anxiety-like behavior in the elevated zero maze (EZM) either immediately or 24 h following stress. Foot shock and optogenetic LC stimulation produced immediate anxiety-like behavior in WT mice, and the effects of foot shock persisted for 24 h. NE-deficient mice were resistant to the anxiogenic effects of acute stress and optogenetic LC stimulation, while mice lacking noradrenergic-derived galanin displayed typical increases in anxiety-like behavior. However, when tested 24 h after foot shock, both Dbh−/− and GalcKO-Dbh mice lacked normal expression of anxiety-like behavior. Pharmacological rescue of NE, but not galanin, in knockout mice during EZM testing was anxiogenic. In contrast, restoring galanin, but not NE, signaling during foot shock normalized stress-induced anxiety-like behavior 24 h later. These results indicate that NE and noradrenergic-derived galanin play complementary, but distinguishable roles in behavioral responses to stress. NE is required for the expression of acute stress-induced anxiety, while noradrenergic-derived galanin mediates the development of more persistent responses following a stressor.
Subject terms: Stress and resilience, Anxiety
Introduction
Stress is an important adaptive mechanism that allows animals to respond appropriately to threatening stimuli in their environment; however, stress is also a primary risk factor in the development of many neuropsychiatric disorders in humans, including anxiety and depression. These disorders are exceedingly common, with both major depressive disorder and all anxiety disorders having a lifetime prevalence rate of ~30% [1–3]. Understanding the underlying neurobiological mechanisms that regulate adaptive and maladaptive behavioral responses to stress and how these mechanisms relate to psychopathology is necessary for the discovery of new, effective treatments for anxiety and depression.
Both the central noradrenergic and galaninergic systems have been implicated in stress-related neuropsychiatric disorders. These two neuromodulators also share a neuroanatomical relationship, as galanin is co-expressed with norepinephrine (NE) in a majority of neurons in the locus coeruleus (LC), the primary source of NE in the brain, across species, including mice, rats, monkeys, and humans [4–9]. Most LC research has focused exclusively on NE, despite the fact that galanin has also been independently implicated in stress-induced behaviors in rodents and stress-related neuropsychiatric disorders in humans [10–13]. Stress strongly activates LC neurons, which broadly innervate the central nervous system (CNS) and play an important role in regulating attention, arousal, and the fight-or-flight response [14]. Because the LC is a major source of both NE and galanin to many stress-responsive brain regions, it is a powerful neuroanatomical substrate for studying the contribution of NE and galanin co-transmission to stress-induced behavior. Neuropeptides, like galanin, have distinct dynamics from small-molecule neurotransmitters like NE due to different mechanisms controlling synthesis, release, and degradation [15]. Neuropeptides are thought to be preferentially released when neuronal firing rates are high, and previous research has suggested that galanin transmission occurs under conditions that strongly activate noradrenergic neurons, such as stress, highlighting the importance of understanding the individual contributions of galanin and NE from the LC in stress-induced behavior [15–17].
Traditional pharmacological and genetic studies allow functional investigation of single or even combinations of neurotransmitters, but they often lack the resolution to manipulate each one independently in a single cell type. On the other hand, application of modern neuroscience tools, such as optogenetics and chemogenetics, can achieve cell type-specificity but modulate neurotransmission indiscriminately, obscuring the potentially unique effects of co-released molecules. To overcome these limitations, we used genetically modified mouse lines that lack either NE (dopamine β-hydroxylase knockout; Dbh−/− mice) or galanin (GalcKO-Dbh mice) specifically in noradrenergic neurons. While these manipulations are not specific to the LC, the LC is the only noradrenergic region in mice where galanin is co-expressed with tyrosine hydroxylase, a marker of noradrenergic neurons [18], and NE from the LC has been shown to be critical for stress-induced anxiety [19]. Importantly, we previously showed that GalcKO-Dbh mice have normal NE levels, and we show here that Dbh−/− mice have normal galanin expression in the LC [18], allowing for the isolation of each neuromodulator’s action. Dbh−/− mice have been extensively studied since their initial creation 25 years ago [20], and do not differ from controls in traditional tests for anxiety-like behavior at baseline [21–23]. Likewise, GalcKO-Dbh mice also show normal anxiogenic behavior at baseline in classic anxiety assays, although have increased active coping behavior in some non-canonical paradigms, suggesting a role for noradrenergic-derived galanin in mediating passive coping behaviors [18]. Here we used these mouse lines to dissect the functional roles of co-released NE and galanin in regulating acute and persistent stress-induced anxiety-like behavior.
Methods and materials
Animals
Because DBH is required for NE synthesis, Dbh−/− mice lack NE completely throughout the body. These mice were generated and maintained on a mixed 129/SvEv and C57BL/6J background, as previously described [20, 24] (see Supplement for details). Dbh+/− littermates were used as controls in experiments with Dbh−/− mice because they are indistinguishable from WT (Dbh+/+) mice in behavior and catecholamine levels [20, 23, 25–27]. While galanin is expressed broadly throughout the CNS and periphery, the GalcKO-Dbh mice used lack galanin expression specifically in noradrenergic neurons. These mice were generated as described previously and maintained on a C57BL/6J background by crossing Dbhcre+;Galflx/flx mice to Dbhcre−;Galflx/flx mice [18]. Because of the difference in background strains, Dbh−/− mice could not be directly compared to GalcKO-Dbh mice, so all experiments were designed to compare Dbh−/− mice to their Dbh+/− littermates, and GalcKO-Dbh mice to their Dbhcre−;Galflx/flx littermates (referred to as WT). Balanced numbers of adult male and female mice (3–9 months) were used for all experiments. No sex differences were seen and data for males and females were combined for all analyses. All procedures related to the use of animals were approved by the Institutional Animal Care and Use Committee of Emory University and were in accordance with the National Institutes of Health guidelines for care and use of laboratory animals.
Stress paradigm
Mice were individually exposed to 20 min of foot shock consisting of 19 shocks randomly interspaced by 30, 60, or 90 s (0.5 ms shocks, 1 mA) in chambers (Coulbourn Instruments, Holliston, MA) with an electric grid shock floor [28]. Control animals were placed in the chamber for 20 min but were not administered shocks. See Supplement for corticosterone (CORT) measurement methods. We have shown previously that long-term (24–48 h) effects of this foot shock stress paradigm are evident in multiple tests for anxiety-like behavior, including the elevated zero maze (EZM), novelty-suppressed feeding, and shock probe defensive burying [28]. We focused on the EZM here because, unlike novelty-suppressed feeding and shock probe defensive burying, GalcKO-Dbh mice have no phenotype in this assay at baseline [18]. This allowed us to assess whether galanin specifically contributes to stress-induced anxiety-like behavior, which would have been confounded in the other tests.
Optogenetics and EZM testing
See Supplement for stereotaxic surgery details. LC optogenetic stimulation was based on a published paradigm used in our lab previously [28, 29]. Photostimulation was delivered to the LC for 30 min (5 Hz tonic stimulation, 10 ms light pulses, 473 nm) in 3 min on/off bins while animals were in the home cage immediately before testing in the EZM. Mice were placed in the EZM and videotaped for 5 min. TopScan software (Clever Sys Inc., Reston, VA) was used to quantify percent time spent in open segments, number of bouts in the open segments, and the total distance traveled. Fecal boli were counted at the end of the test. Some stress and genotype differences were detected for the number of open bouts, distance traveled, and defecation, but they were not consistent across experiments (see Supplement). The EZM was used because it is a validated test for anxiety-like behavior in rodents and is sensitive to LC manipulations [29, 30]. Furthermore, both mutant mouse lines show normal behavior at baseline in this assay, allowing us to observe stress-induced behavioral changes [18, 21]. See Supplement for details on histology in mice used for optogenetics.
Drugs
The non-selective galanin receptor agonist galnon (Bachem, Torrance, CA, USA), which has a half-life of approximately 60 min [31], was dissolved in 0.9% sterile saline with 1% DMSO. Mice were injected with vehicle or galnon (2 mg/kg, i.p.) 20 min prior to either stress or behavioral testing.
The synthetic NE precursor L-3,4-dihydroxyphenylserine (DOPS) was dissolved in distilled water with 2% HCl, 2% NaOH, and 2 mg/kg vitamin C, as described [22, 24]. Dbh−/− mice were injected with vehicle or a cocktail of DOPS (0.5 g/kg, s.c.) and benserazide (250 mg/kg, s.c.) (Sigma-Aldrich), then tested 5 h later when NE levels peak [24, 32]. Benserazide is an aromatic acid decarboxylase inhibitor that cannot cross the blood-brain barrier and was used to prevent the conversion of DOPS to NE in the periphery, thus restricting restoration of NE to the brain [33].
Statistical analysis
Data were found to be normally distributed using the D’Agostino-Pearson test and analyzed by one-way or two-way ANOVA, with Dunnett’s or Sidak’s correction respectively to examine planned comparisons between stress, virus, or drug treatment, where appropriate. Significance was set at p < 0.05, and two-tailed variants of tests were used. Data are presented as mean ± SEM. Calculations were performed, and figures created using Prism Version 8 (GraphPad Software, San Diego, CA).
Additional methods
Additional methods concerning animal care and breeding, stereotaxic surgery, corticosterone measurement, RNAscope, and histology are described in the Supplement.
Results
Galanin mRNA levels in the LC are normal in mice lacking NE
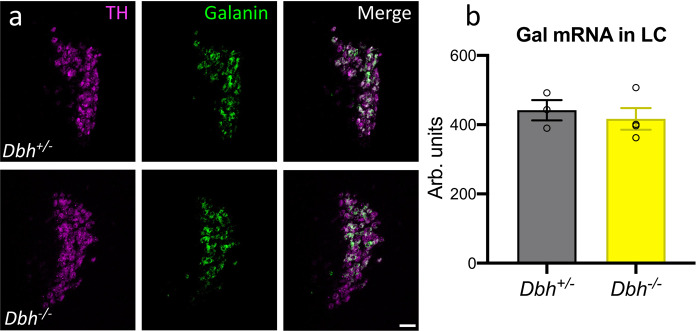
We previously showed that GalcKO-Dbh mice have normal NE levels [18], but to isolate the role of each neuromodulator, we needed to ensure that galanin levels were unchanged in the LC of Dbh−/− mice. Using RNAscope to visualize galanin and TH mRNA transcripts in the LC (Fig. 1a), we found no significant difference between the average galanin signal of Dbh−/− mice and littermate controls (t6 = 0.5583, p = 0.6007) (Fig. 1b).
Fig. 1. Galanin mRNA levels in the LC are normal in mice lacking NE.
Dbh+/− control and Dbh−/− mice have equivalent levels of galanin mRNA in the LC as measured by average fluorescence intensity using RNAscope. a Representative images of TH (magenta) and galanin (green) mRNA expression and overlap in the LC from Dbh+/− and Dbh−/− mice (scale bar, 50 µm). b Quantification of galanin average fluorescence intensity. n = 3–4 mice per group, 2–4 LC sections per mouse. Error bars show SEM.
Dbh−/− mice, but not GalcKO-Dbh mice, are resistant to the acute anxiogenic effects of foot shock stress
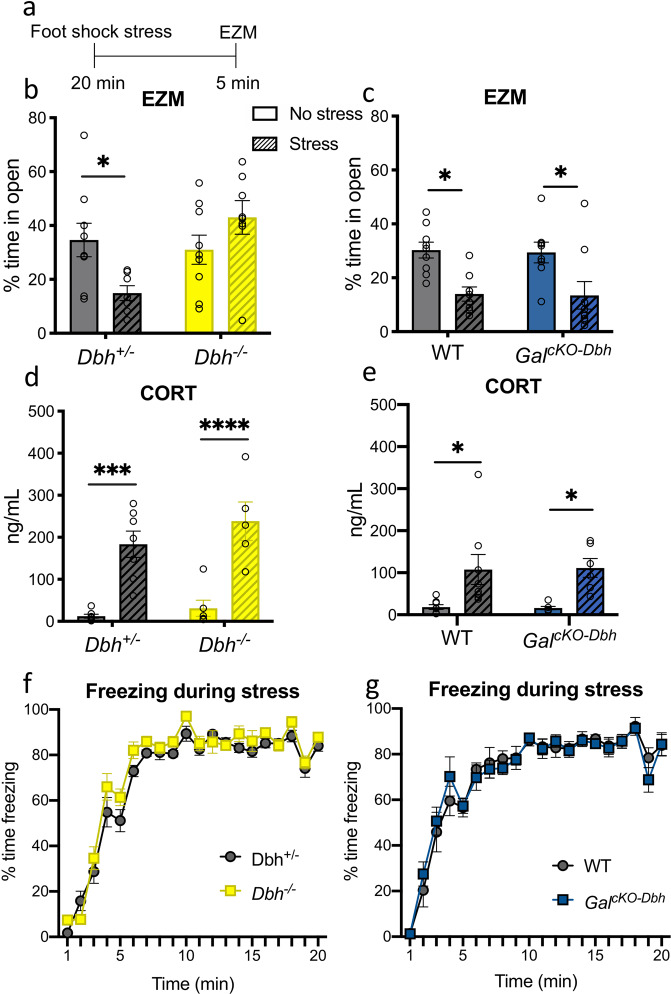
To determine the relative importance of NE and noradrenergic-derived galanin for the acute behavioral response to a stressor, we subjected mice to 20 min of foot shock, and then immediately tested them in the EZM (Fig. 2a). When we analyzed the percentage of time animals spent in the open segments of the EZM, a two-way ANOVA showed a significant genotype x treatment interaction for Dbh−/− mice compared to their Dbh+/− littermates (F1,29 = 8.003, p = 0.0084). Post hoc tests revealed a significant decrease in percent time spent in the open segments for stressed Dbh+/− mice (t29 = 2.444, p = 0.0413), but no difference between stressed and non-stressed Dbh−/− mice (t29 = 1.541, p = 0.2503) (Fig. 2b). For the GalcKO-Dbh line, there was a main effect of treatment (F1,30 = 17.89, p = 0.0002), but no genotype x treatment interaction (F1,30 = 0.00284, p = 0.9580), indicating that stress decreased open segment exploration equally in WT and mutants (WT: t30 = 3.028, p = 0.0100, GalcKO-Dbh: t30 = 2.953, p = 0.0121) (Fig. 2c).
Fig. 2. NE, but not noradrenergic-derived galanin, is required for anxiety-like behavior immediately following foot shock stress.
a Dbh−/−, GalcKO-Dbh, and their respective littermate controls (Dbh+/− and WT) received 20 min of foot shock (“Stress”) or no foot shock (“No stress”) and were tested in the elevated zero maze (EZM) immediately afterward. A separate group of mice received foot shock or no foot shock, and blood was collected immediately afterward for CORT measurements. b Dbh+/−, but not Dbh−/− mice, showed a significant decrease in the percent time spent in the open segments of the EZM immediately after foot shock stress. c Both GalcKO-Dbh and their WT littermates showed a significant decrease in the percent time spent in the open segments in the EZM immediately after foot shock stress. Dbh−/− and GalcKO-Dbh mice showed increases in plasma CORT immediately following the foot shock stress (d, e) and similar freezing behavior during the foot shock stress (f, g) as their respective controls. n = 5–8 mice per group for CORT analysis. n = 8–9 mice per group for behavior. Error bars show SEM. *p < 0.05, ***p < 0.001, ****p < 0.0001.
To confirm that the endocrine stress response was intact in mice lacking NE or noradrenergic-derived galanin, we measure levels of CORT in the blood immediately after exposure to foot shock. For the Dbh−/− mice, there was a significant effect of treatment on CORT levels (F1,21 = 48.49, p < 0.0001), but no effect of genotype (F1,21 = 1.854, p = 0.1877), indicating that both Dbh−/− mice and Dbh+/− controls have similar CORT responses to foot shock stress (Dbh+/−: t21 = 4.759, p = 0.0002, Dbh−/−: t21 = 5.088, p < 0.0001) (Fig. 2d). Similarly, there was a significant effect of treatment on CORT levels for the GalcKO-Dbh mice (F1,24 = 15.87, p = 0.0005), but no effect of genotype (F1,24 = 0.001293, p = 0.9716), indicating that GalcKO-Dbh mice also have a normal CORT response to foot shock (WT: t24 = 2.942, p = 0.0142, GalcKO-Dbh: t24 = 2.722, p = 0.0237) (Fig. 2e). Both the GalcKO-Dbh mice and the Dbh−/− mice also showed normal freezing to the foot shocks compared to their littermate controls during the stress itself (Dbh+/− vs. Dbh−/−: F1,21 = 3.690, p = 0.0684; WT vs. GalcKO-Dbh: F1,10 = 0.000936, p = 0.9925) (Fig. 2f, g).
Dbh−/− mice, but not GalcKO-Dbh mice, show reduced acute anxiogenic effects of optogenetic LC stimulation
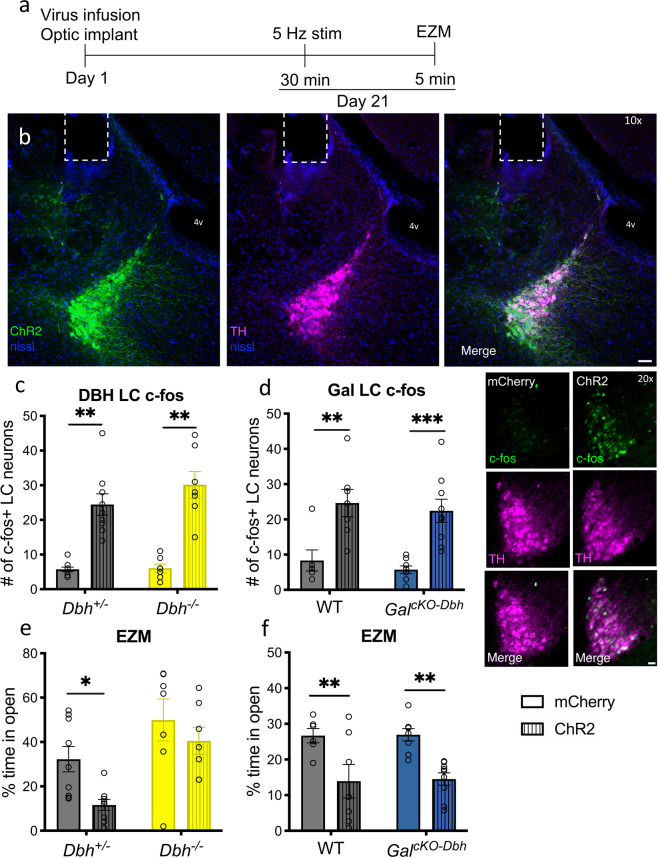
To complement the acute foot shock experiment and more selectively activate NE and galanin co-expressing neurons, we optogenetically stimulated LC neurons in WT, Dbh−/−, and GalcKO-Dbh mice immediately before EZM testing (Fig. 3a). Mice received intra-LC infusion of virus expressing either ChR2-mCherry or mCherry alone driven by the noradrenergic-specific PRSx8 promoter, resulting in high levels of viral expression selectively in the LC. Viral expression and optic ferrule placement were confirmed in all animals by histology (Fig. 3b), and successful activation of the LC following optogenetic stimulation was confirmed via an increase in c-Fos immunoreactivity in ChR2 animals (main effect of virus in Dbh mice: F1,28 = 72.32, p < 0.0001; main effect of virus in Gal mice: F1,26 = 29.68, p < 0.0001) (Fig. 3c, d). For EZM behavior in the Dbh−/− mice, there were significant main effects of both virus (F1,28 = 6.108, p = 0.0198) and genotype (F1,28 = 14.72, p = 0.0006) on the percentage of time mice spent in the open segments, with planned comparisons showing that Dbh+/− ChR2 mice spent significantly less time in the open arms compared to Dbh+/− mCherry mice (t28 = 2.568, p = 0.0314), whereas there was no significant difference between Dbh−/− mice expressing ChR2 or the control virus (t28 = 1.030, p = 0.5263) (Fig. 3e). While a significant genotype x virus interaction was not seen in this experiment (F1,28 = 0.8574, p = 0.3624), the lack of a significant decrease in time spent in the open segments of the EZM for the Dbh−/− ChR2 mice suggests that NE is important for the full expression of optogenetic LC stimulation-induced anxiety-like behavior. For the Gal mice, there was a significant main effect of virus (F1,26 = 21.07, p < 0.0001), but not genotype (F1,26 = 0.0238, p = 0.8786), on the percentage of time spent in open segments of the EZM (Fig. 3f), demonstrating that noradrenergic galanin is dispensable for the normal behavioral response to optogenetic LC activation. Furthermore, we found a significant negative correlation between the number of c-Fos positive LC neurons and the percent time mice spent in the open segments of the EZM (Spearman correlation: p = 0.0169, rs = −0.3024; Supplementary Fig. 3), indicating that higher levels of LC activation were associated with increased anxiety-like behavior.
Fig. 3. NE, but not noradrenergic-derived galanin, is required for anxiety-like behavior immediately following optogenetic LC stimulation.
Dbh−/−, GalcKO-Dbh, and their respective littermate controls (Dbh+/− and WT) expressing either ChR2-mCherry or mCherry alone in the LC were given 30 min of optogenetic LC stimulation (5 Hz tonic, 3 min on/off bins) and then tested in the elevated zero maze (EZM). At least one week following behavioral testing, mice were given 15 min optogenetic LC stimulation and brains were collected 90 min later for c-Fos immunohistochemistry. a Optogenetic surgery and behavior timeline. b Representative image of ChR2 expression (green) and tyrosine hydroxylase (TH, magenta) overlap in the LC and optic fiber ferrule placement (scale bar, 50 µm; 4 v, 4th ventricle). c, d Optogenetic stimulation of LC neurons expressing ChR2 resulted in greater c-Fos expression compared with LC neurons expressing mCherry in all genotypes (TH, magenta; c-Fos, green; scale bar, 20 µm). e Dbh+/− mice expressing ChR2 spent less percent time in the open segments of the EZM following optogenetic simulation compared to mCherry-expressing Dbh+/− mice, while optogenetic LC activation did not impact the behavior of Dbh−/− mice. f Both GalcKO-Dbh and their WT littermates expressing ChR2 showed a significant decrease in percent time spent in the open segments of the EZM following optogenetic stimulation compared to those expressing mCherry. n = 7–9 mice per group. Error bars show SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Both GalcKO-Dbh and Dbh−/− mice are resistant to the persistent anxiogenic effects of foot shock stress
Neuropeptides, such as galanin, have distinct dynamics compared to classic neurotransmitters like NE, and may therefore exert their effects on a different timescale. We have shown previously that anxiogenic-like responses are evident in several behavioral paradigms 24 h following foot shock, including EZM, shock probe defensive burying, and novelty-suppressed feeding [28]. To examine the relative roles of NE and noradrenergic-derived galanin in the persistent behavioral response to a stressor, we subjected mice to 20 min of foot shock, and then tested them in the EZM 24 h later instead of immediately following the stressor (Fig. 4a). We chose the EZM because it revealed the most profound stress-induced change [28], and activation of the LC vigorously and consistently increases anxiety-like behavior in this assay [29]. We found significant genotype x treatment interaction effects between stress and genotype in the percent time spent in the open segments for both Dbh−/− compared to Dbh+/− mice (F1,38 = 6.211, p = 0.0172) (Fig. 4b) and GalcKO-Dbh mice compared to WT controls (F1,38 = 4.694, p = 0.0366) (Fig. 4c). Post hoc analyses revealed that stress-exposed control animals in both experiments spent less time in the open segments of the EZM (Dbh+/−: t38 = 2.503, p = 0.0332, WT: t38 = 2.556, p = 0.0292), while Dbh−/− and GalcKO-Dbh mice were resistant to this effect (Dbh−/−: t38 = 1.054, p = 0.5081, GalcKO-Dbh: t38 = 0.4633, p = 0.8745). These results suggest that both neuromodulators are important for the long-term (24 h) anxiogenic-like effects of foot shock stress. In a separate experiment, we measured freezing to the foot shock stress context 24 h following the stressor and observed high levels of freezing that did not differ between genotypes (Supplementary Fig. 4g, h), confirming that the lack of anxiety-like behavior in the EZM 24 h following stress was not due to an inability of the mutants to remember the stressor.
Fig. 4. Both NE and noradrenergic-derived galanin are required for anxiety-like behavior 24 h following foot shock stress.
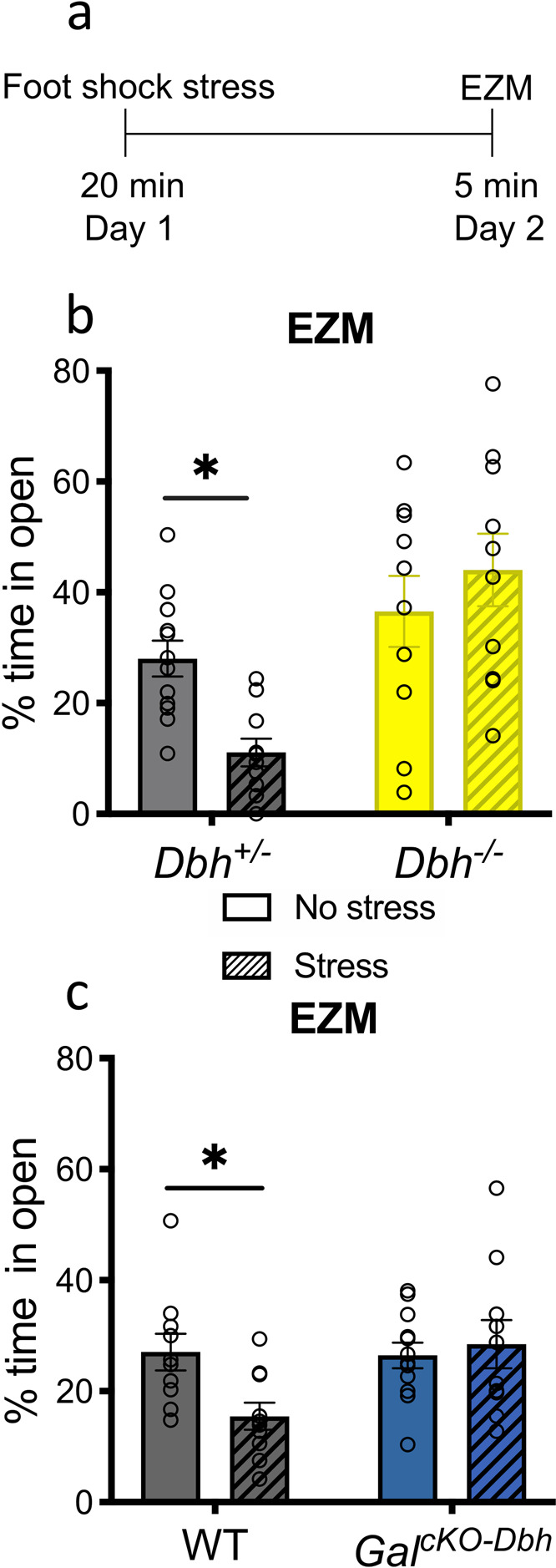
Dbh−/−, GalcKO-Dbh, and their respective littermate controls (Dbh+/− and WT) received 20 min of foot shock (“Stress”) or no foot shock (“No stress”) and were tested in the elevated zero maze (EZM) 24 h later. a Foot shock stress paradigm timeline. b, c Dbh+/− and WT, but not Dbh−/− or GalcKO-Dbh mice, showed a significant decrease in percent time spent in the open segments of the EZM 24 h after foot shock stress. n = 10–12 mice per group. Error bars show SEM. *p < 0.05.
Activation of galanin receptors, but not restoration of NE signaling, during stress exposure rescues normal stress-induced anxiety-like behavior in knockout mice
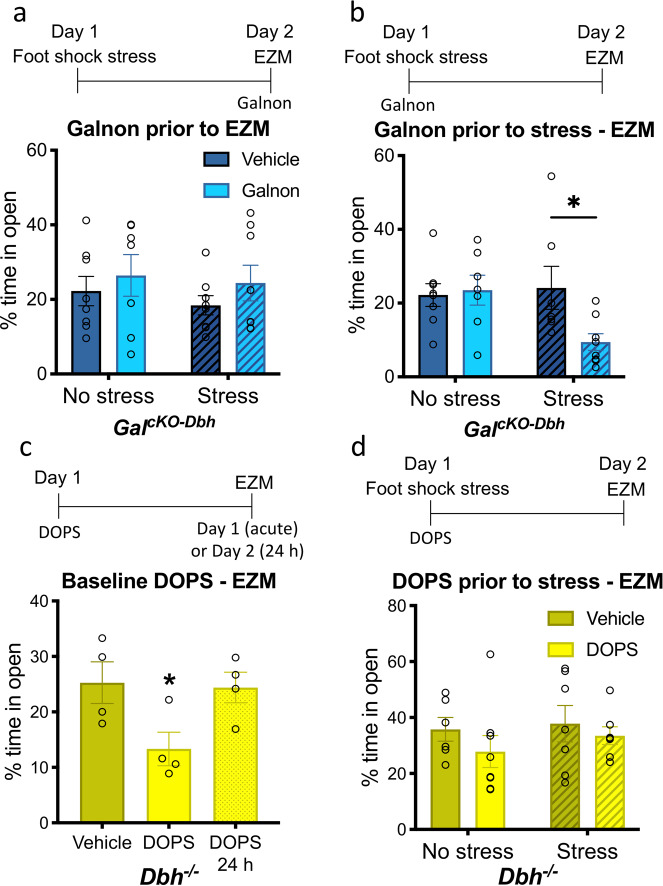
To determine when galanin is acting to influence stress-induced anxiety-like behavior over 24 h, we injected GalcKO-Dbh mice with the non-selective galanin receptor agonist galnon to exogenously activate galanin receptors either during the stressor or during the EZM 24 h later. We found no significant differences in percent time spent in the open segments of the EZM between stressed and non-stressed mice treated with galnon or vehicle during the EZM itself (F1,27 = 0.4267, p = 0.8379) (Fig. 5a); however, when mice were treated with galnon prior to the foot shock, then tested in the EZM 24 h later, we found a significant interaction effect between stress and drug treatment (F1,26 = 4.238, p = 0.0497), with post hoc analysis revealing a significant decrease in percent time spent in the open segments of the EZM for stressed GalcKO-Dbh mice treated with galnon (t26 = 2.558, p = 0.0331) (Fig. 5b). These results demonstrate that galanin transmission during the stressor leads to changes that impact stress-induced behavior 24 h later. In contrast, galanin receptor activation at the time of the behavioral test has no effect on EZM performance.
Fig. 5. Restoration of galanin, but not NE, signaling during stress exposure rescues normal stress-induced anxiety-like behavior 24 h later.
GalcKO-Dbh and Dbh−/− mice were treated with the non-selective galanin receptor agonist galnon (2 mg/kg, i.p.) or the synthetic NE precursor DOPS (0.5 g/kg, s.c.), respectively. a Administration of galnon in GalcKO-Dbh mice prior to EZM testing did not affect 24 h foot shock stress-induced anxiety-like behavior. b Galnon administration prior to the foot shock restored normal stress-induced anxiety-like effect 24 h later. c Acute DOPS treatment in Dbh−/− mice during EZM testing was anxiogenic compared to Dbh−/− mice treated with vehicle, but mice tested 24 h following DOPS administration displayed normal behavior. d Treatment of Dbh−/− mice with DOPS prior to foot shock stress exposure did not alter anxiety-like behavior 24 h later. n = 7–8 mice per group for a, b, d. n = 4 mice per group for c. Error bars show SEM. *p < 0.05.
The synthetic NE precursor DOPS restores 20–30% of wild-type NE levels in the brains of Dbh−/− mice [24] and rescues virtually all described phenotypes in Dbh−/− mice, including those related to stress responses such as fear conditioning [33], novelty-suppressed feeding [34], nestlet shredding [21], and antidepressant response in the forced swim test [35]. DOPS replacement also increases anxiety-like behavior in Dbh−/− in the elevated plus maze [22]. Likewise, we found that pharmacological rescue of NE using DOPS in Dbh−/− mice during EZM testing was acutely anxiogenic compared to Dbh−/− mice treated with vehicle (F2,9 = 4.347, p = 0.0477), while mice tested 24 h following DOPS had normal behavior (Fig. 5c). This indicates that the anxiogenic effects of acute NE restoration in Dbh−/− mice are transient and do not impact anxiety-like behavior 24 h later.
To determine whether NE in combination with stress can influence stress-induced anxiety-like behavior over 24 h, as we found with galanin, we administered DOPS to Dbh−/− mice to restore central NE signaling during foot shock stress, then tested animals in the EZM 24 h later. There was no difference in percent time spent in open segments of the EZM between stressed and non-stressed mice that had been treated with DOPS or vehicle during the stressor (F1,24 = 0.1201, p = 0.7319) (Fig. 5d), suggesting that, unlike galanin, NE signaling during the stressor does not restore normal stress-induced anxiety-like behavior 24 h later in Dbh−/− mice.
Discussion
In this study, we aimed to resolve the roles of the LC co-transmitters NE and galanin in stress-induced behavior by using mice specifically lacking either NE or noradrenergic-derived galanin. This research is important because both of these transmitters regulate behavioral responses to stress in rodents and are implicated in stress-related neuropsychiatric disorders in humans [10–13]. While both Dbh−/− and GalcKO-Dbh mice responded similarly during the stressor itself, with high levels of freezing and increased plasma CORT, we found that that Dbh−/− mice were resistant to both foot shock and optogenetic LC activation-induced anxiety-like behavior in the EZM immediately after the manipulation, whereas the responses of GalcKO-Dbh mice were similar to controls; however, when tested 24 h after foot shock stress exposure, we found that both Dbh−/− and GalcKO-Dbh mice were resistant to the persistent effects of foot shock in EZM performance. Furthermore, we found that pharmacological activation of galanin receptors, but not restoration of NE signaling, in knockout mice during foot shock normalized expression of stress-induced anxiety-like behavior 24 h later. In contrast, rescue of NE signaling in Dbh−/− mice alone at the time of EZM testing induced anxiety-like behavior. These results suggest that NE transmission is important for anxiogenic responses at the time of behavioral testing, while noradrenergic-derived galanin plays no acute role but rather is released during the stressor to promote anxiety-like behavior over the course of hours to days.
Our findings that the Dbh−/− mice were resistant to stress-induced behavioral changes add to growing literature supporting an important role for NE in mediating anxiogenic responses. The inability of NE restoration during foot shock to rescue normal anxiety-like behavior 24 h later in the Dbh−/− mice suggests that acute NE signaling at the time of the behavioral test is required. In support of this idea, administration of DOPS to Dbh−/− alone, in the absence of foot shock, reduced open segment exploration in the EZM (this study) and the related elevated plus maze [22]. We speculate that because Dbh−/− mice lack NE from birth and have a compensatory upregulation of β-adrenergic receptors (βAR) [36], acute NE restoration has anxiogenic properties in the knockouts akin to cocaine-induced increases in WT animals [22]. Recent studies have found that LC projections to the basolateral amygdala (BLA) mediate acute foot shock stress-induced increases in BLA firing through βAR signaling, and local administration of βAR antagonists in the BLA immediately following foot shock leads to decreased freezing behavior in an immediate fear extinction paradigm [37, 38]. Furthermore, LC-NE-mediated βAR transmission in the BLA induced anxiety-like behavior on its own and modulated pain-related anxiety-like behavior [19, 39]. This acute action of NE in the BLA likely explains the acute stress-induced anxiety-like effect in the EZM immediately following foot shock that we observed in WT and GalcKO-Dbh mice, which is absent in the Dbh−/− mice.
A previous study showed that selective optogenetic activation of galanin-containing LC neurons was sufficient to induce avoidance behavior, and this behavioral effect could be prevented by a βAR antagonist, indicating a noradrenergic mechanism;[29] however, the potential contribution from galanin was not explicitly examined. Here we employed the same optogenetic protocol to selectively activate LC neurons in GalcKO-Dbh mutants and observed increased anxiety-like behavior in the EZM similar to WT animals, but this effect was absent in Dbh−/− mice, which had no change in EZM performance. Our results support the previous work by McCall and colleagues by showing that acute optogenetic LC stimulation-induced anxiety-like behavior requires NE, and expand on this finding by showing that noradrenergic-derived galanin is dispensable for this acute behavioral change.
Neuropeptides, such as galanin, have different dynamics than small-molecule neurotransmitters like NE due to distinct mechanisms controlling synthesis, release, and degradation of the molecules [15]. Because of their storage in large, dense core vesicles (LDCV), neuropeptides are thought to be preferentially released when neuronal firing rate and Ca2+ influx are high, and previous research has suggested that galanin transmission occurs under conditions that strongly activate noradrenergic neurons [15–17]. Once released, galanin can act as an inhibitory neuromodulator by signaling acutely through GalR1 and GalR3, which are typically Gi-coupled, but galanin can also act as a neurotrophic factor via GalR2-Gq signaling, leading to changes in neuronal plasticity over time [12]. Our results show that exogenous activation of galanin receptors during the stressor, but not 24 h later during the EZM, can normalize stress-induced anxiety-like behavior in the GalcKO-Dbh, suggesting that galanin is acting through a neurotrophic mechanism in this paradigm to establish persistent anxiety-like behavior. This mechanism could involve changes in dendritic spines or receptor distributions in downstream, stress-sensitive brain regions (e.g. amygdala, prefrontal cortex (PFC)). Alterations in plasticity take time to develop and thus would not change behavior in the EZM test until hours or days after the stress, and would fail to occur in the absence of galanin transmission from the LC in the GalcKO-Dbh mice. Previous research has shown that significant atrophy and remodeling of dendrites occurs in stress-sensitive regions, such as the PFC, as early as 24 h after a single foot shock stress [40, 41], and that chronic intracerebroventricular infusions of galanin prevent foot shock-induced dendritic spine loss in the PFC [42]. Future experiments will be required to identify the mechanism underlying the persistent effects of stress-induced galanin transmission, including determining where galanin is acting in the brain and which receptor mediates its effects.
There are limitations associated with our study. While there are significant benefits to using knockout models like the Dbh−/− and GalcKO-Dbh mice, it is important to note that although these genetic manipulations are specific to noradrenergic neurons, neither is specific to the LC. The LC certainly projects widely to brain regions known to control behavioral responses to stress (e.g., hippocampus, amygdala, PFC), and most LC neurons co-express galanin, making it the most likely source of galanin and NE that mediates the anxiogenic effects of stress; however, A2 noradrenergic neurons in the nucleus of the solitary tract also mediate anxiety-like behavior, although NE transmission from these cells appears to be anxiolytic rather than anxiogenic [43], and they express little if any galanin [18, 44]. Because the knockout mice used here lack expression of NE or galanin throughout development, it is possible that compensatory mechanisms alter the way these systems function, although our findings that acute pharmacological restoration of NE and galanin receptor activation rescues stress responses in the Dbh−/− and GalcKO-Dbh mice, respectively, seem to rule out that caveat in the present experiments. While NE release following foot shock and optogenetic stimulation has been verified using neurochemical methods [19, 45–47], there are currently no available techniques for measuring galanin release in vivo; thus, the behavioral effects we attribute to galanin release from noradrenergic neurons can only be inferred from the phenotypes we observed in the GalcKO-Dbh mice. In addition, galnon was originally shown to act at both GalR1 and GalR2, but subsequent reports have suggested that galnon primarily binds GalR2, not GalR1, as well as several non-galanin receptors, raising the potential for off-target effects [48–50]. The preferential affinity of galnon for GalR2 over GalR1 strengthens our prediction that GalR2-mediated neurotrophic mechanisms underlie the 24 h behavioral effects of galanin found here, and supports the merit of future studies using more selective compounds. Finally, our finding that noradrenergic-derived galanin is required for the persistent anxiogenic effects of stress may appear at first blush to directly contradict our recent report that overexpression of galanin in noradrenergic neurons has the opposite effect and promotes stress resilience [28]. Nonetheless, this inconsistency in the valence of how noradrenergic-derived galanin modulates stress resilience is consistent with the complexity often reported in the galanin literature, and potentially aligns with contrasting roles that galanin plays across different mechanisms (such as engagement of different galanin receptors in different brain regions) and timescales (hours to days versus weeks to months) [12].
Our findings demonstrate that both NE and noradrenergic-derived galanin are involved in mediating aspects of stress-induced anxiety-like behavior and appear to act over different timescales. More broadly, our results may also shed light on how other small molecule and peptide co-transmitter systems function throughout the nervous system. Although some of the complexity created by co-transmission has been dissected with the aid of modern neuroscience techniques, such as using RNAi to knockdown individual co-transmitters in a single cell type [51], this remains an understudied area of research and there is much to discover about the functional effects of co-released neurotransmitters. Our findings on the differential roles of NE and galanin in the LC-NE system point to the importance of examining the roles of co-transmitters over different timescales and may be applicable to other neuromodulatory systems that co-express neuropeptides, such as the serotonergic dorsal raphe neurons which express neuropeptide Y and galanin in some species, or the dopaminergic ventral tegmental area neurons which express high levels of brain-derived neurotrophic factor [52, 53]. Future studies will continue to disentangle the functional relationship between these co-expressed neuromodulators in the LC-NE system and determine the mechanism by which galanin regulates persistent adaptive and maladaptive stress responses, with the ultimate goal of leveraging this knowledge to improve treatments for stress-related disorders.
Funding and disclosures
This work was supported by the National Institutes of Health (NIH) (F31MH116622 to RPT, F31DA044726 to SLF, T32NS007480 to DL, R01NS102306, R01DA038453, R01DA049257, and R01AG061175 to DW). The authors report no financial interests or potential conflicts of interest.
Supplementary information
Acknowledgements
We thank C. Strauss for helpful editing of this manuscript.
Author contributions
RPT and DW conceived, designed, and supervised the project. RNAscope in situ hybridization was performed by SF. Immunohistochemistry experiments were performed by RPT and DL. All behavioral experiments were performed and analyzed by RPT with assistance from KEM. Pharmacological experiments were performed by RPT with assistance from DW. Mouse husbandry and genotyping were performed by LCL. RPT and DW wrote the manuscript with input from co-authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01011-8.
References
- 1.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 2.Kruijshaar MK, B J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression—an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. 2005;20:103–11. doi: 10.1007/s10654-004-1009-0. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melander T, Hokfelt T, Rokaeus A, Cuello A, Oertel W, Verhofstad A, et al. Coexistence of Galanin-like lmmunoreactivity with Catecholamines, 5-Hydroxytryptamine, GABA and Neuropeptides in the Rat CNS. J Neurosci. 1986;6:3640–54. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skofitsch G, Jacobowitz D. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–46. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 6.Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–85. doi: 10.1002/cne.1171. [DOI] [PubMed] [Google Scholar]
- 7.Chan-Palay V, Jentsch B, Lang W, Hochli M, Asan E. Distribution of neuropeptide Y, C-terminal flanking peptide of NPY and galanin coexistence with catecholamine in the locus coeruleus of normal human, Alzheimer’s dementia and Parkinson’s disease brains. Dement Geriatr Cogn Disord. 1990;1:18–31. [Google Scholar]
- 8.Le Maitre E, Barde SS, Palkovits M, Diaz-Heijtz R, Hokfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci USA. 2013;110:E536–45. doi: 10.1073/pnas.1221378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson RM, Holmes A. Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids. 2006;31:231–9. doi: 10.1007/s00726-006-0336-8. [DOI] [PubMed] [Google Scholar]
- 11.Hokfelt T, Barde S, Xu ZD, Kuteeva E, Ruegg J, Le Maitre E, et al. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Front Neural Circuits. 2018;12:106. doi: 10.3389/fncir.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinshenker D, Holmes PV. Regulation of neurological and neuropsychiatric phenotypes by locus coeruleus-derived galanin. Brain Res. 2016;1641:320–37. doi: 10.1016/j.brainres.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borodovitsyna O, Joshi N, Chandler D. Persistent stress-induced neuroplastic changes in the locus coeruleus/norepinephrine system. Neural Plast. 2018;2018:1892570. doi: 10.1155/2018/1892570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentino RJ, Van, Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharm. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hokfelt T, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharm Rev. 2015;67:118–75. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 16.Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Ann Rev Pharm Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- 17.Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36:1965–84. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillage RP, Sciolino NR, Plummer NW, Lustberg D, Liles LC, Hsiang M, et al. Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Struct Funct. 2020;225:785–803. doi: 10.1007/s00429-020-02035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, et al. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife. 2017;6:e18247. doi: 10.7554/eLife.18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–46. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 21.Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacol (Berl) 2020;237:1973–87. doi: 10.1007/s00213-020-05512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63:1007–12. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marino MD, Bourdelat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–76. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SA, Palmiter RD. Disruption of the dopamine p-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci. 1997;111:579–89. doi: 10.1037//0735-7044.111.3.579. [DOI] [PubMed] [Google Scholar]
- 26.Szot P, Weinshenker D, White S, Robbins C, Rust N, Schwartzkroin P, et al. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–92. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. Chronic inhibition of dopamine beta-hydroxylase facilitates behavioral responses to cocaine in mice. PLoS One. 2012;7:e50583. doi: 10.1371/journal.pone.0050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillage RP, Wilson GE, Liles LC, Holmes PV, Weinshenker D. Chronic environmental or genetic elevation of galanin in noradrenergic neurons confers stress resilience in mice. J Neurosci. 2020;40:7464–74. doi: 10.1523/JNEUROSCI.0973-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–20. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacol (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 31.Bartfai T, Wang MW. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharm Sin. 2013;34:880–5. doi: 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci USA. 2007;104:13804–9. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 34.Lustberg D, Tillage RP, Bai Y, Pruitt M, Liles LC, Weinshenker D. Noradrenergic circuits in the forebrain control affective responses to novelty. Psychopharmacol (Berl) 2020;237:3337–55. doi: 10.1007/s00213-020-05615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–91. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res. 2006;1109:45–53. doi: 10.1016/j.brainres.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Giustino TF, Ramanathan KR, Totty MS, Miles OW, Maren S. Locus coeruleus norepinephrine drives stress-induced increases in basolateral amygdala firing and impairs extinction learning. J Neurosci. 2020;40:907–16. doi: 10.1523/JNEUROSCI.1092-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giustino TF, Seemann JR, Acca GM, Goode TD, Fitzgerald PJ, Maren S. Beta-adrenoceptor blockade in the basolateral amygdala, but not the medial prefrontal cortex, rescues the immediate extinction deficit. Neuropsychopharmacology. 2017;42:2537–44. doi: 10.1038/npp.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llorca-Torralba M, Suarez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, et al. Chemogenetic silencing of the locus coeruleus-basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatry. 2019;85:1021–35. doi: 10.1016/j.biopsych.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Nava N, Treccani G, Alabsi A, Kaastrup Mueller H, Elfving B, Popoli M, et al. Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cereb Cortex. 2017;27:694–705. doi: 10.1093/cercor/bhv254. [DOI] [PubMed] [Google Scholar]
- 41.Musazzi L, Tornese P, Sala N, Popoli M. What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Front Pharm. 2018;9:758. doi: 10.3389/fphar.2018.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sciolino NR, Smith JM, Stranahan AM, Freeman KG, Edwards GL, Weinshenker D, et al. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology. 2015;89:255–64. doi: 10.1016/j.neuropharm.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 2017;74:366–75. doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM. Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol. 1987;261:562–82. doi: 10.1002/cne.902610408. [DOI] [PubMed] [Google Scholar]
- 45.Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci. 1999;113:558–66. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- 46.Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–7. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 47.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, et al. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–20. doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Florén A, Sollenberg U, Lundström L, Zorko M, Stojan J, Budihna M, et al. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39:547–58. doi: 10.1016/j.npep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Webling KE, Runesson J, Bartfai T, Langel U. Galanin receptors and ligands. Front Endocrinol (Lausanne) 2012;3:146. doi: 10.3389/fendo.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO. Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Rep. 2019;29:13–21 e4. doi: 10.1016/j.celrep.2019.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hokfelt T, Broberger C, Diez M, Xu ZQ, Shi T, Kopp J, et al. Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm Metab Res. 1999;31:330–4. doi: 10.1055/s-2007-978748. [DOI] [PubMed] [Google Scholar]
- 53.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.