Abstract
Triple-negative breast cancer (TNBC) is characterized by low expression of human epidermal growth factor receptor-2 (HER2), estrogen receptor (ER), and progesterone receptor (PR), which is the most aggressive subtype with poor outcome among breast cancers. The underlying mechanisms of TNBC remain unclear and there is a lack of biomarkers. In this study we conducted an in silico assay and found that FOXC1 was highly expressed in ER−/PR−/HER2− breast cancers, which was confirmed by qRT-PCR, immunohistochemistry, and Western blot analysis. FOXC1 was more highly expressed in TNBCs than the other breast cancers. Kaplan–Meier plotter revealed that expression of FOXC1 was associated with overall survival (OS) of patients with breast cancers. Expression of FOXC1 was reversely associated with level of H3K27me3, which was methylated by EZH2. In MCF-7 and T47D cells, inhibition of EZH2 by DZNeP or GSK343 concentration- and time-dependently increased expression of FOXC1. Finally, we demonstrated that the expression of FOXC1 was associated with resistance of doxorubicin treatment of breast cancer cells. In conclusion, these results suggest that FOXC1 may be a potential biomarker or drug target for TNBCs, and that downregulation of FOXC1 could have therapeutic value in treatment of TNBCs.
Keywords: TNBC, FOXC1, EZH2, H3K27me3, drug resistance
Introduction
Despite decades of research and clinical trials, cancer is still a global health problem [1]. Breast cancer is the most common type of cancer and is the second leading cause of death from cancer in women worldwide [2]. There are three major subtypes of breast cancers according to their molecular profiles: estrogen receptor positive (ER+), human epidermal growth factor receptor-2 positive (HER2+), and triple-negative breast cancer (TNBC). It is well-known that TNBC is a very aggressive breast cancer with a poor prognosis [3]. The molecular mechanisms of TNBC are still unclear.
Forkhead box (FOX) proteins are a superfamily of evolutionarily conserved transcription factors, all of which have a conserved DNA-binding domain 100 amino acids in length that is named for the Drosophila transcription factor forkhead. FOX proteins are involved in many biological processes, including proliferation, development, differentiation, migration, invasion, apoptosis, metabolism, and longevity [4]. FOXC1 (forkhead box C1), also known as FKHL7, is 553 amino acids in length and has a molecular mass of 57 kDa [5]. FOXC1 is associated with Axenfeld–Rieger syndrome and mutated in patients diagnosed with Rieger’s anomaly, iris hypoplasia or Axenfeld’s anomaly. Mutations in FOXC1 result in many glaucoma phenotypes [6–8]. FOXC1 overexpression in basal-like breast cancer (BLBC) was first shown by our laboratory [9, 10]. Additional studies showed that FOXC1 was associated with several types of cancer, such as liver cancer [11], non-small cell lung cancer [12], gastric cancer [13], acute myeloid leukemia [14], melanoma [15], pancreatic ductal adenocarcinoma [16], esophageal squamous cell carcinoma [17], and prostate cancer [18]. However, the roles and underlying mechanisms of FOXC1 expression in TNBC have not yet been worked out.
Epigenetics, especially the methylation and demethylation of DNA [19] and the acetylation/deacetylation and methylation of histones [20], plays an important role in regulating gene expression. Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme encoded by the EZH2 gene that participates in histone methylation and transcriptional repression [21]. EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27 (H3K27me3) through polycomb repressive complex 2 (PRC2) [22, 23]. EZH2 is involved in tumorigenesis, especially in breast cancer [21, 23]. Data from in silico analyses have shown that FOXC1 expression is inversely associated with histone H3 lysine trimethylation, but it is unclear whether expression of FOXC1 is regulated by EZH2.
In this study, we found that FOXC1 was highly expressed in TNBCs and associated with the overall survival of patients with breast cancers. Expression of FOXC1 was also found to be inversely associated with the level of H3K27me3. Overexpression of EZH2 increased the level of H3K27me3 and inhibited expression of FOXC1, whereas the knockdown or inhibition of EZH2 reduced the level of H3K27me3 and increased expression of FOXC1. The expression of FOXC1 was epigenetically regulated by EZH2 rather than enhancer of zeste homolog 1 (EZH1), lysine-specific demethylases 6A (KDM6A) and 6B (KDM6B). The expression of FOXC1 was also associated with resistance to doxorubicin in TNBC cells.
Materials and methods
Cell culture
HMEC, MCF-7, T47D, ZR751, BT474, MDA-MB-468, BT549, HS578T, and HCC1806 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MCF-7 and HS578T cells were cultured in Dulbecco’s modified Eagle’s medium, while T47D, BT474, MDA-MB-468, and BT549 cells were maintained in RPMI-1640 medium, and HCC1806 cells were cultured in minimum Eagle’s medium. The complete culture medium was supplemented with fetal bovine serum to a final concentration of 10%. For HMECs, the complete culture medium was also supplemented with 10 ng/mL epidermal growth factor, 1 µg/mL hydrocortisone, and 10 mM glutamine. All cells were cultured at 37 °C in a humidified incubator containing 5% CO2 [24].
Tumor specimens
The Institutional Review Board of Peking Union Medical College Hospital (Beijing, China) approved the use of human tissues. Immunohistochemistry (IHC) was conducted on paraffin-embedded archival tissue specimens of breast cancers that were diagnosed at Peking Union Medical College Hospital (Beijing, China). Breast cancer tissue arrays (ZL-BRC1021) were purchased from Shanghai Zhuoli Biotech Company Co., Ltd, China.
Cell proliferation
MCF-7 vector, MCF-7 FOXC1, T47D vector, T47D FOXC1, HCC1806 vector, HCC1806 shFOXC1, BT549 vector, and BT549 shFOXC1 cells were seeded into 96-well plates. After overnight incubation, cells were treated with doxorubicin at different doses for 24 h, and cell viability was assessed with a CCK-8 assay (Applygen, Beijing, China).
Transfection
HCC1806 cells, which have low EZH2 expression, were cultured in 60-mm dishes to 75%–85% confluence for 24 h before transfection. Cells were then transfected with EZH2 expression plasmids (OriGene, Rockville, MD, USA) or an empty vector (OriGene, Rockville, MD, USA) using Lipofectamine™ 3000 transfection reagent (Invitrogen, Grand Island, NY, USA) for 24 h. The cells were then incubated with 600 μg/mL geneticin for 3 weeks. HCC1806 cells, with low EZH2 expression, and HCC1806 cells, with high EZH2 expression, are hereafter referred to as HCC1806 vector and HCC1806 EZH2 cells, respectively. MCF-7 and T47D cells were transfected with EZH2 siRNAs and EZH1 siRNAs [10]. FOXC1 shNC and shRNAs were stably infected into HCC1806 and BT549 cells. The cells were then screened with 5 μg/mL puromycin. HCC1806 and BT549 cells transfected with FOXC1 shNC and shRNAs are hereafter referred to as HCC1806 vector, HCC1806 shFOXC1, BT549 vector, and BT549 shFOXC1 cells, respectively [25]. The expression of EZH2 was verified by Western blotting with an anti-EZH2 antibody (#5246, Cell Signaling Technology, MA, USA), an anti-myc antibody (Origene, Rockville, MD, USA) and an anti-DDK antibody (Origene, Rockville, MD, USA). The sequences of FOXC1 shRNA, EZH2 siRNAs, and EZH1 siRNAs are shown in Table 1.
Table 1.
shRNA and siRNAs for FOXC1, EZH1, and EZH2.
| Name | Interference sequence (5′–3′) |
|---|---|
| shFOXC1 | TTCGAGTCACAGAGGATCGGCTTGAACAA |
| siEZH2-1 | GGATACAGCCTGTGCACAT |
| siEZH2-2 | CCACAGTGTTACCAGCATT |
| siEZH2-3 | CCTGACCTCTGTCTTACTT |
| siEZH1-1 | GCAAGCCAACATATGTTAA |
| siEZH1-2 | GCAGTCAAAGAATCACTTA |
| siEZH1-3 | CCAGTTCTTCAGAGGCTAA |
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was extracted from the cells using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA pellet was dissolved in DEPC-treated water, and 1 μg of total RNA was reverse-transcribed into cDNA using a PrimeScript RT Reagent Kit (TaKaRa Clontech, Dalian, China). qRT-PCR was performed on a CFX 96 thermocycler (Bio-Rad, Hercules, CA, USA) by using a SYBR Premix Ex Taq II Kit (TaKaRa Clontech, Dalian, China) to detect the expression of FOXC1 and EZH2. GAPDH was used as a loading control, and assays were carried out three times. Primer sequences are given in Table 2.
Table 2.
Primers for FOXC1 (5′–3′).
| Primer | Forward | Reverse |
|---|---|---|
| FOXC1 | GACTCCAGAAACCATT | AGCAAGGCAGTAAATAATC |
| EZH2 | GGACTCAGAAGGCAGTGGAG | CTTGAGCTGTCTCAGTCGCA |
| FOXC1 (ChIP) | GACCCTCGCGGGCGGGCAGG | CTGCGGGCCGCCCTGCTTCTC |
In silico analysis
The Oncomine database (www.oncomine.org) is widely used for investigating the expression of genes in multiple cancer datasets to validate the relationship between gene expression and cancer. In this study, we investigated the mRNA expression of FOXC1 in breast cancer subtypes using Oncomine. Breast cancer Gene-Expression Miner v4.2 (http://bcgenex.centregauducheau.fr/BC-GEM/GEM-Accueil.php?js=1) was also used to investigate the expression of FOXC1 in ER−, PR−, and HER2− subtype breast cancers and in TNBC.
ENCODE (Encyclopedia of DNA Elements) is a public research consortium aimed at identifying all functional elements in the human and mouse genomes. To confirm whether the expression of FOXC1 is associated with H3K27me3, in silico analysis of ChIP-Seq data on H3K27me3 from MCF-7 cells was carried out using the ENCODE dataset (https://www.ncbi.nlm.nih.gov/genome/gdv/browser/geo/?id=GSM970218).
Immunohistochemistry (IHC)
IHC procedures were performed as described previously [26]. Briefly, 5 µm paraffin-embedded tissue sections were dried, deparaffinized, and rehydrated. For antigen retrieval, the rehydrated sections were immersed in boiled 10 mM sodium citrate buffer (pH 6.0) and maintained at a sub-boiling temperature for 20 min. Afterwards, slides were incubated with 3% hydrogen peroxide for 10 min at room temperature to block the activity of endogenous peroxidase. Sections were blocked in 10% normal goat serum for 30 min to reduce nonspecific binding and then incubated with a primary FOXC1 antibody (1:500 dilution; ab227977, Abcam, Cambridge, MA, USA) in a moist chamber at 4 °C for 12 h. PBS was substituted for the primary antibody as a negative control. The sections were then examined using an IHC detection system (ZSGB-BIO, Beijing, China) according to the manufacturers’ instructions. The slides were incubated in diaminobenzidine (ZSGB-BIO, Beijing, China) until a brown reaction product was observed and then counterstained with hematoxylin. Photographs were taken with equal exposure on a Nikon Eclipse Ti microscope coupled with NIS elements software (Nikon, Melville, NY, USA) for Windows. For quantitation, three visual fields were randomly selected, and scoring was performed independently by two pathologists. Semiquantitative analysis of the stained sections was performed by light microscopy according to the immunoreactive scoring scale of Remmele and Stegner (IRS) [27, 28].
Western blot analysis
Cells were collected and prepared for the detection of target proteins [29]. Cells were lysed in RIPA lysis buffer (P0013C, Applygen, Beijing, China) supplemented with protease inhibitors (Roche, Indianapolis, IN, USA) at 4 °C for 30 min. The cell lysates were centrifuged at 13,000 × g for 15 min at 4 °C, and the supernatants were collected. Protein concentrations were determined using a BCA Kit (CWBIO, Beijing, China). Protein samples were separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Membranes were blocked in 5% fat-free milk in TBST for 1 h and then incubated overnight at 4 °C with primary antibodies against EZH2, H3K27me3 (#9733, Cell Signaling Technology, MA, USA), FOXC1 (#8758, Cell Signaling Technology, MA, USA), KDM6A (#33510, Cell Signaling Technology, MA, USA), KDM6B (#3457, Cell Signaling Technology, MA, USA), EZH1 (#42088, Cell Signaling Technology, MA, USA), β-actin (1:5000, Proteintech Europe), and GAPDH (1:5000, Proteintech, Europe). After washing with TBST, the membrane was incubated with a goat anti-mouse or anti-rabbit HRP-conjugated secondary antibody (1:5000; #7074 Cell Signaling Technology, MA, USA). Signal detection was performed using an eECL Western blotting Kit (CWBIO, Beijing, China) and a Tanon Chemiluminescence Image Analysis System (Shanghai, China).
ChIP assay
ChIP assays were performed using a SimpleChIP Enzymatic ChIP Kit (#9003, Cell Signaling Technology, MA, USA) according to the manufacturer’s instructions. Briefly, 1.2 × 107 MCF-7 cells were fixed with fresh 1% formaldehyde (final concentration) for 10 min at room temperature, and the crosslinking was stopped by the addition of glycine solution at a final concentration of 0.125 M. Cells were then scraped and collected in PBS buffer with a protease inhibitor complex. Chromatin was digested by micrococcal nuclease at 37 °C for 20 min to form DNA fragments with lengths of ~150–900 bp. The DNA fragments were incubated with specific antibodies against H3K27me3 (#9733, Cell Signaling Technology, MA, USA) and normal IgG (#2729, Cell Signaling Technology, MA, USA) at 4 °C overnight with magnetic protein G beads. After washing and elution, the DNA-protein complexes were incubated at 65 °C for 30 min to reverse the crosslinking. Immunoprecipitated and input DNAs were purified using a DNA purification spin column and then analyzed by quantitative real-time PCR with TB Green (Takara Clontech, Dalian, China) [30]. The sequences of the FOXC1 forward and reverse primers are shown in Table 2.
Statistical analysis
The results are presented as the mean ± SD. Statistical significance between different groups was analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
Results
FOXC1 mRNA was highly expressed in TNBC
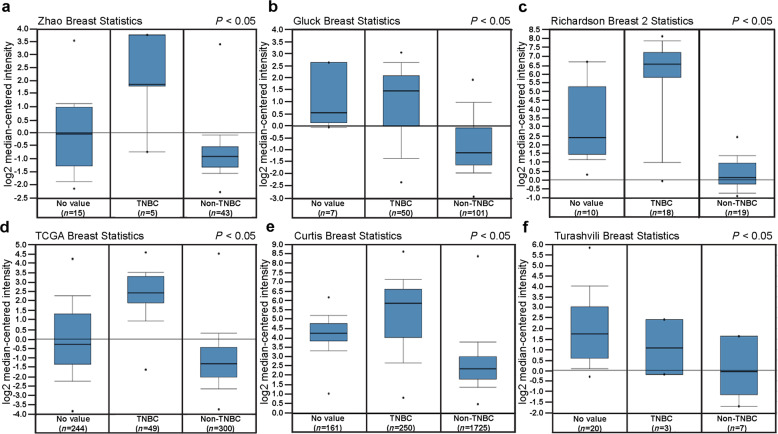
Breast cancer specimens were divided into three major subtypes according to the expression of ER, PR, and HER2: Luminal, HER2, and TNBC. To determine whether the expression of FOXC1 was associated with ER, PR, and HER2, an in silico assay was carried out using data from the Oncomine database (www.oncomine.org) and Breast Cancer Gene-Expression Miner v4.2 (http://bcgenex.centregauducheau.fr/BC-GEM/GEM-Accueil.php?js=1). The FOXC1 expression was significantly higher in TNBC than in non-TNBC according to data from Oncomine (Fig. 1a–f). Similar results were obtained with Breast Cancer Gene-Expression Miner v4.2 (Supplementary Fig. S1a–c). In addition, FOXC1 expression was significantly higher in breast cancers with ER−, PR−, and HER2− than in breast cancers with ER+, PR+, and HER2+ (Supplementary Fig. S1d–f). To validate these in silico results, total RNA was extracted from cell lines, and qRT-PCR was carried out to examine the expression of FOXC1 mRNA. FOXC1 expression was significantly higher in TNBC cell lines (MDA-MB-231, BT549, HS578T, and HCC1806) than in non-TNBC cell lines (HMEC, MCF-7, T47D, BT47D, and ZR751) (Supplementary Fig. S2).
Fig. 1. FOXC1 mRNA was highly expressed in TNBCs.
a–f FOXC1 expression is lower in non-TNBCs than in TNBCs. Data and statistics were obtained from www.oncomine.org (a Zhao et al. (2004); b Gluck et al. (2006); c Richardson 2 et al. (2006); d TCGA (2011); e Curtis et al. (2012); f Turashvili et al. (2007)).
FOXC1 protein was highly expressed in TNBCs
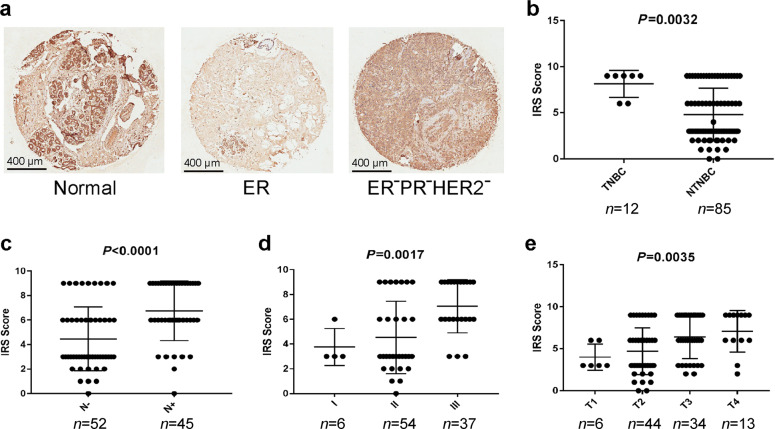
To investigate whether expression of the FOXC1 protein is also highly expressed in TNBCs and associated with the progress of breast cancers, a breast cancer tissue array was purchased, and IHC was carried out on the tissue array. Representative images are shown in Fig. 2a. The expression of the FOXC1 protein was higher in TNBCs than in non-TNBCs (Fig. 2b). Expression of the FOXC1 protein was also higher in breast cancer specimens with positive lymph nodes than in breast cancer specimens with negative lymph nodes (Fig. 2c). In addition, expression of the FOXC1 protein increased with the development of breast cancer (Fig. 2d, e). Together, these results suggest that the FOXC1 protein is highly expressed in TNBCs.
Fig. 2. FOXC1 protein was highly expressed in TNBCs and associated with breast cancer progression.
a Representative images showing the FOXC1 protein in normal tissue, ER+ breast cancer, and TNBC. Scale bar = 400 μm. b Expression of the FOXC1 protein is higher in TNBCs (n = 12) than in non-TNBCs (n = 85). c Expression of the FOXC1 protein is higher in breast cancer with positive lymph nodes (n = 45) than in breast cancer with negative lymph nodes (n = 52). d, e Expression of the FOXC1 protein was increased as breast cancer progressed. I: stage I (n = 6); II: stage II (n = 54); III: stage III (n = 37). T1: tumor T1 (n = 6); T2: tumor T2 (n = 44); T3: tumor T3 (n = 34); T4: tumor T4 (n = 13).
FOXC1 expression was associated with the prognosis of patients with breast cancers
Both FOXC1 mRNA and the FOXC1 protein were overexpressed in TNBCs. To investigate whether the expression of FOXC1 is related to the prognosis of patients with breast cancer, in silico analysis was carried out using data from Kaplan–Meier plotter (http://kmplot.com/analysis/). As shown in Fig. 3a, patients with high FOXC1 expression had a lower overall survival rate than patients with low FOXC1 expression. To validate the results from the in silico analysis, clinical data from patients with breast cancer were analyzed. The results showed that breast cancer patients with high FOXC1 expression had a lower overall survival rate than patients with low FOXC1 expression (Fig. 3b). The results from both the bioinformatics analysis and patients confirmed that expression of FOXC1 is associated with the prognosis of patients with breast cancers.
Fig. 3. Expression of FOXC1 was associated with a poor prognosis in breast cancer patients.
a Patients with high FOXC1 expression had a lower overall survival rate than patients with low FOXC1 expression (in silico analysis) (log-rank P < 0.05). b Analysis of clinical data showed that breast cancer patients with high FOXC1 expression had a lower overall survival rate than patients with low FOXC1 expression (P = 0.0004).
Expression of FOXC1 was reversibly related to H3K27me3 and EZH2
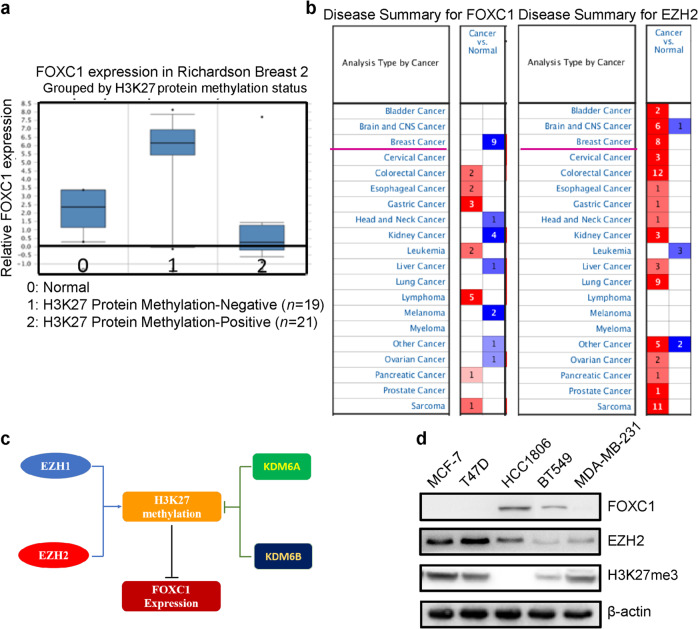
Epigenetics is an important mechanism that regulates the expression of genes. To explore whether expression of FOXC1 is regulated by epigenetics, in silico analysis was carried out using data from Oncomine. The results from the Oncomine datasets showed that the expression of FOXC1 was inversely related to the levels of H3K27me3 (Fig. 4a) and EZH2 (Fig. 4b). EZH2 and EZH1 methylate H3K27, while KDM6A and KDM6B demethylate H3K27me (Fig. 4c). To confirm the relationship between FOXC1 and EZH2 or H3K27me3, Western blot analysis was performed to examine the expression of FOXC1, EZH2, and H3K27me3 using cell lysates from 2 ER-positive breast cancer cell lines (MCF-7 and T47D) and three TNBC cell lines (HCC1806, BT549, and MDA-MB-231). It was shown in Fig. 4d that cells with high FOXC1 expression had lower EZH2 and H3K27me3 expression, whereas cells with low FOXC1 expression had higher EZH2 and H3K27me3 expression.
Fig. 4. Expression of FOXC1 was reversibly related to histone methylation (H3K27me3) by EZH2.
a In silico analysis of the Oncomine datasets showed that FOXC1 expression was inversely related to H3K27me3 expression. b The expression of FOXC1 was inversely related to the expression of EZH2. c Schematic of FOXC1 regulation. EZH2 and EZH1 methylate H3K27 whereas KDM6A and KDM6B demethylate H3K27me. d Expression of FOXC1, EZH2, and H3K27me3 in different breast cancer cell lines. The experiments were performed in triplicate.
EZH2 regulated the expression of FOXC1 by H3K27me3
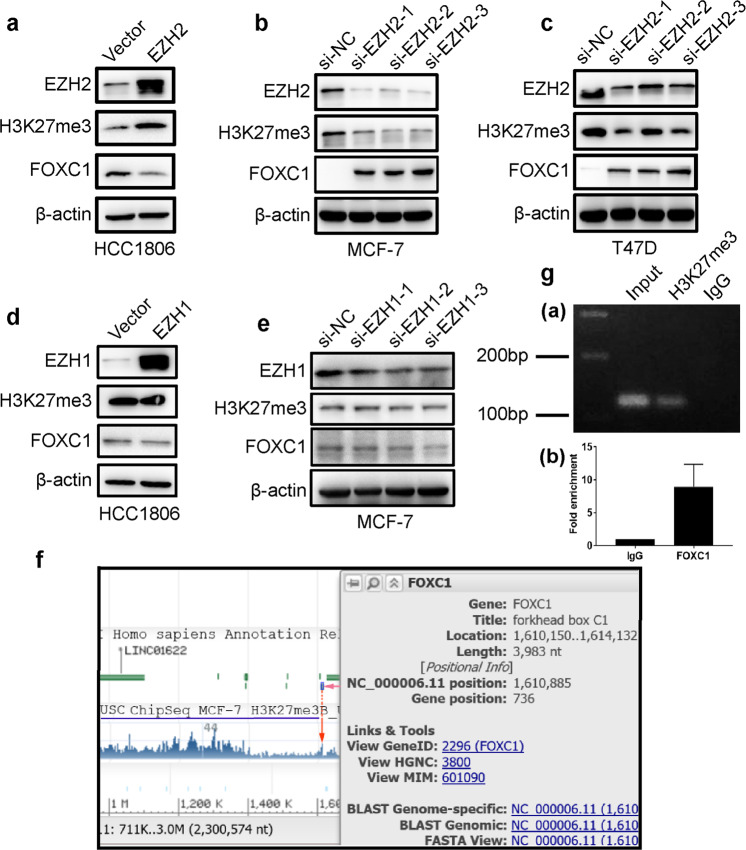
To determine whether EZH2 or EZH1 activity affects the expression of H3K27me3 and FOXC1, stable clones (HCC1806 EZH2 and HCC1806 EZH1) with high EZH2 expression or EZH1 expression were obtained. MCF-7 cells were transfected with EZH2 siRNAs and EZH1 siRNAs, respectively. The expression of EZH2, EZH1, H3K27me3, and FOXC1 was assessed in HCC1806 vector and HCC1806 EZH2, MCF-7 siNC and MCF-7 siEZH2, T47D siNC and T47D siEZH2, HCC1806 vector and HCC1806 EZH1, MCF-7 siNC and MCF-7 siEZH1 cells by Western blotting and qRT-PCR. As shown in Fig. 5a, the upregulation of EZH2 induced H3K27me3 expression and inhibited FOXC1 expression in HCC1806 cells, while the knockdown of EZH2 reduced H3K27me3 expression and increased FOXC1 expression in MCF-7 and T47D cells (Fig. 5b, c). No significant change in either H3K27me3 expression or FOXC1 expression was observed in HCC1806 EZH1 cells compared to control cells (Fig. 5d), and there was no significant change in either H3K27me3 expression or FOXC1 expression in MCF-7 siEZH1 cells compared to control cells (Fig. 5e). It was also found that the mRNA level of FOXC1 was decreased by upregulating EZH2 expression, while after knocking down EZH2, the mRNA level of FOXC1 was increased (Supplementary Fig. S3).
Fig. 5. Overexpression of EZH2 inhibited the expression of FOXC1 by increasing H3K27me3 level.
a Overexpression of EZH2 resulted in the methylation of histone H3 and the formation of H3K27me3, which inhibited the expression of FOXC1. b The knockdown of EZH2 expression reduced the levels of H3K27me3 and increased the expression of FOXC1 in MCF-7 cells. c The knockdown of EZH2 expression reduced the expression of H3K27me3 and increased the expression of FOXC1 in T47D cells. d Neither H3K27me3 nor FOXC1 expression was changed in HCC1806 EZH1 cells overexpressing EZH1 compared to control cells. e Neither H3K27me3 nor FOXC1 expression was changed in HCC1806 EZH1 shRNA cells, in which EZH1 was downregulated compared to control cells. The experiments were performed in triplicate. f In silico analysis from ENCODE showed that the H3K27me3 antibody can bind to the promoter region of the FOXC1 gene. g ChIP with H3K27me3 antibodies or control IgG showed that H3K27me3 antibodies bound to the promoter of the FOXC1 gene.
To explore whether EZH2 regulates the expression of FOXC1 by H3K27me3, in silico analysis of ChIP-Seq data on H3K27me3 from MCF-7 cells was carried out using the ENCODE dataset. The annotated ChIP-Seq data of H3K27me3 from MCF-7 cells in the ENCODE database revealed a peak in the 1 kb upstream region of the FOXC1 gene promoter (Fig. 5f), indicating the presence of H3K27me3 in the FOXC1 gene promoter. To further confirm this result, ChIP with the H3K27me3 antibody was carried out. The results showed that the H3K27me3 antibody could bind to the promoter of the FOXC1 gene (Fig. 5g).
Taken together, these results suggest that EZH2 regulates the expression of FOXC1 by H3K27me3.
Inhibition of EZH2 activity induced expression of FOXC1
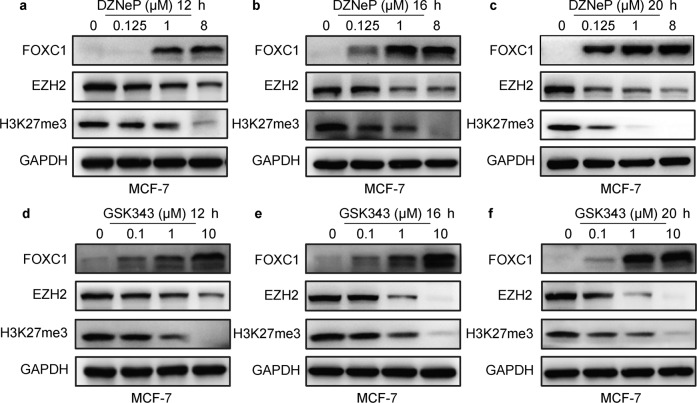
To further investigate whether EZH2 or EZH1 regulates expression of FOXC1 by H3K27me3, DZNeP (a specific inhibitor of EZH2), and GSK343 (a specific inhibitor of EZH2) were added to cells (MCF-7 and T47D), and changes in FOXC1 expression were assessed by Western blot analysis. Fig. 6a–c and Supplementary Fig. S4a–c show that the expression of FOXC1 was significantly increased by treatment with DZNeP in MCF-7 and T47D cells. The results also showed that the expression of FOXC1 was significantly improved by treatment with GSK343 in MCF-7 and T47D cells (Fig. 6d–f and Supplementary Fig. S4d–f). These results suggest that the inhibition of EZH2 induces the expression of FOXC1.
Fig. 6. Inhibition of EZH2 in MCF-7 cells induced the expression of FOXC1.
a–c Western blot analysis showed that the expression of FOXC1 was significantly increased by treatment of DZNeP in MCF-7 cells. d–f The expression of FOXC1 was significantly increased by treatment of GSK343 in MCF-7 cells. The experiments were performed in triplicate.
Inhibition of lysine demethylases KDM6A and KDM6B did not reduce the expression of FOXC1 in breast cancer cells
KDM6A and KDM6B demethylate H3K27me and affect the expression of certain genes. To determine whether KDM6A and 6B affect the expression of FOXC1, expression of FOXC1, KDM6A, KDM6B, EZH2, EZH1, and GAPDH in MCF-7 and T47D cells and HCC1806 cells was examined by Western blot analysis. FOXC1 expression was not associated with expression of KDM6A or KDM6B (Fig. 7a). HCC1806 and BT549 cells, with high FOXC1 expression, were treated with a range of concentrations of GSKJ1, an inhibitor of KDM6A and 6B, and the results showed no change in the expression of FOXC1 (Fig. 7b, c). Therefore, the expression of FOXC1 is not regulated by KDM6A or KDM6B.
Fig. 7. Inhibition of demethylases KDM6A and KDM6B did not alter the expression of FOXC1 in breast cancer cells.
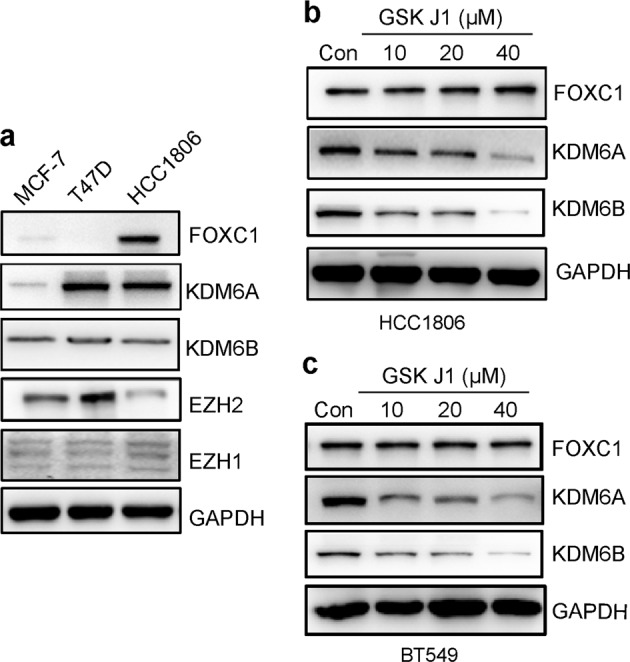
a Expression of FOXC1, KDM6A, KDM6B, EZH2, and EZH1 in MCF-7, T47D, and HCC1806 cells. b Treatment with GSKJ1 did not change the expression of FOXC1 in HCC1806 cells. c Treatment of GSKJ1 did not change the expression of FOXC1 in BT549 cells.
FOXC1 was associated with drug resistance in TNBCs
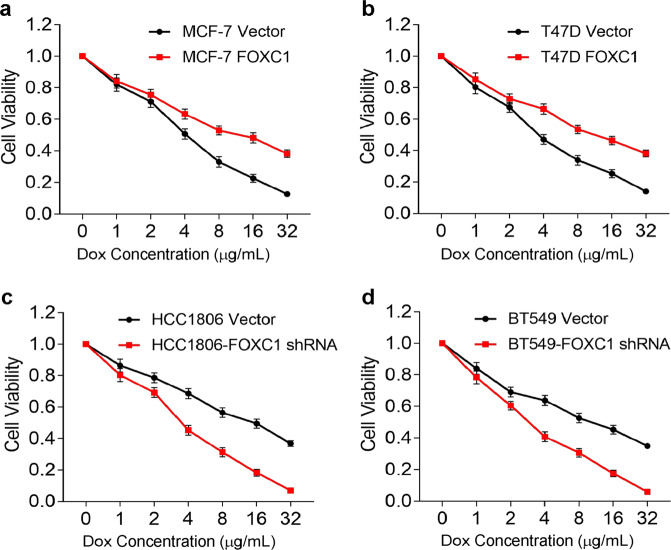
There has been no consistently effective drug for TNBCs. Doxorubicin and paclitaxel have been the main treatments for TNBCs, but some cancers develop resistance to these two drugs. To determine whether FOXC1 expression correlates with doxorubicin resistance, cells with low FOXC1 expression (MCF-7, T47D, HCC1806 FOXC1 shRNA, and BT549 FOXC1 shRNA cells) and cells with high FOXC1 expression (MCF-7 FOXC1, T47D FOXC1, HCC1806, and BT549 cells) were treated with different concentrations of doxorubicin for 48 h, and their proliferation was determined with a CCK-8 assay. Cells with low FOXC1 expression were more sensitive to doxorubicin than cells with high FOXC1 expression (Fig. 8a–d). Taken together, all these results suggest that FOXC1 expression is associated with drug resistance in TNBCs.
Fig. 8. FOXC1 overexpression was associated with doxorubicin (Dox) resistance.
a, b Cells with high FOXC1 expression were less sensitive to doxorubicin than cells with low FOXC1 expression. c, d Cells with low FOXC1 expression were more sensitive to doxorubicin than cells with high FOXC1 expression. Experiments were performed in triplicate.
Discussion
TNBC is an aggressive disease with very poor outcomes characterized by lack of ER, PR, and low expression of HER2 [31]. Tamoxifen is used to treat ER+ breast cancer [32], while Herceptin is used to treat HER2+ breast cancer, but there is no consistently effective drug for TNBCs. Chemotherapy with doxorubicin and paclitaxel has been the primary treatment for TNBCs, but such cancers frequently develop resistance to these drugs [33, 34]. Here, we showed that FOXC1 was highly expressed in TNBC and that the expression of FOXC1 was inversely associated with H3K27me3, which is methylated by EZH2. In addition, the overexpression of FOXC1 was associated with the development of doxorubicin resistance. The expression of FOXC1 was higher in ER− breast cancer than in ER+ breast cancer, confirming a previous report [35]. Our results showed that FOXC1 expression was also higher in PR− and HER2− breast cancers than in receptor-positive breast cancers. More importantly, FOXC1 expression was the highest in TNBCs and may be useful as a biomarker and a potential drug target.
Gene expression can be regulated epigenetically by several mechanisms, such as the methylation of DNA and histones. FOXC1 expression was shown to be inversely associated with the trimethylation of histone 3 on lysine. We also showed that the EZH2 methyltransferase rather than EZH1 was involved in this process. Inhibition of the demethylases KDM6A and KDM6B, which demethylate H3K27me3, was not involved in FOXC1 regulation because FOXC1 expression was not changed by treatment with the inhibitor. Our results suggest that FOXC1 expression is regulated by many mechanisms, including epigenetics [36, 37].
The overexpression of FOXC1 is associated with drug resistance in many cancers [15, 35, 38]. The overexpression of FOXC1 leads to resistance to the BRAF kinase inhibitor PLX4032 in melanoma cells [15]. MCF-7 cells transfected with a FOXC1 vector with high FOXC1 expression are less sensitive to tamoxifen than MCF-7 control cells transfected with an empty vector [35]. The overexpression of FOXC1 negates the effects of Hedgehog inhibitors on xenografted BLBC tumors [38]. Here, we showed that the overexpression of FOXC1 was associated with resistance to doxorubicin in breast cancer cell lines, whereas the knockdown of FOXC1 increased doxorubicin sensitivity. This finding could guide treatment of doxorubicin for patients with TNBC.
Conclusions
In conclusion, we found that FOXC1 was highly expressed in ER−, PR−, and HER2− breast cancer subtypes and TNBCs. Our study revealed that expression of FOXC1 was epigenetically regulated by H3K27me3, which is methylated by EZH2 rather than EZH1. Expression of FOXC1 was associated with resistance to doxorubicin (Fig. 9). These results suggest that FOXC1 might be a potential biomarker and drug target for TNBC.
Fig. 9. Proposed mechanism: EZH2 regulates the expression of FOXC1 by mediating H3K27me3 in breast cancers.
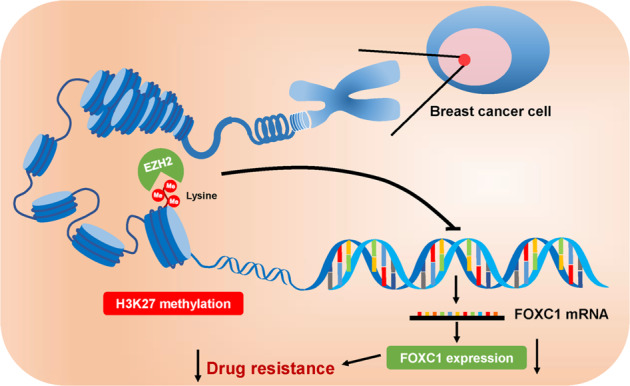
FOXC1 is highly expressed in TNBC. The expression of FOXC1 is epigenetically regulated by H3K27me3 and associated with doxorubicin resistance and a poor prognosis in patients with TNBC.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81573454 to JHW, No. 81703536 to WL) and the Beijing Natural Science Foundation of Beijing Municipality (7172142). This work was also supported by the CAMS Innovation Fund for Medical Sciences (2016-I2M-3-007) and the Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09711001-005-025, 2018ZX09711001-012).
Author contributions
JHW and GHD developed the hypothesis, designed the experiments, and revised the manuscript. XJZ and WL conducted all experiments and wrote the main manuscript. JYL, LWR, SWL, and XMZ collected data and performed the statistical analysis. JY provided the study materials and patients. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiang-jin Zheng, Wan Li
Contributor Information
Jin-hua Wang, Email: wjh@imm.ac.cn.
Guan-hua Du, Email: dugh@imm.ac.cn.
Supplementary information
The online version of this article (10.1038/s41401-020-00543-x) contains supplementary material, which is available to authorized users.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Li W, Zhao Y, Kang FuW, Zheng X, et al. Members of FOX family could be drug targets of cancers. Pharmacol Ther. 2018;181:183–96. doi: 10.1016/j.pharmthera.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Li W, Zheng X, Pang X, Du G. Research progress on the forkhead box C1. Oncotarget. 2018;9:12471–8. doi: 10.18632/oncotarget.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura DY, Swiderski RE, Alward WL, Searby CC, Patil SR, Bennet SR, et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 1998;19:140–7. doi: 10.1038/493. [DOI] [PubMed] [Google Scholar]
- 7.Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 1998;63:1316–28. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatireddy S, Chakrabarti S, Mandal AK, Reddy AB, Sampath S, Panicker SG, et al. Mutation spectrum of FOXC1 and clinical genetic heterogeneity of Axenfeld-Rieger anomaly in India. Mol Vis. 2003;9:43–8. [PubMed] [Google Scholar]
- 9.Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–6. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, et al. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene. 2012;31:4798–802. doi: 10.1038/onc.2011.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–24. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 12.Wei LX, Zhou RS, Xu HF, Wang JY, Yuan MH. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumour Biol J Int Soc Oncodev Biol Med. 2013;34:941–6. doi: 10.1007/s13277-012-0629-3. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Shao QS, Yao HB, Jin Y, Ma YY, Jia LH. Overexpression of FOXC1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2014;64:963–70. doi: 10.1111/his.12347. [DOI] [PubMed] [Google Scholar]
- 14.Somerville TD, Wiseman DH, Spencer GJ, Huang X, Lynch JT, Leong HS, et al. Frequent derepression of the mesenchymal transcription factor gene FOXC1 in acute myeloid leukemia. Cancer Cell. 2015;28:329–42. doi: 10.1016/j.ccell.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Li L, Liu S, Zhao Y, Wang L, Du G. FOXC1 promotes melanoma by activating MST1R/PI3K/AKT. Oncotarget. 2016;7:84375–87. doi: 10.18632/oncotarget.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Gu F, Liu CY, Wang RJ, Li J, Xu JY. High level of FOXC1 expression is associated with poor prognosis in pancreatic ductal adenocarcinoma. Tumour Biol J Int Soc Oncodev Biol Med. 2013;34:853–8. doi: 10.1007/s13277-012-0617-7. [DOI] [PubMed] [Google Scholar]
- 17.Pan F, Yao J, Chen Y, Zhou C, Geng P, Mao H, et al. A novel long non-coding RNA FOXCUT and mRNA FOXC1 pair promote progression and predict poor prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:2838–49. [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103:1574–80. doi: 10.1111/j.1464-410X.2009.08351.x. [DOI] [PubMed] [Google Scholar]
- 19.Sonne SB, Yadav R, Yin G, Dalgaard MD, Myrmel LS, Gupta R, et al. Obesity is associated with depot-specific alterations in adipocyte DNA methylation and gene expression. Adipocyte. 2017;6:124–33. doi: 10.1080/21623945.2017.1320002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasudevan D, Hickok JR, Bovee RC, Pham V, Mantell LL, Bahroos N, et al. Nitric oxide regulates gene expression in cancers by controlling histone posttranslational modifications. Cancer Res. 2015;75:5299–308. doi: 10.1158/0008-5472.CAN-15-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gall Trošelj K, Novak Kujundzic R, Ugarkovic D. Polycomb repressive complex’s evolutionary conserved function: the role of EZH2 status and cellular background. Clin Epigenetics. 2016;8:55. doi: 10.1186/s13148-016-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci. 2012;8:59–65. doi: 10.7150/ijbs.8.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Kuiatse I, Lee AV, Pan J, Giuliano A, Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol Cancer Res MCR. 2010;8:266–77. doi: 10.1158/1541-7786.MCR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J Clin Invest. 2011;121:212–25. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Huang SK, Marzese DM, Hsu SC, Kawas NP, Chong KK, et al. Epigenetic changes of EGFR have an important role in BRAF inhibitor-resistant cutaneous melanomas. J Invest Dermatol. 2015;135:532–41. doi: 10.1038/jid.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Baum RP, et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5:187–94. [PMC free article] [PubMed] [Google Scholar]
- 28.Specht E, Kaemmerer D, Sanger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67:368–77. doi: 10.1111/his.12662. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Guan D, Lei L, Lu J, Liu JQ, Yang G, et al. H6, a novel hederagenin derivative, reverses multidrug resistance in vitro and in vivo. Toxicol Appl Pharmacol. 2018;341:98–105. doi: 10.1016/j.taap.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, et al. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17:197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khosravi-Shahi P, Cabezón-Gutiérrez L, Aparicio Salcedo MI. State of art of advanced triple negative breast cancer. Breast J. 2019;25:967–70. doi: 10.1111/tbj.13369. [DOI] [PubMed] [Google Scholar]
- 32.Praestegaard C, Kjaer SK, Andersson M, Steding-Jensen M, Frederiksen K, Mellemkjaer L. Risk of skin cancer following tamoxifen treatment in more than 16,000 breast cancer patients: a cohort study. Breast Cancer. 2016;23:908–16. doi: 10.1007/s12282-015-0660-5. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Lim W, You D, Jeong Y, Kim S, Lee JE, et al. Chemoresistance in the human triple-negative breast cancer cell line MDA-MB-231 induced by doxorubicin gradient is associated with epigenetic alterations in histone deacetylase. J Oncol. 2019;2019:1345026. doi: 10.1155/2019/1345026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wein L, Loi S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC) Breast. 2017;34(Suppl 1):S27–30. doi: 10.1016/j.breast.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Xu Y, Li L, Wang L, Yao R, Sun Q, et al. FOXC1 is associated with estrogen receptor alpha and affects sensitivity of tamoxifen treatment in breast cancer. Cancer Med. 2017;6:275–87. doi: 10.1002/cam4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang L, Jin J, Xu K, Wang X, Tang J, Guan X. SOX9 interacts with FOXC1 to activate MYC and regulate CDK7 inhibitor sensitivity in triple-negative breast cancer. Oncogenesis. 2020;9:47. doi: 10.1038/s41389-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Huang H, Li M, Zhang X, Liu Y, Wang Y. MicroRNA-374c-5p regulates the invasion and migration of cervical cancer by acting on the Foxc1/snail pathway. Biomed Pharmacother. 2017;94:1038–47. doi: 10.1016/j.biopha.2017.07.150. [DOI] [PubMed] [Google Scholar]
- 38.Han B, Qu Y, Jin Y, Yu Y, Deng N, Wawrowsky K, et al. FOXC1 activates smoothened-independent hedgehog signaling in basal-like breast cancer. Cell Rep. 2015;13:1046–58. doi: 10.1016/j.celrep.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.