Abstract
Background
Clinical trials are a critical source of evidence for oncology care, yet very few patients participate. Among healthcare providers, nurses spend the most time with cancer patients and are the most highly trusted professionals. We developed and evaluated an educational program for oncology nurses targeting knowledge, attitudes, self-efficacy and perceived norms to facilitate discussion about clinical trials and support patient decision making.
Methods
A nationwide sample of oncology nurses were randomly assigned to receive general clinical trials education delivered as text (attention control) vs. tailored video vignettes (intervention) in a web-based continuing education program. Participants completed a baseline assessment and follow up assessments immediately after the educational program and three months later. The primary outcome was intention to discuss clinical trials with patients. Secondary outcomes were knowledge and attitudes about clinical trials, self-efficacy, and perceived norms.
Results
1393 nurses enrolled and completed the educational program and post-intervention assessment (720 control, 673 video). Both text education and tailored video education increased intention to discuss clinical trials with patients, with a greater effect in the video group (p < .0001). Likewise, knowledge, attitudes, perceived behavioral control, and perceived norms were all improved with education in both groups, and the magnitude of benefit was greater (p < .001) for the video group in all outcomes except knowledge.
Conclusion
A one-time online educational program for oncology nurses improves knowledge, attitudes, self-efficacy and intention to engage patients in discussions about clinical trials. A tailored video format was associated with a greater effect than standard text only material.
Keywords: Nurse education, Clinical trials, Communication
1. Introduction
Clinical trials are an essential source of evidence for new approaches to cancer prevention and treatment. Despite the support of government, industry, professional organizations, and patient advocacy groups, participation in clinical research studies remains poor, with estimates that less than 10% of cancer patients take part in therapeutic clinical trials [[1], [2], [3], [4]]. Numerous barriers to patient participation in research studies have been described, including practical impediments that limit access, lack of knowledge, financial concerns, and both patient and provider attitudinal barriers [[5], [6], [7]]. Recommendations for overcoming these barriers have been predominantly directed at physicians [[7], [8], [9], [10], [11]], patients [[12], [13], [14]], and communities [[15], [16], [17]]. Unfortunately, intervention studies to date have not been shown to result in measurable improvement in clinical trial participation at a national level.
Interventions to overcome clinical trial barriers that target oncology care providers other than physicians have not been fully explored. Oncology nurses share a therapeutic relationship with their patients and are estimated to have twice as much contact with patients as their physician counterparts [18,19]. The importance of the role of the clinical trial nurse has been recognized, but there has been less attention to the potential influence of staff nurses and their influence on patient decision making about clinical trials. Given the close involvement of oncology nurses in educating patients and supporting patient decision making about care and treatment [[20], [21], [22]], developing interventions targeting nurses represents an opportunity to further promote trial enrollment.
We previously explored attitudes of non-physician healthcare workers, including nurses, towards clinical trials in two studies. In the first study of 250 nurses, participants generally recognized the importance of clinical trials and benefits to patients; however, attitudes concerning patient participation in trials differed by work setting [23]. In the second small pilot study we found a strong correlation between nurses’ confidence or self-efficacy in talking with patients about trials and their intention to hold these discussions [24]. In preparation for the current study, we conducted in-depth qualitative interviews with 33 nurses regarding their experiences in discussing trials with patients and their identification of barriers to these discussions [25]. In addition to lack of knowledge about trials being a barrier, nurses again described a lack of confidence as an impediment to raising the subject of trial participation. Other challenges included concerns about having the needed skill to effectively communicate in various patient situations and lack of clear norms regarding their role. Based on this work we developed a theory-driven educational program to improve oncology nurse engagement in patient decision making about clinical trials.
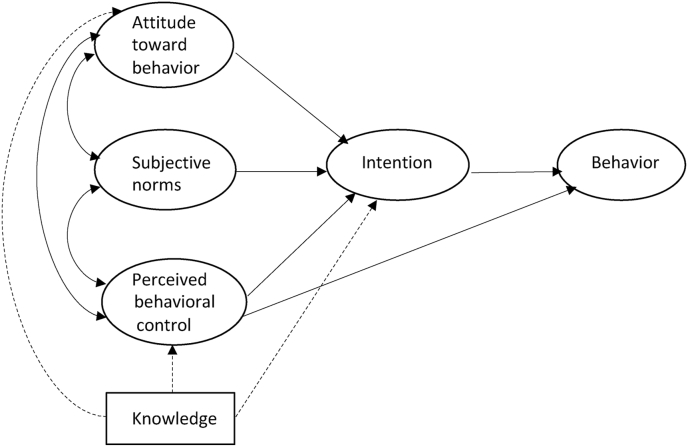
Our previous work with cancer patients demonstrated that an internet-based tailored educational intervention using brief video clips improves knowledge, attitudes and preparation for decision making about clinical trials [14,27]. Guided by the Theory of Planned Behavior [28], we extended this work to develop and test an interactive web-based educational program (Oncology Nurse IMPACT: Improving Communication with Patients about Clinical Trial - NCT02129517) to address nurse clinical trials knowledge, attitudes, subjective normative beliefs, and perceived behavioral control (barriers). Our primary aim and underlying hypothesis was that providing tailored information and skills training in an interactive format that addresses specific nurse barriers would increase nurse intention to discuss clinical trial participation with patients in appropriate clinical settings, when compared with generic print resources. The secondary aim was to assess the impact of the educational program on knowledge, attitudes, subjective normative beliefs, and perceived behavioral control (Fig. 1).
Fig. 1.
Theory of planned behavior model*.
*Azjen's Theory of Planned Behavior is represented in solid lines and the additional relationship of knowledge, tested in this study, is represented in dash lines.
2. Methods
2.1. Participant selection
We conducted a prospective randomized controlled clinical trial that compared the IMPACT educational program to an attention control condition in a nationwide (U.S.) sample of oncology nurses. All eligible members of the Oncology Nursing Society were invited to participate. The Oncology Nursing Society (ONS) is a professional organization of more than 30,000 registered nurses and other healthcare professionals dedicated to excellence in patient care, education, research, and administration in oncology nursing.
Eligibility for IMPACT was based on self-reported demographics in the ONS membership database. Inclusion criteria were: a) currently practicing registered nurse; b) involved in direct patient care; c) self-identify in one of the following primary practice roles: case managers, clinical nurse specialists, nurse practitioners, managers/coordinators, nurse navigators, patient educators, and staff nurses; d) available email address. Research nurses, nurse educators and administrative nurse managers/directors without direct patient care were excluded. Among the ONS membership, 18,995 nurses fulfilled the eligibility criteria for this study.
Potential participants were sent an invitation to participate by US Postal Service, and subsequently received an email invitation with link to the IMPACT website. Nurses were offered Continuing Education credit for participation, $40 gift card, and were entered into a lottery to win an IPAD. All data collection procedures for this study were approved by the Institutional Review Board of University Hospitals Cleveland Medical Center.
2.2. Intervention
IMPACT (video) Arm: Oncology Nurse IMPACT is a theory-driven web-based educational program that delivered information about clinical trials in a series of 2–3 min video scenarios to oncology nurses. The videos addressed essential knowledge of clinical trials as well as potential barriers to discussing trials with patients, identified in our previous work [[23], [24], [25]]. The videos addressed knowledge gaps as well as positive and negative attitudes and beliefs about research in general and clinical trials specifically. For those assigned to the video arm, the selection of educational videos was tailored to each nurse's individual perception of barriers to having such discussions with patients, identified through his/her responses to the baseline survey of knowledge, attitudes, subjective norms, and perceived behavioral control. For example, if the individual indicated on the knowledge survey a lack of understanding of the phases of trials, he/she would be assigned to view the video of a clinician who explained the differences among the phases. If the attitude survey indicated a concern about whether raising the subject would be helpful patients, a video showing one nurse reassuring another that simply offering factual information is generally a benefit would be assigned for viewing. All videos employed scripted vignettes with professional actors. The educational program provided 20–30 min of video content, after which nurses were able to view the entire video library of 17 videos if they wished.
The development of the educational program was based on the Theory of Planned Behavior (TPB) [28] and prior research identifying barrier to clinical trial discussions [14,[24], [25], [26], [27]]. The TPB posits that behavior is a function of intention, which in turn is influenced by attitudes, subjective beliefs about relevant norms, and perceptions about one's ability to determine or control one's behavior (self-efficacy). Because understanding of the conduct, regulatory structure, and various aspects of trials would be required for holding discussions with patients, we added knowledge as a likely influential component to the study model (Fig. 1).
Attention Control Arm: Those randomized to the control condition received on-line untailored text, adapted from the National Cancer Institute website addressing the same general topics as the video arm (phases of trials, informed consent, risks, placebo, human subjects protections, etc.). The text was presented on-line, but subjects were able to print the text if they preferred reading it on paper.
2.3. Measures
Participants completed a baseline survey, a post-intervention follow up survey immediately after viewing the videos or reading the text (IMPACT vs. Control), and a three-month follow up survey. Demographics included age, gender, degree(s) and work setting (e.g. community outpatient, radiation, in-patient academic setting, etc.). Grounded in the TPB, surveys were adapted from prior studies assessing patient barriers to clinical trial accrual [14] and the qualitative interviews with oncology nurses that identified their perceptions and barriers to discussing clinical trials in various settings [25].
In addition to 21 knowledge questions (true/false/don't know), three main concepts were measured: attitudes, perceived behavioral control and subjective norms. Items to assess attitudes towards discussing clinical trials were modified from the work of Francis and colleagues [29], tailored for this study. A total of 21 items assessed attitudes and all had a 7 point Likert response format of strongly disagree to strongly agree. Item content included attitudes about practical issues, such as “If I discuss clinical trials with patients, I will get some of the facts wrong” and potential concerns about the impact on patients, such as “If I bring up clinical trials, patients will think I want them to participate”.
Assessment of perceived behavioral control included 10 items that reflected confidence about discussing clinical trials with patients. Examples of items that asked directly about perceived control included, “I feel confident that I can explain to patients how clinical trials generally work” and “I know where to direct patients to find out more about clinical trials”. Additional indirect items assessed perception of how difficult each action is with response options of difficult, moderately difficult, slightly difficult, neutral, slightly easy, moderately easy, easy.
Items designed to measure subjective norms used seven normative groups identified as potentially influencing nurse behavior: nurse managers, patients, physicians, work institution, other nurses at work, patients’ families and their professional organization. For each normative group two items were assessed as illustrated by the following example: “My nurse manager thinks I should talk about clinical trials with patients” (7 point Likert response format – strongly disagree to strongly agree), and “Doing what my nurse manager think I should do is …” (7-point response format – Not at all important to extremely important). High scores indicate a strong subjective norm to engage in discussing clinical trials with patients.
All items were initially pilot tested with 112 nurses to assess the general performance of items, including readability, clarity of instructions and response formats, and to assess the range of responses. Psychometric evaluation, using the baseline data, was performed and described in detail in a previous report [30]. Internal consistency reliability of each measure was good (Cronbach's alphas: Attitudes = 0.84, PBC = 0.85, Subjective norm = 0.89).
The two follow up surveys, one on completion of the intervention and one 3 months later, assessed the impact of the educational program on knowledge, attitudes, perceived behavioral control, perceived norms, and intention to discuss clinical trials with patients. These were shorter versions of the baseline surveys. In addition, the three-month follow up asked nurses to recall how often they actually engaged patients in clinical trial discussions in the prior 3 months.
2.4. Statistical plan
Eligible patients who completed the baseline assessment were randomized 1:1 to IMPACT or control, stratified by race (white vs non-white), whether the participant's main work setting was inpatient or outpatient, and whether it was an academic or non-academic setting, using varying block sizes of 2 or 4 within strata. Computer-generated randomization lists were prepared by the study statistician and were concealed by using the web interface to perform randomization.
The target sample size for the trial was based on the primary outcome, intention to discuss clinical trials at the post-intervention assessment. Assuming a standard deviation of 6.8 based on pilot data, samples of 433 per group with post-intervention assessment data were needed to provide 90% power to detect a mean difference of 1.5 standard deviation (effect size 0.22), using a 2-sided test at significance level 0.05. Allowing for 15% dropout and rounding up, the target enrollment was set at 515 randomized nurses per group [[31], [32], [33]].
Comparison of outcomes at post-intervention, and at the 3-month assessment used all available cases with the outcome measured at that time point. As a sensitivity analysis, multiple imputation [34] was used to compare intention-to-treat populations consisting of all 1964 randomized participants. Comparability of groups in terms of demographic and clinical characteristics was assessed at baseline, post-intervention, and 3-month follow-up. Measures reported as individual Likert scale items or sums of Likert scale items were analyzed as continuous variables. At each follow-up time, each outcome was compared between groups using analysis of covariance for continuous outcomes, or ordinal logistic regression for ordinal categorical variables, where the model also adjusted for randomization factors, age, education, access to clinical trials, years spent in oncology nursing, and the baseline score of the measure being analyzed. These covariates, pre-specified in the protocol, represented factors expected to be influential. Within-group and between group effect sizes are reported as adjusted mean changes or differences in adjusted means, divided by the baseline standard deviation of the measure. Effect sizes greater than 0.30 were considered clinically significant [33,34]. All reported p-values are two-sided. Statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
Letters were mailed and emails sent to all eligible ONS members (18,995). A total of 2650 oncology nurses registered on the IMPACT website, and of those 2448 consented to participate. Of the 2180 who consented and were eligible, 1964 (9.5% of eligible population) completed the baseline survey and underwent random assignment, 983 to the control condition and 981 to the video arm (Table 1). Ninety-seven percent were female, 87% white, median age was 46 years, and the median duration of oncology nursing was 15 years. Fifty eight percent reported practicing in non-academic settings, and 75% were outpatient nurses. Randomized groups were well balanced with respect to baseline characteristics.
Table 1.
Demographic and clinical characteristics of nurses who completed baseline assessment.
| Variable | Control (N = 983) |
IMPACT Video (N = 981) |
Total (N = 1964) |
p-value* |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age (Mean ± SD) | 719 (46.93 ± 11.33) | 672 (46.50 ± 11.29) | 1391 (46.72 ± 11.31) | 0.48 |
| Sex | ||||
| Male | 26 (3.6) | 10 (1.5) | 36 (2.6) | 0.01 |
| Female | 694 (96.4) | 663 (98.5) | 1357 (97.4) | |
| Race | ||||
| White | 633 (88.0) | 600 (89.4) | 1233 (88.7) | 0.41 |
| Non-White | 86 (12.0) | 71 (10.6) | 157 (11.3) | |
| Hispanic/Latino | ||||
| No | 697 (96.9) | 646 (96.1) | 1343 (96.6) | 0.41 |
| Yes | 22 (3.1) | 26 (3.9) | 48 (3.5) | |
| Highest Nursing Degree | ||||
| Diploma/Associate | 146 (20.3) | 122 (18.1) | 268 (19.2) | 0.57 |
| Bachelor's | 382 (53.1) | 371 (55.1) | 753 (54.1) | |
| Master's/DNP/Ph.D. | 192 (26.7) | 180 (26.8) | 372 (26.7) | |
| Employment | ||||
| Full time | 562 (78.1) | 547 (81.3) | 1109 (79.6) | 0.14 |
| Part time | 158 (21.9) | 126 (18.7) | 284 (20.4) | |
| Direct Care of Patients | ||||
| 1–50% | 89 (12.4) | 95 (14.1) | 184 (13.2) | 0.33 |
| 51–100% | 631 (87.6) | 578 (85.9) | 1209 (86.8) | |
| Primary Position | ||||
| Staff nurse/nurse clinician | 465 (64.6) | 431 (64.0) | 896 (64.3) | 0.98 |
| Nurse practitioner | 93 (12.9) | 89 (13.2) | 182 (13.1) | |
| Othera | 162 (22.5) | 153 (22.7) | 315 (22.6) | |
| Primary Work Setting 1 | ||||
| Academic | 300 (41.7) | 287 (42.6) | 587 (42.1) | 0.72 |
| Non-academic | 420 (58.3) | 386 (57.4) | 806 (57.9) | |
| Primary Work Setting 2 | ||||
| Inpatient | 176 (24.4) | 166 (24.7) | 342 (24.6) | 0.92 |
| Outpatient | 544 (75.6) | 507 (75.3) | 1051 (75.5) | |
| Years of Experience | ||||
| Nursing (Mean ± SD) | 719 (20.96 ± 12.04) | 673 (20.58 ± 11.70) | 1392 (20.57 ± 11.87) | 0.98 |
| Oncology nursing (Mean ± SD) | 717 (15.72 ± 10.03) | 670 (15.09 ± 9.96) | 1387 (15.41 ± 10.00) | 0.24 |
| Familiarity with CTs | ||||
| Not at all familiar | 40 (5.6) | 36 (5.4) | 76 (5.5) | 0.71 |
| Somewhat familiar | 435 (60.4) | 394 (58.5) | 829 (59.5) | |
| Very familiar | 192 (26.7) | 198 (29.4) | 390 (28.0) | |
| Extremely familiar | 53 (7.4) | 45 (6.7) | 98 (7.0) | |
| CTs offered at work place? | ||||
| No | 114 (15.8) | 96 (4.8) | 210 (15.1) | 0.21 |
| Yes | 584 (81.1) | 545 (81.0) | 1129 (81.1) | |
| Don't know | 22 (3.1) | 32 (4.8) | 54 (3.9) | |
*p-values from Chi square/Fisher's exact tests for categorical variables, from two sample t-test for continuous variables.
2Other in primary specialty includes palliative/support care, prevention/detection, surgical oncology, survivorship and other.
Other in primary position includes case manager, clinical nurse specialist, consultant, director/manager/coordinator, genetic counselor, patient educator, staff educator, and other (patient care role).
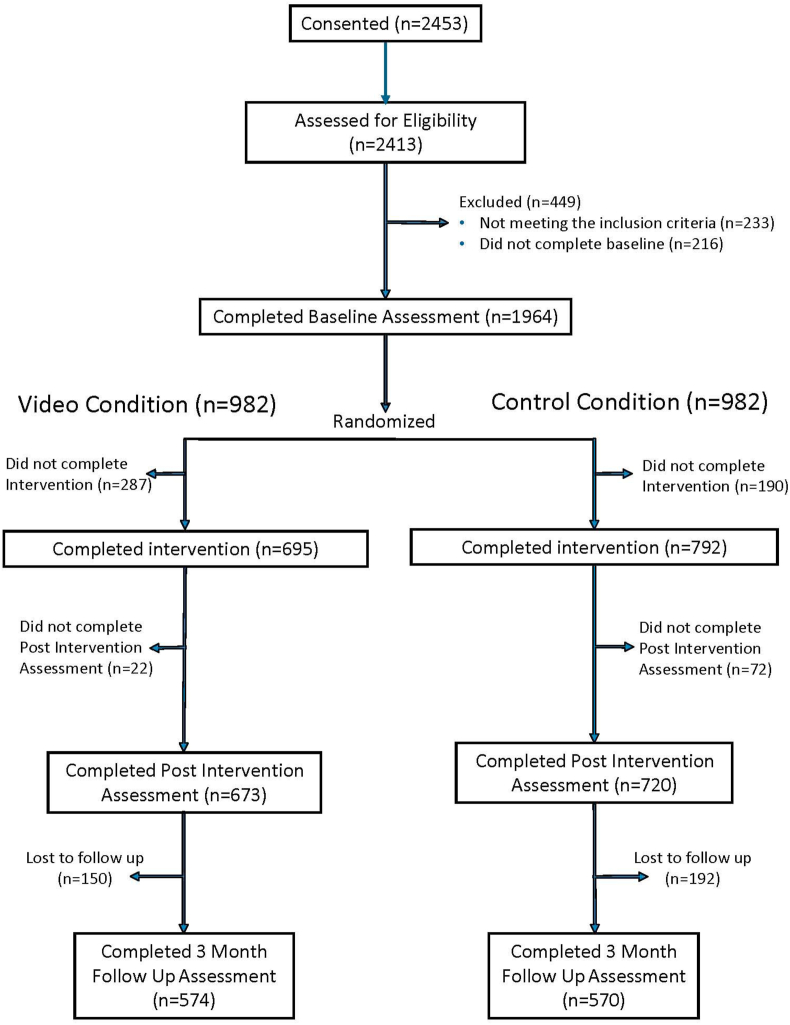
As shown in the CONSORT diagram (Fig. 2), a total of 1488 nurses completed the intervention (793 control, 695 video), and 1393 completed the post-intervention assessment. The 3-month survey was completed by 1161 (78%) participants (576 control, 585 video). Among those completing the post-intervention survey the only significant difference in baseline characteristics between arms was a smaller proportion of males (1.5% vs 3.6%, p = .013) and a smaller proportion having institutions with no one to screen for CT's in the video group compared to controls (10.3% vs. 13.8%, p = .045). Among those completing the 3 month survey, the IMPACT group had a lower percent of males (p = .035) and a lower percent with screening patients for clinical trials (p = .05).
Fig. 2.
CONSORT diagram. Improving Communication with Patients about Clinical Trials.
3.1. Intervention effects
Primary outcome: For the primary outcome of “intention to discuss clinical trials in the next 3 months” both the control and video (IMPACT) educational interventions were associated with substantial increases from baseline to completion of the intervention with effect sizes (ES) 0.47 (control) and 0.72 (video), respectively (Table 2). Compared to controls, the video arm showed larger increases in intention from baseline (difference = 0.46, 95% CI (0.34, 0.58), p < .0001, ES = 0.25).
Table 2.
Comparison of control and IMPACT video groups: Changes in outcomes baseline to post-intervention.
| Controla |
IMPACT Videoa |
Comparison of Video and Controlb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Mean (SD) | 95% CI | Effect sizec | Mean (SD) | 95% CI | Effect sizec | Difference in Mean Change (95% CI) | p-value | Effectsizec | |
| In the next 3 months, I intend to discuss CTs with patients | Pre | 4.27 (1.86) | 4.35 (1.85) | |||||||
| Post | 5.17 (1.49) | 5.66 (1.38) | ||||||||
| Change | 0.89 (1.51) | (0.79, 1.00) | 0.47 | 1.31 (1.66) | (1.18, 1.43) | 0.72 | 0.46 (0.34, 0.58) | <.0001 | 0.25 | |
| Total Intention Score | Pre | 10.14 (8.36) | 10.15 (8.27) | |||||||
| Post | 14.28 (8.94) | 17.14 (8.44) | ||||||||
| Change | 4.14 (6.10) | (3.70, 4.59) | 0.50 | 6.86 (7.53) | (6.27, 7.44) | 0.83 | 2.79 (2.11, 3.47) | <.0001 | 0.34 | |
| Knowledge Score | Pre | 14.80 (3.05) | 14.77 (3.18) | |||||||
| Post | 17.63 (2.25) | 17.33 (2.32) | ||||||||
| Change | 2.81 (3.01) | (2.59, 3.03) | 0.90 | 2.56 (3.13) | (2.32, 2.79) | 0.81 | −0.30 (−0.52, −0.08) | 0.007 | −0.10 | |
| Attitude Score | Pre | 101.7 (15.1) | 102.5 (16.1) | |||||||
| Post | 108.7 (15.1) | 115.9 (16.1) | ||||||||
| Change | 6.9 (12.0) | (6.0, 7.8) | 0.43 | 13.3 (14.8) | (12.2, 14.4) | 0.86 | 6.7 (5.5,8.0) | <.0001 | 0.43 | |
| Indirect Subjective Norms Score | Pre | 144.6 (64.5) | 149.6 (67.2) | |||||||
| Post | 160.4 (69.0) | 176.8 (67.5) | ||||||||
| Change | 15.9 (40.8) | (12.9, 18.9) | 0.23 | 27.7 (48.3) | (24.1, 31.4) | 0.43 | 13.0 (8.5, 17.6) | <.0001 | 0.20 | |
| Direct Perceived Behavioral Control Score | Pre | 17.49 (5.31) | 17.22 (5.33) | |||||||
| Post | 19.62 (4.65) | 21.26 (4.34) | ||||||||
| Change | 2.09 (3.60) | (1.82, 2.35) | 0.41 | 4.03 (4.59) | (3.69, 4.38) | 0.74 | 1.80 (1.44, 2.16) | <.0001 | 0.34 | |
| Indirect Perceived Behavioral Control Score | Pre | 151.9 (63.2) | 156.9 (65.6) | |||||||
| Post | 187.1 (59.7) | 203.0 (60.4) | ||||||||
| Change | 35.6 (43.4) | (32.4, 38.8) | 0.54 | 46.3 (51.3) | (42.4, 50.2) | 0.73 | 12.8 (8.3, 17.2) | <.0001 | 0.20 | |
Control group N = 716–788 due to missing values; Video group N = 673–690 due to missing values.
Comparison of video and control is from an analysis of covariance adjusting for age, race, nursing education, CTs available at work, years of oncology nursing, primary work setting (academic/non-academic), primary work setting2 (Inpatients/Outpatients), and baseline score of measure.
Effect sizes calculated as adjusted mean or difference in adjusted means from analysis of covariance, divided by baseline standard deviation of measure.
At three months, both arms sustained their increased intention to discuss clinical trials with patients compared to baseline, with the video group again showing a greater impact of the educational program (p = .0006). Both study groups also reported an increase in the number of patients with whom the nurses recalled discussing clinical trials over the previous 3 months. However, there was no difference between arms (Table 3).
Table 3.
Comparison of Control and IMPACT Video Groups: Number of patients discussed CT's with in the last 3 months.
| Control |
Video |
Comparison of Video vs. Controla |
|||||
|---|---|---|---|---|---|---|---|
| Pre (N, %) | 3 Month (N, %) | Pre (N, %) | 3 Month (N, %) | OR (95% CI) | p-value | ||
| Approx. how many patients discussed CT's with in past 3 months | 0 | 166 (28.2) | 76 (13.2) | 148 (25.1) | 61 (10.4) | 0.92 (0.74, 1.15) | 0.45 |
| 1–5 | 275 (46.8) | 283 (49.1) | 265 (44.9) | 287 (49.0) | |||
| 6–10 | 65 (11.1) | 107 (18.5) | 84 (14.2) | 114 (19.5) | |||
| 11–15 | 35 (6.0) | 54 (9.4) | 31 (5.3) | 54 (9.2) | |||
| 16–20 | 20 (3.4) | 29 (5.0) | 23 (3.9) | 28 (4.8) | |||
| >20 | 27 (4.69) | 28 (4.9) | 39 (6.6) | 42 (7.2) | |||
| Total | 588 (100.0) | 577 (100.0) | 590 (100.0) | 586 (100.0) | |||
Odds ratio (OR) and p-value are from an ordinal logistic regression model of 3-month response, adjusting for study group, age, race, nursing education, CTs available at work, years of oncology nursing, primary work setting academic or non-academic, primary work setting inpatient or outpatient, baseline value of response. An OR<1 indicates that the number of patients with whom clinical trials were discussed or who enrolled in clinical trials is higher in the video compared to the control group.
Secondary outcome: Both groups showed clinically meaningful improvement in knowledge, attitudes, and self-efficacy. While subjective norms scores showed statistically significant improvements in both groups, the magnitude only met our predefined threshold of clinical significance (i.e. ES ≥ 0.30) in the video group. Across all measures, the IMPACT video intervention was more effective than the attention control text, except for clinical trials knowledge, where the effect size difference of 0.1 favored the control group. Clinically significant effect sizes for the between-group comparison (ES ≥ 0.30) were seen for the total intention score, total indirect attitude score, and the direct perceived behavioral control score.
4. Discussion
This randomized clinical trial demonstrated that IMPACT, an online educational program, resulted in higher oncology nurse intention to discuss clinical trials with patients and an increase in self-reported discussions about clinical trials as compared to a text-based educational intervention. IMPACT resulted in significantly greater increases in attitudes about clinical trials, perceived confidence and ability to have discussions about clinical trials and perceived behavioral norms surrounding such discussions. To our knowledge, this is the largest study to date of an evaluation of an educational program focused on oncology nurses to foster discussions about clinical trials with patients.
The Oncology Nursing Society offers numerous resources to assist nurses in fulfilling their responsibilities in caring for patients considering clinical trial participation [35]. However, we found that even at an NCI-designated comprehensive cancer center, nurses share many of the attitudinal barriers of patients regarding clinical trials [24]. Furthermore, as suggested in our earlier qualitative study of nurses [25], our results confirmed that nurses often do not feel equipped with the tools to discuss clinical trials with patients even in general terms. These barriers include knowledge gaps and attitudes about clinical trials, lack of self-efficacy, and a perception that such discussions are outside of the scope of their role. Thus, we hypothesized that an educational program that specifically targets these barriers with factual content and video role modeling of communication about clinical trials could increase the likelihood that nurses will address clinical trials with patients, ultimately impacting patient decisions regarding clinical trial participation.
It is notable that a clinically and statistically significant impact on educational outcomes was achieved in both arms of our study. Our pragmatic study design allowed us to compare the tailored video approach to provision of readily available information in plain text format from the NCI website. The advantage of this study design is the measure of added impact of the tailored video format rather than merely demonstrating a comparison to no education. The video intervention differed from the control in that video content was tailored based on individual nurse survey responses, and included vignettes that combined didactic information and passive skills training through examples of how to navigate various common interactions with patients, nurses and physicians. Consistent with prior research that supports an interactive, video-based approach to education [14], we found that the tailored intervention had greater impact on nearly all study endpoints, with the exception of knowledge. This suggests that standard text may be adequate to deliver pure factual information to nursing professionals but is less effective regarding desired affective or behavioral outcomes.
Our study was not designed to differentiate between the effect of presenting material in a standardized video format versus tailoring the videos according to individual survey responses. It is possible that the videos were simply more engaging than printed text. However, our previous research demonstrated that individual characteristics play an important role in attitudes and likelihood of engaging in the desired behavior (discussing trials with patients).
Several other researchers have confirmed the importance of directly addressing belief and attitudinal barriers in promoting clinical trials discussions. For example, Ulrich, Zhou, Ratliffe, et al. found that primary care nurse practitioners who expressed comfort with discussing treatment options were more likely to recruit patients in to trials than those who reported discomfort with such discussions [36]. Getz, in a large study of 1255 nurses from all specialties, identified the perception of lack of knowledge, lack of time, and lack of access to trial information as common barriers to referring patients for trial enrollment [37]. The video library we developed included specific videos addressing these issues, including one with a nurse expressing lack of information she could share with a patient and a more senior nurse providing her with the website listing relevant trials and brochures describing trials at that center. Another portrayed nurses preparing medications and talking about lack of time as a barrier to talking about trials with patients; again a more experienced nurse in the video suggested she bring up the subject while performing other tasks, such as changing a central line dressing, thus providing a strategy for accomplishing the desired behavior.
Given the findings our previous findings of the strong supporting role of self-efficacy, we intentionally scripted all videos to convey the message that the nurse was not expected to be able to discuss details of every trial, nor to encourage or convince patients to enroll. Rather, the videos were designed to demonstrate ways in which the nurse could assure that patients had the opportunity to talk about the option of trials, to educate about research in general, and to clarify common misconceptions, such as the concern that placebos are always used – all behaviors that are common aspects of patient education. Similarly, in light of barriers related to uncertainty about the role of the nurse, a video of an officer of the Oncology Nursing Society discussing and reinforcing the importance of clinical trials in cancer care and the essential participation of nurses in educating patients and supporting their access to trials was included in each participant's collection of videos.
Several limitations of this research should be noted. The response rate of nurses to our invitation was very low, and therefore the final sample may not be representative of all oncology nurses. Furthermore, the proportion of male nurses and non-white nurses was low. The sample was representative of the demographic characteristics of the ONS membership, but we could not explore subgroup analyses of the impact of specific demographics on program outcomes. Our comparison of clinical characteristics between nurses who completed and did not complete follow up surveys showed that these groups were largely similar, although there is a possibility that they differed systematically in ways that we did not measure. Finally, this study was not designed to demonstrate an impact of our program on clinical trial accrual. This was not possible given the nationwide participant sample and our inability to track actual numbers of patients enrolled in clinical trials.
In summary, the IMPACT educational program met its primary goal of improving nurse intention to discuss clinical trials with patients, and secondary outcomes supported the proposed underlying behavioral model. Given the complexity of clinical trial decision making, it is likely that multifaceted efforts will be required to impact clinical trial accrual in a meaningful way. In light of the trust patients have in nurses and the amount of time nurses spend with patients, this study suggests that oncology nurse interventions should be included in approaches to improve cancer patient participation in research.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA177574 (Meropol, Daly). The Clinical Trials Registration Number is NCT02129517. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Meropol is an employee of Flatiron Health, Inc., an independent subsidiary of the Roche Group. NJM holds equity interest in Flatiron, and stock in Roche.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Meropol is an employee of Flatiron Health, Inc., an independent subsidiary of the Roche Group. NJM holds equity interest in Flatiron, and stock in Roche.
References
- 1.Go R.S., Frisby K.A., Lee J.A., Mathiason M.A., Meyer C.M., Ostern J.L. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106(2):426–433. doi: 10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- 2.Jiminez R., Zhang B., Jaffe S., Nillson M., Rivera L., Muchler J., Lathan C., Paulk M.E., Prigerson H.G. Clinical trial participation among ethnic/racial minority and majority patients with advanced cancer: what factors most influence recruitment? J. Palliat. Med. 2013;16(3):256–262. doi: 10.1089/jpm.2012.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger J.M., Cook E., Tai E., Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and stratgies. ASCO Educ Book. 2016;36:185–198. doi: 10.14694/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treweek S., Lockhart P., Pitkethly M. Methods to improve recruitment to randomized clinical trials: cochrane systematic review and meta-analysis. BMJ Open. 2013:1–24. doi: 10.1136/bmjopen-2012-002360. http://bmjopen.bmj.com 3e002360. Accessed March 11, 2021 at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong Y.-N., Schluchter M.D., AlbrechtTL, Benson A.B., Buzaglo J., Collins M., Lederman A. Financial concerns about participation in clinical trials among patients with cancer. J. Clin. Oncol. 2016;34(5):479–487. doi: 10.1200/JCO.2015.63.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physical patient barriers to cancer clinical trial participation. J. Natl. Cancer Inst. 2019;111:245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nipp R.D., Hong K., Paskett E.D. Overcoming barriers to clinical trial enrollment. ASCO Educ Book. 2019;39:105–111. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
- 8.Moffitt K., Brogan F., Brown C., Kasper M., Rosenblatt J., Smallridge R. Statewide cancer clinical trial navigation service. J. Oncol. Pract. 2010;6(3):127–132. doi: 10.1200/JOP.200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baer A.R., Michaels M., Good M.J., Schapira L. Engaging referring physicians in the clinical trial process. J. Oncol. Pract. 2012;8(1):e8–e10. doi: 10.1200/JOP.2011.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klabunde C.N., Keating N.L., Ambs A., He Y., Potosky A.L., Hornbrook M.C. A population-based assessment of specialty physician involvement in cancer clinical trials. J. Natl. Cancer Inst. 2011;103(5):384–397. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massett H.A., Parreco L.K., Padberg R.M., Richmond E.S., Rienzo M.E., Leonard C.E.R. AccrualNet: addressing low accrual via a knowledge-based, community of practice platform. J. Oncol. Pract. 2011;7(6):e32–e39. doi: 10.1200/JOP.2011.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter M., Ramaswamy B., Beisler K., Neki P., Single N., Thomas J. A comprehensive program for the enhancement of accrual to clinical trials. Ann. Surg Oncol. 2016;23(7):2146–2152. doi: 10.1245/s10434-016-5091-9. [DOI] [PubMed] [Google Scholar]
- 13.Umutyan A., Chiechi C., Beckett L.A., Paterniti D.A., Turrell C., Gandara D.R. Overcoming barriers to cancer clinical trial accrual. Cancer. 2008;112(1):212–219. doi: 10.1002/cncr.23170. [DOI] [PubMed] [Google Scholar]
- 14.Meropol N.J., Wong Y.-N., Albrecht T., Manne S., Miller S.M., Flamm A.L. Randomized trial of a web-based intervention to address barriers to clinical trials. J. Clin. Oncol. 2016;34(5):469–478. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifer S.D., Michaels M., Collins S. Applying community-based participatory research principles and approaches in clinical trials: forging a new model for cancer clinical research. Prog. Community Health Partnerships. 2010;4(1):37–46. doi: 10.1353/cpr.0.0103. [DOI] [PubMed] [Google Scholar]
- 16.Schutt R.K., Schapira L., Maniates J., Santiccioli J., Henlon S., Bigby J. Community health workers' support for cancer clinical trials: description and explanation. J. Community Health. 2010;35(4):417–422. doi: 10.1007/s10900-010-9267-0. [DOI] [PubMed] [Google Scholar]
- 17.Vuong I., Wright J., Nolan M.B., Eggen A., Bailey E., Strickland R., Traynor A., Downs T. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic trials – a narrative review. J. Canc. Educ. 2020;35:841–849. doi: 10.1007/s13187-019-01650-y. [DOI] [PubMed] [Google Scholar]
- 18.Fasola G., Aprile G., Aita M. A model to estimate human resource needs for the treatment of outpatients with cancer. J. Oncol. Pract. 2012;8(1):13–17. doi: 10.1200/JOP.2011.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiteley C., Vaitekunas D. The 2005 Helene Hudson Memorial Lecture: leaving our imprints: an exploration into the nurse-patient relationship. Can. Oncol. Nurs. J. 2006;16(3):5. doi: 10.5737/1181912x163180184. [DOI] [PubMed] [Google Scholar]
- 20.Sadler G.R., Lantz J.M., Fullerton J.T., Dault Y. Nurses' unique roles in randomized clinical trials. J. Prof. Nurs. 1999;15(2):106–115. doi: 10.1016/s8755-7223(99)80081-7. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy M.D., Cannon L., MacDermott M.L. The professional role for nurses in clinical trials. Semin. Oncol. Nurs. 1991;7(4):268–274. doi: 10.1016/0749-2081(91)90065-w. [DOI] [PubMed] [Google Scholar]
- 22.Jezewski M.A., Meeker M.A., Schrader M. Voices of Oncology Nurses: what is needed to assist patients with advance directives. Canc. Nurs. 2003;26(2):105–112. doi: 10.1097/00002820-200304000-00003. 23. Burnett CB, Koczwara B, Pixley L, Blumenson LE, Yi Ting H, Meropol NJ. Nurses' attitudes toward clinical trials at a comprehensive cancer center. Oncol Nurs Forum. 2001;28(7):1187. [DOI] [PubMed] [Google Scholar]
- 23.Burnett C.B., Koczwara B., Pixley L., Blumenson L.E., Yi Ting H., Meropol N.J. Nurses' attitudes toward clinical trials at a comprehensive cancer center. Oncol. Nurs. Forum. 2001;28(7):1187. [PubMed] [Google Scholar]
- 24.Campagnaro E.L., Margevicius S., Daly B.J., Eads J.R., Kinzy T.G., Liu T.M. Health care worker attitudes about clinical trials at a comprehensive cancer center. J. Clin. Oncol. 2013;31(15 suppl) e20633-e. [Google Scholar]
- 25.Flocke S.A., Antognoli E., Daly B.J., Jackson B., Fulton S.E., Liu T.M. The role of oncology nurses in discussing clinical trials. Oncol. Nurs. Forum. 2017;44(5):547–552. doi: 10.1188/17.ONF.547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller S.M., Hudson S.V., Egleston B.L., Manne S., Buzaglo J.S., Devarajan K. The relationships among knowledge, self-efficacy, preparedness, decisional conflict, and decisions to participate in a cancer clinical trial. Psycho Oncol. 2013;22(3):481–489. doi: 10.1002/pon.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American society of clinical oncology. Welcome to PRE-ACT! https://www.cancer.net/research-and-advocacy/clinical-trials/welcome-pre-act
- 28.Ajzen I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991;50(2):179–211. [Google Scholar]
- 29.Francis J., Eccles M.P., Johnston M., Walker A.E., Grimshaw J.M., Foy R. Centre for Health Services Research; 2004. Constructing Questionnaires Based on the Theory of Planned Behaviour: A Manual for Health Services Researchers. Quality of Life and Management of Living Resources. [Google Scholar]
- 30.Flocke SA, Nock NL, Fulton S, Margevicius S, Manne S, Meropol NJ, et al A national study of oncology nurses discussing cancer clinical trials with patients. West. J. Nurs. Res.. 0(0):0193945919829145. [DOI] [PMC free article] [PubMed]
- 31.Farivar S.S., Liu H., Hays R.D. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Rev. Pharmac. Outcomes. 2004;4(5):515–523. doi: 10.1586/14737167.4.5.515. [DOI] [PubMed] [Google Scholar]
- 32.Kraft M.A. Brown University; 2018. Interpreting Effect Sizes of Education Interventions. Working paper. [Google Scholar]
- 33.Durlak J.A. How to select, calculate, and interpret effect Sizes. J. Pediatr. Psychol. 2009;34(9):917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- 34.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 35.Oncology Nursing Society . 2013. Statement on the Scope and Standards of Oncology Nursing Practice: Generalist and Advanced Practice. Pittsburgh, Pennsylvania. [Google Scholar]
- 36.Ulrich C.M., Zhou Q., Ratcliffe S.J., Ye L., Grady C., Watkins-Bruner D. Nurse Practitioners' attitudes about cancer clinical trials and willingness to recommend research participation. Contemp. Clin. Trials. 2012;33:76–84. doi: 10.1016/j.cct.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getz K.A. US physician and nurse proclivity to refer their patients into clinical trials. Ther. Innov. Regul. Sci. 2020;54(2):404–410. doi: 10.1007/s43441-019-00069-3. [DOI] [PubMed] [Google Scholar]