Abstract
The quality of crude palm oil (CPO) must be maintained since it plays an important role in fulfilling the domestic and global needs (food and non-food). The quality of CPO is determined by the color, Free Fatty Acid (FFA) and carotene. This study was aimed at examining the effect of bentonite concentration (1.5, 2.0, 2.5 and 3.0%) and contact time (15, 30, 45 and 60 min) on the quality of crude palm oil. The refining processes of CPO through degumming, bleaching and distillation were carried out before the analysis on color, FFA and carotene was done. The results showed that the effect of bentonite concentration (1.5, 2.0, 2.5 and 3.0%) experienced the highest percentage in reduction at 3.0% (color 88.7%, FFA 2.99% and carotene 56.37%). Meanwhile, the effect of contact time (15, 30, 45 and 60 min) experienced the highest percentage in reduction at 60 min (color 89.58%, FFA 2.38%, carotene 61.32%). The reduction in the CPO's color, FFA and carotene found in this study indicates that bentonite concentration and contact time are proven to be effective methods for CPO refinery based on the standard set by Malaysian Palm Oil Refiners Association. This study also showed that the removal efficiency and adsorption capacity of FFA and carotene were also affected by contact time and bentonite concentration.
Keywords: Bentonite, Crude palm oil, Color, Carotene, Free fatty acid
Bentonite; Crude palm oil; Color; Carotene; Free fatty acid.
1. Introduction
The fact that palm oil is the most-consumed vegetable oil worldwide explains why its role remains significant (Ndé et al., 2019). Crude palm oil (CPO) is a vegetable oil derived from the pulp of oil palm which consists of solid triacylglyceride or triacylglycerol with lipases, FFAs, pigments, phosphatides, parsia glycerides, coloring agents and carotene (Constant et al., 2017; Guliyev et al., 2018; Ifa et al., 2013; Onwuliri et al., 2011). The carotenoids (pigments found in plants) produce the bright yellow and red colors in palm oil (Ali et al., 2014). However, the carotenoids can also reduce the clarity of palm oil and cause damage to occur sooner when the oil is stored (not in use). The process of oxidation and hydrolysis makes it possible for palm oil to have a harmful impact on human health (Tian et al., 2015).
The quality of CPO is determined by the Free Fatty Acid (FFA) content (Azeman et al., 2015; De Almeida et al., 2013; Fatin et al., 2014) and the Deterioration of Bleachability Index (DOBI) (Lin, 2004). Higher rates on FFA content (>3%) can cause rancidity as well as changes in taste and color of the oil (Cowan et al., 2012).
In general, consumers tend to avoid the use of unrefined crude oil due to its unpleasant colors and odors (Guliyev et al., 2018). Therefore, oil refinery is required to achieve the perfect color, soft taste and good oxidation stability before it can be used in a wide variety of purposes (cooking, food manufacturing, chemicals, pharmaceuticals, cosmetics and soap manufacturing) (Ndé et al., 2019; Sampaio et al., 2011). In this case, physical refining and chemical refining are known to be common methods for oil refinery (Azeman et al., 2015; Riyadi et al., 2016).
Many researchers have reported the refining process of vegetable oil through the adsorption. Although the porosity and the adsorption power in the CPO refinery process can be increased with acid-activated bleaching earth (BE) (Guliyev et al., 2018), high decolorization and chlorophyll produced in only 1.0% of acid-activated bleaching earth can cause strong acid and solid waste pollution and, therefore, require extra costs for environmental neutralization (Chakawa et al., 2019). Abdi, Gharachorloo and Ghavami (2021) studied the use of egg shell powder to remove the metals, oxidation products and unwanted pigments of the soybean oil. Although 2.0% of egg shell powder reduced the carotenoid and chlorophyll content in the soybean oil by 82.56% and 46.33%, the adsorbent efficiency remained lower compared to BE. While acid-activated bleaching earth is the most common adsorbent process for the bleaching of vegetable oil, it can also be used to remove free fatty acids (FFA) in the vegetable oil industry (Cren and Meirelles, 2012). This study used bentonite (easily available natural resource) and made the most out of it to study the effect of its concentration and contact time on the CPO's color, FFA and carotene during the bleaching process.
2. Materials and methods
2.1. Research materials
The main materials used in this study are CPO with four different qualities of FFA: CPO-1 (3.44%), CPO-2 (4.57%), CPO-3 (5.22%), CPO-4 (6.47%). While the bentonite used in this study has the following chemical composition (% w/w): SiO2 (55–80), Al2O3 (5–20), Fe2O3 (2–10), MgO (0–8), CaO (0–5), Na2O (0–2), K2O (0–2), its properties are similar to the properties of BE (moisture content 10%, apparent bulk density 550 kg/m3, surface area 200 m2/g) used by previous study (Silva et al., 2013). Moreover, the materials used for the analysis of the CPO's color, FFA and carotene such as H3PO4 p.a, isooctane p.a, sodium hydroxide p.a, ethyl alcohol p.a, isopropyl alcohol p.a, phenol phthalein p.a, potassium hydrogen pthalate p.a, absolute ethanol p.a, silica gel p.a were obtained from E. Merck.
2.2. Research procedure
The refining process of CPO involves three stages (degumming, bleaching and deodorization) (Jumaah et al., 2019). The three stages are elaborated below:
-
1.
Degumming process
Firstly, 100 mL of CPO was heated to 90 °C. Then, 0.05 mL of 85% H3PO4 was added and stirred for 10 min. Palm oil that has gone through the degumming process is called Degummed Palm Oil (DPO).
-
2.
Bleaching process
One-hundred millimeter of DPO was put into a three-neck flask equipped with a thermometer. Various concentrations of bentonite at 1.5, 2.0, 2.5 and 3.0% (w/v) was then added, stirred and heated to 105 °C with various contact time for 15, 30, 45 and 60 min. The mixture was then put into a büchner funnel (pore size 1 mm, 100 circles, diameter 70 mm, Whatman) before it was filtered under vacuum. DPO oil that has gone through the bleaching process is called Bleached Deodorized Palm Oil (BDPO).
-
3.
Distillation process
BDPO was then distilled (the separation of color pigments and fatty acid content) using a flask equipped with a rotary vacuum pump (Model 2XZ-0.5, speed 1.400 rpm, voltage 220V, 50 Hz). A total of 90 ± 1 g of DBPO was put into a three-neck flask and heated to 280 °C ± 1 °C. After the temperature reached 280 °C, the heating mantle was turned off and the sample was waited to drop to 60 °C before the vacuum pump is turned off again and the three-neck flask was released from the appliance. BDPO that has gone through distillation is known as Refined Bleached Deodorized Palm Oil (RBDPO).
The analysis was carried out based on the procedures proposed by AOCS (American Oil Chemists' Society) and PORIM (Palm Oil Research Institute of Malaysia) which have been modified by PT. Tanjung Sarana Lestari (PT. Astra Agro Lestari Tbk). The analysis of raw materials includes color (AOCS Cc 13b-45), FFA (AOCS Ca 5a-40), carotene (PORIM p.2.9) and DOBI (PORIM p.2.9). DOBI analysis was carried out to test the quality of CPO but not for RBDPO. The analysis of color, FFA and carotene was done with RBDPO.
2.3. Determination of FFA levels
The FFA levels were determined by employing titration method as proposed in the AOCS (1990) with some modifications. Two-point-five gram of preheated CPO and RBDPO (around 50 °C) were weighed in a beaker and mixed with 50 mL of ethyl alcohol. The solution was titrated with 0.1026 N sodium hydroxide for neutralization. The percentage of FFA is calculated as palmitic acid, where the molecular weight of FFA is considered to be 256 based on Eq. (1) (Henry, 2011).
| (1) |
V = the amount of used volume of sodium hydroxide (mL)
m = the amount of used molarity solution of sodium hydroxide (M)
w = the amount of used weight (g)
M = molecular weight of FFA (g/mole)
Eq. (1) can be written as Eq. (2) (Ali et al., 2014; Bahadi et al., 2016; Fatin et al., 2014; Hashim et al., 2019).
| (2) |
V = the amount of used volume of sodium hydroxide (mL)
N = the amount of used normality of sodium hydroxide (0.1026 N)
w = the amount of used weight of CPO (g).
2.4. Determination of the DOBI value
The DOBI value was measured using a Spectrophotometer Shimadzu 1240 UV-Vis. The sample of CPO was first weighed as much as 0.1 g into a 25 mL volumetric flask before it was dissolved using 25 mL of isooctane. The cuvette was filled with sample solution (CPO and isooctane) and the absorbance was measured at a wavelength of 269 nm and 446 nm using isooctane blank (Silva et al., 2014). The DOBI value was calculated according to Eq. (3) (Ribeiro et al., 2018; Silva et al., 2014; Soetaredjo et al., 2021).
| (3) |
The DOBI values ranged between 2.5 and 4.0 indicate a good average quality of crude oil. Meanwhile, the values ranged below 2.0 indicate low quality of crude palm oil (difficult to blanch) (Silva et al., 2014).
2.5. Determination of carotene value
Carotene is commonly used as one of useful parameters to test the effectiveness of an adsorbent. The carotene content, expressed as β-carotene, was determined by measuring the absorbance at a wavelength of 446 nm which was homogenized and diluted in 25 mL of isooctane (Silva et al., 2014). The samples of CPO and RBDPO were weighed as much as 0.1 g into a 25 mL volumetric flask and then dissolved using 25 mL of isooctane (until it reached the meniscus line). The cuvette was filled with sample solutions (CPO or RBDPO and isooctane) and the absorbance was measured at a wavelength of 446 nm using Shimadzu 1240 UV-Vis Spectrophotometer and isooctane blank. The carotene values were calculated according to Eq. (4) which is obtained from the previous study (Hashim et al., 2019).
| (4) |
w = sample weight (g).
The value of 383 is the carotene calibration factor at a wavelength of 446 nm.
2.6. Determination of color
Color was determined according to the AOCS Method Cc 13b-45 using a Lovibond Tintometer Color scale at 70 °C. The analysis was performed using 5″¼ (133.4 mm) glass cells. Since the samples of crude palm oil have darker color than the scale of the glass cell, so smaller scale of 1” (25.4 mm) was used to achieve maximum result (Silva et al., 2014).
2.7. Adsorption capacity and removal efficiency for carotene and FFA
The absorption capacity (qe) of carotene and FFA is determined using Eq. (5) (Elabbas et al., 2016; Naat et al., 2021; Putranti et al., 2018).
| (5) |
Where qe is the absorption capacity (mg/g) of carotene and FFA absorbed by bentonite, Co and Ce are the concentration of carotene and FFA (mg/L) in the solution before and after absorption, V is the volume (L) of the solution and m is mass of bentonite used (g) in the study. The removal efficiency (R) was calculated using Eq. (6) (Anang et al., 2020; Elabbas et al., 2016; Naat et al., 2021; Purwasasmita et al., 2015).
| (6) |
3. Results and discussion
The main raw materials used in this study were CPO with four different qualities of FFA and DOBI. The difference in the initial content of each component will affect the adsorption process when it comes into contact with the adsorbent. Before the four types of CPO were refined, they must be characterized with the value of FFA, DOBI, carotene and color. The CPO used during the bleaching process in this study was representative to all categories, from CPO with a low quality (CPO with a DOBI value < 2.31%) (Lin, 2004) to CPO with a high quality (CPO with a DOBI value > 2.5%) (Silva et al., 2014). The results of the characterization are presented in Table 1.
Table 1.
Characterization of CPO's raw materials.
| Parameter | Raw Materials |
|||
|---|---|---|---|---|
| CPO-1 | CPO-2 | CPO-3 | CPO-4 | |
| FFA (%) | 3.44 | 4.57 | 5.22 | 6.47 |
| DOBI | 2.52 | 2.31 | 2.28 | 1.95 |
| Carotene (ppm) | 486 | 471 | 460 | 434 |
| Color, 1” (R/Y) | 24R/24Y | 22R/22Y | 21R/21Y | 20R/20Y |
The DOBI values ranged between 2.5 and 4.0 indicate a good average quality of crude oil. Meanwhile, the values ranged below 2.0 indicate low quality of crude palm oil (difficult to blanch) (Silva et al., 2014). DOBI value with the absorbance ratio at 446 nm–269 nm (carotene measurement) correlates well with color. A low DOBI value on oil indicates a low carotene.
3.1. The effect of contact time on CPO's color
The color of RBDPO is examined using Red (R) and Yellow (Y) readings. The percentage of oil bleaching for each RBDPO sample was calculated and presented in Tables 2 and 3. Overall, the color of CPO decreased after the bleaching process and continued to decrease with increasing contact time and bentonite concentration.
Table 2.
Effect of contact time on CPO's color at bentonite 3.0%.
| Contact time (minutes) | Color (R/Y) |
|||
|---|---|---|---|---|
| CPO-1 | CPO-2 | CPO-3 | CPO-4 | |
| 0 | 24R/24Y |
22R/22Y |
21R/21Y |
20R/20Y |
| Contact time (minutes) | Color (R/Y) |
|||
| RBDPO-1 | RBDPO-2 |
RBDPO-3 |
RBDPO-4 |
|
| 15 | 4.2R/42Y | 5.1R/51Y | 6.4R/64Y | 7.0R/70Y |
| 30 | 3.6R/36Y | 4.6R/46Y | 6.0R/60Y | 6.6R/66Y |
| 45 | 3.1R/31Y | 4.0R/40Y | 5.2R/52Y | 5.9R/59Y |
| 60 | 2.5R/25Y | 3.4R/34Y | 4.5R/45Y | 5.0R/50Y |
Note: R = Red, Y = Yellow.
Table 3.
Effect of bentonite concentration on all CPO with contact time of 30 min.
| Bentonite concentration (%) | Color (R/Y) |
|||
|---|---|---|---|---|
| CPO-1 | CPO-2 | CPO-3 | CPO-4 | |
| 0 |
24R/24Y |
22R/22Y |
21R/21Y |
20R/20Y |
| Bentonite concentration (%) | Color (R/Y) |
|||
| RBDPO-1 |
RBDPO-2 |
RBDPO-3 |
RBDPO-4 |
|
| 1.5 | 4.3R/43Y | 4.8R/48Y | 6.2R/62Y | 6.7R/67Y |
| 2.0 | 3.9R/39Y | 4.3R/43Y | 5.6R/56Y | 6.2R/62Y |
| 2.5 | 3.1R/31Y | 3.7R/37Y | 5.0R/50Y | 5.7R/57Y |
| 3.0 | 2.7R/27Y | 3.1R/31Y | 4.5R/45Y | 5.1R/51Y |
Note: R = Red, Y = Yellow.
In Table 2, it is shown that the color begins to decrease at 15 min and continued to decrease with increasing contact time in all CPO samples. In general, the longer the contact time between bentonite and CPO, the greater the chance for carotene to be absorbed in the pores of the bentonite. Longer contact time can increase the mass transfer rate through the particulate pores of the bentonite which causes a reduced red color for all types of CPO. The highest percentage of the contact time effect on the decrease of value and color was at 60 min (89.58%). This trend is in line with Kumar et al. (2010) report on the effect of contact time to remove Congo red by cashew nut shells. Similar conditions also occurred in the samples of CPO 2, 3 and 4.
3.2. The effect of bentonite concentration on CPO's color
In Table 3, it is shown that the higher the concentration of bentonite, the more color is absorbed. This finding is in accordance with a previous study conducted by (Acquah et al., 2016; Etim et al., 2016; Kumar et al., 2010). The CPO's ability to bleach correlates with the concentration of bentonite on the percentage of color reduction. If the concentration is low, the active adsorptive site formed may not be sufficient to degrade the color pigment in palm oil. These results are in line with a study conducted by (Tian et al., 2015). As suggested by Wambu, Muthakia, Wa-Thiong'o, & Shiundu (2011), the greater the adsorbent concentration, the greater the amount of color agent absorbed as there is an increase in the adsorption surface available for interaction (Olufemi and Otolorin, 2017; Wambu et al., 2011). The result of this study trend is consistent with Kumar et al. (2010) report on the removal of Congo red by cashew nut shells increased from 56.33 to 99.34% for adsorbent dose increase from 5 to 30 g/L.
In general, Table 3 shows that the red color of all CPO samples decreased with increasing bentonite concentration. The highest percentage of red color reduction was 88.75% (from 24R to 2.7R) with 3.0% of bentonite concentration for the CPO-1 sample. This result is in accordance with a previous study conducted by Chakawa et al. (2019) which suggested that when the concentration of bleaching agent was added with 30 wt. % of CaSO4.2H2O, the percentage of red color reduction was 69.29% (from 14R to 4.4R) (Chakawa et al., 2019).
3.3. The effect of contact time on CPO's carotene
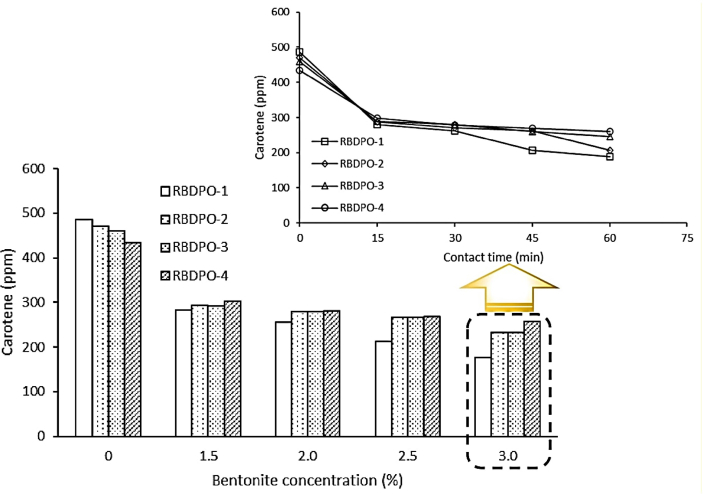
The decrease in carotene levels occurred during the bleaching process with variations in time and bentonite concentration. The increasing contact time and bentonite concentration led to higher percentage of decreased carotene levels which are presented in Tables 4 and 5 and Figure 1.
Table 4.
Effect of contact time on CPO's carotene at bentonite 3.0%.
| Contact time (minutes) | Carotene (ppm) |
|||
|---|---|---|---|---|
| CPO-1 | CPO-2 | CPO-3 | CPO-4 | |
| 0 |
486 |
471 |
460 |
434 |
| Contact time (minutes) | Carotene (ppm) |
|||
| RBDPO-1 |
RBDPO-2 |
RBDPO-3 |
RBDPO-4 |
|
| 15 | 281 | 287 | 290 | 299 |
| 30 | 262 | 272 | 280 | 279 |
| 45 | 206 | 262 | 260 | 270 |
| 60 | 188 | 207 | 247 | 260 |
Table 5.
Effect of bentonite concentration on CPO's carotene with contact time at 30 min.
| Bentonite concentration (%) | Carotene (ppm) |
|||
|---|---|---|---|---|
| CPO 1 | CPO 2 | CPO 3 | CPO 4 | |
| 0 |
486 |
471 |
460 |
434 |
| Bentonite concentration (%) | Carotene (ppm) |
|||
| RBDPO1 |
RBDPO2 |
RBDPO3 |
RBDPO4 |
|
| 1.5 | 282 | 294 | 292 | 302 |
| 2.0 | 256 | 280 | 280 | 281 |
| 2.5 | 212 | 267 | 267 | 268 |
| 3.0 | 177 | 233 | 233 | 257 |
Figure 1.
Effects of contact time and bentonite concentration on CPO's carotene.
Table 4 and Figure 1 shows that the longer the contact time, the lower the carotene value. This is due to the longer the contact time between bentonite and CPO, the greater the chance of carotene to be absorbed in the pores of bentonite which is indicated by the decreasing value of the levels. Similar conditions also occur in samples of CPO-2, CPO-3 and CPO-4. Table 4 and Figure 1 shows that CPO-1 experienced the lowest carotene decrease at 60 min (61.32%). Riyadi et al. (2016) reported that the carotene decreased significantly as the contact time increased and the deodorizing using red palm oil was applied at 150 °C for 2 h Table 4 and Figure 1 indicates that this study has greater percentage of carotene reduction than that of Riyadi et al. (2016) which was only 51.5%.
3.4. The effect of bentonite concentration on CPO's carotene
Table 5 and Figure 1 shows the total of carotenoids in the bleached CPO was originally 486 ppm. The numbers were significantly reduced after the blanching process using various adsorbent concentrations. The decrease in carotenoid content corresponds to higher bentonite concentrations as more and more surface area were provided for adsorption and removal of β-carotene (Abdi et al., 2021). The greater the bentonite concentration, the greater the adsorption of β-carotene which causes CPO to have paler color. The explanation is also applicable to CPO-2, CPO-3 and CPO-4.
Table 5 and Figure 1 shows that the highest percentage of carotene reduction was at 3.0% of bentonite concentration for the CPO-1 (63.58%). This result accords with Silva et al. (2013) which suggested that there was a decrease in the total of carotene as BE concentrations was added. However, the percentage of carotene values reduction in this study was lower than that of Silva et al. (2013) which was 89% (Silva et al., 2013) and Silva et al. (2014) which was 95.5% (from 467 to 21 mg/kg) (Silva et al., 2014). Abdi et al. (2021) recorded the lowest percentage of carotene content reduction (79.05%) using 2.0% of egg shell ash and the highest (83.53%) using 1.0% of acid-activated egg shell powder.
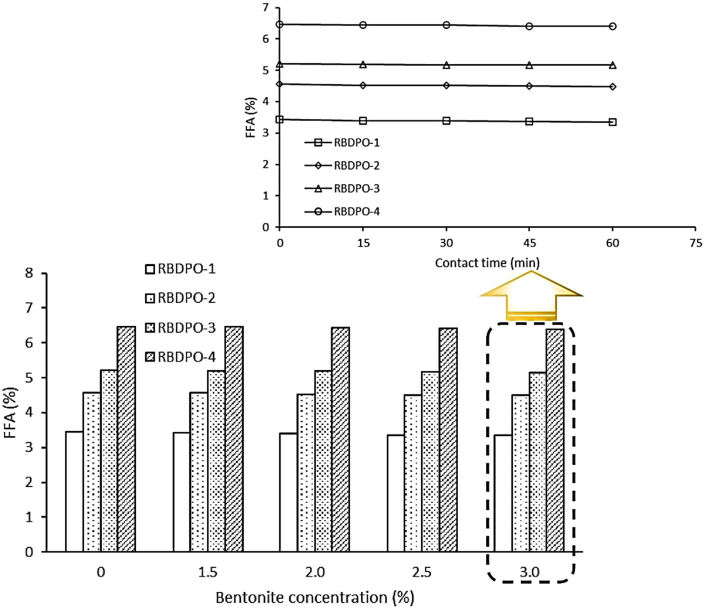
3.5. The effect of contact time on FFA levels
The effect of contact time on FFA levels in CPO after adsorption process is presented in Table 6 and Figure 2. The FFA adsorption was fast at the beginning of the process since the adsorption site on the bentonite surface was still empty. This caused FFA to be quickly absorbed in the pores of bentonite. As the contact time between RBDPO and bentonite increased, the number of empty sites decreased and the adsorption run slowly as it was indicated by the decrease in FFA levels for each sample of the CPO. Table 6 and Figure 2 shows that CPO-1 experiences slightly higher percentage of FFA reduction (2.38%) (from 3.44% to 3.36%) at 60 min of contact time compared to CPO-2, CPO-3 and CPO-4.
Table 6.
Effect of contact time on CPO's reduced FFA at bentonite 3.0%.
| Contact time (minutes) | FFA (%) |
|||
|---|---|---|---|---|
| CPO 1 | CPO 2 | CPO 3 | CPO 4 | |
| 0 |
3.44 |
4.57 |
5.22 |
6.47 |
| Contact time (minutes) | FFA (%) |
|||
| RBDPO1 |
RBDPO2 |
RBDPO3 |
RBDPO4 |
|
| 15 | 3.40 | 4.53 | 5.20 | 6.44 |
| 30 | 3.40 | 4.52 | 5.18 | 6.44 |
| 45 | 3.37 | 4.50 | 5.18 | 6.41 |
| 60 | 3.36 | 4.48 | 5.16 | 6.40 |
Figure 2.
Effects of contact time and bentonite concentration on FFA levels.
Table 6 and Figure 2 shows that FFA levels tend to decrease with increased contact time (from 15 to 60 min) even though it is not significant. This low percentage of reduction in FFA levels is possibly due to impurities and high viscosity in the CPO which can affect the adsorption process. This result accords with Cowan et al. (2012) which also demonstrated that FFA levels tended to decrease with increased contact time. The reduction percentage of FFA levels in CPO with 1.0% of lipase and 2.0% (w/w) of glycerol additions at 14 h was 65.26% (from 4.98 to 1.73%) (Cowan et al., 2012). The same finding was also reported by Riyadi et al. (2016) with a contact time of 2 h (0.32%) at 150 °C (this reduction percentage was slightly greater compared to a contact time of 1 h which was only 0.29%).
3.6. The effect of bentonite concentration on FFA levels
The effect of bentonite concentration on FFA levels in CPO is presented in Table 7 and Figure 2. In general, it shows that the greater the concentration of bentonite, the lower the FFA levels. This finding differs from Silva et al. (2014) which reported that the addition of citrate to BE (from 0.09 to 0.27%) had caused FFA levels to increase by 9% (from 4.61% to 5.0%). Moreover, Abdi et al. (2021) also reported the same finding which suggested that the FFA content of acid-activated blanched oil increased by 96.77% (from 0.31 to 0.61%) while alkaline egg shell powder with totally removed FFA from the oils. The difference between the finding of this study and that of Abdi et al. (2021) lied on the different types of adsorbent used during the research. As the adsorbent used in this study was made of egg shells (alkaline), so the FFA was neutralized. This neutralization is possible as there is a hydrolysis process which turned of triacylglycerol to diacylglycerol which causes FFA to increase. Table 7 and Figure 2 shows that the increase in bentonite concentration causes FFA to be absorbed even though the decrease in FFA levels is relatively low. This is due to the presence of alumina (Al2O3) and silica (SiO2) groups which are known for their reactive response to bentonite. The higher the concentration of bentonite, the greater the amount of SiO2 and Al2O3.
Table 7.
Effect of bentonite concentration on CPO's FFA with contact time of 30 min.
| Bentonite concentration (%) | FFA (%) |
|||
|---|---|---|---|---|
| CPO 1 | CPO 2 | CPO 3 | CPO 4 | |
| 0 |
3.44 |
4.57 |
5.22 |
6.47 |
| Bentonite concentration (%) | FFA (%) |
|||
| RBDPO1 |
RBDPO2 |
RBDPO3 |
RBDPO4 |
|
| 1.5 | 3.42 | 4.56 | 5.20 | 6.45 |
| 2.0 | 3.40 | 4.53 | 5.20 | 6.44 |
| 2.5 | 3.36 | 4.51 | 5.17 | 6.42 |
| 3.0 | 3.34 | 4.50 | 5.15 | 6.39 |
Table 7 and Figure 2 shows that there is no significant effect of the bentonite concentration variations on the reducing levels of FFA. Bentonite with a concentration of 3.0% has higher ability to reduce FFA levels up to 2.99% (from 3.44% to 3.34%). The percentage of reduction in FFA levels found in this study is greater than that of previous study conducted by (Chakawa et al., 2019). For adsorbent concentration of 0–30%, the percentage of FFA remains constant at 0.56% (Chakawa et al., 2019).
3.7. Adsorption capacity and removal efficiency for carotene
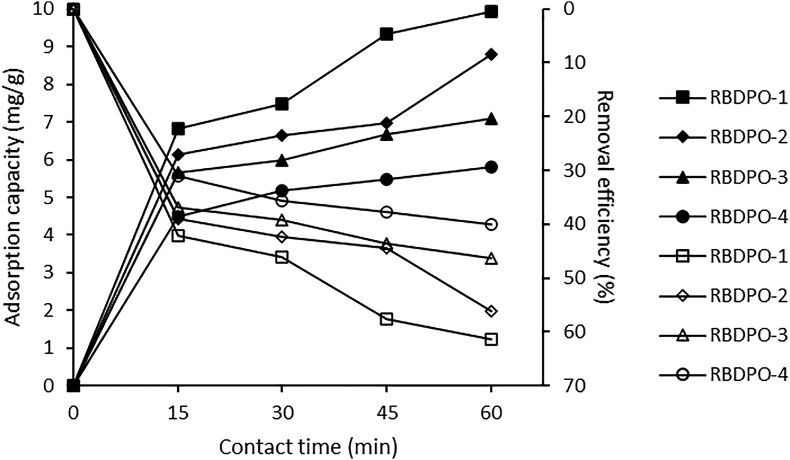
3.7.1. The effect of contact time on CPO's carotene
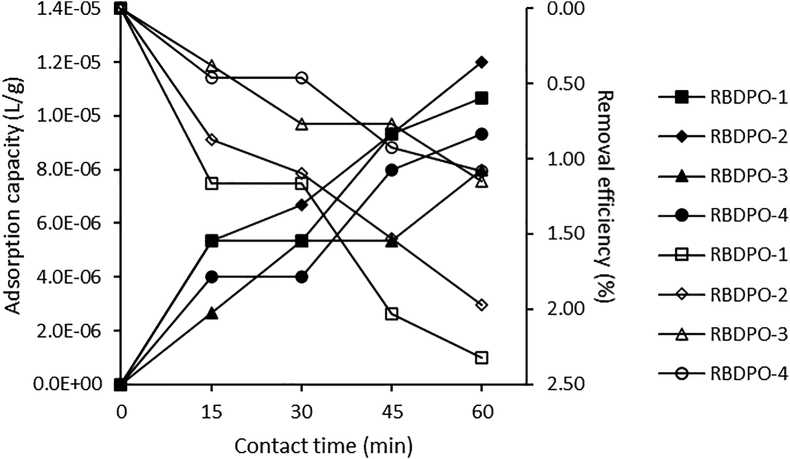
The effect of contact time for carotene removal by bentonite at 15–60 min for 3.0% bentonite concentration of RBDPO1-4 samples showed that carotene adsorption increased rapidly in the first 15 min and, after that, the adsorption capacity slowed down as shown in Figure 3. The longer the contact time, the higher the amount of carotene adsorbed by the bentonite. The highest carotene removal efficiency was at 60 min for the RBDPO-1 sample, which was 61.32%. This result is in line with what Riyadi et al. (2016) reported that total carotene decreased significantly with the increased contact time, using red palm oil at a temperature of 150 °C for 2 h. The efficiency of carotene removal from this study is higher and used shorter time than previous studies using red palm oil, Riyadi et al. (2016), namely 51.5%, and used 2 h (Riyadi et al., 2016).
Figure 3.
Adsorption capacity and removal efficiency for the effect of contact time on CPO’s carotene.
Figure 3 shows that the adsorption capacity increased along with the increased contact time, and the largest adsorption capacity obtained was at 60 min contact time of 9.93 mg/g. The increase in contact time from 45 to 60 min indicates that the adsorption capacity is only about 0.6 (mg/g) compared to what it obtained for contact times of 30–45 min. This trend is in line with previous reports on the effect of contact time on Cu(II) and Pb(II) using silica@mercapto (HS@M) hybrid adsorbent that at a specific time, the percentage of adsorbed metal cations did not increase significantly (Naat et al., 2021). In these conditions, it is said that the adsorption process has reached an equilibrium state. This equilibrium state is achieved when the adsorption rate is the same as the desorption rate, and the active site is less available when the equilibrium phase is reached. This trend is in line with previous reports on the effect of contact time on Cr(III) uptake using cinnamoyl C-phenylcalix [4] resorcinarene (CCPCR) adsorbent (Budiana et al., 2021) and egg shell and powdered marble adsorbents (Elabbas et al., 2016).
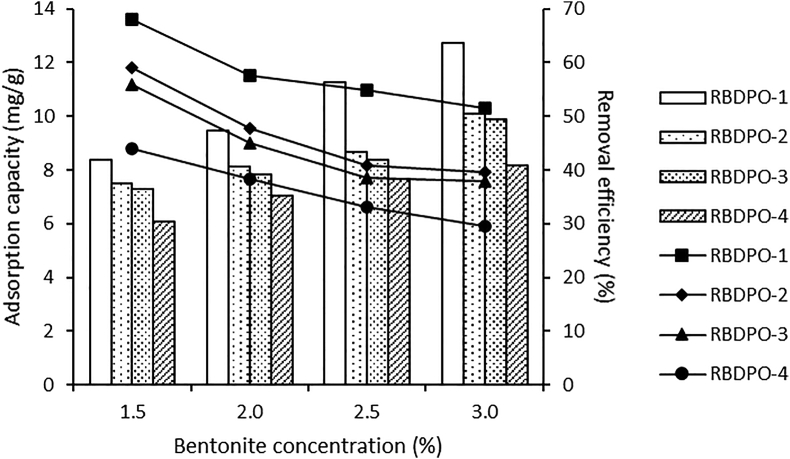
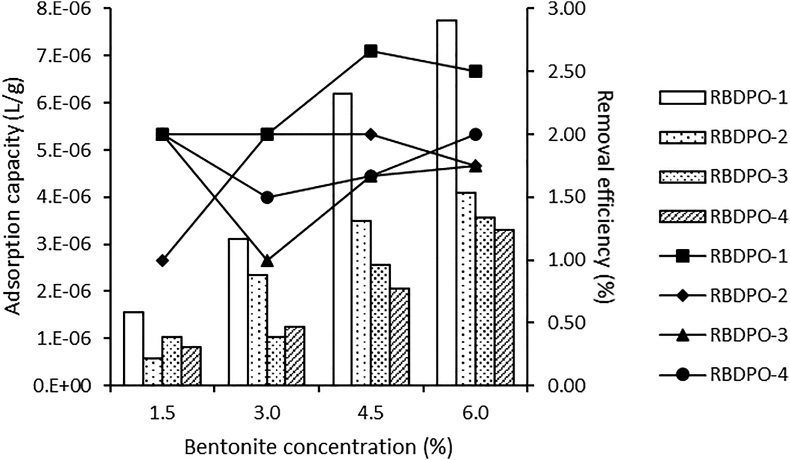
3.7.2. The effect of bentonite concentration on CPO's carotene
Figure 4 shows the relationship between bentonite concentration on adsorption capacity and carotene removal efficiency. From this figure, it is known that the efficiency of carotene removal varies with varying concentrations and increases along with increasing bentonite concentration. This trend is in line with previous reports on the effect of adsorbent dose on the absorption of Cu(II) and Pb(II) using silica@mercapto (HS@M) hybrid adsorbent (Naat et al., 2021) and chromium removal using egg shell adsorbents and powdered marble (Elabbas et al., 2016).
Figure 4.
Adsorption capacity and removal efficiency for the effect of bentonite concentration on CPO’s carotene.
Figure 4 shows that the highest carotene removal efficiency was at the 3.0% bentonite concentration for the RBDPO-1 sample, which was 63.58%. The obtained results match with the research that has been conducted by Silva et al. (2013) and Silva et al. (2014), where there was a decrease in total carotene in the BE concentrate addition. The efficiency of carotene removal results from this study is smaller than the research Silva et al. (2013), which is 89% (Silva et al., 2013), Silva et al. (2014) which is 95.5% (from 467 to 21 mg/kg) (Silva et al., 2014). Abdi et al. (2021) reported that the lowest carotene removal efficiency (79.05%) was using 2.0% egg shell ash, and the highest (83.53%) used 1.0% activated egg shell powder with acid.
In Figure 4, it can be observed that RBDPO1-4 samples have an optimum concentration of 1.5%, showing that the greater the concentration of bentonite used in the adsorption process, the smaller the adsorption capacity be. The adsorption capacity decrease is allegedly caused by the multilayer layer formed above the absorbed adsorbate on the adsorbent surface, which saturates its surface.
3.8. Adsorption capacity and removal efficiency for FFA
3.8.1. The effect of contact time on FFA levels
The rate of FFA removal by bentonite increased along with increased contact time, as shown in Figure 5, showing that the FFA removal efficiency tended to increase with increased contact time but not too significant. The obtained research trends match the research reported (Cowan et al., 2012; Purwasasmita et al., 2015; Salman et al., 2011). According to Cowan et al. (2012), the efficiency of FFA removal aligns with the contact time, where the addition of 1% lipase and 2.0% (w/w) glycerol at 14 h of contact time was 65.26% (from 4.98 to 1.73%). Purwasasmita et al. (2015) reported that the effect of contact time on the obtained efficiency of FFA CPO removal increased along with the longer contact time. At 4 h of contact time, the highest CPO FFA removal efficiency was 98% (Purwasasmita et al., 2015). According to Salman et al. (2011), the longer the interaction between the solution and the adsorbent, the more the amount of adsorbate will be absorbed on the adsorbent surface. The same trend was also reported by Riyadi et al. (2016) with 2 h of contact time; it was found that the FFA removal efficiency was 0.32%, which was slightly higher than the 1 h contact time with an FFA removal efficiency of 0.29%.
Figure 5.
Adsorption capacity and removal efficiency for the effect of contact time on FFA levels.
Figure 5 shows that the FFA adsorption capacity tended to increase with increased contact time (from 15 to 60 min) but not too significant. The low adsorption capacity of FFA (1.07E-05 L/g) is assumed to have an impurity factor in CPO that affects the adsorption process.
3.8.2. The effect of bentonite concentration on FFA levels
Figure 6 shows that, in general, the percentage of FFA removal increased with increased bentonite concentration, which is indicated by the less FFA content contained in RBDPO1-4. The increase of adsorption percentage with the increase in bentonite concentration was due to the increase in the adsorbent's active site, making it easier to penetrate FFA molecules into the adsorption site. A similar trend was observed by (Itodo and Itodo, 2010; Olufemi and Otolorin, 2017; Pérez Marín et al., 2009). As shown in Figure 6, overall (RBDPO1-4), the most significant FFA removal efficiency was obtained at a 6.0% bentonite concentration of 2.91% at 60 min for RBDPO-1. It is clearly shown in Eq. (6) that the removal efficiency is a function of bentonite concentration. Therefore, at low bentonite concentrations, the overall adsorption capacity and removal efficiency will be smaller because it depends on the adsorbent concentration. This trend is in line with previous reports on the effect of adsorbent concentrations on FFA removal (Purwasasmita et al., 2015; Putranti et al., 2018). Purwasasmita et al. (2015) reported that the effect of reactant concentrations was tested by experimenting with NaOH concentrations ranging from 0.1 to 0.5 N, the overall low FFA CPO removal efficiency was obtained at NaOH concentrations below 0.25 N.
Figure 6.
Adsorption capacity and removal efficiency for the effect of bentonite concentration on FFA levels.
Figure 6 shows that, in general, the bigger the bentonite concentration, the greater the efficiency of removing FFA from the used CPO sample. The efficiency of FFA removal in this study (2.33%) was smaller than that reported by Putranti et al. (2018) using alkaline activated zeolites in cooking oil with low FFA levels (0.4%), the FFA removal efficiency was 62.5% (Putranti et al., 2018). The same thing was also reported Abdi et al. (2021) using egg shells obtained an FFA removal efficiency reaches 100% (Abdi et al., 2021). The difference in the efficiency of FFA removal from research results obtained by research (Abdi et al., 2021; Putranti et al., 2018) is in the type of adsorbent used, namely alkaline activated zeolites and alkaline egg shells, so that FFA neutralization occurs.
Figure 6 shows that the adsorption capacity tended to increase from 5.33E-06 to 7.11E-06 L/g with an increase in the concentration of bentonite from 1.5 to 4.5 g. A similar phenomenon was observed in the adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm frond activated carbon (Salman et al., 2011). Raju, Kiran and Rao (2013) also reported a similar trend stated that the metal absorption capacity of the adsorbent increases along with the increasing dosage. It is caused by the number of active sites available for metal uptake will be more because the amount of adsorbent increases. However, the adsorption capacity tended to decrease from 7.11E-06 to 6.67E-06 L/g, with increasing bentonite concentration from 4.5 to 6.0 g. It shows that the optimum concentration is at 4.5%, where the more significant the concentration of bentonite used in the adsorption process, the smaller the adsorption capacity.
4. Conclusion
The process of CPO refinery is carried out through degumming, bleaching and distillation before they are analyzed for FFA, color and carotene value. This study found that, during the bleaching process, 3.0% of bentonite concentration gave the highest percentage of removal efficiency in carotene value 56.37%, color 88.75%, and FFA levels 2.99%. Sixty minutes of contact time gave the highest percentage of removal efficiency in carotene values 61.32%, color 89.58%, and FFA levels of 2.38%. The best adsorption capacity for the effect of bentonite concentration on the carotene value of 13.60 mg/g while FFA levels of 6.67E-06 L/g. The best adsorption capacity for the effect of contact time on carotene values was 61.32% at 60 min, while FFA levels were 2.33%. The adsorption capacity and removal efficiency, color, carotene value and FFA content depend on the concentration of bentonite used and contact time. Therefore, the researcher expects that this study can have a huge impact on the future palm oil industry.
Declarations
Author contribution statement
La Ifa & Heri Septya Kusuma: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lastri Wiyani, Andi Muhammad Triguna Ghalib & Suci Ramadhaniar: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Nurdjannah Nurdjannah: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The researcher would like express his gratitude to the Ministry of Research and Higher Education. In addition, the researcher also would like to thank the Board of Directors of PT. Tanjung Sarana Lestari and Universitas Muslim Indonesia (UMI) for their contributions in providing the facilities needed for this research.
Contributor Information
La Ifa, Email: la.ifa@umi.ac.id.
Heri Septya Kusuma, Email: heriseptyakusuma@gmail.com.
References
- Abdi E., Gharachorloo M., Ghavami M. Investigation of using egg shell powder for bleaching of soybean oil. Food Sci. Technol. 2021;140:1–5. [Google Scholar]
- Acquah C., Sie Yon L., Tuah Z., Ling Ngee N., Danquah M.K. Synthesis and performance analysis of oil palm ash (OPA) based adsorbent as a palm oil bleaching material. J. Clean. Prod. 2016;139:1098–1104. [Google Scholar]
- Ali F.S., Shamsudin R., Yunus R. Vol. 2. Elsevier Srl; 2014. The effect of storage time of chopped oil palm fruit bunches on the palm oil quality; pp. 165–172. (Agriculture and Agricultural Science Procedia). [Google Scholar]
- Anang M.A., Dodoo D., Ntiri B.-S., Zugle R., Fynn G.E. Improving the quality of locally produced vegetable oils in Ghana using zeolite ZSM-11. Chem. Sci. Int. J. 2020;28(4):1–13. [Google Scholar]
- Azeman N.H., Yusof N.A., Othman A.I. Detection of free fatty acid in crude palm oil. Asian J. Chem. 2015;27(5):1569–1573. [Google Scholar]
- Bahadi M.A., Japir A.-W., Salih N., Salimon J. Free fatty acids separation from malaysian high free. Malays. J. Anal. Sci. 2016;20(5):1042–1051. [Google Scholar]
- Budiana I.G.M.N., Jasman J., Neolaka Y.A.B., Riwu A.A.P., Elmsellem H., Darmokoesoemo H., Kusuma H.S. Synthesis, characterization and application of cinnamoyl C-phenylcalix[4]resorcinarene (CCPCR) for removal of Cr(III) ion from the aquatic environment. J. Mol. Liq. 2021;324:114776. [Google Scholar]
- Chakawa D.P., Nkala M., Hlabangana N., Muzenda E. Vol. 35. Elsevier B.V; 2019. The use of calcium sulphate dihydrate (CaSO4.2H2O) as a bleaching agent for crude soya bean vegetable oil; pp. 802–807. (Procedia Manufacturing). [Google Scholar]
- Constant L.-L.-N.B., Godswill N.-N., Frank N.-E.G., Hermine N.-B., Achille N., Martin B.J. A review of main factors affecting palm oil acidity within the smallholder oil palm (Elaeis guineensis Jacq.) sector in Cameroon. Afr. J. Food Sci. 2017;11(9):296–301. [Google Scholar]
- Cowan D., Holm H.C., Yee H.S. Reduction in free fatty acids in crude palm oil by enzymatic remediation. J. Oil Palm Res. 2012;24:1492–1496. [Google Scholar]
- Cren É.C., Meirelles A.J.A. Oleic acid removal from ethanolic solutions by ion exchange. Chem. Eng. J. 2012;184:125–131. [Google Scholar]
- De Almeida D.T., Nunes I.L., Conde P.L., Rosa R.P.S., Rogério W.F., Machado E.R. A quality assessment of crude palm oil marketed in Bahia, Brazil. Grasas Aceites. 2013;64(4):387–394. [Google Scholar]
- Elabbas S., Mandi L., Berrekhis F., Pons M.N., Leclerc J.P., Ouazzani N. Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J. Environ. Manag. 2016;166:589–595. doi: 10.1016/j.jenvman.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Etim U.J., Umoren S.A., Eduok U.M. Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J. Saudi Chem. Soc. 2016;20:S67–S76. [Google Scholar]
- Fatin S., Rosnah S., Yunus R. Effect of chopping oil palm fruit spikelets on the free fatty acid content release rate and its mechanical properties. Int. J. Renew. Energy Technol. 2014;3(1):511–516. [Google Scholar]
- Guliyev N.G., Ibrahimov H.J., Alekperov J.A., Amirov F.A., Ibrahimova Z.M. Investigation of activated carbon obtained from the liquid products of pyrolysis in sunflower oil bleaching process. Int. J. Ind. Chem. 2018;9:277–284. [Google Scholar]
- Hashim H., Yusup S., Arlabosse P. Extraction of crude palm oil (CPO) using thermally assisted mechanical dewatering (TAMD) and their characterization during storage. AIP Conf. Proc. 2019;2124:1–6. [Google Scholar]
- Henry O.H. Monitoring the free fatty acid level of crude palm oil stored under light of different Wavelengths. Am. J. Food Technol. 2011;6(8):701–704. [Google Scholar]
- Ifa L., Nurjannah N., Aladin A., Sabara Z., Jusoff K. Identification of urethane linkage, soft segment polyol and hard segment polyurea in polyuretan from palm oil based polyol. World Appl. Sci. J. 2013;26:50–54. [Google Scholar]
- Itodo H.U., Itodo A.U. Surface coverage and adsorption study of dye uptake by derived acid and base treated mango seed shells. J. Chem. Pharm. Res. 2010;2(3):673–683. [Google Scholar]
- Jumaah M.A., Mohamad Yusoff M.F., Salimon J., Bahadi M. Physical characteristics of palm fatty acid distillate. J. Chem. Pharmaceut. Sci. 2019;12(1):1–5. [Google Scholar]
- Kumar P.S., Ramalingam S., Senthamarai C., Niranjanaa M., Vijayalakshmi P., Sivanesan S. Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination. 2010;261:52–60. [Google Scholar]
- Lin S.W. MPOB Information Eries; 2004. Deterioration of bleachability index; pp. 1–2. [Google Scholar]
- Naat J.N., Neolaka Y.A.B., Lapailaka T., Rachmat Triandi T., Sabarudin A., Darmokoesoemo H., Kusuma H.S. Adsorption of Cu(II) and Pb(II) using silica@mercapto (hs@m) hybrid adsorbent synthesized from silica of Takari sand: optimization of parameters and kinetics. Rasayan J. Chem. 2021;14(1):550–560. [Google Scholar]
- Ndé H.S., Tamfuh P.A., Clet G., Vieillard J., Mbognou M.T., Woumfo E.D. Comparison of HCl and H2SO4 for the acid activation of a cameroonian smectite soil clay: palm oil discolouration and landfill leachate treatment. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02926. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olufemi B.A., Otolorin F. Comparative adsorption of crude oil using mango (Mangnifera indica) shell and mango shell activated carbon. Environ. Eng. Res. 2017;22(4):384–392. [Google Scholar]
- Onwuliri V.A., Igwe C.U., Golu M.D., Agha N.C. Assessment of the quality of some edible vegetable oils consumed in northern Nigeria. Aust. J. Basic Appl. Sci. 2011;5(7):897–905. [Google Scholar]
- Pérez Marín A.B., Aguilar M.I., Meseguer V.F., Ortuño J.F., Sáez J., Lloréns M. Biosorption of chromium (III) by orange (Citrus cinensis) waste: batch and continuous studies. Chem. Eng. J. 2009;155(1–2):199–206. [Google Scholar]
- Purwasasmita M., Nabu E.B.P., Khoiruddin, Wenten I.G. Non dispersive chemical deacidification of crude palm oil in hollow fiber membrane contactor. J. Eng. Technol. Sci. 2015;47(4):426–446. [Google Scholar]
- Putranti M.L.T.A., Wirawan S.K., Bendiyasa I.M. Vol. 299. 2018. Adsorption of free fatty acid (FFA) in low-grade cooking oil used activated natural zeolite as adsorbent; pp. 1–9. (IOP Conference Series: Materials Science and Engineering). [Google Scholar]
- Raju D.S.S.R., Kiran G.A.R., Rao D.V.N. Comparison studies on biosorption of lead (II) from an aqueous solution using Anacardium Occidentale and Carica Papaya. Int. J. Emerg. Trends Eng. Dev. 2013;1(3):273–283. [Google Scholar]
- Ribeiro J.A.A., Almeida E.S., Neto B.A.D., Abdelnur P.V., Monteiro S. Identification of carotenoid isomers in crude and bleached palm oils by mass spectrometry. LWT - Food Sci. Technol. 2018;89:631–637. [Google Scholar]
- Riyadi A.H., Muchtadi T.R., Andarwulan N., Haryati T. Vol. 9. Elsevier Srl; 2016. Pilot plant study of red palm oil deodorization using moderate temperature; pp. 209–216. (Agriculture and Agricultural Science Procedia). [Google Scholar]
- Salman J.M., Njoku V.O., Hameed B.H. Batch and fixed-bed adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm frond activated carbon. Chem. Eng. J. 2011;174(1):33–40. [Google Scholar]
- Sampaio K.A., Ceriani R., Silva S.M., Taham T., Meirelles A.J.A. Steam deacidification of palm oil. Food Bioprod. Process. 2011;89:383–390. [Google Scholar]
- Silva S.M., Sampaio K.A., Ceriani R., Verh R., Stevens C., Greyt W. De, Meirelles A.J.A. Effect of type of bleaching earth on the fi nal color of re fi ned palm oil. LWT - Food Sci. Technol. 2014;59:1258–1264. [Google Scholar]
- Silva S.M., Sampaio K.A., Ceriani R., Verhé R., Stevens C., De Greyt W., Meirelles A.J.A. Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: equilibrium, kinetics and thermodynamics. J. Food Eng. 2013;118:341–349. [Google Scholar]
- Soetaredjo F.E., Laysandra L., Putro J.N., Santoso S.P., Angkawijaya A.E., Yuliana M.…Ismadji S. Ecological-safe and low-cost activated-bleaching earth: preparation, characteristics, bleaching performance, and scale-up production. J. Clean. Prod. 2021;279:123793. [Google Scholar]
- Tian G., Wang W., Mu B., Kang Y., Wang A. Facile fabrication of carbon/attapulgite composite for bleaching of palm oil. J. Taiwan Inst. Chem. Eng. 2015;50:252–258. [Google Scholar]
- Wambu E.W., Muthakia G.K., Wa-Thiong’o J.K., Shiundu P.M. Kinetics and thermodynamics of aqueous Cu(II) adsorption on heat regenerated spent bleaching earth. Bull. Chem. Soc. Ethiop. 2011;25(2):181–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.