Abstract
Background
Nivolumab (NIVO) and irinotecan (IRI) are standard treatments for refractory advanced gastric cancer (AGC); however, it is unclear which drug should be administered first or in which cases. The tumor growth rate (TGR) during preceding treatment is reported to be associated with tumor response in metastatic colorectal cancer patients treated with regorafenib or trifluridine/tipiracil, suggesting that TGR may be useful for drug selection. Therefore, we evaluated the association between TGR during preceding treatment and the tumor response to NIVO or IRI.
Patients and methods
We retrospectively evaluated consecutive AGC patients treated with NIVO or IRI and divided them into slow-growing (Slow) and rapid-growing (Rapid) groups according to TGR and the presence or absence of new lesions (NL+/NL−, respectively) during preceding treatment (Slow group: NL− with low TGR <0.30%/day; Rapid group: NL+ or high TGR ≥0.30%/day).
Results
A total of 117 patients (Rapid/Slow groups, 72/45; NIVO/IRI groups, 32/85) were eligible. All baseline characteristics except peritoneal metastases were similar between patients treated with NIVO and IRI in the Rapid and Slow groups. The response rate was significantly higher in patients treated with NIVO compared with IRI [31%/3%; odds ratio (OR), 13.8; P = 0.01; adjusted OR, 52; P = 0.002] in the Slow group, but there was no difference between patients treated with NIVO and IRI (5%/8%; OR, 0.68; P = 0.73; adjusted OR, 0.94; P = 0.96) in the Rapid group. Disease control rate, progression-free survival, and overall survival were consistent with these results.
Conclusions
Our findings suggest that NIVO treatment is a more favorable option for patients with slow-growing tumors, and NIVO and IRI are similarly recommended for patients with rapid-growing tumors in refractory AGC. TGR and NL emergence during preceding treatment may be helpful for drug selection and warrant further investigation.
Keywords: gastric cancer, predictive marker, retrospective study, chemotherapy, tumor growth rate
Highlights
-
•
NIVO and IRI are standard treatments for refractory AGC, although it is unclear which should be administered first.
-
•
TGR may be useful for drug selection, therefore we evaluated the association between TGR and the tumor response to NIVO or IRI.
-
•
In the Slow group, the response rate (RR) was significantly higher in patients treated with NIVO compared with IRI.
-
•
In the Rapid group, there was no significant difference in RR between the NIVO and IRI groups.
-
•
TGR and NL emergence during preceding treatment may be useful for drug selection.
Introduction
Nivolumab (NIVO) and irinotecan (IRI) are recognized as standard treatments for patients with refractory advanced gastric cancer (AGC). The ATTRACTION-2 trial, which compared NIVO with placebo as a third- or later-line treatment for refractory AGC, concluded that treatment with NIVO was superior to placebo in terms of overall survival (OS) [hazard ratio (HR), 0.63; 95% confidence interval (CI) 0.50-0.78; P < 0.0001]1 and NIVO has been approved in Japan. Pembrolizumab was approved in Western countries based on the results of the KEYNOTE-059 trial.2 In addition, the WJOG4007G trial, which compared paclitaxel with IRI as second-line treatment for AGC, showed that there was no difference in OS for the paclitaxel and IRI groups, and most patients in the paclitaxel group received IRI as third-line treatment.3 Moreover, in the JAVELIN Gastric 300 trial comparing avelumab with physician’s choice of chemotherapy as third-line treatment for AGC, 64.5% of patients received IRI in the chemotherapy group.4 Based on these results, anti-programmed cell death protein 1 (PD-1) antibodies and IRI have been recognized as standard third-line treatments.5 However, in clinical practice, it is unclear which drug should be administered first or in which cases as no trials have directly compared anti-PD-1 antibodies with IRI in AGC.
We previously reported that the tumor growth rate (TGR) during preceding treatment is associated with tumor response in metastatic colorectal cancer patients treated with regorafenib or trifluridine/tipiracil.6 The disease control rate (DCR) was better in patients treated with trifluridine/tipiracil than in those treated with regorafenib among the slow-growing (Slow) group [defined as low TGR and no emergence of new lesion (NL−)] during preceding treatment, although the DCR was similar between patients treated with trifluridine/tipiracil and regorafenib among the rapid-growing (Rapid) group [defined as high TGR and/or emergence of NL (NL+)]. These findings were possibly due to the differing mechanisms of action of regorafenib, which is a multikinase inhibitor, and trifluridine/tipiracil, which targets unspecified DNA of cancer cells. It was suggested that TGR during preceding treatment could be helpful for drug selection. Similarly, NIVO as an immune checkpoint inhibitor and IRI as a cytotoxic drug have different mechanisms of action. Therefore, we hypothesized that TGR during preceding treatment was useful for selecting whether to use NIVO or IRI in refractory AGC.
Patients and methods
Patients
This retrospective study evaluated the association between TGR during preceding treatment and the efficacy of NIVO and IRI in refractory AGC at three institutions. Refractory AGC patients treated with NIVO or IRI at the Aichi Cancer Center Hospital, Shizuoka Cancer Center, and Hokkaido University Hospital from January 2015 to June 2018 were evaluated. The eligibility criteria were: (i) histologically confirmed unresectable gastric adenocarcinoma; (ii) no prior treatment with NIVO and IRI; (iii) refractory or intolerant to fluoropyrimidines and taxanes; (iv) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2; (v) measurable lesion according to RECIST version 1.1; (vi) adequate bone marrow, hepatic, and renal function; and (vii) computed tomography (CT) carried out at least once during preceding chemotherapy and within 30 days before starting NIVO or IRI. Written informed consent was provided by all patients before beginning treatment. The protocol of this retrospective study was reviewed and approved by the Institutional Review Boards of the Aichi Cancer Center Hospital (approval number: 2018-1-287), Shizuoka Cancer Center (approval number: T2020-59-2020-1-3), and Hokkaido University Hospital (approval number: 020-0218).
Treatments
In principle, NIVO (3 mg/kg or 240 mg fixed dose) or IRI (150 mg/m2) was administered intravenously every 2 weeks. Treatments were continued until progressive disease, death, unacceptable toxicity requiring permanent discontinuation of treatment, or patient refusal. Among the patients who received IRI, those who received a reduced initial dose due to the patient’s request or physician’s decision were included in this study.
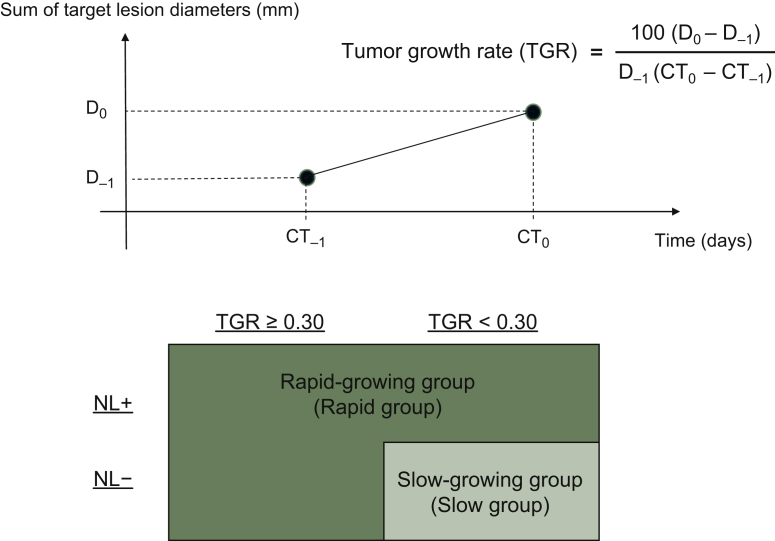
Calculation of TGR and method of classification
TGR was calculated as:
where CT0 represents the date of CT at progressive disease judged by physicians during preceding treatment, CT−1 represents the date of CT directly preceding CT0, and Dn represents the sum of the target lesion diameters at CTn (according to RECIST version 1.1).
We classified patients into two groups according to TGR and whether or not an NL emerged. The TGR cut-off value was defined as 0.30%/day, and was equal to almost 20%/2 months, taking into account the median TGR (0.30%/day) and clinical significance. NL+ was defined as emergence of a lesion at a new site that did not have metastases when the preceding treatment was started. The Slow group was defined as low TGR (<0.30%/day) and NL−, and the Rapid group was defined as high TGR (≥0.30%/day) and NL− and NL+ regardless of TGR (Figure 1).
Figure 1.
Definition of TGR and grouping according to TGR and NL+/NL−.
CT0 represents the date of CT at progressive disease judged by physicians during preceding treatment, CT−1 represents the date of CT directly preceding CT0, and Dn represents the sum of target lesion diameters at CTn. The Slow group was defined as low TGR (<0.30%/day) and NL−, and the Rapid group was defined as high TGR (≥0.30%/day) and NL− and NL+ regardless of TGR.
CT, computed tomography; NL+, emergence of new lesion; NL−, no emergence of new lesion; TGR, tumor growth rate.
The cut-off values of TGR were varied because it was unclear whether the selected TGR cut-off value was appropriate or not.
Evaluation of treatment and statistical analysis
The efficacy of NIVO and IRI was evaluated by responses, which were defined as a complete response or partial response by physicians according to RECIST version 1.1.
Differences in the patients’ characteristics and response rate (RR) between the NIVO and IRI groups were compared using Fisher’s exact test with odds ratio (OR) and 95% CI based on logistic regression analysis. Differences in RR were also evaluated by multivariate analyses using variables selected with P values <0.05 in the univariate analysis and presented as adjusted OR. In the univariate analyses for RR, the following variables were evaluated: age (<65 versus ≥65 years), sex, ECOG PS (0-1 versus 2), histological type (intestinal versus diffuse), resection of primary tumor (no versus yes), human epidermal growth factor receptor 2 status (negative versus positive), peritoneal metastases (no versus yes), liver metastases (no versus yes), lung metastases (no versus yes), number of metastatic sites (1-2 versus ≥3), time from initiation of first-line chemotherapy (<14.4 versus ≥14.4 months), number of prior regimens (<3 versus ≥3), ascites (no versus yes), lactate dehydrogenase (LDH) levels (<240 versus ≥240 IU/l), alkaline phosphatase (ALP) levels (<380 versus ≥380 IU/l), carcinoembryonic antigen levels (<5 versus ≥5 ng/ml), albumin levels (≥3.5 versus <3.5 g/dl), C-reactive protein levels (≤1.0 versus >1.0 ng/ml), platinum administration history in previous chemotherapy (no versus yes), and ramucirumab administration history in previous chemotherapy (no versus yes). We defined the cut-off value of the time from the initiation of first-line chemotherapy as the median time, 14.4 months. Progression-free survival (PFS) was defined as the time from the date of first administration of treatment to the date of the first radiological or clinical observation of disease progression or death due to any cause, whichever occurred first. OS was defined as the time from the first treatment until death due to any cause, with surviving patients censored at the last follow-up date. The median PFS and OS were estimated using the Kaplan–Meier method. HR and 95% CI were estimated using the Cox proportional hazards model.
All statistical analyses were carried out using JMP version 10 (SAS Institute, Cary, NC) and EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan). EZR is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria), and is a modified version of R commander designed to add statistical functions frequently used in biostatistics.7 All statistical tests were two-sided, with P values <0.05 considered statistically significant.
Results
Patient characteristics
Between January 2015 and June 2018, 212 patients with refractory AGC received NIVO or IRI for the first time. We excluded 19 patients who had not received fluoropyrimidines or taxanes, 56 patients without measurable lesions, and 20 patients who had not undergone CT within 30 days before starting NIVO or IRI treatment. Therefore, final totals of 32 and 85 patients who had received NIVO (NIVO group) and IRI (IRI group), respectively, were analyzed as eligible patients. There were 72 patients in the Rapid group, including 19 treated with NIVO and 53 treated with IRI, and 45 patients in the Slow group, including 13 treated with NIVO and 32 treated with IRI (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100179).
In the Rapid group, the proportion of patients with peritoneal metastases was higher in the NIVO group than in the IRI group (79% versus 43%). Almost all other baseline characteristics were similar between the NIVO and IRI groups among the Rapid and Slow groups (Table 1).
Table 1.
Patient characteristics
| Characteristics | Rapid group (n = 72) |
Slow group (n = 45) |
||||
|---|---|---|---|---|---|---|
| NIVO (n = 19) (%) | IRI (n = 53) (%) | P value | NIVO (n = 13) (%) | IRI (n = 32) (%) | P value | |
| Age (years) | ||||||
| <65 | 6 (32) | 20 (38) | 0.78 | 2 (15) | 12 (37) | 0.18 |
| ≥65 | 13 (68) | 33 (62) | 11 (85) | 20 (63) | ||
| Sex | ||||||
| Male | 12 (63) | 39 (74) | 0.40 | 10 (77) | 23 (72) | 1.00 |
| Female | 7 (37) | 14 (26) | 3 (23) | 9 (28) | ||
| ECOG performance status | ||||||
| 0-1 | 14 (74) | 43 (81) | 0.52 | 10 (77) | 28 (88) | 0.39 |
| 2 | 5 (26) | 10 (19) | 3 (23) | 4 (12) | ||
| Histological type | ||||||
| Intestinal | 11 (58) | 36 (68) | 0.58 | 7 (54) | 12 (37) | 0.34 |
| Diffuse | 8 (42) | 17 (32) | 6 (46) | 20 (63) | ||
| Prior gastrectomy | ||||||
| No | 12 (63) | 33 (62) | 1.00 | 6 (46) | 19 (59) | 0.52 |
| Yes | 7 (37) | 20 (38) | 7 (54) | 13 (41) | ||
| HER2 status | ||||||
| Negative | 14 (74) | 30 (57) | 0.27 | 10 (77) | 27 (84) | 0.67 |
| Positive | 5 (26) | 23 (43) | 3 (23) | 5 (16) | ||
| Metastatic sites | ||||||
| Peritoneum | 15 (79) | 23 (43) | 0.01 | 8 (62) | 20 (63) | 1.00 |
| Liver | 8 (42) | 34 (64) | 0.11 | 5 (39) | 13 (41) | 1.00 |
| Lung | 3 (16) | 10 (19) | 1.00 | 3 (23) | 3 (9) | 0.33 |
| Number of metastatic sites | ||||||
| 1-2 | 6 (32) | 12 (23) | 0.54 | 3 (23) | 14 (44) | 0.31 |
| ≥3 | 13 (68) | 41 (77) | 10 (77) | 18 (56) | ||
| Time from initiation of first-line chemotherapy (months) | ||||||
| ≥14.4 | 12 (63) | 28 (53) | 0.59 | 4 (31) | 14 (44) | 0.51 |
| <14.4 | 7 (37) | 25 (47) | 9 (69) | 18 (56) | ||
| Number of prior regimens | ||||||
| <3 | 14 (74) | 42 (79) | 0.75 | 12 (92) | 23 (72) | 0.24 |
| ≥3 | 5 (26) | 11 (21) | 1 (8) | 9 (28) | ||
| Drug administration history | ||||||
| Platinum | 17 (90) | 44 (83) | 0.72 | 9 (69) | 27 (84) | 0.41 |
| Ramucirumab | 14 (74) | 31 (59) | 0.28 | 10 (77) | 21 (66) | 0.72 |
| Ascites | ||||||
| No | 6 (32) | 31 (59) | 0.06 | 5 (38) | 15 (47) | 0.75 |
| Yes | 13 (68) | 22 (41) | 8 (62) | 17 (53) | ||
| LDH (IU/l) | ||||||
| <240 | 12 (63) | 28 (53) | 0.59 | 9 (69) | 22 (69) | 1.00 |
| ≥240 | 7 (37) | 25 (47) | 4 (31) | 9 (28) | ||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (3) | ||
| ALP (IU/l) | ||||||
| <380 | 9 (47) | 34 (64) | 0.28 | 6 (46) | 21 (66) | 0.32 |
| ≥380 | 10 (53) | 19 (36) | 7 (54) | 11 (34) | ||
| CEA (ng/ml) | ||||||
| <5 | 10 (53) | 21 (40) | 0.42 | 2 (15) | 13 (41) | 0.27 |
| ≥5 | 9 (47) | 31 (58) | 9 (70) | 18 (56) | ||
| Unknown | 0 (0) | 1 (2) | 2 (15) | 1 (3) | ||
| Alb (g/dl) | ||||||
| ≥3.5 | 8 (42) | 29 (55) | 0.43 | 6 (46) | 13 (41) | 0.75 |
| <3.5 | 11 (58) | 24 (45) | 7 (54) | 19 (59) | ||
| CRP (mg/dl) | ||||||
| ≤1.0 | 11 (58) | 33 (62) | 0.79 | 8 (62) | 19 (59) | 1.00 |
| >1.0 | 8 (42) | 20 (38) | 5 (38) | 13 (41) | ||
Alb, albumin; ALP, alkaline phosphatase; CEA, carcinoembryonic antigen; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; IRI, irinotecan; LDH, lactate dehydrogenase; NIVO, nivolumab.
There were differences in the intervals between the two scans used to assess TGR since the CT scans were managed according to the local practice by the physicians. We analyzed the numbers allocated to each group with intervals between the scans of ≤6 (Rapid group, n = 8; Slow group, n = 2), 6-12 (Rapid group, n = 51; Slow group, n = 30), and >12 weeks (Rapid group, n = 13; Slow group, n = 13) to assess whether this affected the allocation of patients to the Rapid and Slow groups. The results are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100179. Although the frequency of patients with intervals of ≤12 weeks was higher in the Rapid group than in the Slow group, this was not significantly different.
Comparisons of RR and PFS between NIVO and IRI treatment groups within the Rapid and Slow groups
The intervals from the initiation of NIVO or IRI to the date of the first CT evaluation were similar for patients who received NIVO and IRI among the Rapid and Slow groups (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100179).
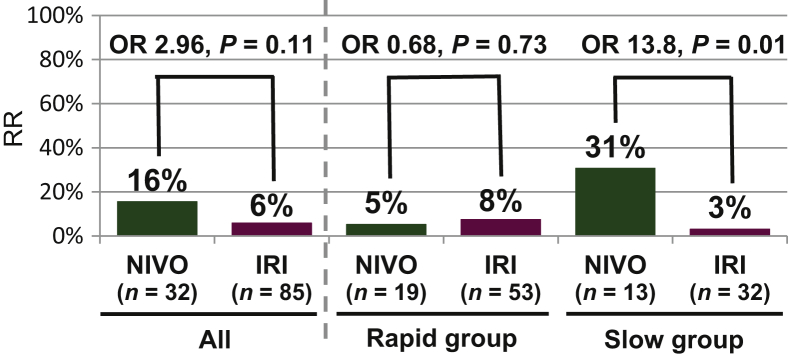
Among the total 117 patients, there was no significant difference in RR between the NIVO and IRI groups (16% versus 6%, respectively; OR, 2.96; 95% CI 0.80-11.00; P = 0.11). In the Slow group, the RR in the NIVO group was significantly higher than that in the IRI group (31% versus 3%; OR, 13.8; 95% CI 1.36-139; P = 0.01). Conversely, in the Rapid group, the RR in the NIVO group was similar to that in the IRI group (5% versus 8%; OR, 0.68; 95% CI 0.07-6.50; P = 0.73). There was a significant interaction between TGR (Rapid/Slow group) and treatment (NIVO/IRI) (P = 0.04).
In the multivariate analysis of predictive factors for obtaining response, the choice of NIVO or IRI was an independent predictive factor for obtaining response in the Slow group (adjusted OR, 52; 95% CI 3.85-2138; P = 0.002) with the covariates of age and ALP levels, but was not a predictive factor in the Rapid group (adjusted OR, 0.94; 95% CI 0.04-7.53; P = 0.96) with the covariates of ALP levels (Figure 2).
Figure 2.
RR between NIVO and IRI groups within the Rapid or Slow groups.
In the Rapid group, there was no difference in RR between the NIVO and IRI groups, whereas in the Slow group, the RR was significantly higher in the NIVO group than in the IRI group.
IRI, irinotecan; NIVO, nivolumab; OR, odds ratio; RR, response rate.
The DCR was similar between the NIVO and IRI groups (31% versus 35%, respectively; OR, 0.83; 95% CI 0.35-1.99; P = 0.68) in the whole population. In the Slow group, the DCR in the NIVO group tended to be higher than that in the IRI group (46% versus 28%, respectively; OR, 2.19; 95% CI 0.58-8.33; P = 0.25). In contrast, in the Rapid group, the DCR in the NIVO group tended to be lower than that in the IRI group (21% versus 40%, respectively; OR, 0.41; 95% CI 0.12-1.39; P = 0.13).
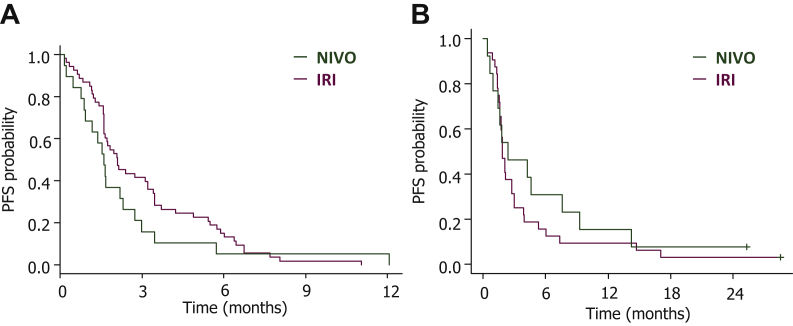
The PFS and OS were similar between the NIVO and IRI groups (median PFS, 1.7 versus 2.1 months, respectively; HR, 1.02; 95% CI 0.67-1.54; P = 0.94; median OS, 6.4 versus 6.3 months, respectively; HR, 0.83; 95% CI 0.52-1.33; P = 0.45) in the whole population. In the Slow group, the NIVO group showed numerically better PFS and OS values than the IRI group, but these were not statistically significant (median PFS, 2.4 versus 1.9 months, respectively; HR, 0.76; 95% CI 0.37-1.45; P = 0.41; median OS, 13.8 versus 6.6 months, respectively; adjusted HR, 0.84; 95% CI 0.35-1.76; P = 0.65; Figure 3). In the Rapid group, the PFS and OS were similar between the NIVO and IRI groups (median PFS, 1.6 versus 2.1 months, respectively; adjusted HR, 1.38; 95% CI 0.78-2.32; P = 0.26; median OS, 6.4 versus 6.2 months, respectively; HR, 0.84; 95% CI 0.45-1.46; P = 0.54).
Figure 3.
PFS between the NIVO and IRI groups.
In the Rapid group (A), there was no difference in PFS between the NIVO and IRI groups, whereas in the Slow group (B), the PFS was longer in the NIVO group than in the IRI group.
IRI, irinotecan; NIVO, nivolumab; PFS, progression-free survival.
RR at various TGR cut-off values
In the Slow group, the NIVO group had a higher RR than the IRI group, irrespective of the TGR cut-off value. However, in the Rapid group, the RRs were similar between the NIVO and IRI groups, even when the TGR cut-off value varied (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100179).
Discussion
In this retrospective study, the RR was higher in the NIVO group than in the IRI group in patients with low TGR and NL− during preceding treatment. This result suggests that patients with slow-growing tumors are better candidates for NIVO than IRI as later-line treatment in refractory AGC. Conversely, in patients with high TGR or NL+ during preceding treatment, the RR was similar between patients treated with NIVO and IRI, suggesting that both drugs could be equally recommended to patients with rapid-growing tumors. The DCR, PFS, and OS were consistent with these results; however, these results did not achieve statistical significance due to a small sample size. To the best of our knowledge, this study is the first report to evaluate the association between NIVO or IRI and TGR during preceding treatment in refractory AGC.
There are some reports supporting the higher RR of NIVO in slow-growing tumors in the present study. Metastatic melanoma patients with lower LDH levels, suggesting indolent tumor, despite being refractory to standard treatments showed a better response to anti-PD-1 antibody.8, 9, 10 In one of them, patients with low LDH levels had a higher RR than those with high LDH levels (43% versus 21%, respectively; OR, 2.52; P < 0.001).8 This report may be consistent with our findings because patients with slow-growing tumors tended to have lower LDH levels in the present study. However, based on the results of univariate and multivariate analyses, low TGR and NL− was a better predictive marker for tumor response to NIVO with superiority to IRI than low LDH levels in the present study. Moreover, in head and neck cancer, patients with low TGR at baseline were reported to respond to NIVO in a better manner and achieve longer survival.11 This report is also consistent with the result of the present study.
NIVO showed an RR of 11%, a median PFS of 1.6 months, and a median OS of 5.3 months in the ATTRACTION-2 trial.1 IRI showed an RR of 3%, a median PFS of 2.3 months, and a median OS of 4.0 months in a retrospective study, and IRI or paclitaxel showed an RR of 4.3%, a median PFS of 2.7 months, and a median OS of 5.0 months in the JAVELIN Gastric 300 trial.4,12 In the present study, efficacies of NIVO and IRI were similar to those observed in the previous reports. The TAGS trial, which compared trifluridine/tipiracil with placebo treatment for refractory AGC, revealed that the trifluridine/tipiracil group exhibited significantly longer survival compared with the placebo group.13 In this study, RR was 4% (95% CI 2% to 8%), median PFS was 2.0 months (95% CI 1.9-2.3 months), and median OS was 5.7 months (95% CI, 4.8-6.2 months) in the trifluridine/tipiracil group. Consequently, NIVO, IRI, and trifluridine/tipiracil have become available for third-line or later-line treatment of gastric cancer. The efficacies of these three drugs are similar, and which drugs to select in the third-line treatment for AGC patients remains an important issue to be resolved. Further studies are required to find a solution to this problem.
The present study has several limitations. First, this was a retrospective non-randomized study with a small sample size which included only patients with a measurable lesion. Therefore, it is difficult to draw definitive conclusions from the results of the present study because of the presence of various biases. In addition, because we could not evaluate patients without measurable lesions in the present study, further investigation is needed to evaluate them. Second, biomarker analyses, such as microsatellite instability (MSI) status, programmed death-ligand 1 (PD-L1) expression, and tumor mutation burden (TMB) status, were not carried out in the whole population, because these tests had not been yet covered by the national health insurance. NIVO may be more effective than IRI for MSI-high, PD-L1-positive, or TMB-high cases, which may have influenced the results of the present study. Third, the CT scan intervals used to assess TGR differed depending on the cases because they were determined by the attending physicians according to their clinical practice. However, there was no significant difference in the CT scan intervals between the Rapid and Slow groups (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100179). Finally, it is unclear whether the TGR cut-off value used in this study was appropriate. We confirmed that the trend of RR did not change despite varied TGR cut-off values. Although further studies are required to confirm the optimum TGR cut-off value for drug selection, our findings provide valuable information for clinical practice. Furthermore, this methodology using TGR and the presence or absence of NLs could be implemented as a stratification factor within the clinical trials in order to select and stratify the patients after additional validation.
In conclusion, our results indicate that treatment with NIVO is a more favorable option for patients with slow-growing tumors, although both NIVO and IRI are equally recommended for patients with rapid-growing tumors in refractory AGC. TGR and the presence or absence of NLs during preceding treatment may help refractory AGC patients and physicians to select a third-line treatment, warranting further investigation.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for English language editing, as well as all the patients who participated in this study and their families.
Funding
None declared.
Disclosure
The authors declare the following conflicts of interest. TM received honoraria from Takeda, Chugai, Merck Serono, Taiho, Bayer, Lilly Japan, Yakult Honsha, Ono, and Sanofi, and research funding from MSD, Daiichi Sankyo, and Ono. YK received honoraria from Taiho, Daiichi Sankyo, Takeda, Chugai, Merck Biopharma, and Eli Lilly Japan. YN received honoraria from Ono, Bristol-Myers Squibb, Eli Lilly, Yakult Honsha, Daiichi Sankyo, and Taiho, and research funding from Ono and Bristol-Myers Squibb. TT received honoraria from Ono, Eli Lilly, Chugai, Yakult Honsha, Taiho, Takeda, and Bayer. SK received honoraria from Chugai, Bristol-Myers Squibb, Ono, Taiho, Merck KGaA, Bayer, Eli Lilly, Daiichi Sankyo, Eisai, and Yakult Honsha, and research funding from Taiho, Chugai, Ono, Eli Lilly, Nobelpharma, and MSD. SY received honoraria from Takeda, Chugai, Bristol-Myers Squibb, Ono, Merck Serono, Taiho, MSD, Bayer, Eli Lilly, Medical & Biological Laboratories, Yakult Honsha, and Sanofi. MT received honoraria from EA Pharma and Olympus and research funding from EA Pharma. NM received honoraria from Ono, Bristol-Myers Squibb, Taiho, Daiichi Sankyo, Yakult Honsha, and Eli Lilly. YK received honoraria from Takeda, Chugai, Bristol-Myers Squibb, Ono, Merck Biopharma, Taiho, Bayer, Lilly, Yakult Honsha, Sanofi, Nipro, Moroo, Asahi Kasei, Mitsubishi Tanabe, Otsuka, Medical Review, and Shiseido, and research funding from MSD, Daiichi Sankyo, NanoCarrier, Eisai, Sysmex, Shionogi, IQVIA, Parexel International, Astellas, Mediscience, Sumitomo Dainippon, A2 Healthcare, Ono, Taiho, Bayer, Yakult Honsha, and Sanofi. KM received honoraria from Takeda, Chugai, Taiho, Bayer, Eli Lilly, Ono, Sanofi, and Bristol-Myers Squibb, and research funding from Parexel International, Merck Serono, MSD, Mediscience Planning, Pfizer, Daiichi Sankyo, Sanofi, Shionogi, Sumitomo Dainippon Pharma, Solasia Pharma, and Takeda. TK received honoraria from Taiho, Takeda, and Chugai. All remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Kang Y.K., Boku N., Satoh T. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs C.S., Doi T., Jang R.W. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hironaka S., Ueda S., Yasui H. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 4.Bang Y.J., Ruiz E.Y., Van Cutsem E. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuishi T., Taniguchi H., Kawakami T. Impact of tumour growth rate during preceding treatment on tumour response to regorafenib or trifluridine/tipiracil in refractory metastatic colorectal cancer. ESMO Open. 2019;4:e000584. doi: 10.1136/esmoopen-2019-000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph R.W., Elassaiss-Schaap J., Kefford R. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24:4960–4967. doi: 10.1158/1078-0432.CCR-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosrati A., Tsai K.K., Goldinger S.M. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116:1141–1147. doi: 10.1038/bjc.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weide B., Martens A., Hassel J.C. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22:5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki C., Kiyota N., Imamura Y. Effect of tumor burden and growth rate on treatment outcomes of nivolumab in head and neck cancer. Int J Clin Oncol. 2020;25:1270–1277. doi: 10.1007/s10147-020-01669-y. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura T., Iwasa S., Nagashima K. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer. 2017;20:655–662. doi: 10.1007/s10120-016-0670-9. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K., Doi T., Dvorkin M. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–1448. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.