Abstract
Microbial secondary metabolites are intensively explored due to their demands in pharmaceutical, agricultural and food industries. Streptomyces are one of the largest sources of secondary metabolites having diverse applications. In particular, the abundance of secondary metabolites encoding biosynthetic gene clusters and presence of wobble position in Streptomyces strains make it potential candidate as a native or heterologous host for secondary metabolite production including several cryptic gene clusters expression. Here, we have discussed the developments in Streptomyces strains genome mining, its exploration as a suitable host and application of synthetic biology for refactoring genetic systems for developing chassis for enhanced as well as novel secondary metabolites with reduced genome and cleaned background.
Keywords: Applications, Biosynthetic cluster of genes, Genome engineering, Heterologous host, Metabolites
Introduction
The genus Streptomyces represents soil-inhabiting Gram-positive and filamentous bacteria. Members belonging to Streptomyces are one of the largest sources of secondary metabolites such as antibiotics, herbicides, parasiticides, immunosuppressive agents, antitumor compounds and several other compounds of pharmaceutical and industrial importance (Bérdy 2005; Lewis 2013; Sharma et al. 2020; Salwan and Sharma 2020). Streptomyces have complex and multicellular lifecycle. The multicellular level of differentiation and ability to produce plethora of secondary metabolites by Streptomyces makes it one of the model prokaryotic system. However, the production level of secondary metabolites by potential Streptomyces strains is often low and often requires process optimization as well as genetic engineering of biosynthetic pathways for enhanced yield (Salas and Méndez 2007; Zabala et al. 2013). The pathway of different secondary metabolites is encoded as a contiguous stretch of DNA known as biosynthetic gene clusters (BGCs) (Osbourn 2010; Bauman et al. 2019). The advancements in genomic tools such as genome mining, genetic engineering and process optimization in last decade have played vital role in unraveling the potential of Streptomyces for different secondary metabolites production (Fig. 1). So far, the complete genome annotation of S. coelicolor A3, S. avermitilis and several other Streptomyces has been done (Ikeda et al. 2003; Ohnishi et al. 2008; Kim et al. 2017; Salwan et al. 2020; Salwan and Sharma 2020).
Fig. 1.
Overview of secondary metabolites: genome mining, genetic engineering and their biosynthetic pathways in Streptomyces
The high-throughput genome sequencing revealed that approximately 90% potential of these microorganisms is still cryptic and underexplored. This is largely due to the fact that many of BGCs are often silent under laboratory conditions and hence their corresponding metabolites remain uncharacterized (Bentley et al. 2002; Omura et al. 2001; Bauman et al. 2019). These BGCs are inexhaustible source of secondary metabolites. However, only a few of these BGCs have been efficiently expressed and a major share of these clusters is difficult to express under common cultivation conditions. The complex and modular organization of BGCs, their tight genetic regulation, complex physiology and environmental stimulus is a challenging task for successful expression (Myronovskyi and Luzhetskyy 2016). For industrial production of different secondary metabolites, several rational and irrational approaches have been tried to enhance the metabolites titers. Using conventional mutagenesis and screening, the significant increase in metabolites production has been achieved. However, in recent times, metabolic engineering emerged methods to obtain higher titers of secondary metabolites have gained special attention compared to the conventional approaches (Rokem et al. 2007). Using metabolic engineering, the entire biosynthetic pathway containing 20 genes encoding operons for nitrogenase, electron transport proteins, chaperones, and the biosynthetic pathway for the iron-molybdenum cofactor (FeMo-co) and other metalloclusters has been expressed successfully for transforming atmospheric N2 to ammonia (Smanski et al. 2014). The breakthrough in metabolic engineering for 6-deoxyerythronolide B (Pfeifer et al. 2001), taxadiene (Ajikumar et al. 2010), artemisinic acid (Paddon et al. 2013) and thiamine or hydrocodone have been achieved successfully in microorganisms (Galanie et al. 2015).
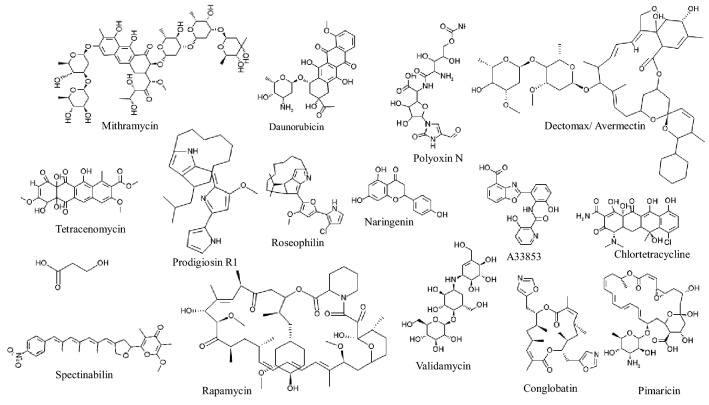
In recent studies, Streptomyces strains undoubtedly have received major attention as heterologous host system due to their high compatibility for heterologous biosynthetic genes cluster of > 80 kb size, better codon usage profile, high number of wobble positions, high G + C content and several other advantages compared to Escherichia coli and Saccharomyces cerevisiae (Sosio et al. 2000; Gustafsson et al. 2004; Yamanaka et al. 2014; Nah et al. 2015; Huang et al. 2016). Besides this, Streptomyces contains abundance of native metabolites (Fig. 2), precursors for the biosynthesis of other metabolites and ability to post-modify the chemical structures (Tan and Liu 2017). Different secondary metabolites have been successfully produced using heterologous host such as Streptomyces lividans (Rajgarhia et al. 2001; Bai et al. 2014; Peng et al. 2018), S. avermitilis (Komatsu et al. 2013, 2010), S. coelicolor (McDaniel et al. 1993) and S. albus J1074 (Zaburannyi et al. 2014) and other strains (Fig. 2; Table 1). These success stories led to the acceptance of Streptomyces as universal chassis for the production of different metabolites. For example, the knock-out developed through a series of native antibiotic pathways, genetic manipulation and fast replication period of about 3.6 h of Streptomyces sp. FR-008 has led to its development as model host for heterologous expression (Liu et al. 2016). Additionally, strains S. hygroscopicus TL01 (Zhou et al. 2011), S. albus BK3-25 (Lu et al. 2016), S. cinnamonensis C730 (Li et al. 2009a, b) and S. erythraea (Huang et al. 2016) have been optimized through mutations for heterologous expression (Pickens et al. 2011). Similarly, the higher titer has been obtained in S. albus Del14 compared to wild types of S. albus J1074 and S. coelicolor (Myronovskyi et al. 2018a, b).
Fig. 2.
Various secondary metabolites obtained from different species of Streptomyces
Table 1.
Examples of identification and expression of BGCs in Streptomyces
| S. no | Species | Metabolite | Host system | Approach | Application | References |
|---|---|---|---|---|---|---|
| 1. | S. albus subsp. chlorinus | Nybomycin | S. albus Del14 | BAC library | Nybomycin along with benzanthric was produced | Estévez et al. (2020) |
| 2. | S. nobilis JCM4274 | Desertomycins | S. lividans TK23 | BAC library | Reported for its antifungal activities | Hashimoto et al. (2020) |
| 3. | S. aureofaciens ATCC 10762 | Chlortetracycline (CTC) | Streptomyces rimosus 461 | Conjugation and integrated at attBφC31 site | Expression of ctc encoding BGC alongwith ctcB activator and two other genes led to higher production of CTC | Wang et al. (2019) |
| 4. | S. seoulensis A01 | Ansaseomycin | BAC containing asm cluster was transformed into S. lividans SBT18, S. coelicolor M1146, and S. albus J1074 | BAC library | Expression of cryptic BCG led to the production of ansaseomycins A (1) and B (2) production | Liu et al. (2019) |
| 5. | S. nanchangensis NS3226, S. avermitilis 3–115 | Ivermectin B1a |
S. lividans as heterologous host S. avermitilis 3–115 genetically engineered |
BAC library | Using PKS module 2 domain set of meilingmycin, a homologue of Avermectin, was used to generate ivermectin biosynthetic gene cluster | Deng et al. (2019) |
| 6. | S. rochei IFO12908 | Macrolactam JBIR-156 | S. avermitilis SUKA | BAC clone | Macrolactam potent cytotoxic activities | Hashimoto et al. (2019) |
| 7. | Streptomyces sp. CNB-091 | Streptophenazine | S. coelicolor M1146 | – | A cryptic hybrid phenazine-type BGC (spz) | Bauman et al. (2019) |
| 8. | S. coelicolor | Actinorhodin biosynthesis | Advanced multiplex site-specific genome engineering (aMSGE) method | Transformation associated recombination | Chromosomal integration of spz BGC into the ϕC31 attachment site | Li et al. (2019) |
| 9. | S. viridochromogenes, S. albus, S. lividans, S. roseosporus | Indigoidine, actinorhodin (ACT) and undecylprodigiosin (RED), Alteramide A | In native strains of Streptomyces | CRISPR–Cas9 | Activation of silent Streptomyces BGCs | Zhang et al. (2017) |
| 10. | S. gilvosporeus | Natamycin | S. gilvosporeus | Iterative random mutagenesis | Three successive mutagenesis led to 110%, 230%, and 340%, increase in the natamycin titer, respectively | Wang et al. (2016d) |
| 11. | S. albus DSM 41398 | Salinomycin | Wild and mutant derived from mutant strain | Overexpression | Combination of cluster deletions and overexpression of ccr gene led to increase in salinomycin titer from 0.60 to 6.60 g/L | Lu et al. (2016) |
| 12. | S. spectabilis, S. orinoci | Spectinabilin gene clusters | De novo cluster assembly | S. lividans | Exhibit antimalarial and antiviral activities | Shao and Zhao (2014) |
| 13. | S. coelicolor M145 | Actinorhodin | CRISPR/Cas9-CodA(sm) combined system | Native strain | Gene editing of actinorhodin ActI-ORF2 led to enhanced biosynthesis of actinorhodin | Zeng et al. (2015) |
| 14. | S. conglobatus ATCC 31005 | Conglobatin | Heterologous expression | S. coelicolor M1154 | Study provides proof of concept of using DNA fragments bearing an intact target gene cluster for rapid in vitro capture by Gibson assembly and direct cloning in E. coli | Zhou et al. (2015) |
| 15. | Streptomyces sp. NRRL12068 | Benzoxazole antibiotics A33853 | Heterologous expression | – | Bioactive against Leishmania | Lv et al. (2015) |
| 16. | S. hygroscopicus 5008 | Validamycin | Tandem deletion | Native strain | Tandem deletion of γ-butyrolactone (GBL) biosynthesis gene afsA and GBL receptor gene arpA enhanced validamycin production and productivity from by 26% and 45%), respectively | Tan et al. (2015) |
| 17. | S. pristinaespiralis | Pristinamycin II | A combinatorial metabolic engineering strategy | S. pristinaespiralis | The combination of PI and PII using Gibson assembly and systemic manipulation of papR1 to papR6 and spbR regulatory elements for higher titer | Li et al. (2015) |
| 18. | S. aureofaciens | Chlortetracycline | Fosmid-based gene inactivation and complementation | S. aureofaciens | Inhibits protein synthesis in bacteria, used as broad spectrum oral active antibiotics | Zhu et al. (2013) |
| 19. | S. orinoci | Spectinabilin | Synthetic biology approach-based “Plug-and-Play Scaffold” | – | The synthetic biology-based approach decouple the expression in native system with sophisticated regulation | Shao et al. (2013) |
| 20. | S. argillaceus M7W1 | Mithramycins | Overexpression of precursor genes | S. argillaceus ATCC12956 | The overexpression pgm or ovmGIH genes of S. coelicolor in S. argillaceus led to increased intracellular pool of glucose-1-phosphate and malonyl-CoA, respectively | Zabala et al. (2013) |
| 21. | S. tsukubaensis NRRL 18488 | FK506 or tacrolimus | A combination of metabolic engineering and Chemobiosynthetic approach | S. tsukubaensis NRRL 18488 | Inactivation of allR gene and addition of allylmalonyl-S-N-acetylcysteamine precursor resulted exclusive production of FK506 | Kosec et al. (2012) |
| 22. | S. aureochromogenes | Polyoxin N and nikkoxin D | Mutation and heterologous expression | S. aureochromogenes | Mutations and heterologous expression led production of four polyoxin-nikkomycin hybrid antibiotics | Zhai et al. (2012) |
| 23. | S. hygroscopicus ATCC 29,253 | Rapamycin | Mutagenesis and rational metabolic engineering | S. hygroscopicus | Immunosuppressive, antifungal, and anticancer activity | Jung et al. (2011) |
| 24. | S. hygroscopicus var. jinggangensis 5008 | Validamycin A and validoxylamine A | UDP-glucose pyrophosphorylase | S.hygroscopicus TL01 | Selective increase in validamycin A and decrease in validoxylamine A was achieved | Zhou et al. (2011) |
| 25. | S. spectabilis | Spectinabilin | fosmid library | S. lividans 66 | The heterologous production of nitrophenyl-substituted polyketide spectinabilin was obtained | Choi et al. (2010) |
| 26. | Streptomyces | Meridamycin | E. coli-Streptomyces shuttle Bacterial Artificial Chromosomal (BAC) | S. lividans | A non-immunosuppressive natural macrolide potential therapeutic applications in a variety of medical treatments | Liu et al. (2009) |
| 27. | S. nodosus | Amphotericin biosynthesis | Gene disruption | S. nodosus | Disruption of PMI and PMM genes resulted production of production of amphotericins and polyene aglycones | Nic and Caffrey (2009) |
| 28. | S. griseoviridis 2464-S5 | Prodigiosin R1 and roseophilin | Overexpression of disrupted the redN gene | S. coelicolor A3(2) | The expression of rphN in S. coelicolor lacking redN restored the prodigiosin production | Kawasaki et al. (2009) |
| 29. | S. cinnamonensis strains | Tetracenomycins | Expression of the entire tetracenomycin (TCM) biosynthetic pathway using an integrative plasmid, pSET154 | S. cinnamonesis and S. lividans TK24 | The high titer production for tetracenomycin obtained after using an integrative plasmid, pSET154 | Li et al. (2009a, b) |
| 30. | S. clavuligerus | Clavulanic acid | Double-reporter-guided mutant selection method | S. clavuligerus | The double-reporter-guided mutant selection method led to improve production of clavulanic acid | Xiang et al. (2009) |
| 31. | S. natalensis | Pimaricin | Gene inactivation or deletion | S. natalensis | Disruption of phoR-phoP revealed phosphate control of pimaricin biosynthesis | Mendes et al. (2007) |
| 32. | S. ansochromogenes | Nikkomycins | sanJ gene inactivation | S. ansochromogene | SanJ, an ATP-dependent picolinate-CoA ligase activate picolinate to give the nikkomycin intermediate | Niu et al. (2006) |
| 33. | S. clavuligerus | Clavulanic acid | Targeted gene disruption | S. clavuligerus | Rational strain improvement using genetically engineering of gene dosage dependent construction of the glycolytic pathway | Li and Townsend (2006) |
| 34. | S. coelicolor M512 | Clorobiocin derivatives and elloramycin | Overexpression | S. coelicolor M512, S. lividans TK64 | Heterologous expression of four genes from the oleandomycin BGC directed the synthesis of dTDP-rhamnose, led to a 26-fold increase of the production of glycosylated aminocoumarins | Freitag et al. (2006) |
| 35. | S. ansochromogenes | Nikkomycin | Duplication of genes-sanU and sanV genes | S. ansochromogenes | A plasmid containing extra copy of sanU and sanV led to 1.8 fold of nikkomycin compared to wild-type strain | Li et al. (2005) |
| 36. | S. avermitilis | Avermectin derivative commercially known as Dectomax™ | Iterative semi-synthetic DNA shuffling | S. avermitilis | Iterative cloning of aveC gene using semi-synthetic DNA shuffling led to improve doramectin production | Stutzman-engwall et al. (2005) |
| 37. | S. coelicolor | Calcium-dependent antibiotic (CDA) | Metabolic flux analysis | S. coelicolor | Investigation through computational metabolic flux and genetic deletions increased CDA production | Kim et al. (2004) |
| 38. | S. clavuligerus | Total β-lactams | Overexpression of expandase gene | Penicillium chrysogenum | Expression of expandase gene led to two-fold increase in the adipoyl-6- aminopenicillanic acid specific productivity | Robin et al. (2003) |
| 39. | Streptomyces sp. strain C5 | Daunorubicin | – | S. lividans TK24, S. coelicolor CH999 | Expression of the dpsABCDEFGdauGI genes resulted first stable chromophore aklanonic acid, for daunorubicin biosynthesis | Rajgarhia et al. (2001) |
| 40. | S. coelicolor | Actinorhodin | Metabolic flux analysis | S. coelicolor | Metabolic flux analysis indicated nitrogen as limited for actinorhodin production | Naeimpoor and Mavituna (2000) |
With the availability of Streptomyces complete genomes, deep insight of biosynthetic pathways and advancements in genetic engineering has resulted in the development of better rational strategies for metabolic engineering (Olano et al. 2008; Papagianni 2012). The secondary metabolite biosynthesis involves precursors derived from glycolysis, tricarboxylic acid cycle (TCA) and pentose–phosphate pathway (PPP) (Rokem et al. 2007). Therefore, metabolic engineering is performed in programmed way via increased flux of precursors towards the targeted metabolite biosynthesis (Olano et al. 2008; Zabala et al. 2013). For example, the clavulanic acid production in S. clavuligerus has been enhanced by the inactivation of the glyceraldehyde-3-phosphate dehydrogenase gene (gap1) (Li and Townsend 2006). Similarly, higher titer of actinorhodin (act) and undecylprodigiosin has been achieved by deactivating the phosphofructokinase encoding gene (pfkA) or glucose-6-phosphate dehydrogenase genes (zwf1 or zwf2) in S. coelicolor and/or S. lividans (Butler et al. 2002; Borodina et al. 2008; Ryu et al. 2006). The expression of specific building blocks has also been deployed for enhanced production of specific metabolites. For example, the overexpression of the acetyl-CoA carboxylase encoding genes in S. coelicolor or in S. cerevisiae resulted higher yields of 6-methylsalicylic acid (Ryu et al. 2006; Wattanachaisaereekul et al. 2008; Zabala et al. 2013). A two-to-threefold increase in clavulanic acid production has been obtained by the overexpression of positive regulatory elements (Paradkar et al. 2001; Li and Townsend 2006), whereas inactivation enhanced secondary metabolites production in the wild type. Besides this, manipulation of UTP–glucose or pentose–phosphate supply is reported to significantly enhance the validamycin production (Zhou et al. 2011; Liao et al. 2009). Still, the activation/expression of cryptic BGCs is a challenging task and of paramount importance for the novel product discovery as well as their enhanced production. Several approaches have been used to activate the metabolic pathways such as optimization of cell-culture conditions, physiological and environmental inducers, modulation of regulatory switches, synthesis and expression of clusters in heterologous hosts and suppression of competitive secondary metabolites encoding clusters (Bode et al. 2002; Rigali et al. 2008; Tanaka et al. 2010; Craney et al. 2012; Olano et al. 2014) (Fig. 1).
The rewiring/refactoring of BGCs using synthetic biology strategies and deploying the recombined modules has been done for efficient production of different secondary metabolites (Rajgarhia et al. 2001; Li et al. 2005; Stutzman-engwall et al. 2005; Freitag et al. 2006; Mendes et al. 2007; Li et al. 2009a, b; Fischbach and Voigt 2010; Medema et al. 2011c; Shao et al. 2013; Zeng et al. 2015; Lu et al. 2016; Zheng et al. 2017; Bauman et al. 2019) (Table 1). The rational method of engineering has been improved using integration of mutagenesis and metabolic modeling (Breitling et al. 2008; Medema et al. 2011a; Nguyen et al. 2012). The poor understanding of biosynthetic pathways of different metabolites and complexity of transcriptional regulation are found to restrict the metabolic engineering of BGCs for efficient and higher production of secondary metabolites (Shao et al. 2013). In addition, lack of information about unexplored metabolites and their biosynthetic pathways limits the rational redesign/strategies. In Streptomyces, the conventional protocols used for cloning BGCs require construction and screening of cosmid or BAC libraries. The genetic manipulation of BGCs with small gene clusters has been performed using either cosmid or BAC clone. Although, the BAC vectors are capable of maintaining large inserts however, low copy number is a major limitation which restricts the genetic manipulation due to low DNA recovery. To overcome this, a new shuttle BAC vector-based system has been developed for E. coli–Streptomyces which can enable the convenient switching from single-copy number to high-copy replication (Liu et al. 2009, 2014). The strategies such as constructing a series of plasmids harboring a subset of the biosynthetic pathway gene followed by the sequential transformation of entire pathway into a heterologous host system (Xue et al. 1999; Tang et al. 2000) or deploying Red/ET recombinant technology by “stitching” several cosmids to assemble the complete pathway into a heterologous host have been used to clone the BGCs containing large gene clusters. For example, using simple restriction digestion of DNA and cloning approach, the complete operon consisting of a neuroprotective polyketide of ∼ 90 kb gene cluster encoding meridamycin has been captured (Newman et al. 2003; Liu et al. 2009). After successful heterologous expression of spectinabilin, 45.3 kb size of the 79C fosmid has been elucidated using transposon approach (Choi et al. 2010). The complete analysis of the fosmid containing sequence revealed open reading frames (spnA-M) for spectinabilin production (Choi et al. 2010).
The large-scale genome sequencing availability and their annotations led to the identification of several cryptic gene clusters which can be explored for novel products (Baltz 2008) by modulating their activation through genetic manipulation (Baltz 2008; Gottelt et al. 2010; Laureti et al. 2011; Gomez-Escribano and Bibb 2012a). Moreover, none of the conventional approaches can be applied for cluster activation which implies the presence of unique regulatory mechanisms that govern the expression of individual natural products. Over the past 30 years, the identification of the chemical structure of different secondary metabolites followed by their correlation to biosynthetic pathways has remarkably increased our understanding. Here, in this review, we have discussed the recent developments in genome mining and the genetic manipulations of Streptomyces species as heterologous host for enhanced production of novel secondary metabolites.
Bioinformatic approaches for mining Streptomyces genome for biosynthetic gene clusters
The identification and characterization of secondary metabolites encoding biosynthetic gene clusters (BGCs) have significantly enhanced the discovery of novel metabolites as well as biosynthetic pathways. The comprehensive view on the evolution and phylogenetic methods of secondary metabolites have been discussed (Schmitt and Barker 2009; Ziemert et al. 2016). Initially, when the complete genome sequence databases were not available, the ketosynthase domain in the BGCs of PKS gene clusters were one of the enzymes studied for phylogenetic analysis and structure depiction (Moffitt and Neilan 2003; Gontang et al. 2010). The evolution of genome-mining tools and refinement of systematic approaches, has led to the identification of approximately 10,000 BGCs. The genome of Streptomyces with high-GC content of 70–75% and a large number of repetitive sequences is often difficult to sequence using Sanger method (Sanger et al. 1977). Although, it is difficult to sequence and assemble the BGCs, still the elucidation of first complete genome of Streptomyces was done by the same method (Cerdeno et al. 2001; Bentley et al. 2002). So far, over 384 genome sequences of Streptomyces including draft assemblies have been done (GenBank database as of 2020). Starting from complete genome sequences of S. coelicolor (Bentley et al. 2002) and S. avermitilis (Ikeda et al. 2003), an unexplored repository of natural products is available in Streptomyces genome. Prior to large-scale high-throughput genome sequencing, reverse genetics-based approaches were primarily used to explore the genetic libraries of potential isolates and then screened for core biosynthetic gene assembly (Ziemert et al. 2016). The “IMG-ABC v.5.0 (Krishnaveni et al. 2020)” and “Integrated Microbial Genomes” Platform of the Joint Genome Institute (JGI IMG-ABC) (Hadjithomas et al. 2015; Chen et al. 2019) and antiSMASH (Blin et al. 2019) alongwith other computational tools are nowadays de facto standard for mining BGCs. The first proprietary pipeline known as DECEIPHER®, developed by Ecopia Biosciences Inc. was reported for automated BGCs mining (Farnet and Zazopoulos 2005; Ziemert et al. 2016). Using Sanger sequencing, Ecopia’s DECIPHER® tool and other databases, BGCs responsible for warhead formation of enediyne were explored (Zazopoulos et al. 2003). Thereafter, strategy was also explored for the biosynthesis of modular type I PKS complex involved in antifungal ECO-02301 biosynthesis (McAlpine et al. 2005). The other genome annotation tools such as PRISM are capable of networking genomic data with chemical structure and resistance genes (Skinnider et al. 2015).
Several other tools have been developed thereafter, for mining secondary metabolite biosynthetic pathways of PKS and/or NRPS (Rausch et al. 2005; Starcevic et al. 2008; Li et al. 2009a, b; Röttig et al. 2011; Skinnider et al. 2015) or RiPPs (de Jong et al. 2006, 2010; van Heel et al. 2013). These tools scan reference genomic data for known and highly conserved biosynthetic domains. The MultiGeneBlast algorithm-based stand-alone software (http://multigeneblast.sourceforge.net/) (Medema et al. 2013) is also used for the identification of similar operons for any given sequence. Till the date of publication, IMG-ABC contains over 4, 12,718 of total gene clusters in JGI's genomic databases and only 60,445 of BGCs have been linked with antiSMASH and the majority of these are orphan in nature (https://img.jgi.doe.gov/cgi-bin/abc-public/main.cgi). On the other side, antiSMASH one of the most comprehensive tool to perform recent version is being used for finished or draft-quality genome sequences (Medema et al. 2011b; Blin et al. 2013; Weber et al. 2015a, b) (https://antismash.secondarymetabolites.org/). Thereafter, additional tools such as BAGEL (de Jong et al. 2006) and CLUSEAN (Weber et al. 2009) have been developed. Comprehensive information of “The Secondary Metabolite Bioinformatics Portal” has been released http://www.secondarymetabolites.org (Weber and Kim 2016).
So far, only 1800 secondary metabolites from different organisms and their cognate BGCs have been characterized and recorded in a repository known as Minimum Information about a Biosynthetic Gene cluster (MIBiG). It can be used for the investigation of newly sequenced genomes. In general, identification of canonical enzyme sequences is done using similarity searches. Although, it is efficient, still the probability of the identification of novel systems is limited. Alternatively, genome-mining tools such as EvoMining (Sélem-Mojica et al. 2019) which is based on phylogenomics method termed as CORe Analysis of Syntenic Orthologs to prioritize Natural product BGCs, or CORASON and Antibiotic Resistant Target Seeker’ (ARTS) exploring the presence of resistant target genes in the proximity of BGC, respectively, have been explored. EvoMining complements conventional genome-mining methods as well as provide insights into secondary metabolite biosynthetic evolution, whereas ARTS help in genome mining and simultaneously linking housekeeping and known resistance genes to BGCs (Sélem-Mojica et al. 2019; Mungan et al. 2020).
Approximately 12.7% of BGCs listed within MIBiG repository has been described as a chemical dark matter (Zhang and Moore 2015). EvoMining uses sequence similarity searches by deploying evolutionary principles (Barona-Gómez et al. 2012; Cruz-Morales et al. 2016) and resulted in an increase of 15 and 26% for antiSMASH predictions in Actinobacteria genomes (Cruz-Morales et al. 2016). According to EvoMining during enzyme evolution, enzyme families may expand due to gene duplication and or horizontal gene transfer. The acquired extra gene copies may evolve into novel enzyme (Jordan et al. 2001) and hence serve for new secondary metabolic pathways. EvoMining have successfully anlaysed 42 core metabolic enzyme families which are conserved in entire actinobacteria, other microorganisms and arsenolipids biosynthesis in Streptomyces (Cruz-Morales et al. 2016). The ARTS-based genome-mining tool has been used to predict the uncharacterized BGCs based on resistance genes which are co-located within or proximity to the antibiotic BGCs (Yan et al. 2018). It detects potential resistant housekeeping genes based on their duplication, co-localization within BGC, and Horizontal Gene Transfer (HGT). The workflow of the ARTS pipeline can be used for bacterial genomes, metagenomes and for the comparative analysis of multiple genomes. In ART, initial screening for BGCs is performed using antiSMASH and simultaneously essential housekeeping genes are identified using TIGR-FAM. After that, the identified core and known resistance genes are evaluated for their location within BGCs (Mungan et al. 2020). Other tool known as ‘biosynthetic gene similarity clustering and prospecting engine’ (BiG-SCAPE) and ‘core analysis of syntenic orthologues to prioritize natural product gene clusters’ (CORASON) provide rapid and interactive similarity analysis of BGCs and gene cluster families. BiG-SCAPE has been validated by metabolomic data across 363 actinobacterial strains, whereas the potential of CORASON has been demonstrated by mapping biosynthetic diversity of detoxin/rimosamide-related gene cluster families which led to the characterization of 7 detoxin analogues (Navarro-Muñoz et al. 2020). Although the discovery of novel biosynthetic pathways based on evolutionary parameters such as EvoMining, CORASON or ARTS platforms are limited, so it is predicted that they can contribute to antibiotics discovery (Chevrette and Currie 2019; Sélem-Mojica et al. 2019).
On an average, Streptomyces genome comprises ~ 30 BGCs (Belknap et al. 2020). However, only limited number of the predicted BGCs and their corresponding products has been characterized. The basic structural organization of BGCs consists of PKS responsible for polyketides biosynthesis, NRPS for biosynthesis of non-ribosomal peptides, post-translationally modified peptides (RiPPs) and aminoglycosides. Recently, the resistance and target-based approaches to identify the BGCs using self-resistant mechanisms of an antibiotic production has been developed. Besides coding for secondary metabolites, the BGCs also code regulatory elements, efflux pumps, degrading enzymes and mechanism for self-resistance to prevent damage to self (Cox and Wright 2013; D'Costa et al. 2011). The use of “Genome Neighborhood Networks (Zhao et al. 2014), an expanded variant of sequence similarity networks (Atkinson et al. 2009) has been used to explore the BGCs such as enediyne biosynthesis (Rudolf et al. 2016).
The biosynthetic pathway of anti-leishmanial antibiotic A33853 isolated from Streptomyces sp. NRRL12068 has been deduced by bioinformatics approach and functional elucidation of different ORFs (Michel et al. 1984; Tipparaju et al. 2008). The gene cluster encoding A33853 revealed group of unusual enzymes such as a ketosynthase (BomK) and their role in amide linkage between 3-hydroxypicolinic and 3-hydroxyanthranilic acid. Thereafter, a putative ATP-dependent coenzyme A ligase (BomJ), and a putative amidohydrolase (BomN) were assigned using deletion study. The availability of complete genome of Streptomyces ambofaciens led to the finding of congocidine and spiramycin biosynthetic clusters of 150 kb size. It encodes 25 genes which includes 9 for type I PKS (Laureti et al. 2011). Presently, the JGI IMG-ABC database represents over 4, 13,028 BGCs and the majority of them are orphan. The presence of high-GC regions is a hallmark of Streptomyces, however, repetitive regions in their genomes still remains a challenge for complete genome sequencing. However, compared to conventional Sanger method, high throughput next generation like Pacbio SMRT, Nanopore sequencing and advanced computational algorithms tools have created high-quality genomic repository as well as resolved the problems of repetitive sequences (Chin et al. 2013) and have mined the rich and diverse reservoir of Streptomyces even for cryptic pathways (Liu et al. 2018).
Regulation and engineering of BGCs in Streptomyces
To overcome the low production of secondary metabolites at industrial level, most of the approaches relies on the use of ‘‘classical’’ methods such as mutations followed by the selection of improved fermentation and downstream processing. Although, the lab methods deploy sophisticated genetic and biochemical tools, the fundamental blueprints of various secondary metabolite gene clusters revealed that synthetic biology approaches persisted from millions of years in nature. The advancements in next-generation sequencing revealed hundreds of high-quality draft genomes and dozens of partial genome sequences of Streptomyces (Wohlleben et al. 2012). Furthermore, using advanced and automated tools such as antiSMASH, these genomes can be anlaysed even for cryptic pathways and other BGCs (Wohlleben et al. 2012). Earlier to this, the identification of key genes using inverse metabolic engineering was one of the preferred methods (Bailey et al. 1996). The role of different genes can be identified by comparative study of wild-type and mutant strains for a trait specific phenotype using genomic, transcriptomics, proteomics, and/or metabolomics profiling. For the identification of key target genes, genetically diverse mutants have been developed (Askenazi et al. 2003). Use of metabolic engineering for targeted expression of the rate-limiting genes and enhanced supply of precursors or deletion of the competitive pathway have been successfully explored in titer improvement (Hung et al. 2007; Li and Townsend 2006; Paradkar et al. 2001). For example, the overexpression of cas2 and ccaR is found to enhance clavulanic acid and cephamycin C biosynthesis (Perez-Llarena et al. 1997; Hung et al. 2007). The expression of BGCs cassette for secondary metabolites production involves a rigorous and complex regulation of various cellular and physiological events as well as impact of environment is often a major limiting factor in the final production (Liu et al. 2013).
For modulating the expression of BGCs and developing approaches for optimized expression, it is essential to understand the biosynthetic pathways. For example, the biosynthesis of FK506 macrolactone is catalyzed by PKS-mediated condensation of shikimic acid-derivative i.e. 4,5-dihydroxycyclohex-1-enecarboxylic acid and the incorporation of extender units such as two malonyl-CoA, five methylmalonyl-CoA, two methoxymalonyl-ACP and one unusual allylmalonyl-CoA extender unit into nascent polyketide chain (Fig. 3b). Thereafter, addition of pipecolic acid derived from lysine and NRPS gene (fkbP) mediated cyclization results in intermediate of FK506 macrolactone. At final step, processing by a specific methyl transferase and oxidoreductase resulted in FK506 biosynthesis (Motamedi et al. 1996; McDaniel et al. 2005; Gregory et al. 2006; Goranovic et al. 2010). Structurally, FK506 differs from FK520 only in the side chain at the carbon 21 where the former contains an allyl residue, while the latter contains an ethyl residue. The structural differences corresponding to extender units for allylmalonyl-CoA or ethylmalonyl-CoA for FK506 or FK520 have been identified and determined for their role by sequencing of BGCs from Streptomyces sp. MA6548 and S. hygroscopicus var. ascomyceticus (Motamedi and Shafiee 1998; Wu et al. 2000). Several genes for FK520 production in S. hygroscopicus ar. ascomyceticus have been identified for their role in a 4-carbon extender unit ethylmalonyl-CoA (Wu et al. 2000). Besides this, another extender unit ethylmalonyl-CoA responsible for acetate assimilation in diverse bacteria has been identified (Erb et al. 2007). In comparison to this, sub-clusters of BGCs of FK520-producing strains S. tsukubaensis NRRL 18,488 has been identified (Goranovic et al. 2010). Using chemobiosynthetic approach, the biosynthesis of FK506 in S. tsukubaensis strain has been successfully achieved after the inactivation of a homologue of crotonyl-CoA carboxylase/gene allR of FK506 biosynthetic cluster (Fig. 3b). The inactivated strains produced neither FK506 nor FK520. However, addition of allylmalonyl-S-N-acetylcysteamine precursor restored FK506 production without formation of side products. The exclusive production of FK506 has been achieved using a combination of chemobiosynthetic and metabolic engineering approach (Mrak et al. 2012).
Fig. 3.
Overview of the secondary metabolites produced by the modulated expression/inactivation of the corresponding genes are indicated by red arrow: a in mithramycin, gene duplication of a phosphoglucomutase (pgm) enhances supply of precursor glucose-1-phosphate, required for mithramycin biosynthesis; b using chemobiosynthetic approach, the biosynthesis of FK506 in S. tsukubaensis strain has been achieved after inactivation of a homologue of crotonyl-CoA carboxylase/gene allR or overexpression of methylmalonyl-CoA of FK506 biosynthetic cluster; and c the biosynthesis of actinorhodin requires a two-component enzyme system containing one flavin-dependent monooxygenase (FMO) ORF known as-ActVA-ORF5 and other ActVB, a flavin:NADH an oxidoreductase. The genes homologous to two-component FMOs are also reported in granaticin, medermycin and other similar antibiotic alnumycin
However, the activation of cryptic BGCs, recruitment of essential building blocks, modulation at the level of transcription and translation of genes and low efficiency of heterologous expression is still challenging (Rutledge and Challis 2015; Bauman et al. 2019). Moreover, the selection of host, limitations of transformation protocols for multiple genes, selection markers for the biosynthesis of natural products are limited to certain hosts. However, the advancements in heterologous expression systems have increased success rate for the bioengineering for natural product discovery. The use of codon optimization, synthetic biology for desired genetic manipulations and manipulation of specific regulatory elements of specific BGCs, optimized expression through promoters and ribosome-binding sites modulation to refactor the native genetic architecture are other probable strategies which can be applied for improved production as well as for the novel compounds’ biosynthesis (Olano et al. 2008; Montiel et al. 2015; Myronovskyi and Luzhetskyy 2016; Freestone et al. 2017). The method for heterologous expression of BGCs in a suitable vector and strain followed by the expression under optimized conditions and then analysis of the final products by comparative metabolites profiling has been described (Pickens et al. 2011; Gomez-Escribano and Bibb 2012b; Myronovskyi and Luzhetskyy 2016).
The understanding of biosynthetic pathways and their regulation has helped in developing both semi-rational and rational strategies for strain improvement. The activation of metabolic pathways to engineer the BGCs for improved production either involves the expression of positive regulatory elements or repressors inactivation. Both the pleiotropic and cluster-type regulators have been identified at transcriptional level for the regulation of secondary metabolism in Actinobacteria (Martin and Liras 2010). Using genomics-based approach, three types of regulatory elements; (a) global regulators involved in morphological differentiation, (b) pleiotropic regulators controlling multiple pathways, and (c) pathway-specific regulators responsible for the titers of a single or multiple compounds from the same gene cluster have been identified within Streptomyces (Chen et al. 2008; Romero-Rodriguez et al. 2015). At cellular level, pathway-specific and global regulators are found to control the production of secondary metabolites in Streptomyces strains. The pathway-specific regulatory elements are positioned on the BGCs of secondary metabolites affecting only the production of corresponding secondary metabolites. On the other side, the global regulatory elements are known for their pleiotropic nature and show multiple effects such as morphological differentiation and metabolite production. The global regulatory elements are positioned outside the BGCs unit and indirectly play role in metabolite production (Bibb 2005; Takano 2006; Martin and Liras 2010). Several pathway-specific regulators such as ActII-ORF4 for actinorhodin (act) biosynthesis of S. coelicolor A3 and DnrI for doxorubicin biosynthesis of S. peucetius belonging to the SARP (Streptomyces antibiotic regulatory protein) family of regulators, whereas other pathway-specific regulators such as the FkbN genes belonging to LAL family belong to other families are already identified in several polyketide biosynthetic gene clusters (Arias et al. 1999; Sheldon et al. 2002; Wilson et al. 2001; Oliynyk et al. 2003; Rascher et al. 2005; Kuščer et al. 2007; He et al. 2008; Hur et al. 2008; Kitani et al. 2009; Mo et al. 2012). A number of global transcriptional regulators including AdpA, PAS–LuxR transcriptional regulators (Santos-Aberturas et al. 2011), two-component PhoP–PhoR system (Mendes et al. 2007), WhiB-like protein WblA (Huang et al. 2017), the nitrogen regulatory protein GlnR (Qu et al. 2015), and the GntR-family transcription regulator DasR (Zhang et al. 2016a, b) have been identified for BGC activation in Streptomyces and other actinobacteria for enhanced metabolite production (Tan et al. 2013; Tan and Liu 2017).
The cluster-situated regulatory genes such as hrmA and hrmB genes are found to modulate the hormaomycin biosynthesis in S. griseoflavus W-38 (Cai et al. 2013). Similarly, the regulatory elements such as sgcR1 and sgrR regulate the biosynthesis of enediyne antitumor antibiotic C-1027 in S. globisporus (Li et al. 2016). The SACE_7301, a TetR family regulator are reported for positive regulation in enhancing erythromycin production in S. erythraea A226 (Wu et al. 2014). The pathway-specific regulatory elements fkbN and tcs7 in Streptomyces sp. KCTC 11604BP are found to enhance the level of FK506 production even in industrial strains (Mo et al. 2012). The different regulators are found to enhance the availability of precursors required for the biosynthesis of targeted metabolites (Tan and Liu 2017). For example, the expression of uridine diphosphate (UDP)-glucose pyrophosphorylase gene (ugp) enhanced UDP-glucose level required for validamycin production in S. hygroscopicus TL01 (Zhou et al. 2011). Furthermore, the duplication of phosphoglucomutase gene (pgm) in S. argillaceus duplication of BGCs for validamycin and pristinamycin led to increased level of precursor glucose-1-phosphate required for anticancerous antibiotic mithramycin biosynthesis (Zabala et al. 2013) (Fig. 3a), validamycin and pristinamycin production in Streptomyces pristinaespiralis (Murakami et al. 2011; Li et al. 2015). On the other side, the deletion of multiple gene clusters of PKS/PKS-NRPS resulted malonyl-CoA and methylmalonyl-CoA for salinomycin biosynthesis in S. albus BK3-25 (Lu et al. 2016).
On the other hand, promoters of inducible nature enable genes and operons regulation. A number of inducers such as tetracycline, thiostrepton, xylose, cumate, glycerol, caprolactame, and resorcinol have been studied for actinobacteria (Holmes et al. 1993; Herai et al. 2004; Rodríguez-García et al. 2005; Baltz 2008; Rudolph et al. 2013; Wang et al. 2016a, b, c, d). In Streptomyces, positive and negative regulatory elements have been characterized for BGCs. By changing the level of positive and decreasing negative regulators, titers of secondary metabolites can be improved (Hong et al. 2007; Yuan et al. 2011; Zhao et al. 2015). For FK506 encoding BGC, fkbN, a positive and tcs7, a negative regulator has been described (Mo et al. 2012). Alternatively, incorporation of multiple copies positive regulators and/or transporter genes (Tu et al. 2018) is also done to achieve high titer production. In general, the regulatory elements associated to BGCs are clustered outside the structural biosynthetic gene cluster and make their activation challenging. So far, only, 30% of BGCs obtained from streptomycetes have been expressed in another Streptomyces. A number of regulatory elements have been characterized so far. For example, within fdm cluster, three regulatory elements fdmR, fdmR1, and fdmR2 have been identified for FDM A production. The inactivation of fdmR1 in S. griseus ATCC 49344 strain was found to suppress the FDM A level, whereas the overexpression of same resulted sixfold titer of ~ 1 g/L (Teijaro et al. 2019). Similarly, the overexpression of a 65-kb mgs gene cluster of S. platensis NRRL 18993 for iso-MGS production in heterologous host such as S. albus J1074, S. coelicolor M512, S. lividans K4-114, S. avermitilis SUKA4, and S. avermitilis SUKA5 resulted variable level of titer in all hosts (Feng et al. 2009; Wu et al. 2010; Yang et al. 2011; Teijaro et al. 2019). The strain has been improved using genetic manipulation of pathway-specific regulators or even global regulators and other rate-limiting enzymes (Adrio and Demain 2006; Olano et al. 2008; Pickens et al. 2011; Baltz 2011; Weber et al. 2015a, b; Zhang et al. 2016a, b). The genetic manipulations have increased the titer in industrial strains such as chlorotetracycline biosynthesis in S. aureofaciens (Zhu et al. 2013), salinomycin synthesis in S. albus (Lu et al. 2016), and natamycin formation in S. chattanoogensis L10 (Jiang et al. 2013; Yu et al. 2014; Liu et al. 2015; Wang et al. 2016a, b, c, d). The refactoring of streptophenazine BGC related four operons under the regulation of actIp, ermEp, sp44 and p21 promoters in S. coelicolor M1146 as heterologous host led to the production of over 100 new streptophenazine active analogues. Similarly, refactoring of bottromycin BGCs in S. lividans, resulted in 5–50-fold increase of bottromycin A2 and production of new bottromycin derivatives (Yang et al. 2019). Alternatively, replacement of native regulatory genes with synthetic regulators has been used for heterologous production of metabolites.
Semi rational methods such as use of promoter of a key biosynthetic gene with reporter gene can be helpful in the selection of improved strains obtained after random mutagenesis (Askenazi et al. 2003; Xiang et al. 2009; Guo et al. 2015). Antibiotic belonging to the benzoisochromanequinone class is produced by S. coelicolor A3(2). The biosynthesis of act require a two-component enzyme system containing one flavin-dependent monooxygenase (FMO) ORF known as—ActVA-ORF5 and other ActVB, a flavin:NADH an oxidoreductase (Fig. 3c). The genes homologous to two-component FMOs are also reported in granaticin, medermycin and other similar antibiotic alnumycin. The functional analysis of ActVA-ORF5, and Gra-ORF21 type of FMO in S. coelicolor and their potential in p-quinone and hydroxylation formation at C-6 and C-8 in the central ring and the lateral ring of a tricyclic substrate, respectively, revealed their bifunctional nature. However, the two-component FMOs system involved in the biosynthesis of act, granaticin, medermycin and alnumycin are found to have distinct functional specificities (Taguchi et al. 2013).
Using combinatorial metabolic engineering, improved production of PII has been achieved by incorporating extra copy of PII BGC. Here, using modified Gibson assembly, large fragments of DNA with high-GC content has been cloned into S. pristinaespiralis HCCB10218 (Li et al. 2015, 2019). The duplication of corresponding BGC enhanced PII titer upto 45%. Besides this, all the cluster-located regulatory genes i.e. papR1, papR6, and spbR have been systematically engineered. Higher titer production has been achieved by the overexpression of activator genes papR4 and papR6 in combination of deletion of repressor genes papR3 or papR5. The combinatorial biosynthesis resulted strains ΔpapR3þR4R6 and ΔpapR5þR4R6 resulted for higher PII production up to 99% and 75%, respectively. The integration of the assembled PII gene cluster (BAC-F1F15) into ΔpapR5þR4R6 approximately increased 1.5-fold higher titer of PII compared to the parent strain (Li et al. 2015).
Using isotope-labeled precursors, the biosynthetic pathway for rapamycin was established in early 1990s (Dumont and Su 1996). It was also established that its macrolactone ring is made up of acetate, methionine and propionate (Paiva et al. 1991). The biosynthesis of chain begins with the addition of 4, 5-dihydroxycyclohex-1-ene carboxylic acid unit derived from shikimate pathway and then elongated in fourteen condensation steps, involving seven units of acetate and propionate (Paiva et al. 1991). Then, a non-ribosomal peptide synthetase mediated addition of a pipecolate residue to linear polyketide results in forming pre-rapamycin (Cheng et al. 1995a; Schwecke et al. 1995; Molnár et al. 1996; Aparicio et al. 1996; Gregory et al. 2004). Furthermore, post-polyketide synthase-meditated modification, cytochrome P450 monooxygenases mediated oxygenation and S-adenosylmethionine-dependent methyl-transferases meditated O-methylations leads to rapamycin synthesis (Schwecke et al. 1995; Aparicio et al. 1996; Molnár et al. 1996). Moreover, Cheng et al. (1995a; b) also demonstrated the roles of various nutrients on rapamycin synthesis in S. hygroscopicus. It was found that a low amount of ammonium without the presence of phenylalanine and methionine enhanced rapamycin production (Cheng et al. 1995a, b; Kim et al. 2002). Furthermore, limited availability of phosphate, magnesium and supplementation with ferrous salt led to enhanced rapamycin production (Cheng et al. 1995a, b). The addition of precursors such as exogenous shikimic doubles rapamycin production (Fang and Demain 1995), whereas addition of proline resulted prolylrapamycin, in trace amount, probably due to competition from endogenous pipecolic acid and proline (Park et al. 2010).
Antibiotic rapamycin biosynthetic platform contains RapA-C, RapP and RapL genes (Park et al. 2010). The rapamycin and its analogs and their applications in medicines have been discussed (Graziani 2009). The rapamycin PKS in S. hygroscopicus contain 3 multifunctional enzymes (RapA, RapB and RapC) with a total of 14 modules. Rapamycin analog produced by S. hygroscopicus, is a macrocyclic compound known for antifungal, immunosuppression, antitumor and other biological anti-aging activities (Harrison et al. 2009). Besides this, RapH and RapG are found to have positive regulatory role in rapamycin biosynthesis (Kuščer et al. 2007). The overexpression of both rapH and rapG under the regulation of the ActII-ORF4/PactI activator/promoter in S. hygroscopicus resulted in 27–55% and 20–32% increased production, respectively. The other genes such as rapY, rapR, and rapS are found to have high similarity to the TetR family transcription regulators, response regulators and two-component systems histidine kinases, respectively. The overexpression of these three genes led to a significant decrease in rapamycin production, whereas in-frame deletion of rapS and rapY in S. rapamycinicus enhanced 4.6- and 3.7-fold rapamycin production, respectively, compared to the wild type (Yoo et al. 2015).
The effect of 35 carbon and nitrogen sources on the rapamycin biosynthesis in S. hygroscopicus such as carbohydrates and organic acids revealed only 12 carbon source such as fructose, galactose, mannose, mannitol, inositol, xylose and cellobiose positive for rapamycin production. A combination of 2% fructose, 0.5% mannose and a combination of 1.5 g/L of aspartic acid, 0.5 g/L of arginine and 0.5 g/L of histidine resulted significant increase in the growth of S. hygroscopicus and rapamycin production (Kojima et al. 1995; Lee et al. 1997; Park et al. 2010). Amino acids such as phenylalanine and methionine have been reported to decrease, whereas lysine increased the rapamycin production. Nitrogen sources of non-amino acids nature such as ammonium derivatives including ammonium chloride, ammonium citrate, urea as well as potassium nitrate stimulated rapamycin production (Lee et al. 1997).
The refactoring of BGCs through codon-randomization of vital structural genes followed by transcriptomic, proteomic and metabolomic approaches could be explored for the systematic identification of rate-liming steps in secondary metabolite biosynthesis. Using bioinformatic pipelines, operons such as nifUSVWZM and nifHDKY of 0.7 Mb size have been optimized which differ substantially from wild type. Researchers also determined the order, orientation and operon occupancy of upto 16 related genes to these operons by the transformation of the rewired cluster from Klebsiella to E. coli, with parallel RBS substitutions across all genes. Similar to this, effective identification of the rate-limiting steps and engineering of optimized regulatory genes involved in various secondary metabolite biosynthesis has been widely explored in actinobacteria (Martin and Liras 2010). In the native S. roseosporus strain, the production of photocyclized alteramide A and a second PTM 2 with structural similarity to dihydromaltophilin has been achieved using knock-out of kasOp element upstream of the first open reading frame and activation of PTM biosynthetic genes (Li et al. 2019). The BGCs are often regulated by different families of regulatory proteins such as SARP family (Streptomyces antibiotic regulatory proteins), two-component regulators, the LAL family (large ATP-binding regulators of the LuxR family) and γ-butyrolactone-binding regulatory proteins. The regulatory elements involved in a pathway-specific or a pleiotropic mode of action affects various morphological and physiological aspects as well as secondary metabolites production (Kuščer et al. 2007).
Precursor biosynthesis through refactoring: analysis and potential for generating diversity
The genetic engineering for precursor biogenesis is a widely utilized approach for the biosynthesis of structurally diverse compounds with novel activities. However, the major drawback of these approaches includes mix biosynthesis of different products which complicates downstream purification and large-scale production of precursors. Therefore, target-specific biosynthesis of desired precursor through mutation and other methods are preferred. The structural organization of majority of BGCs is either polyketide synthase or non-ribosomal peptide synthase in origin or a combination of polyketide synthase and non-ribosomal peptide synthase. The biosynthesis cassette of BGCs is composed of an array of genes for a particular type of chemical compound (Dhakal et al. 2019). Using the uridine diphosphate (UDP)-glucose pyrophosphorylase gene (ugp), enhanced UDP-glucose level for validamycin overproduction in S. hygroscopicus TL01 has been achieved (Zhou et al. 2011). Furthermore, in S. argillaceus, the gene duplication of a phosphoglucomutase (pgm) resulted in increased supply of precursor glucose-1-phosphate required for anticancerous mithramycin biosynthesis (Zabala et al. 2013). The BGC of rapamycin biosynthesis requires an unusual shikimate origin, three PKS synthases, addition of l-pipecolate followed by post-PKS processing. The putative regulatory elements such as rapK, rapR, rapS and rapY involved in rapamycin biosynthesis along with rapH and rapG are found to affect its biosynthesis (Kuščer et al. 2007).
The preference of starter unit, number of extensions, extender units, and subsequent post-processing of the backbone leads to the diversity of polyketide-derived compounds and makes them one of the major sources of pharmaceuticals compounds (Katz and Donadio 1993). In single-step non-iterative reaction, the type I PKS which is composed of a multifunctional and multi-modular enzyme elongates polyketide chain by successive condensation of activated coenzyme A (CoA) thioesters such as acetyl/malonyl/propionyl-CoA. Here, each module through a set of acyl carrier protein (ACP), acyltransferase (AT), and, β-ketoacyl synthase (KS) functional domains elongate the polyketide chain (Dutta et al. 2014; Whicher et al. 2014). During thioester formation, the CoA selected substrate is activated by AT domain and then transferred to ACP domain. The KS domain through decarboxylative and Claisen types of condensation amid substrate and the growing polyketide, form a C–C (carbon–carbon) bond between the extender unit and the thioester carbonyl unit of the ACP-bound acyl chain (Park et al. 2010; Staunton and Weissman, 2001; Chen et al. 2006). Moreover, the additional β-keto processing domains, ketoreductase, dehydratase, and enoyl-reductase act in sequence to reduce the β-keto group into a mature saturated acyl chain (Fischbach and Walsh 2006). Thereafter, the polyketide is off-loaded by thioesterase (Keatinge-Clay 2012; Xu et al. 2013). The research efforts are mostly centric towards manipulation of different polyketide biosynthetic pathways (Chartrain et al. 2000).
Another approach-based combinatorial biosynthesis which involves modification of biosynthetic pathways, is a promising strategy for the biosynthesis of novel compounds with enhanced structural properties. Using combinatorial approach, a number of precursors of different secondary metabolites with diverse activities have been produced in S. venezuelae (Hong et al. 2004; Jung et al. 2007; Han et al. 2011; Shinde et al. 2013). Using deletion of TDP-4-keto-6-deoxy-d-glucose biosynthetic genes of S. venezuelae ATCC 15439, a new strain S. venezuelae YJ003 has been engineered. The deoxy sugar gene cassettes and glycosylation have been expressed in the strain S. venezuelae YJ003 to produce macrolides derivatives narbomycin (Han et al. 2012), olivosyl and quinovosyl (Hong et al. 2004). Similarly, S. venezuelae strain has been crafted by the deletion of entire BGCs of pikromycin PKS and desosamine biosynthesis as suitable host for combinatorial biosynthesis of different analogs of targeted polyketides. In another study, the deoxy sugar pathways of S. venezuelae of thymidine diphosphate (TDP)-d-quinovose or TDP-d-olivose biosynthesis has been engineered in mutant strain. It requires a pair of flexible glycosyltransferase–auxiliary protein DesVII/DesVIII for the biosynthesis of 10-deoxymethynolide, narbonolide and tylactone as precursors for the biosynthesis of quinovose and olivose glycosylated macrolides (Dhakal et al. 2019). The functional diversity of these macrolides is regulated by post-modifications such as methylation, oxidation, glycosylation and hydroxylation which ultimately give rise to distinct chemical structures (Alberti et al. 2017). Besides this, a number of silent BGCs positioned in transcriptionally repressed region of the genome can be activated by the suppression of the histone deacetylase (HDAC) enzymes (Asai et al. 2013; Skellam 2019).
Streptomyces as hosts for secondary metabolite production
For the expression of recombinant proteins, a number of prokaryotic chassis strains such as E. coli, Bacillus and others heterologous hosts due to their simple and economical production have been streamlined at genome level (Cho et al. 2009) and reviewed by Kim and his colleagues. Moreover, the synthetic biology tools such as promoters, ribosome-binding sites and 5′-untranslated regions, choice of expression vectors, other regulatory elements, expression hosts have been developed and optimized. These tools are capable of accommodating the expression of exogenous genetic elements in a regulated way (Khalil and Collins 2010). For the recombinant protein expression, a major share involves redirection of metabolic processes including ATP and other precursors (Hoffmann and Rinas 2004). The genomes of several E. coli strains have been edited and reduced accordingly. The genome reduced strains are found more cellular fit and stable compared to wild parent strains (Pósfai et al. 2006; Park et al. 2014). This is largely achieved after partial or complete removal of mobile DNA elements such as insertion sequences which reduces the probability of IS-mediated mutagenesis (Kim et al. 2020). The recent developments in next-generation DNA sequencing techniques and databases for metabolic engineering strategies such as antiSMASH, EvoMining, kyoto encyclopedia of genes and genomes, StreptomeDB and other tools have played vital role in mining the diversity of secondary metabolites and cryptic BGCs in Streptomycetes (Zhao and Wang 2019). So far, only few of them have been produced under natural or standard fermentation conditions (Zhao and Wang 2019).
Streptomyces, due to high wobble positions, are attractive hosts for the heterologous expression of different secondary metabolites after refactoring/rewiring (Table 1). The expression of genetically engineered cryptic BGCs in heterologous host in a specifically designed and optimized chassis is a potential source of novel metabolites (Baltz 2010; Myronovskyi and Luzhetskyy 2019). A review of different approaches used for genetic engineering has been done by Liu et al. (2018). For native expression, two types of homologous recombination gene disruption methods have been categorized. These include single- and double-crossover recombination. The single-crossover recombination method is found to disrupt target gene with a selectable marker. For the selection of heterologous host, genetically engineered and genome reduced strain after systematic removal of nonessential genomic regions are found better than their wild-type counterparts for recombinant proteins (Morimoto et al. 2008) and secondary metabolite production (Bu et al. 2019; Kim et al. 2020). Furthermore, optimized codon-based heterologous host, expression of rare tRNAs, and synthetic genes (Gustafsson et al. 2004; Fu et al. 2007; Chung and Lee 2012) are found to enhance the overall production of industrially important enzymes, proteins and secondary metabolites (Fu et al. 2007). A number of engineered tools such as TAR, IR and pSBAC vector systems have been developed for the heterologous expression of BGCs in Streptomyces strains. The TAR system based on natural homologous recombination has been successfully used to clone large BGCs (Feng et al. 2010; Kim et al. 2010). Based on yeast, E. coli, and actinobacteria, TAR cloning vector pCAP01 has been developed (Yamanaka et al. 2014). pCAP01 vector system containing oriT and attP-int sites is capable of transferring the desired BGC by conjugation (Nah et al. 2017). The desired BGC can be cloned and then manipulated in yeast and subsequently, shuttled between E. coli and actinobacteria. Using TAR system, 30 kb BGC encoding marinopyrrole was successfully expressed in S. coelicolor (Yamanaka et al. 2014). Another system known as IR system has been developed using 8BT1 integrase-mediated site-specific recombination and Streptomyces genome engineering (Du et al. 2015). The BGCs responsible for actinorhodin, napsamycin and the daptomycin have been expressed using same system (Penn et al. 2006; Du et al. 2015; Nah et al. 2017). Besides this, gene editing tools such as recombinant meganucleases, zinc-finger nuclease (ZFNs), transcription activator-like effector nuclease (TALEN) and clustered regularly interspaced short palindromic repeat and CRISPR-associated proteins (CRISPR/Cas nuclease system) have revolutionized the manipulation of gene/genes in the native hosts. A detail of these technologies has been mentioned in different research articles (Jia et al. 2017; Salwan et al. 2020).
Escherichia coli–Streptomyces shuttle BAC vectors known as pStreptoBAC V and pSBAC have been engineered to transfer BGCs of large size (Miao et al. 2005; Liu et al. 2009). The cloning and heterologous expression of the tautomycetin, pikromycin and meridamycin encoding BGCs (Liu et al. 2009; Nah et al. 2015) based BAC vector cloning have been used for large BGCs insert of more than 200 kb in size. However, generating BAC library of GC rich genomic DNA is laborious. Alternatively, preparation of cosmid library of smaller inserts of up to 40 kb is easy, however, cloning of complete BGCs is impossible. The BGC of 62.7 kb size encoding atratumycin has been expressed in heterologous host using P1-derived artificial chromosome (PAC) library, i.e. pPAC-S1 prepared from S. atratus SCSIO ZH16 using E. coli–Streptomyces artificial chromosome vector system. The PAC clone 434C consisting of complete atr cluster was then transformed into S. coelicolor M1154 by conjugation (Yang et al. 2019).
Furthermore, using a combination of multiple computational tools such as elimination of precursor competing biosynthetic pathways and site-specific recombination system, rational and versatile Streptomyces chassis with minimized genome capable of diverting malonyl-CoA and acetyl-CoA to target metabolites biosynthesis have been obtained (Gomez-Escribano and Bibb 2012b; Nah et al. 2017). The genome reduced chassis S. chattanoogensis L321 are found attractive host for the heterologous expression of secondary metabolites. For S. albus S4, five internal GCs have been removed to generate genetically improved strain compared to parent strain (Fazal et al. 2019). The selection of S. albus G as heterologous host began prior to availability of cloning protocol in Streptomycetes. So far, a number of Streptomyces strains as heterologous host including S. coelicolor, S. lividans, and S. albus J1074 and other have been reviewed for the production of small molecules (Myronovskyi and Luzhetskyy 2019). Chater and Wilde (1980) isolated SalI defective S. albus J1074 strain. It was first used as cloning host for the heterologous expression of streptomycin genes and obtained 10 mg/L purified compound (Rodríquez et al. 1993; Fernández et al. 1996; Gullón et al. 2006; Baltz 2010). Thereafter, S. albus J1074 has been used for the expression of isomigrastatin from S. platensis NRRL18993 thiocoraline pathway of Micromonospora strain (Lombó et al. 2006; Feng et al. 2009). Two other closely related strains S. coelicolor and S. lividans have been explored for the biosynthesis and regulation of secondary metabolites as heterologous host (Kieser et al. 2000). At genome level, S. coelicolor contain five large BGCs of over > 25 kb size and 18 smaller genomic islands which are not reported in S. lividans. Despite close similarity of S. coelicolor and S. lividans, both differ for common secondary metabolite expression. For example, BGCs of actinorhodin, undecyleprodigiosin, and calcium-dependent antibiotic are cryptic in wild-type S. lividans, whereas they are expressed in S. coelicolor. By deleting a set of endogenous BGCs and introduction of additional φC31 attB loci, S. lividans chassis has been engineered (Eustáquio et al. 2004).
For the deployment of genetically engineered and genome reduced strains, the native strains have been engineered to induce metabolites production (Ahmed et al. 2020). Several Streptomyces species such as S. ambofaciens, S. avermitilis, S. avermitilis-derived SUKA, S. coelicolor M1152, M1154, and strains, S. fradiae, S. roseosporus, S. fradiae A54145, S. rhamosus, S. toyocaensis A47934 have been reviewed for secondary metabolite production (Baltz 2010). In genetically engineered and genome reduced strains, endogenous biosynthetic pathways have been deleted to avoid substrate competition and enhance the production of targeted secondary metabolites. The ability of S. lividans to produce blue-colored actinorhodin (act) and red-pigmented undecylprodigiosin (red) can be used to screen genes, fermentation conditions, and metabolites production during expression (Liu et al. 2013). Moreover, the genetically engineered S. lividans strain with simple and clean metabolic chassis showed better growth characteristics compared to wild strain (Ahmed et al. 2020). The mutant strain of S. coelicolor CH999 deficient in act and red biosynthetic genes have been deployed to study type II PKS system. Streptomyces lividans TK24 is one of the widely used chassis for secondary metabolite expression (Ahmed et al. 2020). It is closely related to the S. coelicolor and can accept even methylated DNA and hence preferred as heterologous host for expressing secondary metabolite gene clusters and even recombinant proteins (Eustaquio et al. 2005; Voller et al. 2012; Anne et al. 2014). Two other strains S. lividans K4-114 and K4-155 has been generated after the deletion of entire act gene cluster of S. lividans TK24 (Ziermann and Betlach 1999). The heterologous expression of 6-deoxy-erythronolide B (6-dEB) in S. lividans K4-114 and K4-155 and S. coelicolor CH999 led to erythromycin production (Ahmed et al. 2020). Nine BGCs have been deleted from S. lividans TK24 using φC31 and VWB integrations systems. Initially, for the integration of φC31-based vector system, S. lividans has one major and three pseudo attB sites and later on two additional attB sites were genetically engineered into S. lividans ΔYA9 (Ahmed et al. 2020). Moreover, to increase the expression, rpsL and rsmG mutations have been reported to significantly enhance the expression of act, whereas mutations in rpoB linked to rifampin resistance (RifR) are found to enhance the act, red, and CDA expression. However, it is still unknown that activation of these pathways can activate other cryptic genes or not. Both S. coelicolor and S. lividans have been extensively used as heterologous hosts for the expression of different BGCs. The expression of erythronolide PKS genes resulted upto 50 mg/L of erythronolide production (Kao et al. 1994). For other metabolites, variable yields for S. coelicolor (23–47 mg/L) and S. lividans (0 to more than 450 mg/L) have been recorded. The expression of novobiocin encoding BGCs in S. coelicolor M512 resulted in higher yield compared to S. lividans TK24 (31 mg/L and less than 1 mg/L, respectively). Furthermore, the optimization of fermentation conditions led to increase in yield upto 54 mg/L, whereas the heterologous expression of a StrR-like positive regulatory element novG further enhanced yield upto 163 mg/L (Siebenberg et al. 2009).
The advancements in genomics of Streptomyces led to the development of clean chassis strains with enhanced precursors supply with higher production of desired metabolites. Streptomyces albus J1074 derived strain S. albus Del14, engineered after removal of 15 internal genes clusters is also suitable heterologous host chassis for the expression of secondary metabolite gene cluster (Rodriguez Estevez et al. 2018). The mutant strain Del14 has been used for the expression of cryptic BGC of pyridinopyrone encoding metabolite obtained from metagenomic sample. Besides this, the cryptic gene clusters of salicylic acid and fradiomycin of Frankia alni ACN14a and bhimamycin A and aloesaponarin II of Frankia sp. CcI3 have been expressed in S. albus Del14 (Rodriguez Estevez et al. 2018). The BGCs like streptomycin, cephamycin C and pladienolide have been successfully expressed in genetically engineered S. avermitilis strains including the genome reduced S. avermitilis SUKA5, SUKA17 and SUKA22 strains. The oligomycin, avermectin and filipin biosynthetic gene clusters has been deleted from S. avermitilis SUKA5, whereas extra deletions of pentalenolactone, geosmin and carotenoid-encoding BGCs have been done in SUKA17 and SUKA22 isogenic strains. Other strains such as S. coelicolor M1152 and M1154 have been engineered after deletion of four endogenous BGCs, corresponding to act, red, coelimycin (cpk) and calcium-dependent antibiotic (cda) (Liu et al. 2013). Additionally, rifampicin resistance (rpoB) mutation in M1152 and rpoB and streptomycin resistance (rpsL) M1154 mutations were also done in both of these strains. Other strains of Streptomyces have been used recently for the heterologous expression of BGCs encoding different secondary metabolites (Myronovskyi and Luzhetskyy 2019).
Stretpomyces griseofuscus has also been identified as an attractive heterologous host for bacteriophage titer due to its lack of discernable DNA restriction (Baltz, 2010). It has been used to express the entire puromycin BGC of S. alboniger ATTC12461. Another chassis of S. chattanoogensis L321 have been engineered for heterologous expression of secondary metabolites. Streptomyces chattanoogensis L321 along with L320 were created after the depletion of 0.7 Mb and 1.3 Mb non‑essential genomic regions for optimized expression (Bu et al. 2019). Since the gene clusters of secondary metabolites are large in Streptomyces, therefore, use of cosmid, fosmid and BAC have been used for genomic library preparation and screening for gene clusters and their heterologous expression. So far, a number of Streptomyces–E. coli-shuttle BAC vector systems including pStreptoBAC V and pSBAC have been engineered for large-size BGCs transformation (Miao et al. 2005; Liu et al. 2009;). However, due to their labor-intensive libraries preparation, new protocols of direct cloning such as yeast-based transformation-associated recombination (TAR) cloning, Cas9-assisted targeting of chromosome segments (CATCH), Gibson assembly to clone large regions into BACs, RecET direct cloning, Redαβ recombineering systems, and ExoCET system have been discovered in Streptomyces (Yamanaka et al. 2014; Jiang et al. 2015; Wang et al. 2016b, 2017). These methods are comparatively easy to use and can be applied on a broad range of DNA fragments and genomic complexity. Moreover, genome editing tools such as CRISPR/Cas system are currently purposed for the assembly-line PKSs in Streptomyces to overcome the slow growth rates, and clutter metabolic backgrounds of wild strains (Cobb et al. 2015).
Furthermore, the cryptic gene clusters of Streptomyces are potential source for the biosynthesis of novel products. Genetic engineering either by activating transcriptionally silent cryptic gene clusters or by genetic manipulation of the native organisms or through heterologous expression of cryptic pathways in appropriate host S. coelicolor, S. lividans, and S. albus have been successfully used to some extent (Luo et al. 2015a, b). Streptomyces albus was one of the initial strains developed for the transformation, cloning and overexpression of genes and mutant S. albus J1074 has been widely used (Chater and Wilde 1980; Baltz and Matsushima 1981; Servant et al. 1993; Wendt-Pienkowski et al. 2005; Winter et al. 2007; Baltz 2010; Bilyk et al. 2016; Huang et al. 2011; Lombó et al. 2006; Myronovskyi et al. 2016). The methods for conjugation and transformation of protoplast were already well established (Baltz and Matsushima 1981; Servant et al. 1993). Despite that attempts to improve S. albus J1074 are limited only (Myronovskyi et al. 2014). Recently, first cluster-free chassis has been derived from S. albus J1074 (Myronovskyi et al. 2018a, b). A total to 15 BGCs have been deleted in a series of 14 deletions which resulted S. albus Del14 strain. The mutant strain was not able to produce any metabolites and therefore, greatly explored for heterologous expression. Furthermore, S. albus Del14 has been engineered by the introduction of additional phiC31 attB sites resulting S. albus strains B2P1 and B4. The engineered S. albus B2P1 and B4 strains were better suited for the integration of multiple copies of the BGCs and hence capable of higher production (Myronovskyi et al. 2018a, b).
The activation of BGC for enhanced production of metabolites is essential and rational heterologous expression of genes can overcome the native regulatory mechanisms. However, the expression of entire and large-size biosynthetic pathways, with high G + C content in heterologous hosts often have limitations of regulatory mechanisms. The activation of BGCs in native host can be overcome by methods of improved genetic manipulation after accurate annotations of BGCs and selection of improved heterologous systems. CRISPR–Cas system was discovered as a part of bacterial adaptive immunity against invading viruses and plasmids invasion (Deveau et al. 2008; Makarova et al. 2011). The CRISPR/Cas system, initially discovered as part of bacterial immunity involves Cas9 nuclease, CRISPR RNA (crRNA), spacers in CRISPR arrays which are involved in cleavage of site, recognition of elements, target site recognition and processing, respectively. The CRISPR/Cas systems have been used for genomic manipulations (Cong et al. 2013; Sander and Joung 2014). It uses target-specific guide RNA, one cas9 codon optimized, and two homology-directed repair templates.
It uses target-specific guide RNA, one cas9 codon optimized, and two homology-directed repair templates. The sgRNA of 20 nucleotides guide sequence helps in the recognition of the target DNA, adjacent to PAM (Protospacer Adjacent Motif) region on the target site and followed by binding of scaffold Cas9 mediated by 20 nucleotides guide sequence. Among all, the CRISPR/Cas system for genome editing in Streptomyces species has attained major attention of researchers worldwide. Using CRISPR/Cas9-CodA(sm), a variant of CRISPR–Cas and pKCcas9dO plasmids genome editing in S. coelicolor M145 efficiencies of 60–100% in a single step has been achieved for gene deletion, (actII-ORF4, redD, and glnR) and even for large-size gene cluster deletion, (BGC of act; 21.3 kb, undecylprodigiosin; 31.6 kb and Ca2+-dependent antibiotic (CDA); 82.8 kb), respectively (Zeng et al. 2015). The engineered pCRISPomyces, a type II CRISPR/Cas system for target-specific multiple gene activation and deletions in S. albus, S. lividans, and S. viridochromogenes with gene editing success rate upto 70–100% have been reported (Cobb et al. 2015; Li et al. 2017). Two types of pCRISPomyces systems for genome editing in Streptomyces; pCRISPomyces-1 and pCRISPomyces-2 have been engineered so far. The former is based on tracrRNA and crRNA along with cas9, whereas later one uses a gRNA and cas9 (Zhang et al. 2017). Using CRISPR/Cas9 and pKCcas9dO plasmid, genome editing for S. coelicolor M145 has been developed (Huang et al. 2015). Thereafter, use of CRISPR/Cas9 in activating the pentangular type II polyketide cryptic biosynthetic gene in S. viridochromogenes (Zhang et al. 2017), act biosynthetic pathway refactoring in S. coelicolor A3(2) (Tong et al. 2015) and act polyketide chain length factor (actI-ORF2) activation using CRISPR/Cas9 in combination with D314A cytosine deaminase mutant counter selection system CodA(sm) in S. coelicolor M145 (Zeng et al. 2015) have been effectively used for target-specific gene editing. Besides that, the upstream promoter elements have been modified using CRISPR–Cas 9 compared to ermE promoter of Streptomyces members (Zhang et al. 2017). The refactoring of biosynthetic pathways using CRISPR/Cas9 systems can enhance the discovery of novel metabolites (Liu et al. 2018) (Fig. 4a).
Fig. 4.
a Red/ET recombination tool using crossover step between a construct containing homology arms (hm) and the target site generate recombinant which can be used for deletions or insertions in a BAC or other plasmid vector; and b CRISPR/Cas9-CodA(sm), a variant of CRISPR–Cas and pKCcas9dO plasmids genome editing in S. coelicolor M145 with efficiencies of 60–100% in a single step has been achieved by the deletion
The activation of various antibiotics such as oxytetracycline encoding gene clusters have been achieved successfully using type II CRISPR/Cas9 system of zwf2 and devB genes using single-site and double-site mutations as well as gene disruptions in S. rimosus (Jia et al. 2017; Wang et al. 2016c). In single-site-guided mutation using sgRNA-1 or sgRNA-2, GG was changed to CA, GC was modified to AT, while GG has been changed to CC. Similarly, using same guiding sgRNAs deletions and/or point mutations has been achieved (Fig. 4b). The deletion for eight bases including three bases upstream of the protospacer adjacent motif (PAM), PAM region itself and even two bases downstream to the PAM region has been done. Using single, double and gene disruptions approach, zwf2 and devB encoding glucose 6-phosphate dehydrogenase isozyme of the oxidative pentose phosphate pathway and hydrolysis of 6-phosphogluconolactone, respectively, has been done in S. rimosus (Jia et al. 2017). An intimate relationship of lipid metabolism and polyketide antibiotic biosynthesis has been identified. For example, inactivation of PirA, a negative regulator of vLCAD (AcdB) activity involved in β-oxidation, led to decreased production of polyketide antibiotic. It affected primary cycles of carbon metabolism such as glucose and lipid metabolism, TCA cycle and ethylmalonyl-CoA pathway. The significant changes in the short chain acyl-CoA pools have been observed at intracellular level in the pirA-mutant strain than wild type or pirA complemented strains. This alteration may account for low spiramycin synthesis since timely availability of precursor is essential prerequisite for its production. Therefore, PirA has been identified as a key factor in the regulation of primary carbon metabolism (Talá et al. 2018).
Pathway engineering
The BGC can be activated by the expression of transcription factor or replacing native promoters with constitutive promoters. This approach can also activate the silent BGCs or even enhance titer of a metabolite (Skellam 2019). In another resistance-guided genome, activation of silent BGCs has been achieved in A. nidulans after the identification of inpE, a putative proteasome subunit in its cryptic cluster which encode a proteasome inhibitor (Yeh et al. 2016; Skellam 2019). A genetic de-replication strain has been engineered by the deletion of 244 kbp of the genome which contain eight most highly expressed BGCs (Chiang et al. 2016). On the other side, target-specific inactivation can be used to reveal a specific point in the pathway to reveal intermediate metabolites which are still not characterized. A number of protocols have been deployed to achieve efficient gene targeting (Arazoe et al. 2015; Mizutani et al. 2017; Delmas et al. 2014). The ustiloxin B has been synthesized after heterologous expression of BGC in Ustilaginoidea virens using Cre/loxP marker system. Alternatively, several other BGCs such as phosphatase-2 inhibitor rubratoxin A, mycotoxin cercosporin and halogenated mycotoxin asirochlorine have been elucidated using disruption-based approaches (Skellam 2019). Combinatorial-based biosynthesis can be used for the production of novel or un-natural metabolites. Systematic swapping of biosynthetic pathway modules of benzenediol lactone and azaphilone resulted in the biosynthesis of thirteen novel metabolites such as cytotoxic and heat-shock-inducing, radilarin (Skellam 2019). Chimeric constructs through multifunctional proteins domain swapping of PKSs, iterative NRPSs, NRPS-like enzymes and terpene synthases have been developed. Furthermore, entire BGC domain can be reconstructed either as mono or multi-domains for the production of novel functional enzymes. Use of strategies for programming of the megasynthases in Streptomyces needs to be explored. Highly efficient “Plug-and-play multi-locus and one step systems have been developed after the integration of BGCs in actinomycetes using “multiple integrases-multiple attB sites.” Several systems including ΦC31 (Lomovskaya et al. 1972), R4 (Chater and Carter 1979; Baltz 2012), VWB (Van Mellaert et al. 1998), ΦBT1 (Gregory et al. 2003; Baltz 2012), TG1 (Morita et al. 2009), SV1 (Fayed et al. 2014), ΦK38-1 (Yang et al. 2014), and CBG73463 (Yang et al. 2014), ΦJoe (Fogg et al. 2017) have been developed for actinomycetes. Besides this, based on site-specific integration, unidirectional and stable recombination amid bacteriophage attP and specific attB sites of the streptomycete and other members of actinobacteria has been achieved (Calos 2006; Smith et al. 2010; Kieser et al. 2000; D'Argenio et al. 2016). The ΦC31-based vector system integrates into an attB site of a Pirin-encoding gene which is located close to the origin of replication oriC site, a region containing highest conserved genes (Bentley et al. 2002; Ikeda et al. 2003). Although precise role of pirin is still elusive, however, it is reported to act as transcription regulator or involved in DNA replication/repair. Initially, it was discovered as an interactor of the NF-I/CTF in human (Wendler et al. 1997) and form quaternary type of complexes with Bcl-3 and dimer of NF-κBonNF-κB-binding regions to facilitate the anti-apoptotic genes transcription (Dechend et al. 1999).
Furthermore, activation of cryptic gene clusters of Streptomyces demands novel and efficient genetic engineering tools. The number of biotechnological tools of genetic engineering in Streptomyces is limited compared to Bacillus subtilis or E. coli. The conventional genetic engineering method in Streptomyces uses homologous recombination (HR) and need to screen engineered strain depending upon HR frequency and other parameters such as plasmid clearance. In this context, highly efficient CRISPR/Cas9-based genome editing using plasmids pKCcas9dO and pCRISPomyces for the genetic manipulation of Streptomyces are being developed.
Conclusion
Microbial secondary metabolites have immense applications in pharmaceutical, agricultural and the food industries, due to their diverse biological activities. The emergence of third generation DNA sequencing technologies including Pacbio SMRT and bioinformatics tools such as antiSMASH, MIBiG, EvoMining and PRISM have played major role in mining genomes and understanding the BGCs encoding pathways of different secondary metabolites. So far, the complete genome of S. coelicolor A3, S. avermitilis and several other strains of Streptomyces have been sequenced and elucidated. Streptomyces strains have been reported to contain abundance of native metabolites and precursors for other metabolites biosynthesis. However, the complex regulation of the BGCs and the cryptic nature of various pathways have severely hampered the tapping of natural diversity. The use of irrational approaches to activate such pathways has limited applications. However, the recent developments in genetic engineering, synthetic biology, mass spectrometry and cheminformatics for refactoring the gene cluster and screening of various microorganisms including Streptomyces have been achieved with precision. The coupling of advancements in genome mining, bioinformatics and genetic engineering tools have played major role in developing rational strategies for metabolic engineering and enhancing the speed of natural product discovery in Streptomyces.
Microbial expression systems are continuously explored as preferred heterologous host for recombinant protein production due to ease of genetic manipulation and fast growth rate compared to eukaryotic system. The industrial workhorses including E. coli, B. subtilis and S. cerevisiae have been explored for over years. However, they are not found satisfactory for secondary metabolites production, possibly due to lack of precursors supply. In comparison, the genus Streptomyces has emerged as a model heterologous prokaryotic host system, due to the presence of wobble positions, better codon usage profile, and several other advantages to circumvent the problems of secondary metabolite production. In addition, native Streptomycetes are found to produce plethora of natural products and presently contribute approximate 70% of total antibiotics discovery. Strains of Streptomyces have received major attention in industrial sectors compared to E. coli and S. cerevisiae for heterologous biosynthetic genes cluster of > 80 kb size. However, the low production of secondary metabolites by Streptomyces strains demands process optimization as well as genetic engineering of biosynthetic pathways for enhanced production of targeted metabolites. So far, a number of genome reduced heterologous host such as S. albus J1074, S. coelicolor, S. lividans, S. avermitilis, S. clavuligerus, S. hygroscopicus TL01, S. albus BK3-25, S. cinnamonensis C730 and S. erythraea, Streptomyces sp. FR-008 have been developed as universal chassis for the production of different metabolites.
Future perspectives
Heterologous expression of BGCs encoding targeted metabolite is an attractive strategy used for the expression, reactivation, and even modify the existing pathways for enhanced metabolite production. Still, the activation/expression of cryptic BGCs is a challenging task for natural product discovery. The refactoring of BGCs has gained researcher’s attention. So far, several approaches have been used to activate the metabolic pathways through rewiring/refactoring based on optimization of cell-culture conditions, understanding the role of physiological and environmental inducers, modulation of regulatory switches through mining of genome for secondary metabolites, synthesis and expression of clusters and suppression of competitive secondary metabolites encoding clusters. Although rewiring or refactoring of BGCs is found a suitable platform for several secondary metabolites. However, the problems associated with cloning and efficient overexpression of complete BGC of large size, unbalanced expression such as production of unwanted products and shunt products demand systematically engineered genome reduced heterologous expression platform to ensure target-specific expression and to revitalize secondary metabolite production. Moreover, lack of information for unknown metabolites, their biosynthetic pathways and cryptic gene cluster limits development of rational redesign/strategies in both native producers and heterologous hosts.
The success stories of genome mining to metabolic pathway engineering for secondary metabolites have led to the development of Streptomyces as “suitable production hosts” for whole pathway expression for drug discovery. Despite that, challenges to understand how expression of biosynthetic gene cluster is silenced or activated and role of epigenetic and genomic factors in the transcription of key biosynthetic and regulatory genes and external cues such as nutritional factors to identify bottlenecks for superior performance. The other challenges include mining genome for new structural classes of compounds and linking them to their respective BGCs. A better understanding of the behavior of BGCs, the order of genes in the cluster, operon occupancy and orientation will help to build functional architectures. Keeping in view the diversity of orphan gene clusters in Streptomyces, the challenge is to prioritize and design target-specific BGCs, decipher their modulatory mechanisms and design rational strategies with precision is still a challenge and needs constant efforts.
Acknowledgements
The authors are thankful to Chandigarh University, Gharuan Punjab India and SEED Division, Department of Science and Technology, GOI for providing financial benefits during the completion of this work.
Author contributions
VS and RS collected the literature, and did final editing. RK assisted VS and RS in MS drafting and reference corrections.
Declarations
Conflict of interests
Vivek Sharma, Richa Salwan, and Randhir Kaur declared no conflict of interest. The authors confirm that the no microbial strains have been used (bacteria, fungi, microalgae) used in this work.
Ethical statement
This study was funded by SEED Division, Department of Science and Technology, GOI for providing financial benefits (SP/YO/125/2017) and (SEED-TIASN-023-2018) during the completion of this work.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
The authors Vivek Sharma and Richa Salwan share equal authorship.
Contributor Information
Vivek Sharma, Email: ankvivek@gmail.com.
Richa Salwan, Email: richaihbt332@gmail.com.
References
- Adrio JL, Demain AL. Genetic improvement of processes yielding microbial products. FEMS Microbiol Rev. 2006;30:187–214. doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Ahmed Y, Rebets Y, Estévez MR, Zapp J, Myronovskyi M. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb Cell Fact. 2020 doi: 10.1186/s12934-020-1277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti F, Khairudin K, Venegas ER, Davies JA, Hayes PM, Willis CL, Bailey AM, Foster GD. Heterologous expression reveals the biosynthesis of the antibiotic pleuromutilin and generates bioactive semi-synthetic derivatives. Nat Commun. 2017;8:1831. doi: 10.1038/s41467-017-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne J, Vrancken K, Van Mellaert L, Van Impe J, Bernaerts K. Protein secretion biotechnology in Gram-positive bacteria with special emphasis on Streptomyces lividans. Biochim Et Biophys Acta. 2014;1843(8):1750–1761. doi: 10.1016/j.bbamcr.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Aparicio JF, Molnfir I, Schwecke T, Konig A, Haydock SF, Khaw LE. Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of the enzymatic domains in the modular polyketide synthase. Gene. 1996;169:9–16. doi: 10.1016/0378-1119(95)00800-4. [DOI] [PubMed] [Google Scholar]
- Arazoe T, Ogawa T, Miyoshi K, Yamato T, Ohsato S, Sakuma T, Yamamoto T, Arie T, Kuwata S. Tailor-made TALEN system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol Bioeng. 2015;112:1335–1342. doi: 10.1002/bit.25559. [DOI] [PubMed] [Google Scholar]
- Arias P, Fernández-Moreno MA, Malpartida F. Characterization of the pathway-specific positive transcriptional regulator for actinorhodin biosynthesis in Streptomyces coelicolor A3(2) as a DNA-binding protein. J Bacteriol. 1999;181:6958–6968. doi: 10.1128/JB.181.22.6958-6968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Yamamoto T, Shirata N, Taniguchi T, Monde K. Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression. Org Lett. 2013;15:3346–3349. doi: 10.1021/ol401386w. [DOI] [PubMed] [Google Scholar]
- Askenazi M, Driggers EM, Holtzman DA, Norman TC, Iverson S, Zimmer DP, Boers ME, Blomquist PR, Martinez EJ, Monreal AW, Feibelman TP, Mayorga ME, Maxon ME, Sykes K, Tobin JV, Cordero E, Salama SR, Trueheart J, Royer JC, Madden KT. Integrating transcriptional and metabolite profiles to direct the engineering of lovastatin-producing fungal strains. Nat Biotechnol. 2003;21:150–156. doi: 10.1038/nbt781. [DOI] [PubMed] [Google Scholar]
- Atkinson HJ, Morris JH, Ferrin TE, Babbitt PC. Using sequence similarity networks for visualization of relationships across diverse protein superfamilies. PLoS ONE. 2009;4(2):e4345. doi: 10.1371/journal.pone.0004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T, Yu Y, Xu Z, Tao M (2014) Construction of Streptomyces lividans SBT5 as an efficient heterologous expression host. J Huazhong Agric Univ 33:1–6
- Bailey JE, Sburlati A, Hatzimanikatis V, Lee K, Renner WA, Tsai PS. Inverse Metab Eng: a strategy for directed genetic engineering of useful phenotypes. Biotechnol Bioeng. 1996;52:109–121. doi: 10.1002/(SICI)1097-0290(19961005)52:1<109::AID-BIT11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Baltz RH. Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol. 2008;8(5):557–563. doi: 10.1016/j.coph.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Baltz RH. Streptomyces and Saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J Ind Microbiol Biotechnol. 2010;37:759–772. doi: 10.1007/s10295-010-0730-9. [DOI] [PubMed] [Google Scholar]
- Baltz RH. Strain improvement in actinomycetes in the postgenomic era. J Ind Microbiol Biotechnol. 2011;38:657–666. doi: 10.1007/s10295-010-0934-z. [DOI] [PubMed] [Google Scholar]
- Baltz RH. Streptomyces temperate bacteriophage integration systems for stable genetic engineering of actinomycetes (and other organisms) J Ind Microbiol Biotechnol. 2012;39:661–672. doi: 10.1007/s10295-011-1069-6. [DOI] [PubMed] [Google Scholar]
- Baltz RH, Matsushima P. Protoplast fusion in Streptomyces: conditions for efficient genetic recombination and cell regeneration. J Gen Microbiol. 1981;127:137–146. doi: 10.1099/00221287-127-1-137. [DOI] [PubMed] [Google Scholar]
- Barona-Gómez F, Cruz-Morales P, Noda-García L. What can genome-scale metabolic network reconstructions do for prokaryotic systematics? Antonie Van Leeuwenhoek. 2012;101:35–43. doi: 10.1007/s10482-011-9655-1. [DOI] [PubMed] [Google Scholar]
- Bauman KD, Li J, Murata K, Mantovani SM, Dahesh S, Nizet V, Luhavaya H, Moore BS. Refactoring the cryptic streptophenazine biosynthetic gene cluster unites phenazine, polyketide, and nonribosomal peptide biochemistry. Cell Chem Biol. 2019;26(5):724–736. doi: 10.1016/j.chembiol.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap KC, Park CJ, Barth BM, et al. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci Rep. 2020;10:2003. doi: 10.1038/s41598-020-58904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- Bérdy J. Bioactive microbial metabolites. J Antibiot (tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Bibb MJ. Regulation of secondary metabolism in Streptomycetes. Curr Opin Microbiol. 2005;8:208–215. doi: 10.1016/j.mib.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Bilyk O, Sekurova ON, Zotchev SB, Luzhetskyy A. Cloning and heterologous expression of the grecocycline biosynthetic gene cluster. PLoS ONE. 2016;11:e0158682. doi: 10.1371/journal.pone.0158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E, Weber T. AntiSMASH 2.0: a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013;41(Web Server issue):W204–W212. doi: 10.1093/nar/gkt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. AntiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HB, Bethe B, Höfs R, Zeeck A. Big effects from small changes: possible ways to explore nature’s chemical diversity. ChemBioChem. 2002;3(7):619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Borodina I, Siebring J, Zhang J, Smith CP, van Keulen G, Dijkhuizen L, Nielsen J. Antibiotic overproduction in Streptomyces coelicolor A3 (2) mediated by phosphofructokinase deletion. J Biol Chem. 2008;283:25186–25199. doi: 10.1074/jbc.M803105200. [DOI] [PubMed] [Google Scholar]
- Breitling R, Vitkup D, Barrett MP. New surveyor tools for charting microbial metabolic maps. Nat Rev Microbiol. 2008;6:156–161. doi: 10.1038/nrmicro1797. [DOI] [PubMed] [Google Scholar]
- Bu QT, Yu P, Wang J, Li ZY, Chen XA, Mao XM, Li YQ. Rational construction of genome-reduced and high-efficient industrial Streptomyces chassis based on multiple comparative genomic approaches. Microb Cell Fact. 2019;18:16. doi: 10.1186/s12934-019-1055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MJ, Bruheim P, Jovetic S, Marinelli F, Postma PW, Bibb MJ. Engineering of primary carbon metabolism for improved antibiotic production in Streptomyces lividans. Appl Environ Microbiol. 2002;68:4731–4739. doi: 10.1128/AEM.68.10.4731-4739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Teta R, Kohlhaas C, Crüsemann M, Ueoka R, Mangoni A, Freeman MF, Piel J. Manipulation of regulatory genes reveals complexity and fidelity in hormaomycin biosynthesis. Chem Biol. 2013;20(6):839–846. doi: 10.1016/j.chembiol.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Calos MP. The ϕC31 integrase system for gene therapy. Curr Gene Ther. 2006;6:633–645. doi: 10.2174/156652306779010642. [DOI] [PubMed] [Google Scholar]
- Cerdeno AM, Bibb MJ, Challis GL. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol. 2001;8:817–829. doi: 10.1016/S1074-5521(01)00054-0. [DOI] [PubMed] [Google Scholar]
- Chartrain M, Salmon PM, Robinson DK, Buckland BC. Metabolic engineering and directed evolution for the production of pharmaceuticals. Curr Opin Biotechnol. 2000;11:209–214. doi: 10.1016/s0958-1669(00)00081-1. [DOI] [PubMed] [Google Scholar]
- Chater KF, Carter AT. New, wide host-range, temperate bacteriophage-(R4) of Streptomyces and its interaction with some restriction-modification systems. J Gen Microbiol. 1979;115:431–442. doi: 10.1099/00221287-115-2-431. [DOI] [Google Scholar]
- Chater KF, Wilde LC. Streptomyces albus G mutants defective in the SalGI restriction-modification system. J Gen Microbiol. 1980;116:323–334. doi: 10.1099/00221287-116-2-323. [DOI] [PubMed] [Google Scholar]
- Chen AY, Schnarr NA, Kim CY, Cane DE, Khosla C. Extender unit and acyl carrier protein specificity of ketosynthase domains of the 6-deoxyerythronolide b synthase. J Am Chem Soc. 2006;128:3067–3074. doi: 10.1021/ja058093d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wendt-Pienkowski E, Shen B. Identification and utility of FdmR1 as a Streptomyces antibiotic regulatory protein activator for fredericamycin production in Streptomyces griseus ATCC 49344 and heterologous hosts. J Bacteriol. 2008;190:5587–5596. doi: 10.1128/JB.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IA, Chen IA, Chu K, Palaniappan K, Pillay M, Ratner A, Huang J, Huntemann M, Varghese N, White JR, Seshadri R, Smirnova T, Kirton E, Jungbluth SP, Woyke T, Eloe-Fadrosh EA, Ivanova NN, Kyrpides NC. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 2019;47(D1):D666–D677. doi: 10.1093/nar/gky901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YR, Fang A, Demain AL. Effect of amino acids on rapamycin biosynthesis by Streptomyces hygroscopicus. Appl Microbiol Biotechnol. 1995;43:1096–1098. doi: 10.1007/BF00166931. [DOI] [PubMed] [Google Scholar]
- Cheng YR, Hauck L, Demain AL. Phosphate, ammonium, magnesium and iron nutrition of Streptomyces hygroscopicus with respect to rapamycin biosynthesis. J Ind Microbiol. 1995;14:424–427. doi: 10.1007/BF01569962. [DOI] [PubMed] [Google Scholar]
- Chevrette MG, Currie CR. Emerging evolutionary paradigms in antibiotic discovery. J Ind Microbiol Biotechnol. 2019;46:257–271. doi: 10.1007/s10295-018-2085-6. [DOI] [PubMed] [Google Scholar]
- Chiang YM, Chiang YM, Ahuja M, Oakley CE, Entwistle R, Asokan A, Zutz C, Wang CC, Oakley BR. Development of genetic dereplication strains in Aspergillus nidulans results in the discovery of aspercryptin. Angew Chem Int Ed Engl. 2016;55:1662–1665. doi: 10.1002/anie.201507097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- Cho BK, Zengler K, Qiu Y, Park YS, Knight EM, Barrett CL, Gao Y, Palsson BO. The transcription unit architecture of the Escherichia coli genome. Nat Biotechnol. 2009;27:1043–1049. doi: 10.1038/nbt.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Johannes TW, Simurdiak M, Shao Z, Lu H. Cloning and heterologous expression of the spectinabilin biosynthetic gene cluster from Streptomyces spectabilis w z. Mol Biosyst. 2010 doi: 10.1039/b923177c. [DOI] [PubMed] [Google Scholar]
- Chung BK, Lee DY. Computational codon optimization of synthetic gene for protein expression. BMC Syst Biol. 2012;6:134. doi: 10.1186/1752-0509-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb RE, Wang Y, Zhao H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth Biol. 2015;4(6):723–728. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, Wright GD. Intrinsic antibiotic resistance: mechanisms, origins, challenges and solutions. Int J Med Microbiol. 2013;303(6–7):287–292. doi: 10.1016/j.ijmm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Craney A, Ozimok C, Pimentel-Elardo SM, Capretta A, Nodwell JR. Chemical perturbation of secondary metabolism demonstrates important links to primary metabolism. Chem Biol. 2012;19(8):1020–1027. doi: 10.1016/j.chembiol.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Cruz-Morales P, Kopp JF, Martínez-Guerrero C, Yáñez-Guerra LA, Selem-Mojica N, Ramos-Aboites H, Feldmann J, Barona-Gómez F. Phylogenomic analysis of natural products biosynthetic gene clusters allows discovery of arseno-organic metabolites in model Streptomycetes. Genome Biol Evol. 2016;8(6):1906–1916. doi: 10.1093/gbe/evw125.PMID:27289100;PMCID:PMC4943196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio V, Petrillo M, Pasanisi D, Pagliarulo C, Colicchio R, Talà A, de Biase MS, Zanfardino M, Scolamiero E, Pagliuca C, Gaballo A, Cicatiello AG, Cantiello P, Postiglione I, Naso B, Boccia A, Durante M, Cozzuto L, Salvatore P, Paolella G, Salvatore F, Alifano P. The complete 12 Mb genome and transcriptome of Nonomuraea gerenzanensis with new insights into its duplicated "magic" RNA polymerase. Sci Rep. 2016;6:18. doi: 10.1038/s41598-016-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB, Poinar HN, Wright GD. Antibiotic resistance is ancient. Nature. 2011;477(7365):457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- de Jong A, van Hijum SA, Bijlsma JJ, Kok J, Kuipers OP. BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006;34(Web Server issue):W273–W279. doi: 10.1093/nar/gkl237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A, van Heel AJ, Kok J, Kuipers OP. BAGEL2: mining for bacteriocins in genomic data. Nucleic Acids Res. 2010;38(Web Server issue):W647–W651. doi: 10.1093/nar/gkq365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechend R, Hirano F, Lehmann K, Heissmeyer V, Ansieau S, Wulczyn FG, Scheidereit C, Leutz A. The Bcl-3 oncoprotein acts as a bridging factor between NF-kappaB/Rel and nuclear co-regulators. Oncogene. 1999;18:3316–3323. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- Delmas S, Llanos A, Parrou JL, Kokolski M, Pullan ST, Shunburne L, Archer DB. Development of an unmarked gene deletion system for the filamentous fungi Aspergillus niger and Talaromyces versatilis. Appl Environ Microbiol. 2014;80:3484–3487. doi: 10.1128/AEM.00625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Xiao L, Liu Y, Zhang L, Deng Z, Zhao C. Streptomyces avermitilis industrial strain as cell factory for Ivermectin B1a production. Synth Syst Biotechnol. 2019;4(1):34–39. doi: 10.1016/j.synbio.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau H, Barrangou R, Garneau JE, Labonté J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal D, Sohng JK, Pandey RP. Engineering actinomycetes for biosynthesis of macrolactone polyketides. Microbial Cell Fact. 2019 doi: 10.1186/s12934-019-1184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D, Wang L, Tian Y, Liu H, Tan H, Niu G. Genome engineering and direct cloning of antibiotic gene clusters via phage 8BT1 integrase-mediated site-specific recombination in Streptomyces. Sci Rep. 2015;5:8740. doi: 10.1038/srep08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont FJ, Su Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996;58:373–395. doi: 10.1016/0024-3205(95)02233-3. [DOI] [PubMed] [Google Scholar]
- Dutta S, Whicher JR, Hansen DA, Hale WA, Chemler JA, Congdon GR, Narayan AR, Håkansson K, Sherman DH, Smith JL, Skiniotis G. Structure of a modular polyketide synthase. Nature. 2014;510:512. doi: 10.1038/nature13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb TJ, Berg IA, Brecht V, Muller M, Fuchs G, Alber BE. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc Natl Acad Sci USA. 2007;104:10631–10636. doi: 10.1073/pnas.0702791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez MR, Gummerlich N, Myronovskyi M, Zapp J. Benzanthric acid, a novel metabolite from Streptomyces albus Del14 expressing the nybomycin gene cluster. Front Chem. 2020;7:1–7. doi: 10.3389/fchem.2019.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustáquio AS, Gust B, Li S-M, Pelzer S, Wohlleben W, Chater K, Heide L. Production of 8′-halogenated and 8′-unsubstituted novobiocin derivatives in genetically engineered Streptomyces coelicolor strains. Chem Biol. 2004;11:1561–1572. doi: 10.1016/j.chembiol.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Eustaquio AS, Gust B, Galm U, Li SM, Chater KF, Heide L. Heterologous expression of novobiocin and clorobiocin biosynthetic gene clusters. Appl Environ Microbiol. 2005;71(5):2452–2459. doi: 10.1128/AEM.71.5.2452-2459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang A, Demain AL. Exogenous shikimic acid stimulates rapamycin biosynthesis in Streptomyces hygroscopicus. Folia Microbiol. 1995;40:607–610. doi: 10.1007/BF02818516. [DOI] [PubMed] [Google Scholar]
- Farnet CM, Zazopoulos E (2005) Improving drug discovery from microorganisms. In: Zhang L, Demain AL (eds) Natural Products. Humana Press, Totowa, pp 95–106. 10.1007/978-1-59259-976-9_5
- Fayed B, Younger E, Taylor G, Smith MCM. A novel Streptomyces spp. integration vector derived from the S. venezuelae phage, SV1. BMC Biotechnol. 2014;14:51. doi: 10.1186/1472-6750-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal A, Thankachan D, Harris E, Seipke RF. A chromatogram-simplified Streptomyces albus host for heterologous production of natural products. Antonie Van Leeuwenhoek. 2019;113:511–520. doi: 10.1007/s10482-019-01360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-LeGal MF, Shen B. Engineered production of iso-migrastatin in heterologous Streptomyces hosts. Bioorg Med Chem. 2009;17:2147–2153. doi: 10.1016/j.bmc.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Kim JH, Brady SF. Fluostatins produced by the heterologous expression of a TAR reassembled environmental DNA derived type II PKS gene cluster. J Am Chem Soc. 2010;132:11902–11903. doi: 10.1021/ja104550p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E, Lombó F, Méndez C, Salas JA. An ABC transporter is essential for resistance to the antitumor agent mithramycin in the producer Streptomyces argillaceus. Mol Gen Genet. 1996;251:692–698. doi: 10.1007/BF02174118. [DOI] [PubMed] [Google Scholar]
- Fischbach M, Voigt CA. Prokaryotic gene clusters: a rich toolbox for synthetic biology. Biotechnol J. 2010;5:1277–1296. doi: 10.1002/biot.201000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- Fogg PCM, Haley JA, Stark WM, Smith MCM. Genome integration and excision by a new Streptomyces bacteriophage, Φjoe. Appl Environ Microbiol. 2017;83:02767–2816. doi: 10.1128/AEM.02767-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone TS, Ju K-S, Wang B, Zhao H. Discovery of a phosphonoacetic acid derived natural product by pathway refactoring. ACS Synth Biol. 2017;6:217–223. doi: 10.1021/acssynbio.6b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag A, Me C, Li S, Heide L. Metabolic engineering of the heterologous production of clorobiocin derivatives and elloramycin in Streptomyces coelicolor M512. Metab Eng. 2006;8:653–661. doi: 10.1016/j.ymben.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Fu W, Lin J, Cen P. 5-Aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl Microbiol Biotechnol. 2007;75:777–782. doi: 10.1007/s00253-007-0887-y. [DOI] [PubMed] [Google Scholar]
- Galanie S, Thodey K, Trenchard IJ, Interrante MF, Smolke CD. Complete biosynthesis of opioids in yeast. Science. 2015;349:1095–1100. doi: 10.1126/science.aac9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Escribano JP, Bibb MJ (2012a) Streptomyces coelicolor as an expression host for heterologous gene clusters. natural product biosynthesis by microorganisms and plants Part C, vol 517, 1st edn. Elsevier Inc. 10.1016/B978-0-12-404634-4.00014-0. 10.1016/j.chembiol.2019.02.004 [DOI] [PubMed]
- Gomez-Escribano JP, Bibb MJ. Streptomyces coelicolor as an expression host for heterologous gene clusters. Methods Enzymol. 2012;517:279–300. doi: 10.1016/B978-0-12-404634-4.00014-0. [DOI] [PubMed] [Google Scholar]
- Gontang EA, Gaudêncio SP, Fenical W, Jensen PR. Sequence-based analysis of secondary-metabolite biosynthesis in marine actinobacteria. Appl Environ Microbiol. 2010;76(8):2487–2499. doi: 10.1128/AEM.02852-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goranovic D, Kosec G, Mrak P, Fujs S, Horva J, Kuscer E, Kopitar G, Petkovic H. Origin of the allyl group in FK506 biosynthesis. J Biol Chem. 2010;285:14292–14300. doi: 10.1074/jbc.M109.059600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottelt M, Kol S, Gomez-Escribano JP, Bibb M, Takano E. Deletion of a regulatory gene within the cpk gene cluster reveals novel antibacterial activity in Streptomyces coelicolor A3(2) Microbiology (reading, England) 2010;156:2343–2353. doi: 10.1099/mic.0.038281-0. [DOI] [PubMed] [Google Scholar]
- Graziani EI. Recent advances in the chemistry, biosynthesis and pharmacology of rapamycin analogs. Nat Prod Rep. 2009;26:602–609. doi: 10.1039/b804602f. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Till R, Smith MCM. Integration site for Streptomyces phage phiBT1 and development of site-specific integrating vectors. J Bacteriol. 2003;185:5320–5323. doi: 10.1128/jb.185.17.5320-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MA, Gaisser S, Lill RE, Hong H, Sheridan RM, Wilkinson B, Petkovic H, Weston AJ, Carletti I, Lee HL, Staunton J, Leadlay PF. Isolation and characterization of pre-rapamycin, the first macrocyclic intermediate in the biosynthesis of the immunosuppressant rapamycin by S. hygroscopicus. Angew Chem Int Ed Engl. 2004;43:2551–2553. doi: 10.1002/anie.200453764. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Hong H, Lill RE, Gaisser S, Petkovic H, Low L, Sheehan LS, Carletti I, Ready SJ, Ward MJ, Kaja AL, Weston AJ, Challis IR, Leadlay PF, Martin CJ, Wilkinson B, Sheridan RM. Rapamycin biosynthesis: elucidation of gene product function. Org Biomol Chem. 2006;4:3565–3568. doi: 10.1039/b608813a. [DOI] [PubMed] [Google Scholar]
- Gullón S, Olano C, Abdelfattah MS, Braka AF, Rohr J, Méndez C, Salas JA. Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin. Appl Environ Microbiol. 2006;72:4172–4183. doi: 10.1128/AEM.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Xiang S, Li L, Wang B, Rajasärkkä J, Gröndahl-Yli-Hannuksela K, Ai G, Metsä-Ketelä M, Yang K. Targeted activation of silent natural product biosynthesis pathways by reporter-guided mutant selection. Metab Eng. 2015;28:134–142. doi: 10.1016/j.ymben.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hadjithomas M, Chen IMA, Chu K, Ratner A, Palaniappan K, Szeto E, Huang J, Reddy TBK, Cimermanc P. IMG-ABC: A knowledge base to fuel discovery of biosynthetic gene clusters and novel secondary metabolites. Mbio. 2015;6(4):e00932. doi: 10.1128/mBio.00932-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AR, Park SR, Park JW, Lee EY, Kim DM, Kim BG, Yoon YJ. Biosynthesis of glycosylated derivatives of tylosin in Streptomyces venezuelae. J Microbiol Biotechnol. 2011;21:613–616. doi: 10.4014/jmb.1103.03032. [DOI] [PubMed] [Google Scholar]
- Han AR, Shinde PB, Park JW, Cho J, Lee SR, Ban YH, Yoo YJ, Kim EJ, Kim E, Park SR, Kim BG, Lee DG, Yoon YJ. Engineered biosynthesis of glycosylated derivatives of narbomycin and evaluation of their antibacterial activities. Appl Microbiol Biotechnol. 2012;93:1147–1156. doi: 10.1007/s00253-011-3592-9. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Kozone I, Hashimoto J. Novel macrolactam compound produced by the heterologous expression of a large cryptic biosynthetic gene cluster of Streptomyces rochei IFO12908. J Antibiot. 2019 doi: 10.1038/s41429-019-0265-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kozone I, Hashimoto J. Identification, cloning and heterologous expression of biosynthetic gene cluster for desertomycin. J Antibiot. 2020 doi: 10.1038/s41429-020-0319-0. [DOI] [PubMed] [Google Scholar]
- He W, Lei J, Liu Y, Wang Y. The LuxR family members GdmRI and GdmRII are positive regulators of geldanamycin biosynthesis in Streptomyces hygroscopicus 17997. Arch Microbiol. 2008;189:501–510. doi: 10.1007/s00203-007-0346-2. [DOI] [PubMed] [Google Scholar]
- Herai S, Hashimoto Y, Higashibata H, Maseda H, Ikeda H, Omura S, Kobayashi M. Hyper-inducible expression system for streptomycetes. Proc Natl Acad Sci USA. 2004;101(39):14031–14035. doi: 10.1073/pnas.0406058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Rinas U. Stress induced by recombinant protein production in Escherichia coli. Adv Biochem Eng Biotechnol. 2004;89:73–92. doi: 10.1007/b93994. [DOI] [PubMed] [Google Scholar]
- Holmes DJ, Caso JL, Thompson CJ. Autogenous transcriptional activation of a thiostrepton-induced gene in Streptomyces lividans. EMBO J. 1993;12(8):3183–3191. doi: 10.1002/j.1460-2075.1993.tb05987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JSJ, Park SH, Choi CY, Sohng JK, Yoon YJ. New olivosyl derivatives of methymycin/pikromycin from an engineered strain of Streptomyces venezuelae. FEMS Microbiol Lett. 2004;238:391–399. doi: 10.1111/j.1574-6968.2004.tb09781.x. [DOI] [PubMed] [Google Scholar]
- Hong B, Phornphisutthimas S, Tilley E, Baumberg S, McDowall KJ. Streptomycin production by Streptomyces griseus can be modulated by a mechanism not associated with change in the adpA component of the A-factor cascade. Biotechnol Lett. 2007;29:57–64. doi: 10.1007/s10529-006-9216-2. [DOI] [PubMed] [Google Scholar]
- Huang S, Zhao Y, Qin Z, Wang X, Onega M, Chen L, He J, Yu Y, Deng H. Identification and heterologous expression of the biosynthetic gene cluster for holomycin produced by Streptomyces clavuligerus. Process Biochem. 2011;46:811–816. doi: 10.1016/j.procbio.2010.11.024. [DOI] [Google Scholar]
- Huang H, Zheng G, Jiang W, Hu H, Lu Y. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim Biochim Biophys Sin. 2015;47:231–243. doi: 10.1093/abbs/gmv007. [DOI] [PubMed] [Google Scholar]
- Huang J, Yu Z, Li M-H, Wang J-D, Bai H, Zhou J, Zheng Y-G. High level of spinosad production in the heterologous host Saccharopolyspora erythraea. Appl Environ Microbiol. 2016;82:5603–5611. doi: 10.1128/AEM.00618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ma T, Tian J, Shen L, Zuo H, Hu C, Liao G. wblA, a pleiotropic regulatory gene modulating morphogenesis and daptomycin production in Streptomyces roseosporus. J Appl Microbiol. 2017;123:669–677. doi: 10.1111/jam.13512. [DOI] [PubMed] [Google Scholar]
- Hung TV, Malla S, Park BC, Liou K, Lee HC, Sohng JK. Enhancement of clavulanic acid by replicative and integrative expression of ccaR and cas2 in Streptomyces clavuligerus NRRL3585. J Microbiol Biotechnol. 2007;17:1538–1545. [PubMed] [Google Scholar]
- Hur YA, Choi S-S, Sherman DH, Kim ES. Identification of TcmN as a pathway-specific positive regulator of tautomycetin biosynthesis in Streptomyces sp. CK4412. Microbiology. 2008;154:2912–2919. doi: 10.1099/mic.0.2008/018903-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- Jia H, Zhang L, Wang T, Han J, Tang H, Zhang L. Development of a CRISPR/Cas9-mediated gene-editing tool in Streptomyces rimosus. Microbiology. 2017 doi: 10.1099/mic.0.000501. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang YY, Ran XX, Fan WM, Jiang XH, Guan WJ, Li YQ. Improvement of natamycin production by engineering of phosphopantetheinyl transferases in Streptomyces chattanoogensis L10. Appl Environ Microbiol. 2013;79:3346–3354. doi: 10.1128/AEM.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhao X, Gabrieli T, Lou C, Ebenstein Y, Zhu TF. Cas9-assisted targeting of chromosome segments CATCH enables one-step targeted cloning of large gene clusters. Nat Commun. 2015;6:8101. doi: 10.1038/ncomms9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan IK, Makarova KS, Spouge JL, Wolf YI, Koonin EV. Lineage-specific gene expansions in bacterial and archaeal genomes. Genome Res. 2001;11:555–565. doi: 10.1101/gr.gr-1660r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WS, Han AR, Hong JS, Park SR, Choi CY, Park JW, Yoon YJ. Bioconversion of 12-, 14-, and 16-membered ring aglycones to glycosylated macrolides in an engineered strain of Streptomyces venezuelae. Appl Microbiol Biotechnol. 2007;76:1373–1381. doi: 10.1007/s00253-007-1101-y. [DOI] [PubMed] [Google Scholar]
- Jung WS, Yoo YJ, Park JW, Park SR, Han AR, Ban YH, Kim EJ, Kim E, Yoon YJ. A combined approach of classical mutagenesis and rational metabolic engineering improves rapamycin biosynthesis and provides insights into methylmalonyl-CoA precursor supply pathway in Streptomyces hygroscopicus ATCC 29253. Appl Microbiol Biotechnol. 2011;91(5):1389–1397. doi: 10.1007/s00253-011-3348-6. [DOI] [PubMed] [Google Scholar]
- Kao CM, Katz L, Khosla C. Engineered biosynthesis of a complete macrolactone in a heterologous host. Science. 1994;265:509–512. doi: 10.1126/science.8036492. [DOI] [PubMed] [Google Scholar]
- Katz L, Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Ann Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- Kawasak T, Sakurai F, Nagatsuka SY, Hayakawa Y. Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J Antibiot. 2009;62(5):271–276. doi: 10.1038/ja.2009.27. [DOI] [PubMed] [Google Scholar]
- Keatinge-Clay AT. The structures of type I polyketide synthases. Nat Prod Rep. 2012;29:1050–1073. doi: 10.1039/c2np20019h. [DOI] [PubMed] [Google Scholar]
- Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces genetics. Norwich: The John Innes Foundation; 2000. [Google Scholar]
- Kim WS, Xu L, Souw D, Fang A, Demain AL. An unexpected inhibitory effect of rapamycin against germination of spores of Bacillus brevis strain Nagano. J Antibiot. 2002;55:650–654. doi: 10.7164/antibiotics.55.650. [DOI] [PubMed] [Google Scholar]
- Kim HB, Smith CP, Micklefield J, Mavituna F. Metabolic flux analysis for calcium dependent antibiotic (CDA) production in Streptomyces coelicolor. Metab Eng. 2004;6:313–325. doi: 10.1016/j.ymben.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kim JH, Feng Z, Bauer JD, Kallifidas D, Calle PY, Brady SF. Cloning large natural product gene clusters from the environment: piecing environmental DNA gene clusters back together with TAR. Biopolymers. 2010;93:833–844. doi: 10.1002/bip.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Cho WJ, Song MC, Park SW, Kim K, Kim E, Lee N, Nam SJ, Oh KH, Yoon YJ. Engineered biosynthesis of milbemycins in the avermectin high-producing strain Streptomyces avermitilis. Microb Cell Fact. 2017;16:9. doi: 10.1186/s12934-017-0626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Choe D, Lee D. Engineering biology to construct microbial chassis for the production of difficult-to-express proteins. Int J Mol Sci. 2020;21:990. doi: 10.3390/ijms21030990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani S, Ikeda H, Sakamoto T, Noguchi S, Nihira T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl Microbiol Biotechnol. 2009;82:1089–1096. doi: 10.1007/s00253-008-1850-2. [DOI] [PubMed] [Google Scholar]
- Kojima I, Cheng YR, Mohan V, Demain AL. Carbon source nutrition of rapamycin biosynthesis in Streptomyces hygroscopicus. J Ind Microbiol. 1995;14:436–439. doi: 10.1007/BF01573954. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Uchiyama T, Ōmura S, Cane DE, Ikeda H. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA. 2010;107(6):2646–2651. doi: 10.1073/pnas.0914833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Komatsu K, Koiwai H, Yamada Y, Kozone I, Izumikawa M, Hashimoto J, Takagi M, Omura S, Shin-ya K, Cane DE, Ikeda H. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth Biol. 2013;2(7):384–396. doi: 10.1021/sb3001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosec G, Goranovič D, Mrak P, Ku E, Kopitar G, Petkovic H. Novel chemobiosynthetic approach for exclusive production of FK506. Metab Eng. 2012;14:39–46. doi: 10.1016/j.ymben.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Krishnaveni P, Chen IM-A, Chu K, Ratner A, Seshadri R, Kyrpides NC, Ivanova NN, Mouncey NJ. IMG-ABC v50: an update to the IMG/Atlas of Biosynthetic Gene Clusters Knowledgebase. Nucleic Acids Res. 2020;48(D1):D422–D430. doi: 10.1093/nar/gkz932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuščer E, Coates N, Challis I, Gregory M, Wilkinson B, Sheridan R, Petković H. Roles of rapH and rapG in positive regulation of rapamycin biosynthesis in Streptomyces hygroscopicus. J Bacteriol. 2007;189(13):4756–4763. doi: 10.1128/JB.00129-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureti L, Song L, Huang S, Corre C, Leblond P, Challis GL. Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc Natl Acad Sci USA. 2011;108(15):6258–6263. doi: 10.1073/pnas.1019077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kojima I, Demain AL. Effect on nitrogen source on biosynthesis of rapamycin by Streptomyces hygroscopicus. J Ind Microbiol Biotechnol. 1997;19:83–86. doi: 10.1038/sj.jim.2900434. [DOI] [PubMed] [Google Scholar]
- Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- Li R, Townsend CA. Rational strain improvement for enhanced clavulanic acid production by genetic engineering of the glycolytic pathway in Streptomyces clavuligerus. Metab Eng. 2006;8:240–252. doi: 10.1016/j.ymben.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Ling H, Li W, Tan H. Improvement of nikkomycin production by enhanced copy of sanU and sanV in Streptomyces ansochromogenes and characterization of a novel glutamate mutase encoded by sanU and sanV. Metab Eng. 2005;7:165–173. doi: 10.1016/j.ymben.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Li C, Hazzard C, Florova G, Reynolds KA. High titer production of tetracenomycins by heterologous expression of the pathway in a Streptomyces cinnamonensis industrial monensin producer strain. Metab Eng. 2009;11:319–327. doi: 10.1016/j.ymben.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Li MH, Ung PM, Zajkowski J, Garneau-Tsodikova S, Sherman DH. Automated genome mining for natural products. BMC Bioinform. 2009;10:185. doi: 10.1186/1471-2105-10-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, Ruan L, Yang S, Ge M, Jiang W, Lu Y. A stepwise increase in pristinamycin II biosynthesis by Streptomyces pristinaespiralis through combinatorial Metab Eng. Metab Eng. 2015;29:12–25. doi: 10.1016/j.ymben.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Li X, Lei X, Zhang C, Jiang Z, Shi Y, Wang S, Wang L, Hong B. Complete genome sequence of Streptomyces globisporus C-1027, the producer of an enediyne antibiotic lidamycin. J Biotechnol. 2016;222:9–10. doi: 10.1016/j.jbiotec.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang W, Lu Y. New strategies and approaches for engineering biosynthetic gene clusters of microbial natural products. Biotechnol Adv. 2017;35(8):936–949. doi: 10.1016/j.biotechadv.2017.03.00. [DOI] [PubMed] [Google Scholar]
- Li L, Wei K, Liu X, Wu Y, Zheng G, Chen S, Jiang W, Lu Y. aMSGE: advanced multiplex site-specific genome engineering with orthogonal modular recombinases in actinomycetes. Metab Eng. 2019;52:153–167. doi: 10.1016/j.ymben.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Liao Y, Wei ZH, Bai L, Deng Z, Zhong JJ. Effect of fermentation temperature on validamycin A production by Streptomyces hygroscopicus 5008. J Biotechnol. 2009;142:271–274. doi: 10.1016/j.jbiotec.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Liu H, Jiang H, Haltli B, Kulowski K, Muszynska E, Feng X, Summers M, Young M, Graziani E, Koehn F, Carter GT, He M. Rapid cloning and heterologous expression of the meridamycin biosynthetic gene cluster using a versatile Escherichia coli–Streptomyces artificial chromosome vector, pSBAC. J Nat Prod. 2009 doi: 10.1021/np8006149. [DOI] [PubMed] [Google Scholar]
- Liu G, Chater KF, Chandra G, Niu G, Tan H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wu K, Cheng Y, Lu L, Xiao E, Zhang Y. Engineering an iterative polyketide pathway in Escherichia coli results in single-form alkene and alkane overproduction. Metab Eng. 2014;28:82–90. doi: 10.1016/j.ymben.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Liu SP, Yu P, Yuan PH, Zhou ZX, Bu QT, Mao XM, Li YQ. Sigma factor WhiGch positively regulates natamycin production in Streptomyces chattanoogensis L10. Appl Microbiol Biotechnol. 2015;99:2715–2726. doi: 10.1007/s00253-014-6307-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Xiao L, Zhou Y, Deng K, Tan G, Han Y, Liu X, Deng Z, Liu T. Development of Streptomyces sp. FR-008 as an emerging chassis. Synth Syst Biotechnol. 2016;1:207–214. doi: 10.1016/j.synbio.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Deng Z, Liu T. Streptomyces species: ideal chassis for natural product discovery and overproduction. Metab Eng. 2018;50:74–84. doi: 10.1016/j.ymben.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Liu SH, Wang W, Wang KB, Zhang B, Li W, Shi J, Jiao RH, Tan RX, Ge HM. Heterologous expression of a cryptic giant type I PKS gene cluster leads to the production of ansaseomycin. Org Lett. 2019;21(10):3785–3788. doi: 10.1021/acs.orglett.9b01237. [DOI] [PubMed] [Google Scholar]
- Lombó F, Velasco A, Castro A, de la Calle F, Braña AF, Sánchez-Puelles JM, Méndez C, Salas JA. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two Streptomyces species. Chembiochem Eur J Chem Biol. 2006;7:366–376. doi: 10.1002/cbic.200500325. [DOI] [PubMed] [Google Scholar]
- Lomovskaya ND, Mkrtumian NM, Gostimskaya NL, Danilenko VN. Characterization of temperate actinophage phiC31 isolated from Streptomyces coelicolor A3(2) J Virol. 1972;9:258–262. doi: 10.1128/JVI.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Zhang X, Jiang M, Bai L. Enhanced salinomycin production by adjusting the supply of polyketide extender units in Streptomyces albus. Metab Eng. 2016;35:129–137. doi: 10.1016/j.ymben.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Luo ML, Leenay RT, Beisel CL. Current and future prospects for CRISPR-based tools. Biotechnol Bioeng. 2015;113(5):930–943. doi: 10.1002/bit.25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Li BZ, Liu D, Zhang L, Chen Y, Jia B, Zeng BX, Zhao H, Yuan YJ. Engineered biosynthesis of natural products in heterologous hosts. Chem Soc Rev. 2015;44(15):5265–5290. doi: 10.1039/c5cs00025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Zhao J, Deng Z, Yu Y. Characterization of the biosynthetic gene cluster for benzoxazole antibiotics A33853 reveals unusual assembly logic. Chem Biol. 2015;22(10):1313–1324. doi: 10.1016/j.chembiol.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, Brouns SJJ, Mojica FJM, Wolf YI, Yakunin AF, Van Der Oost J, Koonin EV. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JF, Liras P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr Opin Microbiol. 2010;13:263–273. doi: 10.1016/j.mib.2010.02.008. [DOI] [PubMed] [Google Scholar]
- McAlpine JB, Bachmann BO, Piraee M, Tremblay S, Alarco AM, Zazopoulos E, Farnet CM. Microbial genomics as a guide to drug discovery and structural elucidation: ECO-02301, a novel antifungal agent, as an example. J Nat Prod. 2005;68(4):493–496. doi: 10.1021/np0401664. [DOI] [PubMed] [Google Scholar]
- McDaniel R, Ebert-Khosla S, Hopwood DA, Khosla C. Engineered biosynthesis of novel polyketides. Science. 1993;262:1546. doi: 10.1126/science.8248802. [DOI] [PubMed] [Google Scholar]
- McDaniel R, Welch M, Hutchinson CR. Genetic approaches to polyketide antibiotics. Chem Rev. 2005;105:543–558. doi: 10.1021/cr0301189. [DOI] [PubMed] [Google Scholar]
- Medema MH, Alam MT, Breitling R, Takano E. The future of industrial antibiotic production: from random mutagenesis to synthetic biology. Bioeng Bugs. 2011;2(4):230–233. doi: 10.4161/bbug.2.4.16114. [DOI] [PubMed] [Google Scholar]
- Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39(Web Server issue):W339–W346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, Breitling R, Bovenberg R, Takano E. Exploiting plug-and-play synthetic biology for drug discovery and production in microorganisms. Nat Rev Microbiol. 2011;9:131–137. doi: 10.1038/nrmicro2478. [DOI] [PubMed] [Google Scholar]
- Medema MH, Takano E, Breitling R. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol Biol Evol. 2013;30(5):218–1223. doi: 10.1093/molbev/mst025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes MV, Tunca S, Antón N, Recio E, Sola-Landa A, Aparicio JF, Martín JF. The two-component phoR–phoP system of Streptomyces natalensis: inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metab Eng. 2007;9:217–227. doi: 10.1016/j.ymben.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Miao V, Coeffet-Legal MF, Brian P, Brost R, Penn J, Whiting A, Martin S, Ford R, Parr I, Bouchard M, Silva CJ, Wrigley SK, Baltz RH. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology. 2005;151:1507–1523. doi: 10.1099/mic.0.27757-0. [DOI] [PubMed] [Google Scholar]
- Michel KH, Boeck LD, Hoehn MM, Jones ND, Chaney MO (1984) The discovery, fermentation, isolation, and structure of antibiotic A33853 and its tetraacetyl derivative. J Antibiot 37:441–445 [DOI] [PubMed]
- Mizutani O, Arazoe T, Toshida K, Hayashi R, Ohsato S, Sakuma T, Yamamoto T, Kuwata S, Yamada O. Detailed analysis of targeted gene mutations caused by the platinum-fungal TALENs in Aspergillus oryzae RIB40 strain and a ligD disruptant. J Biosci Bioeng. 2017;123:287–293. doi: 10.1016/j.jbiosc.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Mo S, Yoo YJ, Ban YH, Lee SK, Kim E, Suh JW, Yoon YJ. Roles of fkbN in positive regulation and tcs7 in negative regulation of FK506 biosynthesis in Streptomyces sp. strain KCTC 11604BP. Appl Environ Microbiol. 2012;78(7):2249–2255. doi: 10.1128/AEM.06766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt MC, Neilan BA. Evolutionary affiliations within the superfamily of ketosynthases reflect complex pathway associations. J Mol Evol. 2003;56(4):446–457. doi: 10.1007/s00239-002-2415-0. [DOI] [PubMed] [Google Scholar]
- Molnár I, Aparicio JF, Haydock SF, Khaw LE, Schwecke T, König A, Staunton J, Leadlay PF. Organisation of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of genes flanking the polyketide synthase. Gene. 1996;169:1–7. doi: 10.1016/0378-1119(95)00799-7. [DOI] [PubMed] [Google Scholar]
- Montiel D, Kang H-S, Chang F-Y, Charlop-Powers Z, Brady SF. Yeast homologous recombination-based promoter engineering for the activation of silent natural product biosynthetic gene clusters. Proc Natl Acad Sci USA. 2015;112:8953–8958. doi: 10.1073/pnas.1507606112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, Manabe K, Kanaya S, Ara K, Ozaki K, Ogasawara N. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 2008;15:73–81. doi: 10.1093/dnares/dsn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Yamamoto T, Fusada N, Komatsu M, Ikeda H, Hirano N, Takahashi H. The site-specific recombination system of actinophage TG1. FEMS Microbiol Lett. 2009;297:234–240. doi: 10.1111/j.1574-6968.2009.01683. [DOI] [PubMed] [Google Scholar]
- Motamedi H, Shafiee A. The biosynthetic gene cluster for the macrolactone ring of the immunosuppressant FK506. Eur J Biochem. 1998;256:528–534. doi: 10.1046/j.1432-1327.1998.2560528.x. [DOI] [PubMed] [Google Scholar]
- Motamedi H, Shafiee A, Cai SJ, Streicher SL, Arison BH, Miller RR (1996) Characterization of methyltransferase and hydroxylase genes involved in the biosynthesis of the immunosuppressants FK506 and FK520. J Bacteriol 178(17):5243–5248 [DOI] [PMC free article] [PubMed]
- Mrak P, Ku E, Kopitar G, Petkovic H. Novel chemobiosynthetic approach for exclusive production of FK506. Metab Eng. 2012;14:39–46. doi: 10.1016/j.ymben.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Mungan MD, Alanjary M, Blin K, Weber T, Medema MH, Ziemert N. ARTS 2.0: feature updates and expansion of the antibiotic resistant target seeker for comparative genome mining. Nucleic Acids Res. 2020;48(W1):W546–W552. doi: 10.1093/nar/gkaa374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Burian J, Yanai K, Bibb MJ, Thompson CJ. A system for the targeted amplification of bacterial gene clusters multiplies antibiotic yield in Streptomyces coelicolor. Proc Natl Acad Sci USA. 2011;108:16020–16025. doi: 10.1073/pnas.1108124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myronovskyi M, Luzhetskyy A. Native and engineered promoters in natural product discovery. Nat Prod Rep. 2016;33:1006–1019. doi: 10.1039/C6NP00002A. [DOI] [PubMed] [Google Scholar]
- Myronovskyi M, Luzhetskyy A. Heterologous production of small molecules in the optimized streptomyces hosts. Nat Prod Rep. 2019 doi: 10.1039/c9np00023b. [DOI] [PubMed] [Google Scholar]
- Myronovskyi M, Rosenkränzer B, Luzhetskyy A. Iterative marker excision system. Appl Microbiol Biotechnol. 2014;98:4557–4570. doi: 10.1007/s00253-014-5523-z. [DOI] [PubMed] [Google Scholar]
- Myronovskyi M, Brötz E, Rosenkränzer B, Manderscheid N, Tokovenko B, Rebets Y, Luzhetskyy A. Generation of new compounds through unbalanced transcription of landomycin A cluster. Appl Microbiol Biotechnol. 2016;100:9175–9186. doi: 10.1007/s00253-016-7721-3. [DOI] [PubMed] [Google Scholar]
- Myronovskyi M, Rosenkränzer B, Nadmid S, Pujic P, Normand P, Luzhetskyy A. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab Eng. 2018;49:316–324. doi: 10.1016/j.ymben.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Myronovskyi M, Rosenkränzer B, Nadmid S, Pujic P, Normand P. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab Eng. 2018;49:316–324. doi: 10.1016/j.ymben.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Naeimpoor F, Mavituna F. Metabolic flux analysis in Streptomyces coelicolor under various nutrient limitations. Metab Eng. 2000;2:140–148. doi: 10.1006/mben.2000.0146. [DOI] [PubMed] [Google Scholar]
- Nah H-J, Woo M-W, Choi S-S, Kim E-S. Precise cloning and tandem integration of large polyketide biosynthetic gene cluster using Streptomyces artificial chromosome system. Microb Cell Factor. 2015;14:1. doi: 10.1186/s12934-015-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah HJ, Pyeon HR, Kang SH, Choi SS, Kim ES. Cloning and heterologous expression of a large-sized natural product biosynthetic gene cluster in Streptomyces species. Front Microbiol. 2017;8:394. doi: 10.3389/fmicb.2017.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Muñoz JC, Selem-Mojica N, Mullowney MW, Kautsar SA, Tryon JH, Parkinson EI, De Los Santos ELC, Yeong M, Cruz-Morales P, Abubucker S, Roeters A, Lokhorst W, Fernandez-Guerra A, Cappelini LTD, Goering AW, Thomson RJ, Metcalf WW, Kelleher NL, Barona-Gomez F, Medema MH. A computational framework to explore large-scale biosynthetic diversity. Nat Chem Biol. 2020;16(1):60–68. doi: 10.1038/s41589-019-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66(7):1022–1037. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nguyen Q, Merlo ME, Medema MH, Jankevics A, Breitling R, Takano E. Metabolomics methods for the synthetic biology of secondary metabolism. FEBS Lett. 2012;586(15):2177–2183. doi: 10.1016/j.febslet.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Nic LL, Caffrey P. Phosphomannose isomerase and phosphomannomutase gene disruptions in Streptomyces nodosus: impact on amphotericin biosynthesis and implications for glycosylation engineering. Metabol Eng. 2009;11:40–47. doi: 10.1016/j.ymben.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Niu G, Liu G, Tian Y, Tan H. SanJ, an ATP-dependent picolinate-CoA ligase, catalyzes the conversion of picolinate to picolinate-CoA during nikkomycin biosynthesis in Streptomyces ansochromogenes. Metab Eng. 2006;8:183–195. doi: 10.1016/j.ymben.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, Yamashita A, Hattori M, Horinouchi S. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol. 2008;190:4050–4060. doi: 10.1128/JB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano C, Lombó F, Méndez C, Salas JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng. 2008;10(5):281–292. doi: 10.1016/j.ymben.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Olano C, García I, González A, Rodriguez M, Rozas D, Rubio J, Sánchez-Hidalgo M, Braña AF, Méndez C, Salas JA. Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb Biotechnol. 2014;7(3):242–256. doi: 10.1111/1751-7915.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliynyk M, Stark CB, Bhatt A, Jones MA, Hughes-Thomas ZA, Wilkinson C, Oliynyk Z, Demydchuk Y, Staunton J, Leadlay PF. Analysis of the biosynthetic gene cluster for the polyether antibiotic monensin in Streptomyces cinnamonensis and evidence for the role of monB and monC genes in oxidative cyclization. Mol Microbiol. 2003;49:1179–1190. doi: 10.1046/j.1365-2958.2003.03571.x. [DOI] [PubMed] [Google Scholar]
- Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc Natl Acad Sci USA. 2001;98:12215–12220. doi: 10.1073/pnas.211433198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn A. Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. 2010;26:449–457. doi: 10.1016/j.tig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- Paiva NL, Demain AL, Roberts MF. Incorporation of acetate, propionate, and methionine into rapamycin by Streptomyces hygroscopicus. J Nat Prod. 1991;54:167–177. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- Papagianni M. Recent advances in engineering the central carbon metabolism of industrially important bacteria. Microb Cell Fact. 2012;11:50. doi: 10.1186/1475-2859-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar AS, Mosher RH, Anders C, Griffin A, Griffin J, Hughes C, Greaves P, Barton B, Jensen SE. Applications of gene replacement technology to Streptomyces clavuligerus strain development for clavulanic acid production. Appl Environ Microbiol. 2001;67:2292–2297. doi: 10.1128/AEM.67.5.2292-2297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SR, Yoo YJ, Ban Y, Yoon YJ. Biosynthesis of rapamycin and its regulation: past achievements and recent progress. J Anitbiot. 2010 doi: 10.1038/ja.2010.71. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Yang KS, Jung SC, Lee JH, Kim SC. Enhancing recombinant protein production with an Escherichia coli host strain lacking insertion sequences. Appl Microbiol Biotechnol. 2014;98:6701–6713. doi: 10.1007/s00253-014-5739-y. [DOI] [PubMed] [Google Scholar]
- Peng Q, Gao G, Lü J, Long Q, Chen X, Zhang F, Xu M, Liu K, Wang Y, Deng Z, Li Z, Tao M (2018) Engineered Streptomyces lividans Strains for Optimal Identification and Expression of Cryptic Biosynthetic Gene Clusters. Front Microbiol 10(9):3042. 10.3389/fmicb.2018.03042 [DOI] [PMC free article] [PubMed]
- Penn J, Li X, Whiting A, Latif M, Gibson T, Silva CJ, Brian P. Heterologous production of daptomycin in Streptomyces lividans. J Ind Microbiol Biotechnol. 2006;33:121–128. doi: 10.1007/s10295-005-0033-8. [DOI] [PubMed] [Google Scholar]
- Perez-Llarena FJ, Liras P, Rodriguez-Garcia A, Martin JF. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both beta-lactam compounds. J Bacteriol. 1997;179:2053–2059. doi: 10.1128/jb.179.6.2053-2059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science. 2001;291:1790–1792. doi: 10.1126/science.1058092. [DOI] [PubMed] [Google Scholar]
- Pickens LB, Tang Y, Chooi YH. Metabolic engineeringfor the production of natural products. Annu Rev Chem Biomol Eng. 2011;2:211–236. doi: 10.1146/annurev-chembioeng-061010-114209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pósfai G, Plunkett G, 3rd, Fehér T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, Burland V, Harcum SW, Blattner FR. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- Qu S, Kang Q, Wu H, Wang L, Bai L. Positive and negative regulation of GlnR in validamycin A biosynthesis by binding to different loci in promoter region. Appl Microbiol Biotechnol. 2015;99:4771–4783. doi: 10.1007/s00253-015-6437-0. [DOI] [PubMed] [Google Scholar]
- Rajgarhia VB, Priestley ND, Strohl WR. The product of dpsC confers starter unit fidelity upon the daunorubicin polyketide synthase of Streptomyces sp. strain C5. Metab Eng. 2001;3(1):49–63. doi: 10.1006/mben.2000.0173. [DOI] [PubMed] [Google Scholar]
- Rascher A, Hu Z, Buchanan GO, Reid R, Hutchinson CR. Insights into the biosynthesis of the benzoquinone ansamycins geldanamycin and herbimycin, obtained by gene sequencing and disruption. Appl Environ Microbiol. 2005;71:4862–4871. doi: 10.1128/AEM.71.8.4862-4871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch C, Weber T, Kohlbacher O, Wohlleben W, Huson DH. Specificity prediction of adenylation domains in nonribosomal peptide synthetases (NRPS) using transductive support vector machines (TSVMs) Nucleic Acids Res. 2005;33(18):5799–5808. doi: 10.1093/nar/gki885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP. Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008;9(7):670–675. doi: 10.1038/embor.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin J, Bonneau S, Schipper D, Noorman H, Nielsen J. Influence of the adipate and dissolved oxygen concentrations on the β-lactam production during continuous cultivations of a Penicillium chrysogenum strain expressing the expandase gene from Streptomyces clavuligerus. Metab Eng. 2003;5:42–48. doi: 10.1016/S1096-7176(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García A, Combes P, Pérez-Redondo R, Smith MC, Smith MC. Natural and synthetic tetracycline-inducible promoters for use in the antibiotic-producing bacteria Streptomyces. Nucleic Acids Res. 2005;33(9):e87. doi: 10.1093/nar/gni086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Estevez M, Myronovskyi M, Gummerlich N, Nadmid S, Luzhetskyy A (2018) Heterologous expression of the nybomycin gene cluster from the marine strain Streptomyces albus subsp. chlorinus NRRL B-24108. Mar Drugs 16:E435. 10.3390/md16110435 [DOI] [PMC free article] [PubMed]
- Rodríquez AM, Olano C, Vilches C, Méndez C, Salas JA. Streptomyces antibioticus contains at least three oleandomycin-resistance determinants, one of which shows similarity with proteins of the ABC-transporter superfamily. Mol Microbiol. 1993;8:571–582. doi: 10.1111/j.1365-2958.1993.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Rokem JS, Lantz AE, Nielsen J. Systems biology of antibiotic production by microorganisms. Nat Prod Rep. 2007;24:1262–1287. doi: 10.1039/b617765b. [DOI] [PubMed] [Google Scholar]
- Romero-Rodriguez A, Robledo-Casados I, Sanchez S. An overview on transcriptional regulators in Streptomyces. Biochim Biophys Acta. 2015;1849:1017–1039. doi: 10.1016/j.bbagrm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Röttig M, Medema MH, Blin K, Weber T, Rausch C, Kohlbacher O. NRPSpredictor2-a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res. 2011;39(Web Server issue):W362–W367. doi: 10.1093/nar/gkr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf JD, Yan X, Shen B. Genome neighborhood network reveals insights into enediyne biosynthesis and facilitates prediction and prioritization for discovery. J Ind Microbiol Biotechnol. 2016;43(2–3):261–276. doi: 10.1007/s10295-015-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MM, Vockenhuber MP, Suess B. Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology. 2013;159(Pt 7):1416–1422. doi: 10.1099/mic.0.067322-0. [DOI] [PubMed] [Google Scholar]
- Rutledge PJ, Challis GL. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat Rev Microbiol. 2015;13:509–523. doi: 10.1038/nrmicro3496. [DOI] [PubMed] [Google Scholar]
- Ryu YG, Butler MJ, Chater KF, Lee KJ. Engineering of primary carbohydrate metabolism for increased production of actinorhodin in Streptomyces coelicolor. Appl Environ Microbiol. 2006;72:7132–7139. doi: 10.1128/AEM.01308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas JA, Méndez C. Engineering the glycosylation of natural products in actinomycetes. Trends Microbiol. 2007;15:219–232. doi: 10.1016/j.tim.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Salwan R, Sharma V (2020) Bioactive compounds of Streptomyces. Studies in Natural Products Chemistry Edited by Atta-Ur-Rahman, vol 64, pp 467–491
- Salwan R, Sharma V, Sharma A, Singh A. Molecular imprints of plant beneficial <i>Streptomyces</i> sp. AC30 and AC40 reveal differential capabilities and strategies to counter environmental stresses. Microbiol Res. 2020;235:126449. doi: 10.1016/j.micres.2020.126449. [DOI] [PubMed] [Google Scholar]
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Aberturas J, Payero TD, Vicente CM, Guerra SM, Canibano C, Martin JF, Aparicio JF. Functional conservation of PAS-LuxR transcriptional regulators in polyene macrolide biosynthesis. Metab Eng. 2011;13:756–767. doi: 10.1016/j.ymben.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Schmitt I, Barker FK. Phylogenetic methods in natural product research. Nat Prod Rep. 2009;12:1585–1602. doi: 10.1039/b910458p. [DOI] [PubMed] [Google Scholar]
- Schwecke T, Aparicio JF, Molnár I, König A, Khaw LE, Haydock SF, Oliynyk M, Caffrey P, Cortés J, Lester JB. The biosynthetic gene cluster for the polyketide immunosuppressant rapamycin. Proc Natl Acad Sci USA. 1995;92:7839–7843. doi: 10.1073/pnas.92.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sélem-Mojica N, Aguilar C, Gutiérrez-García K, Martínez-Guerrero CE, Barona-Gómez F. EvoMining reveals the origin and fate of natural product biosynthetic enzymes. Microb Genom. 2019;5(12):e000260. doi: 10.1099/mgen.0.000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant P, Thompson C, Mazodier P. Use of new Escherichia coli/Streptomyces conjugative vectors to probe the functions of the two groEL-like genes of Streptomyces albus G by gene disruption. Gene. 1993;134:25–32. doi: 10.1016/0378-1119(93)90170-8. [DOI] [PubMed] [Google Scholar]
- Shao Z, Zhao H. Manipulating natural product biosynthetic pathways via DNA assembler. Curr Protoc Chem Biol. 2014;6(2):65–100. doi: 10.1002/9780470559277.ch130191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Rao G, Li C, Abil Z, Luo Y, Zhao H. Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth Biol. 2013;2(11):662–669. doi: 10.1021/sb400058n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Sharma A, Malannavar AB, Salwan R (2020) Molecular aspects of biocontrol species of Streptomyces in agricultural crops. In: Molecular aspects of plant beneficial microbes in agriculture. Elsevier. pp 89–109. 10.1016/B978-0-12-818469-1.00008-0
- Sheldon PJ, Busarow SB, Hutchinson CR. Mapping the DNA-binding domain and target sequences ofthe Streptomyces peucetius daunorubicin biosynthesis regulatory protein, DnrI. Mol Microbiol. 2002;44:449–460. doi: 10.1046/j.1365-2958.2002.02886.x. [DOI] [PubMed] [Google Scholar]
- Shinde PB, Han AR, Cho J, Lee SR, Ban YH, Yoo YJ, Kim EJ, Kim E, Song MC, Park JW, Lee DG, Yoon YJ. Combinatorial biosynthesis and antibacterial evaluation of glycosylated derivatives of 12-membered macrolide antibiotic YC-17. J Biotechnol. 2013;168:142–148. doi: 10.1016/j.jbiotec.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Siebenberg S, Bapa PM, Lantz AE, Gust B, Heide L. Reducing the variability of antibiotic production in Streptomyces by cultivation in 24-square deepwell plates. J Biosci Bioeng. 2009;109:230–234. doi: 10.1016/j.jbiosc.2009.08.479. [DOI] [PubMed] [Google Scholar]
- Skellam E. Strategies for engineering natural product biosynthesis in Fungi. Trends Biotechnol. 2019;37(4):416–427. doi: 10.1016/j.tibtech.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Skinnider MA, Dejong CA, Rees PN, Johnston CW, Li H, Webster AL, Wyatt MA, Magarvey NA. Genomes to natural products PRediction Informatics for Secondary Metabolomes (PRISM) Nucleic Acids Res. 2015;43(20):9645–9662. doi: 10.1093/nar/gkv1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smanski MJ, Bhatia S, Zhao D, Park Y, Woodruff L, Giannoukos G, Ciulla D, Busby M, Calderon J, Nicol R, Gordon DB, Densmore D, Voigt CA. Functional optimization of gene clusters by combinatorial design and assembly. Nat Biotechnol. 2014;32:1241–1249. doi: 10.1038/nbt.3063. [DOI] [PubMed] [Google Scholar]
- Smith MCM, Brown WRA, McEwan AR, Rowley PA. Site-specific recombination by ΦC31 integrase and other serine recombinases. Biochem Soc. 2010;38:388–394. doi: 10.1042/BST0380388. [DOI] [PubMed] [Google Scholar]
- Sosio M, Giusino F, Cappellano C, Bossi E, Puglia AM, Donadio S. Artificial chromosomes for antibiotic-producing actinomycetes. Nat Biotechnol. 2000;18:343–345. doi: 10.1038/73810. [DOI] [PubMed] [Google Scholar]
- Starcevic A, Zucko J, Simunkovic J, Long PF, Cullum J, Hranueli D. ClustScan: an integrated program package for the semi-automatic annotation of modular biosynthetic gene clusters and in silico prediction of novel chemical structures. Nucleic Acids Res. 2008;36(21):6882–6892. doi: 10.1093/nar/gkn685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/A909079G. [DOI] [PubMed] [Google Scholar]
- Stutzman-engwall K, Conlon S, Fedechko R, Mcarthur H, Pekrun K, Chen Y, Jenne S, La C, Trinh N, Kim S, Zhang Y, Fox R, Gustafsson C, Krebber A. Semi-synthetic DNA shuffling of aveC leads to improved industrial scale production of doramectin by streptomyces avermitilis. Metab Eng. 2005;7:27–37. doi: 10.1016/j.ymben.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Yabe M, Odaki H, Shinozaki M, Metsä-Ketelä M, Arai T, Okamoto S, Ichinose K. Biosynthetic conclusions from the functional dissection of oxygenases for biosynthesis of actinorhodin and related Streptomyces antibiotics. Chem Biol. 2013;20(4):510–520. doi: 10.1016/j.chembiol.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Takano E. Gamma-butyrolactones: Streptomyces signaling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol. 2006;9:287–294. doi: 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Talà A, Damiano F, Gallo G, Pinatel E, Calcagnile M, Testini M, Fico D, Rizzo D, Sutera A, Renzone G, Scaloni A, De Bellis G, Siculella L, De Benedetto GE, Puglia AM, Peano C, Alifano P. Pirin: a novel redox-sensitive modulator of primary and secondary metabolism in Streptomyces. Metab Eng. 2018;48:254–268. doi: 10.1016/j.ymben.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Tan G, Liu T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab Eng. 2017;39:228–236. doi: 10.1016/j.ymben.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Tan GY, Bai L, Zhong JJ. Exogenous 1,4-butyrolactone stimulates A-factor- like cascade and validamycin biosynthesis in Streptomyces hygroscopicus 5008. Biotechnol Bioeng. 2013;110:2984–2993. doi: 10.1002/bit.24965. [DOI] [PubMed] [Google Scholar]
- Tan G, Peng Y, Lu C, Bai L, Zhong J. Engineering validamycin production by tandem deletion of γ-butyrolactone receptor genes in Streptomyces hygroscopicus 5008. Metab Eng. 2015;28:74–81. doi: 10.1016/j.ymben.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Hosaka T, Ochi K. Rare earth elements activate the secondary metabolite-biosynthetic gene clusters in Streptomyces coelicolor A3(2) J Antibiot (tokyo) 2010;63(8):477–481. doi: 10.1038/ja.2010.53. [DOI] [PubMed] [Google Scholar]
- Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Cloning and heterologous expression of the epothilone gene cluster. Science. 2000;287(5453):640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- Teijaro CN, Adhikari A, Shen B. Challenges and opportunities for natural product discovery, production, and engineering in native producers versus heterologous hosts. J Ind Microbiol Biotechnol. 2019;46(3):433–444. doi: 10.1007/s10295-018-2094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipparaju SK, Joyasawal S, Pieroni M, Kaiser M, Brun R, Kozikowski AP (2008) In pursuit of natural product leads: synthesis and biological evaluation of 2-[3-hydroxy-2-[(3-hydroxypyridine-2-carbonyl)amino]phenyl]benzoxazole-4-c arboxylic acid (A-33853) and its analogues: discovery of N-(2-benzoxazol-2-ylphenyl)benzamides as novel antileishmanial chemotypes. J Med Chem 51:7344–7347 [DOI] [PubMed]
- Tong Y, Charusanti P, Zhang L, Weber T, Lee SY. CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synth Biol. 2015;4:1020–1029. doi: 10.1021/acssynbio.5b00038. [DOI] [PubMed] [Google Scholar]
- Tu J, Li S, Chen J, Song Y, Fu S, Ju J, Li Q. Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802. Microb Cell Fact. 2018;17:28. doi: 10.1186/s12934-018-0875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel AJ, de Jong A, Montalbán-López M, Kok J, Kuipers OP. BAGEL3: automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013;41(Web Server issue):W448–W453. doi: 10.1093/nar/gkt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mellaert L, Mei LJ, Lammertyn E, Schacht S, Anne J. Site-specific integration of bacteriophage VWB genome into Streptomyces venezuelae and construction of a VWB-based integrative vector. Microbiol Sgm. 1998;144:3351–3358. doi: 10.1099/00221287-144-12-3351. [DOI] [PubMed] [Google Scholar]
- Voller GH, Krawczyk JM, Pesic A, Krawczyk B, Nachtigall J, Sussmuth RD. Characterization of new class III lantibiotics–erythreapeptin, avermipeptin and griseopeptin from Saccharopolyspora erythraea, Streptomyces avermitilis and Streptomyces griseus demonstrates stepwise N-terminal leader processing. ChemBioChem. 2012;13(8):1174–1183. doi: 10.1002/cbic.201200118. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang T, Li Y, Li S, Yin S, Styles K, Corre C, Yang K. Development of a synthetic oxytetracycline-inducible expression system for Streptomycetes using de novo characterized genetic Parts. ACS Synth Biol. 2016;5(7):765–773. doi: 10.1021/acssynbio.6b00087. [DOI] [PubMed] [Google Scholar]
- Wang H, Li Z, Jia R, Hou Y, Yin J, Bian X, Li A, Muller R, Stewart AF, Fu J, Zhang Y. RecET direct cloning and Redalphabeta recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat Protoc. 2016;11:1175–1190. doi: 10.1038/nprot.2016.054. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cobb RE, Zhao H (2016c) High-efficiency genome editing of Streptomyces species by an engineered CRISPR/Cas system. In: Methods in enzymology, vol 575, 1st edn. Elsevier Inc. 10.1016/bs.mie.2016.03.014 [DOI] [PubMed]
- Wang Y, Tao Z, Zheng H, Zhang F, Long Q, Deng Z, Tao M. Iteratively improving natamycin production in Streptomyces gilvosporeus by a large operon-reporter based strategy. Metab Eng. 2016;38:418–426. doi: 10.1016/j.ymben.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Li Z, Jia R, Yin J, Li A, Xia L, Yin Y, Muller R, Fu J, Stewart AF, Zhang Y. ExoCET: exonuclease in vitro assembly combined with RecET recombination for highly efficient direct DNA cloning from complex genomes. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yin S, Bai J, Liu Y, Fan K, Wang H, Yuan F, Zhao B, Li Z, Wang W. Heterologous production of chlortetracycline in an industrial grade Streptomyces rimosus host. Appl Microbiol Biotechnol. 2019;103:6645–6655. doi: 10.1007/s00253-019-09970-1. [DOI] [PubMed] [Google Scholar]
- Wattanachaisaereekul S, Lantz AE, Nielsen ML, Nielsen J. Production of the polyketide 6-MSA in yeast engineered for increased malonyl-CoA supply. Metab Eng. 2008;10:246–254. doi: 10.1016/j.ymben.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Weber T, Kim HU. The secondary metabolite bioinformatics portal: computational tools to facilitate synthetic biology of secondary metabolite production. Synth Syst Biotechnol. 2016;1:69–79. doi: 10.1016/j.synbio.2015.1012.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Rausch C, Lopez P, Hoof I, Gaykova V, Huson DH, Wohlleben W. CLUSEAN: a computer-based framework for the automated analysis of bacterial secondary metabolite biosynthetic gene clusters. J Biotechnol. 2009;140(1–2):13–17. doi: 10.1016/j.jbiotec.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Müller R, Wohlleben W, Breitling R, Takano E, Medema MH. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43(W1):W237–W243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Charusanti P, Musiol-Kroll EM, Jiang X, Tong Y, Kim HU, Lee SY. Metabolic engineering of antibiotic factories: new tools for antibiotic production in actinomycetes. Trends Biotechnol. 2015;33:15–26. doi: 10.1016/j.tibtech.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Wendler WM, Kremmer E, Forster R, Winnacker EL. Identification of pirin, a novel highly conserved nuclear protein. J Biol Chem. 1997;272:8482–8489. doi: 10.1074/jbc.272.13.8482. [DOI] [PubMed] [Google Scholar]
- Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B. Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J Am Chem Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- Whicher JR, Dutta S, Hansen DA, Hale WA, Chemler JA, Dosey AM, Narayan AR, Håkansson K, Sherman DH, Smith JL, Skiniotis G. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature. 2014;510:560–564. doi: 10.1038/nature13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DJ, Xue Y, Reynolds KA, Sherman DH. Characterization and analysis ofthe PikD regulatory factor in the pikromycin biosynthetic pathway of Streptomyces venezuelae. J Bacteriol. 2001;183:3468–3475. doi: 10.1128/JB.183.11.3468-3475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JM, Moffitt MC, Zazopoulos E, McAlpine JB, Dorrestein PC, Moore BS. Molecular basis for chloronium-mediated meroterpene cyclization: cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J Biol Chem. 2007;282:16362–16368. doi: 10.1074/jbc.M611046200. [DOI] [PubMed] [Google Scholar]
- Wohlleben W, Mast Y, Muth G, Röttgen M, Stegmann E, Weber T. Synthetic biology of secondary metabolite biosynthesis in actinomycetes: engineering precursor supply as a way to optimize antibiotic production. FEBS Lett. 2012;586(15):2171–2176. doi: 10.1016/j.febslet.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Wu K, Chung L, Revill WP, Katz L, Reeves CD. The FK520 gene cluster of Streptomyces hygroscopicus var. ascomyceticus (ATCC 14891) contains genes for biosynthesis of unusual polyketide extender units. Gene. 2000;251:81–90. doi: 10.1016/s0378-1119(00)00171-2. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang D, Zhu X, Feng Z, Lv Z, Zhang Y, Shen B, Xu Z. Iso-migrastatin titer improvement in the engineered Streptomyces lividans SB11002 strain by optimization of fermentation conditions. Biotechnol Bioprocess Eng. 2010;15:664–669. doi: 10.1007/s12257-009-3129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Chen M, Mao Y, Li W, Liu J, Huang X, Zhou Y, Ye BC, Zhang L, Weaver DT, Zhang B. Dissecting and engineering of the TetR family regulator SACE_7301 for enhanced erythromycin production in Saccharopolyspora erythraea. Microb Cell Fact. 2014;13:158. doi: 10.1186/s12934-014-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SH, Li J, Yin H, Zheng JT, Yang X, Wang HB, Luo JL, Bai H, Yang KQ. Application of a double-reporter-guided mutant selection method to improve clavulanic acid production in Streptomyces clavuligerus. Metab Eng. 2009;11:310–318. doi: 10.1016/j.ymben.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiao K, Tang Y. Structural analysis of protein–protein interactions in type I polyketide synthases. Crit Rev Biochem Mol Biol. 2013;48:98–122. doi: 10.3109/10409238.2012.745476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Ashley G, Hutchinson CR, Santi DV. A multiplasmid approach to preparing large libraries of polyketides. Proc Natl Acad Sci USA. 1999;96(21):11740–11745. doi: 10.1073/pnas.96.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Gonzalez DJ, Nizet V, Dorrestein PC, Moore BS. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc Natl Acad Sci USA. 2014;111(5):1957–1962. doi: 10.1073/pnas.1319584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Liu Q, Zang X, Yuan S, Bat-Erdene U, Nguyen C, Gan J, Zhou J, Jacobsen SE, Tang Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature. 2018;559:415–418. doi: 10.1038/s41586-018-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhu X, Wu X, Fend Z, Huang L, Shen B, Xu Z. Titer improvement of iso-migrastatin in selected heterologous Streptomyces hosts and related analysis of mRNA expression by quantitative RT-PCR. Appl Microbial Biotechnol. 2011;89:1709–1719. doi: 10.1007/s00253-010-3025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Nielsen AAK, Fernandez-Rodriguez J, McClune CJ, Laub MT, Lu TK, Voigtwill CA. Permanent genetic memory with > 1-byte capacity. Nat Methods. 2014;11:1261–1266. doi: 10.1038/nmeth.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wei X, He J, Sun C, Ju J. Characterization of the noncanonical regulatory and transporter genes in atratumycin biosynthesis and production in a heterologous host. Mar Drugs. 2019;10:560. doi: 10.3390/md17100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HH, Ahuja M, Chiang YM, Oakley CE, Moore S, Yoon O, Hajovsky H, Bok JW, Keller NP, Wang CC, Oakley BR. Resistance gene-guided genome mining: serial promoter exchanges in Aspergillus nidulans reveal the biosynthetic pathway for fellutamide B, a proteasome inhibitor. ACS Chem Biol. 2016;11:2275–2284. doi: 10.1021/acschembio.6b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YJ, Hwang J, Shin H, Cui H, Lee J, Oon YJ. Characterization of negative regulatory genes for the biosynthesis of rapamycin in Streptomyces rapamycinicus and its application for improved production. J Ind Microbiol Biotechnol. 2015;42(1):125–135. doi: 10.1007/s10295-014-1546-9. [DOI] [PubMed] [Google Scholar]
- Yu P, Liu SP, Bu QT, Zhou ZX, Zhu ZH, Huang FL, Li YQ. WblAch, a pivotal activator of natamycin biosynthesis and morphological differentiation in Streptomyces chattanoogensis L10, is positively regulated by AdpAch. Appl Environ Microbiol. 2014;80:6879–6887. doi: 10.1128/AEM.01849-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Yin C, Zhu C, Zhu B, Hu Y. Improvement of antibiotic productivity by knock-out of dauW in Streptomyces coeruleobidus. Microbiol Res. 2011;166:539–547. doi: 10.1016/j.micres.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Zabala D, Braña AF, Flórez AB, Salas JA, Méndez C. Engineering precursor metabolite pools for increasing production of antitumor mithramycins in Streptomyces argillaceus. Metab Eng. 2013;20:187–197. doi: 10.1016/j.ymben.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Zaburannyi N, Rabyk M, Ostash B, Fedorenko V, Luzhetskyy A. Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genom. 2014;15:1. doi: 10.1186/1471-2164-15-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazopoulos E, Huang K, Staffa A, Liu W, Bachmann BO, Nonaka K, Ahlert J, Thorson JS, Shen B, Farnet CM. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat Biotechnol. 2003;21(2):187–190. doi: 10.1038/nbt784. [DOI] [PubMed] [Google Scholar]
- Zeng H, Wen S, Xu W, He Z, Zhai G, Liu Y, Deng Z, Sun Y. Highly efficient editing of the actinorhodin polyketide chain length factor gene in Streptomyces coelicolor M145 using CRISPR/Cas9-CodA(sm) combined system. Appl Microbiol Biotechnol. 2015;99(24):10575–10585. doi: 10.1007/s00253-015-6931-4. [DOI] [PubMed] [Google Scholar]
- Zhai L, Lin S, Qu D, Hong X, Bai L, Chen W. Engineering of an industrial polyoxin producer for the rational production of hybrid peptidyl nucleoside antibiotics. Metab Eng. 2012;14(4):388–393. doi: 10.1016/j.ymben.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Moore BS. Digging for biosynthetic dark matter. Elife Genet Genom Microbiol Infect Dis. 2015;4:e06453. doi: 10.7554/eLife.06453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Wang Y, Ang EL, Zhao H. Engineering microbial hosts for production of bacterial natural products. Nat Prod Rep. 2016;33(8):963–987. doi: 10.1039/C6NP00017G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin CY, Li XM, Tang ZK, Qiao J, Zhao GR. DasR positively controls monensin production at two-level regulation in Streptomyces cinnamonensis. J Ind Microbiol Biotechnol. 2016;43(12):1681–1692. doi: 10.1007/s10295-016-1845-4. [DOI] [PubMed] [Google Scholar]
- Zhang MM, Wong FT, Wang Y, Luo S, Lim YH, Heng E, Yeo WL, Cobb RE, Enghiad B, Ang EL, Zhao H. CRISPR-Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat Chem Biol. 2017;13(6):607–609. doi: 10.1038/nchembio.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang L. Recent advances in natural products exploitation in Streptomyces via synthetic biology. Eng Life Sci. 2019 doi: 10.1002/elsc.201800137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sakai A, Zhang X, Vetting MW, Kumar R, Hillerich B, San Francisco B, Solbiati J, Steves A, Brown S, Akiva E, Barber A, Seidel RD, Babbitt PC, Almo SC, Gerlt JA, Jacobson MP. Prediction and characterization of enzymatic activities guided by sequence similarity and genome neighborhood networks. Elife. 2014;3:e03275. doi: 10.7554/eLife.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Li S, Guo Z, Sun M, Lu C. Overexpression of div8 increases the production and diversity of divergolides in Streptomyces sp. W112. RSC Adv. 2015;5:98209–98214. doi: 10.1039/C5RA20083K. [DOI] [Google Scholar]
- Zheng YM, Lin FL, Gao H, Zou G, Zhang JW, Wang GQ, Chen GD, Zhou ZH, Yao XS, Hu D. Development of a versatile and conventional technique for gene disruption in filamentous fungi based on CRISPR–Cas9 technology. Sci Rep. 2017;7:9250. doi: 10.1038/s41598-017-10052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wu H, Li Z, Bai L, Deng Z. Over-expression of UDP-glucose pyrophosphorylase increases validamycin A but decreases validoxylamine A production in Streptomyces hygroscopicus var. jinggangensis 5008. Metab Eng. 2011;13:768–776. doi: 10.1016/j.ymben.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Murphy AC, Samborskyy M, Prediger P, Dias LC, Leadlay PF. Iterative mechanism of macrodiolide formation in the anticancer compound conglobatin. Chem Biol. 2015;22(6):745–754. doi: 10.1016/j.chembiol.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Cheng X, Liu Y, Deng Z, You D. Deciphering and engineering of the final step halogenase for improved chlortetracycline biosynthesis in industrial Streptomyces aureofaciens. Metab Eng. 2013;19:69–78. doi: 10.1016/j.ymben.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Ziemert N, Alanjary M, Weber T. The evolution of genome mining in microbes—a review. Nat Prod Rep. 2016;33(8):988–1005. doi: 10.1039/C6NP00025H. [DOI] [PubMed] [Google Scholar]
- Ziermann R, Betlach MC. Recombinant polyketide synthesis in Streptomyces: engineering of improved host strains. Biotechniques. 1999;26:106–110. doi: 10.2144/99261st05. [DOI] [PubMed] [Google Scholar]