Abstract
The purpose of this study was to determine the dynamic changes of the Nogo-66 receptor 1 (NgR1) pathway during epileptogenesis and the potential beneficial of leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1 (Lingo-1) inhibition on epilepsy rats. The hippocampal changes of the NgR1 pathway during epileptogenesis were determined by western blot analysis of multiple proteins, including neurite outgrowth inhibitor protein A (NogoA), myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), Lingo-1, ras homolog family member A (RhoA) and phosphorylated RhoA (p-RhoA). Lentivirus-mediated short hairpin RNA (shRNA) was used to knockdown the hippocampal expression of Lingo-1. Novel object recognition (NOR) test and Morris Water Maze (MWM) test were employed to determine the cognitive functions of rats. Hematoxylin and eosin (H&E) staining, protein expressions of RhoA, p-RhoA, and myelin basic protein (MBP), as well as convulsion susceptibility test were additionally performed. Our results showed that the NgR1 pathway was activated during epileptogenesis, characterized by up-regulation of NogoA, MAG, OMgp, and Lingo-1, which was especially significant at the chronic phase of epilepsy. The cognitive function, convulsion susceptibility and hippocampal neuronal survival of rats were impaired at the chronic phase of epileptogenesis but all improved by Lingo-1 inhibition; besides, the hippocampal protein expressions of p-RhoA and MBP were significantly decreased at the chronic phase of SC rats but increased after Lingo-1 inhibition. Our results demonstrated that Lingo-1 shRNA can improve epilepsy-induced cognitive impairment, which may be related with the pro-myelination and neuroprotection effects of Lingo-1 inhibition.
Keywords: Status convulsion, Epilepsy, Epileptogenesis, Lingo-1, Cognitive function
Introduction
Epilepsy is a common neurological disorder characterized by spontaneous recurrent seizures, affecting more than 50 million people around the world (Friefeld Kesselmayer et al. 2020). Patients with epilepsy are susceptible to cognitive impairment and neurobehavioral deficits (Holmes 2015), which adversely affect patient’s quality of life. Currently, there are many anti-epileptic drugs (AEDs) available for patients with epilepsy. Unfortunately, AEDs generally inhibit the central nervous system (CNS) of epilepsy patients and enforce significant inhibitory effects on patient’s cognitive function, which further aggravate patient’s situation (Borham et al. 2016); therefore, more effective approaches should be developed to improve the cognitive function of epileptic patients.
The Nogo-66 receptor 1 (NgR1) pathway, initiated by binding of myelin-associated inhibitors (MAIs) to NgR1 and mediated by multiple NgR1 co-receptors such as leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1 (Lingo-1), p75 and Troy, plays important role in regulation of cognitive function (Tong et al. 2012). Although the relationship between components of the NgR1 pathway and epilepsy has been studied, the dynamic change of the NgR1 pathway during epileptogenesis has never been reported.
Lingo-1 is a transmembrane signaling protein selectively expressed in the CNS on both neurons and oligodendrocyte precursor cells (OPCs). As a critical component of the NgR1 receptor complex (i.e., NgR/p75/Lingo-1 or NgR/Troy/Lingo-1), Lingo-1 is involved in a series of physiological and pathological activities such as oligodendrocyte differentiation and myelination, neuronal survival and axonal regeneration, all these are close relevant with epilepsy (Mi et al. 2013). Considering up-regulated of Lingo-1 was reported in the temporal lobe cortex obtained from mesial temporal lobe epilepsy patients (Zhu et al. 2013) and inhibition of Lingo-1 with antibody could attenuate impaired spatial memory deficits by remyelination in 5 × FAD mice (Wu et al. 2018), we speculate that epilepsy-related cognitive impairment may benefit from Lingo-1 inhibition.
To better know the expressions and roles of the NgR1 pathway in epilepsy, we explored the dynamic protein expressions of neurite outgrowth inhibitor protein A (NogoA), myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), Lingo-1, ras homolog family member A (RhoA), and phosphorylated RhoA (p-RhoA) during epileptogenesis by western blot; after then, lentivirus-mediated short hairpin RNA (shRNA) was used to knock down the expression of Lingo-1 to explore whether targeted Lingo-1 inhibition could attenuate epilepsy-induced cognitive impairment.
Materials and methods
Animals
Healthy adult Sprague–Dawley (SD) rats, specific pathogen free (SPF) grade, weighing 200–250 g, were provided by the Laboratory Animal Center of our University. Animals were housed in a well-ventilated room at temperature of 22–24 °C under controlled light cycles (12 h light:dark) with free access to standard food and water. This study protocol was approved by the Ethics Committee of our University (Ethical approval number: 2016032).
Preparation of epilepsy model
Epilepsy model was prepared according to the method previously described (Tan et al. 2019). Briefly, pilocarpine (30 mg/kg, Sigma–Aldrich, MO, USA) was intraperitoneally (i.p.) injected to rats 18–20 h after lithium (i.p., 127 mg/kg, Sigma–Aldrich, MO, USA) injection to make rats onset of status convulsion (SC) (also called status epilepticus, defined as Class 4 or Class 5 seizures lasting for 60 min). 15 min after SC, rats were i.p. injected with atropine sulfate (1 mg/kg, Southwest Pharmaceutical Co., Ltd, Chongqing, China) to decrease the rate of suffocation caused by glandular secretion. 60 min after SC, Diazepam (i.p., 10 mg/kg, Tianjin Jinyao Amino Acid Co., Ltd, Tianjin, China) was used to stop convulsion. Rats that unreached SC or died were drop out from the study and replaced with new rats. All animal experiments were performed according to the guide principles of the Care and Use of Laboratory Animals of National Administration Regulations on Laboratory Animals of China.
Western blotting (WB) analysis
To determine the hippocampal protein expressions of NogoA, MAG, OMgp, Lingo-1, RhoA, and p-RhoA during epileptogenesis, 32 rats were allocated into four groups (n = 8), including the Control group, the acute phase group (1 day after SC), the latent phase group (5 days after SC), and the chronic phase group (20 days after SC). Rats were treated as the above-mentioned methods and the hippocampi of rats were collected and stored at −80 ℃ immediately after dissection from the brain. Total protein was extracted with the Total Protein Extraction Kit (KeyGen, Nanjing, China) and detected by the BCA protein assay kit (Beyotime, Nantong, Jiangsu China). Total cell lysates were prepared in 1 × SDS buffer and separated by SDS–PAGE. Proteins were then transferred onto PVDF membranes (Millipore, CA, USA). After blocked, the PVDF membranes were incubated with the following primary antibodies: NogoA (1:5000; Abcam), MAG (1:5000; EMD Millipore), OMgp (1:5000; EMD Millipore), Lingo-1 (1:5000; Abcam), RhoA (1:5000; Abcam), p-RhoA (1:5000; Sigma–Aldrich) or β-actin (1:5000; Sigma-Aldrich) overnight at 4 °C. The PVDF membranes were then washed and incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG secondary antibody (1:10,000, Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) for 1 h at room temperature. The Pierce ECL Western Blotting Substrate (Thermo, Waltham, MA, USA) was used to detect chemiluminescent signals. Semi-quantitative analysis of proteins was performed using the Image J software (NIH, Washington DC, USA). The ratio of target protein to internal control (β-actin) was calculated.
Interference of the endogenous Lingo-1 gene by lentivirus-mediated Lingo-1 shRNAs
To knockdown the expression of Lingo-1, three specific shRNA sequences were designed by Shanghai GeneChem Co., Ltd. (Shanghai, China). The primers for shRNA3086, shRNA3087, and shRNA3088 were 5'‑GCACAACATCGAAATTGAA‑3', 5'-CCTTCGCCTTCATCTCCAA-3', and 5'-CCTCAACATCAATGCCATA-3', respectively.
48 SD rats were used to determine the interference effects of Lingo-1 shRNAs on the protein expression of Lingo-1. Tested rats were divided into six groups (n = 8 per group) as follows: the Control group, the Empty virus (EV) group, the SC model group, the shRNA3086 group, the shRNA3087 group, and the shRNA3088 group. SC model was established in all rats except for the Control group and the EV group. One day after SC model establishment, 5 μl Lingo-1 shRNA was injected into the lateral ventricles of rats in all shRNA groups according to the method previously described (Song et al. 2018). Rats in the Control group and the EV group were given 5 μl saline and 5 μl empty lentiviral vector, respectively. 20 days after model establishment, western blot was performed to determine the expression of Lingo-1 to choose the optimum shRNA to perform following experiments.
Novel object recognition (NOR) test
40 rats were divided into the Control group, the EV group, the SC group, and the Lingo-1 shRNA group (shRNA group), with 10 in each. Animal intervention was performed as the above-mentioned method. At the chronic phase of epilepsy (i.e., 20 days after SC), NOR test was performed to determine the effect of Lingo-1 inhibition on the memory of rats. The test was taken place at a square and open-topped arena. An overhead camera was used to record the activities of rats. The whole test lasted for 4 days. The initial 2 days formed a familiarization phase, and rats in this phase were placed into the arena for 10 min each day to habituate surrounding environment (day 1) and test objects (day 2, two identical objects, that is A1 and A2, were separately placed near the corners at the same side of the arena). The final 2 days formed a test phase. At day 3, A1 was replaced with a familiar object (A3) and A2 was replaced with a novel object (B1); after then, tested rats were placed into the arena to allow them to explore test objects for 3 min. At day 4, the position of familiar object (A3) and novel object (B1) was displaced and tested rats were placed into the arena again to explore the objects for another 3 min. During day 3 and day 4, the exploration time of tested rats on familiar object and novel object was recorded for subsequent analysis. Exploratory behavior was defined as the animal directing its nose toward the object at a distance of less than 2 cm. The discrimination ratio, used to evaluate the exploratory ability of rats, was calculated using the following equation: Discrimination ratio = exploration time of novel object (s)/exploration time of familiar object(s).
Morris Water Maze (MWM) test
At the chronic phase of epilepsy, MWM test, with same group design and animal intervention as the NOR test (n = 10 per group), was performed to determine the effect of Lingo-1 inhibition on the spatial learning and memory abilities of rats according to the method previously described (Chen et al. 2014). The whole MWM test lasted for six consecutive days: day 1 formed an initial spatial training phase; day 2 to day 5 formed a spatial reversal training phase; and day 6 formed a probe test phase. Escape latency (the time for animal to reach the platform) and escape distance (total swimming distance before escape) at the initial spatial training phase, escape latency at the spatial reversal training phase, and platform passing times at the probe test phase were recorded and analyzed to determine the spatial learning and memory abilities of tested rats. Platform passing time was defined as the platform quadrant area was passed through during a 60 s period.
Convulsion susceptibility test
Convulsion susceptibility test, with the same group design as the NOR test and MWM test (n = 10 per group), was performed to determine the effect of Lingo-1 inhibition on the convulsion susceptibility of tested rats. Briefly, 20 days after SC model establishment, all rats were treated again with the above-motioned method to induce SC. Convulsion latency, defined as the time from pilocarpine injection (at second time of SC induction) to SC occur, was used to evaluate the convulsion susceptibility of tested rats.
Protein expression of RhoA, p-RhoA, and myelin basic protein (MBP)
20 days after SC model establishment, 32 rats (n = 8 per group) were sacrificed by decapitation to determine the protein expression of RhoA, p-RhoA, and MBP. The hippocampi of rats were collected and stored at − 80 ℃ immediately after dissection. Western blot experiment was performed by the above-mentioned method. Primary antibodies of RhoA (1:5000; Abcam), p-RhoA (1:5000; Sigma–Aldrich), MBP (1:1000, SMI99, Covance, Emeryville, CA, USA) and β-actin (1:5000; Sigma–Aldrich), and HRP-conjugated secondary antibody (1:10,000, Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) were used in this experiment.
Pathological staining
Hematoxylin and eosin (H&E) staining (n = 1 per group) was performed to determine the effect of Lingo-1 inhibition on the structural complete of neuronal cells. Briefly, 20 days after SC model establishment, tested rats were anesthetized by i.p injection of 10% chloral hydrate (3 ml/kg), perfused with 100–150 ml 0.9% saline followed by 200 ml 4% paraformaldehyde both at speed of 15 ml/min. Brains were then removed and fixed in 4% paraformaldehyde solution for 24 h and 30% sucrose solution for 2–3 days to prepare paraffin-embedded tissues. Hippocampal sections (4 μm) were cut from the paraffin-embedded tissues for further routine H&E staining. Stained sections were observed by a light microscope (Eclipse 55i; Nikon Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS (version 19.0; SPSS, Inc., Chicago, IL, USA). Platform passing times were analyzed by Wilcoxon signed-rank-sum test. Other data, such as escape length, escape latency, convulsion latency and protein expressions, are expressed as mean ± standard deviation (SD) and compared using the Student’s t test. P < 0.05 was considered significant.
Results
Dynamic changes of proteins during epileptogenesis
To investigate the dynamic changes of NogoA, MAG, OMgp, Lingo-1, RhoA, and p-RhoA during epileptogenesis, western blot was performed. Our results showed that the levels of three MAIs, i.e., NogoA, MAG, and OMgp, were significantly increased at the latent phase and maintained up-regulated at the chronic phase of epilepsy as compared with that of the control (P < 0.05). The level of Lingo-1 immediately increased at the acute phase and maintained up-regulated throughout epileptogenesis (vs. control, P < 0.05). The level of RhoA was not altered throughout epileptogenesis; while, p-RhoA was significantly increased at the acute phase and the latent phase but decreased at the chronic phase (P < 0.05) (Fig. 1a, b).
Fig. 1.
Dynamic changes of the NgR1 pathway in rat’s hippocampus during epileptogenesis. a Representative western blot figures of multiple proteins in the NgR1 pathway; (b) Relative expressions of detected proteins presented using histogram. NgR1 Nogo receptor 1; NogoA neurite outgrowth inhibitor protein A; MAG myelin-associated glycoprotein; OMgp oligodendrocyte-myelin glycoprotein; Lingo-1 leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1; RhoA ras homolog family member A; p-RhoA phosphorylated RhoA. *P < 0.05, vs. the Control group
Lingo-1 protein expression was significantly inhibited by lentivirus-mediated Lingo-1 shRNAs
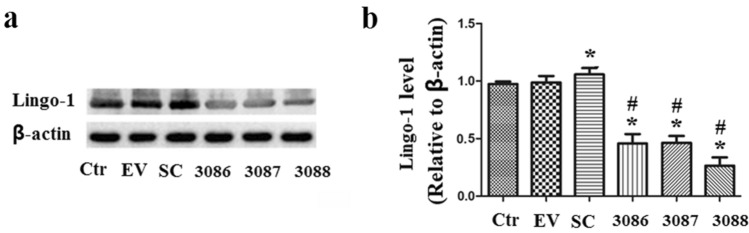
As shown in the Fig. 2a, b, when compared with the Control group, the protein expression of Lingo-1 in the EV group was almost unchanged (P > 0.05); it was significantly increased in the SC model group (P < 0.05) and markedly decreased in the shRNA3086 group, the shRNA3087 group, and the shRNA3088 group as compared with that of the Control group and the SC group (P < 0.05). Among them, shRNA3088 revealed the most significant knockdown effect and, therefore, be chosen to perform following experiments.
Fig. 2.
Protein expression of Lingo-1 was significantly inhibited by lentivirus-mediated Lingo-1 shRNAs. a Representative western blot figures; b Relative expression of Lingo-1 presented using histogram. Ctr Control group; EV Empty virus group; SC Status convulsion group; 3086: Lingo-1 shRNA3086 group; 3087: Lingo-1 shRNA3087 group; 3088: Lingo-1 shRNA3088 group. *P < 0.05, vs. the Control group; #P < 0.05, vs. the SC group
The cognitive function of SC rats was improved after Lingo-1 inhibition
NOR test and MWM test were performed to determine the effect of Lingo-1 inhibition on the cognitive function of SC rats. As shown in the Fig. 3a, at day 3, when a familiar object was replaced by a novel object, the discrimination ratio of the EV group was almost same as that of the Control group (P > 0.05); while, it was significantly decreased in the SC model group when compared with the Control group (P < 0.05). Lingo-1 shRNA significantly increased the discrimination ratio of rats when compared with the SC model group (P < 0.05). At day 4, similar results were observed as day 3 when test objects were displaced (Fig. 3b).
Fig. 3.
The recognition memory ability of chronic phase SC rats was improved by Lingo-1 inhibition determined by novel object recognition test. a Test at day 3 when a familiar object was replaced by a novel object; b Test at day 4 when test objects were displaced. At day 3 and day 4, the discrimination ratio of SC rats was significantly decreased but increased after Lingo-1 inhibition. Ctr Control group; EV Empty virus group; SC Status convulsion group; shRNA Lingo-1 shRNA3088 group. *P < 0.05, vs. the Control group. #P < 0.05, vs. the SC group
In the MWM test, no significant differences were observed with regard to both the escape latency (Fig. 4a) and distance (Fig. 4b) between the four groups at the initial spatial training phase (day 1) (P > 0.05); during the spatial reversal training phase (day 2–day 5), the escape latency was gradually decreased in all four groups. At each day in this phase, the escape latency of the SC model group was significantly longer than that of the Control group and the EV group; at day 4 and day 5, Lingo-1 shRNA significantly decreased the escape latency of rats as compared with the SC model group (Fig. 4c; P < 0.05). At the probe test phase (day 6), the platform passing times of the SC model group was significantly lower than that of other three groups (Fig. 4d, P < 0.05). Collectively, our results indicate that the spatial learning and memory ability of rats were severely impaired following SC and improved by Lingo-1 inhibition.
Fig. 4.
The spatial learning and memory ability of chronic phase SC rats was improved by Lingo-1 inhibition determined by Morris Water Maze test. a Escape latency of day 1; b Escape distance of day 1; c Escape latency of day 2 to day 5; d Platform passing times of day 6. Ctr Control group; EV Empty virus group; SC Status convulsion group; shRNA Lingo-1 shRNA3088 group. *P < 0.05, vs. the Control group; #P < 0.05, vs. the SC group
Convulsion latency, p-RhoA, and MBP expressions were increased after Lingo-1 inhibition
As shown in the Fig. 5a, the convulsion latency of the SC model group was significantly decreased than that of the Control group (P < 0.05) and outstandingly increased after injection with Lingo-1 shRNA (vs. the SC group, P < 0.05), which indicate that targeted Lingo-1 inhibition can improve the convulsion susceptibility of rats.
Fig. 5.
Convulsion susceptibility test and hippocampal protein expressions of RhoA, p-RhoA, and MBP. a Convulsion susceptibility test. Convulsion latency of rats was significantly decreased at the SC group but increased at the shRNA group, suggesting that the convulsion susceptibility of SC rats was improved by Lingo-1 inhibition; b Representative western blot figures and histograms of RhoA, p-RhoA, and MBP. The hippocampal protein expressions of p-RhoA and MBP were significantly decreased at the SC group but outstandingly increased at the shRNA group. RhoA ras homolog family member A; p-RhoA phosphorylated RhoA; MBP myelin basic protein; Ctr Control group; EV Empty virus group; SC Status convulsion group; shRNA: Lingo-1 shRNA3088 group. *P < 0.05, vs. the Control group; #P < 0.05, vs. the SC group
Western blot results showed that, the hippocampal protein level of RhoA was not altered in the EV, SC, and shRNA groups as compared with that of the Control group (P > 0.05); while, for p-RhoA and MBP, both of them were significantly decreased in the SC group compared with that of the Control group (P < 0.05) and remarkably increased in the shRNA group which was even higher than that of the Control group (P < 0.05) (Fig. 5b).
Better neuronal survival was achieved after Lingo-1 inhibition
H&E staining showed that the neuronal cells in the CA1 and CA3 pyramidal layers of the Control group (Fig. 6a) and the EV group (Fig. 6b) arranged regularly with normal morphology and structure. Neuronal cells in same regions of the SC model group (Fig. 6c) were significantly reduced and arranged irregularly; nuclei were shrunk accompanied by cell swelling. On the contrary, the shRNA group showed a relatively complete CA1 and CA3 pyramidal layer structure with the regular arrangement of neurons (Fig. 6d).
Fig. 6.
Better neuronal survival was achieved by Lingo-1 inhibition. a H&E staining figure of the Control group; b H&E staining figure of the EV group; c H&E staining figure of the SC group; (d) H&E staining figure of the shRNA group. In the Control group, EV group, and shRNA group, neuronal cells in the CA1 and CA3 pyramidal layers arranged regularly with normal morphology and structure; while, in the SC model group, neuronal cells in the same regions were significantly reduced and arranged irregularly; nuclei were shrunk accompanied by cell swelling. EV Empty virus; SC Status convulsion; shRNA Lingo-1 shRNA3088. Black arrow indicates the CA1 pyramidal layer; Red arrow indicates the CA3 pyramidal layer. Scale bars = 100 µm
Discussion
Cognitive impairment is an important pathologic sequel of epilepsy (Avanzini et al. 2013; Lopes et al. 2016), which was found both in convulsive status epilepticus patients (Legriel et al. 2010) and in epilepsy animals (Gao et al. 2014; Kalemenev et al. 2015). In recent years, the negative regulatory function of the NgR1 pathway on cognitive function has been explored and established in conditions such as aging (VanGuilder Starkey et al. 2013) and traumatic brain injury (Marklund et al. 2009). While, nearly no study had focused on the roles of the NgR1 pathway on epilepsy-related cognitive impairment. To better know the expressions and roles of the NgR1 pathway on epilepsy, we used lithium‐pilocarpine model to imitate the process of epileptogenesis and determined the dynamic changes of multiple components of the NgR1 pathway, including three MAIs (NogoA, MAG, OMgp) and Lingo-1, in the hippocampi of rats. Our results showed that all three MAIs and Lingo-1 were increased at the latent phase and maintained up-regulated at the chronic phase of epilepsy. In addition to MAIs and Lingo-1, the dynamic changes of RhoA and p-RhoA during epileptogenesis was also determined because evidence had suggested that Lingo-1 can activate downstream signaling such as the RhoA/ROCK pathway to modulate relevant physiological and pathological activities (Mi et al. 2013). In the present study, the expression of RhoA kept unchanged during epileptogenesis; while, the expression of p-RhoA was significantly increased at the acute and latent phases but significantly decreased at the chronic phase. Since the phosphorylation of RhoA on 188S deactivates RhoA (Collins et al. 2014), we suspect that up-regulated of p-RhoA at the acute and latent phases of epileptogenesis may partially counteracts the negative regulatory signals of the NgR1 pathway; while, down-regulated of p-RhoA at the chronic phase may activates the RhoA pathway to aggravate the cognitive impairment induced by epilepsy.
As one of key co-receptors that transmit negative regulatory signals of the NgR1 pathway, accumulating evidences indicate that Lingo-1 is closely involved with cognitive function. For example, Wu et al. demonstrated that inhibition of Lingo-1 with antibody can attenuate impaired spatial memory deficits by remyelination in 5XFAD mice (Wu et al. 2018). Niu et al. reported that the improvement effect of epimedium flavonoids on chronic cerebral hypoperfusion-induced cognitive impairment is relevant with inhibition of the Lingo-1/Fyn/ROCK pathway and activation of the BDNF/NRG1/PI3K pathway (Niu et al. 2020). Therefore, the focus of this study was then shifted to find out whether inhibition of Lingo-1 by shRNA can improve epilepsy-induced cognitive impairment. Our results showed that the recognition memory ability and the learning and memory ability of rats were impaired at the chronic phase of epilepsy and significantly improved by Lingo-1 inhibition. Besides, the convulsion susceptibility of SC rats was improved by Lingo-1 inhibition. Taken together, these results suggest that Lingo-1 inhibition can improve epilepsy-induced cognitive impairment.
Abnormal myelination is related to several demyelinating neurological diseases such as Alzheimer’s disease (Kavroulakis et al. 2018), Parkinson’s disease (Dean et al. 2016), and epilepsy (Drenthen et al. 2019). Recently, some studies suggest that abnormal myelination has close relationship with cognitive impairment. For instance, Ma et al. reported that quetiapine attenuates cognitive impairment and decreases seizure susceptibility in a rat model of malformations of cortical development possibly through promoting myelin development (Ma et al. 2015). Demyelination has been suggested to be involved in the depression comorbidity of a chronic epilepsy rat model via dysregulation of Olig2/LINGO-1 and disturbance of calcium homeostasis (Ma et al. 2019). As previous evidences had demonstrated the Lingo-1 negatively regulates myelination partially via activating RhoA pathway (Mi et al. 2013), we further determined the protein expressions of RhoA and p-RhoA after Lingo-1 inhibition. As expected, the p-RhoA was down-regulated in the SC model but up-regulated after Lingo-1 inhibition, which indicated that RhoA pathway may be activated after SC but deactivated by Lingo-1 inhibition. MBP, accounts for almost 1/3 of the total myelin proteins, is one of the major proteins that constitute myelin, which makes it a surrogate to evaluate the degree of myelin damage (Zhou et al. 2015). Our previous study confirmed that the level of MBP was gradually decreased and the severity of the myelin sheath damage was increased throughout epileptogenesis (Luo et al. 2015). In the present study, we found the level of MBP was decreased after SC and increased after Lingo-1 inhibition. Taken together, these evidences suggested that the improvement effect of Lingo-1 inhibition on epilepsy-induced cognitive impairment may be relevant with its role on pro-myelination via deactivating the RhoA pathway.
Recently, evidences also indicate that neuronal loss in hippocampi directly correlates with cognitive deficits (Hei et al. 2019; Li et al. 2013). The results of the present study clearly showed that neuronal injury, characterized by neuronal loss in hippocampal CA1 and CA3 regions, was perceived in the SC rats. While, neuronal injury was relieved by Lingo-1 inhibition, this phenomenon was also observed in the study performed by Wu et al., in which authors demonstrated that targeted Lingo-1 inhibition can promote the survival of neurons in a spinal cord injury model (Wu et al. 2013).
However, there are some limitations in the present study. First, only western blot was used to determine the dynamic changes of NgR1 pathway related proteins, it would be better if other experiments such as reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescence were added; Second, the number of rats used in the H&E staining is too little, more rats are warranted to support the pathological observations of this study.
Conclusions
Our results demonstrated that the NgR1 pathway was activated during epileptogenesis, especially at the chronic phase of epilepsy. The cognitive functions and convulsion susceptibility of SC rats at the chronic phase were all improved by Lingo-1 shRNA, which may be related with the pro-myelination and neuroprotection effects of Lingo-1 inhibition.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (grant number 81371452) and Chongqing Municipal Planning Commission of science and Research Fund (grant number 2015XMSB000712).
Author contributions
RH: conceptualization, methodology, investigation, formal analysis, visualization, and writing original draft. WH, XJS, LC, and HSC: methodology, resources, investigation, and validation. LJ: conceptualization, methodology, supervision, funding acquisition, review, and editing manuscript. All the authors have read and agreed to the published version of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Rong He, Email: 652055610@qq.com.
Li Jiang, Email: li_jiang0920@163.com.
References
- Avanzini G, Depaulis A, Tassinari A, De Curtis M. Do seizures and epileptic activity worsen epilepsy and deteriorate cognitive function? Epilepsia. 2013;54:14–21. doi: 10.1111/epi.12418. [DOI] [PubMed] [Google Scholar]
- Borham LE, Mahfoz AM, Ibrahim IA, Shahzad N, Lrefai AA, Labib AA, Sef BB, Alshareef A, Khan M, Milibary A. The effect of some immunomodulatory and anti-inflammatory drugs on Li-pilocarpine-induced epileptic disorders in Wistar rats. Brain Res. 2016;1648:418–424. doi: 10.1016/j.brainres.2016.07.046. [DOI] [PubMed] [Google Scholar]
- Chen D-L, Zhang P, Lin L, Zhang H-M, Deng S-D, Wu Z-Q, Liu S-H, Wang J-Y. Protective effects of bajijiasu in a rat model of Aβ25-35-induced neurotoxicity. J Ethnopharmacol. 2014;154:206–217. doi: 10.1016/j.jep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Collins C, Osborne LD, Guilluy C, Chen Z, O’Brien ET, Reader JS, Burridge K, Superfine R, Tzima E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat Commun. 2014;5:3984. doi: 10.1038/ncomms4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, III, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, Theisen F, Johnson SC, Alexander AL, Gallagher CL. Alterations of myelin content in Parkinson’s disease: a cross-sectional neuroimaging study. PLoS ONE. 2016;11:e0163774. doi: 10.1371/journal.pone.0163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenthen GS, Fonseca Wald ELA, Backes WH, Debeij-Van Hall MHJA, Hendriksen JGM, Aldenkamp AP, Vermeulen RJ, Klinkenberg S, Jansen JFA. Lower myelin-water content of the frontal lobe in childhood absence epilepsy. Epilepsia. 2019;60:1689–1696. doi: 10.1111/epi.16280. [DOI] [PubMed] [Google Scholar]
- Friefeld Kesselmayer R, McMillan T, Lee B, Almane D, Hermann BP, Jones JE. Psychosocial and functional outcomes in young adults with childhood-onset epilepsy: a 10-year follow-up. Dev Med Child Neurol. 2020 doi: 10.1111/dmcn.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Gao Y, Liu Y-f, Wang L, Li Y-j. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsych Dis Treat. 2014;10:2139. doi: 10.2147/NDT.S73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei Y, Chen R, Mao X, Wang J, Long Q, Liu W. Neuregulin1 attenuates cognitive deficits and hippocampal CA1 neuronal apoptosis partly via ErbB4 receptor in a rat model of chronic cerebral hypoperfusion. Behav Brain Res. 2019;365:141–149. doi: 10.1016/j.bbr.2019.02.046. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17:101–116. doi: 10.1684/epd.2015.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalemenev S, Zubareva O, Frolova E, Sizov V, Lavrentyeva V, Lukomskaya NY, Kim KK, Zaitsev A, Magazanik L. Impairment of exploratory behavior and spatial memory in adolescent rats in lithium-pilocarpine model of temporal lobe epilepsy. Dokl Biol Sci. 2015;463:175–177. doi: 10.1134/S0012496615040055. [DOI] [PubMed] [Google Scholar]
- Kavroulakis E, Simos PG, Kalaitzakis G, Maris TG, Karageorgou D, Zaganas I, Panagiotakis S, Basta M, Vgontzas A, Papadaki E. Myelin content changes in probable Alzheimer's disease and mild cognitive impairment: associations with age and severity of neuropsychiatric impairment. J Magn Reson Imaging. 2018;47:1359–1372. doi: 10.1002/jmri.25849. [DOI] [PubMed] [Google Scholar]
- Legriel S, Azoulay E, Resche-Rigon M, Lemiale V, Mourvillier B, Kouatchet A, Troché G, Wolf M, Galliot R, Dessertaine G. Functional outcome after convulsive status epilepticus. Crit Care Med. 2010;38:2295–2303. doi: 10.1097/CCM.0b013e3181f859a6. [DOI] [PubMed] [Google Scholar]
- Li G, Cheng H, Zhang X, Shang X, Xie H, Zhang X, Yu J, Han J. Hippocampal neuron loss is correlated with cognitive deficits in SAMP8 mice. Neurol Sci. 2013;34:963–969. doi: 10.1007/s10072-012-1173-z. [DOI] [PubMed] [Google Scholar]
- Lopes MW, Lopes SC, Santos DB, Costa AP, Gonçalves FM, de Mello N, Prediger RD, Farina M, Walz R, Leal RB. Time course evaluation of behavioral impairments in the pilocarpine model of epilepsy. Epilepsy Behav. 2016;55:92–100. doi: 10.1016/j.yebeh.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Luo Y, Hu Q, Zhang Q, Hong S, Tang X, Cheng L, Jiang L. Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain Res. 2015;1627:154–164. doi: 10.1016/j.brainres.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Ma L, Yang F, Zhao R, Li L, Kang X, Xiao L, Jiang W. Quetiapine attenuates cognitive impairment and decreases seizure susceptibility possibly through promoting myelin development in a rat model of malformations of cortical development. Brain Res. 2015;1622:443–451. doi: 10.1016/j.brainres.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Ma T, Li B, Le Y, Xu Y, Wang F, Tian Y, Cai Q, Liu Z, Xiao L, Li H. Demyelination contributes to depression comorbidity in a rat model of chronic epilepsy via dysregulation of Olig2/LINGO-1 and disturbance of calcium homeostasis. Exp Neurol. 2019;321:113034. doi: 10.1016/j.expneurol.2019.113034. [DOI] [PubMed] [Google Scholar]
- Marklund N, Morales D, Clausen F, Hånell A, Kiwanuka O, Pitkänen A, Gimbel DA, Philipson O, Lannfelt L, Hillered L, Strittmatter SM, McIntosh TK. Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice. Neuroscience. 2009;163:540–551. doi: 10.1016/j.neuroscience.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27:493–503. doi: 10.1007/s40263-013-0068-8. [DOI] [PubMed] [Google Scholar]
- Niu H-m, Wang M-y, Ma D-l, Chen X-p, Zhang L, Li Y-l, Zhang L, Li L. Epimedium flavonoids improve cognitive impairment and white matter lesions induced by chronic cerebral hypoperfusion through inhibiting the Lingo-1/Fyn/ROCK pathway and activating the BDNF/NRG1/PI3K pathway in rats. Brain Res. 2020 doi: 10.1016/j.brainres.2020.146902. [DOI] [PubMed] [Google Scholar]
- Song X-J, Han W, He R, Li T-Y, Xie L-L, Cheng L, Chen H-S, Jiang L. Alterations of hippocampal myelin sheath and axon sprouting by status convulsion and regulating Lingo-1 expression with RNA interference in immature and adult rats. Neurochem Res. 2018;43:721–735. doi: 10.1007/s11064-018-2474-2. [DOI] [PubMed] [Google Scholar]
- Tan X, Tu Z, Han W, Song X, Cheng L, Chen H, Tu S, Li P, Liu W, Jiang L. Anticonvulsant and neuroprotective effects of dexmedetomidine on pilocarpine-induced status epilepticus in rats using a metabolomics approach. Med Sci Monitor. 2019;25:2066. doi: 10.12659/MSM.912283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Liu W, Wang X, Han X, Hyrien O, Samadani U, Smith DH, Huang JH. Inhibition of Nogo-66 receptor 1 enhances recovery of cognitive function after traumatic brain injury in mice. J Neurotraum. 2012;30(4):247–258. doi: 10.1089/neu.2012.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder Starkey HD, Sonntag WE, Freeman WM. Increased hippocampal NgR1 signaling machinery in aged rats with deficits of spatial cognition. Eur J Neurosci. 2013;37:1643–1658. doi: 10.1111/ejn.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-F, Cen J-S, Zhong Q, Chen L, Wang J, Deng DY, Wan Y. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials. 2013;34:1686–1700. doi: 10.1016/j.biomaterials.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Wu D, Tang X, Gu LH. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in the early stage of 5XFAD mice. CNS Neurol Disord Drug Targets. 2018;16:51–64. doi: 10.2174/1871527315666160915150754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Li W, Qu L-H, Tang J, Chen S, Rong X. Relationship of plasma S100B and MBP with brain damage in preterm infants. Int J Clin Exp Med. 2015;8:16445. [PMC free article] [PubMed] [Google Scholar]
- Zhu K-J, Li S, Xu G-Z, Liu S-Y, An N, Yang H. Expression of Nogo-A signaling pathway in temporal lobe cortex from mesial temporal lobe epilepsy patients. J Third Military Med Univ. 2013;11:1076–1079. [Google Scholar]