Abstract
A new matrix formulation was devised for catalase immobilization. Carrageenan-alginate beads different ratios were developed and soaked into different ratios of CaCl2–KCl as a hardening solution. The best formulation for loading capacity was selected, treated with polyethylene imine followed by glutaraldehyde and further studied. The best concentration of catalase for immobilization was 300U/ml and the best loading time was 6 h. The catalytic properties increased after immobilization and the immobilized catalase achieved optimum activity at a temperature range of 30–50 °C that was compared to the optimum activity of free catalase which occurred at 40 °C. Higher catalytic activity of immobilized catalase occurred at alkaline pHs than the free one which achieved optimum catalytic activity at neutral pH. A comparison between the kinetic parameters of immobilized and free catalase showed variation. The KM and Vmax of the immobilized catalase were 2.4 fold and six times higher than those of free catalase. The results of the study indicate that the formulated matrix can be used as a good matrix for catalase enzyme in various industrial applications.
Keywords: Biotechnology, Biopolymers, Enzymes, Immobilized catalase and grafted gel beads
Introduction
The application of enzymes in industrial processes has many advantages, such as high-efficiency reaction, chemostereo selectivity and without toxic byproducts (Labus et al. 2020). However, using enzymes in their native free form has many disadvantages such as difficult separation from reaction mixture, which limits reusability. They cannot resist harsh environmental conditions such as high temperature or adverse pH values (Yassin and Gad 2020). Therefore, there is always a need to immobilize enzymes for different applications (Ali et al. 2017; Hassan et al. 2019a, b).
Enzyme immobilization can be defined as trapanning of an enzyme to restrict its movement in space, thus imparting the enzyme molecule with various advantages such as reusability of enzyme in the reaction several times, ease of separation of the immobilized enzyme from the reaction mixture and enhansing the thermal and pH stability (Hassan et al. 2019a, b).
Catalase enzyme (EC 1.11.1.6) is an oxidoreductase enzyme that enables cells to eleminate reactive oxygen species (ROS) which causes oxidative stress and becomes cytotoxic. Hydrogen peroxide is ROS byproduct of aerobic respiration. Catalase acts as an antioxidant and protects cells from oxidative stress by breaking down hydrogen peroxide into water and molecular oxygen (Vincent et al. 2021).
Immobilized catalase has several industrial applications, including biotechnology, biochemical analysis, sensor applications, food packaging, wastewater treatment, pharmaceuticals and textile bleaching (Sel et al. 2020).
Various matrices have been developed to Catalase immobiliztion in different studies; for example, Haiyan et al. immobilized catalase using the PAES-C polymer for wastewater treatment (Bi et al. 2021), Kadir et al. used poly (HEMA-GMA) cryogel as a matrix for immobilization of catalase enzyme which enhanced the loading capacity of catalase (Erol et al. 2019). Also, Monier et al. immobilized the catalase enzyme using entrapment and encapsulation technique for U.V. curing with a photo-cross-linkable material based on styrylpyridine modified gellan gum (Monier et al. 2020). Danial et al. formed another matrix from alginate beads by encapsulating catalase and superoxide dismutase and enhanced their stability (Danial and Alkhalaf 2020). In this study, two naturally occurring hydrogels, carrageenan (car-) and alginate (alg-) were used as a matrix for the immobilization of the catalase enzyme, as they are nutritionally and pharmaceutically safe and cost-effective (Wahba et al. 2020).
Carrageenan is a linear polysaccharide extracted from red seawee. (Rhodophycae sp.) (Yassin et al. 2019). There are three different types of carrageenan, Kappa-carrageenan (κ-) which usually forms a strong firm/rigid gel in the presence of potassium ions; lamda-carrageenan (λ-) which is used as a thickening agent and iota-carrageenan (ί-) which requires calcium ions to form soft elastic gels (Ramdhan et al. 2020; Yassin et al. 2018). This study utilized kappa-carrageenan (κ-) as it forms a strong gel and when combined with alginate, it forms matrix beads for catalase immobilization.
Alginate is a linear anionic polysaccharide extracted from bacteria and brown algae, which consists of repeating units of β-1,4-linked D-mannuronic acid (M) and l-guluronic acid (G) in different ratios. To form a hard gel network, alginate can form rigid hydrogels with divalent cations, such as Ca2+ (Zhang and Zhao 2020).
In this study, two naturally occurring hydrogels, carrageenan (car) and alginate (alg) were modified by polyethylene imine followed by glutaraldehyde and used as a matrix for the immobilization of the catalase enzyme as they are nutritionally and pharmaceutically safe as well as being cost-effective. Comparison of catalytic parameters such as temperature, pH and kinetic parameters between immobilized and free enzyme were evaluated.
Methods
Materials
Carrageenan and sodium alginate were purchased from Fluka, Switzerland. The Glutaraldehyde solution (GA) and polyethyleneimine (PEI) were purchased from Sigma-Aldrich, Germany. Catalase enzyme extracted from bovine liver was purchased from Sigma-Aldrich with the activity of 2 U/µg. All other reagents were of analytical grade.
Methods
Preparation of carrageenan-alginate beads in different concentration ratios
Carrageenan-alginate beads were prepared via the ionotropic gelation method (Villamar and Langdon 1993) as follows:
A solution of 2.5% carrageenan and 2.5% alginate was prepared. Different ratios of these polymers were mixed together to form different formulations (10:90, 20:80, 30:70, 40:60 and 50:50) as the percent volume. Each formulation was dropped into different ratios of hardening solution (0.3 M KCl and 3% CaCl2) as the volume ratio (1:1, 1:2 and 1:3). Beads were then allowed to harden overnight. The effect of these variables on the catalase loading capacity was studied.
Treatment of gel beads
Beads were filtered, washed and soaked in 4% of polyethyleneimine pH 9 for 2 h. After that, beads were filtered, washed trice with distilled water and finally soaked in 2% glutaraldehyde solution for another 2 h, and also washed with distilled water to remove any traces of glutaraldehyde (Wahba and Hassan 2017).
Immobilization of the catalase enzyme
Approximately 40U/ml of catalase solution was prepared in 50 mM sodium phosphate buffer pH 7. The preperd beads were filtered and washed, of which approximately 0.5 gm was soaked in 5 ml of the above-prepared enzyme solution. The immobilization process spanned approximately 16 h. Beads were then filtered and washed with phosphate buffer pH 7 in preparation for the immobilized catalase activity assay (Elnashar et al. 2013).
Catalase activity assay
Catalase activity was determined by preparing 30 mM of H2O2 as a substrate (using 50 mM phosphate buffer pH 7). Then 0.5 gm of immobilized catalase beads were soaked in 3 ml of substrate. and the reaction was processed for 3 min. Next, the reaction was stopped by removing beads from the reaction mixture. The absorbance of the substrate was measured spectrophotometrically at the start and end of the reaction at 240 nm (Chance and Maehly 1955). The activity of immobilized catalase was calculated according to the following equation:
Optimization of the loading conditions
Effect of the enzyme concentration on loading capacity
Different concentrations of the catalase enzyme ranging from (20 to 800 U/ml) were prepared. Then 0.5 gm of treated beads was immersed in 2.5 ml of the prepared enzyme concentration. The immobilization reaction was processed for 3 h. After which, the beads were filtered, washed with buffer. and prepared for assay (Alptekin et al. 2010).
Effect of time on the loading capacity
To test the effect of time on the loading capacity, 0.5 gm of treated beads was immersed in 2.5 ml of 300 U/ml of catalase enzyme. The immobilization reaction was processed in different intervals of time as (2, 4, 6, 8, 12, and 16 h). After each time interval, the beads were filtered and washed, then used for the assay of immobilized catalase activity (Alptekin et al. 2009).
Catalytic parameters
Effect of temperature on the catalytic activity
Both immobilized and free catalase enzymes were assayed at different temperatures (4–80 °C) as described previously in the section on the determination of catalase activity. The activity at an optimum temperature was taken as the 100% activity, an other activities at other temperatures were expressed as a percentage of the total (100%) activity (Alptekin et al. 2010).
Effect of pH on the catalytic activity
Both the immobilized and free catalase enzyme were assayed in different pH values of buffer (pH 3–9) as was described in the section on the determination of catalase activity. Different buffers of different pHs were used as the citrate buffer (pH range = 3–6), phosphate buffer (pH range = 6–8) and tris buffer (pH range = 8–9).The activity measured at optimum pH was considered to be 100% activity, and other activities at other pH values were expressed as a percentage of this optimum 100% activity (Alptekin et al. 2010).
Kinetic parameters
The kinetic parameters for immobilized and free catalase were investigated using the Michaelis—Menten equation. Different concentrations of hydrogen peroxide substrate (10–80 mM) were used in the buffer solution to determine the KM and Vmax (Wagner 1976).
Reusability study
The optimized beads were applied to catalyze a hydrogen peroxide decomposition reaction for several runs. After each run, the beads were washed three times with distilled water and used to catalyze another hydrogen peroxide decomposition reaction under the same conditions. The reusability study was performed for approximately 23 runs. The obtained relative activities were plotted against the number of cycles (Doğaç and Teke 2013).
Results and discussion
Effect of bead composition and hardening solution ratio on catalase loading capacity
Carrageenan-alginate beads of different ratios were developed and soaked into different ratios of a combined CaCl2/KCl hardening solution. The best formulation for loading capacity was selected and treated with polyethylene imine followed by glutaraldehyde. As illustrated in Fig. 1, the formulation 50:50 percent volume of carrageenan to alginate solution forming beads was optimal for obtaining high catalase loading capacity and the hardening solution of 1:1 percent volume of KCl to CaCl2 was selected due to the strength that it imparted for beads.
Fig. 1.
Effect of bead composition and hardening solution ratio on catalase loading capacity
Optimization of loading conditions
Effect of the enzyme concentration on loading capacity
As shown in Fig. 2, the loading capacity on beads increases up to 300 U/ml enzyme concentration, as the enzyme concentration increases. After ward, there is no obvious effect of the increasing enzyme concentration on loading capacity, which can be interpreted as the active site of catalase molecules might be restricted due to a high enzyme concentration (Doğaç et al. 2015). These observations support the use of 300U/ml enzyme concentration as the optimal concentration for immobilization.
Fig. 2.

Effect of catalase concentration on loading capacity
Effect of time on the loading capacity
Figure 3 reveals the direct relationship between the time and activity of catalase immobilized beads. Six hours incubation of beads with the catalase enzyme achieved the highest activity of immoblized catalase. After ward the activity gradually decreased. Thus a time of 6 h was selected as the optimum time for catalase immobilization.
Fig. 3.
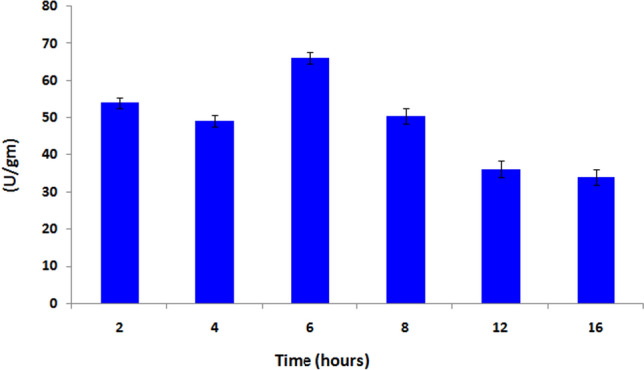
Effect of time on loading capacity of immobilized catalase
Catalytic parameters
Effect of temperature on catalytic activity
As shown in Fig. 4, the activity of both free and immobilized catalase increases as the temperature increases. For free catalase, the optimum relative activity was obtained at 40 °C. After which, the activity gradually decreased untill it reached its minimum activity at 80 °C. On the other hand, the relative activity of the immobilized enzyme also increased as temperature increased and it reached its optimum activity at 30–50 °C to form a broadband at these temperatures, thus becoming more stable than the free enzyme. Jyoti Kaushal et al. studied the effect of temperature on the activity of free and chitosan immobilized catalase and found that optimum activity was obtained at 40 °C. After which, the activity obviously decreased (Kaushal et al. 2018). In addition, Monier et al. found that both soluble and immobilized catalase retained their optimum activity levels at 35 °C and their activity lessened as the temperature increased (Monier et al. 2020). These findings could be interpreted as a temperature induced denaturation, it causes denaturation of protein-based catalase macromolecule leading to a loss of enzyme activity (Monier et al. 2020).
Fig. 4.

Effect of temperature on catalytic activity of immobilized catalase
Effect of pH on the catalytic activity
As indicated in Fig. 5, the relative activity for both free and immobilized catalase increased as pH increased untill it reached the optimum value of pH 6. However, at pH 7 and 8, the relative activity of immobilized catalase was higher than free catalase. The free catalase showed higher activity at acidic pH (3–6) than immobilized catalase. On the contrary, this immobilized catalase showed higher activity at alkaline pH. This behavior could be attributed to conformational changes of the catalase enzyme including changes in the charge and structure of the matrix that affects catalase activity (Inanan et al. 2018).
Fig. 5.
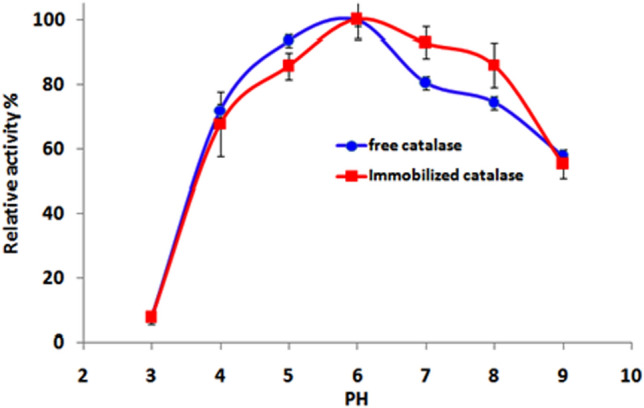
Effect of pH on catalytic activity of immobilized catalase
Kinetic parameters
The kinetic parameters (KM and Vmax) of free and immobilized catalase are shown in Fig. 6 and tabulated in Table 1. As shown in Fig. 6, the KM for the free catalse enzyme was 28 mM which is in agrrement with the common value for KM of catalase in the literature (25 mM) (Bradford 2007). The KM of immobilized catalase was 2.35 times higher than that of the free catalase while it was only 1.5 time when immobilized on chitosan beads as reported by Arabaci et al. (2013). A higher KM reflects the decreased affinity of immobilized catalase for its substrate which could be due to the steric effect and diffusion limitations caused by enzyme immobilization (Doğaç et al. 2015). In addition, the immobilized catalase Vmax was approximately six times higher than that of free catalase while it decreased by fourfold when immobilized onto poly(HEMA-GMA) cryogels with different amount of GMA as reported by Erol and his group (Erol et al. 2019). This behavior of inceament could be due to changes in the catalase configuration and molecular crowding caused by immobilization (Ibrahim et al. 2016; Ahmed et al. 2019).
Fig. 6.
Kinetic parameters of free and immobilized catalase
Table 1.
Kinetic parameters of free and immobilized catalase
| Enzyme | Km (mM) | Vmax (µmole/min.mg) |
|---|---|---|
| Free catalase | 28 ± 0.5 | 12 ± 0.8 |
| Immobilized catalase | 66 ± 2.5 | 73 ± 1.5 |
Reusability study
The immobilized catalase beads maintained approximately 88% of relative activity after the 5th run and approximately 50% at the 19th run of catalytic reactions. After which, the relative activity gradually decreased untill it reached 22% relative activity at cycle 23 (Fig. 7).
Fig. 7.

Reusability study of immobilized catalase beads
Kadir Erol et al. obtained 81% of the total immobilized catalase activity at the end of five cycles. At the end of the 10th use, activity decreased by 45% (Erol et al. 2019). As a comaprison, Nazar Hamzah maintained approximately 75% of the activity when it was reused eight times and reached 20% of activity at the 14th run (Hamzah 2020).
Conclusions
Catalase was covalently immobilized onto polyethyleneimine–glutaraldehyde activated carrageenan-alginate beads. Parameters affecting the loading capacity were studied and optimized. The immobilization process using this formulated matrix enhanced catalase catalytic activity at different temperatures and pH levels. Surprisingly, the Vmax of the immobilized catalase significantly increased compared to free catalase which is considered to be an advantage for this formulated matrix. In addition, the beads maintained approximately 88% of its relative activity after the 5th run and approximately 50% at the 19th run of catalytic reactions. The developed immobilized catalase is believed to be a promising biocatalyst for various catalase industrial applications.
Acknowledgements
The authors would like to gratefully aknowledge Dr/Mohamed Elsayed Ali Hassan, Dr/Mohamed Ahmed Hussein Yassin, Dr/Roqaya Ibrahim Bassuny and Dr/Asmaa El-Shershaby for their scientific and technical support.
Author contributions
All authors have given consent to publish this work in 3 Biotech.
Funding
This work received no external funding. This work was funded internally by the National Research Centre and is a part of the doctoral thesis of Ali O. Ali.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
This research studies conducted for this article did not use human participants, vertebrates or higher invertebrates.
References
- Ahmed SA, Saleh SAA, Abdel-Hameed SAM, Fayad AM. Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics. Heliyon. 2019;5(5):e01674. doi: 10.1016/j.heliyon.2019.e01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali KA, Hassan ME, Elnashar MMM. Development of functionalized carrageenan, chitosan and alginate as polymeric chelating ligands for water softening. Int J Environ Sci Technol (tehran) 2017;14(9):2009–2014. doi: 10.1007/s13762-017-1298-y. [DOI] [Google Scholar]
- Alptekin Ö, Tükel S, Yildirim D, Alagöz D. Characterization and properties of catalase immobilized onto controlled pore glass and its application in batch and plug-flow type reactors. J Mol Catal B-Enzym. 2009;58:124–131. doi: 10.1016/j.molcatb.2008.12.004. [DOI] [Google Scholar]
- Alptekin Ö, Tükel SS, Yıldırım D, Alagöz D. Immobilization of catalase onto Eupergit C and its characterization. J Mol Catal b Enzym. 2010;64(3):177–183. doi: 10.1016/j.molcatb.2009.09.010. [DOI] [Google Scholar]
- Arabaci G, Usluoglu A. Catalytic properties and immobilization studies of catalase from Malva sylvestris L. J Chem. 2013 doi: 10.1155/2013/686185. [DOI] [Google Scholar]
- Bi H, Tian J, Fu Y, Wang D, Cai W. Immobilization of catalase using PAES-C polymer for wastewater biological treatment research. IOP Conf Ser Earth Environ Sci. 2021;661(1):012004. doi: 10.1088/1755-1315/661/1/012004. [DOI] [Google Scholar]
- Bradford BU. Role of peroxisomes in the swift increase in alcohol metabolism. J Gastroenterol Hepatol. 2007;22(s1):S28–S30. doi: 10.1111/j.1440-1746.2006.04641.x. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Methods in enzymology. Cambrigde: Academic Press; 1955. Assay of catalases and peroxidases; pp. 764–775. [Google Scholar]
- Danial EN, Alkhalaf MI. Co-immobilisation of superoxide dismutase and catalase using an in vitro encapsulation protocol. J King Saud Univ Sci. 2020;32(4):2489–2494. doi: 10.1016/j.jksus.2020.04.003. [DOI] [Google Scholar]
- Doğaç Yİ, Teke M. Immobilization of bovine catalase onto magnetic nanoparticles. Prep Biochem Biotechnol. 2013;43(8):750–765. doi: 10.1080/10826068.2013.773340. [DOI] [PubMed] [Google Scholar]
- Doğaç YI, Çinar M, Teke M. Improving of catalase stability properties by encapsulation in alginate/Fe3O4 magnetic composite beads for enzymatic removal of H2O2. Prep Biochem Biotechnol. 2015;45(2):144–157. doi: 10.1080/10826068.2014.907178. [DOI] [PubMed] [Google Scholar]
- Elnashar M, Hassan M, Awad G. Grafted carrageenan gel disks and beads with polyethylenimine and glutaraldehyde for covalent immobilization of Penicillin G acylase. Colloid Sci Biotechnol. 2013;2:27–33. doi: 10.1166/jcsb.2013.1029. [DOI] [Google Scholar]
- Erol K, Cebeci BK, Köse K, Köse DA. Effect of immobilization on the activity of catalase carried by poly(HEMA-GMA) cryogels. Int J Biol Macromol. 2019;123:738–743. doi: 10.1016/j.ijbiomac.2018.11.121. [DOI] [PubMed] [Google Scholar]
- Hamzah N. Charaterization of free and immobilized catalase purified from Convulvulus arvensis L. Plant Arch. 2020;20:2399–2404. [Google Scholar]
- Hassan ME, Yang Q, Xiao Z. Covalent immobilization of glucoamylase enzyme onto chemically activated surface of κ-carrageenan. Bull Natl Res Cent. 2019;43(1):102. doi: 10.1186/s42269-019-0148-0. [DOI] [Google Scholar]
- Hassan ME, Yang Q, Xiao Z, Liu L, Wang N, Cui X, Yang L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 2019;9(12):440. doi: 10.1007/s13205-019-1969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Al-Salamah AA, El-Toni AM, Almaary KS, El-Tayeb MA, Elbadawi YB, Antranikian G (2016) Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres. Int J Mol Sci 17 (2). doi:10.3390/ijms17020184 [DOI] [PMC free article] [PubMed]
- Inanan T, Tüzmen N, Karipcin F. Oxime-functionalized cryogel disks for catalase immobilization. Int J Biol Macromol. 2018;114:812–820. doi: 10.1016/j.ijbiomac.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Kaushal J, Seema SG, Arya SK. Immobilization of catalase onto chitosan and chitosan–bentonite complex: a comparative study. Biotechnol Rep. 2018;18:e00258. doi: 10.1016/j.btre.2018.e00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus K, Wolanin K, Radosiński Ł. Comparative study on enzyme immobilization using natural hydrogel matrices—experimental studies supported by molecular models analysis. Catalysts. 2020;10(5):489. doi: 10.3390/catal10050489. [DOI] [Google Scholar]
- Monier M, Shafik AL, El-Mekabaty A. Designing and investigation of photo-active gellan gum for the efficient immobilization of catalase by entrapment. Int J Biol Macromol. 2020;161:539–549. doi: 10.1016/j.ijbiomac.2020.06.079. [DOI] [PubMed] [Google Scholar]
- Ramdhan T, Ching SH, Prakash S, Bhandari B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci Technol. 2020;106:150–159. doi: 10.1016/j.tifs.2020.10.002. [DOI] [Google Scholar]
- Sel E, Ulu A, Ateş B, Köytepe S. Comparative study of catalase immobilization via adsorption on P(MMA-co-PEG500MA) structures as an effective polymer support. Polym Bull. 2020 doi: 10.1007/s00289-020-03233-0. [DOI] [Google Scholar]
- Villamar DF, Langdon CJ. Delivery of dietary components to larval shrimp (Penaeus vannamei) by means of complex microcapsules. Mar Biol. 1993;115(4):635–642. doi: 10.1007/BF00349371. [DOI] [Google Scholar]
- Vincent A, Thauvin M, Quévrain E, Mathieu E, Layani S, Seksik P, Batinic-Haberle I, Vriz S, Policar C, Delsuc N. Evaluation of the compounds commonly known as superoxide dismutase and catalase mimics in cellular models. J Inorg Biochem. 2021 doi: 10.1016/j.jinorgbio.2021.111431. [DOI] [PubMed] [Google Scholar]
- Wagner M. Enzyme kinetics, behavior and analysis of rapid equilibrium and steady-state enzyme systems (Segel, Irwin H.) J Chem Educ. 1976;53(11):A472. doi: 10.1021/ed053pA472. [DOI] [Google Scholar]
- Wahba MI, Hassan ME. Agar-carrageenan hydrogel blend as a carrier for the covalent immobilization of β-d-galactosidase. Macromol Res. 2017;25(9):913–923. doi: 10.1007/s13233-017-5123-8. [DOI] [Google Scholar]
- Wahba MI, Hassan ME, Ali KA. Chitosan-glutaraldehyde activated carrageenan-alginate beads for β-D-galactosidase covalent immobilisation. Biocatal Biotransformation. 2020 doi: 10.1080/10242422.2020.1832476. [DOI] [Google Scholar]
- Yassin MA, Gad AAM. Immobilized enzyme on modified polystyrene foam waste: a biocatalyst for wastewater decolorization. J Environ Chem Eng. 2020;8(5):104435. doi: 10.1016/j.jece.2020.104435. [DOI] [Google Scholar]
- Yassin MA, Naguib M, Abdel Rehim MH, Ali KA. Immobilization of β-galactosidase on carrageenan gel via bio-inspired polydopamine coating. J Text Color Polym Sci. 2018;15(1):85–93. doi: 10.21608/jtcps.2018.6267.1012. [DOI] [Google Scholar]
- Yassin MA, Gad AAM, Ghanem AF, Abdel Rehim MH. Green synthesis of cellulose nanofibers using immobilized cellulase. Carbohydr Polym. 2019;205:255–260. doi: 10.1016/j.carbpol.2018.10.040. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhao X. Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol. 2020;162:1414–1428. doi: 10.1016/j.ijbiomac.2020.07.311. [DOI] [PubMed] [Google Scholar]




