Western blotting (WB) is an antibody-based experimental technique used to detect and quantify target proteins, which are often within a complex mixture extracted from cells or tissue. Although there are many new alternative technologies, such as enzyme-linked immunosorbent assay (ELISA), immunofluorescence, and mass spectrometry (MS), they all have their own limitations to some extent. ELISA lacks loading controls, immunofluorescence is an in situ technique and is semiquantitative, while MS is expensive and depends on the experimental technique and conditions. Therefore, WB remains the most commonly used methodology in the lab for protein detection. However, concerns about WB have been voiced by many scientific journals in an effort to reduce potential mistakes and increase reproducibility [1]. Here, we will focus on some essential caveats during the WB experiment. This guide, therefore, aims to provide an updated and more concise and useable reference for future experiments and paper writing.
WB includes the following steps. First, proteins are separated from the mixture by sodium dodecyl sulfate–polyacrylamide gel electrophoresis according to their molecular weights. Next, the separated proteins are transferred and bound to a solid membrane. Then, the target protein on the membrane is detected by the immunological method. The identification of a specific protein is based on two parameters: molecular weight and signal intensity. Molecular weight could be estimated by prestained molecular weight markers. The signal is determined by a secondary antibody following the addition of primary antibodies to detect the protein blotted onto the membrane.
Since WB involves multiple steps for detection of different proteins, there is no one particular set of optimal conditions suitable for all proteins. Researchers usually spend considerable time optimizing the conditions to obtain the best signal-to-noise ratios, yet difficulties persist in obtaining consistent and high-quality results. Many specific techniques used in the experiment influence the result of WB, among which the experimental controls, the characterization of antibodies, the choice of loading controls, and the image processing and presentation are the most noticeable challenges. Next, we will discuss those important aspects of WB.
Sample preparation will directly affect the quality of the results, so the choice of correct lysis buffer is a critical step. In general, lysis buffers containing nonionic detergents such as NP-40 or Triton X-100 are sufficient to release proteins from cells, while ionic detergents such as SDS and sodium deoxycholate can be considered for harsh extraction conditions. Thus, the most commonly used commercial lysis buffers are radioimmunoprecipitation assay buffer containing SDS and NP-40 buffer without SDS. In special cases, guanidine-HCl, a chaotropic agent, can be added into lysis buffer to denature oligomerized proteins into their native conformations. Moreover, proteolysis could be inhibited by protease inhibitors, such as PMSF, pepstatin, and EDTA; and protein dephosphorylation could be prevented by phosphatase inhibitors, such as NaF and Na3VO4. Thus, the appropriate commercial protease inhibitor cocktail could be used according to specific needs.
After sample lysis, the protein concentration is measured before the next procedure. Various methods for protein concentration detection can be applied, including the Bradford assay, Lowry assay, and bicinchoninic assay (BCA). The Bradford assay is based on the absorption of the dye Coomassie Blue G-250 by proteins. The principles of the Lowry assay and BCA assay are similar and rely on color development from the Biuret reaction based on the concentrations of the proteins dissolved in samples. The advantages of the Bradford assay are that it is easy and quick to perform with one reagent, while the advantages of the Lowry assay and BCA assay are their extreme sensitivity and improved compatibility with a wide range of detergents (SDS, Triton X-100, Tween 20, etc.).
It is important to set both positive and negative controls for the detected proteins to validate the WB results. Genetically modified animal tissue or cells are suggested as choices for controls. We can verify the correct molecular weight by comparing the wild-type sample with the knockout or knockdown animal or cell sample. Controls lacking the primary antibody or the blocking peptide of the antibody can verify the specificity of the antibody used in WB. Although the above-mentioned controls may not always be available for all proteins, positive and negative controls need to be included as much as possible.
The selectivity of antibodies directly affects WB results, and poor selectivity may lead to the misinterpretation of the results. There are currently databases that can be used for choosing characterized antibodies with high selectivities, such as Antibodypedia (https://www.antibodypedia.com/), the Human Protein Atlas (http://www.proteinatlas.org/), and the Antibody Registry (https://antibodyregistry.org/). For unvalidated antibodies, there are suggested methodologies to validate the selectivity of the antibodies [2]. These methods include detection of whether the signal is eliminated or significantly reduced after genetic knockout or knockdown of the target gene; analysis of the correlation between WB signals and signals of other detection methods (e.g., MS) in a set of different samples with variable expression of the target protein; analysis of the correlation of protein levels by using two or more independent antibodies targeting different epitopes of the same protein; expression of the target protein with a tag, and analysis of the correlation between antibody labeling and the detection of the tag. If the results are highly correlated, then the antibody is validated for WB analysis.
Usually, there are multiple secondary antibodies suitable for the subsequent detection of the target protein, and the selection can be optimized in specific experiments. When selecting a secondary antibody, both the type of primary antibody and the requirements of subsequent detection schemes should be considered comprehensively:
Species source of the primary antibody: the reactivity of the secondary antibody should be consistent with the species source of the primary antibody used. For example, if the primary antibody is a mouse-derived monoclonal antibody, an anti-mouse secondary antibody (goat anti-mouse or rabbit anti-mouse) should be selected.
Type of primary antibody: the secondary antibody must match the class or subclass of the primary antibody. This is usually applicable for monoclonal antibodies. Polyclonal antibodies are mainly IgG immunoglobulins, so the corresponding secondary antibodies are anti-IgG antibodies. If the primary antibody is mouse IgM, then the corresponding secondary antibody should be anti-mouse IgM. If the primary monoclonal antibody is of a certain subclass of mouse IgG (IgG1, IgG2a, IgG2b, or IgG3), then almost all anti-mouse IgG can bind to it, or the secondary antibody can be selected to specifically target this subclass. If the type of the primary antibody is not clear, IgG against the corresponding species can be used.
Species source of the secondary antibody: there is usually no predictable connection between species source and the quality of the secondary antibody. However, the use of secondary antibodies from the same species as the primary antibodies should be avoided, especially in double-labeling experiments. If one of the primary antibodies is derived from goat, whereas the other is derived from mice, the corresponding secondary antibodies must be anti-goat and anti-mouse secondary antibodies, respectively. The secondary antibody cannot be derived from goat or mice.
Coupling of probes to the secondary antibody: probes coupled to secondary antibodies mainly include enzymes (such as horseradish peroxidase and alkaline phosphatase), fluorescent molecules (FITC, rhodamine, Texas Red, PE, Dylight, etc.), biotin, and gold particles. The probes can be selected according to the detection system used for WB. For WB and ELISA, the most commonly used secondary antibody is an enzyme-labeled secondary antibody, while cell or tissue labeling experiments (cellular immunochemistry, histoimmunochemistry, and flow cytometry) usually use fluorescent molecule-labeled secondary antibodies.
Another critical issue is the selection of the loading control, which has been widely used in the normalization of WB results to adjust for systematic differences between samples or even between experiments. Housekeeping proteins, such as β-actin and GAPDH, have been commonly used as loading controls. However, the expression of these proteins can change under certain conditions [3, 4]. The selected housekeeping proteins need to be proven stable under the experimental conditions. An alternative to the use of a specific protein as the loading control is the staining of total protein. Some methods used for staining of total protein on the membrane, such as Ponceau S [5] and Fast Green [6], have been found to be reliable as loading controls. Ponceau S is the most commonly used removable stain and can be conveniently used before immunodetection, but it is relatively insensitive. Fast Green is a more permanent dye used for staining in histology and electrophoresis. It cannot be easily removed and may inhibit subsequent immunodetection. Alternatively, staining with Fast Green after immunodetection has been used in some recent publications. Moreover, some housekeeping proteins are also used as markers for subcellular compartments according to their intracellular distribution [7] (Table 1).
Table 1.
Markers for subcellular compartments.
| Compartments | Markers |
|---|---|
| Nucleus | Histone H4, Lamin B1, TCF4, RanBP3 |
| Cytosol | GAPDH |
| Cytoskeleton | Actin, Tubulin, Vimentin, α-actinin |
| Plasma membrane | Caveolin, Cadherins, LRP6, Flotillin |
| Lysosome, Late endosome | Lapm1 |
| Early endosome | EEA1 |
| Golgi | Golgin97 |
| Mitochondria | Tom20, COX IV, VDAC1 |
| Endoplasmic reticulum | Calreticulin, Calnexin |
Therefore, to achieve reproducible WB results, the following information should be provided in “Materials and Methods” of a paper:
The primary antibody species (for monoclonal or polyclonal antibodies), isotype (IgG, IgY, etc.), and epitopes generated.
Secondary antibody species, isotype, and labeling.
Source of the primary and secondary antibodies; catalog and lot numbers are needed if they were obtained from a commercial company.
Dilution and incubation conditions of the primary and secondary antibodies.
Type of blotting membrane (nitrocellulose, polyvinylidene fluoride, etc.).
Blocking agents (bovine serum albumin (0.2%–5.0%), nonfat milk, casein, gelatin, etc.).
The most critical rule for image processing and presentation is to maximally preserve the integrity of the original immunoblots. Full scans or images of uncropped blots should be provided (as supplementary files) to reviewers and editors during the submission of papers. If precut blots are used for the antibody treatment, this must be clearly stated and justified by the authors in the Methods section or figure legends. In addition, the number of repetitions performed for the same WB experiment (usually more than two) should also be stated, especially when representative images from only one experiment are shown.
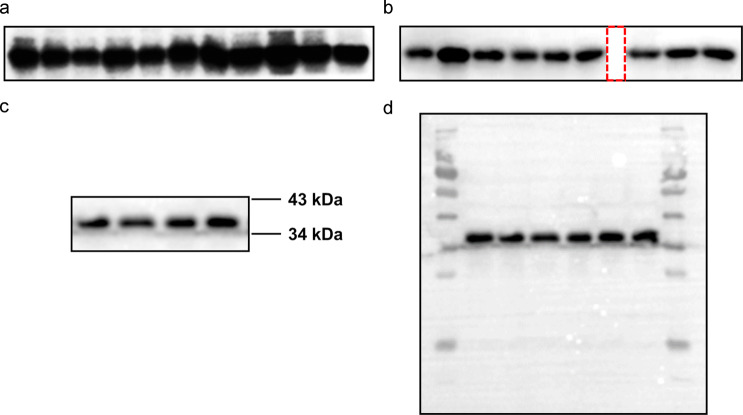
Oversaturated exposure of blots should be avoided to maintain the band signal intensity (expressed either as optical density or fluorescence units) in the linear range for quantitation. Trial experiments aiming to generate a standard curve are recommended, especially when new antibodies or methods are employed. Fig. 1a shows an example of oversaturated bands, which may have masked or at least reduced the differences among samples.
Fig. 1. Representative WB bands.
a Oversaturated bands in the WB panel. b The space (red dotted line) indicates blot splicing. c The cut blot. d The full blot.
Comparisons (whether statistical or not) between bands and normalization to loading controls should only be conducted on the same blot. In case the number of samples exceeds the capacity of one single gel, the same control sample in the exact same amount can be included on separate gels. However, comparison with this control sample should still be limited to samples within the same blot.
Separate blots should never be merged into one image. If multiple blots are organized side by side in one figure panel, there should be clearly visible space between them (as shown in Fig. 1b). If certain lanes contain data not relevant to the topic, they can be cut out from the blot, but the full blot should still be provided to editors or reviewers according to the journals’ requirements (Fig. 1c, d). However, if these irrelevant lanes were located in the middle of a blot, they should not be simply removed. In this case, the gel should be rerun with reorganized samples. The positions of molecular weight markers should be shown or marked on all the blot images. If the blots have been cropped horizontally, at least two neighboring marker positions (i.e., above and below the bands) should be indicated, as shown in Fig. 1c.
Any image adjustments (e.g., brightness, contrast, rotation, and resizing) should be applied to the whole blot (not just a certain portion of it) to ensure that no specific feature of the original data is eliminated or misrepresented. Figures in TIFF format are preferred. For WB images, a minimum resolution of 300 dpi is required.
Competing interests
The authors declare no competing interests.
Contributor Information
Lu Tie, Email: tielu@bjmu.edu.cn.
Han Xiao, Email: xiaohan@bjmu.edu.cn.
Da-lei Wu, Email: dlwu@sdu.edu.cn.
References
- 1.Alexander SPH, Roberts RE, Broughton BRS, Sobey CG, George CH, Stanford SC, et al. Goals and practicalities of immunoblotting and immunohistochemistry: a guide for submission to the British. Br J Pharmacol. 2018;175:407–11. doi: 10.1111/bph.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, et al. A proposal for validation of antibodies. Nat Methods. 2016;13:823–7. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigelso A, Dybboe R, Hansen CN, Dela F, Helge JW, Guadalupe Grau A. GAPDH and beta-actin protein decreases with aging, making stain-free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol. 2015;118:386–94. doi: 10.1152/japplphysiol.00840.2014. [DOI] [PubMed] [Google Scholar]
- 4.Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L. Housekeeping genes; expression levels may change with density of cultured cells. J Immunol Methods. 2010;355:76–9. doi: 10.1016/j.jim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Thacker JS, Yeung DH, Staines WR, Mielke JG. Total protein or high-abundance protein: which offers the best loading control for Western blotting? Anal Biochem. 2016;496:76–8. doi: 10.1016/j.ab.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Luo S, Wehr NB, Levine RL. Quantitation of protein on gels and blots by infrared fluorescence of coomassie blue and fast green. Anal Biochem. 2006;350:233–8. doi: 10.1016/j.ab.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Fagotto F. A method to separate nuclear, cytosolic, and membrane-associated signaling molecules in cultured cells. Sci Signal. 2011;4:pl2. doi: 10.1126/scisignal.2002165. [DOI] [PubMed] [Google Scholar]