Abstract
While results from clinical trials are important in determining the efficacy of treatment, restrictive eligibility criteria may limit generalizability to patient populations in the real-world setting. Real-world analyses can therefore identify subgroups of patients who may respond differently to specific therapeutic regimens. This supplementary data is supportive to the research article entitled “Real-world outcomes of immunotherapy–based regimens in first-line advanced non-small cell lung cancer” [1]. Using electronic health records data from a large demographically and geographically diverse oncology database, we present real-world progression-free survival (rwPFS) outcomes for patients with advanced non-small cell lung cancer in the United States treated with either first-line immunotherapy as monotherapy or single-agent immunotherapy combined with chemotherapy. rwPFS was estimated for patients in each treatment group using Kaplan-Meier methods; analyses were conducted separately for patients with squamous and non-squamous histology and stratified by Eastern Cooperative Oncology Group performance status, tumor programmed death ligand-1 expression, and presence of brain metastases.
Keywords: Non-small cell lung cancer, Real-world outcomes, Immunotherapy, Immune checkpoint inhibitors
Specifications Table
| Subject | Cancer Research |
| Specific subject area | Real-world retrospective analysis of treatment outcomes in advanced lung cancer |
| Type of data | Table Figure |
| How data were acquired | Electronic health records in the Flatiron Health oncology database Instruments: SAS Enterprise Guide 7.1 and R 3.6.2 |
| Data format | Analyzed |
| Parameters for data collection | Electronic health records for patients aged ≥18 years with confirmed stage III–IV non-small cell lung cancer who received either first-line immunotherapy monotherapy or single-agent immunotherapy combined with chemotherapy on or after January 1, 2016 were analyzed; patients were excluded if they had <2 documented visits on/after initial non-small cell lung cancer diagnosis, EGFR-/ALK-positive tumors, or tumors with unknown histology. |
| Description of data collection | Death information was derived from 3 data sources (electronic health records, Social Security Death Index, and commercial death dataset). Progression data were abstracted from unstructured clinician notes using Flatiron-defined methodology. Disease progression was defined as a distinct episode in which the treating physician concluded there had been growth or worsening in disease, as determined by clinic visit notes. The start date of the 1L therapy was defined as the first episode of an eligible therapy that was given after or up to 14 days before the date of diagnosis with advanced NSCLC. |
| Data source location | Institution: Flatiron Health, Inc. Country: United States |
| Data accessibility | The data for this analysis were provided by Flatiron Health and are not publicly available. Interested parties are encouraged to contact DataAccess@flatiron.com regarding license agreements and methods to obtain the deidentified data. |
| Related research article | D. Waterhouse, J. Lam, K.A. Betts, L. Yin, S. Gao, Y. Yuan, J. Hartman, S. Rao, S. Lubinga, D. Stenehjem, Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer, Lung Cancer. 156 (2021) 41-49. DOI:https://doi.org/10.1016/j.lungcan.2021.04.007 |
Value of the Data
-
•
These data present real-world progression-free survival outcomes in patients with advanced non-small cell lung cancer who receive first-line immunotherapy-agent–based regimens. In contrast to pivotal phase 3 clinical trials, the current analysis included few restrictions on eligibility criteria to ensure that the analysis population was closely representative of the real-world patient population in the United States.
-
•
Health care professionals involved in the treatment of patients with lung cancer can benefit from these data.
-
•
These results may be used to guide future studies aimed at identifying subgroups of patients less likely to benefit from treatment with current immunotherapy regimens.
1. Data Description
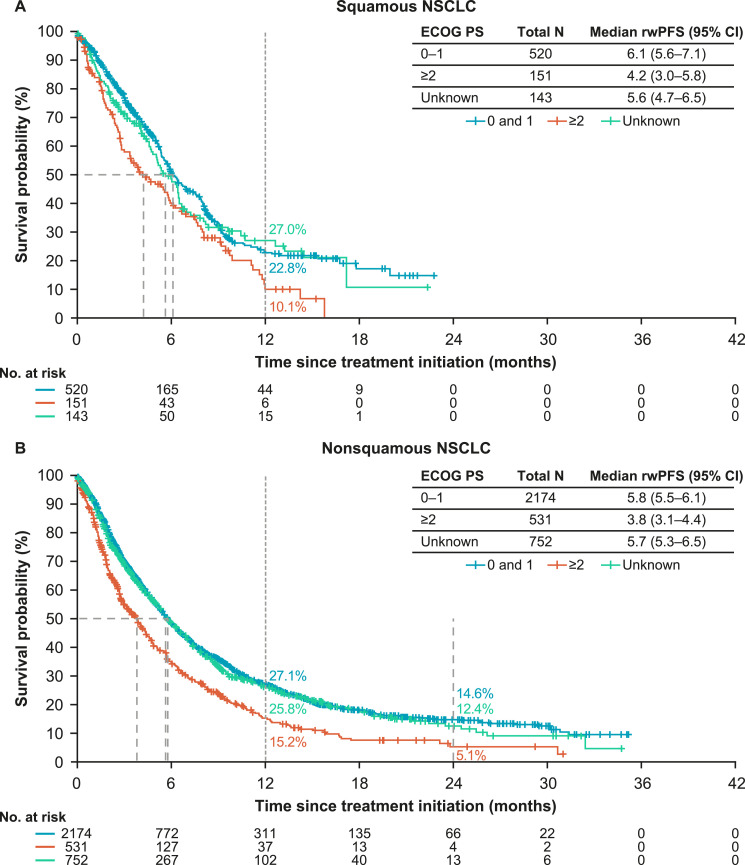
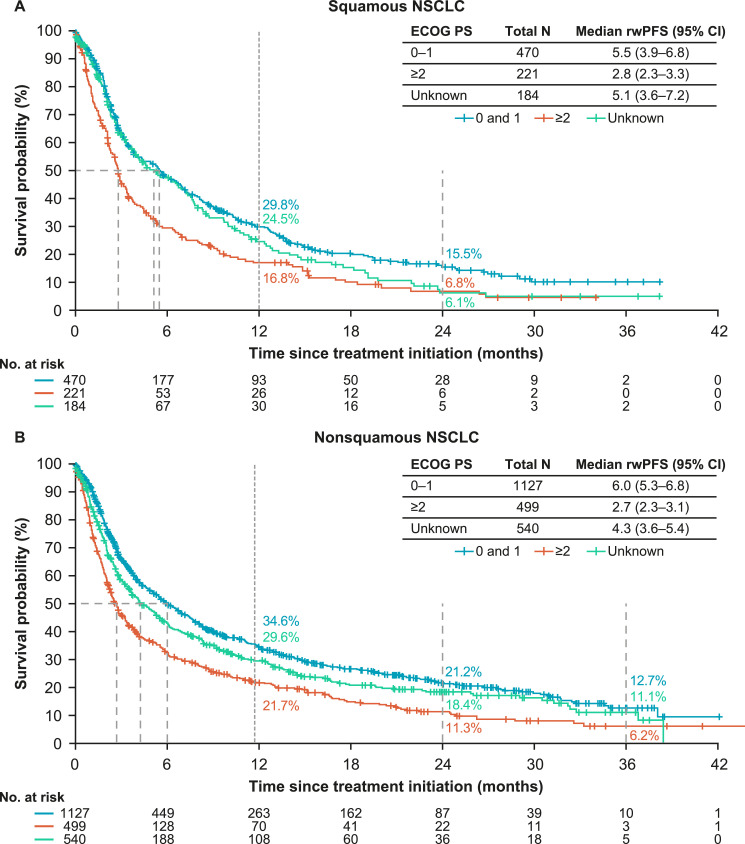
The provided data are supplementary to the associated research article. Data presented here include real-world progression-free survival (rwPFS) in a cohort of patients with advanced non-small cell lung cancer (NSCLC) who received either first-line (1L) immunotherapy (I-O) monotherapy or single-agent I-O combined with chemotherapy. Median rwPFS and landmark rwPFS rates are presented in Table 1 for patients with squamous and non-squamous histology treated with I-O plus chemotherapy and stratified by subgroups (age, tumor programmed death ligand-1 [PD-L1] expression, and presence of brain metastases). Median rwPFS and landmark rwPFS rates are presented in Table 2 for patients with squamous and non-squamous histology treated with I-O monotherapy and stratified by subgroups. Fig. 1 presents Kaplan-Meier curves for rwPFS in patients with squamous and non-squamous histology treated with I-O plus chemotherapy and stratified by ECOG performance status. Fig. 2 presents Kaplan-Meier curves for rwPFS in patients with squamous and non-squamous histology treated with I-O monotherapy and stratified by ECOG performance status.
Table 1.
rwPFS With I-O Plus Chemotherapy Stratified by Subgroup.
| Squamous NSCLC |
Non-squamous NSCLC |
||||||
|---|---|---|---|---|---|---|---|
| n | Median rwPFS (95% CI), Months | 12-Month rwPFS Rate, % | n | Median rwPFS (95% CI), Months | 12-Month rwPFS Rate, % | 24-month rwPFS Rate, % | |
| All patients | 814 | 5.9 (5.4–6.4) | 21.3 | 3457 | 5.5 (5.2–5.7) | 25.1 | 12.9 |
| Age, years | |||||||
| <65 | 230 | 5.4 (4.6–6.5) | 18.5 | 1230 | 5.1 (4.8–5.7) | 26.3 | 14.3 |
| ≥65 to <75 | 318 | 6.4 (5.3–8.0) | 26 | 1296 | 5.8 (5.4–6.2) | 25.1 | 13.1 |
| ≥75 | 266 | 5.9 (5.3–6.4) | 17.9 | 931 | 5.3 (4.8–5.7) | 23.5 | 11.1 |
| Tumor PD-L1 expression | |||||||
| <1% | 209 | 5.4 (5.0–6.0) | 20.9 | 1064 | 4.7 (4.3–5.0) | 19 | 8.3 |
| 1–49% | 252 | 5.9 (5.1–6.7) | 17 | 967 | 5.7 (5.2–6.2) | 23.7 | 11.8 |
| ≥50% | 120 | 6.5 (5.0–8.3) | 29.5 | 679 | 7.5 (6.5–8.7) | 36.1 | 21.8 |
| Unknown | 233 | 6.0 (5.1–7.5) | 22.1 | 747 | 5.1 (4.6–5.9) | 24.6 | 11.8 |
| Brain metastases | |||||||
| With | 42 | 2.6 (1.3–5.0) | 8.3 | 468 | 4.2 (3.8–4.8) | 19.9 | 9.5 |
| Without | 772 | 6.0 (5.5–6.5) | 22 | 2989 | 5.7 (5.4–5.9) | 25.9 | 13.4 |
CI, confidence interval; I-O, immuno-oncology; NSCLC, non-small cell lung cancer; PD-L1, programmed death ligand 1; rwPFS, real-world progression-free survival.
Table 2.
rwPFS With I-O Monotherapy Stratified by Subgroup.
| Squamous NSCLC |
Non-squamous NSCLC |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Median rwPFS (95% CI), Months | 12-Month rwPFS Rate, % | 24-Month rwPFS Rate, % | n | Median rwPFS (95% CI), Months | 12-Month rwPFS Rate, % | 24-month rwPFS Rate, % | |
| All patients | 875 | 4.2 (3.6–5.2) | 25.4 | 11.2 | 2166 | 4.6 (4.1–5.2) | 30.3 | 18.2 |
| Age, years | ||||||||
| <65 | 167 | 2.9 (2.3–4.9) | 19.5 | 8.4 | 534 | 4.3 (3.5–5.9) | 33.1 | 22 |
| ≥65 to <75 | 302 | 5.1 (3.5–6.2) | 27.9 | 13.4 | 673 | 4.1 (3.6–5.0) | 27.8 | 19.6 |
| ≥75 | 406 | 4.3 (3.4–5.4) | 26 | 10.6 | 959 | 5.1 (4.2–5.7) | 30.6 | 15.1 |
| Tumor PD-L1 expression | ||||||||
| <1% | 52 | 4.2 (2.8–10.0) | 27.5 | 16.8 | 102 | 4.2 (3.4–6.1) | 24.5 | 14.3 |
| 1%–49% | 157 | 3.1 (2.7–4.4) | 23.2 | 8.2 | 239 | 2.8 (2.3–3.4) | 20.5 | 10.9 |
| ≥50% | 536 | 5.2 (3.9–5.9) | 27.6 | 12.3 | 1582 | 5.1 (4.2–5.7) | 31.9 | 19.5 |
| Unknown | 130 | 3.3 (2.6–4.9) | 17.5 | 7 | 243 | 4.9 (3.4–6.1) | 31.2 | 17.9 |
| Brain metastases | ||||||||
| With | 42 | 2.5 (1.3–3.4) | 13.4 | 8.9 | 317 | 3.5 (2.7–4.6) | 27.8 | 15.8 |
| Without | 833 | 4.5 (3.8–5.4) | 26 | 11.4 | 1849 | 4.8 (4.2–5.4) | 30.8 | 18.6 |
CI, confidence interval; I-O, immuno-oncology; NSCLC, non-small cell lung cancer; PD-L1, programmed death ligand 1; rwPFS, real-world progression-free survival.
Fig. 1.
rwPFS in patients with squamous and non-squamous histology treated with I-O plus chemotherapy and stratified by ECOG performance status.
Fig. 2.
rwPFS in patients with squamous and non-squamous histology treated with I-O monotherapy and stratified by ECOG performance status.
2. Experimental Design, Materials and Methods
This retrospective, observational study used patient-level de-identified data from the Flatiron Health electronic health record database, a diverse repository nationally representative of the US oncology setting [2]. Of the 63,051 patients in the database with a confirmed diagnosis of advanced NSCLC, 62,243 had ≥2 documented visits on or after initial diagnosis. A total of 7312 patients were aged ≥18 years, received 1L I-O monotherapy or single-agent I-O combined with chemotherapy on or after January 1, 2016 and were without EGFR-positive/ALK-positive tumors, or tumors with unknown histology. Of these, 3041 patients received 1L I-O monotherapy at the index date (non-squamous histology: 2166 patients; squamous histology: 875 patients) and 4271 patients received I-O combined with chemotherapy at the index date (non-squamous histology: 3457 patients; squamous histology: 814 patients).
The initiation of 1L systemic therapy was defined as the index date. Patients were tracked from the index period until the last documented follow-up or the end of study period (June 30, 2020), whichever occurred first. rwPFS was estimated using Kaplan-Meier methods, and was defined as time from index date until disease progression or death from any cause. Patients without an event were censored at the date of the last clinical note from the progression data. Disease progression was defined based on the treating physician's clinic visit notes as a distinct episode indicating growth or worsening in disease. All rwPFS estimations were conducted separately for the I-O plus chemotherapy and I-O monotherapy treatment groups by histology, including in subgroups stratified by ECOG PS (0–1, ≥2, and unknown), age (<65, 65–74, and ≥75 years), tumor PD-L1 expression (<1%, 1%–49%, ≥50%, and unknown), and brain metastases (with and without). No statistical comparisons between groups were conducted.
Ethics Statement
Ethics approval was not required because this was a retrospective, non-interventional study using routinely collected de-identified data.
CRediT Author Statement
David Waterhouse: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; Jenny Lam: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; Keith A. Betts: Investigation, Formal analysis, Writing - Original draft preparation, Writing - Reviewing and Editing; Lei Yin: Investigation, Formal analysis, Writing - Original draft preparation, Writing - Reviewing and Editing; Sophie Gao: Investigation, Formal analysis, Writing - Original draft preparation, Writing - Reviewing and Editing; Yong Yuan: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; John Hartman: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; Sumati Rao: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; Solomon Lubinga: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing; David Stenehjem: Conceptualization, Writing - Original draft preparation, Writing - Reviewing and Editing.
Declaration of Competing Interest
Keith Betts, Sophie Gao and Lei Yin are employees of Analysis Group which received research funding for the work under consideration. David Stenehjem has received personal fees from Bristol Myers Squibb, Dracen Pharmaceuticals, Iterion Therapeutics, Molecular Templates, and Salarius Pharmaceuticals; grants from Bristol Myers Squibb, AstraZeneca, Bayer, Novartis, and Jazz Pharmaceuticals. David Waterhouse has received personal fees from Abbvie, Amgen, AstraZeneca, AZTherapies, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, Janssen Oncology, Jazz Pharmaceuticals, MCGivenny Global, Merck, Pfizer, Mirati Therapeutics, and Seattle Genetics. John Hartman, Jenny Lam, Solomon Lubinga and Sumati Rao are employees of Bristol Myers Squibb.
Acknowledgments
Medical writing and editorial support were provided by Stefanie Puglielli, PhD, CMPP, and Brooke Middlebrook, CMPP, Evidence Medical Affairs (Philadelphia, PA, USA) and was funded by Bristol Myers Squibb.
References
- 1.Waterhouse D., Lam J., Betts K.A., Yin L., Gao S., Yuan Y., Hartman J., Rao S., Lubinga S., Stenehjem D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer. 2021;156:41–49. doi: 10.1016/j.lungcan.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Berger M.L., Curtis M.D., Smith G., Harnett J., Abernethy A.P. Opportunities and challenges in leveraging electronic health record data in oncology. Fut. Oncol. 2016;12:1261–1274. doi: 10.2217/fon-2015-0043. [DOI] [PubMed] [Google Scholar]