Abstract
Actin dynamics in dendritic spines can be associated with the neurobiological mechanisms supporting the comorbidity between stress exposure and cocaine increase rewards. The actin cytoskeleton remodeling in the nucleus accumbens (NA) has been implicated in the expression of stress-induced cross-sensitization with cocaine. The present study evaluates the involvement of cofilin, a direct regulator of actin dynamics, in the impact of stress on vulnerability to cocaine addiction. We assess whether the neurobiological mechanisms that modulate repeated-cocaine administration also occur in a chronic restraint stress-induced cocaine self-administration model. We also determine if chronic stress induces alterations in dendritic spines through dysregulation of cofilin activity in the NA core. Here, we show that the inhibition of cofilin expression in the NA core using viral short-hairpin RNA is sufficient to prevent the cocaine sensitization induced by chronic stress. The reduced cofilin levels also impede a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor surface expression enhancement and promote the reduction of head diameter in animals pre-exposed to stress after a cocaine challenge in the NA core. Moreover, downregulation of cofilin expression prevents facilitation of the acquisition of cocaine self-administration (SA) in male rats pre-exposed to chronic stress without modifying performance in sucrose SA. These findings reveal a novel, crucial role for cofilin in the neurobiological mechanisms underpinning the comorbidity between stress exposure and addiction-related disorders.
Keywords: Cofilin, Dendritic spines, Chronic stress, Cocaine self-administration, Actin dynamic, AMPA receptor
Highlights
-
•
Cofilin is crucial for the development of substance use disorders induced by stress.
-
•
Cofilin downregulation prevents stress-induced sensitization to cocaine.
-
•
Cofilin modulates stress-induced structural plasticity in the nucleus accumbens core.
-
•
Cofilin regulates AMPA receptor expression in stress-induced sensitization to cocaine.
1. Introduction
There is converging epidemiological evidence of high comorbidity between stressful life events and posttraumatic stress disorder (PTSD) with substance use disorders (SUDs) (Boden et al., 2012; Clark et al., 2001; Ouimette et al., 1998; Sinha, 2001). Stressful experiences play a critical role throughout the cycle of addiction, from the development of addictive disorders to maintenance, relapse, and recovery from SUDs, suggesting that the brain circuitry regulating reward and reinforcement is a critical hub for the effects of stress on behavior ((Boyson et al., 2014, Garcia-Keller et al., 2016, Miczek et al., 2008, Piazza and Moal, 1998). In rodent models, it has been shown that exposure to stress is able to promote the capacity of psychostimulants to release dopamine in the nucleus accumbens (NA) (Kalivas and Stewart, 1991; Pierce and Kalivas, 1997) and thus to enhance the locomotor response to a psychostimulant, a phenomenon known as cross-sensitization (Marinelli and Piazza, 2002; Pacchioni et al., 2007; Sorg and Kalivas, 1991). Our previous reports in animal models of stress and substance use showed enduring neurobiological adaptations at glutamatergic synapses in the NA core, which underlie the development of behavioral sensitization (Esparza et al., 2012; Garcia-Keller et al., 2013) and facilitate the acquisition of cocaine, alcohol and heroin self-administration (SA) (Carter et al., 2020; Garcia-Keller et al., 2016, 2019b).
Behavioral alterations associated with sensitization are mediated by enduring structural modifications in NA neurons. Indeed, long-term exposure to cocaine increases dendritic complexity and spine density and induces changes in the spine morphology of NA medium spiny neurons (MSNs) (Russo et al., 2010) that are responsible, in part, for the enhanced cocaine reward and also promote behavioral responses (Dietz et al., 2012; Shen et al., 2009; Toda et al., 2006; Verma et al., 2019). Modifications in dendritic spines depend on actin cytoskeleton remodeling (Dietz et al., 2012; Matus, 2005; Russo et al., 2010; Shen et al., 2009; Toda et al., 2006; Wang et al., 2005). Thus, actin cycling is crucial for the correct physiology of the spine (Rust, 2015) and is controlled by actin-binding proteins that maintain equilibrium between monomeric globular G-actin and filamentous F-actin (Dos Remedios et al., 2003).
Cofilin is a low molecular weight actin-modulating protein, a member of the actin depolymerizing protein family, an essential regulator of spine morphology and functional aspects of synaptic plasticity (Kruyer et al., 2019; Rust, 2015). The actin-binding of cofilin is controlled via phosphorylation (inactivation, p-cofilin) and dephosphorylation (activation) of a conserved serine residue at position 3 (Ser3). This phosphorylation cycle of cofilin is a critical event that leads to reorganization of the actin cytoskeleton, and is also involved in the post-synaptic transport and membrane transport of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) in synaptic plasticity (Bamburg, 1999; Kruyer et al., 2019; Lu et al., 2019; Mizuno, 2013; Rust, 2015).
Previous studies proposed that chronic cocaine administration regulates actin turnover in the NA associated with changes in F-actin levels and the phosphorylated state of cofilin (Dietz et al., 2012; Toda et al., 2006). Like cocaine administration, repeated exposure to stressors induces enduring adaptations in dendritic branching, shape and the number of spines in NA MSNs (Qiao et al., 2016; Ruisoto and Contador, 2019). However, the molecular mechanisms mediating the long-lasting changes in behavioral responses to cocaine induced by stress are not yet completely understood. Previously, we reported that chronic stress is associated with long-term changes in p-cofilin protein levels, regulating the actin cytoskeleton in the NA core (Esparza et al., 2012).
Here, we explore molecular and cellular events that are implicated in the reorganization of the actin cytoskeleton in dendritic spines and AMPA receptor expression during sensitization to cocaine induced by chronic stress. Moreover, we characterize the critical role of cofilin in the stress-induced facilitation of acquisition of cocaine self-administration behavior and we establish a crucial role of cofilin in the vulnerability to develop cocaine addiction. This work contributes to the hypothesis that modifications in the tight regulation of actin dynamics in dendritic spines may be associated with the neurobiological mechanisms underpinning the comorbidity between stress exposure and drug reward.
2. Materials and methods
2.1. DNA construct and viral vectors
The cofilin short-hairpin RNA (shRNA) plasmid was constructed in psilencer 1.0 U6 vector. 5'gggcaaggagattctggtagg and 3'cctaccagaatctccttgccc were used as targeting sequences following experimental procedures described by (Chuang et al., 2013). The DNA fragments were generated following procedures described by (Bisbal et al., 2008) and were used for developing pseudotyped lentiviral (LV) vectors named shCtrl (empty) and shcofilin (shRNA cofilin).
2.2. Animal housing
Young adult male Sprague-Dawley rats (two months old, 250–300g; CIPReB Facultad de Ciencias Médicas, Universidad Nacional de Rosario) were housed in cages of 12x30x50 cm in the Facultad de Ciencias Químicas vivarium under a 12:12h dark/light cycle, with free access to food and water. All experiments were performed in the light cycle, except in the case of SA experiments. All animal experiments were approved by the Animal Care and Use Committee of the Facultad de Ciencias Quimicas (CICUAL), Universidad Nacional de Córdoba (RES HCD 1220/18), which is consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. Stress protocol
Animals were randomly assigned to NS (non-stress) and S (chronic stress) groups. The animals assigned to the chronic stress group were restrained daily for 2 h in a Plexiglass restraining device for seven consecutive sessions between 10 a.m. and 2 p.m. The Plexiglass cylinders were designed to allow the rats’ tails to emerge from the rear. The animals appeared healthy, as shown by their coat texture and minimal, reversible changes in body weight (Cancela et al., 1996; Esparza et al., 2012). Control animals remained undisturbed in their home cages for the full period, except for weekly weighing and regular husbandry. Animals were randomly assigned to the behavioral, biochemical or neurochemical studies.
2.4. Intracranial viral infusion
The day after the last stress session (day 8), all animals underwent stereotaxic surgery to perform bilateral microinjections of shCtrl or shcofilin in the NA core according to coordinates: +1.6 mm anterior; ±1.5 mm lateral (at an angle of 6° from vertical) and −6.6 mm ventral from bregma (Paxinos G & Watson C., 2007). The microinjections at the NA core site were verified for all experimental procedures.
2.5. Cocaine-induced locomotion
Three weeks after the intracranial injection of LV particles, locomotor activity was monitored in automated activity boxes equipped with photocells and quantified as total photocell counts (interruption of a beam that resulted in a photocell count). The animals were allowed to habituate in the activity chambers for 30 min, then 1 h was registered in response to saline (Sal) and 2 h in response to cocaine (Coc, 15 mg/kg i.p.). The behavior was recorded for 120 min over 10 min interval-bins (Esparza et al., 2012).
2.6. Operant conditioning chambers
Experiments were conducted in operant response test chambers (Med Associates, USA), each placed in a ventilated, sound-attenuating cubicle. Each operant chamber was equipped with two levers: one active and one inactive. Depression of the active lever activated the infusion pump or a food container trough onto which liquid sucrose was delivered. Responses on an inactive lever were counted but had no consequences. A cue light and a speaker were above the active lever. The house light was turned on at the start of each test session. Scheduling of experimental events and data collection was accomplished using Med Associates software.
2.7. Cocaine self-administration
Cocaine SA was performed as described in detail previously (Garcia-Keller et al., 2016). Briefly, eight days after implantation of permanent catheters in the right jugular vein, rats began daily 2 h SA training on a fixed radio (FR1), in which one press on the active lever was reinforced with an infusion of cocaine (0.3 mg/kg/infusion, followed by 10 s timeout period), paired with a white cue light above the active lever and a discrete tone cue. Rats were trained for 10 days and they reached the SA criterion when they obtained >15 infusions of cocaine on at least two consecutive days. The exclusion criteria used during the SA experiment was if the animal pressed the inactive lever >30 times (<10% exclusion).
2.8. Oral sucrose self-administration
On day 16, eight days after intracranial microinjections of LV in the NA core, rats began daily 1 h sucrose oral SA on a FR1, in which pressing the active lever was reinforced with a sucrose delivery (0.1 ml of 10% sucrose solution available for 10 s in a well on the side of the chamber, followed by 10 s timeout period), paired with a white cue light above the active lever and a discrete tone cue. Rats were trained for 10 days and they reached the SA criterion when they obtained >15 intakes on at least two consecutive days. The exclusion criteria used was if the animals during the SA experiment pressed the inactive lever >30 times (<10% exclusion) (You et al., 2018; Zhang et al., 2015).
2.9. Experimental design
All experimental designs are detailed in the supplementary material section.
2.10. Surface biotinylation and western blotting
Surface biotinylation and western blotting experiments were performed as described in detail previously (Esparza et al., 2012). Briefly, animals were decapitated, and the NA core was dissected. The bilateral slices were pooled. Bilateral NA tissue was homogenized, and protein concentrations were determined using a protein assay (Bio-Rad, Hercules, CA, USA). Then the soluble fraction was mixed with SDS sample buffer protein and loaded onto precast Tris–HCl polyacrylamide gel. The samples were transferred to PVDF membrane and blocked in blocking buffer. Blocked membranes were incubated overnight at 4 °C with primary antibodies and then for 1 h with secondary antibodies. The bands were visualized using chemiluminescent peroxidase substrate. Bands were quantified with ImageJ Software and normalized to ɑ-tubulin to control for equal loading. Antibody data are detailed in the supplementary material.
2.11. Tissue preparation and DiI labeling
Morphological analysis and spine type classification were performed as previously published (Calfa et al., 2012; Giachero et al., 2015). Briefly, animals received a challenge injection of saline or cocaine and, 24 h later, animals were deeply anesthetized before being perfused transcardially. Sections of the brain were made with a vibratome. Dendritic portions of NA core cells were stained with saturated solution of lipophilic dye (DiI, Invitrogen Carlsbad, CA, USA) in fish oil (Pozzo-Miller et al., 1999) by microinjection with a patch pipette and positive pressure application. Z-sections from labeled dendritic segments were collected using a confocal microscope (Carl Zeiss LSM 800 inverted microscope).
2.12. Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad software, La Jolla, CA). Behavioral, biochemical and neurochemical data were analyzed by one-way or two-way analysis of variance (ANOVA). Bonferroni's post-hoc test was applied for multiple comparisons and p < 0.05 was considered statistically significant. Data were expressed as mean ± SEM. Results of dendritic spines were analyzed by Rstudio software (version 3.5.3) and the statistical package nlme (Pinheiro et al., 2020) version 3.1–148. To analyze the differences between spine density means, a three-factor linear model was fitted. Differences in distribution were evaluated using the Kolmogorov-Smirnov (K–S) test. The p-values were adjusted using the Bonferroni correction method when applicable.
3. Results
3.1. Time course analysis of protein expression after lentiviral injections in the NA core
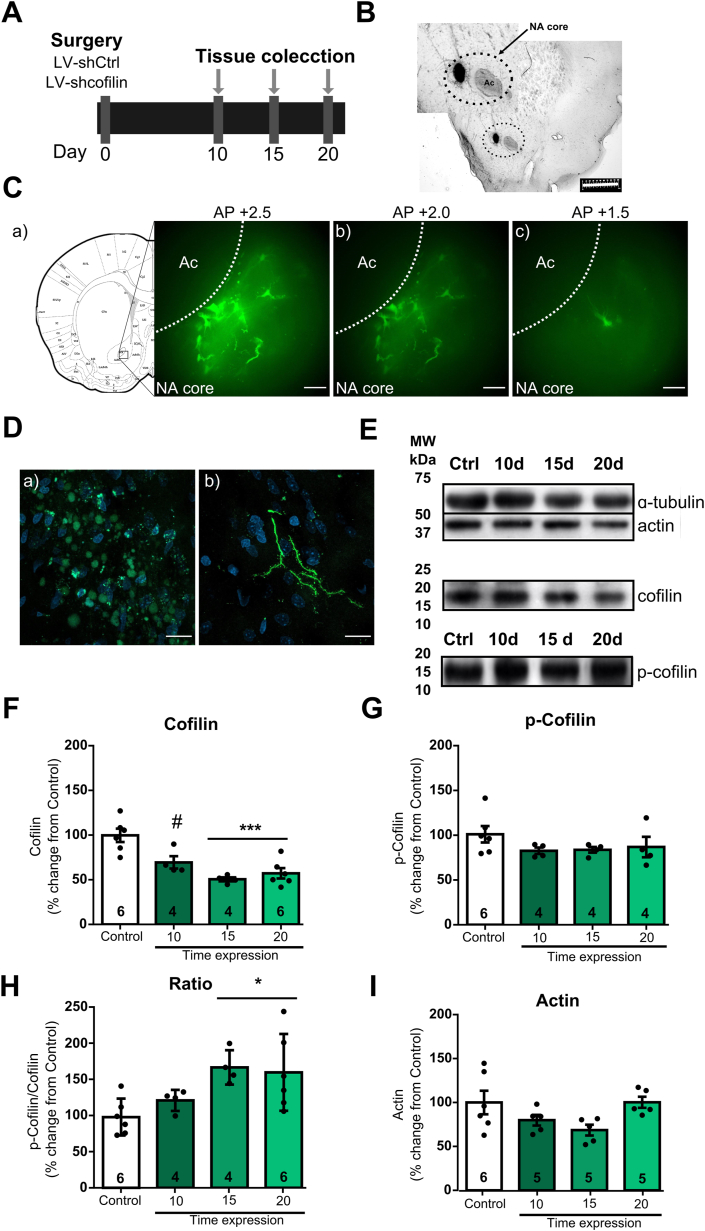
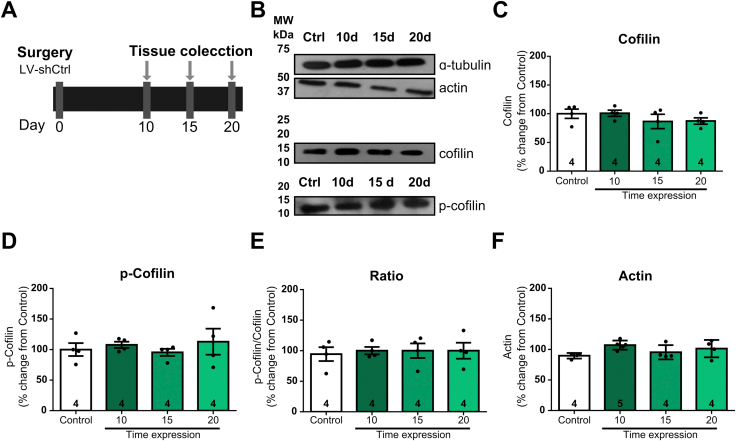
To characterize the involvement of cofilin in the vulnerability to develop cocaine addiction induced by stress, we inhibited cofilin expression with a LV short-hairpin RNA, named shcofilin, in the NA core and verified the downregulation of cofilin expression at 10, 15 and 20 days after LV microinjection, since all our experiments were performed three weeks after the last stressful event. The experimental timeline showing animals treated with shCtrl (empty virus), denominated as control, represents the basal levels of protein expression (Fig. 1A). Fig. 1B shows a representative image of the LV particles infusion site stained with cresyl violet and a magnification of the dotted line representing the NA core area (arrow). Fig. 1C (a-c) shows coronal sections of the NA core infected with shcofilin-GFP across the different anterior-posterior axis. Fig. 1D (a-b) shows confocal images of infected cells expressing shcofilin-GFP (Green) and nuclear staining (DAPI in Blue) at NA core. Fig. 1E shows representative western blots of drug-naïve NA core tissue samples. Fig. 1F–H shows the levels of expression of total cofilin (cofilin), p-cofilin, p-cofilin/cofilin ratio and actin proteins after the microinjection of shcofilin in the NA core.
Fig. 1.
Time course analysis of protein expression after lentiviral injections in NA core.
Data are expressed as mean ± SEM of the number of determinations depicted in each graphic. The results were analyzed by one-way ANOVA followed by Bonferroni's post-hoc. (A) Experimental design. (B) Representative image of the lentiviral particles infusion site stained with cresyl violet. The dotted line represents the NA core area (arrow). (C) (a,b,c) Representative images of a coronal sections of the NA core with shcofilin-infected cells expressing GFP across the different anterior-posterior axis. Scale bar = 50 μm. (D) (a,b) Confocal images showing shcofilin infected cells expressing GFP (Green) and DAPI (Blue) at NA core. Scale bar = 5 μm. (E) Representative western blots of each protein. ɑ-tubulin was used as loading control for Western blot experiments. The standard molecular weight band coincides with the expected molecular weight of each protein studied. A single band was observed for ɑ-tubulin (top), cofilin (middle) and phosphorylated cofilin, p-cofilin (bottom). (F) Cofilin expression levels after intra-NA core injection of shcofilin, n = 4–6 in each group. (G) p-Cofilin expression levels after intra-NA core injection of shcofilin, n = 4–6 in each group. (H) p-Cofilin/Cofilin ratio after intra-NA core injection of shcofilin, n = 4–6 in each group. (I) Actin expression levels after intra-NA core injection shcofilin, n = 4–6 in each group. *p < 0.05, compared with control. #p < 0.05, compared with control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Our results revealed a decrease in cofilin expression levels in the NA core 10, 15 and 20 days after the microinjection of shcofilin LV particles (one-way ANOVA followed by Bonferroni's post-hoc. Treatment F(3-16) = 12.39 p = 0.0002) (Fig. 1F). Additionally, we examined p-cofilin expression levels and we observed no changes in its expression in the NA core after infection with shcofilin (one-way ANOVA followed by Bonferroni's post-hoc. Treatment F(3-14) = 1.218 p = 0.3397) (Fig. 1G), but we report an increase in p-cofilin/cofilin ratio at 15 and 20 days after shcofilin microinjection (one-way ANOVA followed by Bonferroni's post-hoc. Treatment F(3-16) = 4.534 p = 0.0175) (Fig. 1H). Total actin levels remained unchanged during the time measured (one-way ANOVA followed by Bonferroni's post-hoc. Treatment F(3-17) = 2.813 p = 0.0705) (Fig. 1I). These data are consistent with the ability of shcofilin in vivo to induce long-lasting changes in cofilin protein expression. Moreover, rats microinjected with shCtrl revealed no changes in protein levels compared with naive rats at any timepoint (Fig. S1).
3.2. Cofilin downregulation inhibits stress-induced sensitization to cocaine
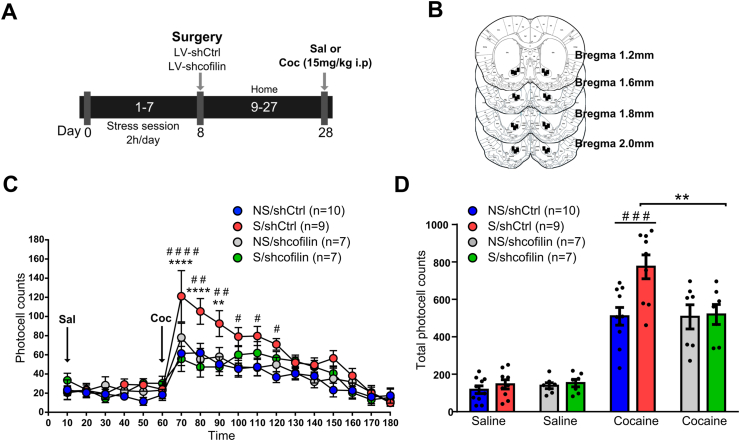
It is well known that acute stress, chronic stress or cocaine exposure increase the locomotor response to an acute cocaine injection (Esparza et al., 2012; Garcia-Keller et al, 2013, 2016; Pacchioni et al., 2007), all leading to structural and functional synaptic alterations in NA MSNs (Anderson and Self, 2017; Garcia-Keller et al, 2016, 2019a, 2020; Khibnik et al., 2016; Qiao et al., 2016; Verma et al., 2019). However, several questions remain unanswered regarding how stress induces changes in the neuronal architecture and plasticity during cross-sensitization between stress and cocaine. Cofilin has been proposed as a target involved in plastic changes related to cocaine and stress (Esparza et al., 2012; Kruyer et al., 2019; Toda et al., 2006). Thus, we proposed to determine whether cofilin is necessary for stress-induced vulnerability to developing cocaine addiction and its association with the structural changes occurring in MSNs of NA. To achieve this, we followed the timeline described in Fig. 2A. Microinjection sites in the NA core are depicted in Fig. 2B.
Fig. 2.
Cofilin downregulation inhibits stress-induced sensitization to cocaine.
(A) Experimental design. (B) Location of lentiviral microinjection in the NA core of rats included in the data analyses. Schematic line drawings, adapted from Paxinos and Watson (2007). Numbers to the right indicate millimeters from bregma. (C) Time course of horizontal photocell counts after saline and cocaine (15 mg/kg i.p.) injections. Values represent the mean ± SEM of horizontal photocell counts over 10 min periods. Timeframe from 10 to 60 min corresponds to saline administration at 0 min, and timeframe from 70 to 180 min to cocaine administration. The results were analyzed by two-way ANOVA with repeated measures (RM) over time and Bonferroni's post-hoc. *p < 0.05, compared with NS/shcofilin and S/shcofilin, #p < 0.05, compared with NS/shCtrl (n = 7–10 in each group). (D) Total horizontal photocell counts 120 min as mean ± SEM, after the saline or cocaine injection analyzed by two-way ANOVA and Bonferroni's post-hoc. *p < 0.05, compared with NS/shcofilin and S/shcofilin. #p < 0.05, compared with NS/shCtrl and remaining groups after saline injection, n = 7–10 in each group.
Our results revealed that the expression of cross-sensitization between stress and cocaine was prevented by an intra-accumbens microinjection of shcofilin three weeks before the cocaine challenge. Shcofilin did not alter the locomotor response elicited by acute saline in either prior chronic stress or non-stress animals, nor did it alter the motor response elicited by acute cocaine in non-stress animals (two-way ANOVA with repeated measures (RM) over time (non-stress⁄stress x shCtrl/shcofilin): time, F(17-493) = 22.75, p < 0.0001; non-stress/stress, F(3-29) = 7.326, p = 0.0008; interaction, F(51-493) = 1.719, p = 0.0022) (Fig. 2C). Bonferroni's post-hoc comparisons indicated that the stress shCtrl group shows significantly higher photocell counts at 70, 80 and 90 min than the stress shcofilin and all the remaining groups (Fig. 2C). These comparisons also showed that counts at between 70 and 150 min in the stress shCtrl group were significantly higher than those of the non-stress shCtrl group (Fig. 2C). The total photocell counts over 120 min after cocaine injection also showed a higher response of the stress shCtrl group compared with all the remaining groups (two-way ANOVA (non-stress⁄stress x shCtrl/shcofilin): treatment F(3-58) = 74.21, p < 0.0001; non-stress/stress F(1-58) = 6.621 p = 0.0127; interaction F(3-58) = 4.352, p = 0.0078). Bonferroni's post-hoc comparisons indicated a significant difference (p < 0.05) in the total cumulative counts between the stress shCtrl group and all the remaining groups after cocaine injection (Fig. 2D).
3.3. Cofilin downregulation modulates structural plasticity in response to cocaine in stress animals
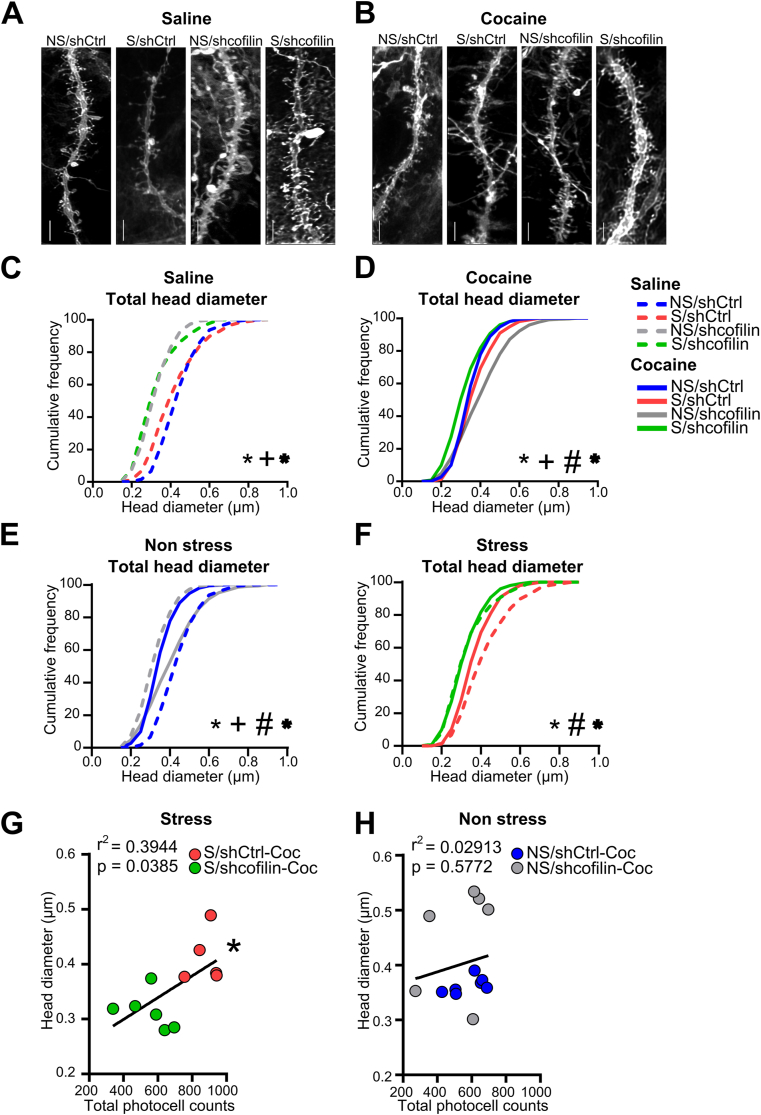
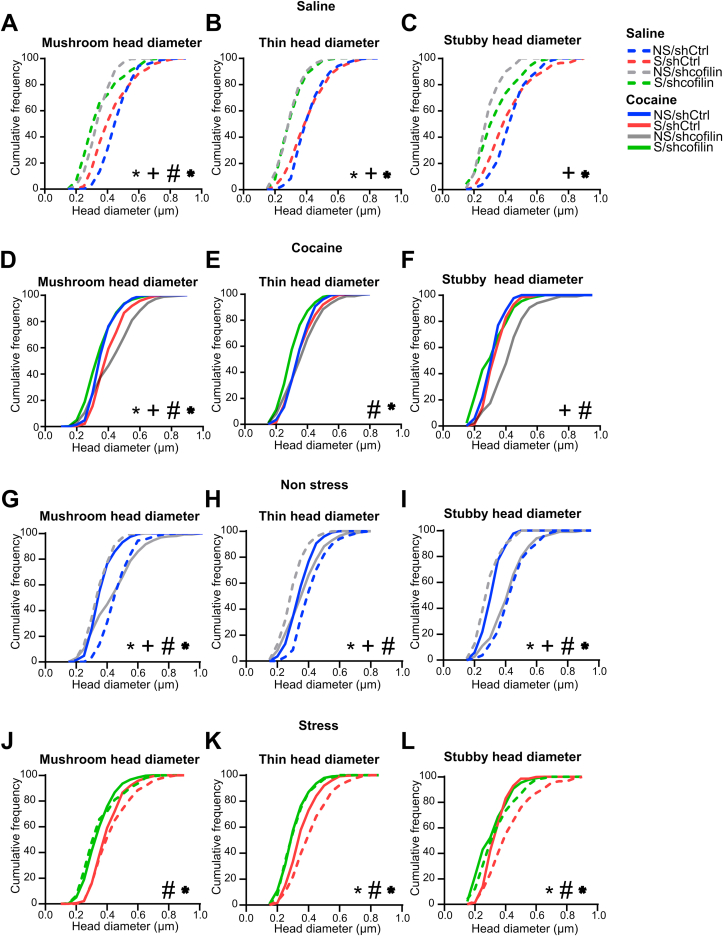
In order to assess the influence of cofilin in spine morphology associated with stress-induced sensitization to cocaine we analyzed cumulative plots of head diameter (dh) of total spines (Fig. 3) and separately mushroom, thin and stubby spines (Fig. S2) following a single cocaine administration after 1 day of withdrawal. Thus, MSNs in the NA core brain slices were injected with DiI to fill the cells and quantify dendritic spine morphology. Fig. 3A–B shows dendritic segments from each saline (A) or cocaine (B) treatment groups of MSNs in the NA core.
Fig. 3.
Cofilin downregulation modulates structural plasticity in response to cocaine in stress animals.
(A) Representative dendritic segments from each saline treatment group of MSNs in the NA core. Scale bar = 5 μm. (B) Representative dendritic segments from each cocaine treatment group of MSNs in the NA core. Scale bar = 5 μm. (C) Cumulative frequency plot of total spine head diameter (dh) 24 h after saline exposure analyzed by Kolmogorov-Smirnov (K–S) test. Total data analyzed 800–1000 μm dendritic length per group, n = 5–7 rats in each group. *NS/shCtrl vs S/shCtrl; +NS/shCtrl vs NS/shcofilin; *S/shCtrl vs S/shcofilin. (D) Cumulative frequency plot of total spine dh 24 h after cocaine exposure analyzed by Kolmogorov-Smirnov (K–S) test. Total data analyzed 800–1000 μm dendritic length per group, n = 5–7 rats in each group. *NS/shCtrl vs S/shCtrl; +NS/shCtrl vs NS/shcofilin; #NS/shcofilin vs S/shcofilin; *S/shCtrl vs S/shcofilin. (E) Cumulative frequency plot of total spine dh of non-stressed animals 24 h after saline or cocaine exposure analyzed by Kolmogorov-Smirnov (K–S) test. Total data analyzed 800–1000 μm dendritic length per group, n = 5–7 rats in each group. *NS/shCtrl-Sal vs NS/shCtrl-Coc; +NS/shcofilin-Sal vs NS/shcofilin-Coc; #NS/shCtrl-Sal vs NS/shcofilin-Sal and *NS/shCtrl-Coc vs NS/shcofilin-Coc (F) Cumulative frequency plot of total spine dh of stressed animals 24 h after saline or cocaine exposure analyzed by Kolmogorov-Smirnov (K–S) test. Total data analyzed 800–1000 μm dendritic length per group, n = 5–7 rats in each group. *S/shCtrl-Sal vs S/shCtrl-Coc; +S/shcofilin-Sal vs S/shcofilin-Coc; #S/shCtrl-Sal vs S/shcofilin-Sal and *S/shCtrl-Coc vs S/shcofilin-Coc. (G) Correlation between total spine dh and total photocell counts in S/Coc groups, n = 5–6 in each group. (H) Correlation between total spine dh and total photocell counts in NS/Coc groups, n = 6–7 in each group.
We found that chronic stress in animals microinjected with shCtrl (S/shCtrl-Sal, dotted pink line vs NS/shCtrl-Sal, dotted blue line) induced a leftward shift in the cumulative curve in total (Fig. 3C, p < 0.0004), mushroom (Fig. S2A, p < 0.0004) and thin dh (Fig. S2B, p = 0.02), with no significant changes in stubby dh (Fig. S2C, p = 0.1136). While, after cocaine, stress exposure (S/shCtrl-Coc, pink line vs NS/shCtrl-Coc, blue line) produced a rightward shift in the cumulative curve in total (Fig. 3D, p < 0.0252) and mushroom dh (Fig. S2D, p < 0.0252), with no changes in thin dh (Fig. S2E, p = 1.5496) or stubby dh (Fig. S2F, p = 1.0972). Collectively, these spine data indicated that changes in structural plasticity associated with stress-induced sensitization to cocaine were related to an enhancement of total dh correlated with augmented mushroom dh spines in NA MSNs after cocaine. Then, we examined the effect of cofilin downregulation on non-stress rats, after saline or cocaine injection. We found that shcofilin (NS/shcofilin-Sal, dotted gray line vs NS/shCtrl-Sal, dotted blue line) induced a leftward shift in the cumulative curve of total spine dh (Fig. 3C, p < 0.0004), as well as in all three type of spines (Fig. S2A-C, p < 0.0004). Nevertheless, non-stress rats microinjected with shcofilin and exposed to cocaine showed a rightward shift in the cumulative curve of total compared with the control shCtrl group (Fig. 3D, p < 0.0004, NS/shCtrl-Coc, blue line vs NS/shcofilin-Coc, gray line) and all three types of spines dh (Fig. S2D-F, p < 0.0004). This rightward shift was better understood when we analyzed the effect of cocaine in non-stress groups microinjected with shCtrl or shcofilin (Fig. 3E and Fig. S2G-I). Cocaine exposure in non-stress groups microinjected with shCtrl led to a leftward shift in the cumulative distribution in total spine dh (Fig. 3E, p < 0.0004, NS/shCtrl-Sal (dotted blue line) vs NS/shCtrl-Coc (blue line), and in all three types of spines dh (Fig. S2G-I, p < 0.0004). However, in non-stress animals microinjected intra-accumbens with shcofilin (NS/shcofilin-Coc, gray line) cocaine injection induced a rightward shift in the cumulative distribution compared with the NS/shcofilin-Sal group (dotted gray line) in total spine dh and in all three types of spines dh (Fig. 3E and Fig. S2G-I, p < 0.0004), almost reaching the basal levels observed in the control group (NS/shCtrl-Sal), thus explaining why the behavioral response remained unaffected (Fig. 2C–D).
Interestingly, when we examined the consequences of cofilin downregulation on stress-mediated spine morphology, we found that the shcofilin in stress rats (S/shCtrl-Sal, dotted pink line vs S/shcofilin-Sal, dotted green line) produced a leftward shift in total spine dh (Fig. 3F, p = 0.0048), mushroom, thin (Fig. S2J-K, p < 0.0004) and stubby dh (Fig. S2L, p = 0.0048). Similarly, after cocaine, the cumulative plots in chronic stress rats microinjected with shcofilin (S/shcofilin-Coc, green line vs NS/shcofilin-Coc, gray line) showed a leftward shift in total spine dh (Fig. 3D, p < 0.0004) across all three types of spines dh (mushroom, thin and stubby, Fig. S2D-F, p < 0.0004). However, the adaptive structural plasticity occurring after chronic stress and a single cocaine administration (S/shcofilin-Coc, green line vs S/shCtrl-Coc, pink line) revealed that cofilin downregulation induced a decrease in total spine dh (Fig. 3D, p < 0.0004), as well as in mushroom dh (Fig. S2D, p < 0.0004), thin dh (Fig. S2E, p < 0.0004) and stubby dh (Fig. S2F, p = 0.0088), suggesting a persistent reduction on spine dh in stressed animals given by the reduction of cofilin expression in NA core after cocaine injection.
Furthermore, supporting a relationship between cofilin downregulation and dh in regulating cocaine sensitization induced by stress, we were able to positively correlate the diminution of behavioral response to cocaine with smaller dh, when we compared both stress groups exposed to cocaine (Fig. 3G, r2 = 0.3944, p = 0.0385), while these changes were not observed in non-stress groups (NS-shCtrl vs NS-shcofilin, Fig. 3H, r2 = 0.02913, p = 0.5772). These results highlight the role of cofilin in the maintenance of structural plasticity during stress-induced sensitization, as we are able to report modifications in spine head diameter after a cocaine challenge only in non-stress animals, while this response is blunted in previously stressed animals.
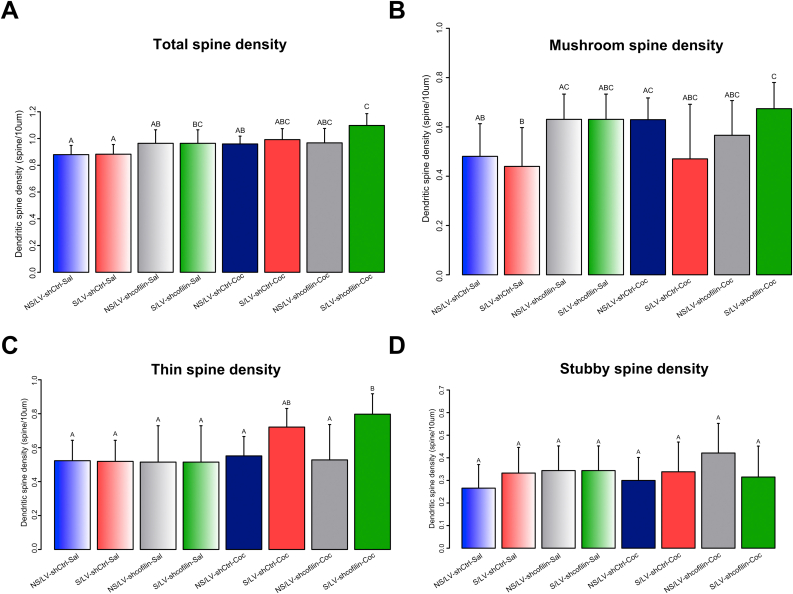
Moreover, we analyzed differences in spine density for each type of treatment, using a three-factor linear mixed model by Rstudio software (Fig. S3). These results revealed a three-factor interaction only in mushroom spine density (Table 1. SM, Three-factor linear model: cocaine F(1-145) = 3.36 p = 0.069, lentivirus (LV) F(1-40) = 11.77 p = 0.001, stress F(1-40) = 1.92 p = 0.173. stress*LV F(1-40) = 3.97 p = 0.053, stress*cocaine F(1-145) = 0.25 p = 0.615, LV*cocaine F(1-145) = 1.21 p = 0.275 and stress*LV*cocaine F(1-145) = 5.97 p = 0.016). Furthermore, modifications in total spine density are dependent on cocaine and lentivirus effect and not due to stress exposure (Table 1. SM, cocaine F(1-145) = 9.45 p = 0.003, LV F(1-40) = 10.80 p = 0.002 and stress F(1-40) = 4.02 p = 0.052. stress*LV F(1-40) = 1.55 p = 0.221, stress*cocaine F(1-145) = 1.84 p = 0.178, LV*cocaine F(1-145) = 0.83 p = 0.363 and stress*LV*cocaine F(1-145) = 054 p = 0.464). Indeed, we noticed an independent effect on stress and cocaine in thin spines, with a trend toward increased density in thin spines in stress animals after cocaine (Table 1Table 1. SM, cocaine F(1-145) = 5.24 p = 0.023, LV F(1-40) = 1.53 p = 0.223, stress F(1-40) = 9.67 p = 0.003. stress*LV F(1-40) = 1.43 p = 0.239, stress*cocaine F(1-145) = 2.51 p = 0.115, LV*cocaine F(1-145) = 0.10 p = 0.755 and stress*LV*cocaine F(1-145) = 0.00 p = 0.988), and no changes in stubby spine density.
Thus, our results suggest that cofilin downregulation modulates actin remodeling in MSN, influencing the chronic stress-induced sensitization to psychostimulant response to cocaine.
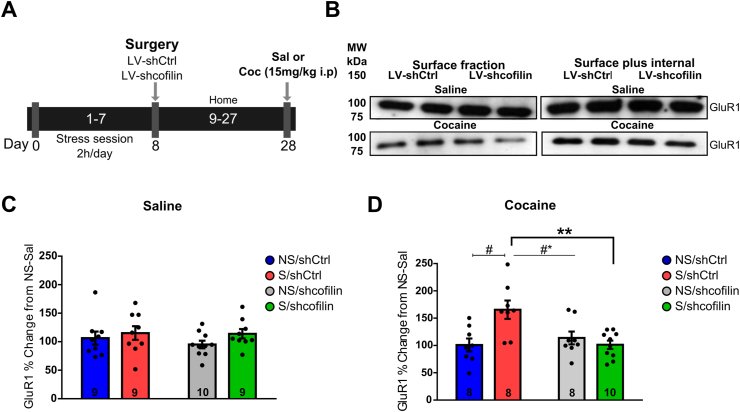
3.4. Inhibition of Cofilin in the NA core prevents the enhancement of AMPAR surface expression in stressed rats after cocaine
Glutamate signaling in the NA plays an important role in the behavioral and molecular plasticity observed in behavioral sensitization to cocaine (Loweth et al., 2014; Wolf and Ferrario, 2010; Wolf and Tseng, 2012). Our previous report showed that actin reorganization promotes an increase in AMPAR surface expression in the NA core in stress animals after cocaine (Esparza et al., 2012). To examine the role of cofilin in the NA core in the expression of the AMPAR in stressed animals, we performed an intra-accumbens microinjection with shcofilin and, three weeks after chronic stress, surface expression of the AMPAR subunit GluR1 was evaluated 45 min after a challenge with cocaine or saline (Fig. 4A). Representative Western blots of the surface fraction and surface plus internal fractions are shown in Fig. 4B. Non-stress⁄stress shCtrl and non-stress⁄stress shcofilin that received an injection of saline did not show modifications in the surface expression of the AMPAR subunit GluR1 (two-way ANOVA (non-stress⁄stress x shCtrl-Sal/shcofilin-Sal) revealed no effect of non-stress vs stress group F(1-33) = 2.020, p = 0.1646, interaction F(1-33) = 0.2593, p = 0.6140 and shcofilin vs shCtrl F(1-33) = 0.4025 p = 0.5302) (Fig. 4C). However, shCtrl animals pre-exposed to stress showed an enhancement in surface expression of the GluR1 containing AMPAR after a cocaine challenge, while the remaining groups were unchanged (two-way ANOVA (non-stress⁄stress x shCtrl-Coc/shcofilin-Coc) revealed effect of non-stress vs stress F(1-30) = 4.643, p = 0.0393, interaction F(1-30) = 10.17, p = 0.0033 and shcofilin vs shCtrl F(1-30) = 4.528 p = 0.0417) (Fig. 4D). These findings suggest that cofilin downregulation impedes the GluR1 surface enhancement in the NA core described in stressed animals after a cocaine challenge.
Fig. 4.
Cofilin downregulation in NA core prevents the enhancement of AMPAR surface expression in stressed rats exposed to cocaine.
(A) Experimental design. (B) Representative western blots of GluR1 surface fraction and GluR1 surface plus internal. ɑ-tubulin was used as loading control for Western blot experiments. (C) AMPAR subunit GluR1 in rats pretreated with shCtrl or shcofilin into the NA core, 45 min after a saline injection, analyzed by two-way ANOVA and Bonferroni's post-hoc comparison, n = 9–10 in each group. (D) AMPAR subunit GluR1 in rats pretreated with shCtrl or shcofilin into the NA core, 45 min after a cocaine injection, analyzed by two-way ANOVA and Bonferroni's post-hoc, n = 8–10 in each group. Data are expressed as mean ± SEM of the number of determinations depicted in each graphic. *p < 0.05, compared with S/shcofilin. #*p < 0.05, compared with NS/shcofilin. #p < 0.05, compared with NS/shCtrl.
3.5. Inhibition of cofilin expression in the NA core prevents the facilitatory influence of stress during the acquisition of cocaine self-administration
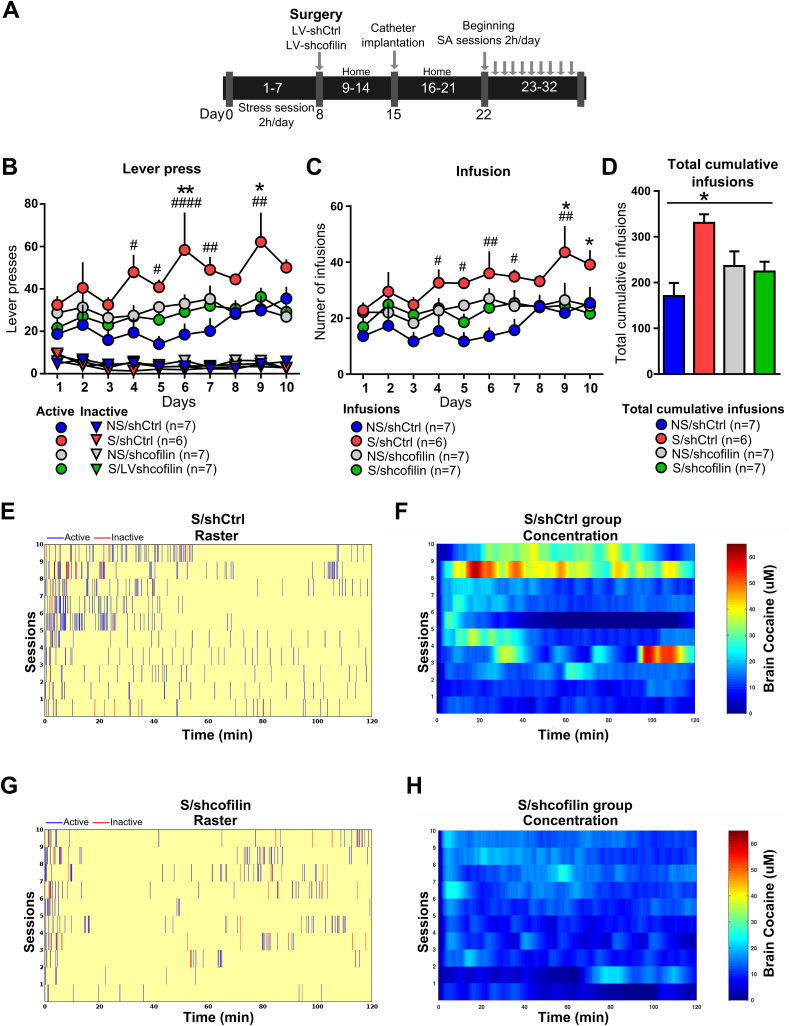
Stress plays a powerful role in the initiation, escalation, and relapse to drug abuse (Garcia-Keller et al, 2016, 2019a; Kim et al., 2009; Shaham et al., 2000; Sinha, 2007, 2009). Here, we show that cofilin, has a crucial role in the vulnerability to develop SUDs. One week after microinjection of LV particles, non-stress and stress rats underwent surgery for implantation of jugular catheters and, eight days later, began daily 2 h cocaine SA on a FR1 for 10 days (Fig. 5A). Three weeks after chronic stress, the acquisition of cocaine SA was facilitated in the group stress shCtrl (Fig. 5B). Stress-induced facilitation was significant when quantified by the number of active lever presses (two-way ANOVA with RM over time (non-stress ⁄ stress x LV-shCtrl/shcofilin) revealed no effect of interaction F (27-207) = 0.8880 p = 0.6288, but significant effects in: non-stress/stress F(3-23) = 7.592 p = 0.0011 and time F(9-207) = 3.097 p = 0.0016) (Fig. 5B) or cocaine infusions (two-way ANOVA with RM over time (non-stress ⁄stress x shCtrl/shcofilin) revealed no effect of interaction F(27-207) = 0.6837 p = 0.8796 but significant effects in non-stress/stress F(3-23) = 6.118 p = 0.0032 and time F(9-207) = 3.235 p = 0.0011) (Fig. 5C). Bonferroni's post-hoc comparisons indicated that the stress shCtrl group shows significantly higher performance on active lever presses between day 4 and day 9 of SA compared with the non-stress shCtrl group and all the remaining groups (Fig. 5B). Also, these differences were observed in cocaine infusions between stress/non-stress shCtrl groups during the same period of time (Fig. 5C). These comparisons showed no significant differences between the non-stress shCtrl and non-stress/stress shcofilin groups in the number of active lever presses or cocaine infusions during the 10 days of SA (Fig. 5B and C). Differences between the stress shCtrl and stress shcofilin groups in the number of active lever presses at day 6 and day 9 were significant (Fig. 5B). In addition, significant differences in the cocaine infusions were evidenced at 9 and 10 days of SA (Fig. 5C). Further, these differences were also evidenced as the total cumulative number of infusions taken by each treatment group (two-way ANOVA (non-stress⁄stress x shCtrl/shcofilin)) revealed effect of interaction (F(1-23) = 11.08 p = 0.0029) and non-stress vs stress (F(1-23) = 9.438 p = 0.0054, with no effect of treatment (F(1–23) = 0.8539 p = 0.3651) (Fig. 5D). The inactive lever pressing rate (two-way ANOVA with RM over time (non-stress⁄stress x shCtrl/shcofilin)) showed no effect of interaction (F(27-207) = 0.8078 p = 0.7392) or treatment (F(3-23) = 0.3498 p = 0.7896), but significant effect of time (F(9-207) = 2.430 p < 0.0121) (Fig. 5B).
Fig. 5.
Cofilin downregulation in NA core prevents the facilitatory influence of stress during the acquisition of cocaine self-administration.
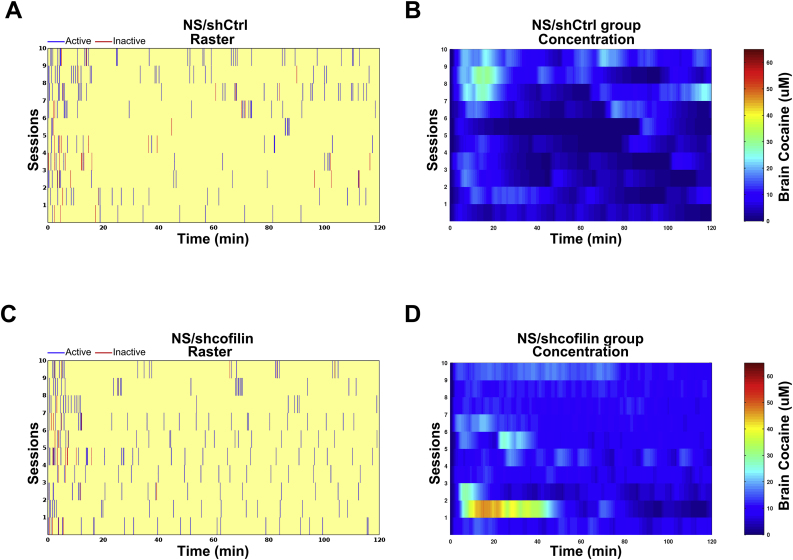
(A) Experimental design. (B) Number of active lever and inactive presses during acquisition of cocaine self-administration 2 h daily on a fixed ratio (FR1) over 10 days. The results were analyzed by two-way ANOVA with repeated measures (RM) over time followed by Bonferroni's post-hoc, n = 7–8 in each group. #p < 0.05, compared with NS/shCtrl. *p < 0.05, compared with S/shcofilin. Number of inactive lever presses revealed no significant differences among groups. (C) Number of cocaine infusions during acquisition of cocaine self-administration under FR1 schedule (two-way ANOVA with repeated measures (RM) over time followed by Bonferroni's post-hoc, n = 7–8 in each group. #p < 0.05, compared with NS/shCtrl. *p < 0.05, compared with S/shcofilin). (D) Total cumulative number of infusions taken by each treatment group after 10 days of cocaine self-administration (two-way ANOVA followed by Bonferroni's post-hoc. *p < 0.05, compared with all the remaining groups). (E, G) Example raster plots of the best performing stress animal microinjected with shCtrl (E) or shcofilin (G). Presses on the active (blue) and inactive (red) lever. (F, H) Heat maps of average modeled brain cocaine concentration (uM) in stress animals microinjected with shCtrl (F) or shcofilin (H). Data are shown as mean (±S.E.M.). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To further investigate the effect of the inhibition of cofilin on the acquisition of cocaine SA induced by stress, we analyzed the lever presses of individual rats of each group (Fig. 5E,G and Fig. S4 A and C) and the brain cocaine concentration across self-administration sessions was averaged within treatment groups (Fig. 5F,H and Fig. S4B,D). These findings revealed a faster lever-pressing response rate and stabilization of response for cocaine in stress shCtrl animals (Fig. 5E,G). The heat maps of the average modeled brain cocaine concentration showed stable brain cocaine levels during the first days with the highest levels on day 9, continuing with high stable brain cocaine concentration in the following days (Fig. 5F). Non-stress shCtrl animals, however, stabilized their lever-pressing response on day 7, reaching lower brain cocaine levels than the stress shCtrl group (Fig. S4A-B). The analysis of lever presses of individual rats and average brain concentrations of cocaine over time during SA showed no apparent differences between the non-stress shCtrl group (Fig. S4A-B) and non-stress/stress shcofilin groups (Fig. S4C-D). Nevertheless, differences were evidenced between stressed shCtrl and shcofilin groups (Fig. 5E,G). Interestingly, both groups of animals stabilized their lever-pressing response rate around day 6 but, in the following days, the stress shCtrl animals reached much higher brain levels of cocaine than those treated with shcofilin (Fig. 5F,H).
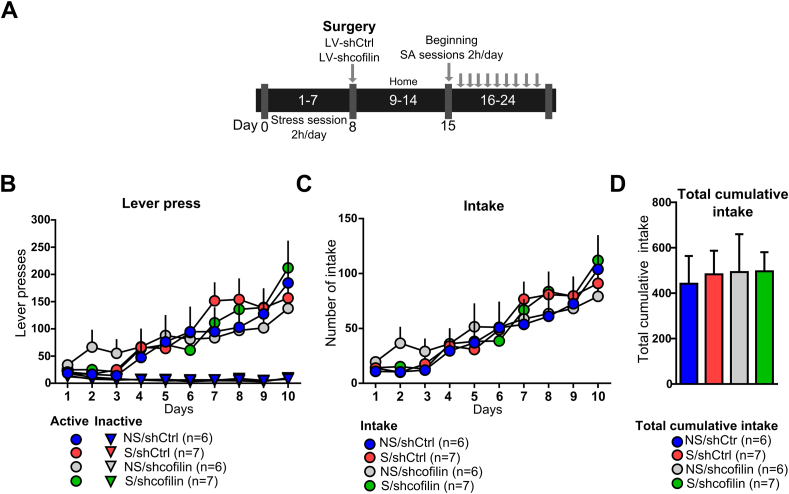
Additionally, the same experimental groups were evaluated under a model of acquisition of oral sucrose SA (Fig. 6A). The sucrose SA acquisition after 10 sessions of training did not differ among the different groups (Fig. 6B–D). In these experiments, the active lever pressing rate during 1 h/day, under the FR1 schedule (two-way ANOVA with RM over time (non-stress⁄stress x shCtrl/shcofilin)) revealed no effect of interaction (F (27-198) = 0.9093 p = 0.5982) or treatment (F (3-22) = 0.05047 p = 0.9846), but a significant effect of time (F (9-198) = 19.38 p < 0.0001) (Fig. 6B). Furthermore, the analyses of sucrose intake revealed no effect of interaction (F (27-198) = 0.6134 p = 0.9338) or treatment (F (3-22) = 0.04324 p = 0.9877), but a significant effect of time analyzed by a two-way ANOVA with RM over time (non-stress⁄stress x shCtrl/shcofilin) time (F (9-198) = 21.70 p < 0.0001) (Fig. 6C). The analysis of total sucrose intake for each treatment group revealed no significant differences among groups (Fig. 6D). Further, the inactive lever pressing rate (two-way ANOVA with RM over time (non-stress⁄stress x shCtrl/shcofilin)) showed no effect of interaction (F (27-198) = 0.3239 p = 0.9995) or treatment (F (3-21) = 0.9087 p = 0.4536), but significant effect of time (F (9-198) = 5.827 p < 0.0001) (Fig. 6B). Together, these data support the idea of a differential, specific role of cofilin in the acquisition of cocaine SA, given that all groups of animals are capable of self-administering sucrose independently of the treatment.
Fig. 6.
Inhibition of cofilin expression in NA core did not affect sucrose self-administration.
(A) Experimental design. (B) Number of active and inactive lever presses during acquisition of sucrose self-administration 1
h daily on a fixed ratio (FR1) over 10 days. The results were analyzed by two-way ANOVA with repeated measures (RM) over time followed by Bonferroni's post-hoc, n = 6–7 in each group. (C) Number of intakes during acquisition of sucrose self-administration under the FR1 schedule (two-way ANOVA with RM over time followed by Bonferroni's post-hoc, n = 6–7 in each group). (D) Total cumulative sucrose intake for each treatment group after 10 days of sucrose self-administration. The results were analyzed by two-way ANOVA followed by Bonferroni's post-hoc. Data are shown as mean ± S.E.M.
4. Discussion
These results provide a novel characterization of the role of cofilin in stress-induced cocaine sensitization and the potentiation of cocaine SA using molecular, biochemical assays and behavioral approaches. Considering that cofilin is involved in spine structural plasticity regulating actin remodeling and AMPAR trafficking and accumulation within synapses in a dynamic sequence of activation and inactivation during long-term potentiation (LTP) (Bosch et al., 2014; Gu et al., 2010; Noguchi et al., 2016; Rust et al., 2010), we hypothesized that cofilin activity is critical for the development of SUDs induced by chronic stress.
We report that cofilin downregulation prevents stress-induced cocaine sensitization (Fig. 2C–D), and facilitation of cocaine self-administration (Fig. 5B–D). The specificity of this process was clearly demonstrated by the fact that sucrose administration remained unmodified in shcofilin-exposed animals (Fig. 6B–D). Furthermore, after cofilin downregulation in chronically stressed rats, the prevention of cross-sensitization to cocaine was accompanied by a smaller spine head diameter, allowing us to establish a positive correlation between the reduced behavioural response to cocaine and smaller spine head diameter. Moreover, cofilin downregulation prevents the AMPAR enhancement in the NA core described in stressed animals after a cocaine challenge. Our previous study showed that chronic stress downregulates p-cofilin expression and decreases F-actin levels in the NA core, and that, after a single cocaine injection in stressed animals the levels of F-actin returns to normal, accompanied with decreased levels of p-cofilin and p-cortactin protein expression (Esparza et al., 2012). Here, we report that pretreatment with shcofilin induces persistent cofilin downregulation in the NA core (Fig. 1F), suggesting enhanced stabilization of actin dynamics, due to increased p-cofilin/cofilin ratio (Fig. 1H), after stress exposure preventing cocaine sensitization (Fig. 2C–D).
4.1. Cofilin and dendritic spine plasticity
Chronic cocaine administration and stress exposure are able to regulate actin turnover in the NA (Dietz et al., 2012; Golden and Russo, 2012; Shen et al., 2009; Toda et al, 2006, 2010). Exposure to either or both has been associated with an increase in spine density and reorganization of the dendritic structure of NA MSNs (Brown et al., 2011; Christoffel et al., 2011; Dietz et al., 2012; Dumitriu et al., 2012; Khibnik et al., 2016; Robinson and Kolb, 2004). Toda et al. (2006) reported that non-contingent acute cocaine administration enhanced p-cofilin, but withdrawal from repeated cocaine was associated with a decrease in p-cofilin, downregulated LIMK1 and therefore increased actin dynamics influencing cocaine-induced reinstatement of drug seeking. Similarly, downregulation of Rac1 promotes alterations in cofilin activity and changes in dendritic spine morphology (Dietz et al., 2012). Also, it was shown that p-cofilin upregulation through focal adhesion kinase (FAK) is required for cue-induced cocaine reinstatement in D1-MSN in the NA core (Garcia-Keller et al., 2020). Several studies reported no changes (Francis et al., 2017) or enhanced immature spine density in NA MSNs after social defeat stress (CSDS) through a Rac1-dependent mechanism involving the redistribution of synaptic cofilin in the NA (Golden et al., 2013). Further, Fox et al. (2018) report that dendritic atrophy of D1, but not D2 receptor containing MSNs is strongly associated with social avoidance after CSDS caused by upregulation of the GTPase RhoA and its downstream effector Rho-kinase (Fox et al., 2018). Likewise, after CSDS, D2-MSNs exhibit increased spine density, mostly thin and stubby, and this increased spine density negatively correlates with social interaction behavior (Fox et al., 2020). Additionally, in a previous publication (Garcia-Keller et al., 2016) we showed that an acute stress exposure was associated with increases in spine density, increased AMPA/NMDA currents and potentiation in acquisition of cocaine self-administration in the stress group. The fact that a single stressor produced such long-lasting alterations at glutamatergic synapses in the NA core and facilitated the acquisition of cocaine self-administration suggests possible common substrates that may predispose individuals suffering from stress disorders to develop cocaine addiction. Here, after chronic restraint exposure, we report unchanged spine density in stress animals after cocaine, although there was a trend toward increased density in thin spines of stress animals after cocaine (Fig. S3A and C). Interestingly, we did not find modifications in mushroom spine density at this time point, but we found a three-factor interaction: chronic stress, the shcofilin and cocaine, only in this spine subtype, pointing up the importance of cofilin in the chronic stress-induced sensitized cocaine response (Fig. S3B ). Moreover, we reported a reduction in dh in the stress shCtrl group after saline (Fig. 3C and Fig. S2A-B), which may be associated with the decreased levels of p-cofilin and F-actin in the NA core described in our previous report (Esparza et al., 2012). Further, we found that cofilin downregulation promoted reduced behavioral response to cocaine in stress rats and this reduced response was positively correlated with a smaller spine dh, supporting a possible causal association between spine head enlargement and stress-induced sensitization to cocaine. Our results highlight the importance of structural and functional changes induced by stress and, more importantly, provide evidence suggesting the involvement of cofilin in this process, as we show that cofilin downregulation impedes the actin remodeling necessary for rapid changes in spine morphology in response to cocaine and thereby prevents the sensitized response. In line with our observations, several evidences demonstrate that cofilin and its upstream effectors Rac1 and LIMK1 seemed to be implicated in the synaptic plasticity induced by different paradigms of chronic stress in NA (Golden et al., 2013), ventro-medial prefrontal cortex (vmPFC) (Fan et al., 2018), hippocampus (Chen et al., 2008; Lu et al., 2019) and mPFC (Gao et al., 2020). In addition, the RhoA-ROCK signalling pathway mediates depression-like behaviours, via dendritic remodeling of NA D1-MSNs (Fox et al., 2018). However, other articles have reported that chronic stress-induced modifications were not directed by cofilin, but rather by myosin phosphatase targeting protein 1 (MYPT1), downstream of RhoA–ROCK kinases, underlying the spine loss in hippocampal neurons (Castañeda et al., 2015; Garcia Rojo et al., 2017). Further studies are needed to explain the divergent results regarding MSNs morphological changes induced by stress, specifically evaluating the role of DA receptor expression in relation to stress-induced drug-seeking behavior.
4.2. Cofilin and AMPAR plasticity
In MSNs of the NA, the cell surface expression enhancement of AMPARs is required for cocaine-seeking behavior, and synaptic levels of AMPARs are upregulated after withdrawal from cocaine (Kalivas and Volkow, 2005; Wolf, 2010; Wolf and Ferrario, 2010; Wolf and Tseng, 2012). Here, we prevented the enhancement of AMPAR surface expression in the NA core (Fig. 4) previously observed in stressed rats exposed to cocaine by inhibiting cofilin expression (Esparza et al., 2012; Garcia-Keller et al., 2013). It has been reported that cofilin controls AMPAR surface expression via an actin-dependent mechanism, and that cofilin controls synaptic strength via AMPAR mobility (Bosch et al., 2014; Rust et al., 2010), evidencing an important function of cofilin in both structural and functional plasticity (Borovac et al., 2018). Moreover, changes in structural plasticity suggest a temporal sequence of cofilin activation and inactivation during LTP, relevant for initial spine enlargement and synaptic AMPAR accumulation (both dependent on cofilin activation), followed by consolidation of structural changes (dependent on cofilin inactivation) (Gu et al., 2010; Noguchi et al., 2016; Stefen et al., 2016). Previous literature described that repeated cocaine changes in AMPAR surface expression are paralleled by increases in AMPA currents, AMPA/NMDA ratio and the density of dendritic spines in accumbens MSNs directed by cofilin-induced changes in actin dynamics (Dietz et al., 2012; Kourrich et al., 2007; Robinson and Kolb, 2004; Shen et al., 2009; Verma et al., 2019). Here, we found that the reduced behavioral response to cocaine in pre-stress rats exposed to shcofilin with a smaller spine dh also showed reduced levels of AMPAR surface expression (Fig. 4D) probably explained by the stabilization of actin remodeling and the reduction of AMPAR mobility (Rust, 2015; Rust et al., 2010). This result is in agreement with our previous observation describing an enhancement in AMPAR surface expression during the sensitization between stress and cocaine and increased PSD (Esparza et al., 2012).
4.3. Conclusion
In conclusion, we have shown that cofilin is involved in the enhanced vulnerability to cocaine abuse induced by stress, as we were able to prevent it by diminishing cofilin levels of expression, thereby altering its precise regulation during behavioral sensitization to cocaine and the acquisition of cocaine SA.
Our work highlights the role of cofilin as molecular mediator of spine morphological changes and AMPAR surface expression, reinforcing the cocaine sensitization and cocaine SA induced by previous chronic stress exposure. Thus, our research provides new directions for the comprehension of the neurobiological basis of comorbidity between stress exposure and cocaine addiction and provides a platform for further studies on approaches to therapy for this dual pathology.
Funding and disclosure
This work was supported by Argentine grants from ISN-CAEN 1B (to F.B.), FONCyT BID PICT 2013-958 and PICT 2016-674 (to F.B.), FONCyT BID PICT 2015-1622 (to L.M.C.), PUE 22920180100029CO (to L.M.C.) and SECyT Res. 212/16, 411/18, 472/18 (to L.M.C.) and 411/18 (to F.B.). The authors report no biomedical financial interests or potential conflicts of interest.
Data accessibility
Data collected for this manuscript can be provided upon request from the corresponding author.
CRediT authorship contribution statement
Daiana Rigoni: Conceptualization, Methodology, Resources, Writing – original draft, Visualization. Maria P. Avalos: Methodology, Resources. Maria J. Boezio: Methodology, Resources. Andrea S. Guzmán: Methodology, Resources. Gaston D. Calfa: Methodology, Resources. Eduardo M. Perassi: Resources, Formal analysis. Silvia M. Pierotti: Resources, Formal analysis. Mariano Bisbal: Methodology, Resources, Writing – review & editing. Constanza Garcia-Keller: Resources, Writing – review & editing, Visualization. Liliana M. Cancela: Methodology, Investigation, Resources, Writing – review & editing, Project administration, Funding acquisition. Flavia Bollati: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Visualization, Project administration, Funding acquisition.
Declaration of competing interest
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
The authors would like to thank Estela Salde, Lorena Mercado and Alberto Leandro Oliveros for their technical laboratory support, Javier Alejandro Reparaz, Yanina Altamirano, Nicolas Jaime and Walter Requena for their technical assistance in laboratory animal care, Dr. Cecilia Sanpedro, Dr. Carlos Mas and Dr. Gonzalo Quassolo from CEMINCO (Centro de micro y nanoscopia de Córdoba, Córdoba, Argentina) for their microscopy technical support and Joss Heywood for English technical assistance. We would also like to thank to Dr James Bamburg of Colorado State University, Fort Collins, CO, USA and Dr Arturo G. Romano of IFIByNE, UBA - CONICET for kindly providing cofilin and p-cofilin antibodies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100349.
Contributor Information
Daiana Rigoni, Email: daiana.rigoni@unc.edu.ar.
Maria P. Avalos, Email: mp.avalos@unc.edu.ar.
Maria J. Boezio, Email: jboezio@unc.edu.ar.
Andrea S. Guzmán, Email: andrea.guzman@unc.edu.ar.
Gaston D. Calfa, Email: gaston.calfa@unc.edu.ar.
Eduardo M. Perassi, Email: eduardo.perassi@unc.edu.ar.
Silvia M. Pierotti, Email: silvia.pierotti@unc.edu.ar.
Mariano Bisbal, Email: mbisbal@immf.uncor.edu.
Constanza Garcia-Keller, Email: garciake@musc.edu.
Liliana M. Cancela, Email: lcancela@unc.edu.ar.
Flavia Bollati, Email: fbollati@unc.edu.ar, fbollati@unc.edu.ar.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
Data availability
Data will be made available on request.
References
- Anderson E.M., Self D.W. It's only a matter of time: longevity of cocaine-induced changes in dendritic spine density in the nucleus accumbens. Curr. Opin. Behav. Sci. 2017;13:117–123. doi: 10.1016/j.cobeha.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg J.R. Proteins OF the ADF/cofilin family: essential regulators of actin dynamics. Cell Dev. Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bisbal M., Conde C., Donoso M., Bollati F., Sesma J., Quiroga S., Malhotra V., Marzolo M.P., Cáceres A. Protein kinase D regulates trafficking of dendritic membrane proteins in developing neurons. J. Neurosci. 2008;28:9297–9308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden M.T., Kimerling R., Jacobs-Lentz J., Bowman D., Weaver C., Carney D., Walser R., Trafton J.A. Seeking Safety treatment for male veterans with a substance use disorder and post-traumatic stress disorder symptomatology. Addiction. 2012;107:578–586. doi: 10.1111/j.1360-0443.2011.03658.x. [DOI] [PubMed] [Google Scholar]
- Borovac J., Bosch M., Okamoto K. Molecular and Cellular Neuroscience Regulation of actin dynamics during structural plasticity of dendritic spines: signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018;91:122–130. doi: 10.1016/j.mcn.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Bosch M., Castro J., Takeo S., Hitomi M., Mriganka S., Yasunori H. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson Christopher O., Holly Elizabeth N., Shimamoto Akiko, Albrechet-Souza Lucas, Weiner Lindsay A., DeBold Joseph F., Miczek Klaus A. Social stress and CRF-dopamine interactions in the VTA: Role in long-term escalation of cocaine self-administration. Journal of Neuroscience. 2014;34(19):6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.E., Lee B.R., Mu P., Ferguson D., Dietz D., Ohnishi Y.N., Lin Y., Suska A., Ishikawa M., Huang Y.H., Shen H., Kalivas P.W., Sorg B.A., Suzanne Zukin R., Nestler E.J., Dong Y., Schlüüter O.M. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J. Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G., Chapleau C.A., Campbell S., Inoue T., Morse S.J., Lubin F.D., Pozzo-Miller L. Hdac activity is required for bdnf to increase quantal neurotransmitter release and dendritic spine density in CA1 pyramidal neurons. Hippocampus. 2012;22:1493–1500. doi: 10.1002/hipo.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela L.M., Volosin M., Molina V.A. Gangliosides attenuate stress-induced changes on body weight , motor activity and on the behavioral response to 5-methoxy-N , N-Dimethyltryptamine. Brain Res. Bull. 1996;40:105–110. doi: 10.1016/0361-9230(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Carter J.S., Kearns A.M., Vollmer K.M., Garcia-Keller C., Weber R.A., Baker N.L., Kalivas P.W., Reichel C.M. Long-term impact of acute restraint stress on heroin self-administration, reinstatement, and stress reactivity. Psychopharmacology (Berl) 2020;237:1709–1721. doi: 10.1007/s00213-020-05486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda P., Muñoz M., García-Rojo G., Ulloa J.L., Bravo J.A., Márquez R., García-Pérez M.A., Arancibia D., Araneda K., Rojas P.S., Mondaca-Ruff D., Díaz-Véliz G., Mora S., Aliaga E., Fiedler J.L. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J. Neurosci. Res. 2015;93:1476–1491. doi: 10.1002/jnr.23602. [DOI] [PubMed] [Google Scholar]
- Chen Y., Dubé C.M., Rice C.J., Baram T.Z. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Dumitriu D., Robison A.J., Janssen W.G., Ahn H.F., Krishnan V., Reyes C.M., Han M.H., Ables J.L., Eisch A.J., Dietz D.M., Ferguson D., Neve R.L., Greengard P., Kim Y., Morrison J.H., Russo S.J. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.-Z., Yeh T.-Y., Bollati F., Conde C., Canavosio F., Caceres A., Sung C. The Dynein light chain tctex-1 has a Dynein-independent role in actin remodeling during neurite outgrowth. Dev. Cell. 2013;9:1–21. doi: 10.1016/j.devcel.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H.W., Masson C.L., Delucchi K.L., Hall S.M., Sees K.L. Violent traumatic events and drug abuse severity. J. Subst. Abuse Treat. 2001;20:121–127. doi: 10.1016/S0740-5472(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Dietz D.M., Sun H., Lobo M.K., Cahill M.E., Chadwick B., Gao V., Koo J.W., Mazei-robison M.S., Dias C., Maze I., Dietz K.C., Scobie K.N., Ferguson D., Ohnishi Y., Hodes G.E., Zheng Y., Neve R.L., Klaus M. Essential role for Rac1 in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat. Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios C.G., Chhabra D., Kekic M., Dedova I.V., Tsubakihara M. Actin binding Proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Dumitriu D., Laplant Q., Grossman Y.S., Dias C., Janssen W.G., Russo S.J., Morrison J.H., Nestler E.J. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J. Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza M.A., Bollati F., Garcia-Keller C., Virgolini M.B., Lopez L.M., Brusco A., Shen H.W., Kalivas P.W., Cancela L.M. Stress-induced sensitization to cocaine: actin cytoskeleton remodeling within mesocorticolimbic nuclei. Eur. J. Neurosci. 2012;36:3103–3117. doi: 10.1111/j.1460-9568.2012.08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Zhu X., Song Q., Wang P., Liu Z., Yu S.Y. MiR-134 modulates chronic stress-induced structural plasticity and depression-like behaviors via downregulation of Limk1/cofilin signaling in rats. Neuropharmacology. 2018;131:364–376. doi: 10.1016/j.neuropharm.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Fox M.E., Chandra R., Menken M.S., Larkin E.J., Nam H., Engeln M., Francis T.C., Lobo M.K. Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol. Psychiatr. 2018;25:1022–1034. doi: 10.1038/s41380-018-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.E., Figueiredo A., Menken M.S., Lobo M.K. Dendritic spine density is increased on nucleus accumbens D2 neurons after chronic social defeat. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-69339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T.C., Chandra R., Gaynor A., Konkalmatt P., Metzbower S.R., Evans B., Engeln M., Blanpied T.A., Lobo M.K. Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol. Psychiatr. 2017;22:1512–1519. doi: 10.1038/mp.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T.T., Wang Y., Liu L., Wang J.L., Wang Y.J., Guan W., Chen T.T., Zhao J., Jiang B. LIMK1/2 in the mPFC plays a role in chronic stress-induced depressive-like effects in mice. Int. J. Neuropsychopharmacol. 2020;23:821–836. doi: 10.1093/ijnp/pyaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C., Kupchik Y.M., Gipson C.D., Brown R.M., Spencer S., Bollati F., Esparza M.A., Roberts-Wolfe D.J., Heinsbroek J.A., Bobadilla A.C., Cancela L.M., Kalivas P.W. Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Mol. Psychiatr. 2016;21:1063–1069. doi: 10.1038/mp.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C., Martinez S., Esparza A., Bollati F., Kalivas P., Cancela L. Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur. J. Neurosci. 2013;37:982–995. doi: 10.1111/ejn.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C., Neuhofer D., Bobadilla A.C., Spencer S., Chioma V.C., Monforton C., Kalivas P.W. Extracellular matrix signaling through β3 integrin mediates cocaine cue–induced transient synaptic plasticity and relapse. Biol. Psychiatr. 2019;86:377–387. doi: 10.1016/j.biopsych.2019.03.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C., Scofield M.D., Neuhofer D., Varanasi S., Reeves M.T., Anderson E., Richie C.T., Mejias-Aponte C., Pickel J., Hope B.T., Harvey B.K., Cowan C.W., Kalivas P.W. Relapse-associated transient synaptic potentiation requires integrin-mediated activation of focal adhesion kinase and cofilin in D1-expressing neurons. J. Neurosci. 2020 doi: 10.1523/JNEUROSCI.2666-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Keller C., Smiley C., Monforton C., Melton S., Kalivas P.W., Gass J. N-Acetylcysteine treatment during acute stress prevents stress-induced augmentation of addictive drug use and relapse. Addiction Biol. 2019;25:1–12. doi: 10.1111/adb.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Rojo G., Fresno C., Vilches N., Díaz-Véliz G., Mora S., Aguayo F., Pacheco A., Parra-Fiedler N., Parra C., Rojas P.S., Tejos M., Aliaga E., Fiedler J.L. The ROCK inhibitor Fasudil prevents chronic restraint stress-induced depressive-like behaviors and dendritic spine loss in rat hippocampus. Int. J. Neuropsychopharmacol. 2017:1–38. doi: 10.1093/ijnp/pyw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachero M., Calfa G.D., Molina V.A. Hippocampal dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus. 2015;25:545–555. doi: 10.1002/hipo.22409. [DOI] [PubMed] [Google Scholar]
- Golden S.A., Christoffel D.J., Heshmati M., Hodes G.E., Magida J., Davis K., Cahill M.E., Dias C., Ribeiro E., Ables J.L., Kennedy P.J., Robison A.J., Gonzalez-Maeso J., Neve R.L., Turecki G., Ghose S., Tamminga C.A., Russo S.J. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden Sam A., Russo Scott J. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Lee C.W., Fan Y., Komlos D., Tang X., Sun C., KuainYu, Hartzell H.C., Chen G., Bamburg J.R., Zheng J.Q. ADF/Cofilin-Mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat. Neurosci. 2010;13:1280. doi: 10.1038/nn.2634. 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W., Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatr. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Khibnik L.A., Beaumont M., Doyle M., Heshmati M., Slesinger P.A., Nestler E.J., Russo S.J. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatr. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.Y., Shin S.R., Kim S., Jeon S., Kim J.H. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience. 2009;163:501–505. doi: 10.1016/j.neuroscience.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Kourrich S., Rothwell P.E., Klug J.R., Thomas M.J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A., Ball L.E., Townsend D.M., Kalivas P.W., Uys D. Post-translational S-glutathionylation of cofilin increases actin cycling during cocaine seeking. PloS One. 2019;14:1–15. doi: 10.1371/journal.pone.0223037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth J.A., Kuei Y. Tseng, Wolf M.E. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;23:1–7. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Sun G., Yang F., Guan Z., Zhang Z., Zhao J., Liu Y., Chua L., Pei L. Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.109054. [DOI] [PubMed] [Google Scholar]
- Marinelli M., Piazza P.V. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Matus A. Growth of dendritic spines: a continuing story. Curr. Opin. Neurobiol. 2005;15:67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Miczek Klaus A., Yap Jasmine J., Iii Herbert E. Covington III. Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacology Ther. 2008;120(2):102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell. Signal. 2013;25:457–469. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Noguchi J., Hayama T., Watanabe S., Ucar H., Yagishita S., Takahashi N., Kasai H. State-dependent diffusion of actin-depolymerizing factor/cofilin underlies the enlargement and shrinkage of dendritic spines. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep32897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette P.C., Brown P.J., Najavits L.M. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addict. Behav. 1998;23:785–795. doi: 10.1016/S0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Pacchioni A.M., Cador M., Bregonzio C., Cancela L.M. A glutamate – dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 6th ed. Elsevier Inc; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Piazza Pier Vincenzo, Moal Michel Le. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19(February):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pierce R.C., Kalivas P.W. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/S0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., Saikat D., Sarkar D., Siem H., Bert Van W., R-core . ’; 2020. Package ‘ nlme. [Google Scholar]
- Pozzo-Miller L.D., Inoue T., Murphy D.D. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J. Neurophysiol. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- Qiao H., Li M., Xu C., Chen H., An S., Ma X. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016:20–24. doi: 10.1155/2016/8056370. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.E., Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ruisoto P., Contador I. The role of stress in drug addiction. An integrative review. Physiol. Behav. 2019:62–68. doi: 10.1016/j.physbeh.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Dietz D.M., Dumitriu D., Malenka R.C., Nestler E.J. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.B. ADF/cofilin: a crucial regulator of synapse physiology and behavior. Cell. Mol. Life Sci. 2015;72 doi: 10.1007/s00018-015-1941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.B., Gurniak C.B., Renner M., Vara H., Morando L., Görlich A., Sassoè-Pognetto M., Banchaabouchi M. Al, Giustetto M., Triller A., Choquet D., Witke W. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/S0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shen H., Toda S., Moussawi K., Bouknight A., Zahm D.S., Kalivas P.W. Altered dendritic spine plasticity in cocaine-withdrawn rats. J. Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol. Psychiatr. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr. Psychiatr. Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sorg B.A., Kalivas P.W. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1991;53:695–703. doi: 10.1016/0306-4522(93)90617-O. [DOI] [PubMed] [Google Scholar]
- Stefen H., Chaichim C., Power J., Fath T. Regulation of the postsynaptic compartment of excitatory synapses by the actin cytoskeleton in Health and its Disruption in Disease. Neural Plast. 2016 doi: 10.1155/2016/2371970. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S., Shen H., Kalivas P.W. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox. Res. 2010;18:410–415. doi: 10.1007/s12640-010-9193-z. [DOI] [PubMed] [Google Scholar]
- Toda S., Shen H., Peters J., Cagle S., Kalivas P.W. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J. Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Bennett J., Ayse Merve Ö., Elena P., Brian R. Cocaine addicted to cytoskeletal change and a fibrosis high. Cytoskeleton. 2019;1–9 doi: 10.1002/cm.21510. [DOI] [PubMed] [Google Scholar]
- Wang H., Lu F., Jin I., Udo H., Kandel E.R., Vente J. De, Walter U., Lohmann S.M., Hawkins R.D., Antonova I. Presynaptic and postsynaptic roles of NO , cGK , and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wolf M.E. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotox. Res. 2010;18:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.E., Ferrario C.R. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.E., Tseng K.Y. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front. Mol. Neurosci. 2012;5:1–27. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z., Bi G., Galaj E., Kumar V., Cao J., Gadiano A., Rais R., Slusher B.S., Gardner E.L., Xi Z., Newman A.H. Dopamine D 3 R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology. 2018;116:1–10. doi: 10.1038/s41386-018-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Bi G., Li X., Li J., Qu H., Zhang S., Li C., Onaivi E.S., Gardner E.L., Xi Z.-X., Qing-Rong L. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropharmacology. 2015:1037–1051. doi: 10.1038/npp.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this manuscript can be provided upon request from the corresponding author.
Data will be made available on request.