Highlights
-
•
Increase in upper limb function post task specific training in chronic stroke.
-
•
Motor improvements were not accompanied by changes in white matter integrity.
-
•
Integrity in contralesional fibers predicted larger motor recovery in Responders.
-
•
Non-responders had more severe damage of transcallosal fibers than Responders.
Abbreviations: ARAT, Action Research Arm Test; CL, contralesional; CST, corticospinal tract; FA, Fractional anisotropy; IL, ipsilesional; MCID, minimal clinically important difference; nRESP, non-responder; RESP, responder; TBSS, tract-based spatial statistics; TST, task specific training; UL, upper limb; WM, white matter
Keywords: Diffusion tensor imaging, Stroke rehabilitation, Upper limb, Task specific training, Neuroimaging biomarker, Transcallosal fibers
Abstract
Objective
To investigate white matter (WM) plasticity induced by intensive upper limb (UL) task specific training (TST) in chronic stroke.
Methods
Diffusion tensor imaging data and UL function measured by the Action Research Arm Test (ARAT) were collected in 30 individuals with chronic stroke prior to and after intensive TST. ANOVAs tested the effects of training on the entire sample and on the Responders [ΔARAT ≥ 5.8, N = 13] and Non-Responders [ΔARAT < 5.8, N = 17] groups. Baseline fractional anisotropy (FA) values were correlated with ARATpost TST controlling for baseline ARAT and age to identify voxels predictive of response to TST. Results.
While ARAT scores increased following training (p < 0.0001), FA changes within major WM tracts were not significant at p < 0.05. In the Responder group, larger baseline FA of both contralesional (CL) and transcallosal tracts predicted larger ARAT scores post-TST. Subcortical lesions and more severe damage to transcallosal tracts were more pronounced in the Non-Responder than in the Responder group.
Conclusions
The motor improvements post-TST in the Responder group may reflect the engagement of interhemispheric processes not available to the Non-Responder group. Future studies should clarify differences in the role of CL and transcallosal pathways as biomarkers of recovery in response to training for individuals with cortical and subcortical stroke. This knowledge may help to identify sources of heterogeneity in stroke recovery, which is necessary for the development of customized rehabilitation interventions.
1. Introduction
Despite advances in acute management, stroke remains the leading cause of chronic motor disability in adults worldwide (Benjamin et al., 2019). The global age-adjusted mortality rates for ischemic and hemorrhagic stroke decreased between 1990 and 2015 (Krishnamurthi et al., 2013, Benjamin et al., 2019). This scenario resulted in substantial increase in the already large number of stroke survivors experiencing motor disability (Krishnamurthi et al., 2013). Current rehabilitation strategies, though they can improve upper limb (UL) motor outcomes, are still lacking as response to training remain suboptimal and highly heterogeneous (Takeuchi and Izumi, 2013, Van Vliet et al., 2012). Studies investigating the neural correlates of specific therapies are scarce. These studies help to clarify how the therapies work, what aspects of brain organization can/cannot be modified, and who is likely to respond.
Lesion location and integrity of motor tracts are key factors influencing motor recovery and response to training post-stroke (Riley et al., 2011, Dromerick and Reding, 1995). For instance, past studies have provided unprecedented insights showing that stroke resulting in extensive CST damage, and larger asymmetries of CST integrity, are associated with more severe motor impairments, and reduced global motor function, motor learning, and hand dexterity (Schaechter et al., 2009, Qiu et al., 2011, Stinear et al., 2007). Likewise, individuals with lesion confined to subcortical structures (e.g. basal ganglia) are known to experience worse recovery and are more resistant to treatment than those with cortical lesions (Miyai et al., 1997, Shelton and Reding, 2001). More recently, a larger body of studies has shown evidence for post-stroke brain reorganization in other motor tracts and subsystems extending beyond the CST (Li et al., 2015, Lindenberg et al., 2012, Wadden et al., 2019, Plow et al., 2016). Indeed, the presence of parallel distributed processing in the motor system gives the advantage of multiple options to reconfigure UL motor function (Baker et al., 2015). However, it remains unclear which white matter (WM) tracts critically support UL motor gains following training. Basic information on structural-behavioral correlations post-stroke is crucial to the development of more targeted rehabilitation approaches.
Our main goal was to investigate WM plasticity induced by UL intensive task specific training (TST) in chronic stroke. Stroke patients in this study received higher doses of training than standard conventional training ranging from 3200 to 9600 repetitions for 8 weeks to test for the presence of a dose–response of TST on UL function. Tract-based spatial statistics (TBSS) (Smith et al., 2006) was computed to study potential remodeling of major WM tracts. We hypothesized that improvement in UL function following TST would be correlated with increased WM tract integrity. In addition, given that in our sample some subjects had large motor gains while others did not improve at all, we hypothesized that larger baseline WM integrity would predict increased UL function in response to TST. In addition to our data-driven analysis we performed an ROI-based analysis of transcallosal fibers to further explore the role of WM integrity and interhemispheric interactions in the response to TST.
2. Materials and methods
2.1. Study population
This report includes data from participants with chronic stroke recruited for a phase II, single-blinded, dose–response trial performed at a single site (NCT 01146379) previously published in (Lang et al., 2016). They were included if they met the following inclusion criteria: (1) Ischemic or hemorrhagic stroke as determined by a stroke neurologist; (2) right-handed by self-report; (3) time post stroke of at least 6 months; (4) cognitive skills to actively participate, quantified by scores of 0 to 1 on items 1b and 1c of the National Institutes of Health Stroke Scale (NIHSS); (5) unilateral UL weakness, quantified by a score of 1 to 3 on item 5 (arm item) on the NIHSS; and (6) mild-to-moderate motor function of the affected UL, quantified by a score of 10 to 48 on the Action Research Arm Test (ARAT) (Yozbatiran et al., 2008, Lyle, 1981). They were excluded if they met the following conditions: (1) unavailability for 2-month follow-up; (2) inability to follow two-step commands; (3) psychiatric diagnoses; (4) current participation in other UL stroke treatments; (5) other neurological diagnoses; (6) participants living further than 1 h away and unwilling to travel for assessment and treatment sessions; and (7) pregnancy.
In total, 85 S patients were enrolled in the clinical trial. Thirty-one of these patients underwent diffusion tensor imaging (DTI) scans before training. Out of the 31 patients assessed prior training, two did not repeated the scans after the training. Structural imaging from one participant scanned both prior and after training failed registration and was excluded from the whole analysis. This left 28 participants with DTI measures before and after the training [mean age: 59.43 ± (SD) 10.0 with range of 37 – 82] used for analysis involving changes in UL motor function and DTI post-TST and 30 participants with DTI measures before training [mean age: 59.87 ± (SD) 10.36 with range of 37 – 82] used for predictive analysis of stroke recovery. This research was approved by the Washington University Human Research Protection Office and all participants gave written informed consent prior to participation.
2.2. Study design
Participants were scanned and assessed behaviorally before and after an intervention of TST. The training involved a variety of UL movements including reaching for, moving/manipulating, and releasing objects during a 1 h session, 4 days/week for 8 weeks. Patients were assigned to one of four dose groups with different total number of repetitions of UL tasks: 3,200 (100 repetitions/session; N = 8), 6,400 (200 repetitions/session, N = 5), 9,600 (300 repetitions/session, N = 7), or individualized maximum repetitions (300 repetitions/session and sessions continuing until a stopping criteria was met, N = 10). A detailed description of the study design and the TST has been provided in a previous publication (Lang et al., 2016).
2.3. Outcome measures
The primary outcome measure assessing the UL function following TST was the ARAT test) (Yozbatiran et al., 2008, Lyle, 1981). The ARAT quantifies limitation of the UL function using 19 items divided into four subscales: grasp, grip, pinch, and gross movement. The maximum score of 57 indicates normal performance. The ARAT test was chosen because it is a reliable and valid method to evaluate UL function following stroke and has been widely used in research (Hsieh et al., 1998). Other information reported are: demographic and clinical characteristics: age, sex, race, whether the dominant of the non-dominant side was affected, stroke location, and presence of hemispatial neglect quantified by the unstructured version of the Mesulam Cancellation test (Rengachary et al., 2009).
2.4. Neuroimaging data collection
Scanning was performed with a Siemens 3 T Tim-Trio whole body MRI scanner with a 12-channel head coil including: structural and DTI scans. Structural scans consisted of magnetization prepared rapid gradient echo (MPRAGE) T1-weighted images (TR = 1950 ms, TE = 2.26 ms, flip angle = 9°, voxel size = 1 × 1 × 1 mm, slice thickness = 1 mm) and T2-weighted images (TR = 2500 ms, TE = 435 ms, flip angle = 120°, voxel-size = 1 × 1 × 1 mm, slice thickness = 1.00 mm). The DTI imaging parameters were: single-shot echo planar imaging (EPI), repetition time = 9,200 ms, echo time = 92 ms, single average (NEX = 1), field of view (FOV) = 256 × 256 mm, voxel size = 2 × 2 × 2 mm, 64 contiguous axial slices, flip angle = 90°, 63 directions with b = 1000 s/mm2. Two sequences with b = 0 were also collected.
2.5. Lesion segmentation
Stroke lesions were manually segmented on the high resolution MPRAGE of each subject’s space using MRIcron software (Robb and Hanson, 1991). All segmentations were reviewed by a board-certified neurologist (AC). Then, the segmented lesions were transformed to Montreal Neurological Institute (MNI) standard and summed at the voxel-wise level to display the number of individuals with structural damage for each voxel at that location (lesion overlay map).
2.6. DTI
DTI images were processed with FMRIB Software Library (FSL, University of Oxford, FSL v5.0.1, www.fmrib.ox.ac.uk/fsl). Standard FSL diffusion processing pipeline (https://fsl.fmrib.ox.ac.uk/fslcourse/lectures/practicals/fdt1/index.html) was used to compute the voxelwise maps of four diffusion indices: fractional anisotropy (FA), mean diffusivity (MD), axonal diffusivity (AD), and radial diffusivity (RD). Most DTI studies of stroke recovery measure FA to estimate general WM integrity, however, other parameters such as AD and RD have also been used to investigate axonal damage and myelin injury respectively (Fox et al., 2011, Song et al., 2003). MD is an averaged tridimensional measure of water diffusion related to the amount of water in the extracellular space and detection of edema and necrosis (O’Donnell and Westin, 2011).
2.7. Tract-based spatial statistics (TBSS)
Voxel-based statistical analysis of DTI images was performed using TBSS, part of FSL (Smith et al., 2006). First, brain images of participants that received training in their left arm were flipped right-to-left, thereby placing all lesions in the image on the left. Then, the FA volume from each participant was aligned to the within-group target volume that was closest to the group mean using a nonlinear registration algorithm, followed by a 12-degrees of freedom (DOF) affine registration from the target FA volume to the MNI152 template. Residual misalignments between subjects that may happen after the initial nonlinear registrations were minimized by computing a WM skeleton. This process involves morphological thinning of the inter-subject mean FA, and projection of individual’s FA values onto a common FA skeleton of major white matter tracts (Smith et al., 2006). The mean FA skeleton representing the centers of all tracts common to the group was generated by setting a map of threshold for voxels with FA values ≥ 0.2. Aligned FA data for each participant were projected onto the standard skeletonized FA image by searching the area perpendicular to each tract for the highest local FA value and assigning this value to the skeleton. A mean FA skeleton was compiled by averaging aligned FA maps for each participant. Finally, the non-linear warps and skeleton projection obtained were applied to MD, AD, and RD.
2.8. ROI-based DTI analysis
We performed a ROI-based DTI analysis to further quantify diffusion characteristics in transcallosal regions. The transcallosal mask was manually drawn using the thickened FA skeleton computed in the TBSS analysis. The mask was divided into 5 parcels using Hofer segmentation (Hofer and Frahm, 2006) with a customized Matlab code. Then, the mean FA was extracted for each parcel of the Hofer segmentation. According to this segmentation regions I-V contain fibers projecting respectively into prefrontal region (region I), to premotor and supplementary motor cortical areas (region II), primary motor cortex (region III), primary sensory cortex (region IV), and finally transcallosal parietal, temporal, and occipital fibers (region V) (Hofer and Frahm, 2006).
2.9. WM tract template
To estimate the percentage of streamlines of specific WM tracts that were lesioned by the stroke, we used a tractography atlas constructed with data from 842 Human Connectome Project (HCP-842 template in MNI152 space) participants available at www.dsi-studio.labsolver.org. The atlas used deterministic fiber tracking (Yeh et al., 2013) to extract 550,000 streamline trajectories that were then reviewed and labeled by experts. The streamline trajectories were segmented into 65 neuroanatomically defined WM tracts. Because we expected that different transcallosal segments might show different relationships to UL function, the transcallosal tract was split into 5 segments based on the Freesurfer’s WM segmentation algorithm (Dale et al., 1999) included in DSI_studio. Also, because the reticulospinal tract has been associated with stroke recovery (Owen et al., 2017), the right and left cortico-reticulo-spinal tract were added from the DSI_studio support group. Therefore, there were a total of 72 WM tracts grouped into five categories: projection, transcallosal, association, brainstem, and cerebellar pathways (cranial nerves were not included).
2.10. Statistical analysis
The statistical analysis was divided in three main parts: response to TST (dose–response and overall response to TST), prediction of response to TST, and analyses to compare the RESP and nRESP groups. All statistical analyses were performed in RStudio Version 1.2.1335 and significance was assumed at p < 0.05. Normality assumptions were tested and Greenhouse-Geisser correction was applied as needed.
For the dose–response analysis, we performed an ANCOVA with factor dose-group (3,200, 6,400, 9,600, and individualized maximum repetition) for ΔARAT controlling for the effects of baseline ARAT. Then, given the absence of dose–effect on ΔARAT, mean differences between baseline ARAT and ARAT post-TST were compared using paired t-test. Finally, to investigate brain changes associated with TST, we performed a Pearson correlation between ΔFA and ΔARAT controlling for age and prediction of response to TST.
For the predicted response to TST, the main analysis was performed at the voxel-wise level. A Pearson correlation was conducted as a follow up analysis to determine the strength of this correction. Then a ROI-based analysis was conducted to determine whether transcallosal fibers were predictive of response to TST. At the voxel-wise level (within the region defined by the TBSS FA skeleton), the relationship between baseline DTI and ARATpost (indicating response to TST) was examined using Pearson correlation with baseline ARAT and age as covariates using the FSL Glm statistical model, similarly to previous research (Cassidy et al., 2018). Multiple comparison corrections were applied using a permutation based statistical approach (N = 5000) within FSL “randomize” program using the threshold-free cluster enhancement method (Smith et al., 2006). For the follow up analysis, the average FA at baseline of all predictive voxels from the prior analysis was calculated for each subject. The strength of the relationship between these baseline FA values and ΔARAT was expressed by calculating a Pearson correlation coefficient. Lastly, we defined transcallosal ROIs using the Hoffer parcellation and computed Spearman correlations between baseline FA and 1) baseline ARAT, 2) ARAT post-TST, and 3) ΔARAT.
To investigate differences between the RESP and nRESP groups at baseline, we performed two analyses. In the first one, we compared the demographic and clinical characteristics of the RESP and nRESP groups using either Chi-square or t-tests, and their lesion distribution. In the second one, we calculated the percentage of streamlines that intersected with lesioned voxels by each subject’s stroke. The streamlines and category of tracts was determined based on the HCP842 tractography (Yeh et al., 2018) atlas. The mean percentage of streamlines with lesioned voxels was computed for each tract category and divided into four bins : 0–25%, 26–50%, 51–75%, and 76–100% to indicate the severity of tract damage in the RESP and nRESP groups at baseline and post-training.
3. Results
3.1. Participant characteristics
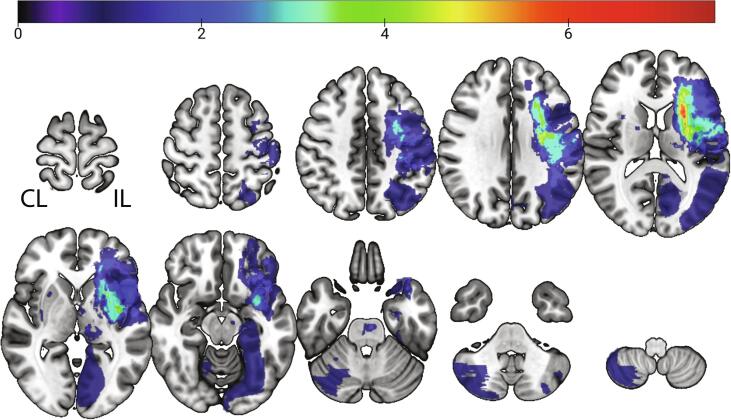
Participants’ characteristics are shown in Table 1. Fig. 1 shows a voxel-wise average of the segmented lesions normalized to a standard MNI-152 brain template. Seventy percent of strokes were subcortical (N = 18/26) localized in the ipsilesional (IL) basal ganglia, thalamus, and WM fibers. In 28% of the cases, lesions were both subcortical and cortical (N = 8/26) and occurred predominantly within the middle cerebral artery distribution. In four individuals no lesion could be identified. The average lesion volume was 15,262 mm3 (1,908 voxels of 2 × 2 × 2 mm).
Table 1.
Participant’s characteristics.
| ID | Age | Sex | Race | Education | Arm Trained | Mesulam | Baseline ARAT | ARATpost | ΔARAT | Lesion Volume (mm3) | Lesion Location | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 03 | 60 | F | Afr. Amer. | NA | Dom | 1 | 39 | 57 | 18 | 2452 | L CR & BG / R BG |
| 2 | 22 | 54 | M | Afr. Amer. | HS | Dom | 1 | 41 | 57 | 16 | 29,178 | Bil Cerebellar Hemisphere (R > L) |
| 3 | 34 | 53 | F | Caucasian | < College | Dom | 0 | 40 | 55 | 15 | NA | Not identifiable |
| 4 | 01 | 37 | M | Afr. Amer. | HS | Dom | 15 | 27 | 40 | 13 | 48,240 | L Frontal/Parietal/Insula/Occipital (C + S); L thalamic & BG lacune / Bilateral cerebellar lacune |
| 5 | 24 | 82 | M | Caucasian | NA | Dom | 0 | 16 | 27 | 11 | 90,095 | L Frontal/Parietal/Temporal/Insula (C + S); L CR and BG |
| 6 | 44 | 48 | F | Afr. Amer. | < College | Dom | 2 | 40 | 49 | 9 | 482 | L CR, Thalamus, BG, genu of the internal capsule;R PLIC lacune |
| 7 | 02 | 60 | F | Caucasian | < College | Dom | 0 | 31 | 40 | 9 | 35,646 | L Frontal/Parietal (C + S); L Insula (C) |
| 8 | 19 | 61 | M | Afr. Amer. | HS | Dom | 0 | 39 | 47 | 8 | 5124 | L Frontal (S), CC, CR, BG, thalamus, ALIC, L middle cerebellar peduncle / R VA thalamus lacune; pons |
| 9 | 38 | 59 | F | Caucasian | < College | Dom | 1 | 48 | 55 | 7 | 61 | L genu of the internal capsule |
| 10 | 08 | 60 | M | Afr. Amer. | < College | Non-Dom | 3 | 39 | 46 | 7 | 434 | R CR & BG / L CR & cerebellum |
| 11 | 43 | 56 | M | Caucasian | HS | Non-Dom | 1 | 37 | 44 | 7 | 13,118 | R parietal/posterior insula (C + S) |
| 12 | 35 | 65 | M | Afr. Amer. | < College | Dom | 26 | 32 | 38 | 6 | 1562 | L prefrontal (S), multiple bilateral thalamic and BG lacunes and L pons |
| 13 | 12 | 58 | M | Afr. Amer. | HS | Dom | 0 | 30 | 36 | 6 | 463 | L Frontal/Parietal (C + S) / R Frontal/Temporal (C + S) |
| 14 | 41 | 54 | M | Caucasian | HS | Non-Dom | 2 | 38 | 43 | 5 | 72,119 | R Frontal/Parietal/Temporal/Insula (C + S), thalamus, and basal ganglia |
| 15 | 18 | 70 | M | Afr. Amer. | HS | Dom | 5 | 37 | 42 | 5 | 7108 | L frontal (S), CR, PLIC, thalamus, and BG |
| 16 | 30 | 56 | M | Caucasian | < College | Dom | 0 | 25 | 30 | 5 | 134 | L PLIC |
| 17 | 33 | 50 | M | Caucasian | HS | Dom | 0 | 38 | 42 | 4 | 7209 | L CR, BG, subinsula |
| 18 | 27 | 42 | F | Caucasian | HS | Non-Dom | 2 | 33 | 37 | 4 | 12,757 | R CR, BG, insula (C + S), medial temporal lobe |
| 19 | 40 | 79 | NA | NA | < College | Dom | 2 | 31 | 35 | 4 | NA | Not identifiable lesion |
| 20 | 17 | 77 | F | Afr. Amer. | NA | Dom | 12 | 10 | 14 | 4 | 386 | L pontine / R BG and thalamic lacune |
| 21 | 10 | 52 | M | Afr. Amer. | HS | Non-Dom | 0 | 13 | 15 | 2 | 12,631 | R frontal (S) CR & BG, superior occipital frontal fasciculus / L cerebellar lacune / L thalamic lacune |
| 22 | 13 | 60 | F | Afr. Amer. | HS | Non-Dom | 0 | 10 | 12 | 2 | 981 | R CR & BG |
| 23 | 37 | 66 | F | Afr. Amer. | < College | Non-Dom | 0 | 38 | 39 | 1 | 884 | R thalamus |
| 24 | 26 | 60 | M | Caucasian | < College | Non-Dom | 1 | 38 | 39 | 1 | 413 | L Thalamus / PLIC |
| 25 | 20 | 53 | F | Afr. Amer. | NA | Dom | 3 | 37 | 38 | 1 | NA | Not identifiable lesion |
| 26 | 23 | 66 | F | Caucasian | VS/TS | Dom | 1 | 36 | 37 | 1 | 910 | L thalamus & BG lacune, cerebral peduncle |
| 27 | 21 | 54 | M | Caucasian | < College | Non-Dom | 8 | 39 | 39 | 0 | NA | Not identifiable lesion |
| 28 | 45 | 79 | M | Caucasian | NA | Non-Dom | 6 | 17 | 17 | 0 | 791 | L insular cortex (S), CR |
| 29 | 09 | 64 | F | Afr. Amer. | HS | Dom | 5 | 33 | 31 | −2 | 38,940 | L parietal/temporal/occipital (C + S), cerebellum, thalamus-pulvinar, and PLIC / R internal capsule |
| 30 | 11 | 61 | M | Caucasian | < College | Non-Dom | 0 | 32 | 29 | −3 | 233 | L pontomedulary junction / inferior cerebellar peduncle |
| Summary Mean ± SD | 59.87 ± 10.36 | 17 M, 12F, 1NA | 15 Afr. American 14 Caucasian 1NA | 12 HS 1 VS/TS 12 < College 5NA | 21 Dom, 9 Non-Dom | 3.23 ± 5.63 | 32.13 ± 9.80 | 37.67 ± 11.86 | 5.53 ± 5.20 | 14705.81 ± 23449.36 | 18 (S), 8 (C + S), 4NA | |
Legend: Afr. Amer. = African American, Dom = Dominant, Non-Dom: Non-Dominant, IM = Individualized Maximum, HS = High School, VS/TS = Vocational School / Technical School. R = Right, L = Left, MCA = Middle Cerebral Artery, PCA = Posterior Cerebral Artery, PLIC = Posterior Limb of the Internal Capsule.
C = cortical, S = subcortical, CR = corona radiata, BG = basal ganglia, ALIC = anterior limb of the internal capsule, PLIC = posterior limb of the internal capsule, VA = ventral anterior nucleus.
Fig. 1.
Overlap of lesion location for all participants. Images of participants that received training in their left arm were flipped around the midsagittal plane. In 4 participants (20, 21, 34, and 40 subject’s IDs in Table 1), no lesion was identified in the images, N = 26. Images are in Neurological convention. IL = ipsilesional, CL = contralesional.
3.2. Response to TST
3.2.1. Dose-Response to TST
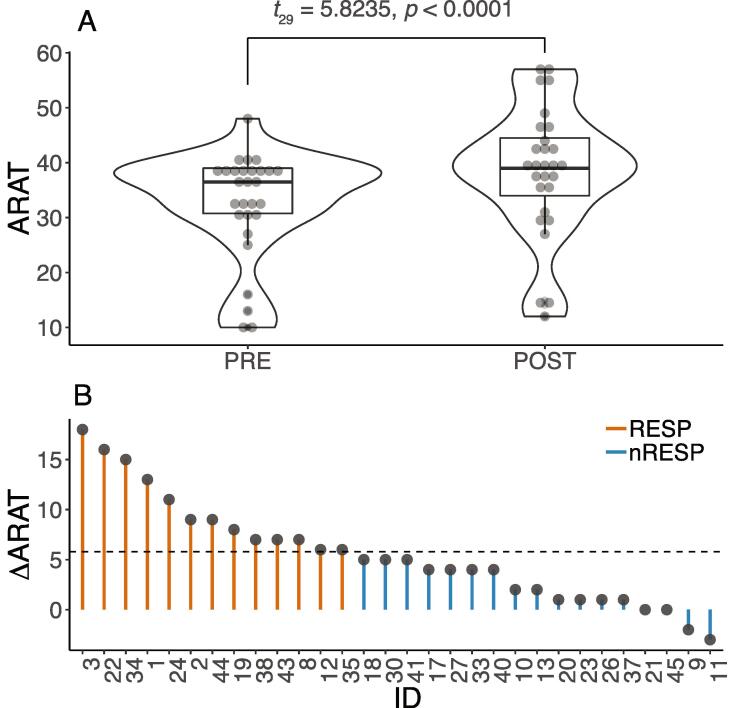
The ANCOVA showed no significant dose–response for ΔARAT (F3,1 = 0.880, p = 0.466, Supplementary Fig. 1). The mean ± SD change in ARAT score for each intervention group was: 3,200: 4.43 ± 3.15; 6,400: 4.40 ± 2.88; 9,600: 5.86 ± 6.41, and Individualized Maximum: 7.89 ± 6.41. These values were slightly below those reported for the larger cohort that ranged between 5.1 and 8.4 points (Lang et al., 2016). When the dose groups were combined, the mean ARAT score increased significantly following TST (PRE: 32.23 ± 9.80 and POST: 37.67 ± 12.06, t29 = 5.8235; p < 0.0001, 95%CI: 3.59–7.48, Fig. 2A). This mean increase of 5.53 ± (SD) 5.20 points is close to the MCID value of 5.8.
Fig. 2.
A) Box plots with an outline of the probability density showing values of ARAT score pre- and post- task specific therapy for all participants (N = 30). B) Values of changes in ARAT scores following task-specific therapy for each participant illustrating the variability of response from therapy. The dotted line indicates the minimal clinically important difference (MCID) of 5.8 for ARAT score that was used to classify the participants into Responders (greater than5.8, N = 13) and Non-Responders (<5.8, N = 17).
3.2.2. Overall response to TST
The focus of this research was to investigate brain changes either associated with or predictive of response to TST. Thus, given the absence of a significant dose–effect on ARAT score, we did not investigate effects of dose of TST on DTI-related measures. The overall effect of the TST on UL function was not accompanied by changes in FA, MD, AD, and RD at p = 0.05 as quantified by TBSS. In summary, there was an increase in ARAT, but no change in DTI measures.
3.3. Prediction of response to TST
3.3.1. Baseline FA in TBSS predicts UL response to TST in the RESP groups
The response to TST was highly heterogeneous. As can be observed in Fig. 2B, while ΔARAT was<5.8 for 17 participants (nRESP group), it was larger than 5.8 for 13 participants (RESP group). Our next question was whether the integrity in specific WM tracts was predictive of UL response to TST. Because our main research goal was to understand and predict response to training, we performed further analysis with the RESP group.
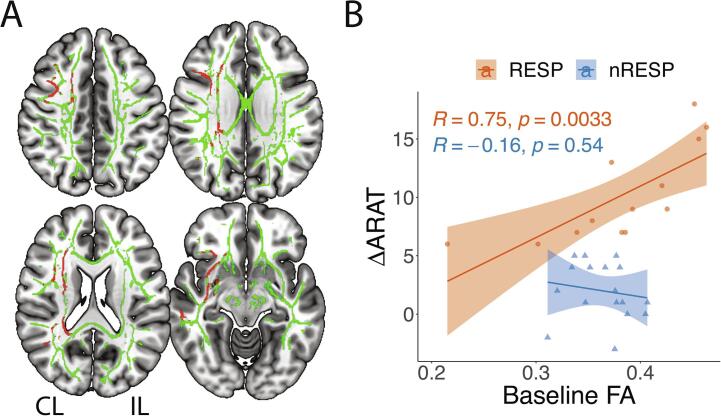
At the whole brain voxel-wise level, voxels significantly associated with response to TST for the RESP group is shown in both Fig. 3A for FA and Supplementary Fig. 2A for MD, AD, and RD. In summary, larger baseline FA, and smaller baseline MD, AD, and RD significantly predicted improvement in UL function quantified by ARATpost controlling for baseline ARAT and age. Predictive voxels were located in the CL (untrained) hemisphere and in transcallosal tracts. Fig. 3B shows that the linear regression between baseline FA and ΔARAT was significant (R = 0.75, p-value = 0.0033) for the RESP group and not significant for the nRESP group (R = -0.16, p-value = 0.54). In the RESP group, the pathways with the largest percentage of streamlines containing voxels whose baseline FA was predictive of response to TST included CL association pathways (inferior fronto-occipital cortex, middle and superior longitudinal fasciculus, arcuate fasciculus, extreme capsule, and uncinate fibers), projection pathways (optic radiation, corticostriatal, and fronto, parieto, temporo, and occipito-pontine fibers), and transcallosal pathways (Supplementary Fig. 2B).
Fig. 3.
A) Voxels in green represent the white matter skeleton for Responders on a MNI-152 template. Voxels in red represent significant correlation (corrected p < 0.05) between baseline FA and ARATpost scores controlling for baseline ARAT and age for Responders (N = 13). B) Pearson, R, correlation between averaged baseline FA values and changes in ARAT score (ΔARAT) for both Responders (RESP, N = 13) and for non-Responders (N = 17). For both groups, averaged baseline FA values was extracted from all significant voxels in the TBSS analysis (shown in red in Fig. 3A). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3.2. Baseline FA in some transcallosal ROIs correlated with UL post TST in RESP
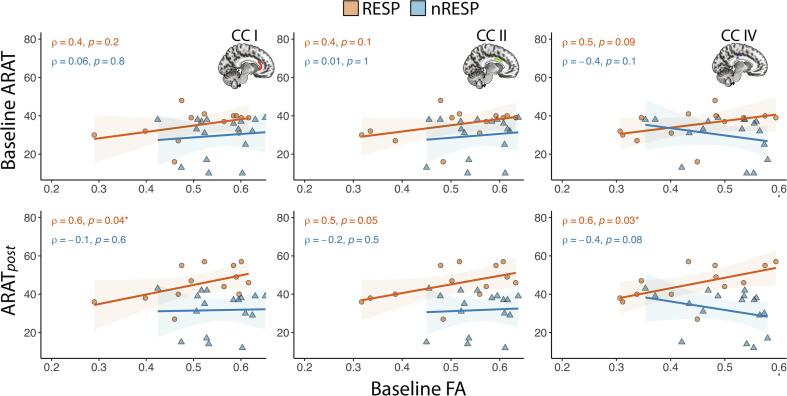
The principle pathway by which the CL hemisphere can exert its influence on the hemiparetic UL is by transcallosal fibers. We performed a correlational analysis between baseline FA and 1) baseline ARAT, 2) ARATpost, and 3) ΔARAT in 5 transcallosal ROIs. Results from CCI, CCII, and CCIV are illustrated in Fig. 4, and results from CCIII and CCV are not shown. At baseline, ARAT score did not correlate with FA in any CC region (Fig. 4, upper row). However, for the RESP group, a correlation developed after training in that a larger ARATpost following TST was correlated with larger baseline FA values within CCI, CCII, and CCIV (Fig. 4, lower row). Baseline FA values within CCIII were not significantly correlated with baseline ARAT (RESP: ρ = 0.3, p = 0.4; nRESP: ρ = -0.02, p = 0.9) or with ARATpost (RESP: ρ = 0.3, p = 0.3; nRESP: ρ = -0.2, p = 0.5). Similarly, baseline FA values within CCV were not significantly correlated with baseline ARAT (RESP: ρ = 0.3, p = 0.3; nRESP: ρ = -0.2, p = 0.4) or with ARATpost (RESP: CCV: ρ = 0.5, p = 0.1; nRESP: ρ = -0.3, p = 0.2). Also, baseline FA was not significantly predictive of ΔARAT at p < 0.05. These findings suggest a role for interhemispheric interactions in UL function post training. However, they do not support transcallosal FA in isolation as a prognostic biomarker of response to TST or in distinguishing the RESP from the nRESP group at baseline.
Fig. 4.
Spearman (ρ) correlation between baseline FA values extracted from transcallosal ROIs and both baseline ARAT score and ARATpost for Responders (RESP, N = 13) and Non-Responders (nRESP, N = 17). ROIs were defined using Hofer segmentation, regions I, II and IV contain fibers projecting respectively into prefrontal region (region I), to premotor and supplementary motor cortical areas (region II), and to primary sensory cortex (region IV) (Hofer and Frahm 2006).
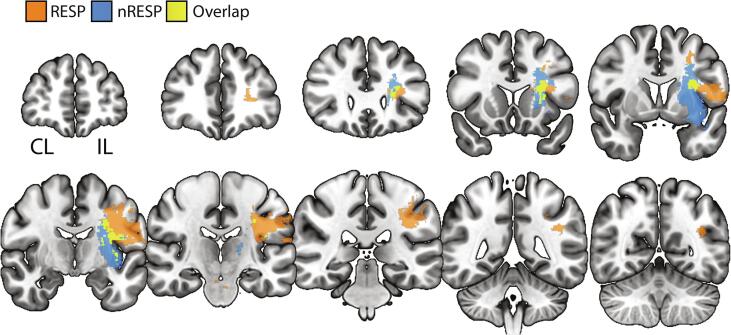
3.4. Lesion location and lesioned WM tracts may distinguish the RESP from the nRESP group.
The RESP and nRESP groups were well matched in terms of age, sex, race, level of education, whether the stroke affected the dominant or the non-dominant arm, the level of UL function quantified by the ARAT score, and lesion volume (Table 2). Fig. 5 shows the contrast and overlap images of binarized lesion location with a threshold ≥ 1 for the RESP and nRESP groups. While the RESP group had predominantly rostral/cortical lesions, the nRESP group had predominantly caudal WM and subcortical lesions affecting thalamus, basal ganglia, and internal capsule. In Table 3 we present the mean % of streamlines with lesioned voxels for the RESP and nRESP groups across WM tract category and for IL CST. Transcallosal pathways were more extensively lesioned in the nRESP than in the RESP group (Table 3 and Supplementary Fig. 3). The % streamlines of IL CST was not different in both the RESP and nRESP groups which may be expected given the comparable ARAT scores between groups at baseline.
Table 2.
Comparison between the Responder (RESP) and non-Responder (nRESP) groups.
| RESP (n = 13) | nRESP (n = 17) | p-value* | |
|---|---|---|---|
| Age (yrs) | 57.92 ± 10.14 | 61.35 ± 10.59 | 0.376 |
| Sex | 8 M, 5F | 9 M, 7F, 1NA | 1.000 |
| Race | 8 Afr. American 5 Caucasian |
7 Afr. American 9 Caucasian 1NA |
0.462 |
| Dom vs. Non-Dom | 11 Dom, 2 NDom | 10 Dom, 7 NDom | 0.229 |
| Education | 5 HS 0 VS/TS 6 < College 2NA |
7 HS 1 VS/TS 6 < College 3NA |
1.000 |
| Mesulam | 3.86 ± 7.78 | 2.76 ± 3.42 | 0.646 |
| Baseline ARAT | 35.31 ± 8.07 | 29.71 ± 10.53 | 0.110 |
| ΔARAT | 10.15 ± 4.08 | 2.20 ± 2.54 | <0.001 |
| Lesion Volume | 18904.58 ± 27773.48 | 11106.86 ± 20416.37 | 0.209 |
*From comparisons between groups using Chi square tests for categorical data and t-tests for continuous data.
Legend: M = Male, F = Female, Afr. American = African American, Dom = Dominant, Non-Dom: Non-Dominant, IM = Individualized Maximum, HS = High School, VS/TS = Vocational School / Technical School. R = Right, L = Left, RESP: Responder, nRESP: Non-Responders. N = 30.
Fig. 5.
Contrast and overlap images of binarized lesion location for Responders (RESP) and non-Responders (nRESP) thresholded at ≥ 1. RESP had a more rostral/cortical lesions and nRESP had a more caudal white matter and subcortical lesions affecting thalamus, basal ganglia, and internal capsule. Images are in Neurological convention, N = 26.
Table 3.
Descriptive table with mean percentage of streamlines interrupted by lesioned voxels. Tracts count are in parenthesis. RESP: Responder (N = 12) and nRESP: non-Responder (N = 14). Total number of subjects is 26 because 4 subjects had non-identifiable lesion in Table 1.
| Lesioned Streamlines | Group | Projection | Transcallosal | Association | Brainstem | Cerebellum | CST, IL |
|---|---|---|---|---|---|---|---|
| 0% | RESP | 0.00 (2 2 9) | 0.00 (70) | 0.00 (2 4 5) | 0.00 (1 4 7) | 0.00 (69) | 0.00 (7) |
| nRESP | 0.00 (2 5 7) | 0.00 (76) | 0.00 (2 8 6) | 0.00 (1 6 6) | 0.00 (93) | 0.00 (5) | |
| 1–25% | RESP | 9.24 (32) | 10.75 (16) | 5.76 (21) | 9.95 (21) | 6.28 (18) | 0.00 |
| nRESP | 9.21 (38) | 7.47 (17) | 10.00 (18) | 9.20 (28) | 3.09 (11) | 9.67 (3) | |
| 26–50% | RESP | 49.52 (15) | 32.20 (5) | 37.34 (6) | 28.54 (11) | 46.00 (1) | 31.50 (2) |
| nRESP | 44.71 (21) | 38.86 (7) | 37.28 (7) | 29.92 (11) | 33.00 (1) | 41.75 (4) | |
| 51–75% | RESP | 58.80 (5) | 0.00 | 64.00 (6) | 60.00 (1) | 65.50 (2) | 57.67 (3) |
| nRESP | 66.17 (6) | 69.67 (3) | 62.00 (6) | 68.00 (2) | 0.00 | 63.50 (2) | |
| 76–100% | RESP | 87.80 (5) | 0.00 | 87.75 (8) | 78.00 (1) | 80.00 (1) | 76.00 (1) |
| nRESP | 89.11 (9) | 94.00 (2) | 91.60 (3) | 0.00 | 0.00 | 80.00 (2) |
4. Discussion
Individuals with chronic stroke experienced gains in UL function after intensive TST. However, these motor gains were not accompanied by microstructural changes within major WM pathways. We observed a heterogeneous response to TST, which led to the post-hoc hypothesis that baseline WM microstructure predicts motor response following TST. TBSS analysis showed that larger UL gains were predicted by larger baseline WM integrity of fibers located predominantly within the CL hemisphere, but only in the subgroup of RESP. While it may seem that this relationship is an artifact necessarily resulting from focusing only on RESP, the null hypothesis was that baseline FA values would be randomly distributed with respect to response to therapy. Our results disprove this null hypothesis. Importantly, while TBSS and ROI analyses did not distinguish the RESP and nRESP groups at baseline, a qualitative analysis of the lesion distribution suggested that the category of damaged tracts may be predictive of response to training. More specifically, our data suggests that the nRESP group had more subcortical lesions and more severe damage to transcallosal tracts than the RESP group, which may have interfered with additional gains post-TST. Below we discuss the major implications of the findings from this study.
4.1. Increase in UL function post-TST
UL function measured with the ARAT increased significantly following 8 weeks of TST. The number of repetitions in this study represents a very large increase in dose compared to the number of repetitions documented during standard rehabilitation (Carey et al., 2002, Boyd and Winstein, 2006). However, the ARAT increase was still only on the order of the MCID, suggesting that if a dose response curve does exist, even 10,000 repetitions may lie within the flattened lower range of a likely sigmoid-shaped dose–response curve. This amount of response to TST is similar to previous reports on UL interventions provided at the chronic stage (Bundy et al., 2017), but smaller than more recent studies that tested very high doses of training (Daly et al., 2019, McCabe et al., 2015). Alternatively, lack of dose–response could reflect a ceiling effect, however more recent studies that tested very high doses of training in chronic stroke (Daly et al., 2019, McCabe et al., 2015) suggest that larger behavioral improvements are possible with larger doses of therapy.
4.2. Mechanisms of improved UL function in response to TST
We investigated whether UL motor response to TST was associated with underlying plasticity of the WM microstructure. Structural changes in WM have been previously shown after training (Draganski and May, 2008). Training-induced plasticity associated with arm function in individuals with stroke may involve the use of alternate motor, transcallosal, or even non-motor fibers (Choudhury et al., 2019, Baker et al., 2015). In subacute stroke, larger FA in CST, alternate motor fibers (such as cortico-rubro-spinal and cortico-reticulo-spinal tract) and transcallosal fibers were positively correlated with larger treatment response (Song et al., 2015, Young et al., 2016). By contrast, in chronic stroke, the current literature lacks evidence for structural plasticity following motor training (Borich et al., 2014, Rickards et al., 2014, Sterr et al., 2014). For instance, two studies showed gains in the use of the UL following constraint-induced movement therapy that were not associated with changes in CST integrity (Rickards et al., 2014, Sterr et al., 2014). Similarly, we failed to show an association between UL response to TST and microstructural WM changes. However, improvements following intensive speech therapy in chronic stroke have been associated with training-induced WM plasticity (Schlaug et al., 2009, Allendorfer et al., 2012, Wan et al., 2014). In Wan et al. (2014), FA decrease in right-hemisphere regions was associated with improvement in speech production. Earlier report showed correlations of speech production with larger fiber number and volume in the left arcuate fasciculus (Schlaug et al., 2009). Thus, conclusions from the positive results following training of other systems even at the chronic stage should not be extrapolated to the motor system. Although brain structure measured with DTI is claimed to be a stronger predictor of chronic recovery than inter-hemispheric functional connectivity (Lin et al., 2018, Qiu et al., 2011), the absence of WM change post-training suggests that the major mechanism targeted by TST and underlying observed motor gains is non-structural as detected with MRI. This should be investigated further.
4.3. Prediction of response to TST
The finding that most voxels predictive of the size of response to TST lie CL to the damaged hemisphere for the RESP group was not expected. However, this observation is not without precedent as the CL hemisphere has long been hypothesized to be involved in stroke recovery (Simis et al., 2015, Dodd et al., 2017, Buetefisch, 2015, Chen et al., 2019). For instance, CL PLIC FA was significantly correlated with multiple dimensions of motor recovery (e.g. level of physical impairment, grip strength, and hand dexterity) in chronic stroke (Borich et al., 2012). Progressive activation of the CL hemisphere has been recently associated with the expression of atypical flexor synergies (McPherson et al., 2018) and the use of use alternate motor pathways to support UL function (Owen et al., 2017). There has been a heated debate in the field on whether the CL hemisphere is associated with positive or negative response to training, and for which subgroups of stroke patients (Li et al., 2019, Dodd et al., 2017, Buetefisch, 2015). Our results agree with the notion that the CL hemisphere supports UL recovery for a subset of chronic stroke patients.
In addition to the TBSS evidence implicating CL hemispheric and transcallosal pathways, our ROI-based analysis supports an involvement of transcallosal fibers in the RESP group. Reduced integrity of transcallosal fibers has been frequently associated with poor arm recovery (Wang et al., 2012, Stewart et al., 2017, Hayward et al., 2017, Jang et al., 2009, Li et al., 2015). However, many fewer studies have examined the value of transcallosal pathways in predicting the motor response to training (Lindenberg et al., 2012). Lindenberg et al. (2012) showed that integrity of transcallosal fibers are better predictors of UL gains following training than IL CST (Lindenberg et al., 2012). Although our ROI analysis failed to show that transcallosal ROIs were predictive of the response to therapy, we observed the development of a significant correlation between FA and ARAT in 3 of 5 segments of the corpus callosum post treatment which was not present prior to training. This observation supports the interpretation that interhemispheric communication is important for UL motor function in TST. ROIs CC-I, CC-II, and CC-IV contain fibers from prefrontal, premotor and supplementary motor areas, and primary sensory pathways respectively suggesting a possible role for executive function, motor planning, and sensory based-feedback in the response to TST.
The result of a predictive role for non-motor CL hemispheric tracts (Fig. 2 and Supplementary Fig. 2) raises the possibility that other WM pathways are facilitating TST-related improvement. More specifically, in addition to the transcallosal fibers, several association fibers classically related to language function were predictive of UL motor gain such as superior longitudinal fasciculus and arcuate fasciculus, the uncinate fasciculus, extreme capsule, middle longitudinal fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus (Dick and Tremblay, 2012). While this finding was unexpected, activity of higher-order visuo-motor control is suggested to be independent of that of the primary motor cortex (Gregory Króliczak et al., 2016). Moreover, homotopic recruitment is a well-known mechanism of recovery post-stroke. It is possible that homotopic language pathways also support praxis, given the strong correlation between these two fundamental cognitive abilities that may share common cerebral specialization (Grzegorz Króliczak et al., 2020). Importantly, we also reported streamlines with predictive voxels in WM tracts connecting pons and striatum to frontal, parietal, temporal, and occipital cortex. The full repertoire of UL behavior depends on cortico-subcortical circuits that consist not only of projection fibers from primary motor cortex, but also tracts supporting higher-order cognition associated with feedforward and feedback-based control (Xu et al., 2015). Integration of higher-order pathways and subcortical regions from the intact hemisphere may facilitate motor learning strategies targeted by TST rehabilitation. The predictive power of baseline DTI for magnitude of motor gain post-TST in the RESP group may seem incongruent with its inability to distinguish the RESP from the nRESP group. This discrepancy might be explained by the level of engagement of CL pathways in these two groups. It may be only under conditions involving CL recruitment that CL WM integrity comes into play.
4.4. Lesion anatomy and response to TST
Both the RESP and the nRESP groups were well matched in terms of demographic characteristics including their baseline ARAT score, age, and sociodemographics. Therefore, information acquired from brain imaging might be invoked to explain their failure to respond to TST. A qualitative evaluation of stroke anatomic lesion distribution in our sample shows a striking difference between the RESP group with more rostral/cortical lesions, and the nRESP group with more caudal WM and subcortical lesions affecting the thalamus, basal ganglia and internal capsule. In Table 3 and Supplementary Fig. 3 we showed that transcallosal fibers were more extensively lesioned in the nRESP group than in the RESP group.
A growing body of evidence suggests that patients with subcortical lesions have deficits that are more severe and longer lasting than do individuals with cortical lesions (Miyai et al., 1997, Shelton and Reding, 2001). For example, a neuroimaging study by Shelton and Reding (2001) demonstrated that recovery of isolated UL movements was more likely for individuals with purely cortical stroke (involving supplementary motor area, premotor cortex or primary motor cortex) compared with a subcortical or mixed cortical/subcortical group. Likewise, subcortical stroke participants of the ICARE clinical trial who received a higher dose of UL training achieved 2–3 points less recovery from impairment than controls (Edwardson et al., 2019). In contrast, those with cortical or mixed lesions experienced ~ 7 points more recovery than controls (Edwardson et al., 2019). Thickbroom et al. (2015) showed that larger UL impairment was associated with increased M1 CL excitability for patients with subcortical stroke, which was not observed for cortical stroke. In their study that did not include imaging, it was the relationship between behavior and electrophysiology rather than their baseline values that differentiated both groups. All together, these findings suggest that patients with subcortical WM lesions may be less responsive to task-based therapies alone. Patients with this type of lesion may require adjunct interventions such as non-invasive brain stimulation or pharmacological agents or may respond to completely different interventions such as training with a brain-computer interface driven by signals from the CL hemisphere (Bundy et al., 2017). It is also likely that some individuals with damage to critical pathways will not experience gains in UL function with any therapy. Whether resistance to training is due to damage to their WM as opposed to the effects of damage to thalamus and basal ganglia deserves further investigation.
4.5. Study limitations
Due to the nature of stroke, it is possible that some of the variability observed in baseline WM integrity may reflect diffuse changes due to vascular compromise (Hamanaka et al., 2018a, Hamanaka et al., 2018b). In four individuals no lesion could be identified. In the chronic stage of stroke, it can be difficult to distinguish what were initially small lacunar strokes from the non-specific changes typical of small vessel disease that are of unclear behavioral significance. In addition, the hematoma of a hemorrhagic stroke, while obvious at the acute stage, is resorbed in time sometimes leaving behind only a fraction of the volume of the initial lesion which can blend in with the other changes seen in small vessel disease. Residual hemosiderin is not identifiable in T1-weighted or standard T2 MRI scans, and a T2*-weighted sequence used to depict hemosiderin was not acquired in this study. It is unlikely that the presence of these “lesion-negative” subjects would have influenced the interpretation of our results as lesioned voxels were not excluded from WM analysis and as the predictive value of baseline FA was restricted to the non-lesioned hemisphere and transcallosal tracts. It is possible that our failure to observe training-dependent structural changes in WM integrity relates to factors affecting the signal to noise ratio such as small sample size, stroke heterogeneity within our sample, and type of analysis. Our sample size was small with heterogeneous characteristics (cortical and subcortical lesions, variable lesion sizes, participants with previous history of stroke) and likely contributed significantly to inter-individual variability in response to TST. The small sample size also limited exploring statistical tests with robust models including multiple ROIs of interest and their interactions. Also, we did not use an impairment level measure such as the UL Fugl-Meyer or arm kinematics and indices of motor coordination which may have been better correlated with our structural measures of WM integrity. Outcome measures of UL motor function with a higher level of granularity may be used to distinguish motor from non-motor pathways supporting UL function. This may be necessary to further investigate pathways specifically associated with atypical synergies or dexterity. Finally, delivering an even larger dose of training (Supplementary Fig. 1), possibly by using a protocol similar to Daly et al. (2019), might have facilitated the detection of neural correlates of improvement by achieving an even greater effect size on behavior and increasing the number of subjects in the RESP group.
5. Conclusion
In this study of the neural correlates of motor improvement after TST of the UL in chronic stroke, we found no evidence of WM plasticity. We suggest that neural correlates of larger doses of treatment should be investigated. There was significant heterogeneity in the response to TST allowing for the characterization of the RESP and nRESP groups. In the RESP group, larger motor gains following TST were associated with larger baseline WM integrity within CL hemisphere motor and non-motor pathways and transcallosal fibers. In addition, transcallosal tracts were more frequently lesioned in the nRESP group. These findings suggest that the response to TST in the RESP group may reflect the engagement of interhemispheric processes to which the nRESP group does not have access. Future studies should include CL and transcallosal FA as biomarkers to better characterize spontaneous recovery and response to training in cortical and subcortical stroke. This knowledge may help to identify sources of heterogeneity post-stroke, which is a necessary condition for the development of customized rehabilitation interventions.
CRediT authorship contribution statement
Daniela J.S. Mattos: Conceptualization, Formal analysis, Writing - original draft, Visualization. Jerrel Rutlin: Software, Data curation. Xin Hong: Investigation. Joshua S. Shimony: Investigation. Alexandre R. Carter: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by R01 HD068290 and the Department of Neurology at Washington University School of Medicine. The authors thank Dr. Catherine Lang, PT, PhD, Professor of Physical Therapy, Neurology, and Occupational Therapy at Washington University School of Medicine and her team for conducting the training and collecting all the behavioral data. JSS was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102710.
Contributor Information
Daniela J.S. Mattos, Email: juncked@wustl.edu.
Jerrel Rutlin, Email: rutlinj@wustl.edu.
Xin Hong, Email: xhong@wustl.edu.
Joshua S. Shimony, Email: shimonyj@wustl.edu.
Alexandre R. Carter, Email: alexandre.carter@wustl.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Allendorfer J.B., Storrs J.D.M., Szaflarski J.P. Changes in White Matter Integrity Follow Excitatory RTMS Treatment of Post-Stroke Aphasia. Restor. Neurol. Neurosci. 2012;30(2):103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.N., Zaaimi B., Fisher K.M., Edgley S.A., Soteropoulos D.S. Pathways mediating functional recovery. Prog. Brain Res. 2015;218(April):389–412. doi: 10.1016/bs.pbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O’Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10) doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Borich Michael R., Brown Katlyn E., Boyd Lara A. Motor Skill Learning Is Associated with Diffusion Characteristics of White Matter in Individuals with Chronic Stroke. Journal of Neurologic Physical Therapy. 2014 doi: 10.1097/NPT.0b013e3182a3d353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich M.R., Mang C., Boyd L.A. Both Projection and Commissural Pathways Are Disrupted in Individuals with Chronic Stroke: Investigating Microstructural White Matter Correlates of Motor Recovery. BMC Neuroscience. 2012;13(1):107. doi: 10.1186/1471-2202-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Lara A., Winstein Carolee J. Explicit Information Interferes with Implicit Motor Learning of Both Continuous and Discrete Movement Tasks after Stroke. Journal of Neurologic Physical Therapy. 2006 doi: 10.1097/01.NPT.0000282566.48050.9b. [DOI] [PubMed] [Google Scholar]
- Buetefisch C.M. Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front. Neurol. 2015;6(OCT):1–10. doi: 10.3389/fneur.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy David T., Souders Lauren, Baranyai Kelly, Leonard Laura, Schalk Gerwin, Coker Robert, Moran Daniel W., Huskey Thy, Leuthardt Eric C. Contralesional brain-computer interface control of a powered exoskeleton for motor recovery in chronic stroke survivors. Stroke. 2017;48(7):1908–1915. doi: 10.1161/STROKEAHA.116.016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey James R., Kimberley Teresa J., Lewis Scott M., Auerbach Edward J., Dorsey Lisa, Rundquist Peter, Ugurbil Kamil. Analysis of FMRI and Finger Tracking Training in Subjects with Chronic Stroke. Brain. 2002 doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Cassidy J.M., Tran G., Quinlan E.B., Cramer S.C. Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke. 2018;49(2):433–438. doi: 10.1161/STROKEAHA.117.018844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Yen Ting, Shengai Li, Craig Ditommaso, Ping Zhou, and Sheng Li. 2019. “Possible Contributions of Ipsilateral Pathways from the Contralesional Motor Cortex to the Voluntary Contraction of the Spastic Elbow Flexors in Stroke Survivors: A TMS Study.” In American Journal of Physical Medicine and Rehabilitation, 98:558–65. Lippincott Williams and Wilkins. https://doi.org/10.1097/PHM.0000000000001147. [DOI] [PMC free article] [PubMed]
- Choudhury S., Shobhana A., Singh R., Sen D., Anand S.S., Shubham S., Baker M.R., Kumar H., Baker S.N. The Relationship Between Enhanced Reticulospinal Outflow and Upper Limb Function in Chronic Stroke Patients. Neurorehabilitation and Neural Repair. 2019;33(5):375–383. doi: 10.1177/1545968319836233. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daly J.J., McCabe J.P., Holcomb J., Monkiewicz M., Gansen J., Pundik S. Long-Dose Intensive Therapy Is Necessary for Strong, Clinically Significant, Upper Limb Functional Gains and Retained Gains in Severe/Moderate Chronic Stroke. Neurorehabilitation and Neural Repair. 2019;33(7):523–537. doi: 10.1177/1545968319846120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick Anthony Steven, Tremblay Pascale. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012 doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Dodd K.C., Nair V.A., Prabhakaran V. Role of the Contralesional vs. Ipsilesional Hemisphere in Stroke Recovery. Front. Hum. Neurosci. 2017;11(September):1–9. doi: 10.3389/fnhum.2017.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., May A. Training-Induced Structural Changes in the Adult Human Brain. Behav. Brain Res. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Dromerick A.W., Reding M.J. Functional Outcome for Hemiparesis Hemihypesthesia and Hemianopsia, Does Lesion Location Matter. Pdf. Stroke. 1995;26(11):2023–2026. doi: 10.1161/01.str.26.11.2023. [DOI] [PubMed] [Google Scholar]
- Edwardson M.A., Ding L.i., Park C., Lane C.J., Nelsen M.A., Wolf S.L., Winstein C.J., Dromerick A.W. Reduced Upper Limb Recovery in Subcortical Stroke Patients with Small Prior Radiographic Stroke. Front. Neurol. 2019;10(MAY):1–10. doi: 10.3389/fneur.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R.J., Cronin T., Lin J., Wang X., Sakaie K., Ontaneda D., Mahmoud S.Y., Lowe M.J., Phillips M.D. Measuring myelin repair and axonal loss with diffusion tensor imaging. Am. J. Neuroradiol. 2011;32(1):85–91. doi: 10.3174/ajnr.A2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka G., Ohtomo R., Takase H., Lok J., Arai K. Role of oligodendrocyte-neurovascular unit in white matter repair. Neurosci. Lett. 2018;684(June):175–180. doi: 10.1016/j.neulet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Hamanaka G., Ohtomo R., Takase H., Lok J., Arai K. White-Matter Repair: Interaction between Oligodendrocytes and the Neurovascular Unit. Brain Circulation. 2018;4(3):118. doi: 10.4103/bc.bc_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward K.S., Neva J.L., Mang C.S., Peters S., Wadden K.P., Ferris J.K., Boyd L.A. Interhemispheric Pathways Are Important for Motor Outcome in Individuals with Chronic and Severe Upper Limb Impairment Post Stroke. Neural Plasticity. 2017;2017:1–12. doi: 10.1155/2017/4281532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S., Frahm J. Topography of the Human Corpus Callosum Revisited-Comprehensive Fiber Tractography Using Diffusion Tensor Magnetic Resonance Imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hsieh C.L., Hsueh P., Chiang F.M., Lin P.H. Inter-rater reliability and validity of theaction research arm test in strokepatients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- Jang S.H., Park K.A., Ahn S.H., Cho Y.W., Byun W.M., Son S.M., Choi J.H., Kwon Y.H. Transcallosal Fibers from Corticospinal Tract in Patients with Cerebral Infarct. NeuroRehabilitation. 2009;24(2):159–164. doi: 10.3233/NRE-2009-0464. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi R.V., Feigin V.L., Forouzanfar M.H., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L.M., Truelsen T., O'Donnell M., Venketasubramanian N., Barker-Collo S., Lawes C.M.M., Wang W., Shinohara Y., Witt E., Ezzati M., Naghavi M., Murray C. Global and Regional Burden of First-Ever Ischaemic and Haemorrhagic Stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. The Lancet Global Health. 2013;1(5):e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Króliczak G., Piper B.J., Frey S.H. Specialization of the Left Supramarginal Gyrus for Hand-Independent Praxis Representation Is Not Related to Hand Dominance. Neuropsychologia. 2016;93:501–512. doi: 10.1016/j.neuropsychologia.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Króliczak Grzegorz, Piper Brian J., Potok Weronika, Buchwald Mikołaj, Kleka Paweł, Przybylski Łukasz, Styrkowiec Piotr P. Praxis and language organization in left-handers. Acta Neuropsychologica. 2020 doi: 10.5604/01.3001.0013.9738. [DOI] [Google Scholar]
- Lang C.E., Strube M.J., Bland M.D., Waddell K.J., Cherry‐Allen K.M., Nudo R.J., Dromerick A.W., Birkenmeier R.L. Dose Response of Task-Specific Upper Limb Training in People at Least 6 Months Poststroke: A Phase II, Single-Blind, Randomized, Controlled Trial. Ann. Neurol. 2016;80(3):342–354. doi: 10.1002/ana.v80.310.1002/ana.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen Y.T., Francisco G.E., Zhou P., Rymer W.Z. A Unifying Pathophysiological Account for Post-Stroke Spasticity and Disordered Motor Control. Front. Neurol. 2019;10(MAY):1–8. doi: 10.3389/fneur.2019.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wu P., Liang F., Huang W., Yao D. The Microstructural Status of the Corpus Callosum Is Associated with the Degree of Motor Function and Neurological Deficit in Stroke Patients. PLoS ONE. 2015;10(4):e0122615. doi: 10.1371/journal.pone.0122615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.Y., Ramsey L., Metcalf N.V., Rengachary J., Shulman G.L., Shimony J.S., Corbetta M., Boltze J. Stronger Prediction of Motor Recovery and Outcome Post-Stroke by Cortico-Spinal Tract Integrity than Functional Connectivity. PLoS ONE. 2018;13(8):e0202504. doi: 10.1371/journal.pone.0202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R., Zhu L.L., Rüber T., Schlaug G. Predicting Functional Motor Potential in Chronic Stroke Patients Using Diffusion Tensor Imaging. Hum. Brain Mapp. 2012;33(5):1040–1051. doi: 10.1002/hbm.v33.510.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle R.C. A Performance Test for Assessment of Upper Limb Function in Physical Rehabilitation Treatment and Research. Int. J. Rehabil. Res. 1981;4(4):483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- McCabe Jessica, Monkiewicz Michelle, Holcomb John, Pundik Svetlana, Daly Janis J. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015;96(6):981–990. doi: 10.1016/j.apmr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- McPherson J.G., Chen A., Ellis M.D., Yao J., Heckman C.J., Dewald J.P.A. Progressive Recruitment of Contralesional Cortico-Reticulospinal Pathways Drives Motor Impairment Post Stroke. J. Physiol. 2018;596(7):1211–1225. doi: 10.1113/tjp.2018.596.issue-710.1113/JP274968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai Ichiro, Blau Alan D., Reding Michael J., Volpe Bruce T. Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts. Neurology. 1997;48(1):95–101. doi: 10.1212/WNL.48.1.95. [DOI] [PubMed] [Google Scholar]
- O’Donnell L.J., Westin C.-F. An Introduction to Diffusion Tensor Image Analysis. Neurosurg. Clin. N. Am. 2011;22(2):185–196. doi: 10.1016/j.nec.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, Meriel, Carson Ingo, and Julius P.A. Dewald. 2017. “Upper Extremity Motor Impairments and Microstructural Changes in Bulbospinal Pathways in Chronic Hemiparetic Stroke.” Frontiers in Neurology 8 (JUN). https://doi.org/10.3389/fneur.2017.00257. [DOI] [PMC free article] [PubMed]
- Plow E.B., Sankarasubramanian V., Cunningham D.A., Potter-Baker K., Varnerin N., Cohen L.G., Sterr A., Conforto A.B., Machado A.G. Models to Tailor Brain Stimulation Therapies in Stroke. Neural Plasticity. 2016;2016:1–17. doi: 10.1155/2016/4071620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Darling W.G., Morecraft R.J., Ni C.C., Rajendra J., Butler A.J. White Matter Integrity Is a Stronger Predictor of Motor Function than BOLD Response in Patients with Stroke. Neurorehabilitation and Neural Repair. 2011;25(3):275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J., d'Avossa G., Sapir A., Shulman G.L., Corbetta M. Is the Posner Reaction Time Test More Accurate Than Clinical Tests in Detecting Left Neglect in Acute and Chronic Stroke? Arch. Phys. Med. Rehabil. 2009;90(12):2081–2088. doi: 10.1016/j.apmr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards T., Sterling C., Taub E., Perkins-Hu C., Gauthier L., Graham M., Griffin A., Davis D., Mark V.W., Uswatte G. Diffusion Tensor Imaging Study of the Response to Constraint-Induced Movement Therapy of Children with Hemiparetic Cerebral Palsy and Adults with Chronic Stroke. Arch. Phys. Med. Rehabil. 2014;95(3):506–514.e1. doi: 10.1016/j.apmr.2013.08.245. [DOI] [PubMed] [Google Scholar]
- Riley J.D., Le V.u., Der-Yeghiaian L., See J., Newton J.M., Ward N.S., Cramer S.C. Anatomy of Stroke Injury Predicts Gains from Therapy. Stroke. 2011;42(2):421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R.A., Hanson D.P. Software System for Interactive and Quantitative Visualization of Multidimensional Biomedical Images. Australas. Phys. Eng. Sci. Med. 1991 [PubMed] [Google Scholar]
- Schaechter J.D., Fricker Z.P., Perdue K.L., Helmer K.G., Vangel M.G., Greve D.N., Makris N. Microstructural Status of Ipsilesional and Contralesional Corticospinal Tract Correlates with Motor Skill in Chronic Stroke Patients. Hum. Brain Mapp. 2009;30(11):3461–3474. doi: 10.1002/hbm.v30:1110.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G., Marchina S., Norton A. Evidence for Plasticity in White-Matter Tracts of Patients with Chronic Broca’s Aphasia Undergoing Intense Intonation-Based Speech Therapy. Ann. N. Y. Acad. Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton Fátima De N.A.P., Reding Michael J. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32(1):107–112. doi: 10.1161/01.STR.32.1.107. [DOI] [PubMed] [Google Scholar]
- Simis M., Doruk D., Imamura M., Anghinah R., Morales-Quezada L., Fregni F., Battistella L.R. Neurophysiologic Predictors of Motor Function in Stroke. Restor. Neurol. Neurosci. 2015;34(1):45–54. doi: 10.3233/RNN-150550. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-Based Spatial Statistics: Voxelwise Analysis of Multi-Subject Diffusion Data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song J., Nair V.A., Young B.M., Walton L.M., Nigogosyan Z., Remsik A., Tyler M.E., Farrar-Edwards D., Caldera K.E., Sattin J.A., Williams J.C., Prabhakaran V. DTI Measures Track and Predict Motor Function Outcomes in Stroke Rehabilitation Utilizing BCI Technology. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ju W.-K., Lin S.-J., Cross A.H., Neufeld A.H. Diffusion Tensor Imaging Detects and Differentiates Axon and Myelin Degeneration in Mouse Optic Nerve after Retinal Ischemia. NeuroImage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Sterr A., Dean P.J.A., Szameitat A.J., Conforto A.B., Shen S. Corticospinal Tract Integrity and Lesion Volume Play Different Roles in Chronic Hemiparesis and Its Improvement through Motor Practice. Neurorehabilitation and Neural Repair. 2014;28(4):335–343. doi: 10.1177/1545968313510972. [DOI] [PubMed] [Google Scholar]
- Stewart, Jill Campbell, Pritha Dewanjee, George Tran, Erin Burke Quinlan, Lucy Dodakian, Alison McKenzie, Jill See, and Steven C. Cramer. 2017. “Role of Corpus Callosum Integrity in Arm Function Differs Based on Motor Severity after Stroke.” NeuroImage: Clinical 14: 641–47. https://doi.org/10.1016/j.nicl.2017.02.023. [DOI] [PMC free article] [PubMed]
- Stinear C.M., Alan Barber P., Smale P.R., Coxon J.P., Fleming M.K., Byblow W.D. Functional Potential in Chronic Stroke Patients Depends on Corticospinal Tract Integrity. Brain. 2007;130(1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Takeuchi, Naoyuki, and Shin-ichi Izumi. 2013. “Rehabilitation with Poststroke Motor Recovery.Pdf” 2013. [DOI] [PMC free article] [PubMed]
- Thickbroom Gary W., Cortes Mar, Rykman Avrielle, Volpe Bruce T., Felipe Fregni H., Krebs Igo, Pascual-Leone Alvaro, Edwards Dylan J. Stroke subtype and motor impairment influence contralesional excitability. Neurology. 2015;85(6):517–520. doi: 10.1212/WNL.0000000000001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet P., Carey L., Nilsson M. Targeting Stroke Treatment to the Individual. International Journal of Stroke. 2012;7(6):480–481. doi: 10.1111/j.1747-4949.2012.00867.x. [DOI] [PubMed] [Google Scholar]
- Wadden, K. P., S. Peters, M. R. Borich, J. L. Neva, K. S. Hayward, C. S. Mang, N. J. Snow, et al. 2019. “White Matter Biomarkers Associated with Motor Change in Individuals with Stroke: A Continuous Theta Burst Stimulation Study.” Neural Plasticity 2019 (di). https://doi.org/10.1155/2019/7092496. [DOI] [PMC free article] [PubMed]
- Wan C.Y., Zheng X., Marchina S., Norton A., Schlaug G. Intensive Therapy Induces Contralateral White Matter Changes in Chronic Stroke Patients with Broca’s Aphasia. Brain Lang. 2014;136(July):1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.E., Tittgemeyer M., Imperati D., Diekhoff S., Ameli M., Fink G.R., Grefkes C. Degeneration of Corpus Callosum and Recovery of Motor Function after Stroke: A Multimodal Magnetic Resonance Imaging Study. Hum. Brain Mapp. 2012;33(12):2941–2956. doi: 10.1002/hbm.v33.1210.1002/hbm.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Jing, Adrian M. Haith, and John W. Krakauer. 2015. “Motor Control of the Hand before and after Stroke.” In Clinical Systems Neuroscience. https://doi.org/10.1007/978-4-431-55037-2_14.
- Yeh F.C., Panesar S., Fernandes D., Meola A., Yoshino M., Fernandez-Miranda J.C., Vettel J.M., Verstynen T. Population-Averaged Atlas of the Macroscale Human Structural Connectome and Its Network Topology. NeuroImage. 2018;178:57–68. doi: 10.1016/j.neuroimage.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F.-C., Verstynen T.D., Wang Y., Fernández-Miranda J.C., Tseng W.-Y., Zhan W. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE. 2013;8(11):e80713. doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.M., Stamm J.M., Song J., Remsik A.B., Nair V.A., Tyler M.E., Edwards D.F., Caldera K., Sattin J.A., Williams J.C., Prabhakaran V. Brain-Computer Interface Training after Stroke Affects Patterns of Brain-Behavior Relationships in Corticospinal Motor Fibers. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozbatiran N., Der-Yeghiaian L., Cramer S.C. A Standardized Approach to Performing the Action Research Arm Test. Neurorehabilitation and Neural Repair. 2008;22(1):78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.