Abstract
Purpose
Breast cancer outcomes in sub-Saharan Africa is reported to be poor, with an estimated five-year survival of 50% when compared to almost 90% in high-income countries. Although several studies have looked at the effect of HIV in breast cancer survival, the effect of ARTs has not been well elucidated.
Methods
All females newly diagnosed with invasive breast cancer from May 2015–September 2017 at Charlotte Maxeke Johannesburg Academic and Chris Hani Baragwanath Academic Hospital were enrolled. We analysed overall survival and disease-free survival, comparing HIV positive and negative patients. Kaplan-Meier survival curves were generated with p-values calculated using a log-rank test of equality while hazard ratios and their 95% confidence intervals (CIs) were estimated using Cox regression models.
Results
Of 1019 patients enrolled, 22% were HIV positive. The overall survival (95% CI) was 53.5% (50.1–56.7%) with a disease-free survival of 55.8% (52.1–59.3) after 4 years of follow up. HIV infection was associated with worse overall survival (HR (95% CI): 1.50 (1.22–1.85), p < 0.001) and disease-free survival (OR (95% CI):2.63 (1.71–4.03), p < 0.001), especially among those not on ART at the time of breast cancer diagnosis. Advanced stage of the disease and hormone-receptor negative breast cancer subtypes were also associated with poor survival.
Conclusion
HIV infection was associated with worse overall and disease-free survival. HIV patients on ARTs had favourable overall and disease-free survival and with ARTs now being made accessible to all the outcome of women with HIV and breast cancer is expected to improve.
Keywords: SURVIVAL, BREAST CANCER, HIV, ART
Highlights
-
•
22% of participants were HIV positive.
-
•
The 4 years overall survival was 53.5%.
-
•
The 4 years disease-free survival was 55.8%.
-
•
HIV infection was associated with poor overall and disease-free survival.
-
•
ART use was associated with better overall and disease-free survival.
1. Introduction
Breast cancer is the commonest cancer and cause of cancer-related mortality among women globally [1]. In South Africa, it remains the commonest malignancy among women, accounting for approximately 22% of all malignancies [2]. The lifetime risk (LR) for developing breast cancer in South Africa is estimated to be 1 in 25 women, however, this varies with self-reported race, which is lower than what is reported in the USA (1 in 8 women) [2,3].
In 2018, about 38 million people were reported to be living with HIV/AIDS (PLWHIV) worldwide with 68% of them receiving anti-retroviral therapy (ART) [4]. In South Africa, 7.52 million PLWHIV were reported with 62% of them being on ARTs in 2018 [4,5]. Due to the implementation of the universal “test and treat” strategy for all PLWHIV in 2016, the number of patients on ART is expected to rise [6]. So far, the success of the ART program is evidenced by a significant decrease in AIDS-related mortality from 37.2% reported in 2002 to 22.06% reported in 2018 [5]. The ART regime previously used included, tenofivir disoprostil fumarate & emtricitabine/lamivudine and efavirenz, but has recently been changed to a new regime: tenofovir, lamivudine and dolutegravir [7,8].
Breast cancer survival rates vary globally with high income countries (HICs) having better survivals than low-middle income countries (LMICs). In the United States, the reported 5-year overall survival is 89.9%, compared to 52.3% reported in the sub-Saharan Africa [3,9]. Some of the contributing factors to the poor overall survival in sub-Saharan Africa include young age at breast cancer diagnosis, advanced clinical stage at presentation, co-morbid disease, black race and aggressive pathological characteristics of breast cancer [[9], [10], [11], [12], [13]].
Multiple studies were conducted looking at the effect of HIV on breast cancer survival. In a study done in Soweto, South Africa, HIV did not affect the overall survival of breast cancer after 2 years although 42% of patients were lost to follow up [14]. In Botswana, the 2 years overall survival was very low among HIV positive patients than the HIV negative ones (57% vs 73%, P < 0.001) [15]. Similarly, in the USA, a high cancer-specific mortality among HIV positive patients with breast cancer [HR 2.64; 96% CI 1.86–3.73] was reported [16]. A recently published meta-analysis also demonstrated that HIV positive patients had the worst overall survival when compared to HIV negative patients [17]. But none of the published studies specifically looked at the effect of ART use on the survival of breast cancer in HIV positive patients. This study, therefore, aims to describe the effect of HIV infection, duration of HIV positivity, ART use, as well as the duration of ART use on the outcomes of breast cancer in South Africa.
2. Methodology
This descriptive study is a sub-study of the South African Breast Cancer and HIV Outcome (SABCHO) study [18], which was conducted at two public hospitals, Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) Surgical Breast Unit and the Batho Pele Breast Unit of the Chris Hani Baragwanath Academic Hospital (CHBAH). Both these surgical breast units have an estimated HIV prevalence of 22% and diagnose between 250 and 350 new patients with breast cancer every year [18,19]. The study participants were all consenting women newly diagnosed with breast cancer from 15 May 2015 through 31 September 2017.
An electronic breast cancer database was used to extract the following data: demographics, clinical stage at presentation, co-morbidities, level of education and the status of the patient when last seen (alive with no evidence of disease, alive with stable disease, alive with disease progression or dead). Histopathological characteristics (histological type, tumor grade, estrogen (ER) and progesterone receptor (PR) statuses, human epidermal growth factor receptor 2 (HER-2) and Ki67 expression) was obtained from the National Health Laboratory Service (NHLS). An Allred score was used to determine the positivity of both oestrogen and progesterone receptors, with the score of more than 3 regarded as positive [20]. HER2 was regarded positive when the test showed 3+, with the use of fluorescence in situ hybridization (FISH) reserved for tests with equivocal results(HER2 2+) [20]. Receptor subtypes were defined as: Luminal A (ER +, PR +, HER2 -, Ki 67 < 14%), Luminal B (ER +, PR +/−, HER2 -, Ki 67 > 14% or ER +, PR +/−, HER-2 +, any Ki 67), HER2-enriched (ER/PR -, HER2 +, any Ki 67) and Triple negative (ER/PR/HER2 -, any Ki 67) [21]. The American Joint Committee on Cancer (AJCC) system, 7th edition was used for clinical TNM staging at diagnosis [22].
Patients with unknown HIV status were counselled and an informed consent for testing was obtained. Post-test counselling was offered to all those newly diagnosed with HIV and blood samples for CD 4 counts and HIV viral load testing were drawn. We recorded the year of HIV diagnosis and when ARTs were initiated, with a cut-off period of one year used to compare those who were on ARTs for a longer period with those who have recently started ARTs (less than a year). Those newly diagnosed with HIV and not on ART were referred to the ART clinic for commencement of ARTs (tenofivir disoprostil fumarate& emtricitabine/lamivudine and efavirenz. For HIV positive patients, CD 4 counts and HIV viral loads were obtained from the NHLS. The viral load below detectable limit was defined as a viral load ≤50 copies/ml.
Following the completion of prescribed breast cancer treatment, patients were followed up at three-to six-month intervals. If follow-up was missed, patients were called by the research navigators. Completion of chemotherapy and radiation treatment was defined as patients who received the full prescribed number of doses. The first line chemotherapy regime included: Adriamycin (Doxorubicin), Cyclophosphamide and Taxanes (AC-T). At the time of the study, Trastuzumab was not yet available at these two study sites. Overall survival was defined as survival from the date of breast cancer diagnosis to the date of death for any cause while disease free survival was defined as survival from the date of breast cancer diagnosis to the date of disease recurrence or death for any cause. Patients with disease recurrence included all patients with radiologically and histologically confirmed breast cancer recurrence. Patients with stage IV disease at presentation were excluded in the analysis of disease free survival. The site of disease recurrence was sub-classified into loco-regional (ipsilateral chest wall, ipsilateral breast following breast conserving surgery and/or regional lymph nodes) and distant metastases (visceral and non-visceral sites). The time to and site of disease recurrence were documented. For patients who died, the date of death, as documented in the medical record or as provided by the next-of-kin, was also documented.
3. Statistical analysis
Demographic and clinico-pathological data were represented as medians and interquartile ranges for continuous data or as frequencies and percentages for categorical data. Categorical comparisons between HIV positive and HIV negative patients were performed using a χ [2] test; age and body mass index (BMI) were compared using a Mann-Whitney test. Patients were censored on the last date they were known to be alive with an administrative censoring date of 31 July 2020 for those still alive at the time of data extraction. Patients censored before the 31 July 2020 were lost to follow up if they could not follow up for more than 9 months and their status could not be ascertained via the ‘Verify ID’ portal. Kaplan-Meier survival curves were generated assuming non-informative losses to follow-up; p-values were calculated using a log-rank test of equality. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox regression models. Both unadjusted (crude) HRs and manually adjusted HRs (adjusted for age at diagnosis (continuous), BMI (continuous), stage at diagnosis (2 categories), receptor subtype (4 categories), employment status (2 categories) and education (3 categories)) were calculated. Logistic regression analyses, adjusting for age at diagnosis (2 categories), stage at diagnosis (2 categories) and receptor subtype (4 categories), was used to calculate odds ratios for disease recurrence or death. Data were analysed using STATA v14.2 and the stset suite of commands.
4. Results
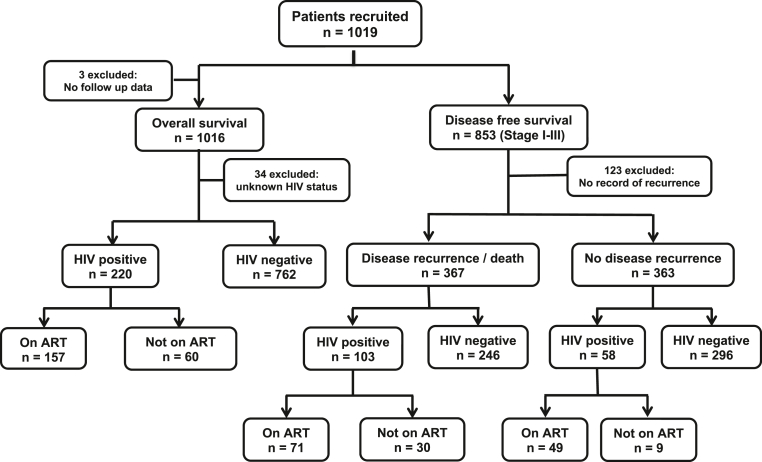
1019 women newly diagnosed with breast cancer were followed up with a median follow up time of 36.9 months (Interquartile range (IQR) 16.8–46.5 months). The HIV status was unknown in 3.33% of patients and 36 (3.5%) were lost to follow up, with a median time to loss to follow up of 16.5 months (IQR 8.3–28.9 months) (Fig. 1). Many of our patients (55.8%) presented in stage III & IV of the disease. Basic demographics, clinical and pathological characteristics of all patients are shown in Table 1.
Fig. 1.
Flowchart showing the total number on recruited patients, included and excluded from the analysis.
Table 1.
Demographics, clinical and pathological characteristics of 1019 HIV positive and negative women diagnosed with breast cancer at Chris Hani Baragwanath and Charlotte Maxeke Johannesburg Academic Hospitals between May 2015 and September 2017.
| Overall (n = 1019) | HIV-positive (n = 221) | HIV-negative (n = 764) | p-valuea | |
|---|---|---|---|---|
| Patient Demographics | ||||
| Age at diagnosis (median (IQR)) | 54 (44–64) | 44 (39–52) | 57 (46–67) | <0.001 |
| Self-Reported Ethnicity | ||||
| Black | 854 (84.0%) | 214 (97.3%) | 614 (80.5%) | |
| White | 85 (8.4%) | 1 (0.5%) | 80 (10.5%) | |
| Mixed Ancestry | 58 (5.7%) | 5 (2.3%) | 52 (6.8%) | |
| Asian | 20 (2.0%) | 0 (0%) | 17 (2.2%) | |
| BMI (median (IQR)) | 30.5 (25.5–35.8) | 27.3 (22.9–33.9) | 31.6 (26.7–36.2) | <0.001 |
| Co-morbiditiesb | ||||
| Yes | 484 (47.9%) | 78 (35.8%) | 387 (51.0%) | |
| No | 527 (52.1%) | 140 (64.2%) | 372 (49.0%) | <0.001 |
| Losses to follow-up | 36 (3.5%) | 7 (3.2%) | 28 (3.7%) | 0.725 |
| Educationc | ||||
| Primary or lower | 238 (23.8%) | 42 (19.3%) | 186 (24.8%) | |
| Secondary | 669 (66.8%) | 156 (71.6%) | 491 (65.6%) | |
| Tertiary | 94 (9.4%) | 20 (9.6%) | 72 (9.6%) | 0.206 |
| Employment | ||||
| Employed | 553 (55.0%) | 109 (49.8%) | 428 (56.9%) | |
| Unemployed | 452 (45.0%) | 110 (50.2%) | 324 (43.1%) | 0.061 |
| Tumor characteristics | ||||
| Clinical stage at diagnosis | ||||
| Stage 1 | 60 (6.1%) | 6 (2.7%) | 54 (7.1%) | |
| Stage 2 | 372 (38.1%) | 77 (35.0%) | 295 (39.0%) | |
| Stage 3 | 390 (39.9%) | 99 (45.0%) | 291 (38.4%) | |
| Stage 4 | 155 (15.9%) | 38 (17.3%) | 117 (15.5%) | 0.040 |
| Early Stage (1 and 2) | 447 (44.3%) | 83 (37.7%) | 349 (46.1%) | |
| Advanced Stage (3 and 4) | 563 (55.7%) | 137 (62.3%) | 408 (53.9%) | 0.028 |
| Tumour grade | ||||
| Grade 1 | 55 (5.7%) | 11 (5.3%) | 42 (5.8%) | |
| Grade 2 | 486 (50.6%) | 113 (54.1%) | 356 (49.4%) | |
| Grade 3 | 420 (43.7%) | 85 (40.7%) | 323 (44.8%) | 0.490 |
| Receptor subtyped | ||||
| Luminal A | 135 (14.1%) | 23 (11.1%) | 106 (14.8%) | |
| Luminal B | 615 (64.3%) | 135 (64.9%) | 460 (64.1%) | |
| Her2-enriched | 58 (6.1%) | 12 (5.8%) | 43 (6.0%) | |
| Triple negative | 149 (15.6%) | 38 (18.3%) | 109 (15.2%) | 0.459 |
| Treatments | ||||
| Completed Chemotherapy | 655 (90.9%) | 164 (92.1%) | 478 (90.9%) | 0.608 |
| Completed Radiation | 413 (99.8%) | 83 (100%) | 318 (99.7%) | 0.610 |
| HIV positive patients: | ||||
| Detectable viral loade | 89 (47.1%) | |||
| CD4 count (median (IQR)) | 477 (295–677) | |||
| Duration HIV positivity | ||||
| ≤1 year | 66 (29.9%) | |||
| >1 year | 155 (70.1%) | |||
| On ART | 158 (72.5%) | |||
| On ART at diagnosis | 131 (60.1%) | |||
| Duration ART usage | ||||
| ≤1 year | 30 (19.0%) | |||
| >1 year | 128 (81.0%) |
Note: 34 patients had unknown HIV status. Missing values as follows: ethnicity n = 2, BMI n = 78, co-morbidities n = 8, education n = 18, employment n = 14, stage n = 9, grade n = 58, receptor subtype n = 62, viral load n = 32, CD4 count n = 12, art n = 3, duration of ART n = 63. 298 participants did not receive chemotherapy and 605 participants did not receive radiation therapy.
P-values compare HIV-positive to HIV-negative patients using a chi-squared test except for age, BMI and follow-up time which use a Mann-Whitney test.
Co-morbidities included hypertension (77.7%), diabetes (18.8%), arthritis (17.6%), tuberculosis (11.6%), asthma/COPD (11.2%), heart disease (5.0%), stroke (3.9%).
Primary education refers to the first 7 years of school education and secondary as the first 12 years.
Receptor subtypes were defined by immunohistochemistry as follows: Luminal A (ER +, PR +, HER2 -, Ki 67 < 14%), Luminal B (ER +, PR +/−, HER2 -, Ki 67 > 14% or ER +, PR +/−, HER-2 +, any Ki 67), HER2-enriched (ER/PR -, HER2 +, any Ki 67) and Triple negative breast cancer (ER/PR/HER2 -, any Ki 67).
Viral loads were detectable when >50 copies/ml.
5. Overall survival
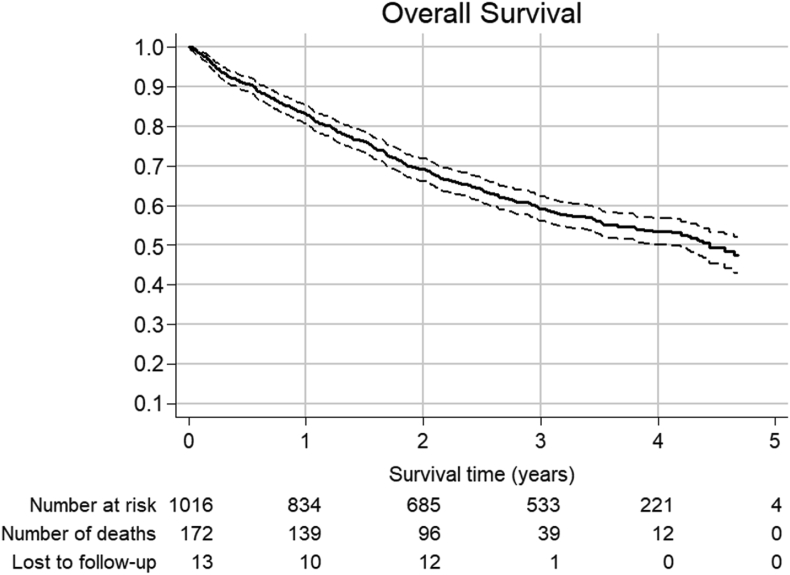
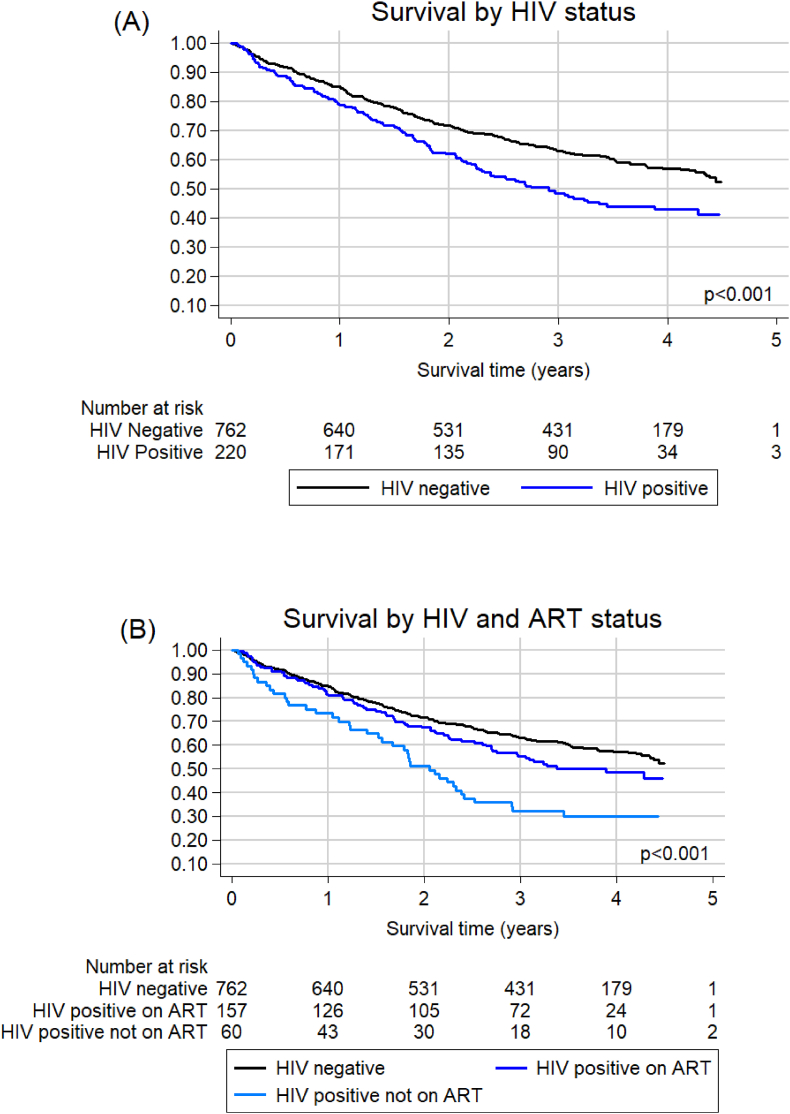
After 4 years, there were documented 446 deaths (43.8%) with a median (IQR) time to death of 15.7 months (6.9–25.9). The source of deaths was predominantly next of kin (72.0%), followed by the ‘Verify ID’ portal (16.4%) and hospital records (9.5%). Two percent of patients (n = 11) had no record of the source of death. The overall survival rate (95% CI) was 53.5% (50.1–56.7%) (Fig. 2) after 4 years. Age at breast cancer diagnosis did not affect the overall survival (p = 0.326) while HIV positive status, and those not on ART, was associated with poor overall survival (p < 0.001). (Fig. 3, Table 2). Early stage of the disease at diagnosis, hormone receptor positive tumours and a luminal A breast cancer subtype were associated with more favourable outcomes (Table 2).
Fig. 2.
Overall survival and 95% confidence interval for 1016 patients. At 1 year, survival (95% CI) was 83% (81–85%), at 2 years it was 69% (66–72%), at 3 years it was 59% (56–62%) and at 4 years it was 53% (50–57%).
Fig. 3.
(A) Overall survival by HIV status for 982 patients with known HIV status. Unadjusted HR (95% CI): 1.50 (1.22–1.85), p < 0.001. (B) Overall survival by HIV status for 979 patients with known HIV and ART status diagnosed with breast cancer. Unadjusted HR (95% CI) compared to HIV negative: HIV positive on ART 1.28 (1.00–1.63), p = 0.054; HIV positive not on ART 2.10 (1.52–2.91), p < 0.001.
Table 2.
Unadjusted and adjusted Hazard Ratios for overall survival in patients diagnosed with breast cancer at Chris Hani Baragwanath and Charlotte Maxeke Johannesburg Academic Hospitals between May 2015 and September 2017.
| Unadjusted |
Adjusted∗ | |||||
|---|---|---|---|---|---|---|
| Number at risk | Number of deaths | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Age at diagnosis (years) | 1016 | 457 | ||||
| <50 | 1.00 (Reference) | 1.00 (Reference) | ||||
| ≥50 | 1.10 (0.91–1.33) | 0.326 | 1.09 (0.87–1.36) | 0.450 | ||
| Linear trend | 1.01 (1.00–1.01) | 0.067 | 1.01 (1.00–1.02) | 0.015 | ||
| HIV status | 982 | 437 | ||||
| Negative | 1.00 (Reference) | 1.00 (Reference) | ||||
| Positive | 1.50 (1.22–1.85) | <0.001 | 1.77 (1.37–2.28) | <0.001 | ||
| Stage at Diagnosis | 1007 | 456 | ||||
| Early stage (Stage I/II) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Advanced Stage (Stage (III/IV) | 3.85 (3.09–4.79) | <0.001 | 3.71 (2.92–4.72) | <0.001 | ||
| Receptor Statusa | ||||||
| ER positive | 986 | 449 | 0.68 (0.55–0.83) | <0.001 | 0.71 (0.57–0.88) | 0.002 |
| PR positive | 984 | 448 | 0.65 (0.54–0.78) | <0.001 | 0.74 (0.60–0.90) | 0.003 |
| Her2-positive | 964 | 439 | 1.16 (0.94–1.43) | 0.166 | 0.94 (0.75–1.18) | 0.601 |
| Receptor Subtypeb | 954 | 436 | ||||
| Luminal A | 1.00 (Reference) | 1.00 (Reference) | ||||
| Luminal B | 1.84 (1.32–2.58) | <0.001 | 1.88 (1.30–2.72) | 0.001 | ||
| Her2-enriched | 1.76 (1.06–2.93) | 0.029 | 1.46 (0.84–2.56) | 0.182 | ||
| Triple negative | 2.52 (1.72–3.69) | <0.001 | 2.65 (1.75–4.01) | <0.001 | ||
| BMI (kg/m2) | 939 | 411 | 0.99 (0.98–1.00) | 0.206 | 0.99 (0.98–1.00) | 0.178 |
| Employment status | 1002 | 447 | ||||
| Employed | 1.00 (Reference) | 1.00 (Reference) | ||||
| Unemployed | 1.13 (0.94–1.37) | 0.182 | 1.17 (0.96–1.44) | 0.129 | ||
| Educationc | 998 | 445 | ||||
| Primary or lower | 1.00 (Reference) | 1.00 (Reference) | ||||
| Secondary | 0.65 (0.53–0.81) | <0.001 | 0.75 (0.59–0.95) | 0.017 | ||
| Tertiary | 0.49 (0.34–0.73) | <0.001 | 0.78 (0.51–1.20) | 0.256 | ||
∗Adjusted for age at diagnosis (continuous), BMI (continuous), stage at diagnosis (early vs advanced), receptor subtype (luminal A vs luminal B, Her2-enriched, triple negative), employment status (employed vs unemployed) and education (primary vs secondary, tertiary). Receptor Status adjusted for age at diagnosis (continuous), BMI (continuous), stage at diagnosis (early vs late), employment status (employed vs unemployed) and education (primary vs secondary, tertiary).
Reference for each receptor is the receptor negative.
Receptor subtypes were defined by immunohistochemistry as follows: Luminal A (ER +, PR +, HER2 -, Ki 67 < 14%), Luminal B (ER +, PR +/−, HER2 -, Ki 67 > 14% or ER +, PR +/−, HER-2 +, any Ki 67), HER2-enriched (ER/PR -, HER2 +, any Ki 67) and Triple negative breast cancer (ER/PR/HER2 -, any Ki 67).
Primary education refers to the first 7 years of school education and secondary as the first 12 years.
6. Disease free survival
Out of 730 patients diagnosed with stage I-III, 160 (21.9%) patients had disease recurrence and 207 (28.4%) had died. One hundred and twenty-three stage 1–3 patients had no record of whether they had disease recurrence. The overall median (IQR) time to disease recurrence was 18.9 (12.0–27.1) months with the time being shorter among those who presented with recurrence at distant sites (17.2 (10.1–26.2) months) compared to loco-regional sites (22.4 (14.5–29.7) months). Majority presented with recurrence at distant sites (55.7%) with lung and pleura combined (35.4%) being the commonest site followed by bone (33.5%), liver (25.3%) and brain (12.0%). Thirty-seven (23.4%) patients presented with disease recurrence loco-regionally while 20.9% had both loco-regional and distant sites of recurrence on follow up. One hundred and seven (66.9%) patients with disease recurrence subsequently died.
HIV positive status, an advanced stage at diagnosis, hormone receptor negative tumours and patients who did not completed their prescribed course of chemotherapy had greater odds of disease recurrence or death (Table 3). Disease free survival (95% CI) was 87.7% (85.3%–89.7%) after 1 year, 72.8% (69.7%–75.7%) after 2 years, 61.5% (58.0%–64.7%) after 3 years and 55.8 (52.1%–59.3%) after 4 years.
Table 3.
Characteristics of those with disease recurrence or death compared to those with no evidence of disease recurrence.
| Disease Recurrence or death | No disease recurrence | Odds Ratio (95% CI)a | p-value | |
|---|---|---|---|---|
| Total | 367 (50.3%) | 363 (49.7%) | ||
| Age at Presentation | ||||
| <50 | 149 (40.6%) | 139 (38.3%) | 1.00 (Ref) | |
| ≥50 | 218 (59.4%) | 224 (61.7%) | 1.00 (0.72–1.38) | 0.982 |
| HIV status | ||||
| Negative | 246 (70.5%) | 296 (83.6%) | 1.00 (Ref) | |
| Positive | 103 (29.5%) | 58 (16.4%) | 2.63 (1.71–4.03) | <0.001 |
| Stage at diagnosis | ||||
| Early Stage (Stage I/II) | 133 (36.2%) | 246 (67.8%) | 1.00 (Ref) | |
| Advanced Stage (Stage III) | 234 (63.8%) | 117 (32.2%) | 3.88 (2.80–5.36) | <0.001 |
| Receptor Statusb | ||||
| ER positive | 254 (70.8%) | 282 (80.6%) | 0.66 (0.46–0.95) | 0.027 |
| PR positive | 205 (57.1%) | 253 (72.3%) | 0.58 (0.42–0.81) | 0.001 |
| Her2 positive | 94 (26.6%) | 79 (23.2%) | 1.03 (0.71–1.50) | 0.881 |
| Receptor subtypec | ||||
| Luminal A | 37 (10.5%) | 60 (17.8%) | 1.00 (Ref) | |
| Luminal B | 222 (63.3%) | 220 (65.3%) | 1.43 (0.89–2.30) | 0.141 |
| Her2-enriched | 22 (6.3%) | 13 (3.9%) | 2.23 (0.96–5.21) | 0.063 |
| Triple negative | 70 (19.9%) | 44 (13.1%) | 2.13 (1.19–3.84) | 0.011 |
| Completed Chemotherapy | 234 (85.4%) | 273 (97.7%) | 0.13 (0.05–0.31) | <0.001 |
| Completed Radiation | 123 (100%) | 219 (99.6%) | ||
| HIV positive patients: | ||||
| Duration HIV positivity | ||||
| ≤1 year | 29 (28.2%) | 15 (25.9%) | 1.00 (Ref) | |
| >1 year | 74 (71.8%) | 43 (74.1%) | 1.03 (0.46–2.32) | 0.936 |
| On ART at diagnosis | 62 (61.4%) | 38 (65.5%) | 0.96 (0.45–2.02) | 0.905 |
| Duration ART usage | ||||
| ≤1 year | 10 (14.1%) | 11 (22.5%) | 1.00 (Ref) | |
| >1 year | 61 (85.9%) | 38 (77.6%) | 1.87 (0.65–5.36) | 0.243 |
Odds ratios were adjusted for age at diagnosis (continuous), stage at diagnosis (early (I/II) vs advanced (III)) and receptor subtype (luminal a vs luminal B, Her2-enriched, triple negative).
Reference for each receptor is the receptor negative.
Receptor subtypes were defined by immunohistochemistry as follows: Luminal A (ER +, PR +, HER2 -, Ki 67 < 14%), Luminal B (ER +, PR +/−, HER2 -, Ki 67 > 14% or ER +, PR +/−, HER-2 +, any Ki 67), HER2-enriched (ER/PR -, HER2 +, any Ki 67) and Triple negative breast cancer (ER/PR/HER2 -, any Ki 67).
7. Breast cancer IN HIV positive patients
Of the 985 patients with known HIV status, 22% were HIV positive with 71.4% of them already diagnosed with HIV before the time of breast cancer diagnosis. Moreover, 60.1% of HIV positive patients were already on ARTs at the time of breast cancer diagnosis. (Table 1). HIV positive patients were younger than the HIV negative patients with a median (IQR) age of 44 (39–52), when compared to the HIV negative patients with the median age of 57 years (IQR: 46–67, p < 0.001). Moreover, the HIV positive patients presented more often with an advanced stage of the disease at the time of breast cancer diagnosis than the HIV negative patients (62.3% vs 53.9%, p = 0.028).
Regarding overall survival, HIV infection was strongly associated with a worse overall survival (p < 0.001, Fig. 3, Table 2). Adjusted hazard ratios (95% CI) for overall survival were 1.77 (1.37–2.28) for HIV positive versus HIV negative patients, with age and stage at diagnosis accounting for many of the differences between the unadjusted and adjusted ratios. Among the HIV positive patients, patients who were on ARTs had a better survival when compared to those who were not on ARTs. [HR 0.60 (0.41–0.88), p = 0.008; Fig. 3B]. Duration of ART use did not however affect overall survival or disease recurrence or death. (Table 4).
Table 4.
Hazard ratios for survival from breast cancer in HIV positive patients.
| Unadjusted |
Adjusteda | |||||
|---|---|---|---|---|---|---|
| Number at risk | Number of deaths | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Duration of known HIV positivity | 220 | 122 | ||||
| Less than or equal to 1 year | 1.00 (Reference) | 1.00 (Reference) | ||||
| More than 1 year | 0.76 (0.53–1.12) | 0.169 | 0.80 (0.54–1.18) | 0.262 | ||
| Taking ART | 217 | 119 | ||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||
| Yes | 0.60 (0.41–0.88) | 0.008 | 0.74 (0.50–1.10) | 0.137 | ||
| Duration of ART use | 157 | 78 | ||||
| Less than or equal to 1 year | 1.00 (Reference) | 1.00 (Reference) | ||||
| More than 1 year | 1.05 (0.59–1.87) | 0.880 | 0.94 (0.51–1.71) | 0.833 | ||
| Stage at diagnosis | 219 | 122 | ||||
| Early stage (Stage I/II) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Advanced Stage (Stage (III/IV) | 3.16 (2.06–4.82) | <0.001 | 3.50 (2.24–5.46) | <0.001 | ||
| Receptor Subtypeb | 207 | 119 | ||||
| Luminal A | 1.00 (Reference) | 1.00 (Reference) | ||||
| Luminal B | 2.27 (1.05–4.92) | 0.038 | 1.95 (0.90–4.24) | 0.091 | ||
| Her2-enriched | 3.08 (1.12–8.50) | 0.030 | 2.10 (0.75–5.86) | 0.158 | ||
| Triple negative | 2.96 (1.28–6.83) | 0.011 | 2.88 (1.25–6.66) | 0.013 | ||
Adjusted for age at diagnosis (continuous), stage at diagnosis (early vs advanced) and receptor subtype (luminal A vs luminal B, Her2-enriched, triple negative).
Receptor subtypes were defined by immunohistochemistry as follows: Luminal A (ER +, PR +, HER2 -, Ki 67 < 14%), Luminal B (ER +, PR +/−, HER2 -, Ki 67 > 14% or ER +, PR +/−, HER-2 +, any Ki 67), HER2-enriched (ER/PR -, HER2 +, any Ki 67) and Triple negative breast cancer (ER/PR/HER2 -, any Ki 67).
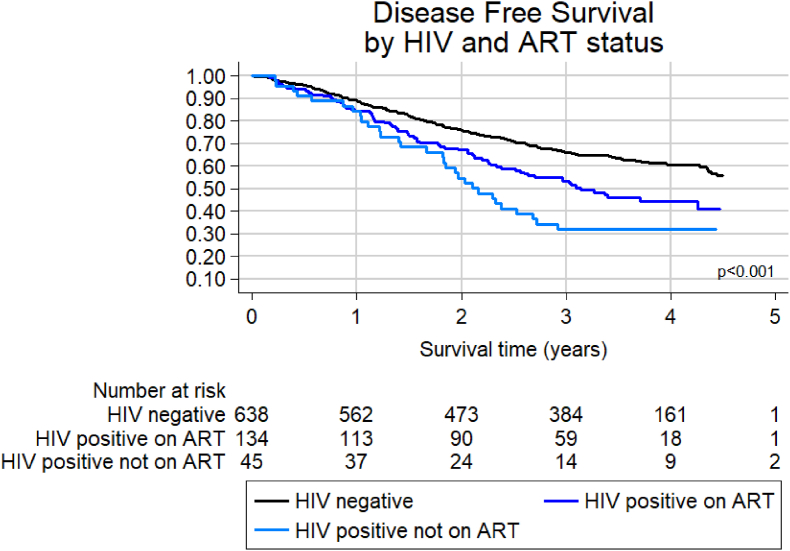
HIV infection increased the odds of disease recurrence or death (29.5% vs 16.4%, OR (95% CI): 2.63 (1.71–4.03), p < 0.001; Table 3). Disease free survival by HIV status for those on and not on ART is shown in Fig. 4. After adjusting for age, stage and receptor subtypes, compared to HIV negative patients, hazard ratios (95% CI) in HIV positive patients on ART were 1.93 (1.44–2.58) and HIV positive patients not on ART they were 2.33 (1.57–3.48).
Fig. 4.
Disease free survival by HIV and ART status for 817 patients diagnosed with stage I–III breast cancer. Unadjusted HR (95% CI) compared to HIV negative: HIV positive on ART 1.60 (1.23–2.08), p < 0.001; HIV positive not on ART 2.18 (1.49–3.19), p < 0.001.
8. Discussion
HIV infection was associated with poorer breast cancer overall survival and greater odds for disease recurrence. A study from Botswana showed that women living with HIV had a lower breast cancer survival than those who were HIV negative [15]. Other studies have also shown that cancer-associated mortality is increased among HIV-positive patients when compared to HIV-negative patients [16,23,24]. In the United States, a double-fold increase in mortality among HIV positive patients with breast cancer was reported, with the hazard ratio range between 1.20 (95% CI, 1.14–1.26) and 2.93 (95% CI, 2.08–4.13) [25]. The recently published meta-analysis also revealed a poor overall survival among HIV positive women with breast cancer [17].
Possible causes for poor breast cancer survival among HIV positive patients remain unclear. One contributory factor is the advanced stage at presentation, which correlates with poorer overall survival [10,11,[26], [27], [28]]. In this study, an advanced disease stage at presentation was strongly associated with a poorer survival compared to an early stage of the disease (p < 0.001), even after adjusting for other known risk factors for poor survival. More of HIV positive patients presented with an advanced stage (Stage III/IV) of the disease at the time of breast cancer diagnosis compared to the HIV negative patients (62.3% vs 53.9%, p = 0.028). Similarly, other studies have shown that PLWHIV are more likely to present with an advanced stage of disease at the time of cancer diagnosis [14,19,24]. These findings were, however, contrasted by the findings of a study conducted in Uganda, which showed that HIV positive patients were less likely to present at an advanced stage of disease at the time of cancer diagnosis possibly due to an indirect benefit of being part of the healthcare system for ART [29].
Another contributory factor is age at breast cancer diagnosis. Women younger than 40 years of age and elderly patients (70–89 years old) are reported to have worse outcomes [[10], [11], [12]]. The mortality rate of breast cancer in young women is reported to be twice the rate of women aged 50–59 [11]. Factors associated with worse prognosis among young women with breast cancer include triple negative breast cancer, luminal B HER-2 enriched and HER-2 enriched breast cancer subtypes and advanced disease stage at the time of diagnosis [10,11,30]. Furthermore, it has also been shown that despite adequate treatment, the risk of young women dying of breast cancer remains higher compared to the middle-aged women [31]. However, in this study, there was no association between age and worse overall or disease-free survival.
Among HIV positive patients, studies have shown improved survival of patients with both AIDS-defining and non-AIDS defining malignancies with the use of ART [32], [33], [34]. However, there was no reported association between the use of ART and survival of patients with breast and lung cancers [24]. In this study, we observed an improved breast cancer overall and disease-free survival among HIV positive patients who were on ART compared to those who were not on ART. This could be explained by the effectiveness of ART use in decreasing HIV-associated deaths and not necessarily the breast-cancer specific mortality. Moreover, ART use is also associated with improved immune system and level of CD4 cells lymphocytes, thus improved level of tumour infiltrating lymphocytes which are associated with favourable breast cancer survival [35]. However, the actual effect of ART use on breast cancer specific mortality still needs to be further explored.
Other contributory factors include triple negative breast cancer and HER-2 enriched subtypes [[10], [11], [12], [13]]. A study conducted in Soweto, South Africa showed a 2.5 and 3-fold increase in mortality among patients with HER- 2 enriched and triple negative breast cancer subtypes, respectively [14]. Similarly, in this study, triple negative, luminal B and HER-2 enriched breast cancer subtypes were significantly associated with worse breast cancer outcomes.
The overall survival was poor at 55.6% after 4 years of follow-up in this study, an outcome which remains low compared to other geographical regions. In China, an overall survival of 88.64% after 3 years of follow up was reported [12]. In HICs, the overall survival is reported to be higher with the 5-year overall survival of more than 90% [26]. An advanced stage at breast cancer diagnosis is one of the contributing factors for poor survival in LMICs. Studies have shown that more than 75% of patients from several countries in the sub-Saharan Africa have an advanced stage of diseases (stage III/IV) at the time of breast cancer diagnosis [34,[36], [37], [38]]. This is in comparison with patients from HICs, from which less than 20% of breast cancer patients present with an advanced stage of breast cancer [39]. Several other patient-dependent and health care system-dependent factors have been shown to contribute significantly towards late presentation [34,36,37].
The limitations of this study include short follow up of patients and lack of information to determine the breast cancer-specific mortality rate. Routine staging CT scan is not done therefore, it is possible that we might have under-staged our patients. Moreover, we did not adjust for history of AIDS-defining diseases and CD4+ count at the time of diagnosis, which may play a role in the overall outcome. The severe acute respiratory syndrome coronavirus-2 outbreak disrupted both the clinical care and research not related to COVID-19 globally [40]. In South Africa, the country was put on lockdown from the 26 March 2020, entailing strict movement of individuals except those rendering essential services [41]. During this period, most patients could not come for physical follow-ups mainly due to the increased fear of contracting the virus at the hospital, however telephonic follow-up of these patients was made. Therefore, it is possible that the number of patients with disease recurrence may be underestimated.
9. Conclusion
HIV infection was associated with poor overall and disease-free survival. Patients on ARTs had favourable overall and disease-free survival and with ARTs now being made accessible to all people living with HIV, the overall survival of women with HIV and breast cancer is expected to improve beyond what it is now.
Ethics clearance
Ethics clearance for this study was obtained from the Human Research Ethics Committee (Medical) at the University of Witwatersrand (clearance number: M161130 and M150351).
Disclaimer
The authors declare no conflicts of interest.
Acknowledgement
NIH of USA National Cancer Institute (Grant No: R01-CA192627 and P30-CA13696): Drs J Jacobson, M Joffe, A Neugut and P Ruff (2015–2020).
University of Witwatersrand/South African Medical Research Council Common Epithelial Cancer Research Centre Grant (CECRC): Prof P Ruff (2015–2023).
The Physician Partnerships Trust- Netcare (Prof Bongani Mayosi Clinical scholarship): 1 year scholarship.
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018:1–31. doi: 10.3322/caac.21492. 0. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Registry. Summary statistics of cancer diagnosed histologically in 2016. Female- All population groups combined. https://www.nicd.ac.za/wp-content/uploads/2020/04/NCR_2016_Report_updated_14April2020.pdf.
- 3.Cancer Stat Facts Female breast cancer. National cancer Institute. Surveillance, epidemiology and end results program. 2016. https://seer.cancer.gov/statfacts/html/breast.html
- 4.HIV/AIDS Data & Statistics WHO| data & statistics. 2019. https://www.who.int/hiv/data/en/
- 5.HIV stats in South Africa mid-year population . Statistical release; 2018. Statistics South Africa. P0302. [Google Scholar]
- 6.Implementation of the universal test & treat strategy for HIV positive patients and differentiated care for stable patients. Department of Health circular, Republic of South Africa; 2016. [Google Scholar]
- 7.Meintjes G., Moorhouse M.A., Carmona S. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med. 2017;18(1):a776. doi: 10.4102/sajhivmed.v18i1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low-and middle-income countries at reduced price. 2019. WHO.int/hiv. [Google Scholar]
- 9.Joko-Fru W.Y., Miranda-Filho A., Soerjomataram I. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study. Int J Canc. 2020;146:1208–1218. doi: 10.1002/ijc.32406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allahpour S., Navaneelan T., De P., Borgo A. Breast cancer survival by molecular subtype: a population based analysis of cancer registry data. CMAJ Open. 2017 doi: 10.9778/cmajo.20170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson A.L.V., Trewin C.B., Hjerkind K.V. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Canc. 2019;144:1251–1261. doi: 10.1002/ijc.31950. [DOI] [PubMed] [Google Scholar]
- 12.Dong G., Wang D., Liang X. Factors related to survival rates for breast cancer patients. Int J Clin Exp Med. 2014;7(10):3719–3724. www.ijcem.com/ISSN:1940-5901/IJCEM0001997 [PMC free article] [PubMed] [Google Scholar]
- 13.Poorolajal J., Nafissi N., Akbari M.E. Breast cancer survival analysis based on immunohistochemistry Subtypes (ER/PR/HER2): a retrospective Cohort Study. Arch Iran Med. 2016;19(10):680–686. [PubMed] [Google Scholar]
- 14.Cubasch H., Dickens C., Joffe M. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009–11. Cancer Epidemiol. 2018;52:120–127. doi: 10.1016/j.canep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadigh K.S., Hodgeman M.R., Tapela N. Conference on Retroviruses and opportunistic infections. 2019. HIV is associated with decreased breast cancer survival: a prospective, cohort study.http://www.croiwebcasts.org/p/2019croi/16 [Google Scholar]
- 16.Coghill A.E., Shiels M.S., Suneja G., Engels A.E. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandão M., Bruzzone M., Franzoi M.A. Impact of HIV infection on baseline characteristics and survival of women with breast cancer. AIDS. 2021 Mar 15;35(4):605–618. doi: 10.1097/QAD.0000000000002810. PMID: 33394680. [DOI] [PubMed] [Google Scholar]
- 18.Cubasch H., Ruff P., Joffe M. South African breast cancer and HIV outcomes study: methods and baseline assessment. J Glob Oncol. 2016 doi: 10.1200/JGO.2015.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phakathi B., Cubasch H., Nietz S. Clinico-pathological characteristics among South African women with breast cancer receiving anti-retroviral therapy for HIV. Breast. 2019 Feb;43:123–129. doi: 10.1016/j.breast.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgibbons P.L., Dillon D.A., Alsabeh R. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014;138:595–601. doi: 10.5858/arpa.2013-0566-CP. [DOI] [PubMed] [Google Scholar]
- 21.Goldhirsch A., Wood W.C., Coates A.S. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy or early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Joint cancer staging manual, seventh ed.
- 23.Marcus J.L., Chao C., Leyden A.W. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Canc Epidemiol Biomark. Prev. 2015;24(8):1167–1173. doi: 10.1158/1055-9965.EPI-14-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotti D., Raffetti E., Albini L. Survival in HIV-infected patients after a cancer diagnosis in the cART era: results of an Italian multicenter study. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0094768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coghill A.E., PhD, Han X., Suneja G. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer. 2019;125:2868–2876. doi: 10.1002/cncr.32158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Cancer Society . American Cancer Society; Atlanta, Ga: 2020. Cancer facts & figures 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balabram D., Turra C.M., Gobbi H. Survival of patients with operable breast cancer (Stages I-III) at a Brazilian public hospital - a closer look into cause-specific mortality. BMC Canc. 2013;13:434. doi: 10.1186/1471-2407-13-434. http://www.biomedcentral.com/1471-2407/13/434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howlader N., Cronin A.K., Kurian A.W., Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Canc Epidemiol Biomark. Prev. 2018;27(6):619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 29.Menon M.P., Coghill A., Mutyaba I. Association between HIV infection and cancer stage at presentation at the Uganda Cancer Institute. J Glob Oncol. 2017 doi: 10.1200/JGO.17.00005. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anders C.K., Hsu D.S., Broadwater G. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 31.Fredholm H., Eaker S., Frisell J. Breast cancer in young women: poor survival despite intensive treatment. PloS One. 2009;4(11) doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spagnuolo V., Galli L., Salpietro S. Ten-year survival among HIV-1-infected subjects with AIDS or non-AIDS-defining malignancies. Int J Canc. 2012;130:2990–2996. doi: 10.1002/ijc.26332. [DOI] [PubMed] [Google Scholar]
- 33.Pinzone M.R., Fiorica F., Di Rosa M M. Non-AIDS-defining cancers among HIV-infected people. Eur Rev Med Pharmacol Sci. 2012;16:1377–1388. [PubMed] [Google Scholar]
- 34.Dickens C., Joffe M., Jacobson J. Stage at breast cancer diagnosis and distance from diagnostic hospital in a peri-urban setting: a South African public hospital case series of over 1000 women. Int J Canc. 2014;135(9):2173–2181. doi: 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bense R.D., Sotiriou C., Piccart-Gebhart M.J. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer, JNCI. J Natl Cancer Inst. 2017;109(1) doi: 10.1093/jnci/djw192. January, djw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace L.E., Mounga T., Hategekimana V. Delays in breast cancer presentation and diagnosis at two rural cancer referral centres in Rwanda. Oncol. 2015;20:780–788. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joffe M., Ayeni O., Norris S.A. Barriers to early-stage presentation of breast cancer among women in Soweto, South Africa. PloS One. 2018;13(2) doi: 10.1371/journal.pone.0192071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iqbal J., Ginsburg O., Rochon P. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. J Am Med Assoc. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 39.Ries L, Eisner M, Kosary C et al. SEER Cancer statistics review.1975-2001.
- 40.Bailey C., Black J.R.M., Swanton C. Cancer Research: the lessons to learn from COVID-19. Canc Discov. 2020;10:1263–1266. doi: 10.1158/2159-8290.CD-20-0823. [DOI] [PubMed] [Google Scholar]
- 41.South African government: Regulations and guidelines- Coronavirus COVID-19 https://www.gov.za/covid-19/resources/regulations-and-guidelines-coronavirus-covid-19.