Abstract
Background
It is important to continually reevaluate the risk/benefit calculus of internal mammary node irradiation (IMNI) in the era of modern systemic therapy. We aimed to investigate the effect of IMNI on survival in node-positive breast cancer treated with mastectomy and anthracycline plus taxane-based chemotherapy.
Methods
We analyzed 348 patients who underwent mastectomy and anthracycline plus taxane-based chemotherapy for node-positive breast cancer between January 2006 and December 2011. All patients received postoperative radiation therapy (RT) with IMNI (n = 105, 30.2%) or without IMNI (n = 243, 69.8%). The benefit of IMNI for disease-free survival (DFS) and overall survival (OS) was evaluated using multivariate analysis and inverse probability of treatment weighting (IPTW) to adjust for unbalanced covariates between the groups.
Results
After a median follow-up of 95 months, the 10-year locoregional recurrence-free survival rate, DFS, and OS in all patients were 94.8%, 77.4%, and 86.2%, respectively. The IPTW-adjusted hazard ratio (HR) for the association of IMNI (vs. no IMNI) with DFS and OS was 0.208 (95% confidence intervals (CI) 0.045–0.966) and 0.460 (95% CI, 0.220–0.962), respectively. In multivariate analysis, IMNI was a favorable factor for DFS (HR, 0.458; P = 0.021) and OS (HR 0.233, P = 0.018).
Conclusions
IMNI was associated with improved DFS and OS in node-positive patients treated with mastectomy, post-mastectomy RT, and taxane-based chemotherapy, although the rate of locoregional recurrence was low.
Keywords: Regional lymph node irradiation, Internal mammary node, Radiation
Highlights
-
•
We evaluated the benefit of IMNI in modern era.
-
•
In taxane-based chemotherapy era, locoregional recurrence was very low (5.2%) in node-positive breast cancer.
-
•
IMNI was associated with improved survival.
1. Introduction
The benefit of post-mastectomy radiation therapy (PMRT) in node-positive breast cancer has been established in several randomized trials [[1], [2], [3], [4]]. In these previous studies, PMRT consisted of chest wall and comprehensive regional nodal irradiation (RNI). The European Organization for Research and Treatment of Cancer (EORTC) 22922 and MA 20 trials recently demonstrated the benefit of RNI on the rate of distant metastases as well as locoregional recurrence in positive axillary lymph node (LN) or high risk, node-negative breast cancer [5,6]. Although comprehensive inclusion of axillary LN, supraclavicular node (SCN), and internal mammary node (IMN) are recommended in international guidelines, there is significant inter-physician variability in real-world practice, especially in IMN as IMN irradiation can increase doses to the heart and lungs [[7], [8], [9]].
During the past decades, there have been several important advances in the field of systemic treatments for breast cancer. The use of taxane-based chemotherapy, in addition to anthracyclin-based chemotherapy, was proven to improve survival in breast cancer patients and has become the standard adjuvant chemotherapy for node-positive breast cancer [[10], [11], [12]]. The advent of anti-HER2 target agents has revolutionized the treatment of HER2-positive disease with improved survival [13]. Whether applying RNI, specifically irradiating IMN, can provide the same benefit shown in the EORTC 22022 (1996–2004) and MA 20 (2000–2007) trials in the context of modern adjuvant therapies including anthracycline/taxane-based chemotherapy and anti-HER2 therapy, which were not administered in these studies, is a clinically relevant question.
We previously reported retrospective studies investigating the role of IMNI in the 1990s cohort (mostly received cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy) and the 2000s cohort (in the setting of preoperative systemic therapy) [14,15]. In this study, we aimed to investigate the effect of IMNI in patients who received PMRT and adjuvant modern systemic therapy for node-positive breast cancer.
2. Materials and methods
2.1. Patients
This study was approved by the Institutional Review Board at the Samsung Medical Center and Yonsei Cancer Center, and informed consent was waived. From January 2006 to December 2011, we identified 348 patients diagnosed with node-positive breast cancer who underwent a mastectomy followed by adjuvant taxane-based chemotherapy and radiation therapy (RT). We excluded patients who had clinical involvement of IMN or SCN, did not receive taxane-based chemotherapy, underwent preoperative systemic therapy, or had a history of previous or concurrent malignancy, including contralateral breast cancer.
2.2. Treatment
All patients underwent a mastectomy, followed by taxane-based adjuvant chemotherapy. The most common regimen was four cycles of anthracycline and cyclophosphamide (AC), followed by four cycles of taxane (T) (n = 234, 67.2%), followed by eight cycles of anthracycline plus taxane (AT) (n = 103, 29.6%). Adjuvant endocrine therapy was administered to 98.5% (269/273) of estrogen receptor (ER)-positive or progesterone receptor (PR)-positive patients, and targeted therapy was administered to 85.0% (68/80) of the HER2-positive patients. For axillary management, 345 (99.1%) patients underwent axillary LN dissection, and three (0.9%) patients underwent sentinel LN biopsy only. The median number of dissected LNs was 22 (range, 3–59). PMRT was indicated in patients with pN2 and high risk N1 [[16], [17], [18]]. Irradiation to the ipsilateral SCN was performed in 328 (94.3%) patients, and the median radiation dose to the chest wall and SCN was 50.4 Gy (range, 45–54 Gy) with 1.8.2.0 Gy per fraction. IMNI was performed based on the physician's discretion, as described in previous studies [14,15]. Computed tomography-based treatment planning and three-dimensional conformal RT were used in all patients. The ipsilateral IMN area, including the 1st to 3rd intercostal space, was delineated for IMN treatment. In the no IMNI group, two tangential photon beam fields for the chest wall and a single anterior photon beam field for the SCNs were used with a single isocenter. In the IMNI group, the SCN and lateral chest wall were treated by the photon beam reverse hockey stick field, and the IMN and medial chest wall were treated by an anterior electron beam field. An individual custom-made step bolus and reverse hockey stick technique were used to compensate for differences in chest wall thickness to reduce the irradiation dose to the lung/heart. The heart block was not routinely applied.
2.3. Analysis
The characteristics according to IMNI, were compared using χ2 or Fisher's exact test. To minimize the effects of imbalanced baseline characteristics between the groups, the propensity score inverse probability of treatment weighting (IPTW) was used. Propensity scores were calculated using a multivariate logistic regression model, including laterality (left vs. right), grade (I−II vs. III), T stage (T1 vs. T2 vs. T3–T4), and N stage (N1 vs. N2 vs. N3). Each patient was then assigned an estimated propensity score based on the patient's baseline characteristics.
Disease-free survival (DFS) was defined as the time from surgery to any recurrence or death, and overall survival (OS) was defined as the time from surgery to death from any cause. DFS and OS rates were estimated using the Kaplan-Meier method and compared using log-rank tests, and IPTW-adjusted Kaplan-Meier curves were also estimated. Local recurrence was defined as disease recurrence within the ipsilateral chest wall, and regional recurrence was defined as recurrence within the axillary, supraclavicular, or internal mammary LNs. Any symptomatic event after irradiation was investigated to evaluate the pulmonary and cardiac toxicities. Radiation pneumonitis was graded using common terminology criteria for adverse events version 5.0. Any cardiac toxicities included coronary artery disease, cardiomyopathy, pericardial disease, and conduction abnormalities. Cox proportional hazards regression models in univariate and multivariate analyses for all patients to calculate adjusted hazard ratios (HRs) and 95.0% confidence intervals (CIs). A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics version 20 (IBM, USA).
3. Results
3.1. Patient characteristics
The median age of all patients was 47 years (range, 27–74 years). Of all patients, 105 (30.2%) patients received IMNI and 243 (69.8%) did not. Patient characteristics, according to IMNI before and after IPTW, are described in Table 1. Before applying IPTW, patients in the no IMNI group had left breast cancer and unfavorable factors more frequently, including grade III, advanced pT stage, and advanced pN stage, than those in the IMNI group. Other characteristics, including age, menopausal status, location, and ER, PR, and HER2-positivity, were evenly distributed between the groups. After applying IPTW, the distribution of variables between the groups attained a reasonable balance.
Table 1.
Patient characteristics by internal mammary node irradiation before and after using inverse probability treatment weighting method.
| Characteristics | Before IPTW |
After IPTW |
|||||
|---|---|---|---|---|---|---|---|
| Total | No IMNI | IMNI | P value | No IMNI | IMNI | P value | |
| Age | 0.338 | 0.145 | |||||
| <50 | 217 (62.4%) |
156 (64.2%) |
61 (58.1%) |
167.1 (63.5%) |
82.3 (56.2%) |
||
| ≧50 | 131 (37.6%) |
87 (35.8%) |
44 (41.9%) |
95.9 (36.5%) |
64.1 (43.8%) |
||
| Menopausal status | 0.558 | 0.75 | |||||
| Pre | 219 (62.9%) |
150 (61.7%) |
69 (65.7%) |
164.3 (62.5%) |
93.8 (64.1%) |
||
| Post | 129 (37.1%) |
93 (38.3%) |
36 (34.3%) |
98.7 (37.5%) |
52.6 (35.9%) |
||
| Laterality | 0.055 | 0.751 | |||||
| Left | 178 (51.1%) |
133 (54.7%) |
45 (42.9%) |
133.7 (50.8%) |
72.5 (49.5%) |
||
| Right | 170 (48.9%) |
110 (45.3%) |
60 (57.1%) |
129.3 (49.2%) |
73.9 (50.5%) |
||
| Location | 0.989 | 0.606 | |||||
| Inner/central | 197 (56.6%) |
137 (56.4%) |
60 (57.1%) |
148.1 (56.3%) |
85.9 (58.7%) |
||
| Outer | 151 (43.4%) |
106 (43.6%) |
45 (42.9%) |
114.9 (43.7%) |
60.5 (41.3%) |
||
| Grade | 0.013 | 0.954 | |||||
| I-II | 209 (60.1%) |
135 (55.6%) |
74 (70.5%) |
160.3 (61.0%) |
88.8 (60.6%) |
||
| III | 139 (39.9%) |
108 (44.4%) |
31 (29.5%) |
102.7 (39.0%) |
57.6 (39.4%) |
||
| Pathologic T stage | 0.001 | 0.967 | |||||
| pT1 | 78 (22.4%) |
43 (17.7%) |
35 (33.3%) |
62.6 (23.8%) |
33.5 (22.9%) |
||
| pT2 | 182 (52.3%) |
128 (52.7%) |
54 (51.4%) |
132.7 (50.4%) |
76.1 (52.0%) |
||
| pT3-4 | 88 (25.3%) |
72 (29.6%) |
16 (15.2%) |
67.7 (25.7%) |
36.8 (25.1%) |
||
| Pathologic N stage | <0.001 | 0.941 | |||||
| pN1 | 64 (18.4%) |
22 (9.1%) |
42 (40.0%) |
49.7 (18.9%) |
27.4 (18.7%) |
||
| pN2 | 154 (44.3%) |
120 (49.4%) |
34 (32.4%) |
116.0 (44.1%) |
66.6 (45.5%) |
||
| pN3 | 130 (37.4%) |
101 (41.6%) |
29 (27.6%) |
97.3 (37.0%) |
52.4 (35.8%) |
||
| ER | 0.118 | 0.173 | |||||
| Negative | 80 (23.0%) |
62 (25.5%) |
18 (17.1%) |
66.2 (25.2%) |
28.3 (19.3%) |
||
| Positive | 268 (77.0%) |
181 (74.5%) |
87 (82.9%) |
196.8 (74.8%) |
118.1 (80.7%) |
||
| PR | 0.624 | 0.701 | |||||
| Negative | 94 (27.0%) |
68 (28.0%) |
26 (24.8%) |
70.7 (26.9%) |
42.1 (28.7%) |
||
| Positive | 254 (73.0%) |
175 (72.0%) |
79 (75.2%) |
192.3 (73.1%) |
104.3 (71.3%) |
||
| Her2 | 1.000 | 0.856 | |||||
| Negative | 268 (77.0%) |
187 (77.0%) |
81 (77.1%) |
205.9 (78.3%) |
113.7 (77.7%) |
||
| Positive | 80 (23.0%) |
56 (23.0%) |
24 (22.9%) |
57.1 (21.7%) |
32.7 (22.3%) |
||
IMNI, internal mammary node irradiation; ER, estrogen receptor; PR, progesterone receptor.
3.2. Survival
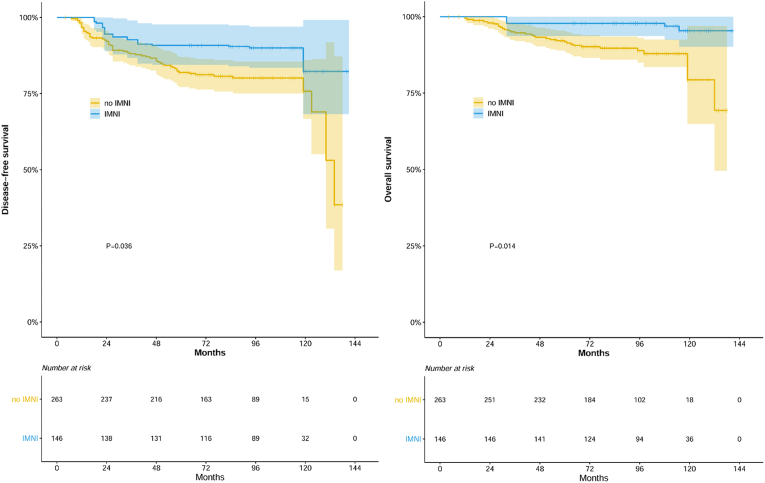
During the median follow-up of 95 months (range, 4–141 months), 66 patients experienced recurrence and 34 patients died of disease progression (n = 32) or without disease (n = 2). For the whole cohort, DFS and OS were 83.8% and 93.2% at 5-year and 77.4% and 86.2% at 10-year, respectively. In univariate analysis, the IMNI group showed better DFS (79.9% and 92.4% at 5 years, HR 0.370 [95% CI 0.193–0.710], P = 0.002) and OS (90.5% vs. 99.0% at 5 years, HR 0.178 [95% CI 0.054–0.584], P = 0.001) compared to the no IMNI group. In a multivariate analysis for the unadjusted cohort, IMNI remained as a significant factor for DFS (HR, 0.458, 95% CI 0.235–0.891, P = 0.021) and OS (HR 0.233, 95% CI 0.070–0.778, P = 0.018, Table 2). In addition to IMNI, advanced pT stage (pT3–T4, HR 1.727, 95% CI 1.021–2.920, P = 0.042) and advanced pN stage (pN3, HR 2.666, 95% CI 1.602–4.438, P < 0.001) were unfavorable factors for DFS, and advanced pN stage (pN3, HR 2.313, 95% CI 1.134–4.716, P = 0.021) was an unfavorable factor for OS in the multivariate analysis. In the IPTW-adjusted cohort, IMNI was also associated with improved DFS (HR 0.420, 95% CI 0.045–0.966, P = 0.036) and OS (HR 0.208, 95% CI 0.045–0.966, P = 0.014), respectively (Fig. 1).
Table 2.
Multivariate analysis for disease-free survival and overall survival in the unadjusted cohort.
| Characteristics | Disease-free survival |
Overall survival |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| IMNI | ||||
| No | 1 | 1 | ||
| Yes | 0.458 (0.235–0.891) | 0.021 | 0.233 (0.070–0.778) | 0.018 |
| Age | ||||
| <50 | 1 | 1 | ||
| ≧50 | 0.921 (0.533–1.592) | 0.768 | 1.020 (0.478–2.174) | 0.960 |
| Laterality | ||||
| Left | 1 | 1 | ||
| Right | 0.972 (0.594–1.592) | 0.911 | 0.977 (0.490–1.946) | 0.946 |
| Location | ||||
| Inner/central | 1 | 1 | ||
| Outer | 0.944 (0.568–1.567) | 0.823 | 0.880 (0.429–1.809) | 0.729 |
| Grade | ||||
| I-II | 1 | 1 | ||
| III | 1.056 (0.616–1.812) | 0.843 | 1.134 (0.526–2.447) | 0.748 |
| pT stage | ||||
| pT1-2 | 1 | 1 | ||
| pT3-4 | 1.727 (1.021–2.920) | 0.042 | 1.974 (0.960–4.056) | 0.064 |
| pN stage | ||||
| pN1-2 | 1 | 1 | ||
| pN3 | 2.666 (1.602–4.438) | <0.001 | 2.313 (1.134–4.716) | 0.021 |
| ER | ||||
| Negative | 1 | 1 | ||
| Positive | 0.381 (0.114–1.269) | 0.116 | 0.222 (0.047–1.060) | 0.059 |
| PR | ||||
| Negative | 1 | 1 | ||
| Positive | 1.789 (0.555–5.771) | 0.330 | 1.306 (0.275–6.191) | 0.737 |
| Her2 | ||||
| Negative | 1 | 1 | ||
| Positive | 0.676 (0.348–1.311) | 0.247 | 0.628 (0.263–1.502) | 0.296 |
HR, hazard ratio; CI, confidence interval; IMNI, internal mammary node irradiation; ER, estrogen receptor; PR, progesterone receptor.
Fig. 1.
Disease-free survival and overall survival with or without internal mammary node irradiation in adjusted cohorts using the inverse probability treatment weighting method.
3.3. Patterns of failure
Table 3 shows the first relapse site according to the IMNI. The most common failure was distant recurrence (61 patients, 17.5%) in all patients. The rate of distant recurrence was higher in the no IMNI group (21.0% vs. 9.5%, P = 0.010), and regional recurrence was higher in the no IMNI group (5.8% vs. 1.9%, P = 0.115). In the IMNI group, there was no IMN recurrence, while four patients developed IMN recurrence in the no IMNI group. Patterns of failure in the IPTW-adjusted cohort revealed that the regional recurrence rate was higher in the no IMNI group than in the IMNI group (5.6% vs. 1.4%, P = 0.035, Table 3).
Table 3.
Patterns of failure in the unadjusted cohort.
| No IMNI | IMNI | P value | |
|---|---|---|---|
| Patterns of failure in the unadjusted cohort | |||
| Local | 2 (0.8%) | 2 (1.9%) | 0.385 |
| Regional | 14 (5.8%) | 2 (1.9%) | 0.115 |
| Axilla | 4 | 0 | |
| SCN | 2 | 2 | |
| IMN | 4 | 0 | |
| Axilla & SCN | 3 | 0 | |
| SCN & IMN | 1 | 0 | |
| DM | 51 (21.0%) | 10 (9.5%) | 0.010 |
| LRR only | 2 (0.8%) | 1 (1.0%) | 0.082 |
| DM only | 39 (16.0%) | 8 (7.6%) | |
| LRR + DM | 12 (4.9%) | 2 (1.9%) | |
| Death | 31 (12.8%) | 3 (2.9%) | 0.004 |
| Death without disease | 2 | 0 | |
| Patterns of failure in the inverse probability treatment weighting-adjusted cohort | |||
| Local | 2.0 (0.8%) | 3.4 (2.3%) | 0.254 |
| Regional | 14.7 (5.6%) | 2.1 (1.4%) | 0.035 |
| DM | 49.6 (18.8%) | 16.2 (11.1%) | 0.034 |
IMNI, internal mammary node irradiation; SCN, supraclavicular node; IMN, internal mamary node; LRR, locoregional recurrence; DM, distant metastasis.
3.4. Toxicities
Grade 1–2 radiation pneumonitis was observed in six patients, and cardiac toxicities developed in nine patients after radiation. There was no significant difference in toxicities between the groups (Table 4).
Table 4.
Toxicities.
| No IMNI | IMNI | p value | |
|---|---|---|---|
| Grade 1–2 Radiation pneumonitis | 4 (1.6%) | 2 (1.9%) | 0.865 |
| Any cardiac toxicities | 6 (2.5%) | 3 (2.9%) | 0.834 |
IMNI, internal mammary node irradiation.
3.5. Subgroup analysis
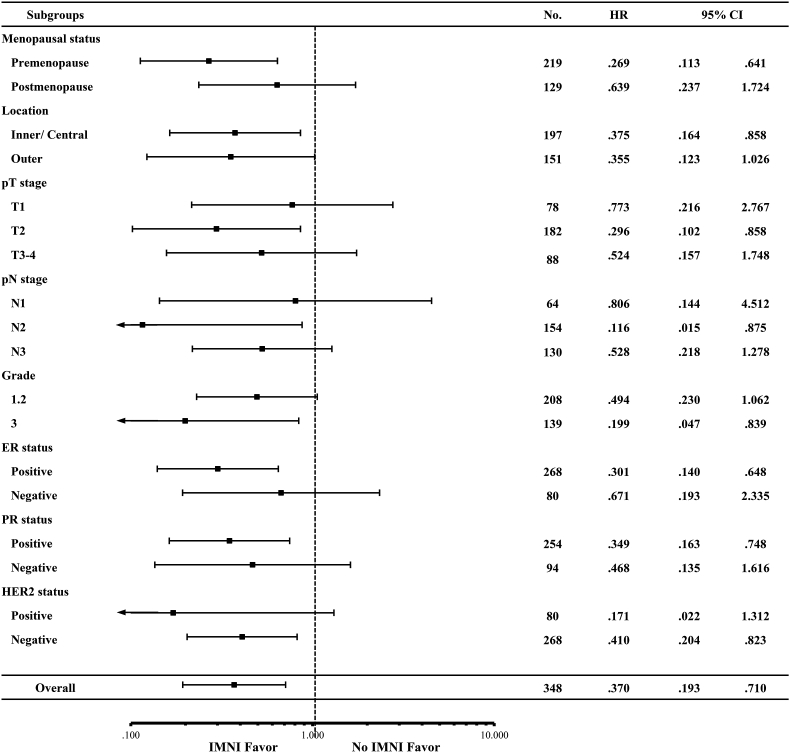
The effect of IMNI was evaluated in various subgroups including menopausal status, laterality, location, grade, pT stage, pN stage, and ER, PR, and HER2-positivity. The effect of IMNI on DFS was more evident in premenopausal women (HR, 0.269, 95% CI 0.113–0.641, P = 0.003), pT2 disease (HR 0.296, 95% CI 0.102–0.858, P = 0.025), pN2 disease (HR 0.116, 95% CI 0.015–0.875, P = 0.037), grade III (HR 0.199, 95% CI 0.047–0.839, P = 0.028), ER-positive (HR 0.301, 95% CI 0.140–0.648, P = 0.002), PR-positive (HR 0.349, 95% CI 0.163–0.748, P = 0.007), and HER2-negative patients (HR 0.410, 95% CI 0.204–0.823, P = 0.012, Fig. 2).
Fig. 2.
Hazard ratio of internal mammary node irradiation on disease-free survival in different subgroups.
4. Discussion
In our study, we sought to explore whether the benefit of IMNI observed in EORTC22922/MA20 trials also applies to breast cancer patients treated with modern chemotherapy, including taxane-based chemotherapy and/or anti-HER2-targeted therapies. Unlike other retrospective studies in which IMNI was applied to patients with a higher risk [19,20], the IMNI group of this study included less advanced tumors than the no IMNI group. This imbalance is primarily attributable to the different policies between the physicians, one of whom treated IMNs in all RNI cases while the other physician treated IMNs only when the tumor was involved. To assess the independent effect of IMNI with our imbalanced retrospective data, we conducted a Cox proportional multivariate analysis and an IPTW-adjusted analysis. Even after adjustment for imbalanced factors, IMNI was a significant factor for higher DFS and OS.
After the final reports of MA 20 and the EORTC trial demonstrated the benefit of comprehensive nodal irradiation, guidelines and patterns of practice have changed toward supporting its routine use [21]. However, these trials did not determine whether IMNI specifically contributed to the survival benefit. The first randomized trial to assess the role of IMNI, which was initiated by the French group in 1991, was published in 2013 and showed no statistical differences in 10-year OS between IMNI and no IMNI [22]. Second, the DBCG-IMN study, a prospective, population-based cohort study in Denmark, reported a statistically significant improvement in 8-year OS with IMNI (HR, 0.82) [23]. Recently, a multi-center prospective trial (KROG 08–06), which randomly assigned 747 patients to the IMNI or no IMNI group was presented in the 2020 annual meeting of the Korean Society for Radiation Oncology, and found that the increase in 7-year DFS (IMNI vs. no IMNI, 85% vs. 82%) was not statistically significant and the pre-defined endpoint (10% difference) was not reached [24].
These conflicting data can be explained by the incidental doses of IMN in the no IMNI group and the insufficient doses in the IMNI group in each study. In the quality assurance (QA) study of KROG 08–06, 37% of the patients in the no IMNI group received 60% or more of the prescribed dose, and only 53% of the patients in the IMNI group received the recommended dose of the IMNI [25]. In the French trial, treatment planning was two-dimensional, and quality control was lacking. Without a rigorous RT QA program, it would be difficult to detect the effect of IMNI in multi-center trials. In DBCG-IMN, although not randomly assigned, patients were recommended to undergo IMNI in right-sided tumors and were recommended not to receive IMNI in left-sided tumors as per national guidelines, based on the presumption of potentially increased heart toxicity by IMNI in left-sided tumors. In this context, we speculated that the consistent discrepancy in the RT technique and indication between the two physician groups (IMNI vs. no IMNI) makes our findings [14,15] more similar to those from the DBCG-IMN study.
The serial reports of our group demonstrated an improvement in survival outcomes over the study period (10-year DFS 61%, 70%, and 77% in the study by Chang et al. Kim et al. and this study, respectively) [14,15], which probably reflects the benefit of advanced systemic treatment (Supplementary Fig. 1). Compared to the DBCG-IMN study, the OS rate in our study was much higher (86.2% at 10 years vs. 72.2–75.9% at 8 years), even though our cohort included more advanced N stage (pN2, 82% vs. 40%). Although there are concerns about the possible diminished role of RT in the context of modern chemotherapy [26], we found that the contribution of IMNI to DFS was higher, or at least similar in the current study (HR 0.46) than in our previous studies (HR 0.58–0.70, Supplementary Table 1) [14,15]. This may be in line with the lessons of the EBCTCG analysis, which showed that the effect of locoregional control on survival is higher under effective systemic treatment and modern RT [27].
The benefit of IMNI was more evident in premenopausal, pT2 disease, pN2 disease, and grade III, ER-positive, PR-positive, and HER2-negative disease. This is similar to the DBCG-IMN study, which showed that the benefit of IMNI was more pronounced in the pN2 and premenopausal subgroup [23]. Our group also previously reported that the benefit of IMNI was the most obvious in pN2 patients [15]. Interestingly, ER-positive, PR-positive, and HER2-negative patients showed a higher benefit of IMNI, which is similar to a study by Wang et al. [20]. They investigated the effect of IMNI in 872 patients treated with breast-conserving surgery or mastectomy in the modern systemic treatment era. They also showed that IMNI improved both DFS and OS even after propensity score matching. Our results, together with these previous studies, support the selective use of IMNI for patients at high risk of recurrence.
One of the major concerns regarding IMNI is that pulmonary and cardiac toxicities could offset the effect of IMNI. In our study, IMNI did not increase the incidence of adverse events. We used an electron beam with an individualized custom-made step bolus for the IMN and medial chest wall irradiation in the IMNI group, which might reduce lung and cardiac toxicities. Less lifestyle-associated risk factors and younger age at cancer diagnosis of Asian women might have attributed to less risk of radiation-related cardiac toxicity [28,29]. Previous studies in the Korean population reported that the incidence of coronary events did not differ between women treated with RT for left and right breast cancer, and the risk of cardiac-related death did not differ between breast cancer survivors and the general population [30,31]. Advances in cardiac-sparing RT techniques, including deep inspiration breath hold, prone, proton, and/or IMRT, which were not used in this study, might further reduce the risk of cardiac and lung toxicities [32]. Continued efforts to reduce RT-related toxicities will be needed to maximize the benefit of IMNI by widening the therapeutic window.
This study has several limitations. First, the baseline characteristics between the IMNI and no IMNI groups were imbalanced. Although we conducted a multivariate analysis, bias may still exist because of the retrospective nature of this study. Possible underreporting of late cardiac and pulmonary toxicities is another limitation of this study. Nevertheless, this study included a large number of patients treated with protocol-based planning, which would have minimal variation in RT dose distribution.
In conclusion, our study suggests that IMNI has a statistically significant impact on DFS and OS in node-positive breast cancer patients treated with mastectomy, axillary LN dissection, PMRT, and taxane-based chemotherapy, although the rate of locoregional recurrence in this population is very low. Given that modern systemic therapies dramatically impact the risk of locoregional recurrence and modern RT techniques can substantially reduce heart/lung doses, the risk/benefit calculus of IMNI should be continually reevaluated.
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health &Welfare, Republic of Korea (grant number: HA17C0043).
Ethical approval and informed consent
Methods for data collection and analyses were in accordance with the 1964 Helsinki declaration and its later amendments. This study was approved by the institutional review board of the principal investigator's (W Park) institution (SMC 2020-06-058-001), and informed consent of the participants was waived.
Availability of data and material
Data are available from the authors upon reasonable request.
Declaration of competing interest
There are no conflicts of interest relevant to this article to report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.breast.2021.05.012.
Contributor Information
Won Park, Email: wonro.park@samsung.com, wonp68@skku.edu.
Chang Ok Suh, Email: suhchangok@cha.ac.kr.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/s0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overgaard M., Jensen M.B., Overgaard J., Hansen P.S., Rose C., Andersson M. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M., Hansen P.S., Overgaard J., Rose C., Andersson M., Bach F. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 4.Park S.H., Lee J., Lee J.E., Kang M.K., Kim M.Y., Park H.Y. Local and regional recurrence following mastectomy in breast cancer patients with 1-3 positive nodes: implications for postmastectomy radiotherapy volume. Radiat Oncol J. 2018;36:285–294. doi: 10.3857/roj.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poortmans P.M., Collette S., Kirkove C., Van Limbergen E., Budach V., Struikmans H. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 6.Whelan T.J., Olivotto I.A., Parulekar W.R., Ackerman I., Chua B.H., Nabid A. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dess R.T., Liss A.L., Griffith K.A., Marsh R.B., Moran J.M., Mayo C. Ischemic cardiac events following treatment of the internal mammary nodal region using contemporary radiation planning techniques. Int J Radiat Oncol Biol Phys. 2017;99:1146–1153. doi: 10.1016/j.ijrobp.2017.06.2459. [DOI] [PubMed] [Google Scholar]
- 8.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 9.Harris E.E., Correa C., Hwang W.T., Liao J., Litt H.I., Ferrari V.A. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 10.Albain K., Anderson S., Arriagada R., Barlow W., Bergh J., Bliss J. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson I.C., Berry D.A., Demetri G.D., Cirrincione C.T., Goldstein L.J., Martino S. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Version 4.2020.
- 13.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.H., Noh J.M., Kim Y.B., Chang J.S., Keum K.C., Huh S.J. Does internal mammary node irradiation affect treatment outcome in clinical stage II-III breast cancer patients receiving neoadjuv ant chemotherapy? Breast Canc Res Treat. 2015;152:589–599. doi: 10.1007/s10549-015-3505-1. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.S., Park W., Kim Y.B., Lee I.J., Keum K.C., Lee C.G. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys. 2013;86:867–872. doi: 10.1016/j.ijrobp.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Chang J.S., Lee J., Kim K.H., Sohn J.H., Kim S.I., Park B.W. Do recent advances in diagnostic and therapeutic procedures negate the benefit of postmastectomy radiotherapy in N1 patients with a low risk of locoregional recurrence? Medicine (Baltim) 2015;94:e1259. doi: 10.1097/MD.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J.I., Park W., Huh S.J., Choi D.H., Lim Y.H., Ahn J.S. Determining which patients require irradiation of the supraclavicular nodal area after surgery for N1 breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1135–1141. doi: 10.1016/j.ijrobp.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Yu J.I., Park W., Shin K.H., Lee N.K., Choi D.H., Huh S.J. Prophylactic supraclavicular radiotherapy after surgery in high-risk n1 breast cancer. Oncology. 2013;85:14–20. doi: 10.1159/000352002. [DOI] [PubMed] [Google Scholar]
- 19.Luo J., Jin K., Chen X., Wang X., Yang Z., Zhang L. Internal mammary node irradiation (IMNI) improves survival outcome for patients with clinical stage II-III breast cancer after preoperative systemic therapy. Int J Radiat Oncol Biol Phys. 2019;103:895–904. doi: 10.1016/j.ijrobp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang X., Luo J., Jin K., Chen X., Zhang L., Meng J. Internal mammary node irradiation improves 8-year survival in breast cancer patients: results from a retrospective cohort study in real-world setting. Breast Cancer. 2020;27:252–260. doi: 10.1007/s12282-019-01015-9. [DOI] [PubMed] [Google Scholar]
- 21.Belkacemi Y., Kaidar-Person O., Poortmans P., Ozsahin M., Valli M.C., Russell N. Patterns of practice of regional nodal irradiation in breast cancer: results of the European Organization for Research and Treatment of Cancer (EORTC) NOdal Radiotherapy (NORA) survey. Ann Oncol. 2015;26:529–535. doi: 10.1093/annonc/mdu561. [DOI] [PubMed] [Google Scholar]
- 22.Hennequin C., Bossard N., Servagi-Vernat S., Maingon P., Dubois J.B., Datchary J. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86:860–866. doi: 10.1016/j.ijrobp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Thorsen L.B., Offersen B.V., Dano H., Berg M., Jensen I., Pedersen A.N. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320. doi: 10.1200/JCO.2015.63.6456. [DOI] [PubMed] [Google Scholar]
- 24.Byun H.K. The 2020 annual meeting of Korean society for radiation Oncology. 2020. Internal mammary node irradiation in patients with node-positive breast cancer: a prospective, randomized, phase 3 trial (KROG-0806) [Google Scholar]
- 25.Yoon H.I., Yoon J., Chung Y., Nam C.M., Cha H., Choi J. Individual case review in a phase 3 randomized trial to investigate the role of internal mammary lymph node irradiation for breast cancer: Korean Radiation Oncology Group 08-06 study. Radiother Oncol. 2017;123:15–21. doi: 10.1016/j.radonc.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Cady B. Local therapy and survival in breast cancer. N Engl J Med. 2007;357:1051–1052. author reply 2. [PubMed] [Google Scholar]
- 27.Poortmans P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet. 2014;383:2104–2106. doi: 10.1016/S0140-6736(14)60192-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang J.S., Shin J., Park E.C., Kim Y.B. Risk of cardiac disease after adjuvant radiation therapy among breast cancer survivors. Breast. 2019;43:48–54. doi: 10.1016/j.breast.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Chang J.S., Kim Y.B., Shin J. In regard to Cahlon et al. Int J Radiat Oncol Biol Phys. 2018;100:1298–1299. doi: 10.1016/j.ijrobp.2018.01.058. 10.1016/j.ijrobp.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Chang J.S., Ko B.K., Bae J.W., Yu J.H., Park M.H., Jung Y. Radiation-related heart disease after breast cancer radiation therapy in Korean women. Breast Canc Res Treat. 2017;166:249–257. doi: 10.1007/s10549-017-4398-y. [DOI] [PubMed] [Google Scholar]
- 31.Cho W.K., Park W., Choi D.H., Cha H., Nam S.J., Kim S.W. Which patients with left breast cancer should be candidates for heart-sparing radiotherapy? J Breast Cancer. 2018;21:206–212. doi: 10.4048/jbc.2018.21.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee B.M., Chang J.S., Kim S.Y., Keum K.C., Suh C.O., Kim Y.B. Hypofractionated radiotherapy dose scheme and application of new techniques are associated to a lower incidence of radiation pneumonitis in breast cancer patients. Front Oncol. 2020;10:124. doi: 10.3389/fonc.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon reasonable request.