Highlights
-
•
Dendritic complexity and presynaptic density are reduced in the 4R-tauopathies of PSP and CBD.
-
•
The reduction in ODI and UCB-J binding is coupled across the cortical mantle as well as subcortically.
-
•
The relationship between ODI and UCB-J binding is not attributed to atrophy.
-
•
ODI may be a useful biomarker of synaptic pathology in neurodegenerative diseases.
Keywords: Neurodegeneration, [11C]UCB-J, PET, NODDI, Tauopathy, Synapse
Abbreviations: PSP-RS, Progressive Supranuclear Palsy – Richardson's Syndrome; CBD, Corticobasal degeneration; PET, Positron Emission Tomography; NODDI, Neurite Orientation and Dispersion Imaging
Abstract
Understanding the cellular underpinnings of neurodegeneration remains a challenge; loss of synapses and dendritic arborization are characteristic and can be quantified in vivo, with [11C]UCB-J PET and MRI-based Orientation Dispersion Imaging (ODI), respectively. We aimed to assess how both measures are correlated, in 4R-tauopathies of progressive supranuclear palsy – Richardson's Syndrome (PSP-RS; n = 22) and amyloid-negative (determined by [11C]PiB PET) Corticobasal Syndrome (Cortiobasal degeneration, CBD; n =14), as neurodegenerative disease models, in this proof-of-concept study. Compared to controls (n = 27), PSP-RS and CBD patients had widespread reductions in cortical ODI, and [11C]UCB-J non-displaceable binding potential (BPND) in excess of atrophy. In PSP-RS and CBD separately, regional cortical ODI was significantly associated with [11C]UCB-J BPND in disease-associated regions (p < 0.05, FDR corrected). Our findings indicate that reductions in synaptic density and dendritic complexity in PSP-RS and CBD are more severe and extensive than atrophy. Furthermore, both measures are tightly coupled in vivo, furthering our understanding of the pathophysiology of neurodegeneration, and applicable to studies of early neurodegeneration with a safe and widely available MRI platform.
1. Introduction
Abnormal dendritic morphology, and synaptic pathology are increasingly recognized as hallmarks of neurodegeneration, prior to cell death and atrophy, and correlate closely with cognitive dysfunction (Clare et al., 2010; DeKosky and Scheff, 1990; Herms and Dorostkar, 2016; Terry et al., 1991). However, quantification of synaptic pathology in vivo has until recently posed a challenge. Much of our knowledge about synaptic loss in neurodegeneration stems from animal and postmortem studies. For example, with frontotemporal lobar degeneration pathology (Coyle-Gilchrist et al., 2016) including Frontotemporal lobe Dementia (FTD), Progressive Supranuclear Palsy (PSP) and Corticobasal Degeneration (CBD), there is 30%–50% loss of synapses, as determined by postmortem synaptophysin assays (Bigio et al., 2001; Lipton et al., 2001).
To quantify this pathophysiological change in vivo and uncover the early stages of neurodegeneration, would enable better design of clinical trials, accurate measurement of therapeutic response, and early intervention. In vivo cerebral glucose metabolism measured with [18F]FDG Positron Emission Tomography (PET), has often been interpreted as a synaptic density marker. Reductions are clinically relevant, for example, there are hippocampal reductions of [18F]FDG uptake in mild cognitive impairment (Mosconi et al., 2005) and reduced frontotemporal uptake in frontotemporal lobar degeneration (Blin et al., 1992; Juh et al., 2004); however, [18F]FDG is an indirect marker. In contrast, the recently, developed radioligand [11C]UCB-J ((R)-1-((3-(methyl-11C)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyr-rolidin-2-one), allows direct quantification of synaptic density based on its affinity for the ubiquitously expressed presynaptic vesicle glycoprotein SV2A (Finnema et al., 2017, 2016). This ligand shows up-to 40% loss of hippocampal synapses in patients with Alzheimer's disease (Chen et al., 2018), and a 20%–50% global reduction in PSP-RS/CBD (Holland et al., 2020) correlating with cognitive dysfunction. However, PET has limited scalability in diagnostics and trials, because of cost, specialist facilities required, and radiation limitations on repeated assessment.
Here we propose that with the loss of synapses, presynaptic vesicle density would correlate with changes in postsynaptic dendritic complexity. While diffusion tensor imaging (DTI) has proved to be a valuable noninvasive technique to probe microstructural white matter integrity, it has limited utility to characterize such changes in the cortex due to the isotropic water diffusion of the grey matter. Therefore, there is a need for biomarkers capable of evaluating functionally relevant microstructure noninvasively. To address the limitations of DTI, Neurite Orientation Dispersion Imaging (NODDI) is a multi-compartment biophysical diffusion imaging model that is capable of disentangling the diffusion signal arising from distinct tissue compartments, such as extracellular, intracellular water and cerebrospinal fluid (Zhang et al., 2012). This multicompartmentalization of the diffusion signal enables metrics such as Neurite Density Index and Orientation Dispersion Index (ODI) to characterize the amount of neurites as well as the variability of neurite orientations respectively. Both the clinical feasibility and relevance of NODDI alterations have been successfully demonstrated in normal ageing and dementia (Cox et al., 2016; Slattery et al., 2017, 2015; Zhang et al., 2012). However, the biological substrates of NODDI are still unclear due to limited evidence of cross-validation among NODDI metrics and alternative proxies of grey matter microstructure. Recent studies have shown that NODDI Orientation Dispersion Index (ODI) is correlated with histologically-derived indices of neurite dispersion (Grussu et al., 2017; Schilling et al., 2018), highlighting its potential viability as a proxy of underlying biological changes in grey matter microstructure. Of particular relevance to our current study, reductions in cortical ODI has been found in a tau-transgenic mice model, entirely consistent with the severe extent of dendritic degeneration (Colgan et al., 2016). To the best of our knowledge, there has not been any attempt to characterize the coupling of cortical ODI with measurements of synaptic pathology (with [11C]UCB-J PET imaging). Compared to PET imaging, the ODI from NODDI would have the advantage of wide scalability in multicenter studies, repeatable assays without ionizing radiation, and lower cost within a clinically feasible acquisition time (~15 minutes).
In this proof-of-concept study, we focus on 2 rapidly progressive neurodegenerative tauopathies, progressive supranuclear palsy – Richardson's Syndrome (PSP-RS) and corticobasal degeneration (CBD) as disease models where grey matter atrophy although present, is not extensive unlike in Alzheimer's disease where we would expect generalized atrophy; this allows us to examine cortical microstructure without the influence of atrophy. Pathologically, both PSP-RS and CBD are associated with accumulation of both cortical and subcortical 4-repeat tau (Dickson et al., 2011; Kovacs et al., 2020; Rösler et al., 2019; Schofield et al., 2012), and clinically present with a movement disorder (Armstrong et al., 2013; Höglinger et al., 2017; Litvan et al., 1996) and cognitive decline (Burrell et al., 2014). Both conditions are associated with cortical and subcortical atrophy (Jabbari et al., 2019) but we have previously shown significant widespread presynaptic loss extending beyond atrophic areas (Holland et al., 2020) concordant with findings from studies utilizing electromagnetoencephalography where impairment in cortical physiology is seen in both atrophic and minimally atrophic regions (Cope et al., 2018; Hughes et al., 2014; Sami et al., 2018). Having illustrated presynaptic loss as a potential explanation for impairment in cortical physiology, we aimed to investigate the synaptic bouton at large by also focusing on the postsynaptic compartment, by utilizing a non-invasive MRI method such as ODI, as an index of dendritic complexity.
There is a very high clinicopathological correlation between 4R-tauopathy and the classical presentation of PSP-RS. The clinical spectrum of CBD is diverse; the pathology of CBD is a major cause of the corticobasal syndrome (CBS), which is often mimicked by Alzheimer's disease or sometimes PSP-RS pathology (Alexander et al., 2014). For this reason, CBD generally refers to the pathology and CBS to the clinical syndrome. This strategy reduces the likelihood of mixing amyloid pathology from Alzheimer's disease with the primary 4R-tauopathy of PSP-RS and CBD. We therefore infer that patients with CBS in whom Alzheimer's disease is excluded (with [11C]PiB PET) have probable CBD, but acknowledge that other pathologies are also likely.
We test the hypothesis that the functionally relevant loss of presynaptic density caused by PSP-RS and CBD is correlated to the changes in postsynaptic dendritic complexity, independent of changes in grey matter atrophy.
2. Materials and methods
2.1. Experimental design
Patients were recruited from the Cambridge tertiary neurology clinic with for PSP-RS and CBS/CBD. Healthy volunteers were recruited from the UK National Institute for Health Research Join Dementia Research (JDR) register. Patients had either probable PSP-RS–Richardson Syndrome (Höglinger et al., 2017), or CBS with possible/probable CBD (Armstrong et al., 2013). Participants underwent a clinical and cognitive assessment including measures of disease severity (Table 1), 3T MRI, and [11C]UCB-J PET. Patients with CBD underwent amyloid PET imaging using Pittsburgh Compound B ([11C]-PiB). Only those with a negative amyloid status are included in the subsequent analysis, as determined by a [11C]-PiB standardized uptake value ratio (SUVR; 50-70 minutes post injection; whole cerebellum reference tissue) less than 1.21 (Centiloid scale 19 (Jack et al., 2017)); on this basis 12 out of 26 CBS patients were excluded. The research protocol was approved by the Cambridge Research Ethics Committee and the Administration of Radioactive Substances Advisory Committee. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Demographics and clinical variables.
| Control (N = 27) | CBD (N = 14) | PSP-RS (N = 22) | p | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 69.0 (7.34) | 70.0 (7.91) | 70.9 (8.69) | 0.715 |
| Median [Min, Max] | 72.0 [52.0, 84.0] | 70.0 [58.0, 87.0] | 73.0 [51.0, 85.0] | |
| Gender | ||||
| Female | 11 (40.7%) | 5 (35.7%) | 12 (54.5%) | 0.474 |
| Male | 16 (59.3%) | 9 (64.3%) | 10 (45.5%) | |
| Education (years) | ||||
| Mean (SD) | 14.9 (3.65) | 12.4 (3.03) | 12.1 (4.33) | 0.005 |
| Median [Min, Max] | 16.0 [10.0, 22.0] | 12.0 [7.00, 18.0] | 11.0 [9.00, 29.0] | |
| ACER | ||||
| Mean (SD) | 95.9 (3.81) | 78.2 (18.9) | 80.4 (12.7) | <0.001 |
| Median [Min, Max] | 97.0 [83.0, 100] | 83.0 [24.0, 95.0] | 81.5 [37.0, 97.0] | |
| PSPRS | ||||
| Mean (SD) | 0.148 (0.534) | 26.9 (7.88) | 33.3 (9.86) | <0.001 |
| Median [Min, Max] | 0 [0, 2.00] | 27.0 [12.0, 45.0] | 33.5 [14.0, 52.0] |
Key: ACER, Addenbrooke's cognitive examination-revised; CBD, corticobasal degeneration defined as amyloid negative corticobasal syndrome; PSP-RS, progressive supranuclear palsy - Richardson's syndrome; PSPRS, progressive supranuclear palsy rating scale. Parameters are expressed as mean ± standard deviation (SD). p values are shown for analysis of variance across groups or chi-squared test as appropriate.
2.2. Neuroimaging processing
2.2.1. T1-weighted MRI
T1-MPRAGE was acquired on the Siemens Magnetom 3T PRISMA scanner (TE = 2.93 ms, TR = 2s, slice thickness = 1.1 mm, resolution = 1.1 mm3 isotropic, 208 slices). The data were processed using Computational Anatomy Toolbox in SPM12. The T1-MPRAGE images were then segmented into grey matter, white matter, and CSF images by using a unified tissue segmentation technique after image intensity nonuniformity correction was performed. Regional cortical thickness was derived in CAT12 based on the projection-based thickness method (Dahnke et al., 2013) which uses topology correction (Yotter et al., 2011a) and spherical mapping (Yotter et al., 2011b). Previous studies have shown that CAT12 produces reliable estimates of cortical thickness, yielding larger effect sizes in case-control comparisons (Seiger et al., 2018). All segmentations were visually inspected by 3 authors (E.M, A.L. and M.M). Three PSP-RS subjects were excluded due to sub-optimal contrast between grey and white matter tissue, resulting in unsatisfactory segmentations.
2.2.2. Diffusion-weighted MRI
Diffusion scans were acquired on Siemens Magnetom Prisma scanner (TE = 75.6 ms, TR = 2.4s , slice thickness = 1.75 mm, 98 directions, 104 slices, bvals = 300, 1000, 2000). Diffusion datasets were preprocessed with FSL-FDT (FMRIB's Diffusion Toolbox). First, the DWI data were stripped of nonbrain tissue using the Brain Extraction Tool. The resulting brain masks were visually inspected for anatomic fidelity. Eddy currents and head movements were corrected with “eddy” in FSL (Version 6.0.1). TOPUP was applied to correct for estimating and correcting susceptibility induced distortions. The b0 volume from the reversed phase-encode blip was used in TOPUP for the estimation and correction of susceptibility induced distortion. Quantitative identification of slices with signal loss was performed in “eddy” and these volumes were replaced by nonparametric predictions using the Gaussian process (Andersson et al., 2016).The b-matrix was subsequently reoriented by applying the rotational part of the affine transformation used during eddy correction (Leemans and Jones, 2009). Next, ODI maps were derived from the eddy-corrected datasets using the Microstructural Diffusion Toolbox (MDT) (Harms et al., 2017). There has been recent debate over the validity of NODDI's assumption of a fixed intrinsic diffusivity across the brain, especially in the grey matter. Therefore, to optimize analyses of ODI, we set the intrinsic diffusivity to 1.1 (0.1) x 10−3 mm2/s as previously proposed (Fukutomi et al., 2018; Genç et al., 2018).
2.2.3. Positron emission tomography
A subset of patients underwent dynamic PET imaging (Controls = 19, PSP-RS = 20, CBD = 12) on a GE SIGNA PET/MR (GE Healthcare, Waukesha, USA) for 90 minutes starting immediately after [11C]UCB-J injection (median injected activity: 351 ± 107 MBq, injected mass ≤ 10 µg), with attenuation correction including the use of a multi-subject atlas method (Burgos et al., 2014; Prados et al., 2016) and also improvements to the MRI brain coil component (Manavaki et al., 2019). Each emission image series was aligned using SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12/) to ameliorate the effect of patient motion, then rigidly registered to a T1-weighted MRI acquired during PET data. To quantify SV2A density, parametric maps of [11C]UCB-J non-displaceable binding potential (BPND) was determined using a basis function implementation of the simplified reference tissue model (Wu and Carson, 2002), with the reference tissue defined in the centrum semiovale (Koole et al., 2019; Rossano et al., 2019).
2.2.4. Extraction of regional measurements from T1, ODI and [11C]UCB-J BPND
ODI and [11C]UCB-J maps were coregistered to the skull-stripped T1 brain image using rigid registrations in Advance Normalization Tools (ANTS; http://stnava.github.io/ANTs/). The accuracy of all coregistrations was visually inspected. The coregistered ODI and [11C]UCB-J volumes were projected to the surface for cortical analyses. To that end, we applied a weighted-mean method that uses a Gaussian kernel for mapping along the normal, based on the recommended settings in CAT12. Finally, region-of-interest (ROI) extraction was performed within the surface space using the Desikan Killiany atlas. We used a modified Hammers atlas to extract measurements from the subcortical regions and midbrain. The atlas was subsequently transposed into the native spaces of ODI and [11C]UCB-J respectively using the inverse of transformations from the coregistrations between ODI or [11C]UCB-J and T1 MPRAGE. Mean regional ODI, [11C]UCB-J and grey matter volumes were extracted using fslstats in FSL. Finally, each subject has the following imaging measurements: regional cortical thickness, cortical ODI, cortical [11C]UCB-J BPND, as well as subcortical and midbrain grey matter volumes, ODI and [11C]UCB-J BPND.
2.3. Statistical analysis
We used R to compare demographic variables between the diagnostic groups using ANOVA, Kruskal Wallis and chi-square tests where appropriate. To investigate regional group differences of grey matter, ODI and [11C]UCB-J BPND, we used nonparametric permutation-based inference, implemented using Permutation Analysis of Linear Models (PALM; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM) in MATLAB, adjusted for age (5000 permutations) (Winkler et al., 2014). A key advantage of permutation-based approach is the robustness of statistics to heteroscedasticity and its minimal assumptions on data distributions. Next, nonparametric permutation tests for Biological Parametric Mapping (BPM) were used to assess whole-brain inter-regional associations between ODI and [11C]UCB-J BPND across the patient sample, as well as within CBS and PSP-RS groups separately. Correlational analyses were adjusted for age and local grey matter thickness / volume. Statistical results were adjusted using the False Discovery Rate (FDR) correction across the cortical, subcortical regions and the midbrain (81 ROIs). Mean values, standard deviations, P values and Cohen's D were overlaid on 2D brain templates using the ggseg package in R (Mowinckel and Vidal-Piñeiro, 2019). The derived data that support the findings of this study are available from the corresponding author, upon reasonable request for academic (noncommercial) purposes.
3. Results
3.1. Demographics
Clinical and demographic information are summarized in Table 1. Patients and controls were matched in age and gender although education years were significantly lower in PSP-RS relative to controls (Post-hoc Dunn's Test after Kruskal-Wallis test; p < 0.001). Patients with PSP-RS and CBD had lower total ACE-R scores compared to controls (p < 0.001).
3.2. Group comparisons of grey matter atrophy, ODI and [11C]UCB-J BPND
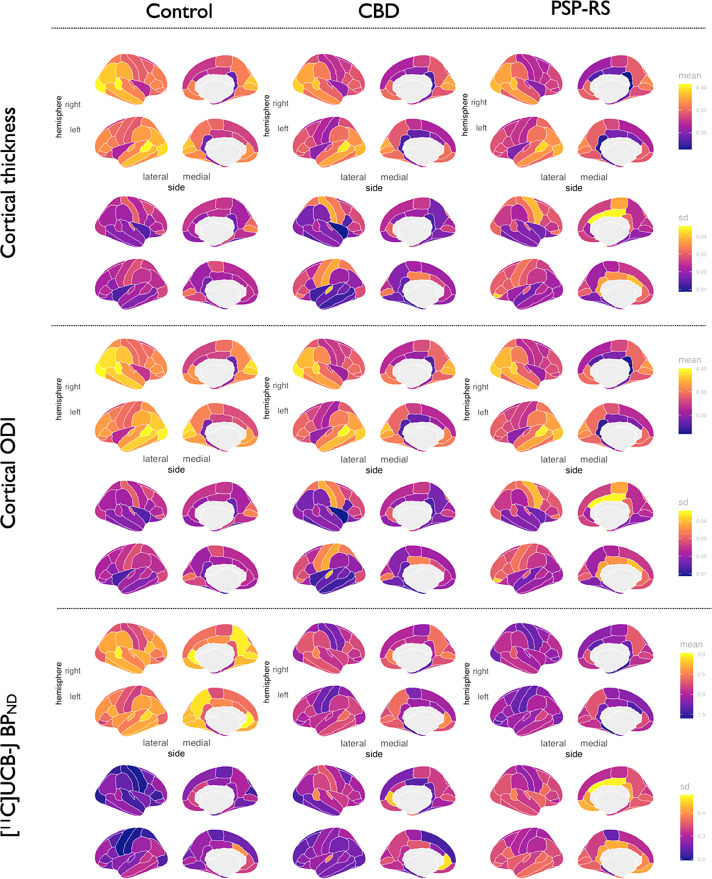
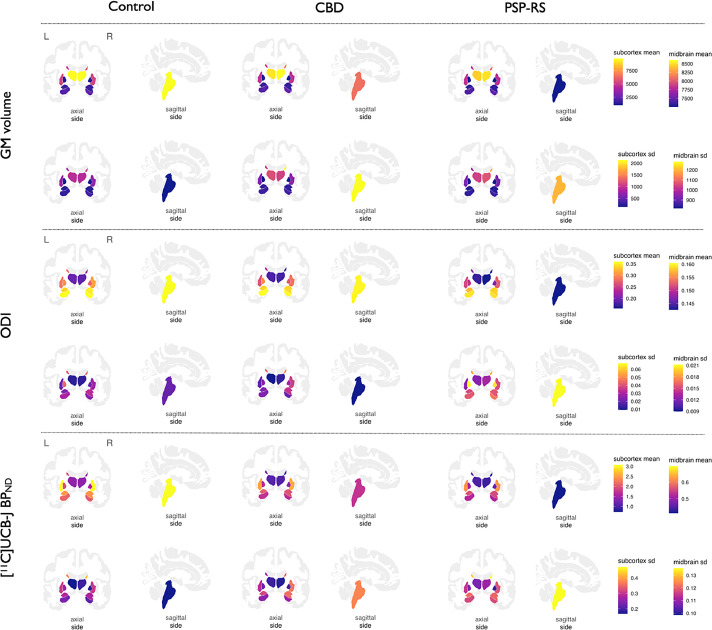
For all groups, mean ± standard deviation (SD) distributions of regional cortical thickness, subcortical grey matter volumes, ODI and [11C]UCB-J BPND are overlaid on brain templates in Fig. 1 and Fig. 2. Permutation-based statistical comparisons across all imaging measurements are reported for PSP-RS and CBD relative to controls, adjusted for age and FDR corrected in Fig. 3 and Fig. 4.
Fig. 1.
Regional distributions of cortical thickness, cortical ODI and cortical [11C]-UCB-J BPND across controls, CBD, and PSP-RS. Abbreviations: CBD, amyloid negative corticobasal syndrome; ODI, Orientation Dispersion Index; PSP-RS, progressive supranuclear palsy-Richardson's syndrome; sd, standard deviation. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Subcortical distributions of grey matter volumes, ODI and [11C]-UCB-J BPND across controls, CBD, and PSP-RS. Abbreviations: CBD, amyloid negative corticobasal syndrome; GM, grey matter; ODI, Orientation Dispersion Index; PSP-RS, progressive supranuclear palsy-Richardson's syndrome; sd, standard deviation. For ease of visualization the colors in the brainstem represent the midbrain only. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
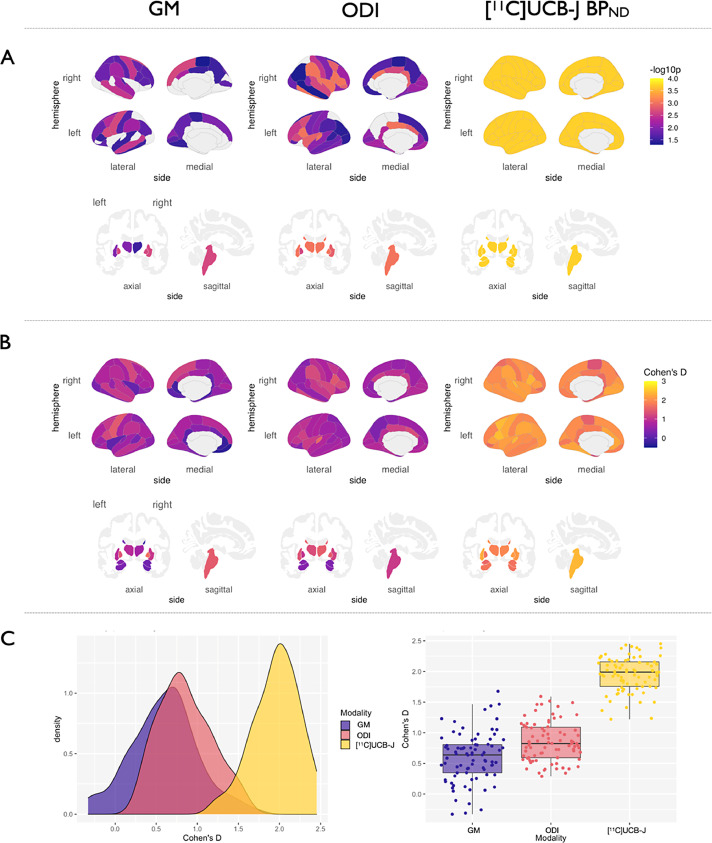
Fig. 3.
GM atrophy, reduced ODI and [11C]UCB-J BPND in PSP-RS vs. Controls. A: P-values of regions surviving FDR correction are visualized on cortical and subcortical brain templates. B: Density plots of distributions of effect sizes. C: Boxplots of effect sizes of grey matter atrophy, ODI and [11C]UCB-J BPND from the group comparisons. Abbreviations: FDR, false discovery rate; GM, grey matter ODI, Orientation Dispersion Index; PSP-RS, progressive supranuclear palsy-Richardson's syndrome. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
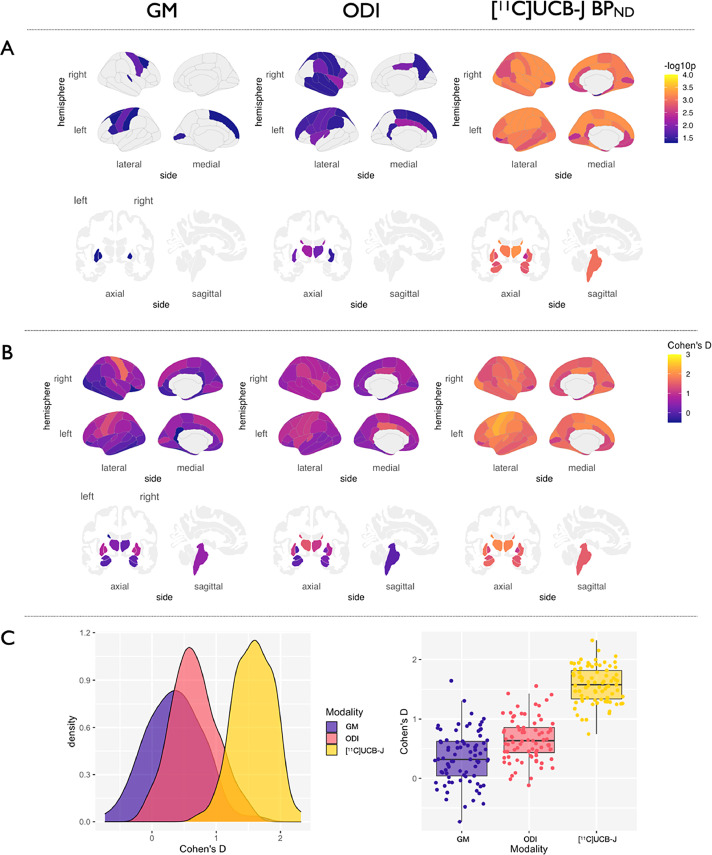
Fig. 4.
GM atrophy, reduced ODI and [11C]UCB-J BPND in CBD vs. Controls. (A) p-values of regions surviving FDR correction are visualized on cortical and subcortical brain templates. (B) Density plots of distributions of effect sizes. (C) Boxplots of effect sizes of grey matter atrophy, ODI and [11C]UCB-J BPND from the group comparisons. Abbreviations: CBD, amyloid negative corticobasal syndrome; FDR, false discovery rate; GM, grey matter; ODI, Orientation Dispersion Index. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.1. Progressive supranuclear palsy – Richardson's syndrome
Statistical results and corresponding effect sizes from the comparisons between PSP-RS and controls illustrated in Fig. 3A-C. Nonparametric permutation-based tests showed that, relative to controls, the PSP-RS group exhibited significant cortical thinning particularly in the motor cortex and frontal cortices; subcortical atrophy was found in the thalamus, putamen and pallidum and midbrain. In PSP-RS, significant reductions in cortical ODI were more widespread than areas affected by atrophy, including multiple areas within the cortical mantle and also subcortically including the basal ganglia. Post-hoc paired T-Tests of the regional Fisher's transformed Cohen's D indicated that the regional effect sizes were significantly larger for ODI compared to grey matter atrophy (Fig. 3C). As expected, the PSP-RS group showed extensive and severe reductions of [11C]UCB-J BPND across the cortex and subcortical regions compared to controls, replicating our previous findings in a smaller sample (Holland et al., 2020). Across the whole brain, reductions in [11C]UCB-J BPND yielded the largest effect size relative to both ODI and grey matter atrophy.
3.2.2. Corticobasal degeneration
Statistical results and corresponding effect sizes from the comparisons between CBD and controls are illustrated in Fig. 4A-C. Relative to controls, the CBD group showed focal cortical thinning in motor cortex, superior frontal cortex and the occipital lobe, as well as atrophy in the left putamen and bilateral pallidum. In contrast, widespread ODI reductions were found extending beyond the atrophy-affected motor cortices to other regions that were relatively preserved from atrophy, notably the temporo-parietal and cingulate cortices. In addition, there was bilateral significant ODI reductions in the caudate and putamen, both of which showed no significant grey matter atrophy. Accordingly, regional Cohen's D of ODI was significantly larger than that of grey matter atrophy (Fig. 4C). Finally, [11C]UCB-J BPND comparisons revealed a generalized and widespread extent of significantly reduced [11C]UCB-J BPND across the cortex and subcortical regions compared to controls.
3.3. Regional associations of ODI with [11C]UCB-J BPND
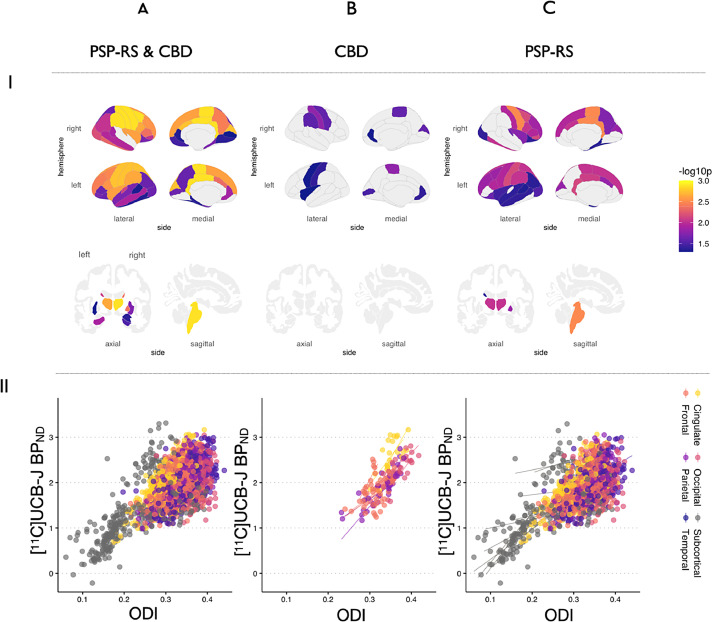
Nonparametric permutation models were used to assess the inter-regional associations between ODI and [11C]UCB-J BPND across the full patient sample, while sensitivity analyses were conducted for PSP-RS and CBD groups separately. To visualize the spatial patterns of these regional correlations, accounting for age and regional grey matter atrophy, we projected the p values on brain templates (Fig. 5). Within the total sample of CBD and PSP-RS patients, multiple cortical and subcortical regions demonstrated local positive associations where [11C]UCB-J BPND locally predicted ODI, that is, across subjects, both presynaptic density and dendritic arborization showed reciprocal associations within the same region (Fig. 5A). Scatter plots for each significant local association are shown in Supplementary Figure 1. The regions showing the strongest correlations between ODI and [11C]UCB-J BPND were primarily in bilateral pre and postcentral gyri and the prefrontal cortex, but also extended to subcortical areas including the basal ganglia, thalamus and the midbrain. When the analyses were restricted to the CBD sample, the spatial extent of local associations was markedly attenuated, although significant associations were still present within the bilateral pre and post central gyri, and isolated regions in the frontal and occipital lobe (Fig. 5B). Within the PSP-RS group, [11C]UCB-J BPND was significantly correlated with ODI in a widespread spatial pattern similar to that of the total sample. Peak correlations were identified within the motor cortex and cingulate regions (Fig. 5C), but also included the thalami, midbrain and parts of the basal ganglia. These analyses did not include the control group and are thus not indicative of a group effect. Nevertheless, to determine the specificity of the coupling between ODI and [11C]UCB-J BPND, we ran the same nonparametric permutation model on the controls, accounting for age and regional grey matter atrophy. This analysis did not yield any significant local associations that retained statistical significance after FDR correction.
Fig. 5.
Local associations between ODI and [11C]UCB-J BPND in (A) total sample of patients, (B) CBD and (C) PSP-RS separately. I: Statistical p values for regions surviving FDR correction are overlaid on cortical and subcortical brain templates. II: Scatter plots showing the relationships between ODI and [11C]UCB-J BPND for all regions surviving FDR correction, colored by lobes and subcortex. Abbreviations: CBD, amyloid negative corticobasal syndrome; ODI, orientation dispersion index; PSP-RS, progressive supranuclear palsy-Richardson's syndrome. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
This study tested the hypothesis that changes in synaptic density are closely linked to MRI measures of cortical microstructure, in the neurodegenerative tauopathies of CBD and PSP-RS. The main insights are that (i) in the neurodegenerative tauopathies of CBD and PSP-RS, widespread changes in grey matter dendritic complexity are observed even in areas without significant atrophy; and (ii) NODDI ODI, an MRI-based measure of postsynaptic dendritic structure and complexity, correlates with the loss of presynaptic density estimated by the PET radioligand [11C]UCB-J; this effect is not a result of changes in grey matter atrophy. Our findings extend previous investigations of ODI microstructural changes in PSP-RS and CBS/CBD (Mitchell et al., 2019), demonstrating the in vivo coupling of dendritic complexity to presynaptic density, in line with preclinical models of tauopathies (Hoffmann et al., 2014; Rocher et al., 2010).
Grey matter atrophy within the motor cortex, as well as the basal ganglia and brainstem are common MRI findings in PSP-RS and CBD; the pattern of atrophy shown in Fig. 1 and Fig. 2 concords with previous studies (Jabbari et al., 2019). In this study however we show that, both within areas of the brain where there is atrophy, and in areas with absent atrophy, there is significant and more severe loss of dendritic complexity and, as previously shown (Holland et al., 2020) and replicated here, presynaptic density in the patient cohort. The widespread pattern of cortical ODI and presynaptic deficits in PSP-RS and CBD matches the expected pattern of tau pathology, both in the subcortical and cortical area (Dickson et al., 2011; Kovacs et al., 2020). Loss of dendritic complexity and presynaptic loss, in nonatrophied areas of the brain, for example the occipital lobes, potentially reflects early changes in synaptic function, in response to tau pathology (Kovacs et al., 2020), or toxic oligomers of tau, that later in the disease process progress to atrophy. Indeed, in preclinical models, pathological tau oligomers induce synaptic degeneration (Usenovic et al., 2015) and interfere with synaptic function and density (Yoshiyama et al., 2007), in the absence of neuronal loss.
The effect size for the comparison between ODI and [11C]UCB-J BPND in patients relative to controls, was stronger than for atrophy. Together, the larger extent of ODI reductions relative to grey matter atrophy indicate that NODDI can reveal new aspects of the cellular pathology of tauopathies, in keeping with the recent demonstration that changes in ODI parameters closely reflect complex histological changes (Grussu et al., 2017). Notwithstanding the cross-sectional design of this study, this observation highlights the early structural changes in disease pathogenesis that may underlie the emergence of cognitive and motor symptoms not attributable to atrophy. Indeed studies of cortical physiology in PSP-RS have illustrated abnormal electrophysiology in the absence of atrophy (Hughes et al., 2013; Sami et al., 2018). Our findings of reduced ODI beyond atrophy are in agreement with those published from the Alzheimer's disease literature (Parker et al., 2018) which is a related tauopathy to PSP-RS and CBD, where there is a stronger relationship between cognitive function and synaptic density than with atrophy (Terry et al., 1991; Masliah et al., 1992; Scheff et al., 2006).
Our observation of a tight coupling between dendritic complexity, as measured with ODI, and presynaptic density, as measured with [11C]UCB-J, in the patient cohort, echo preclinical findings in animal models of tauopathy (Harris and Kater, 1994), and postmortem studies (Bigio et al., 2001; Lipton et al., 2001). There are 2 potential explanation for this tight coupling: first, tau protein is enriched in axons and associated with axonal growth and transport; the synaptic toxicity associated with pathological tau is observed both in pre- and postsynaptic structures (see review by Wang & Mendelkow 2015 (Wang and Mandelkow, 2016)), leading to both a reduction in dendritic complexity and presynaptic density. Second, while a cross-sectional design precludes inferences of causality, alterations in synaptic function in CBD and PSP-RS (Holland et al., 2020) are expected to induce dendritic morphological alterations with early loss of dendritic spines and reduced dendritic branching. This has been illustrated in animal studies, where postsynaptic dendritic morphology correlates with the numbers of presynaptic vesicles and synaptic strength (Schikorski and Stevens, 1999). It is possible that primary dendritic degeneration in CBD and PSP-RS causes a reduction in the density of the presynaptic contacts on the distal dendrites. These alternative accounts are not mutually exclusive and could act in parallel to impair effective transneuronal connectivity, and subsequently motor and cognitive function.
The downstream effect of the above is a loss of functional connectivity. Indeed in PSP-RS there is reduced resting state connectivity between cortical and subcortical areas - reviewed in (Filippi et al., 2019). In CBD, the evidence is mixed as, thus far, functional MRI studies in this disease have not differentiated between amyloid positive and amyloid negative participants (also reviewed in (Filippi et al., 2019); in the former where Alzheimer's pathology is the most likely finding at post mortem, functional connections are strengthened; however in the latter where a 4R tauopathy is likely, functional connections may become inefficient as seen in PSP-RS (Cope et al., 2018).
Synaptic pathology is a common feature in many neurodegenerative diseases (Clare et al., 2010; Herms and Dorostkar, 2016; Kweon et al., 2017). In the related tauopathy of Alzheimer's disease, widespread reduction in synapses has been shown using [11C]UCB-J PET in patients (Mecca et al., 2020), as well as changes to dendritic complexity seen both in vivo as reduced ODI (Parker et al., 2018) and in animal models of Alzheimer's disease (Dorostkar et al., 2015). Similar pre- and postsynaptic losses are seen in the substantia nigra of patients with Parkinson's disease (Bellucci et al., 2016; Reeve et al., 2018), and within the hippocampi of patients with Lewy Body Dementia (Revuelta et al., 2008). The tight coupling of the presynaptic and postsynaptic compartments shown in our study, may therefore extend to pathologies other than primary 4R-tauopathies, where synaptic dysfunction is one of the earliest stages of disease, regardless of the culprit protein aggregate in question. If so, the opportunity to utilize both MRI and PET to understand disease pathogenesis would greatly facilitate cohort studies of individual phenotypic differences, or repeat testing in clinical trials monitoring. Furthermore, the in vivo observation of the tight coupling between the pre- and postsynaptic compartments, offer a new angle of interpretation for future studies utilizing either of these methods in isolation; for example if cost, scalability and resources are limited for larger cohort studies, MRI measures of dendritic complexity can offer a close surrogate of presynaptic density, although we appreciate it is not a substitute given the significantly larger effect sizes seen with [11C]UCB-J (Figs. 3C and 4C). This latter observation, which replicates our previous study in a smaller cohort of patients, may indicate a more severe toxic effect at the presynapse compared to dendritic pathology, although longitudinal studies are required to confirm this. However, it is important to note that ODI should not be taken as a direct representation of the postsynaptic density seen in histological studies. Rather, it provides complementary information to the PET data, with support from histological studies that have shown ODI to be a close surrogate (Grussu et al., 2017).
There are several strengths of this study. First, our NODDI resolution of 1.75 mm was specifically optimized for the investigation of cortical microstructure, and is higher than previous NODDI studies in early onset AD (2.5 mm) (Parker et al., 2018), Parkinson's disease (5 mm) (Kamagata et al., 2016) and PSP-RS (2.0 mm) (Mitchell et al., 2019). Second, we use amyloid imaging to exclude patients with CBS likely due to Alzheimer's disease. This helps in reducing the potential underlying pathologies at play in the CBS cohort to 4R tauopathy, although we acknowledge other pathologies are possible but less likely (Alexander et al., 2014) – in this regard it is reassuring that the spatial patterns of reductions in ODI and [11C]UCB-J binding are not substantially different between the PSP-RS and CBD cohorts. Thirdly, the use of nonparametric permutation analyses also confer additional robustness to our statistical results, since this approach is not reliant on data distributions (Winkler et al., 2014).
We acknowledge the limitations of our study including the relatively small sample size in our CBD cohort. However, given the large effect sizes seen of ODI and [11C]UCB-J binding reduction in both our patient groups, our study was sufficiently powered to examine the relationships between both imaging markers. Secondly, we acknowledge that the MRI measure of ODI is not a measure of postsynaptic density but rather dendritic complexity; PET radioligands targeting the postsynaptic density may therefore provide further useful insights into postsynaptic pathology. Furthermore, although we illustrate a tight correlation between our 2 measures of synaptic health, we do not show causality in this cross-sectional study design, but are working towards this aim through our longitudinal studies. Lastly, we highlight the limitations of clinicopathological correlations, but take reassurance from previous validation studies illustrating a high consistency in the clinicopathological correlations in PSP-RS and in amyloid negative CBS.
Understanding the pathological processes that precede atrophy in neurodegeneration is key not only in expanding our knowledge of the pathophysiology of disease but also in informing the design of clinical trials both in terms of the imaging options for measuring disease-related changes and the optimal timing of intervention in the disease process. Our data implicate correlated changes in dendritic microstructure and synaptic density in patients with primary degenerative tauopathies including PSP-RS and CBD. Further cross-validation of ODI with [11C]UCB-J BPND may help further our understanding of the pathophysiology of neurodegeneration, applicable to future studies of early neurodegeneration with a safe and widely available MRI platform.
Author contributions
Elijah Mak: Conceptualisation, Methodology, Software, Validation, Formal analysis, Writing Original Draft, Writing – Review & Editing, Visualisation. Negin Holland: Conceptualisation, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing Original Draft, Writing – Review & Editing, Visualisation, Project administration P. Simon Jones: Validation, Writing – Review & Editing. George Savulich: Data Curation, project administration, Writing – Review & Editing. Audrey Low: Validation, Writing – Review & Editing. Maura Malpetti: Validation, Writing – Review & Editing. Sanne S Kaalund: conceptualization, Writing – Review & Editing. Luca Passamonti: conceptualization, Writing – Review & Editing, Timothy Rittman: conceptualisation, Writing – Review & Editing. Rafael Romero-Garcia: Methodology, Software, Validation, Formal analysis, Writing – Review & Editing. Roido Manavaki: Formal analysis, Data Curation, Investigation. Guy B. Williams: Methodology. Young T. Hong: Methodology, Software, Formal analysis, Resources. Tim D. Fryer: Methodology, Software, Resources, Writing – Review & Editing. Franklin I. Aigbirhio: Resources, Writing – Review & Editing. John T O'Brien: Conceptualisation, Writing – Review & Editing, Supervision, Funding acquisition. James B Rowe: Conceptualisation, Writing – Review & Editing, Supervision, Funding acquisition.
Disclosures
James B Rowe serves as an associate editor to Brain and is a nonremunerated trustee of the Guarantors of Brain, Darwin College and the PSP Association (UK). He provides consultancy to Asceneuron, Biogen, UCB, Althira, Astex, SVHealth and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK. John T. O'Brien has no conflicts related to this study; unrelated to this work he has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai, has acted as a consultant for Roche, has received research support from Alliance Medical and Merck.
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
All authors have reviewed and approved the submission of the data. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding
The study was funded by the Cambridge University Centre for Parkinson-Plus (RG95450); the National Institute for Health Research Cambridge Biomedical Research Centre (146281; BRC-1215-20014); the Wellcome Trust (103838) and the Association of British Neurologists, Patrick Berthoud Charitable Trust (NH: RG99368) and Medical Research Council (SUAG 051/G101400). EM is supported by Alzheimer's Society Junior Research Fellowship (443 AS JF 18017). RRG is supported by the Guarantors of Brain Fellowship (RNAG/474). AL is supported by the Lee Kuan Yew Fitzwilliam Scholarship and the Tan Kah Kee Scholarship.
Declaration of interests
James B Rowe serves as an associate editor to Brain and is a nonremunerated trustee of the Guarantors of Brain, Darwin College and the PSP-RS Association (UK). He provides consultancy to Asceneuron, Biogen, UCB, Althira, Astex, SVHealth and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK. John T. O'Brien has no conflicts related to this study; unrelated to this work he has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai, has acted as a consultant for Roche, has received research support from Alliance Medical and Merck. TR has received honoraria from Biogen, unrelated to this work.
Acknowledgements
The authors thank the research participants and caregivers, the staff at the Wolfson Brain Imaging Centre, and at the Cambridge Centre for Parkinson-Plus. We thank UCB Pharma for providing the precursor for UCB-J synthesis. Infrastructure support was provided by the High Performance Hubs for Clinical Informatics (HPHI), funded by the MRC Research Infrastructure Award (MR/M009041/1).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2021.01.016.
Appendix. Supplementary materials
References
- Alexander S.K., Rittman T., Xuereb J.H., Bak T.H., Hodges J.R., Rowe J.B. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J. Neurol. Neurosurg. Psychiatry. 2014;85:923–927. doi: 10.1136/jnnp-2013-307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Graham M.S., Zsoldos E., Sotiropoulos S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B., Boxer A.L., Dickson D.W., Grossman M., Hallett M., Josephs K.A., Kertesz A., Lee S.E., Miller B.L., Reich S.G., Riley D.E., Tolosa E., Tröster A.I., Vidailhet M., Weiner W.J. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci A., Mercuri N.B., Venneri A., Faustini G., Longhena F., Pizzi M., Missale C., Spano P. Review: Parkinson's disease: from synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016;42:77–94. doi: 10.1111/nan.12297. [DOI] [PubMed] [Google Scholar]
- Bigio E.H., Vono M.B., Satumtira S., Adamson J., Sontag E., Hynan L.S., White C.L., Baker M., Hutton M. Cortical synapse loss in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2001;60:403–410. doi: 10.1093/jnen/60.5.403. [DOI] [PubMed] [Google Scholar]
- Blin J., Vidailhet M.-J., Pillon B., Dubois B., Feve J.-R., Agid Y. Corticobasal degeneration: decreased and asymmetrical glucose consumption as studied with PET. Mov. Disord. 1992;7:348–354. doi: 10.1002/mds.870070409. [DOI] [PubMed] [Google Scholar]
- Burgos N., Cardoso M.J., Thielemans K., Modat M., Pedemonte S., Dickson J., Barnes A., Ahmed R., Mahoney C.J., Schott J.M., Duncan J.S., Atkinson D., Arridge S.R., Hutton B.F., Ourselin S. Attenuation correction synthesis for hybrid PET-MR scanners: application to brain studies. IEEE Trans. Med. Imaging. 2014;33:2332–2341. doi: 10.1109/TMI.2014.2340135. [DOI] [PubMed] [Google Scholar]
- Burrell J.R., Hodges J.R., Rowe J.B. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov. Disord. 2014;29:684–693. doi: 10.1002/mds.25872. [DOI] [PubMed] [Google Scholar]
- Chen M.K., Mecca A.P., Naganawa M., Finnema S.J., Toyonaga T., Lin S.F., Najafzadeh S., Ropchan J., Lu Y., McDonald J.W., Michalak H.R., Nabulsi N.B., Arnsten A.F.T., Huang Y., Carson R.E., Van Dyck C.H. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 2018;75:1215–1224. doi: 10.1001/jamaneurol.2018.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare R., King V.G., Wirenfeldt M., Vinters H.V. Synapse loss in dementias. J. Neurosci. Res. 2010 doi: 10.1002/jnr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan N., Siow B., O'Callaghan J.M., Harrison I.F., Wells J.A., Holmes H.E., Ismail O., Richardson S., Alexander D.C., Collins E.C., Fisher E.M., Johnson R., Schwarz A.J., Ahmed Z., O'Neill M.J., Murray T.K., Zhang H., Lythgoe M.F. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer's disease. Neuroimage. 2016;125:739–744. doi: 10.1016/j.neuroimage.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope T.E., Rittman T., Borchert R.J., Jones P.S., Vatansever D., Allinson K., Passamonti L., Vazquez Rodriguez P., Bevan-Jones W.R., O'Brien J.T., Rowe J.B. Tau burden and the functional connectome in Alzheimer's disease and progressive supranuclear palsy. Brain. 2018;141:550–567. doi: 10.1093/brain/awx347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S.R., Ritchie S.J., Tucker-Drob E.M., Liewald D.C., Hagenaars S.P., Davies G., Wardlaw J.M., Gale C.R., Bastin M.E., Deary I.J. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat. Commun. 2016;7:13629. doi: 10.1038/ncomms13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Gilchrist I.T.S., Dick K.M., Patterson K., Rodríquez P.V., Wehmann E., Wilcox A., Lansdall C.J., Dawson K.E., Wiggins J., Mead S., Brayne C., Rowe J.B. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahnke R., Yotter R.A., Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. doi: 10.1016/j.neuroimage.2012.09.050. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T., Scheff S.W. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Dickson D.W., Kouri N., Murray M.E., Josephs K.A. Springer; 2011. Neuropathology of Frontotemporal Lobar Degeneration-Tau (FTLD-Tau), in: Journal of Molecular Neuroscience; pp. 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostkar M.M., Zou C., Blazquez-Llorca L., Herms J. Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Sarasso E., Agosta F. Resting-state functional MRI in Parkinsonian syndromes. Mov. Disord. Clin. Pract. 2019;6:104–117. doi: 10.1002/mdc3.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema S.J., Nabulsi N.B., Eid T., Detyniecki K., Lin S.F., Chen M.K., Dhaher R., Matuskey D., Baum E., Holden D., Spencer D.D., Mercier J., Hannestad J., Huang Y., Carson R.E. Imaging synaptic density in the living human brain. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- Finnema S.J., Nabulsi N.B., Mercier J., Lin S., Chen M.-K., Matuskey D., Gallezot J.-D., Henry S., Hannestad J., Huang Y., Carson R.E. Kinetic evaluation and test–retest reproducibility of [11 C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J. Cereb. Blood Flow Metab. 2017 doi: 10.1177/0271678X17724947. 0271678X1772494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi H., Glasser M.F., Zhang H., Autio J.A., Coalson T.S., Okada T., Togashi K., Van Essen D.C., Hayashi T. Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. Neuroimage. 2018;1–12 doi: 10.1016/j.neuroimage.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç E., Fraenz C., Schlüter C., Friedrich P., Hossiep R., Voelkle M.C., Ling J.M., Güntürkün O., Jung R.E. Diffusion markers of dendritic density and arborization in gray matter predict differences in intelligence. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-04268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grussu F., Schneider T., Tur C., Yates R.L., Tachrount M., Ianuş A., Yiannakas M.C., Newcombe J., Zhang H., Alexander D.C., DeLuca G.C., Gandini Wheeler-Kingshott C.A.M. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann. Clin. Transl. Neurol. 2017;4:663–679. doi: 10.1002/acn3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms R.L., Fritz F.J., Tobisch A., Goebel R., Roebroeck A. Robust and fast nonlinear optimization of diffusion MRI microstructure models. Neuroimage. 2017;155:82–96. doi: 10.1016/j.neuroimage.2017.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.M., Kater S.B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994 doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Herms J., Dorostkar M.M. Dendritic spine pathology in neurodegenerative diseases. Annu. Rev. Pathol. Mech. Dis. 2016;11:221–250. doi: 10.1146/annurev-pathol-012615-044216. [DOI] [PubMed] [Google Scholar]
- Hoffmann N.A., Dorostkar M.M., Blumenstock S., Goedert M., Herms J. Impaired plasticity of cortical dendritic spines in P301S tau transgenic mice. Acta Neuropathol. Commun. 2014;2:82. doi: 10.1186/2051-5960-1-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G.U., Respondek G., Stamelou M., Kurz Carolin, Josephs K.A., Lang A.E., Mollenhauer B., Müller U., Nilsson C., Whitwell J.L., Arzberger T., Englund E., Gelpi E., Giese A., Irwin D.J., Meissner W.G., Pantelyat A., Rajput A., van Swieten J.C., Troakes C., Antonini A., Bhatia K.P., Bordelon Y., Compta Y., Corvol J.C., Colosimo C., Dickson D.W., Dodel R., Ferguson L., Grossman M., Kassubek J., Krismer F., Levin J., Lorenzl S., Morris H.R., Nestor P., Oertel W.H., Poewe W., Rabinovici G., Rowe J.B., Schellenberg G.D., Seppi K., van Eimeren T., Wenning G.K., Boxer A.L., Golbe L.I., Litvan I., Boxer A.L., Rajput A., Pantelyat A., Antonini A., Lang A.E., Giese A., Mollenhauer B., Colosimo C., Kurz Caroline, Nilsson C., Troakes C., Irwin D.J., Dickson D.W., Gelpi E., Krismer F., Schellenberg G.D., Respondek G., Rabinovici G., Wenning G.K., Höglinger G.U., Morris H.R., Litvan I., Rowe J.B., Kassubek J., Corvol J.C., Whitwell J.L., Levin J., van Swieten J., Bhatia K.P., Josephs K.A., Seppi K., Golbe L.I., Grossman M., Nestor P., Dodel R., Lorenzl S., van Eimeren T., Arzberger T., Müller U., Meissner W.G., Poewe W., Oertel W.H., Compta Y., Bordelon Y. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N., Jones P.S., Savulich G., Wiggins J.K., Hong Y.T., Fryer T.D., Manavaki R., Sephton S.M., Boros I., Malpetti M., Hezemans F.H., Aigbirhio F.I., Coles J.P., O'Brien J., Rowe J.B. Synaptic loss in primary tauopathies revealed by [11 C] UCB-JPositron Emission Tomography. Mov. Disord. mds. 2020 doi: 10.1002/mds.28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.E., Ghosh B.C.P., Rowe J.B. Reorganisation of brain networks in frontotemporal dementia and progressive supranuclear palsy. NeuroImage Clin. 2013;2:459–468. doi: 10.1016/j.nicl.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.E., Rowe J.B., Ghosh B.C.P., Carlyon R.P., Plack C.J., Gockel H.E. The binaural masking level difference: cortical correlates persist despite severe brain stem atrophy in progressive supranuclear palsy. J. Neurophysiol. 2014;112:3086–3094. doi: 10.1152/jn.00062.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari E., Holland N., Chelban V., Jones P.S., Lamb R., Rawlinson C., Guo T., Costantini A.A., Tan M.M.X., Heslegrave A.J., Roncaroli F., Klein J.C., Ansorge O., Allinson K.S.J., Jaunmuktane Z., Holton J.L., Revesz T., Warner T.T., Lees A.J., Zetterberg H., Russell L.L., Bocchetta M., Rohrer J.D., Williams N.M., Grosset D.G., Burn D.J., Pavese N., Gerhard A., Kobylecki C., Leigh P.N., Church A., Hu M.T.M., Woodside J., Houlden H., Rowe J.B., Morris H.R. Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C.R., Wiste, H.J., Weigand, S.D., Therneau, T.M., Lowe, V.J., Knopman, D.S., Gunter, J.L., Senjem, M.L., Jones, D.T., Kantarci, K., Machulda, M.M., Mielke, M.M., Roberts, R.O., Vemuri, P., Reyes, D.A., Petersen, R.C., 2017. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's Dement. 13, 205–216. https://doi.org/10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed]
- Juh R., Kim J., Moon D., Choe B., Suh T. Different metabolic patterns analysis of Parkinsonism on the 18 F-FDG PET. Eur. J. Radiol. 2004;51:223–233. doi: 10.1016/S0720-048X(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Kamagata K., Hatano T., Okuzumi A., Motoi Y., Abe O., Shimoji K., Kamiya K., Suzuki M., Hori M., Kumamaru K.K., Hattori N., Aoki S. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur. Radiol. 2016;26:2567–2577. doi: 10.1007/s00330-015-4066-8. [DOI] [PubMed] [Google Scholar]
- Koole M., van Aalst J., Devrome M., Mertens N., Serdons K., Lacroix B., Mercier J., Sciberras D., Maguire P., Van Laere K. Quantifying SV2A density and drug occupancy in the human brain using [11 C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:396–406. doi: 10.1007/s00259-018-4119-8. [DOI] [PubMed] [Google Scholar]
- Kovacs G.G., Lukic M.J., Irwin D.J., Arzberger T., Respondek G., Lee E.B., Coughlin D., Giese A., Grossman M., Kurz C., McMillan C.T., Gelpi E., Compta Y., van Swieten J.C., Laat L.D., Troakes C., Al-Sarraj S., Robinson J.L., Roeber S., Xie S.X., Lee V.M.-Y., Trojanowski J.Q., Höglinger G.U. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020:1–21. doi: 10.1007/s00401-020-02158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon J.H., Kim S., Lee S.B. The cellular basis of dendrite pathology in neurodegenerative diseases. BMB Rep. 2017;50:5–11. doi: 10.5483/BMBRep.2017.50.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lipton A.M., Munro Cullum C., Satumtira S., Sontag E., Hynan L.S., White C.L., Bigio E.H. Contribution of asymmetric synapse loss to lateralizing clinical deficits in frontotemporal dementias. Arch. Neurol. 2001;58:1233–1239. doi: 10.1001/archneur.58.8.1233. [DOI] [PubMed] [Google Scholar]
- Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., Goetz C.G., Golbe L.I., Grafman J., Growdon J.H., Hallett M., Jankovic J., Quinn N.P., Tolosa E., Zee D.S. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology. 1996 doi: 10.1212/WNL.47.1.1. [DOI] [PubMed] [Google Scholar]
- Manavaki R., Hong Y.T., Fryer T.D. Brain MRI coil attenuation map processing for the GE SIGNA PET/MR: impact on PET image quantification and uniformity. 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC) 2019:1–2. doi: 10.1109/NSS/MIC42101.2019.9059867. [DOI] [Google Scholar]
- Masliah E., Ellisman M., Carragher B., Mallory M., Young S., Hansen L., DeTeresa R., Terry R.D. Three-dimensional analysis of the relationship between synaptic pathology and neuropil threads in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1992;51:404–414. doi: 10.1097/00005072-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Mecca A.P., Chen M., O'Dell R.S., Naganawa M., Toyonaga T., Godek T.A., Harris J.E., Bartlett H.H., Zhao W., Nabulsi N.B., Wyk B.C.Vander, Varma P., Arnsten A.F.T., Huang Y., Carson R.E., Dyck C.H. in vivo measurement of widespread synaptic loss in Alzheimer's disease with SV2A PET. Alzheimer's Dement. 2020;alz.12097 doi: 10.1002/alz.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T., Archer D.B., Chu W.T., Coombes S.A., Lai S., Wilkes B.J., McFarland N.R., Okun M.S., Black M.L., Herschel E., Simuni T., Comella C., Xie T., Li H., Parrish T.B., Kurani A.S., Corcos D.M., Vaillancourt D.E. Neurite orientation dispersion and density imaging (NODDI) and free-water imaging in Parkinsonism. Hum. Brain Mapp. 2019;40:5094–5107. doi: 10.1002/hbm.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L., Tsui W.H., De Santi S., Li J., Rusinek H., Convit A., Li Y., Boppana M., De Leon M.J. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- Mowinckel, A.M., Vidal-Piñeiro, D., 2019. Visualisation of brain statistics with R-packages ggseg and ggseg3d. arXiv. doi: 10.1177/2515245920928009. [DOI]
- Parker T.D., Slattery C.F., Zhang J., Nicholas J.M., Paterson R.W., Foulkes A.J.M., Malone I.B., Thomas D.L., Modat M., Cash D.M., Crutch S.J., Alexander D.C., Ourselin S., Fox N.C., Zhang H., Schott J.M. Cortical microstructure in young onset Alzheimer's disease using neurite orientation dispersion and density imaging. Hum. Brain Mapp. 2018;39:3005–3017. doi: 10.1002/hbm.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados, F., Cardoso, M.J., Burgos, N., Wheeler-Kingshott, C., Ourselin, S., Angela, C., Gandini, M., Ourselin, S., 2016. NiftyWeb: web based platform for image processing on the cloud, in: 24th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine (ISMRM) Abstract available from https://discovery.ucl.ac.uk/id/eprint/1476510/.
- Reeve, A.K., Grady, J.P., Cosgrave, E.M., Bennison, E., Chen, C., Hepplewhite, P.D., Morris, C.M., 2018. Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson's disease. npj Park. Dis. 4, 1–10. doi: 10.1038/s41531-018-0044-6. [DOI] [PMC free article] [PubMed]
- Revuelta G.J., Rosso A., Lippa C.F. Neuritic pathology as a correlate of synaptic loss in dementia with Lewy bodies. Am. J. Alzheimers. Dis. Other Demen. 2008;23:97–102. doi: 10.1177/1533317507310565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher A.B., Crimins J.L., Amatrudo J.M., Kinson M.S., Todd-Brown M.A., Lewis J., Luebke J.I. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp. Neurol. 2010;223:385–393. doi: 10.1016/j.expneurol.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler T.W., Tayaranian Marvian A., Brendel M., Nykänen N.P., Höllerhage M., Schwarz S.C., Hopfner F., Koeglsperger T., Respondek G., Schweyer K., Levin J., Villemagne V.L., Barthel H., Sabri O., Müller U., Meissner W.G., Kovacs G.G., Höglinger G.U. Four-repeat tauopathies. Prog. Neurobiol. 2019 doi: 10.1016/j.pneurobio.2019.101644. [DOI] [PubMed] [Google Scholar]
- Rossano S., Toyonaga T., Finnema S.J., Naganawa M., Lu Y., Nabulsi N., Ropchan J., De Bruyn S., Otoul C., Stockis A., Nicolas J.M., Martin P., Mercier J., Huang Y., Maguire R.P., Carson R.E. Assessment of a white matter reference region for 11C-UCB-J PET quantification. J. Cereb. Blood Flow Metab. 2019 doi: 10.1177/0271678X19879230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S., Williams N., Hughes L.E., Cope T.E., Rittman T., Coyle-Gilchrist I.T.S., Henson R.N., Rowe J.B. Neurophysiological signatures of Alzheimer's disease and frontotemporal lobar degeneration: pathology versus phenotype. Brain. 2018;141:2500–2510. doi: 10.1093/brain/awy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S.W., Price D.A., Schmitt F.A., Mufson E.J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schikorski T., Stevens C.F. Quantitative fine-structural analysis of olfactory cortical synapses. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4107–4112. doi: 10.1073/pnas.96.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K.G., Janve V., Gao Y., Stepniewska I., Landman B.A., Anderson A.W. Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2017.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield E.C., Hodges J.R., Bak T.H., Xuereb J.H., Halliday G.M. The relationship between clinical and pathological variables in Richardson's syndrome. J. Neurol. 2012;259:482–490. doi: 10.1007/s00415-011-6205-8. [DOI] [PubMed] [Google Scholar]
- Seiger R., Ganger S., Kranz G.S., Hahn A., Lanzenberger R. Cortical thickness estimations of FreeSurfer and the CAT12 toolbox in patients with Alzheimer's disease and healthy controls. J. Neuroimaging. 2018;28:515–523. doi: 10.1111/jon.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery C.F., Zhang J., Paterson R.W., Foulkes A.J.M., Carton A., Macpherson K., Mancini L., Thomas D.L., Modat M., Toussaint N., Cash D.M., Thornton J.S., Henley S.M.D., Crutch S.J., Alexander D.C., Ourselin S., Fox N.C., Zhang H., Schott J.M. ApoE influences regional white-matter axonal density loss in Alzheimer's disease. Neurobiol. Aging. 2017;57:8–17. doi: 10.1016/j.neurobiolaging.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery C.F., Zhang J., Paterson R.W., Foulkes A.J.M., Mancini L., Thomas D.L., Modat M., Toussaint N., Cash D.M., Thornton J.S., Alexander D.C., Ourselin S., Fox N.C., Zhang H., Schott J.M. Neurite orientation dispersion and density imaging (NODDI) in young-onset Alzheimer's disease and its syndromic variants. Alzheimer's Dement. 2015;11:P91. doi: 10.1016/j.jalz.2015.06.156. [DOI] [Google Scholar]
- Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Usenovic M., Niroomand S., Drolet R.E., Yao L., Gaspar R.C., Hatcher N.G., Schachter J., Renger J.J., Parmentier-Batteur S. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 2015;35:14234–14250. doi: 10.1523/JNEUROSCI.1523-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016 doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Carson R.E. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. Cereb. Blood Flow Metab. 2002 doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M.Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Yotter R.A., Dahnke R., Thompson P.M., Gaser C. Topological correction of brain surface meshes using spherical harmonics. Hum. Brain Mapp. 2011;32:1109–1124. doi: 10.1002/hbm.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotter R.A., Thompson P.M., Gaser C. Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J. Neuroimaging. 2011;21:1–14. doi: 10.1111/j.1552-6569.2010.00484.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.