Abstract
As a potential chemo-therapeutic agent, all-trans retinoic acid (ATRA) can significantly reverse epithelial-mesenchymal transition (EMT) of hepal-6 hepatocarcinoma cell line in vitro, but the mechanism is unclear. The expression profile of microRNA-200 (miR-200) families is different in hepatocellular carcinoma. In this study, we found that ATRA treatment could up-regulate the expression of miR-200a-3p, 200c-3p, and 141-3p, which were involved in ATRA regulated proliferation and apoptosis of hepal-6 cell, but not colony formation. Meanwhile, miR-200a-3p, 200c-3p, and 141-3p could recovery ATRA inhibited migration and invasion abilities of hepal-6 cells at various levels. miR-200a-3p and 200c-3p prevented ATRA from inducing the differentiation and hepatic functions of hepal-6 cells. Antagomir specific for miR-200a-3p and 200c-3p down-regulated the expression of CK18, but only miR-200a-3p antagomir played prominent role in regulating the expression of these mesenchymal markers, N-Cadherin, Snail and Twist. The transcriptional activities of 8 transcription factors were up-regulated and 35 transcription factors were down-regulated by ATRA. Compared with ATRA group, inhibition of miR-200a-3p, 200c-3p, and 141-3p significantly strengthened the expression of Fra1/Jun (AP1), Ets1/PEA3, Brn3, and Zeb1/AREB6 at varying degrees. Therefore, this result suggested that ATRA may suppress EMT through down-regulating miR-200a-3p, 200c-3p and 141-3p related transcription factors. miR-200 and their downstream genes might be the potentially specific targets for the treatment of hepatocarcinoma.
Keywords: All-trans retinoic acid, Differentiation, Epithelial–mesenchymal transition, Hepatocellular carcinoma cells, microRNA 200
Introduction
All-trans retinoic acid (ATRA), an active form of vitamins A, has shown important physiological functions in embryonic development, morphogenesis, cell growth and differentiation.1, 2, 3 As a prospective anti-tumor drug, ATRA has anti-tumor effects on malignant hematological diseases and many other malignant solid tumors.4, 5, 6 Different researches have revealed the anti-liver cancer effect of ATRA, ATRA can inhibit the proliferation of liver cancer cells, restore the function of normal tissues, and reduce the recurrence rate of liver cancer patients after chemotherapy.7,8 Our previous studies have also shown that ATRA reverses epithelial-mesenchymal transition (EMT) of mouse hepal-6 hepatocarcinoma cell line by inhibiting proliferation, migration, invasion and inducing differentiation and hepatic function.9 However, the mechanisms of how ATRA affects the benign fate of hepatocellular carcinoma cells are not entirely clear.
MicroRNA is an endogenous non-coding small RNA molecule, play important roles in the regulation of cell development, differentiation and tumorgenesis.10 A large number of studies have shown that the retinoic acid signal mediated by ATRA is involved in the regulation of miRNA in glioma cells, breast cancer cells, acute leukemia cells, liver cancer cells and other tumor cells.11, 12, 13 We previously found that ATRA could induce mature differentiation of hepatic progenitor cells (HPCs),14 and widely regulated regulates the miRNA expression profile of HPCs, especially miR-200 family members. Compared with control group, the expression of miR-200a-3p, 200c-3p and 141-3p in ATRA treated HPCs increased 52.53, 46.95 and 196.76 times, respectively. Disruptive differentiation theory holds a point of view that the abnormal differentiation of hepatic stem/progenitor cells might be the reason of hepatocellular carcinoma (HCC) occurrence.15,16 Therefore, we try to investigate whether ATRA can upregulate the expression of miR-200 family to affect the biological character of hepal-6 cells and reverse EMT process?
Herein, we demonstrated that ATRA could inhibit the growth, migration, and invasion of hepal-6 cells, even promote its apoptosis and induce differentiation. ATRA may reverse EMT by regulating miR-200a-3p, miR-200c-3p, and miR-141-3p and some downstream transcriptional factors. Our finding may help to reveal the possible mechanism of the biological effect of ATRA on hepatocarcinoma, and provide a new thinking for the research on its clinical application for liver disease.
Materials and methods
Grouping and treatment of cells
The mouse hepatocarcinoma cell line hepal-6 was purchased from the American Type Culture Collection (Manassas, VA, USA). The miRNA antagomirs were used to specifically suppress the biological effect of miR-200 members. miRNA antagomir (miR03101, miR30000519, miR30000617, miR312418142000) were all purchased from RiboBio (Guangzhou, China).
The hepal-6 cells were divided into the following groups: control group, ATRA group, negative control antagomir (NC) + ATRA group, miR-200a-3p antagomir + ATRA group, miR-200c-3p antagomir + ATRA group, and miR-141-3p antagomir + ATRA group. The complete DMEM medium containing miRNA antagomir (100 nmol/L) were used to incubate cells for 24 h, and then changed into DMEM medium containing 10.0 μmol/L of ATRA.
miRNA quantitative real-time PCR (qRT-PCR) analysis
miRNAs were extracted and reverse transcribed using All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia, Guangzhou, China). The reaction mixture was incubated at 37 °C for 60 mins, then at 85 °C for 5 mins to inactivate the enzyme. Then qPCR was performed using the All-in-One miRNA qRT-PCR Primer and SYBR Green I with standard 3-step method. Relative expressions were determined by the 2–ΔΔCt method. The expression levels of miRNAs were normalized against the corresponding expression levels of RNU6. The primers were obtained from GeneCopoeia and shown in Table 1.
Table 1.
miRNA Primers for qRT-PCR.
| Catalog# | Mature ID |
|---|---|
| MmiRQP0298 | mmu-miR-200a-3p |
| MmiRQP0297 | mmu-miR-200a-5p |
| MmiRQP0300 | mmu-miR-200b-3p |
| MmiRQP0299 | mmu-miR-200b-5p |
| MmiRQP0302 | mmu-miR-200c-3p |
| MmiRQP0301 | mmu-miR-200c-5p |
| MmiRQP0184 | mmu-miR-141-3p |
| MmiRQP0885 | mmu-miR-141-5p |
| MmiRQP0999 | mmu-miR-429-3p |
| MmiRQP3192 | mmu-miR-429-5p |
| MmiRQP9002 | RNU6 |
MTT assay
Hepal-6 cells (1 × 104 cells/well) were seeded onto 96-well plates and treated as above. At day 1 and day 3 of ATRA treatment, 50 μL of 1 × MTT was added to each well. After 4 h incubation, the supernatant was aspirated and 150 μL of DMSO was added to each well. At last, the absorbance was measured at wavelengths of 570 nm by a microplate reader (BioTek, VT, USA).
Annexin V-FITC/PI staining assay
Hepal-6 cells were seeded in 6-well plates with cell density starting at 15% confluence. After 3 days of ATRA treatment, cells were trypsin-released, washed with cold PBS, re-suspended in binding buffer (100 μL) containing Annexin V-FITC (5 μL) and PI (5 μL), and incubated for 15 mins. The samples were analyzed using a fluorescence-activated cell sorting flow cytometer (BD Bioscience, CA, USA). Data analysis was performed using the FlowJo Software Program.
Colony formation assay
Briefly, hepal-6 cells (200 cells/well) were planted in 12-well plates. After treatment for 21 days, cells were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. Cell colonies containing more than 50 cells were counted and the plate clone-forming efficiency was calculated as follows: (number of colonies/number of cells seeded) × 100%.9
Wound healing assay
Hepal-6 cells were plated in 6-well plates reached to 90% confluent cell monolayer. A consistent gap in the surface of confluent cells was created by a pipette tip across the cell layer. Cells were incubated with different treatment. Bright-field images of the same wound field were captured at 1, 2, and 3 days to assess cell migration across the gap. Each assay was done in triplicate.9
Transwell assay for cell migration and invasion (Matrigel coating)
At 3 days after different treatments, cells were harvested and re-suspended in DMEM without FBS. Cell suspension (1 × 105 cells in 200 μl per well) was seeded in the upper Transwell chamber (8.0 μm, Polycarbonate, Corning, NY, USA). Complete DMEM medium containing 10% FBS was added to the lower chamber. Following 48 h of incubation, the invaded cells on the lower membrane were fixed with 4% paraformaldehyde for 20 mins and stained with 0.1% crystal violet (Beyotime, Shanghai, China) for 15 mins. The number of invasive cells in at least five random fields of view was counted. The stain was dissolved by 33% acetic acid and absorbance of each well was measured at 570 nm with a micro plate reader (Thermo Scientific, MA, USA). In invasion assay, matrigel was coated in upper chamber in advance. The procedure was repeated independently for three times with triplicate in each group.
Indocyanine green (ICG) uptake and release
Hepal-6 cells were seeded in 24-well plates and differently treated for 12 days. As previously described,17,18 cells were washed and incubated in complete DMEM medium containing 1 mg/ml freshly prepared ICG reagent (Sigma) at 37 °C for 1 h. At least 10 non-overlapping vision fields were captured under the microscope and cells with green stained nucleus were calculated as positive. After that, ICG-free complete medium was changed to incubate cells at 37 °C for more than 6 h to detect the ICG release function.
Periodic acid-schiff (PAS) staining
Hepal-6 cells were seeded in 24-well plates. After differently treated for 12 days, cells were fixed with 4% paraformaldehyde for 10 mins and stained with 0.5% periodic acid solution for 5 mins. Then cells were stained with Schiff's reagent (Sigma) for 15 mins, followed by counterstaining with hematoxylin solution for 2 mins. All steps were carried out at room temperature, and tap water rinsed cells after each step. More than 10 non-overlapping vision fields in each group were recorded using a microscope and cells with purple-red stained cytoplasm were calculated as positive.18,19
qRT-PCR analysis
Total RNA extraction and qRT-PCR analysis of hepatic markers were performed as previously described.17 The total RNA of different treated hepal-6 cells were extracted by Trizol. 10 μg of total RNA were reversely transcripted by using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) to generate cDNA templates. PCR primers were designed by using the Primer3 program to amplify the gene of interest (Table 2). All samples were normalized by endogenous levels of GAPDH.
Table 2.
qRT-PCR Primers.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| CK18 | CTGGGCTCTGTGCGAACT | ACAGAGCCACCCCAGACA |
| E-cadherin | CAAGGACAGCCTTCTTTTCG | TGGACTTCAGCGTCACTTTG |
| N-cadherin | CTGGGACGTATGTGATGACG | TGATGATGTCCCCAGTCTCA |
| Vimentin | CAGATGCGTGAGATGGAAGA | TCCAGCAGCTTCCTGTAGGT |
| Snail | AAACCCACTCGGATGTGAAG | GAAGGAGTCCTGGCAGTGAG |
| twist | CAGCGGGTCATGGCTAACG | CTTGTCCGAGGGCAGCGT |
| Jun | GCGCATGAGGAACCGCATT | TGAGCATGTTGGCCGTGGA |
| Fra-1 | CTCCAGGACCCGTACTTGAACC | TGCTGCTACTCTTTCGGTGAGC |
| Zeb1 | GTGCCAACCCCATAAACA | AGGGCTCACAGTAGCTGAAT |
| Etv4 | GCTGCGATACTATTATGAGAAAGG | AGCTGGACGTTGATTATCTGG |
| Pou4f1 | CTCGCTCAGCCAGAGCACCAT | GGAAGTCCGCTTGCGCTTCTT |
| Klf12 | ACTATTGTTGTACCGCTCCTG | GTTTCATTAACGCTATCTAAGGTC |
| GAPDH | GGCTGCCCAGAACATCAT | CGGACACATTGGGGGTAG |
Western blot
At different time points, cells were lysed to extract total proteins. Approximately 20 μg of total protein per lane were separated on a 10% SDS-PAGE (Beyotime) and electrophoretically transferred to polyvinylidene fluoride (PVDF) membrane (Milipore, Billerica, MA, USA). After blocking, the membrane were incubated at 4 °C overnight with primary antibodies against CK18 (Santa Cruz, Dallas, TX, USA), E-cadherin (Wanleibio, Shenyang, China), N-Cadherin (Wanleibio), Fra1/Jun (BosterBio, Wuhan, China), Ets1/PEA3 (BosterBio), Brn3 (BosterBio), Zeb-1/AREB6 (BosterBio), Klf12 (Proteintech Inc, IL, USA), and β-actin (Santa Cruz), After washing by TBST, the membranes were probed with the appropriate second antibody at room temperature for 1 h. The presence of the proteins were visualized by using enhanced Chemiluminescent Substrate (Kaiji, Nanjing, China) and exposed under the Syngene GBOX Imaging System (Cambridge, UK).
TransSignal Protein/DNA array
Approximately 1 × 107 cells were seeded in 100-mm dishes and treated with ATRA for 3 days. Nuclear proteins (25 μg) were extracted by using NE-PER Nuclear Protein Extraction Kit (Pierce, Rockford, IL, USA), and followed by incubation with biotin-labeled DNA binding probes for 30 mins at 15 °C. The eluted bound probes were hybridized to the TransSignal array membrane containing 345 of transcription factor consensus binding sites (Catalog no. MA1215, Panomics, Freemont, CA, USA) for 24 h at 42 °C. After washed by blocking buffer, the membrane was incubated with HRP-conjugated streptavidin solution for 5 mins and visualized by using HRP Substrate Working Solution (Millipore, Billerica, MA, USA). The images of the chemiluminescent signal were captured and quantitated with ScanAlyze software.
Prediction of target transcription factors
Compared with control group, transcription factor in ATRA group with more than 2-fold changes of up or down-regulation of transcriptional activity was considered to be different. The mirbase, miranda and targetscan bioinformatics software's were used to predict the possible genes that might be targeted by 10 miR-200 members via binding to their 3′-UTR sequences. The genes in the overlapping of the three databases were listed.
Statistical analysis
The data are presented as mean SD and analyzed using the SPSS 19.0 software. The two-tailed student's t test was used to evaluate the difference between two groups, while One-Way ANOVA and a post hoc SNK's test were used to evaluate significant differences among more than three groups. P < 0.05 was considered to be statistically significant.
Results
ATRA up-regulated miR-200s expression of hepal-6 cells
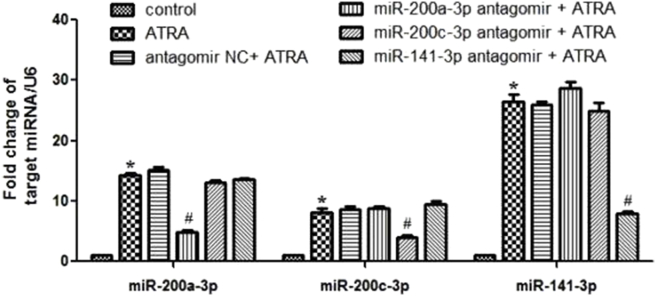
Compared with control, the expression of miR-200a-3p, miR-200c-3p and miR-141-3p significantly increased by more than 16-fold, 9-fold and 28-fold after 3 days of ATRA treatment, while the expression of other seven miR-200 subtypes showed no statistical difference between ATRA and control groups (Fig. 1).
Figure 1.
The mRNA expression of miR-200s. hepal-6 cells were treated with 10.0 μmol/L ATRA for 3 days (*P < 0.05 vs. control). miRNA was extracted and transcripted by using All-in-One™ miRNA qRT-PCR detection kit. The miRNA expression of 10 miR-200 members was measured by real-time PCR analysis. The results were confirmed in at least 3 batches of independent experiments, and representative results were shown.
miR-200s inhibited the effects of ATRA on proliferation and apoptosis of hepal-6 cells
Consistent with previous results,9 with ATAR treatment, the proliferation and colony formation abilities of hepal-6 cells were significantly inhibited, whereas the apoptosis rate of hepal-6 cells increased (Fig. 2, P < 0.05). miR-200a-3p, 200c-3p, 141-3p antagomirs could specifically suppress the ATRA increased expression of miR-200a-3p, 200c-3p, 141-3p (Fig. S1). The proliferation capability of hepal-6 cells was remarkably recovery and the apoptosis rate decreased in all these three groups compared with antagomir negative control + ATRA (NC + ATRA) group (Fig. 2A and C, P < 0.05). The cell proliferation of miR-141-3p antagomir + ATRA group showed statistically less than that of miR-200a-3p/200c-3p antagomir (Fig. 2A, P < 0.05). However, the colony formation of hepal-6 cells in the three antagomir + ATRA treated groups appeared no significant difference compared with NC + ATRA group (Fig. 2B). The results suggested that miR-200a-3p, miR-200c-3p, miR-141-3p may be involved in the regulatory effects of ATRA on proliferation and apoptosis of hepal-6 cell, but not the colony formation.
Figure 2.
miR-200a-3p, 200c-3p, 141-3p could affect ATRA regulated cell proliferation and cell apoptosis but not colony formation. (A) Cell proliferation was measured by MTT assay (*, P < 0.05 vs. control; #, P < 0.05 vs. NC + ATRA; &, P < 0.05 vs. miR-200a/200c antagomir + ATRA groups). (B) Colony formation assay. Three independent experiments were performed in duplicate, and representative results are shown. (C) Hepal-6 cells were treated with 10.0 μmol/L ATRA and different miRNA antagomirs for 3 days. Cells stained with Annexin V-FITC/PI were evaluated by flow cytometry (*, P < 0.05 vs. control group; #, P < 0.05 vs. NC + ATRA group).
miR-200s weakened the suppression effect of ATRA on migration and invasion of hepal-6 cells
The migration and invasion abilities of hepal-6 cells were all significantly inhibited by ATRA treatment (Fig. 3, P < 0.05). The healing rates of hepal-6 cells in miR-200a-3p, 200c-3p, 141-3p antagomir + ATRA groups were all higher than that in NC + ATRA group (P < 0.05). Meanwhile, the cell scratches in miR-200a-3p/200c-3p antagomir + ATRA groups healed faster than that in miR-141-3p antagomir + ATRA group (P < 0.05). The results indicated that miR-200a-3p, miR-200c-3p and miR-141-3p could weaken the suppression effect of ATRA on horizontal migration ability of hepal-6 cells, whereas the cell proliferation of hepal-6 cells in miR-141-3p antagomir + ATRA group was slower than that in the other two groups, which may affect the result of wound healing assay. In transwell migration assay, the number of vertical migrated cells in miR-200a-3p/200c-3p antagomir + ATRA group was significantly higher than that in NC + ATRA group (Fig. 4A, P < 0.05), while there was no difference between miR-141-3p antagomir + ATRA and NC + ATRA groups (p > 0.05). Nevertheless, similarly to the horizontal migration, the numbers of invaded cells in miR-200a/200c/141-3p antagomir + ATRA groups were all increased compared with NC + ATRA group, especially miR-200c-3p antagomir + ATRA group (Fig. 4B, P < 0.05). These above results suggested that miR-200a-3p, 200c-3p and 141-3p could resume ATRA inhibited migration and invasion abilities of hepal-6 cells at various levels.
Figure 3.
miR-200a-3p, 200c-3p, 141-3p could reverse ATRA inhibited cell horizontal migration ability. Hepal-6 cells were treated with 10.0 μmol/L ATRA and different miRNA antagomirs. The migration of hepal-6 cells were determined by wound-healing assay(*, P < 0.05 vs. control; #, P < 0.05 vs. NC + ATRA, &, P < 0.05 vs. miR-200a/200c antagomir + ATRA groups).
Figure 4.
miR-200a-3p, 200c-3p, 141-3p could reverse ATRA inhibited cell vertical migration and invasion abilities in various degrees. Hepal-6 cells planted in non-marigel-coated transwells. (A) or marigel-coated transwells. (B) were treated with 10.0 μmol/L ATRA and different miRNA antagomirs for 48 h. Cell were fixed and stained with crystal violet (*, P < 0.05 vs. control; #, P < 0.05 vs. NC + ATRA, &, P < 0.05 vs. miR-200a/200c antagomir + ATRA groups).
MiR-200a-3p and miR-200c-3p prevented ATRA from inducing the differentiation and maturation of hepal-6 cells
ALB and CK18 are two of the representative markers of mature hepatocytes. ICG uptake and PAS staining are commonly used to estimate the differentiation and function of hepatocytes. As shown in Fig. 5, ATRA treatment significantly increased the mRNA expression of ALB and CK18, as well as the number of ICG uptake and PAS stain positive cells (P < 0.05). After treated with the corresponding miRNA200 antagomirs, the expression of ALB and CK18 of hepal-6 cells in miR-200a/200c-3p antagomir + ATRA group was remarkably attenuated compared with in NC + ATRA group (P < 0.05), yet the expression of ALB and CK18 of hepal-6 cells had no statistical significance between miR-141-3p antagomir + ATRA and NC + ATRA groups (P > 0.05). The same tendency was found in the ICG uptake and PAS staining assay. All these results suggested that miR-200a-3p and miR-200c-3p may prevent ATRA from inducing differentiation and mature of hepal-6 cells.
Figure 5.
miR-200a-3p and miR-200c-3p prevented ATRA from inducing the hepal-6 cells differentiation and mature. Hepal-6 cells were treated with 10.0 μmol/L ATRA and different miRNA antagomirs for 7 days. (A) Real-time PCR was used to detect mRNA expression of the mature hepatocyte markers ALB and CK18. (B) Indocyanine green (ICG) uptake assay: cells with a green-stained nucleus are the positive-stained cells. (C) Periodic acid-Schiff (PAS) staining, purple color in cell plasma indicates glycogen accumulation (*, P < 0.05 vs. control; #, P < 0.05 vs. NC + ATRA).
MiR-200s regulated ATRA related EMT markers
It has been demonstrated that the regulation effect of ATRA on hepal-6 cells was associated with EMT. Next, we investigated whether miR-200s were associated in regulating EMT markers. CK18 is an epithelial marker of hepatocyte, E-cadherin plays an important role in maintaining epithelial cell–cell adhesion and polarity. Compared with control group, the expression of CK18 and E-cadherin of hepal-6 cells exhibited a remarkable increase after ATRA treatment. Then ATRA improved CK18 expression was significantly inhibited by miR-200a-3p and 200c-3p antagomir, but not miR-141-3p antagomir. However, no statistical significance was found among three antagomir + ATRA groups. The expression of mesenchymal markers N-Cadherin, vimentin, Snail and Twist was significantly repressed by ATRA treatment. Compared with NC + ATRA group, miR-200a-3p antagomir increased the expression of N-Cadherin, Snail and Twist, miR-200c-3p antagomir only increased the expression of N-Cadherin, while miR-141-3p antagomir increased the expression of N-Cadherin and Twist. There was no difference in Vimentin expression among three antagomir + ATRA groups (Fig. 6A). The protein expression of CK18, E-cadherin, and N-Cadherin exhibited the similar tendency (Fig. 6B). Therefore, these results indicated that miR-200a-3p, miR-200c-3p, and 141-3p might be associated with ATRA regulated EMT in hepatocarcinoma.
Figure 6.
miR-200s regulated ATRA related EMT markers. Hepal-6 cells were treated with 10.0 μmol/L ATRA and different miRNA antagomirs for 3 days. (A) The mRNA expression of EMT markers including CK18, E-cadherin, N-Cadherin, vimentin, Snail and Twist were measured by real-time PCR analysis. The results were confirmed in at least 3 batches of independent experiments, and representative results were shown. (B) The protein expression of CK18, E-cadherin, and N-Cadherin exhibited the similar tendency.
The genes of transcription factors regulated by ATRA are associated with miRNA200s
As TransSignal Protein/DNA array result showed (Fig. 7), the activity of 8 transcription factors were upregulated and 35 were down-regulated after ATRA induction. Bioinformatics analyses were performed to analyze the relationships between genes related to these 43 discrepant transcription factors and ten miR-200 family members by using miranda, mirbase and Targetscan databases. Jun, Etv4, Pouf1, Zeb1 and klf12 genes, which corresponded to transcription factors Fra1/Jun (AP1), Ets1/PEA3, Brn-3, AREB6, and AP2, might be regulated by miR-200a-3p, miR-200c-3p, and miR-141-3p. CP1B and HLF were eliminated because of their extremely low transcriptional activity. While other miR-200 subtypes appeared no correlation with these discrepant transcription factors. More interestingly, this analysis result was consistent with the real-time PCR result that ATRA only increased the expression of miR-200a-3p, miR-200c-3p, and miR-141-3p among 10 miR-200 subtypes (Fig. 1).
Figure 7.
Functional analysis of transcription factors by Protein/DNAarray. Hepal-6 cells were treated with 10.0 μmol/L ATRA for 3 days. Nuclear proteins were extracted and mixed with biotin-labeled DNA binding probes. The eluted bound probes were hybridized to a membrane which contained an array of 345 transcription factor consensus binding sequences. Bioinformatics analysis reveals a possible regulatory circuitry between the 10 miR-200 family members and the ATRA induced transcriptional factors.
ATRA and miR-200a-3p/200c-3p/141-3p were involved in regulating the mRNA and protein expression of discrepant transcription factors and corresponding target genes
The mRNA expression of Fra1/Jun, Etv4, Pouf1 and Zeb1 was down-regulated and Klf12 was up-regulated with ATRA treatment (Fig. 8A, P < 0.05). In addition, at 3 days of biological inhibition of miR-200a-3p/200c-3p/141-3p by specifically corresponding antagomirs, the protein expression of Fra1/Jun (AP1), Ets1/PEA3, Brn3, and Zeb1/AREB6 of hepal-6 cells was significantly strengthened at varying degrees compared with NC + ATRA control group, while no statistic significance of Klf12 expression was observed among different treated groups (Fig. 8B). Therefore, we considered that ATRA treatment down-regulated these related transcription factors by upregulating miR-200a-3p, 200c-3p and 141-3p, then stimulating suppression of EMT in hepatocarcinoma.
Figure 8.
The mRNA and protein expression of selected transcription factors. Hepal-6 cells were treated with 10.0 μmol/L ATRA and different miRNA antagomirs for 3 days. (A) The mRNA expression of Jun, Etv4, Pou4f1, Zeb1, Klf12 was measured by real-time PCR analysis and analyzed with GAPDH normalization. Real-time PCR results were confirmed in at least three batches of independent experiments and representative results were shown (*, P < 0.05 vs. control). (B) Cell lysates of each group were subjected to SDS-PAGE and western blotting by using Fra1/Jun, Ets1/PEA3, Brn3, Zeb1/AREB6, Klf12 antibodies with β-actin normalization.
Discussion
Hepatocarcinoma is one of the most common malignant tumors in clinic. Hepatocellular carcinoma cells can easily invade the surrounding capsule and blood vessels, and then can lead to the local diffusion and distant metastasis of tumor cells.20 At present, clinical therapy of HCC mainly focuses on early surgery, supplemented with radiotherapy and chemotherapy, but the overall effect is not ideal. The rapid development, high recurrence and metastasis of hepatocarcinoma are the key factors to affect the curative effect and long-term survival of patients.21,22
EMT refers to the transdifferentiation of epithelial cells to mesenchymal cells in specific cases. Decreased cell adhesion and enhanced cell motility are the basis of tumor invasion and metastasis. This EMT phenomenon frequently occurs in the invasion and metastasis of primary hepatocarcinoma.23,24 In our previous studies, ATRA has been demonstrated to reverse EMT of mouse hepal-6 hepatocarcinoma cell lines by inhibiting its proliferation, migration, invasion, and inducing differentiation.9 Nevertheless, a clear understanding of the molecular mechanisms is necessary for the development of ATRA on the potential treatment for HCC.
MicroRNA is a class of endogenous non-coding RNA found in eukaryotes, which are about 19–25 nucleotides long in size. The expression and function of miRNAs are affected by promoter transcription, epigenetics, post transcriptional splicing, and RNA editing.25 A large number of studies have shown that ATRA mediated retinoic acid signaling is involved in the regulation of miRNA. Retinoic acid signaling can affect the expression levels of multiple miRNA in acute leukemia HL-60 cells, in which, miR-663 is closely related to ATRA induced differentiation.26 Some miRNA promoter regions contain predicted sites of retinoic acid receptor RARα, and heterodimer of RARα and RXRα can directly bind to miR-210, 23a, and 24–2 promoters to participate in transcriptional regulation.27 We previously found that ATRA could widely alter the miRNA expression profile of HPCs. The analysis results showed that the miRNA200 family raised significantly, miR-200a-3p, 200b-3p, 200c-3p, 141-3p were increased by 52.53, 6.28, 46.95, 196.76 times respectively after ATRA treatment.
According to the nucleotide sequence of seed region, the miRNA-200s are divided into two different genomic clusters. MiR-200a, 200b and 429 are located on chromosome 1 of human chromosome 1p36.33, miR-200c and 141 are located on chromosome 12 of human chromosome 12p13.31. These two regions often appear chromosome point mutation, deletion or translocation in tumor.28 MiR-200 family is well known as key factors to inhibit the epithelial–mesenchymal transition, but few studies are reported in hepatocellular carcinoma.29,30 Down-regulation of miR-200a can enhance the proliferation and migration of hepatoma cells, probably through TGF-β regulation and histone acetylation.31 MiR-200b also plays a role of tumor suppressor to affect tumor cell migration, invasion by upregulating E-cadherin.32 Based on the similar effect of ATRA on hepatic progenitor cells and hepatocellular carcinoma cells,9,14 we assume whether miR-200 family is related to ATRA induced reversal of EMT in hepatocellular carcinoma cells. We firstly detected 10 miR-200 isoforms in hepal-6 cells and found that the expression of miR-200a-3p, 200c-3p and 141-3p was significantly up-regulated.
EMT is the process by which tumor cells lose their epithelial characteristics and obtain mesenchymal phenotypes.33,34 Next, we confirmed that miR-200a-3p, 200c-3p and 141-3p were involved in cell proliferation, migration and invasion inhibited by ATRA, at the same time, miR-200a-3p and 200c-3p might be involved in the longitudinal migration inhibited by ATRA as well as maturation and differentiation induced by ATRA. CK18 is a hepatocyte-specific marker and represent epithelial phenotype. The expression of CK18 decreased with down-regulation of miR-200a-3p and 200c-3p. As a key protein in cell polarity and epithelial organization, the expression of E-cadherin had no statistic changes with 3 days of ATRA and miRNA antagomir treatment, which might be due to time-dependent regulation of ATRA on E-cadherin. Mesenchymal markers N-Cadherin, vimentin, Snail, and Twist are closely linked to metastasis and invasion in a variety of human malignancies.35, 36, 37 ATRA treatment significantly suppressed the expression of N-Cadherin, vimentin, Snail and Twist in hepal-6 cells. However, only miR-200a-3p antagomir played prominent role in regulating the expression of these mesenchymal markers. The biological function of different miR-200 members is diverse; the same subgroup members have similar target genes and biological effects.38,39 In the present study, the subpopulation of miR-200a and miR-141 is relatively weaker in inhibition of invasion of HCC cells by ATRA. MiR-200a-3p and 200c-3p have stronger regulation effect on proliferation and parallel migration than miR-141-3p, while miR-200a-3p and 200c-3p were also related to longitudinal migration and maturation differentiation of HCC cells.
A large number of computer analysis results suggest that most transcription factors are potential target genes regulated by miRNA.40, 41, 42 The transcription factor chip results showed that ATRA regulates transcriptional activity of 43 transcriptional factors. Bioinformatics was used to analyze the relationship between 3′-UTR of candidate transcription factors and 10 miR-200 members. MiR-200a-3p, 200c-3p, 141-3p were considered to be related to Klf12, Zeb1, Pou4f1, Ets1, Jun genes, which correspond to AP2, AREB1, Brn3, Ets1/PEA3 and Fra1/Jun transcription factor respectively. Other 7 miR-200 isoforms are not associated to ATRA regulated transcriptional. This result was consistent with that only miR-200a-3p, 200c-3p and 141-3p were upregulated after ATRA induction. Fra-1/Jun, Etv4, Pou4f1, and ZEB1 have great importance to proliferation, apoptosis, migration, invasion and differentiation of various tumor cells, which are involved in the maintenance of tumor stem cell characteristics and the formation of EMT.43,44
miR-200a-3p, 200c-3p and 141-3p were associated with Zeb1 gene, and our experiments found that the three miR-200s were involved in the effect of ATRA on cell proliferation, parallel migration, invasion and apoptosis. With treatment of specific miRNA antagomir for miR-200a-3p, 200c-3p, 141-3p, the protein expression of ZEB1 significantly increased, and the expression of Pou4f1, Ets1, Jun increased at different degree despite they are related to only one miR-200 member. AP2α is an important regulator of gene expression during carcinogenesis.45 Klf12 is a member of the Kruppel-like zinc finger protein family and can bind to a specific site in AP2α gene promoter to repress its expression.46 However, the protein expression of Klf12 has no obvious change after antagomir treatment. ATRA plays a regulatory role on the transcription activity of these transcription factors, might be attributable to other signals besides miRNA200.
In summary, we explored the biological effects and particular molecular mechanisms of ATRA in the prognosis of hepatocarcinoma from the perspectives of miRNA. ATRA may play the reversal effect of epithelial mesenchymal transition in hepatocellular carcinoma cells by upregulating miR-200a-3p, 200c-3p and 141-3p. All of the three miR-200s are involved in the inhibition of cell proliferation, parallel migration and invasion, and the promotion of cell apoptosis regulated by ATRA. Moreover, miR-200a-3p and miR-200c-3p are involved in the inhibition of vertical migration and induction of cell differentiation regulated by ATRA. Target genes Jun, Pou4f1, Eva4, and Zeb1 have been suggested to be related to ATRA regulated EMT of hepatocarcinoma. MiR-200 and their downstream genes might be the potentially specific targets for the treatment of hepatocarcinoma. Our study provides an important experimental basis and theoretical basis for the combination of ATRA and other anticancer drugs in clinical treatment of hepatocarcinoma.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
The reported work was supported by a research grant from the Natural Science Foundation of Chongqing City [grant numbers cstc2018jcyjAX0111 to YH, csct2016jcyjA0228 to YB] and the Program for Innovation Team Building at Institutions of Higher Education in Chongqin [grant number CXTDX201601015 to NT].
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.12.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
References
- 1.Mason J.B., Sanders D., Greiner T. Vitamin A deficiency: policy implications of estimates of trends and mortality in children. Lancet Glob Health. 2016;4(1):e21. doi: 10.1016/S2214-109X(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 2.Bi Y., Gong M., He Y. AP2α transcriptional activity is essential for retinoid-induced neuronal differentiation of mesenchymal stem cells. Int J Biochem Cell Biol. 2014;46:148–160. doi: 10.1016/j.biocel.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Connolly R.M., Nguyen N.K., Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res. 2013;19(7):1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X.J., He M.J., Zhou G. All-trans retinoic acid induces anti-tumor effects via STAT3 signaling inhibition in oral squamous cell carcinoma and oral dysplasia all-trans retinoic acid induces anti-tumor effects via STAT3 signaling inhibition in oral squamous cell carcinoma and oral dysplasia. J Oral Pathol Med. 2019;48(9):832–839. doi: 10.1111/jop.12931. [DOI] [PubMed] [Google Scholar]
- 5.Li M., Sun Y., Guan X. Advanced progress on the relationship between RA and its receptors and malignant tumors. Crit Rev Oncol Hematol. 2014;91(3):271–282. doi: 10.1016/j.critrevonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Arisi M.F., Starker R.A., Addya S. All trans-retinoic acid (ATRA) induces re-differentiation of early transformed breast epithelial cells. Int J Oncol. 2014;44(6):1831–1842. doi: 10.3892/ijo.2014.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasekaran D., Srivastava J., Ebeid K. Combination of nanoparticle-delivered siRNA for astrocyte elevated gene-1 (AEG-1) and all-trans retinoic acid (ATRA): an effective therapeutic strategy for hepatocellular carcinoma (HCC) Bioconjug Chem. 2015;26(8):1651–1661. doi: 10.1021/acs.bioconjchem.5b00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanki K., Akechi Y., Ueda C. Biological and clinical implications of retinoic acid-responsive genes in human hepatocellular carcinoma cells. J Hepatol. 2013;59(5):1037–1044. doi: 10.1016/j.jhep.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Cui J., Gong M., He Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepal-6 cells through reversing EMT in vitro. Int J Oncol. 2016;48(1):349–357. doi: 10.3892/ijo.2015.3235. [DOI] [PubMed] [Google Scholar]
- 10.Malla B., Zaugg K., Vassella E. Exosomes and exosomal MicroRNAs in prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98(5):982–995. doi: 10.1016/j.ijrobp.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang R., Liu C. All-trans retinoic acid therapy induces asymmetric division of glioma stem cells from the U87MG cell line. Oncol Lett. 2019;18(4):3646–3654. doi: 10.3892/ol.2019.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Ren W., Chen K. miR-34a promotes apoptosis and inhibits autophagy by targeting HMGB1 in acute myeloid leukemia cells. Cell Physiol Biochem. 2017;41(5):1981–1992. doi: 10.1159/000475277. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z., Liu J., Yang Z. MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting Sox7 involving Wnt/β-catenin signaling pathway. Oncotarget. 2016;7(19):28000–28012. doi: 10.18632/oncotarget.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J., Bi Y., Zhu G.H. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int. 2009;29(10):1569–1581. doi: 10.1111/j.1478-3231.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y., Wang Y., Gong J. High expression of MAGE-A9 contributes to stemness and malignancy of human hepatocellular carcinoma. Int J Oncol. 2018;52(1):219–230. doi: 10.3892/ijo.2017.4198. [DOI] [PubMed] [Google Scholar]
- 16.Liu K., Lee J., Kim J.Y. Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell. 2017;68(2):281–292. doi: 10.1016/j.molcel.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui H., Ma W.J., Cui J. Periodic acid-Schiff staining method for function detection of liver cells is affected by 2% horse serum in induction medium. Mol Med Rep. 2017;16(6):8062–8068. doi: 10.3892/mmr.2017.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y., He T.C., Bi Y. Comparison of proliferation and differentiation potential between mouse primary hepatocytes and embryonic hepatic progenitor cells in vitro. Int J Mol Med. 2013;32(2):476–484. doi: 10.3892/ijmm.2013.1413. [DOI] [PubMed] [Google Scholar]
- 19.He Y., Cui J., Bi Y. 5-azacytidine promotes terminal differentiation of hepatic progenitor cells. Mol Med Rep. 2015;12(2):2872–2878. doi: 10.3892/mmr.2015.3772. [DOI] [PubMed] [Google Scholar]
- 20.Alcedo K.P., Guerrero A., Basrur V. Tumor-selective altered glycosylation and functional attenuation of CD73 in human hepatocellular carcinoma. Hepatol Commun. 2019;3(10):1400–1414. doi: 10.1002/hep4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huan H.B., Wu L.L., Lau W.Y. Curative treatment for hepatocellular carcinoma: a re-analysis of meta-analyses of individual patients' data. Oncotarget. 2017;8(52):90291–90300. doi: 10.18632/oncotarget.18853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon H., Choi J.E., Lee I.J. All-treatment array of hepatocellular carcinoma from initial diagnosis to death: observation of cumulative treatments. J Cancer Res Clin Oncol. 2017;143(11):2327–2339. doi: 10.1007/s00432-017-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gils N., Verhagen H.J.M.P., Smit L. Reprogramming acute myeloid leukemia into sensitivity for retinoic-acid-driven differentiation. Exp Hematol. 2017;52:12–23. doi: 10.1016/j.exphem.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Boutzen H., Saland E., Larrue C. Isocitrate dehydrogenase 1 mutations prime the all-trans retinoic acid myeloid differentiation pathway in acute myeloid leukemia. J Exp Med. 2016;213(4):483–497. doi: 10.1084/jem.20150736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schirle N.T., Sheu-Gruttadauria J., MacRae I.J. Structural basis for microRNA targeting. Science. 2014;346(6209):608–613. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jian P., Li Z.W., Fang T.Y. Retinoic acid induces HL-60 cell differentiation via the upregulation of miR-663. Hematol Oncol. 2011;4:e20. doi: 10.1186/1756-8722-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima L., de Melo T.C.T., Marques D. Modulation of all-trans retinoic acid-induced miRNA expression in neoplastic cell lines: a systematic review. BMC Canc. 2019;19(1):e866. doi: 10.1186/s12885-019-6081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein D.M. Special delivery: microRNA-200-containing extracellular vesicles provide metastatic message to distal tumor cells. J Clin Investig. 2014;124(12):5107–5108. doi: 10.1172/JCI79191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhayat S.A., Mardin W.A., Köhler G. The microRNA-200 family-a potential diagnostic marker in hepatocellular carcinoma? J Surg Oncol. 2014;110(4):430–438. doi: 10.1002/jso.23668. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Riascos Z.V., Ginesta M.M., Fabregat J. Expression and role of MicroRNAs from the miR-200 family in the tumor formation and metastatic propensity of pancreatic cancer. Mol Ther Nucleic Acids. 2019;17:491–503. doi: 10.1016/j.omtn.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong C., Li M.Y., Chen Z.Y. MicroRNA-200a inhibits epithelial-mesenchymal transition in human hepatocellular carcinoma cell line. Int J Clin Exp Pathol. 2015;8(9):9922–9931. [PMC free article] [PubMed] [Google Scholar]
- 32.Hung C.S., Liu H.H., Liu J.J. MicroRNA-200a and -200b mediated hepatocellular carcinoma cell migration through the epithelial to mesenchymal transition markers. Ann Surg Oncol. 2013;20(Suppl 3):S360–S368. doi: 10.1245/s10434-012-2482-4. [DOI] [PubMed] [Google Scholar]
- 33.Nallanthighal S., Elmaliki K.M., Reliene R. Pomegranate extract alters breast cancer stem cell properties in association with inhibition of epithelial-to-mesenchymal transition. Nutr Cancer. 2017;69(7):1088–1098. doi: 10.1080/01635581.2017.1359318. [DOI] [PubMed] [Google Scholar]
- 34.Attar-Schneider O., Drucker L., Gottfried M. Migration and epithelial-to-mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI. Lab Investig. 2016;96(9):1004–1015. doi: 10.1038/labinvest.2016.77. [DOI] [PubMed] [Google Scholar]
- 35.Cohen D.J., Gloerich M., Nelson W.J. Epithelial self-healing is recapitulated by a 3D biomimetic E-cadherin junction. Proc Natl Acad Sci U S A. 2016;113(51):14698–14703. doi: 10.1073/pnas.1612208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markopoulos G.S., Roupakia E., Marcu K.B. Epigenetic regulation of Inflammatory Cytokine-Induced epithelial-to-mesenchymal cell transition and cancer stem cell generation. Cells. 2019;8(10):E1143. doi: 10.3390/cells8101143. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z., Jin Z.Y., Liu C.H. MicroRNA-21 regulates biological behavior by inducing EMT in human cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8(5):4684–4694. [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Zhang J., Xie B. MicroRNA-200FamilyProfile: a promising ancillary tool for accurate cancer diagnosis. Am J Therapeut. 2016;23(2):e388–e397. doi: 10.1097/MJT.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S.K., Kim H.S., Jin T. Overexpression of themiR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Canc. 2016;16:e570. doi: 10.1186/s12885-016-2620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong J., Dong H., Dai W. Functional DNA hexahedron for real-time detection of multiple microRNAs in living cells. Anal Chim Acta. 2019;1078:176–181. doi: 10.1016/j.aca.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Preca B.T., Bajdak K., Mock K. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer. 2015;137(11):2566–2577. doi: 10.1002/ijc.29642. [DOI] [PubMed] [Google Scholar]
- 42.Ma Z., Li Y., Xu J. MicroRNA-409-3p regulates cell invasion and metastasis by targeting ZEB1 in breast cancer. IUBMB Life. 2016;68(5):394–402. doi: 10.1002/iub.1494. [DOI] [PubMed] [Google Scholar]
- 43.Mi X.G., Song Z.B., Sun L.G. Cardamonin inhibited cell viability and tumorigenesis partially through blockade of testes-specific protease 50-mediated nuclear factor-kappaB signaling pathway activation. Int J Biochem Cell Biol. 2016;73:63–71. doi: 10.1016/j.biocel.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Keenan M.M., Liu B., Tang X. ACLY and ACC1 regulate hypoxia-induced apoptosis by modulating ETV4 via α-ketoglutarate. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W., Chen C., Liang Z. AP-2α inhibits hepatocellular carcinoma cell growth and migration. Int J Oncol. 2016;48(3):1125–1134. doi: 10.3892/ijo.2016.3318. [DOI] [PubMed] [Google Scholar]
- 46.Roth C., Schuierer M., Günther K. Genomic structure and DNA binding properties of the human zinc finger transcriptional repressor AP-2rep (KLF12) Genomics. 2000;63(6):384–390. doi: 10.1006/geno.1999.6084. [DOI] [PubMed] [Google Scholar]