Abstract
Cardiac Troponin I (cTnI) is a subunit of the thin filament involved in regulation of heart contraction. Mutated cTnI accounts for most genetic mutations associated with restrictive cardiomyopathy (RCM). We previously found phosphodiesterase 4D (PDE4D) decreased in RCM mice with cTnIR193H mutation and the mutant cTnI might be involved in PDE4D reduction. This study aims to elucidate a novel role of cTnIR193H mutant as a gene regulator. Overexpression of cTnIR193H mutant in cardiomyocytes showed decrease in PDED4D protein expression, while the enrichment of histone deacetylase 1 (HDAC1) was increased along with decreases in acetylated lysine 4 (acH3K4) and 9 (acH3K9) levels in the PDE4D promoter. HDAC1 overexpression could also downregulate PDE4D via reducing acH3K4 and acH3K9 levels. Co-IP assays showed that cTnIR193H mutant owed increased binding ability to HDAC1 compared with wild type cTnI. EGCG as a HDAC1 inhibitor could diminish the strength of cTnIR193H-HDAC1 interactions and alleviate the reduction in PDE4D expression. Together, our data indicated that cTnIR193H mutant could repress PDE4D expression in cardiomyocytes through HDAC1 associated histone deacetylation modification. Unlike the typical function of cTnI in cytoplasm, our study suggested a novel role of cTnI mutants in nuclei in regulating gene expression.
Keywords: cTnIR93H, EGCG, HDAC1, Histone modifications, PDE4D reduction
Introduction
Restrictive cardiomyopathy (RCM) is characterized by ventricular stiffness that limits ventricular filling and leads to cardiac dysfunction. Not as common as hypertrophic or dilated cardiomyopathy, RCM occurs with an extremely poor prognosis, and the suffering patients in a large number die in their childhood.1, 2, 3 No effective treatment is available for RCM other than heart transplantation.4 Although most RCM cases are described as idiopathic, myofibril gene mutations are widely accepted to play principle roles.5 Mutated cTnI, a part of the troponin complex regulating cardiac muscle contraction, accounts for most genetic mutations pertaining to RCM.6 Lots of studies support that RCM is caused by cTnI mutations that increase Ca2+ sensitivity and lead to cardiac dysfunction,7, 8, 9 and green tea extract flavonoid (−)-epigallocatechin gallate (EGCG) has been proved to be capable of reducing sarcomeric Ca2+ hypersensitivity and restoring normal diastolic function.10, 11, 12 However, the genetic basis of RCM still remains largely unknown for the reason that mutations in cTnI genes can cause hypertrophic cardiomyopathy as well due to shared etiology of increased Ca2+ sensitivity and hypercontractility,9,13 but the clinic feature of hypertrophic cardiomyopathy is quite opposite to RCM. This phenotypic variation suggests the presence of major genetic modifiers or activation of multiple genetic pathways that additively contribute to the phenotypes.14 Emerging evidence has revealed that epigenetic regulation is related to the development of cardiomyopathies.15, 16, 17 There is a critical need to understand whether cTnI mutations, as RCM causal factors, contribute to any epigenetic effects on the etiology of RCM. We previously found PDE4D decreased in RCM mice with cTnIR193H mutations, and HDAC1 associated histone deacetylation modification might be involved in its regulation.18 However, the role of cTnIR193H in regulating PDE4D remains unclear.
In this study, we carried out the experiments in cultured primary neonatal cardiomyocytes overexpressing wild type cTnI, cTnIR193H mutant or HDAC1 to test the epigenetic role of cTnIR193H in repressing PDE4D expression. Transcriptional activities of wild type cTnI and cTnIR193H mutant were detected by luciferase assay in 293T cells expressing cTnI or cTnIR193H. The effects of wild type cTnI, cTnIR193H mutant and HDAC1 on PDE4D expression were detected by western blotting. The epigenetic relationship of cTnIR193H mutant and PDE4D expression was determined by chromatin immunoprecipitation (ChiP) assay. Co-immunoprecipitation (Co-IP) assay could also explain the way how cTnIR193H mutant together with HDAC1 regulate PDE4D expression. We also investigated whether EGCG, an HDAC1 inhibitor, alleviates cTnIR193H induced PDE4D reduction and its underlying mechanisms. Our results demonstrated that cTnIR193H, as a myofibril protein, could act as a gene regulator in an epigenetic way. We hope our study could provide new directions to help understanding why mutations in a single gene can result in various cardiomyopathy phenotypes.
Materials and methods
Cell culturing, transfection and treatment
Culturing of primary neonatal cardiomyocytes from mice was performed as previously described.19 The cells were divided into control group, HDAC1 overexpression group, cTnI overexpression group, cTnIR193H group and cTnIR193H + EGCG group. Cardiomyocytes were transfected with empty vector or overexpression adenovirus. Cardiomyocytes in cTnIR193H + EGCG group were treated with EGCG (20 uM) right after transfection with cTnIR193H overexpression adenovirus. At 48 h after transfection or treatment, cells were harvested and collected for further analyses.
Luciferase reporter gene assay
Promoter regions (2000 bp) of mouse PDE4D were amplified via PCR from genomic DNA of C57BL/6 mouse and cloned into PGL3-basic. The TNNI3 expression vector was prepared by cloning wild-type mouse TNNI3 cDNA (661 bp) into a pcDNA3.1(−) vector. After gene G578 were interchanged with “A”, mutant vectors of pcDNA3.1(−)-cTnIR193H were constructed. The 293T cell lines were seeded into a 24-well plate and cultured to reach 90–95% confluent. PGL3-PDE4D promoter fused firefly luciferase (300 ng per well), TK-renilla luciferase (2 ng per well) and pcDNA3.1(−)-cTnI (300 ng per well)/pcDNA3.1(−)-cTnIR193H/pcDNA3.1(−)-null were transfected using Lipofectamine™ 2000 Transfection Reagent (No.1769821; Invitrogen, USA). Luciferase activity was measured via Promega™ E1500 Luciferase Assay System, with firefly luciferase activity normalized to renilla activity.
Immunofluorescence microscopy
Primary neonatal cardiomyocytes were seeded at a density of 1 × 105 cells per ml into a 24-well culture plate with coverslips. After 48 h, cells were fixed in ice-cold 4% formaldehyde solution for 20 min prior to incubation for 15 min at room temperature with 0.05% Triton-X. Then the coverslips of each group cells were blocked with normal goat serum for 30 min. After three times washes, the coverslips of cells were incubated overnight at 4 °C with mouse cTnI antibody (1: 300) and rabbit HDAC1 antibody (1: 200) for detecting cTnI and HDAC1 respectively. Cells were then incubated for 60 min at room temperature with appropriate secondary Cy-3-conjugated anti-mouse and anti-rabbit antibodies, followed by three times of washing. The cells were incubated with DAPI (5 μg/ml, 10236276001; Roche) for 15 min at room temperature for identifying cell nuclei. Coverslips were mounted on glass slides using DAPI Fluoromount-GTM (36308ES11; YEASEN, Shanghai) and examined with confocal microscope (Nikon, Tokyo, Japan). Image analysis was performed using NIHI ImageJ software.
Quantitative real-time PCR
Total RNA was extracted using an RNA Extraction kit (Bioteck, Beijing, China). Single strand cDNA was reversely transcribed from 500 to 1000 ng RNA by using oligo dT-Adaptor primers and AMV reverse transcriptase kit (TaKaRa, Otsu, Japan). cDNA was detected using quantitative real-time PCR assay with a SYBR Green RealMasterMix kit (Tiangen, Beijing, China). GAPDH was used as an endogenous ‘housekeeping’ gene to normalize RNA levels across samples. The primer sequences for genes of interest and controls were listed in supporting table.
Western blotting
Western blotting was performed as previously described. Proteins bound to the PDVF membrane were analyzed by western blotting using primary antibodies against PDE4d (Abcam, USA), GAPDH (Arigo, Taiwan), HDAC1 (CST, USA) and DYKDDDK Tag (GenScript, China). Band intensity was analyzed and quantified using a G-BOX imaging system (Syngene, UK).
Chromatin immunoprecipitation assay
ChiP assay was conducted using a ChIP assay kit (ChIP Kit-one step, Abcam, USA). Protein-DNA complexes were incubated at room temperature for 3 h using a monoclonal antibody against acH3K4, acH3K9, HDAC1 or DYKDDDK Tag. Mouse IgG was used as a negative control. Total column input also served as a positive control. Primers of the −1000bp upstream regions of PDE4D used for PCRs were listed in supporting table.
Co-immunoprecipitation assay
Co-IP kits (Touch and Release, Millipore, USA) were used to perform Co-IP assays to identify protein–protein interactions. Tests were conducted as per the manufacturer's instructions. Each reaction contains 500 μg protein diluted in 500 μl IP-incubation buffer and mixed with 5 μg DYKDDDK Tag antibody or IgG control antibody. The sample/antibody mixture was incubated in a spin column overnight at 4 °C on a rotator. Afterward, each immunoprecipitation supernatant was collected for western blotting, while potential Flag-binding proteins were pulled down in the spin column. Columns were washed with immunoprecipitation washing buffer three times, and then the denatured protein was eluted from the column. Protein solution (10 ul) was then used in western blotting.
HDAC1 activity assay
HDAC1 activity assay was performed using a HDAC1 Immunoprecipitation & Activity Assay Kit (Biovision). Proteins were extracted as per the manufacturer's instructions and the protein concentration was determined using the Bradford Assay. A total of 100 ug protein for each immunoprecipitation was used with 6 μl HDAC1 antibody to Samples and 6 μl IgG to Control groups. The volume of the protein texture was added to 500 μl with PBS containing protease inhibitors, followed by incubation overnight at 4 °C on a rotary mixer. After incubation, 25 μl of the protein-A/G bead slurry was added to each tube and incubated for 1 h at 4 °C. The beads were collected by centrifugation at 14,000 g for 10 s at 4 °C. The beads were washed three times with 1 ml PBS, by centrifuging at 14,000 g for 10s and aspirating the supernatant in between washes. For each reaction, 168 μl Reaction Mix containing HDAC Assay Buffer and HDAC Substrate was prepared and added to each sample or control tube, followed by mixing and incubation at 37 °C for 2 h. The Positive Control, Developer and Standard Curve was prepared as per the manufacturer's instructions. The Positive control, Control and sample tubes were centrifuged at 14,000 g for 2 min at room temperature. 100 μl of each reaction supernatant was transferred to individual wells in a white plate. The fluorescence was read at Ex/Em = 380/500 nm, followed by plotting the AFC Standard Curve. The Background Control reading was subtracted from all Sample readings. The corrected sample reading was applied to the AFC Standard Curve to get B pmol of AFC in the sample wells. Sample HDAC Activity = 2 × B/TS = pmol·min−1·mg−1 = mU, where: B = AFC amount from the Standard Curve (pmol), 2 = sample dilution factor, T = reaction time (120 min), and S = sample amount (0.1 mg).
Statistical analysis
All the data were analyzed using the SPSS 21.0 software package (SPPS Inc., Chicago, II, U.S.A). All data were expressed as mean ± standard deviation (SD) and analyzed by one-way ANOVA. P values < 0.05 was considered statistically significant.
Results
cTnIR193H down-regulates PDE4D
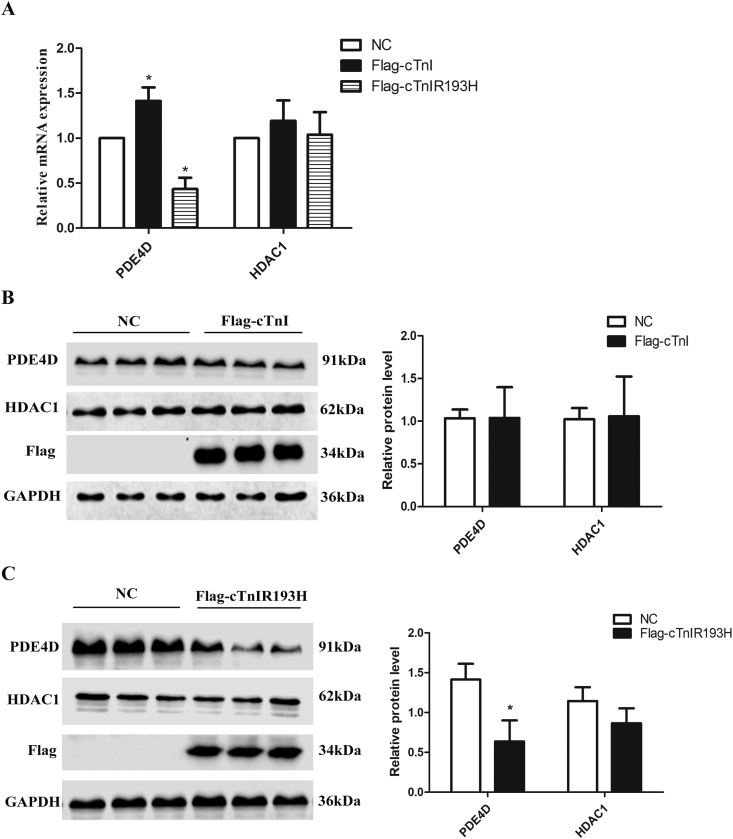
PDE4D was previously found decreased in cTnIR193H mice, and HDAC1 might repress PDE4D expression by histone deacetylation modification.18 In this study, we detected PDE4D expression in primary neonatal cardiomyocytes overexpressing cTnI and cTnIR193H respectively to identify their functions on PDE4D regulation. Although cTnI could promote PDE4D mRNA expression, it could not promote PDE4D protein expression. cTnIR193H could reduce PDE4D expression at both mRNA and protein levels. However, both cTnI and cTnIR193H had no influences on the expression of HDAC1, suggesting that the content of HDAC1 do not contribute to cTnIR193H induced decrease in PDE4D expression (Fig. 1).
Figure 1.
The effects of cTnI or cTnIR193H on PDE4D expression. mRNA or protein were extracted from primary neonatal cardiomyocytes overexpressing cTnI and cTnIR193H respectively (A) PDE4D mRNA levels in control group (NC), cTnI overexpression group (Flag-cTnI) and cTnIR193H overexpression group (Flag-cTnIR193H). (B) PDE4D protein level in NC and Flag-cTnI groups and quantification of western blotting results. (C) PDE4D protein level in NC and Flag-cTnIR193H groups and quantification of western blotting results. Statistical significance was determined by ANOVA followed by Least-Significant Difference (LSD) tests. *P < 0.05 relative to NC; n = 6 per group.
Nuclear translocation and transcriptional activity of cTnIR193H compared to cTnI
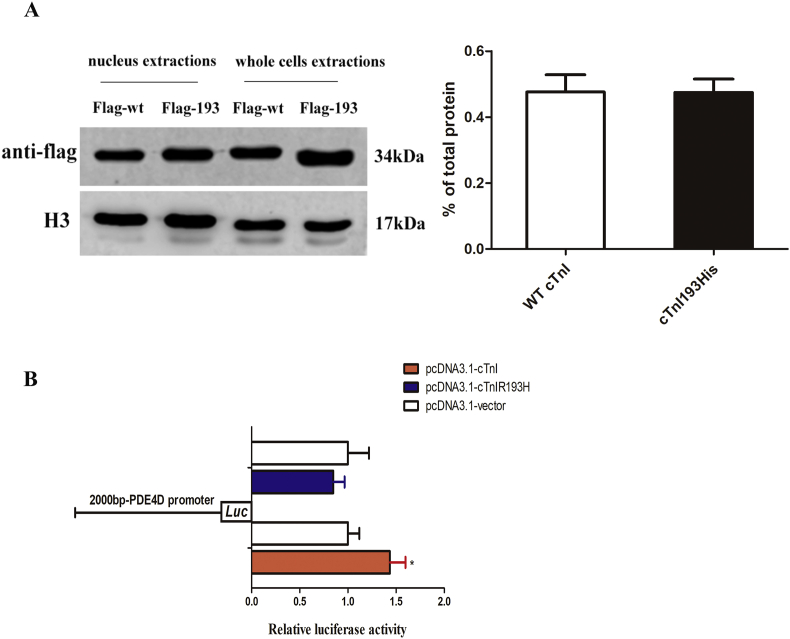
The content of transcription factors in nuclei determines the ability of transcriptional activity. To understand the molecular mechanisms for cTnIR193H to regulate PDE4D expression, we first compared the nuclear translocation ability of cTnI and cTnIR193H in primary neonatal cardiomyocytes overexpressing cTnI and cTnIR193H respectively. The ratio of nuclear protein to total protein was used to reflect the ability of cTnI or cTnIR193H entering into the nuclei. As shown in Fig. 2A, the ratio for cTnI (0.427) is almost equal to the ratio for cTnIR193H (0.431), indicating the same ability of entering the nuclei. This suggests that the ability of cTnIR193H nuclear translocation is not the key factor to downregulate PDE4D. Luciferase reporter gene assays were performed with 293T cells expressing cTnI or cTnIR193H to detect the transcriptional activity of cTnI or cTnIR193H to PDE4D. As shown in Fig. 2B, compared with the control group, the luciferase activity was elevated in the cells transfected with pcDNA3.1-cTnI plasmid. However, the luciferase activity presented with no significant change in cells with pcDNA3.1-cTnIR193H plasmid.
Figure 2.
Nuclear translocation of cTnIR193H/cTnI and their transcriptional activities to PDE4D. (A) Western blotting for detecting the nuclear protein and total cTnI or cTnIR193H in primary neonatal cardiomyocytes overexpressing cTnI or cTnIR193H, and column graph for the ratios of nuclear to total protein reflecting the translocation ability. 30ug nuclei and 100ug whole cells extraction were used to make H3 similar (B) 293T cells expressing cTnI or cTnIR193H for dual-luciferase reporter gene assays to test cTnI or cTnIR193H transcriptional activities to PDE4D. *P < 0.05 relative to vector group; n = 6 per group.
cTnIR193H might contribute to repression of PDE4D via HDAC1
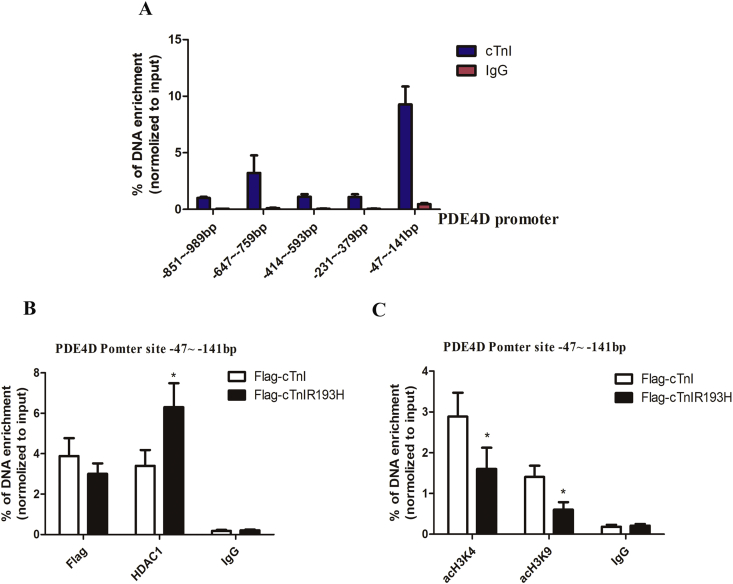
On transcriptional activity of cTnI, we then ask if cTnI and its mutant with different transcriptional activities could bind to PDE4D promoter like transcriptional factors and regulate PDE4D expression. We detected the enrichment level of cTnI among the −1000bp upstream regions of PDE4D in primary neonatal cardiomyocytes. As shown in Fig. 3A, there is sufficient cTnI in the nuclei highly enriched at −47∼-141bp of PDE4D promoter. Then we compared cTnI and cTnIR193H enrichment in this region in primary neonatal cardiomyocytes with overexpression cTnI or cTnIR193H. Compared with IgG, we observed significant enrichment of both cTnI and cTnIR193H, but with no significant difference between them in enrichment (Fig. 3B), suggesting that binding level to PED4D promoter was not a key factor for cTnIR193H to reduce PDE4D. Interestingly, we observed HDAC1 enrichment was enhanced and histone markers associated with transcription activation including acH3K4 and acH3K9 were repressed in cTnIR193H overexpression group compared with cTnI overexpression group (Fig. 3B and C). This suggests that cTnIR193H might downregulate PDE4D via HDAC1 induced deacetylation of H3K4 and H3K9 in PDE4D promoter region.
Figure 3.
cTnIR193H enhances the enrichment of HDAC1 and reduces acH3K4 and acH3K9 levels in PDE4D promoter. (A) ChiP assays for identifying the region of PDE4D promoter with highest enrichment of cTnI in the −1000bp upstream regions of PDE4D in primary neonatal cardiomyocytes. (B) The enrichment of cTnI, cTnIR193H and HDAC1 in the promoter region of −47∼-141bp in primary neonatal cardiomyocytes with overexpression of cTnI or cTnIR193H. (C) The enrichment of acH3K4 and acH3K9 in the promoter region of −47∼-141bp in primary neonatal cardiomyocytes with overexpression of cTnI or cTnIR193H. The results are expressed as mean ± SD. Statistical significance was determined by ANOVA followed by LSD tests. *P < 0.05 relative to Flag-cTnI group; n = 6 per group.
HDAC1 represses PDE4D expression via histone deacetylation
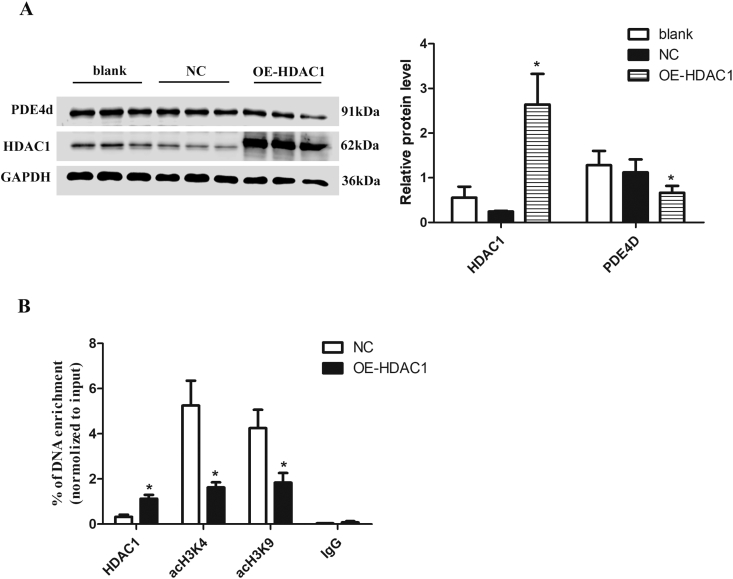
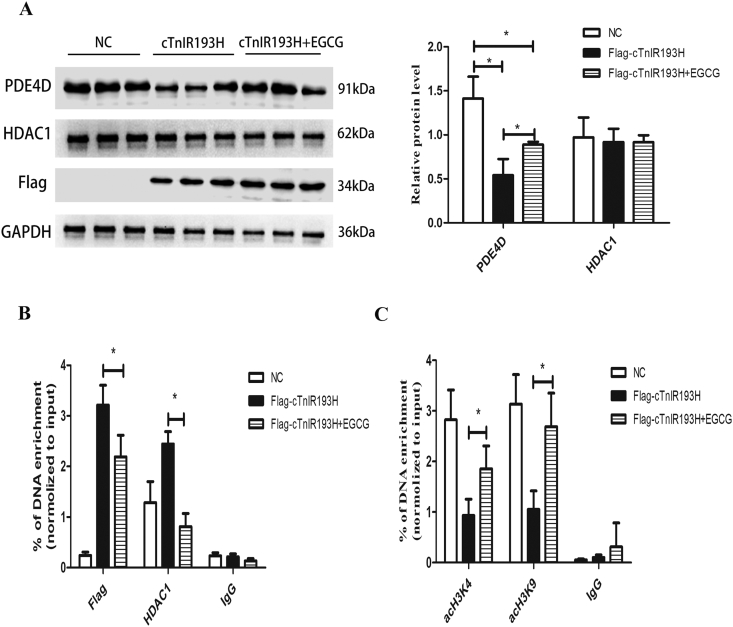
To directly verify the regulatory effects of HDAC1 on PDE4D, primary neonatal cardiomyocytes overexpressing HDAC1 were cultured in vitro. As shown in Fig. 4A, PDE4D expression was reduced in HDAC1 overexpression group compared with NC group. The enrichment of HDAC1 in the promoter region of PDE4D was increased, and acH3K4 and acH3K9 levels were decreased in HDAC1 overexpression group compared with NC group (Fig. 4B), indicating negative regulatory effect of HDAC1 on PDE4D expression and confirming that cTnIR193H could downregulate PDE4D via HDAC1 induced histone deacetylation modifications.
Figure 4.
HDAC1 represses PDE4D expression via histone deacetylation. Primary neonatal cardiomyocytes overexpressing HDAC1 were used for these experiments. (A) PDE4D protein level in blank group, NC group, and HDAC1 overexpression group (OE-HDAC1). (B) ChiP assays for detecting the enrichment of HDAC1, acH3K4 and acH3K9 among the three groups. The results are expressed as mean ± SD. Statistical significance was determined by ANOVA followed by LSD tests. *P < 0.05 as relative to NC group; n = 6 per group.
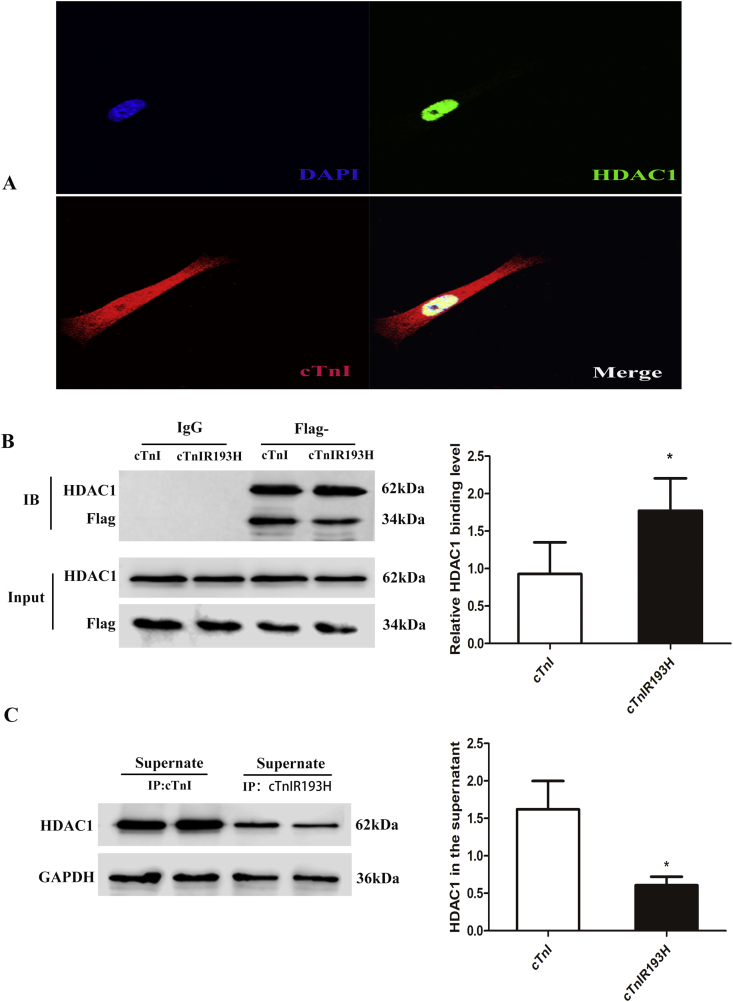
cTnIR193H mutant showed increased binding to HDAC1 compared with cTnI
To understand the molecular mechanism for cTnIR193H to enhance the enrichment of HDAC1, our strategy was to identify cTnIR193H-HDAC1 interacting strength, because we previously confirmed that HDAC1 is a cTnI interacting protein.18 cTnI and HDAC1 coexisting in nuclei of primary neonatal cardiomyocytes also indicated their potential interaction (Fig. 5A). Then we compared the interactions between HDAC1 and cTnI or cTnIR193H in primary neonatal cardiomyocytes with overexpression of cTnI or cTnIR193H. As shown in Fig. 5B, both cTnI and cTnIR193H could interact with HDAC1, but the ratio of immunoprecipitated-HDAC1 to -cTnIR193H was higher than that of immunoprecipitated-HDAC1 to -cTnI, indicating increased binding of cTnIR193H mutant to HDAC1. The content of HDAC1 that are not immunoprecipitated in the supernatant were decreased in cTnIR193H group compared with cTnI group, which could confirm the conclusion from the reverse side (Fig. 5C). These data could explain why cTnIR193H causes an increase in HDAC1 at the promoter region of PDE4D.
Figure 5.
cTnIR193H mutant shows increased binding to HDAC1 compared with cTnI. (A) Immunofluorescence shows cTnI and HDAC1 coexist in the nuclei of primary neonatal cardiomyocytes. (B) Co-IP assays for detecting the interaction between HDAC1 and cTnI or cTnIR193H in Primary neonatal cardiomyocytes overexpressing cTnI or cTnIR193H. The ratio of immunoprecipitated-proteins reflecting the interaction strength shows with column graph. (C) Western blotting for detecting the free HDAC1 in the supernatant of the cells overexpressing cTnI or cTnIR193H. The results are expressed as mean ± SD. Statistical significance was determined by ANOVA followed by LSD tests. *P < 0.05 as compared with each other; n = 6 per group.
EGCG alleviates PDE4D low expression via inhibiting cTnIR193H-HDAC1 interactions
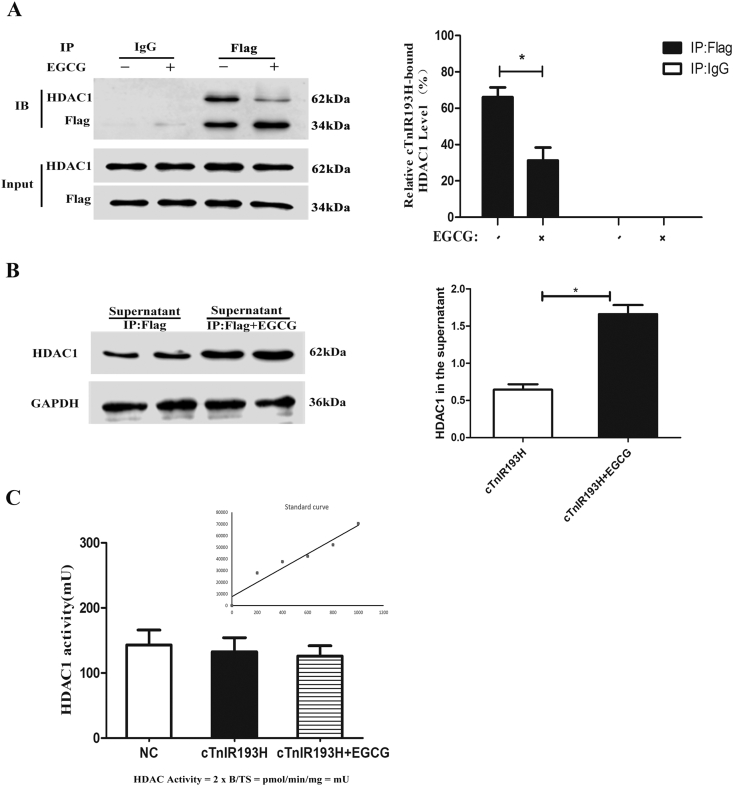
EGCG was proved to be HDAC1 inhibitor, we examined if EGCG treatment could alleviate the cTnIR193H-HDAC1-induced decrease of PDE4D. We again observed reduced PDE4D expression by cTnIR193H in primary neonatal cardiomyocytes with overexpression of cTnIR193H, while EGCG treatment significantly relieved the low expression of PDE4D induced by cTnIR193H (Fig. 6A). We then examined the enrichment level of cTnIR193H and HDAC1 in the PDE4D promoter. The results showed that EGCG treatment could reduce the enrichment of cTnIR193H and HDAC1 and the subsequent improvement of acH3K4 and acH3K9 were observed compared to cTnIR193H group (Fig. 6B). We still examined the interaction between cTnIR193H and HDAC1 with EGCG treatment. We observed the attenuation in strength of cTnIR193H-HDAC1 interaction after EGCG treatment (Fig. 7A and B). We also detect HDAC1 activity in these cells with or without EGCG treatment. However, no significant differences in HDAC1 activity were observed in NC, cTnIR193H and cTnIR193H + EGCG groups (Fig. 7C), suggesting that cTnIR193H do not affect HDAC1 activities to regulate PDE4D expression.
Figure 6.
EGCG alleviates cTnIR193H induced PDE4D low expression. Primary neonatal cardiomyocytes overexpressing cTnIR193H with or without EGCG treatment were used for these experiments. (A) PDE4D protein level in NC group, cTnIR193H group and cTnIR193H + EGCG group. (B) ChiP assays for detecting the enrichment of cTnIR193H and HDAC1 with or without EGCG treatment. (C) ChiP assays for detecting the enrichment of acH3K4 and acH3K9 with or without EGCG treatment. The results are expressed as mean ± SD. Statistical significance was determined by ANOVA followed by LSD tests. *P < 0.05; n = 6 per group.
Figure 7.
EGCG inhibits cTnIR193H-HDAC1 interactions but not HDAC1 activity. Primary neonatal cardiomyocytes overexpressing cTnIR193H with or without EGCG treatment were used for these experiments. (A) CoIP assays for detecting the interaction between HDAC1 and cTnIR193H in the presence and absence of EGCG, and column graph for the ratio of immunoprecipitated-proteins reflecting the interaction strength. (B) Western blot assays for detecting the free HDAC1 in the supernatant. (C) HDAC1 activities. The results are expressed as mean ± SD. Statistical significance was determined by ANOVA followed by LSD tests. *P < 0.05; n = 6 per group.
Discussion
Cardiomyopathies represent a heterogeneous group of diseases and its genetic basis remains extremely unknown because of the phenotypic variation caused by mutations in myofibril genes. In recent years, epigenetic regulation has been shown to be related to cardiomyopathies, including hypertrophic, dilated, and diabetic cardiomyopathies, for example, mutations in cTnT could cause dilated cardiomyopathy via epigenetic mechanisms that underlie the compromised β-adrenergic signaling.17 Like cTnT, cTnI is well known as a thin filament component binding to actin-tropomyosin and inhibiting the troponin complex to regulate muscle contraction.20 Although the nuclear translocation of cTnI or cTnI mutant was observed,21, 22, 23, 24, 25 their nuclear functional implication is not understood. We previously wonder that PDE4D reduction might be associated with HDAC1 in RCM mice with cTnIR193H mutations. In this study, we firstly verified this hypothesis via overexpressing HDAC1 in primary cultured cardiomyocytes, and found down-regulation on PDE4D expression, with increase in HDAC1 enrichment at the PDE4D promoter and decreases in acH3K4 and acH3K9 levels. These results confirmed the epigenetic role of HDAC1 in PDE4D regulation in cardiomyocytes. However, it is unclear whether and how cTnIR193H, the main causative factor for RCM, can lead to reduction on PDE4D expression. Therefore, we overexpressed cTnIR193H mutant and wild type cTnI in primary cultured cardiomyocytes. We found both PDE4D mRNA and protein decreased in cTnIR193H cardiomyocytes but cTnI could just enhance PDE4D mRNA expression. These data strongly suggest that cTnIR193H could reduce PDE4D expression but the mechanisms remain unclear. We believe the expression level of HDAC1 had no effect in this progress because cTnIR193H did not affect HDAC1 expression in this study. Since cTnI could enter cardiomyocyte nuclei, we wonder whether cTnIR193H mutant is more likely to enter the nuclei and affect PDE4D expression. However, our western blotting results showed the same nuclear localization of cTnIR193H mutant and wild type cTnI, suggesting the content of nuclear cTnIR193H mutant is not the key factor to negatively regulate PDE4D expression. Since we observed the transcriptional activity of cTnI, we wonder whether cTnI or cTnIR193H mutant affects PDE4D expression by binding to the promoter region of PDE4D. ChiP assays showed that both cTnI and cTnIR193H could bind to −47∼-141bp regions of PDE4D promoter but with no difference in their binding levels, suggesting that the binding level of cTnIR193H to PDE4D promoter was not the key factor to regulate PDE4D expression.
Histone acetylation is a dynamic process and is mediated by histone acetylase and HDACs. HDAC1 can regulate most of the observed changes in histone acetylation, especially the acH3K9.26, 27, 28 Our data demonstrated that the enrichment of HDAC1 in −47∼-141bp regions of PDE4D promoter was increased in cardiomyocytes overexpressing cTnIR193H mutant, and the levels of acH3K4 and acH3K9, markers for gene activation, were decreased, suggesting cTnIR193H might down-regulate PDE4D via HDAC1 induced deacetylation of H3K4 and H3K9 in the PDE4D promoter regions. However, a question that still remains unclear is how cTnIR193H mutant could affect HDAC1 binding to PDE4D promoter regions. Considering the confirmed protein interaction of cTnI and HDAC1, we asked whether cTnIR193H mutant induces stronger interactions with HDAC1. Co-IP assays demonstrated that compared with cTnI, cTnIR193H is more likely to interact with HDAC1 with stronger interaction between cTnIR193H and HDAC1, which might enhance the enrichment of HDAC1 in the promoter regions of PDE4D leading to hypoacetylation of H3K4 and H3K9 and PDE4D reduction.
EGCG has been shown to have multiple effects on cardiovascular diseases.29,30 It also plays a considerable role in epigenetic regulation. EGCG can inhibit class I histone deacetylases to increase levels of acetylated histone and inhibit DNA methyltransferase.31, 32, 33 Furthermore, EGCG can inhibit HDAC1 expression and activities in aging heart,34 and improve cardiac diastolic dysfunction in RCM mice with cTnIR193H mutation.35 In this study, we select EGCG as an inhibitor of HDAC1 to detect whether EGCG reverses or alleviates cTnIR193H induced PDE4D reduction. Our data showed that EGCG treatment could alleviate the reduction of PDE4D induced by cTnIR193H mutant. Enrichment of HDAC1 in the PDE4D promoter regions was reduced and acH3K4 and acH3K9 levels were elevated after EGCG treatment. However, in our study, EGCG had no effects on HDAC1 expression and activity, indicating other mechanisms for EGCG to improve PDE4D expression exist. Finally, Co-IP assays showed the strength of interactions between cTnIR193H and HDAC1 was diminished after EGCG treatment, which could explain why EGCG improved the reduction of PDE4D caused by cTnIR193H. The mechanisms for EGCG to inhibit the interactions between cTnIR193H and HDAC1 still need to be studied.
Conclusion
Our present work revealed a novel role of cTnI and its mutant in epigenetic regulation. Our data demonstrated that cTnIR193H mutants can down-regulate PDE4D expression via protein interactions with HDAC1 and recruiting HDAC1 to the promoter regions of PDE4D and then reduce acetylation of H3K4 and H3K9. It is still unclear whether cTnI or HDAC1 could bind to the PDE4D promoter alone, or the binding to the promoter requires their own complex forms. Although PDE4D defects or inhibition could accelerate heart relaxation frequencies and exacerbate diastolic dysfunction,36 it should be pointed out that the exact roles of PDE4D in etiology of RCM remain unclear. However, our novel findings can provide us with new research directions to elucidate the pathogenesis of RCM or other inherited cardiomyopathies caused by cTnI mutations.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
This study was supported by research grants from National Natural Science Foundation of China [grant number 81700214].
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.01.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Y., Charles P.Y., Nan C. Correcting diastolic dysfunction by Ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol. 2010;49(3):402–411. doi: 10.1016/j.yjmcc.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ligi I., Fraisse A., Chabrol B. Restrictive cardiomyopathy due to myofibrillar myopathy. Arch Pediatr. 2003;10(5):432–435. doi: 10.1016/s0929-693x(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 3.Palka P., Lange A., Ward C. A fatal case of idiopathic restrictive cardiomyopathy. Cardiol Young. 2003;13(5):469–471. [PubMed] [Google Scholar]
- 4.Russo L.M., Webber S.A. Idiopathic restrictive cardiomyopathy in children. Heart. 2005;91(9):1199–1202. doi: 10.1136/hrt.2004.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Zhang L., Pacciulli D. Restrictive cardiomyopathy caused by troponin mutations: application of disease animal models in translational studies. Front Physiol. 2016;7 doi: 10.3389/fphys.2016.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dadson K., Hauck L., Billia F. Molecular mechanisms in cardiomyopathy. Clin Sci (Lond) 2017;131(13):1375–1392. doi: 10.1042/CS20160170. [DOI] [PubMed] [Google Scholar]
- 7.Dvornikov A.V., Smolin N., Zhang M., Martin J.L., Robia S.L., de Tombe P.P. Restrictive cardiomyopathy troponin I R145W mutation does not perturb myofilament length-dependent activation in human cardiac sarcomeres. J Biol Chem. 2016;291(41):21817–21828. doi: 10.1074/jbc.M116.746172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Zhang L., Jean-Charles P.Y. Dose-dependent diastolic dysfunction and early death in a mouse model with cardiac troponin mutations. J Mol Cell Cardiol. 2013;62:227–236. doi: 10.1016/j.yjmcc.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen Y., Xu Y., Wang Y., Pinto J.R., Potter J.D., Kerrick W.G. Functional effects of a restrictive-cardiomyopathy-linked cardiac troponin I mutation (R145W) in transgenic mice. J Mol Biol. 2009;392(5):1158–1167. doi: 10.1016/j.jmb.2009.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson I.M., Li M.X., Sykes B.D. Solution structure of human cardiac troponin C in complex with the green tea polyphenol, (-)-epigallocatechin 3-gallate. J Biol Chem. 2009;284(34):23012–23023. doi: 10.1074/jbc.M109.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Zhang Z., Wu G. Green tea extract catechin improves internal cardiac muscle relaxation in RCM mice. J Biomed Sci. 2016;23(1):51. doi: 10.1186/s12929-016-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Nan C., Chen Y. Calcium desensitizer catechin reverses diastolic dysfunction in mice with restrictive cardiomyopathy. Arch Biochem Biophys. 2015;573:69–76. doi: 10.1016/j.abb.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Wen Y., Pinto J.R., Gomes A.V. Functional consequences of the human cardiac troponin I hypertrophic cardiomyopathy mutation R145G in transgenic mice. J Biol Chem. 2008;283(29):20484–20494. doi: 10.1074/jbc.M801661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rindler T.N., Hinton R.B., Salomonis N., Ware S.M. Molecular characterization of pediatric restrictive cardiomyopathy from integrative genomics. Sci Rep. 2017;7 doi: 10.1038/srep39276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asrih M., Steffens S. Emerging role of epigenetics and miRNA in diabetic cardiomyopathy. Cardiovasc Pathol. 2013;22(2):117–125. doi: 10.1016/j.carpath.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Haas J., Frese K.S., Park Y.J. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol Med. 2013;5(3):413–429. doi: 10.1002/emmm.201201553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H., Lee J., Vincent L.G. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell. 2015;17(1):89–100. doi: 10.1016/j.stem.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W., Wu X., Wang Z. Epigenetic regulation of phosphodiesterase 4d in restrictive cardiomyopathy mice with cTnI mutations. Sci China Life Sci. 2019;63(4):563–570. doi: 10.1007/s11427-018-9463-9. [DOI] [PubMed] [Google Scholar]
- 19.Sreejit P., Kumar S., Verma R.S. An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim. 2008;44(3-4):45–50. doi: 10.1007/s11626-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 20.Tobacman L.S. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- 21.Asumda F.Z., Chase P.B. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation. 2012;83(3):106–115. doi: 10.1016/j.diff.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann O., Zdunek S., Alkass K., Druid H., Bernard S., Frisén J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res. 2011;317(2):188–194. doi: 10.1016/j.yexcr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Kajstura J., Urbanek K., Perl S. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107(2):305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sahota V.K., Grau B.F., Mansilla A., Ferrús A. Troponin I and Tropomyosin regulate chromosomal stability and cell polarity. J Cell Sci. 2009;122(Pt 15):2623–2631. doi: 10.1242/jcs.050880. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H., Huang H., Ji Z. A double heterozygous mutation of TNNI3 causes hypertrophic cardiomyopathy in a han Chinese family. Cardiology. 2016;133(2):91–96. doi: 10.1159/000440877. [DOI] [PubMed] [Google Scholar]
- 26.Loponte S., Segré C.V., Senese S. Dynamic phosphorylation of Histone Deacetylase 1 by Aurora kinases during mitosis regulates zebrafish embryos development. Sci Rep. 2016;6 doi: 10.1038/srep30213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Večeřa J., Bártová E., Krejčí J. HDAC1 and HDAC3 underlie dynamic H3K9 acetylation during embryonic neurogenesis and in schizophrenia-like animals. J Cell Physiol. 2018;233(1):530–548. doi: 10.1002/jcp.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q., Li S., Li N. miR-34a targets HDAC1-regulated H3K9 acetylation on lipid accumulation induced by homocysteine in foam cells. J Cell Biochem. 2017;118(12):4617–4627. doi: 10.1002/jcb.26126. [DOI] [PubMed] [Google Scholar]
- 29.Chu C., Deng J., Man Y., Qu Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res Int. 2017;2017 doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eng Q.Y., Thanikachalam P.V., Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. 2018;210:296–310. doi: 10.1016/j.jep.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Yuan Y.Y., Meeran S.M., Tollefsbol T.O. Synergistic epigenetic reactivation of estrogen receptor-α (ERα) by combined green tea polyphenol and histone deacetylase inhibitor in ERα-negative breast cancer cells. Mol Cancer. 2010;9 doi: 10.1186/1476-4598-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandakumar V., Vaid M., Katiyar S.K. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32(4):537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakur V.S., Gupta K., Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41(1):353–361. doi: 10.3892/ijo.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan B., Quan J., Liu L. Epigallocatechin gallate reverses cTnI-low expression-induced age-related heart diastolic dysfunction through histone acetylation modification. J Cell Mol Med. 2017;21(10):2481–2490. doi: 10.1111/jcmm.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean-Charles P.Y., Li Y.J., Nan C.L., Huang X.P. Insights into restrictive cardiomyopathy from clinical and animal studies. J Geriatr Cardiol. 2011;8(3):168–183. doi: 10.3724/SP.J.1263.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beca S., Helli P.B., Simpson J.A. Phosphodiesterase 4D regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ Res. 2011;109(9):1024–1030. doi: 10.1161/CIRCRESAHA.111.250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.